Abstract
Tetanus neurotoxin (TeNT) is an exotoxin produced by Clostridium tetani that causes paralytic death to hundreds of thousands of humans annually. TeNT cleaves vesicle-associated membrane protein-2, which inhibits neurotransmitter release in the central nervous system to elicit spastic paralysis, but the molecular basis for TeNT entry into neurons remains unclear. TeNT is a ∼150-kDa protein that has AB structure-function properties; the A domain is a zinc metalloprotease, and the B domain encodes a translocation domain and C-terminal receptor-binding domain (HCR/T). Earlier studies showed that HCR/T bound gangliosides via two carbohydrate-binding sites, termed the lactose-binding site (the “W” pocket) and the sialic acid-binding site (the “R” pocket). Here we report that TeNT high affinity binding to neurons is mediated solely by gangliosides. Glycan array and solid phase binding analyses identified gangliosides that bound exclusively to either the W pocket or the R pocket of TeNT; GM1a bound to the W pocket, and GD3 bound to the R pocket. Using these gangliosides and mutated forms of HCR/T that lacked one or both carbohydrate-binding pocket, gangliosides binding to both of the W and R pockets were shown to be necessary for high affinity binding to neuronal and non-neuronal cells. The crystal structure of a ternary complex of HCR/T with sugar components of two gangliosides bound to the W and R supported the binding of gangliosides to both carbohydrate pockets. These data show that gangliosides are functional dual receptors for TeNT.
Tetanus is an acute, often fatal disease of humans that was first described by Hippocrates over 24 centuries ago (1). Tetanus is characterized by generalized increased rigidity and convulsive spasms of skeletal muscles. Tetanus is caused by exposure to tetanus neurotoxin (TeNT)3 produced by the spore-forming bacterium Clostridium tetani. TeNT is delivered from the bloodstream to the peripheral nervous system, from where TeNT traffics to the central nervous system to cleave vesicle-associated membrane protein-2 (VAMP2), which inhibits neurotransmitter release and elicits spastic paralysis (2). Although prevented by vaccination, tetanus is responsible for hundreds of thousands of deaths per year in countries where vaccination is not common (3).
TeNT is produced as a ∼150-kDa protein that is cleaved to a di-chain protein, comprising an N-terminal light chain (∼50 kDa) and a C-terminal heavy chain domain (∼100 kDa) linked through a single disulfide bond (4). TeNT light chain is a zinc metalloprotease that cleaves the neuronal SNARE protein VAMP2 (2). The TeNT heavy chain contains two functional domains: a translocation domain and a C-terminal receptor-binding domain (HCR/T, ∼50 kDa).
The first step in TeNT action involves binding to a receptor(s) on the presynaptic membrane of α-motor neurons. Although the molecular basis for TeNT entry remains undetermined, an unambiguous role for gangliosides has been demonstrated (5–9). Current models implicate a dual receptor mechanism for the binding of the clostridial neurotoxins to neurons, which includes a ganglioside-binding component (10). Complex gangliosides are sialic acid-containing glycosphingolipids that are located on the outer leaflet of cell membranes and contain a common “core” (GA1) consisting of Gal(β1–3)GalNAc(β1–4)Gal(β1–4)Glc(β1–1)Cer to which one or more N-acetylneuraminic acids (sialic acids) are bound, yielding “a” and “b” series gangliosides (11, 12). Numerous structural and biochemical studies have established that HCR/T contains two carbohydrate-binding sites: a lactose-binding site and a sialic acid-binding site (13). Previous studies showed that Trp1289 is the key residue for the lactose-binding site, and Arg1226 is the key residue for the sialic acid-binding site (14). In this study, we denote the lactose-binding site as the “W” pocket and the sialic acid-binding site as the “R” pocket. Binz and co-workers (14) showed that functional R and W binding sites were required for TeNT toxicity (7). These biochemical and cellular studies were supported by a co-crystal structure of HCR/T bound to a GT1b-β anomer analog, which showed that the W and R carbohydrate-binding pockets were located at different regions of TeNT (7). We recently reported that the W pocket binds gangliosides via the GA1 core structure, whereas the R pocket binds gangliosides via di- or tri-sialic acid moieties (15) where simultaneous binding of TeNT to two gangliosides was synergistic (see Fig. 1a). In the current study, gangliosides were identified that bound exclusively to either the W pocket or R pocket, which allowed the characterization of the role of ganglioside binding to the W and R pockets as dual receptors for TeNT entry into neurons.
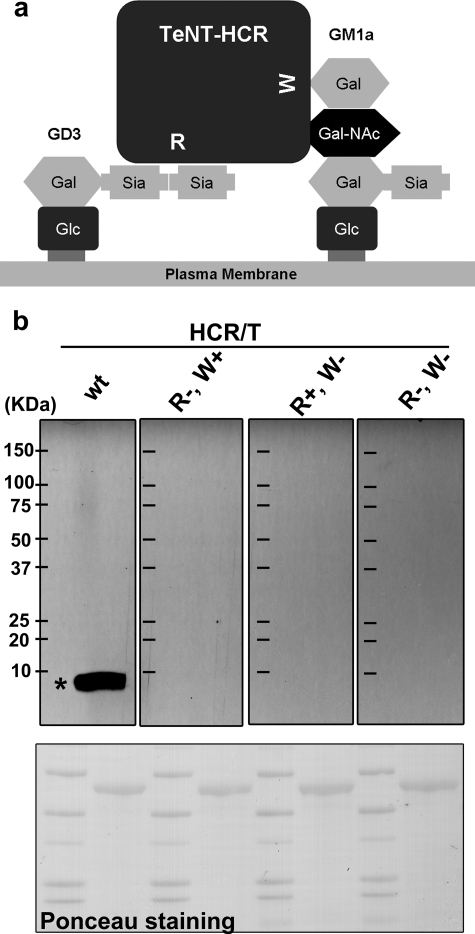
FIGURE 1.
Interaction of the HCR domain of TeNT with its putative cellular receptor. a, HCR/T has two ganglioside-binding sites. The W pocket binds to the terminal GalNAc-Gal of the ganglioside (illustrated by GM1a). The R pocket binds to the di-sialic acid of the ganglioside (illustrated by GD3). b, alternating lanes of molecular mass marker proteins and cortical neuron lysates were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was stained for protein with Ponceau S (bottom panel), and then the membrane strips were incubated with 10 nm of the indicated HCR/T (HCR/T wild type (wt), HCR/T (R+, W−), HCR/T (R−, W+), or HCR/T (R−, W−)) followed by HRP-conjugated α-FLAG antibody. The bands were visualized with SuperSignal; exposed film is shown (upper panel). The asterisk denotes the position of purified gangliosides resolved under identical conditions. Migration of the molecular mass marker proteins is indicated (kDa) in the left-most lane in the upper panel.
MATERIALS AND METHODS
HCR Expression and Purification
3×FLAG-tagged wild type and mutated forms of tetanus receptor-binding domain (HCR/T) were expressed and purified as described previously (15).
Protein-Glycan Interaction Screen
HCRs were labeled with Alexa 488 succinimidyl ester as described by the manufacturer (Invitrogen). Labeled HCRs were screened by core H of the Consortium for Functional Glycomics using array version 3.1. Proteins (∼200 μg/ml for wild type, for R−, W+, and for R+, W−; ∼100 μg/ml for R−, W−) were bound to the matrix in 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 2 mm CaCl2, 2 mm MgCl2, 0.05% Tween 20, and 1% bovine serum albumin (16). Solid phase HCR binding to ganglioside GD3 was performed as described previously (15).
Far Western Analysis
Rat E18 cortical neurons (BrainBits) were cultured for 4 weeks, washed, and lysed. Clarified lysates and protein molecular weight markers (Bio-Rad) were subjected to gradient SDS-PAGE, transferred to polyvinylidene difluoride membrane, and stained for protein with Ponceau S (Sigma). The membrane was cut into strips and incubated with 10 nm of the indicated HCR/T bound to HRP-conjugated α-FLAG antibody. The membranes were washed, incubated with SuperSignal (Pierce), and exposed to x-ray film.
HCR/T Binding to Cultured Cells
Gangliosides GD3, GM1a, or GT1b (100 μg/ml) were sonicated in serum-free medium supplemented with 50 nm nonfat bovine serum albumin (Sigma) for 20 min and then applied to Neuro2a (CCL-131), PC12 (CRL-1721), or HeLa (CCL-2) cells (ATCC) for 24 h. The cells were washed and incubated with 100 nm HCR and 10 μg/ml of Alexa 488-Transferrin at 37 °C for 30 min. The cells were washed and permeabilized, and bound HCR was detected by immunofluorescence (15). PC12 cells were also cultured with 25 μm d,l-threo-l-phenyl-2-hexadecanoylamino-3-morpholino-propanol (PPMP; Sigma) for 48 h when gangliosides were added for an additional 24 h with PPMP. The cells were washed and incubated with 100 nm HCR and 10 μg/ml of Alexa 488-Transferrin at 37 °C for 30 min. HCR binding and internalization was visualized by immunofluorescence using anti-FLAG antibody (15). A schematic of several gangliosides described in this study are shown in Fig. 2.
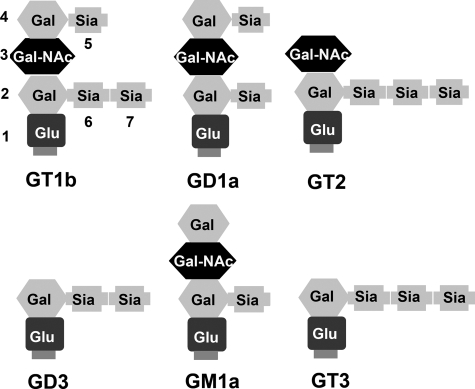
FIGURE 2.
Schematic of gangliosides described in the study. Gangliosides are composed of ceramide (not shown, but located at the bottom of each structure) linked by a glycosidic bond to an oligosaccharide chain containing hexoses and N-acetylneuraminic acid(s) (sialic acid). Sia, sialic acid; Glu, glucose; Gal, galactose; Gal-NAc, N-acetylgalactosamine.
TeNT Cleavage of VAMP2 in PC12 Cells
PC12 cells were incubated alone or with 100 μg/ml gangliosides for 24 h. The cells were washed and incubated with 30 nm TeNT for 48 h. The cells were washed with phosphate-buffered saline and lysed with radioimmune precipitation assay buffer (Sigma). The cell lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and probed for VAMP2, using mouse α-Syb2 (clone 69.1, Synaptic Systems) and goat α-mouse IgG-HRP and SuperSignal (15).
Statistical Analysis
HCR binding to cultured cells alone or in the presence of added gangliosides was quantified by two-tailed Student's t test, p values <0.05 at the 95% confidence level are indicated by two asterisks (see Fig. 7) for lined analyses.
FIGURE 7.
Gangliosides reconstitute entry of HCR/T into PC12 cells treated with the glycosphingolipids synthesis inhibitor PPMP. a, PC12 cells were incubated with 25 μm PPMP for 48 h and then loaded with the indicated ganglioside(s) in the presence of PPMP for an additional 24 h. The cells were washed and incubated with 100 nm HCR/T and 10 μg/ml Alexa Fluor 488-Transferrin (Tfn) at 37 °C. Cell-associated HCR was visualized as described above. b, fluorescence was quantified in 20 random fields, averaged, and presented as a fluorescence intensity ratio of bound HCR/Transferrin with standard deviation. Background fluorescence intensities in the absence of exogenous ganglioside loading were subtracted from the ganglioside treated groups. Two-tailed Student's t test, p values <0.05 at the 95% confidence level are indicated by (**) for lined analyses.
HCR/T Binding to Proteinase K-treated PC12 Cells
PC12 cells were incubated with 100 μg/ml GT1b for 24 h. The cells were washed and suspend in Dulbecco's phosphate buffered saline (without calcium and magnesium). The cells were then pellet and treated with or without 4 units of proteinase K (Sigma) for 30 min at 4 °C in serum-free medium. The cells were washed thoroughly with 10 ml of cold Dulbecco's phosphate buffered saline four times and then incubated with 100 nm HCR/T or HCR/T (R−, W−) for 1 h at 4 °C in serum-free medium. The cells were lysed in radioimmune precipitation assay buffer after two washes. Cell lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and probed for FLAG epitope using HRP-conjugated anti-FLAG antibody (Sigma), anti-epidermal growth factor receptor antibody (Clone sc-3; Santa Cruz Biotechnology), and anti-β actin antibody (clone AC-74; Sigma).
Crystallization of HCR/T
Purified HCR/T was dialyzed against 20 mm Tris-HCl buffer, pH 7.9, containing 100 mm NaCl and was concentrated to a final concentration of 10 mg/ml. The concentrated protein solution was incubated with the carbohydrate moiety of GT2 at 1:8 molar ratio (hereafter referred to as GT2) for several hours prior to the crystallization setup. The crystals were obtained by vapor diffusion using the hanging drop method. Hanging drops containing 2 μl each of protein solution (0.1 mm HCR/T and 0.8 mm GT2) and well solution (100 mm bis(trispropane) buffer, pH 7.0, 25% polyethylene glycol 2000 and 300 mm ammonium sulfate) were equilibrated against 0.5 ml the well solution at 19 °C. The HCR/T crystals belong to the orthorhombic space group P212121 with cell dimensions of a = 67.0, b = 72.7, and c = 119.1 Å. There is one monomer/asymmetric unit with a Matthews coefficient of 2.9 Å3/Da. For the ternary complex of HCR/T with GT2 and lactose, the co-crystals of HCR/T with GT2 were soaked in 80 mm lactose for 4 h prior to data collection. Both data sets were collected at −175 °C using an in-house R-AXIS IV++. HKL2000 (17) was used for data processing (Table 1).
TABLE 1.
Data collection and refinement statistics
| Data collection | HCR/T-GT2 | HCR/T-GT2-Lactose |
| Resolution/highest resolution shell | 27.2-2.0/2.07-2.0 (Å) | 30-2.1/2.18-2.10 (Å) |
| No. of total reflections | 276,437 | 238,739 |
| No. of unique reflections | 39,755/3,848 | 34,207/3,289 |
| Redundancy | 7.0/6.4 | 7.0/6.2 |
| Completeness (%) | 99.2/97.7 | 98.2/96.3 |
| No. of crystals used | 1 | 1 |
| I/σ(I) | 30.0/3.6 | 17.8/2.6 |
| Cell dimensions (Å) a, b, c | 67.0, 72.7, 119.1 | 67.0, 72.7, 119.4 |
| Space group | P212121 | P212121 |
| Rmerge | 0.076/0.517 | 0.109/0.619 |
| Vm, solvent content | 2.9 Å3/Da, 56% | 2.9 Å3/Da, 56% |
| Monomer in an asymmetric unit | 1 | 1 |
| Refinement | ||
| No. of protein atoms | 3575 | 3575 |
| No. of GT2 atoms | 61 (3 sialic acids) | 41 (2 sialic acids) |
| No. of lactose atoms | 12 (galactose) | |
| No. of water molecules | 371 | 192 |
| B average of main chain atoms (Å2) | 25.1 | 26.8 |
| B average of side chain (Å2) | 26.9 | 28.5 |
| B average of GT2 molecule(Å2) | 43.6 | 51.9 |
| B average of lactose molecule (Å2) | 43.1 | |
| B average of water molecules (Å2) | 32.5 | 30.3 |
| Rcrystal | 0.202 | 0.213 |
| Rfree | 0.236 | 0.256 |
| Root mean square deviation | ||
| Bond length (Å) | 0.006 | 0.006 |
| Bond angle (°) | 1.3 | 1.3 |
The crystal structure of HCR/T was solved by the molecular replacement method with MOLREP within the CCP4 program suite (18) using the data within the resolution range of 20–4.0 Å. The monomer of HCR/T (Protein Data Bank code 1fv2 (7)) was used as the search model. The initial structure from molecular replacement was refined using the program CNS (19). Each round of refinement contained rigid body minimization, positional refinement, and slow cooling simulated annealing protocol beginning at 3000 K and a second positional refinement. The topology and parameter files of GT2 were generated by modifying those of GT1b (Protein Data Bank code 1fv2), and the coordinates of lactose (Protein Data Bank code 1F31) (20) were used to generate its parameter and topology files. Only three sialic acids of the GT2 molecule were visible in the Fo − Fc difference Fourier map at the 2 σ cut-off. The structure was further refined with alternating manual adjustments using the TURBO-FRODO Program package (21). The structure of the ternary complex structure was solved by the difference Fourier method using the binary complex structure as the starting model. Only the galactose moiety of lactose was visible from the Fo − Fc difference map. The structure was refined using CNS and Turbo-Frodo using the same protocol as in the refinement of the binary complex structure. The final models were completed with a crystallographic Rcrystal/Rfree of 0.202/0.236 for the binary complex with GT2 and 0.210/0.279 for the ternary complex with GT2 and lactose. Data collection and refinement statistics are given in Table 1 (Protein Data Bank codes 3HMY for binary complex and 3HN1 for ternary complex).
RESULTS
Dual Carbohydrate-binding Pockets Are Required for High Affinity Binding of HCR/T to Rat Cortical Neuron Lysates
To identify potential cellular receptors for TeNT, a far Western blot analysis was performed using cultured rat cortical neuron lysates. 10 nm HCR/T bound to the cultured rat cortical neuron lysates near the dye front (Fig. 1b), whereas neither of the mutated forms of HCR/T that were deficient in one of the two carbohydrate-binding pockets, HCR/T (R−, W+) or HCR/T (R+, W−), bound, indicating that TeNT utilized both carbohydrate-binding pockets for high affinity binding (Fig. 1b). One hundred nm of the mutated forms of HCR/T did not show detectable binding to the lysate (data not shown). Control experiments showed that gangliosides co-migrated with the site of HCR/T binding to rat cortical neuron lysates by far Western, as indicated by the asterisk in Fig. 1b (wt lane). These data showed that TeNT utilized both carbohydrate-binding pockets to preferentially associate with a low molecular weight molecule within the cultured rat cortical neuron lysates that co-migrated with purified gangliosides (data not shown and Ref. 8).
Wild Type HCR/T Binds GD3 via the R Carbohydrate-binding Pocket
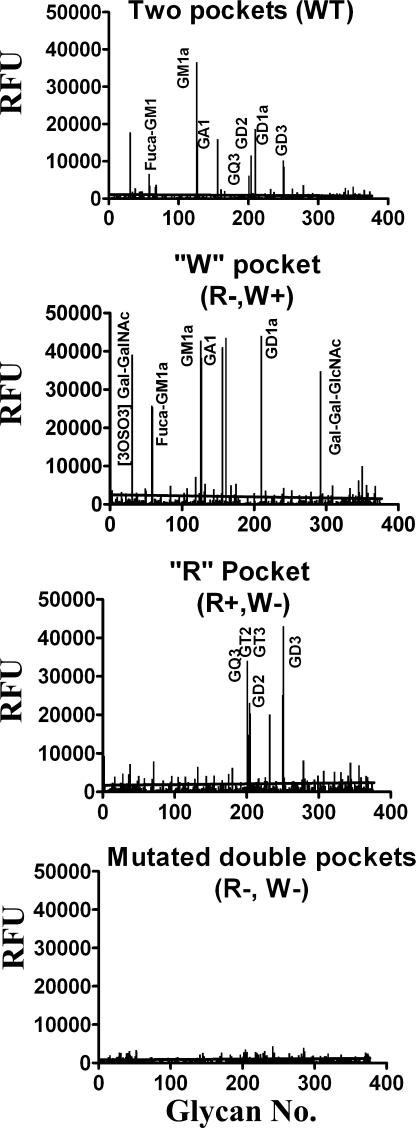
To address the specificity of TeNT binding to gangliosides, HCR/Ts were tested for the ability to bind a glycan array composed of natural and synthetic carbohydrate moieties (Fig. 3, Consortium for Functional Glycomics array version 3.1 containing 377 carbohydrate structures) (16). HCR/T (R−, W+) bound GM1a, GD1a, GA1, and Fuca-GM1a, whereas HCR/T (R+, W−) bound GD3 and GT2. Wild type HCR/T had a composite binding profile relative to HCR/T (R−, W+) and HCR/T (R+, W−). HCR/T (R−, W−) did not bind carbohydrates in the array. This showed that the R pocket of HCR/T bound di- or tri-sialic acid moieties of gangliosides, whereas the W pocket of HCR/T bound the GA1 core of gangliosides. The glycan array version 3.1 does not contain GT1b and GD1b moieties; therefore, GT1b or GD1b were not identified by the array screen. Solid phase ganglioside binding assays confirmed the specificity of the array analysis where GD3 bound only wild type HCR/T and HCR (R+, W−) (Fig. 4). Earlier studies showed that wild type HCR/T and HCR/T (R−, W+) bound GM1a with similar affinities (15). Thus, GM1a binds only the W pocket of HCR/T, whereas GD3 binds only the R pocket of HCR/T, making these gangliosides useful tools to determine the role of the W and R pockets in TeNT binding to neurons. Previous studies also showed the simultaneous binding of two GT1b molecules to TeNT via the R and W binding sites (14), which was supported by the subsequent observation that both GT1b or GD1b can bind independently to the R or W pockets (15). This suggests that the observed binding to these gangliosides represents the simultaneous occupancy of both the W and R pockets of TeNT by two molecules of GT1b or GD1b.
FIGURE 3.
Glycan array analysis of wild type and mutated HCR/T. Alexa Fluor 488-labeled 200 μg/ml HCR/T, HCR/T (R−, W+), HCR/T (R+, W−), and 100 μg/ml HCR/T (R−, W−) were screened on a protein-glycan array version 3.1 by the Consortium for Functional Glycomics. Glycan array version 3.1 contained 377 derivatives, and HCR/T mainly interacts with ganglioside derivatives that are shown on the graph. The complete data sets of these analyses are available at the Consortium for Functional Glycomics website.
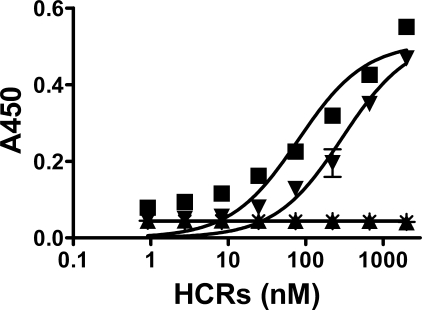
FIGURE 4.
Binding of wild type and mutated HCR/T domains to ganglioside GD3. Immobilized ganglioside GD3 (250 ng/well) was incubated with the indicated HCR domains and assayed for HCR binding as described previously (15). The data were plotted using GraphPad Prism version 5 (GraphPad Software Inc.). ■, HCR/T wild type; ▾, HCR/T (R+, W−); ▴, HCR/T (R−, W+); *, HCR/T (R−, W−).
Occupation of Two Carbohydrate-binding Pockets by Gangliosides Enhances Binding of HCR/T and VAMP2 Cleavage by TeNT in Cultured Neuronal Cells
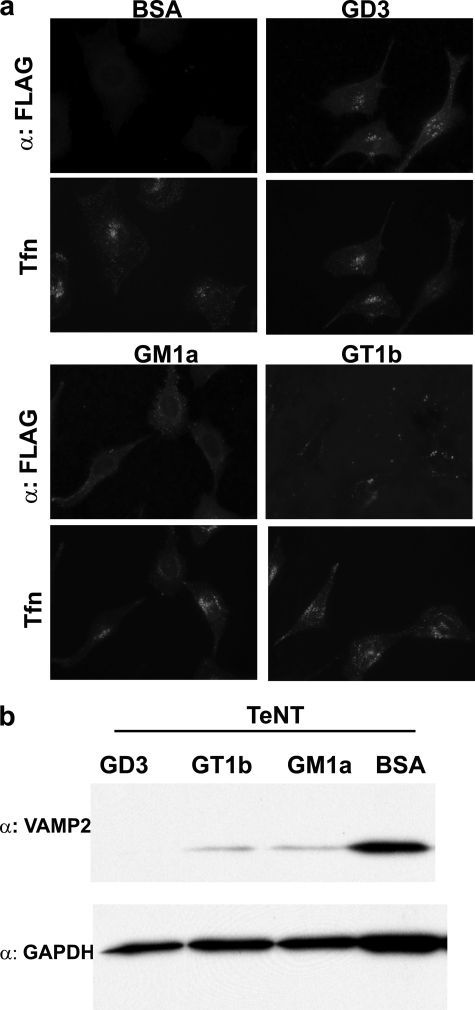
PC12 cells were used to determine whether gangliosides alone mediated functional binding and entry of TeNT. PC12 cells are enriched in b-series gangliosides GD1b, GT1b, and GQ1b and express low levels of a-series ganglioside Fuca-GM1a but do not express GD1a (22, 23). HCR/T did not bind to untreated PC-12 cells but bound PC12 cells preloaded with GD3, GM1a, or GT1b (Fig. 5a). Moreover, PC12 cells loaded with these exogenous gangliosides enhanced the ability of TeNT to cleave VAMP2 (Fig. 5b). This confirmed that exogenous gangliosides GD3, GM1a, or GT1b enhanced the binding of HCR/T and functional entry of TeNT into PC12 cells and that ganglioside composition and amount are a limiting step in the functional entry of TeNT into PC12 cells.
FIGURE 5.
Ganglioside loading of PC12 cells enhances the uptake of TeNT. PC12 cells were loaded with the indicated ganglioside. a, cells were washed and incubated with 100 nm HCR/T and 10 μg/ml of Alexa Fluor 488-Transferrin (Tfn) for 30 min at 37 °C. HCR/T was visualized by immunofluorescence using a mouse α-FLAG antibody followed by Alexa-coupled secondary IgG. b, cells were incubated with 30 nm TeNT for an additional 48 h. VAMP2 cleavage was visualized by Western blotting, using a mouse α-Syb2 antibody, which recognizes only full-length VAMP2. BSA, bovine serum albumin.
The ability of gangliosides to mediate cellular entry of TeNT was extended to Neuro2a neuroblastoma cells. Neuro2a cells are enriched in a-series gangliosides such as GM1a and GD1a but do not express b-series gangliosides such as GD1b and GT1b (24). HCR/T did not bind to untreated Neuro2a cells or cells preloaded with GM1a, a ganglioside that binds only within the TeNT W carbohydrate-binding pocket (supplemental Fig. S1), whereas HCR/T bound and entered Neuro2a cells preloaded with either GD3 or GT1b. Thus, ganglioside enhanced TeNT binding, and uptake into Neuro2a cells required ganglioside binding to both the R and W pockets of TeNT.
Ganglioside-mediated High Affinity Binding to PC12 Cells Is Proteinase K-independent
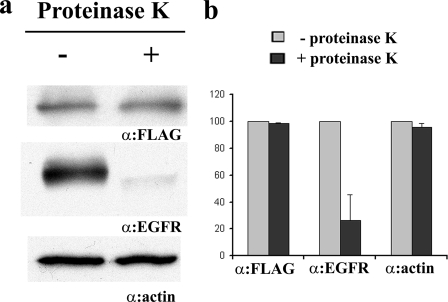
To test whether a protein could be implicated in TeNT binding to neuronal cells, PC12 cells were loaded with exogenous ganglioside GT1b, treated with proteinase K to degrade extracellular protein components, and then tested for the ability to bind HCR/T. Proteinase K treatment reduced surface-bound epidermal growth factor receptor but did not reduce HCR/T binding nor the amount of intracellular actin in the proteinase K-treated cell (Fig. 6). This experiment did not implicate a role of cell surface proteins as a component of ganglioside-mediated high affinity binding of TeNT.
FIGURE 6.
Proteinase K-independent binding of HCR/T on PC12 cells. a, PC12 cells were loaded with 100 μg/ml GT1b for 24 h and treated with or without proteinase K for 30 min at 4 °C. The cells were washed and incubated with 100 nm HCR/T for 1 h at 4 °C. The cells were washed and lysed with radioimmune precipitation assay buffer. HCR/T binding was visualized by Western blot using HRP-conjugated anti-3×FLAG antibody. As control, the extracellular protein epidermal growth factor receptor (EGFR) was visualized by α-epidermal growth factor receptor antibody, and the intracellular protein actin was visualized by α-β actin antibody. b, two independent experiments were quantified using Alpha Innotech Fluorchem system. Immunoblots of the cells treated with proteinase K were presented as a relative level compared with the immunoblots of cells not treated with proteinase K, which were set at 100. The data were presented as a mean with standard error.
Occupation of Two Carbohydrate-binding Pockets by Gangliosides Is Required for High Affinity Binding of HCR/T into PC12 Cells
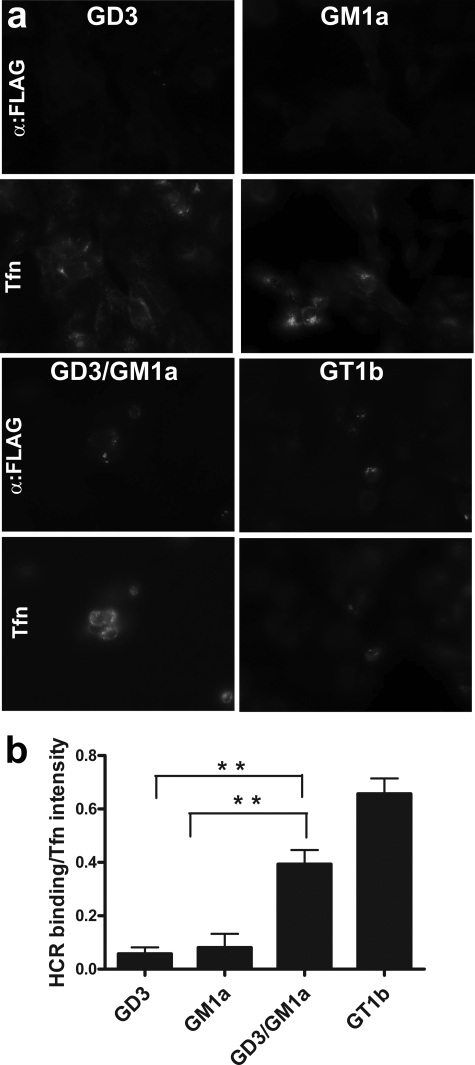
To assess directly the role of gangliosides in TeNT binding to neuronal cells, endogenous gangliosides of PC12 cells were depleted by treatment with the glucosyl ceramide synthase inhibitor PPMP (25). Depletion of endogenous gangliosides was measured as a reduction of cholera toxin B subunit binding, a GM1a-dependent process (26). Cholera toxin B subunit binding to PPMP-treated PC12 cells was rescued by preloading cells with GM1a (supplemental Fig. S2a). Controls showed that exogenous GD3 and GT1b were incorporated into PPMP-treated PC12 cells (supplemental Fig. S2b).
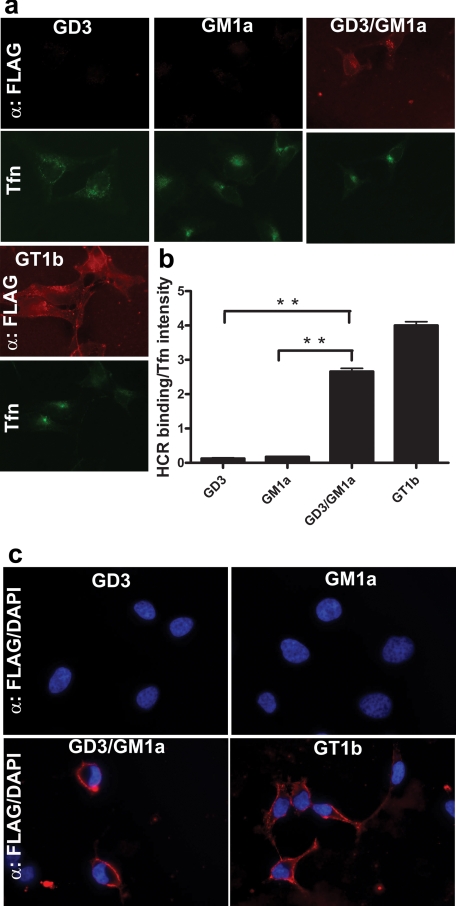
Previous studies showed that GD3 bound the R pocket, and GM1a bound the W pocket, whereas GT1b binds to both carbohydrate pockets of TeNT (15). Thus, preloading PPMP-treated PC12 cells with GD3 and GM1a tested the role of the individual carbohydrate-binding pockets of TeNT in cell binding and entry. Although HCR/T did not bind to PPMP-treated PC12 cells preloaded with either GD3 or GM1a alone, HCR/T bound PPMP-treated PC12 preloaded with a mixture of GD3/GM1a (1:1), showing that HCR/T bound cells with high affinity only in the presence of gangliosides that together can bind both the R and W carbohydrate-binding pockets (Fig. 7). HCR/T also bound with high affinity PPMP-treated PC12 cells that were preloaded with GT1b. This suggests the simultaneous binding of 2 mol of GT1b to TeNT via the R and W pockets (9, 14). Attempts to deplete endogenous gangliosides with PPMP in PC12 cells, reload exogenous gangliosides, and then measure VAMP cleavage were not successful because of PPMP toxicity to PC12 cells during extended incubations (data not shown).
Occupation of Two Carbohydrate-binding Pockets by Gangliosides Mediates HCR/T Binding and Entry into Non-neuronal Cells
HeLa cells contain limited amounts of GM1a and GM3 and lack more complex gangliosides (27). HCR/T did not enter untreated HeLa cells or HeLa cells preloaded with either GD3 or GM1a alone (Fig. 8, a and b) but entered HeLa cells preloaded with either a GD3/GM1a mixture or GT1b alone. At 4 °C, HCR/T bound but remained on the periphery of cells preloaded with a GD3/GM1a mix or GT1b alone (Fig. 8c). This demonstrated that gangliosides mediated binding and entry of TeNT into cells independent of neuron-specific proteins.
FIGURE 8.
Ganglioside-dependent binding of HCR/T to non-neuronal (HeLa) cells. HeLa cells were loaded with the indicated ganglioside. a and c, the cells were washed and incubated with 100 nm HCR/T and 10 μg/ml of Alexa 488-Transferrin (Tfn) either at 37 °C (a) or 4 °C (c). HCR/T was visualized as described above. b, HCR/T fluorescence from a was normalized to transferrin binding, quantified, and processed as described in the legend to Fig. 7 (n = 10).
HCR/T Simultaneously Binds Carbohydrate Components of Gangliosides in the W and R Pockets of TeNT
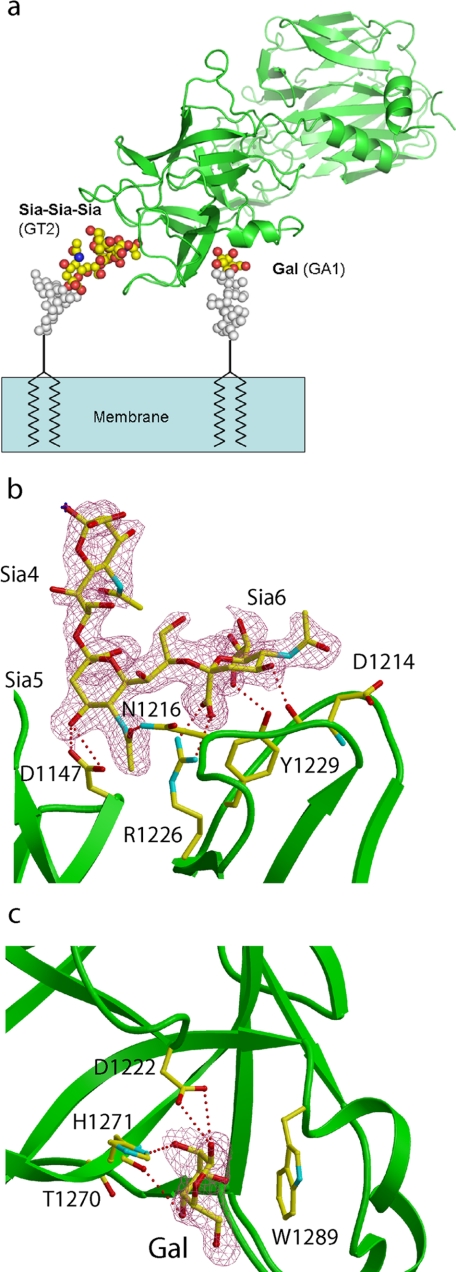
To further substantiate the role of gangliosides in mediating TeNT binding and entry, the ternary complex of structure of HCR/T with the oligosaccharide component of ganglioside GT2 and lactose was determined (Fig. 9a).
FIGURE 9.
Structure of HCR/T in complex with ganglioside GT2 and Lactose. a, proposed binding mode of HCR/T on the membrane surface. The structures of gangliosides GT2 and GA1 were modeled onto the complex of HCR/T using the coordinates of HCR/T bound to a GT2 analog and lactose. The HCR is colored green, GT2 analog and galactose are shown in atom coloring, and modeled sugar moieties are shown in gray. b, electron density map (2Fo − Fc map contoured at 1 σ level) around GT2, overlaid with the final refined model (atom coloring, GT2; green, HCR/T). HCR/T residues binding to GT2 are shown in atom coloring. c, electron density map (2Fo − Fc contoured at 1 σ) around galactose, overlaid with the final refined structure (atom coloring, galactose; green, HCR/T). HCR/T residues binding to galactose are shown in atom coloring.
The GT2-OS binding site is located at the distal tip of the HCR (Fig. 9b). The shallow binding pocket involves residues Asp1147, Asp1214, Asn1216, Arg1226, and Tyr1229 and overlaps with the described previously R pocket involved in HCR/T binding to a GT1b-β anomer analog and the oligosaccharide moiety of GD3 (7, 28). Only three sialic acids (of six sugar residues of GT2) are visible in the structure, and two of them make direct interactions with HCR/T. Sia5 forms hydrogen bonds between its 4-OH and the carboxylate of Asp1147 and Sia5 O-4 and between the amide nitrogen of Asn1216 and the N-acetyl carbonyl of Sia5. Sia6 forms more contacts with the protein than Sia5. The major interaction is a salt bridge formed between the Sia6 carboxylate and Arg1226. This is consistent with previous structural and biochemical studies demonstrating a critical role for this residue in TeNT toxicity. In addition, hydrogen bonds are formed between 4-OH and the carbonyl oxygen of Asp1214 and between 8-OH and the Tyr1229 hydroxyl group. A role for Asp1214 and Asn1216 in TeNT toxicity was implicated previously when a HCR/T deletion construct (HCR/ T-ΔAsp1214–Asn1219) that retained neuronal binding failed to undergo retrograde transport (29).
Crystals of the complex of HCR/T with GT2 were then soaked in a solution of lactose. GT2 was used based upon availability of the glycan component from the Consortium for Functional Glycomics. Density corresponding to the glucose ring was not observed within the complex. In contrast, the hydrophobic face of the galactose moiety was observed to be packed against the indole ring of Trp1289 (distance ∼4 Å). In addition, hydrogen bonds were formed between the galactose hydroxyls and the side chains of His1271 and Asp1222 and the main chain amide group of Thr1270 (Fig. 9c).
DISCUSSION
The requirement for gangliosides as a component of the neuronal receptor(s) for TeNT is well established (9, 10, 29). Although numerous structural and biochemical studies have described the interactions of TeNT with gangliosides in vitro, few studies have addressed ganglioside function in vivo. The current study demonstrates high affinity binding, and entry of TeNT into cultured cells require the simultaneous interaction with two molecules of ganglioside, represented herein by b-series gangliosides for the TeNT R carbohydrate-binding pocket and a-series gangliosides for the TeNT W carbohydrate-binding pocket.
This model is supported by several lines of evidence including the crystal structure of HCR/T in complex with both the sugar moiety of ganglioside GT2 and lactose. The terminal sialic acid moiety of GT2 (Sia6) is coordinated primarily through a salt bridge to Arg1226, which defines the R carbohydrate-binding pocket of TeNT. Similarly, the galactose moiety of lactose is packed against the indole ring of Trp1289, which defines the W carbohydrate pocket of TeNT. The observed interactions are consistent with previous co-crystal structures showing the di-sialic acid moiety of the ganglioside GD3 oligosaccharide bound to the R pocket (28) or lactose binding to the W pocket (13). The demonstration that TeNT can simultaneously bind two oligosaccharides within the carbohydrate-binding pockets is further supported by mass spectroscopy data showing that TeNT simultaneously bound two molecules of GT1b and that mutation within either carbohydrate-binding pocket reduced TeNT toxicity (14). Moreover, the co-crystal structure of HCR/T with a GT1b-β anomer analog demonstrated that ganglioside induced cross-linking two HCR/T molecules by binding to the R site of one molecule and the W site of the other (7). The observed interactions of the two terminal sialic acids of GT2 with R pocket amino acids also explains an earlier observation showing that b-series gangliosides bound more tightly to the R pocket than the a-series ganglioside GD1a, because the terminal di-sialic acids NeuAc(2–8)NeuAc moiety of b-series gangliosides would have more contacts to R pocket amino acids than the terminal NeuAc(2–3)Gal disaccharide of GD1a (15).
Although GT1b mediated HCR/T binding and internalization, GT1b cannot be used to study ganglioside binding to individual carbohydrate-binding pockets of TeNT, because GT1b binds to both the W and R carbohydrate-binding pockets (14). In the current study, GD3 was found to bind only to the R pocket, whereas GM1a binds only to W pocket of TeNT (15). This provided novel reagents to study the role of the individual carbohydrate-binding pockets for TeNT-receptor interactions. This analysis showed that TeNT bound and entered cultured cells only when both carbohydrate-binding pockets were occupied by ganglioside. This is the first study to directly show that TeNT high affinity binding requires both carbohydrate-binding pockets to be occupied by gangliosides.
Studies in mice with a disruption of the α-2,8-sialyltransferase gene (GD3 synthase) displayed an ∼60-fold decrease in sensitivity to TeNT relative to wild type littermates (30), highlighting the key role of b-series gangliosides in TeNT toxicity. Furthermore, mice lacking all complex gangliosides through disruption of the β1,4-N-acetylgalactosaminyltransferase gene (GM2/GD2 synthase) displayed an ∼600-fold decrease in sensitivity to TeNT relative to wild type littermates. In contrast, the related botulinum neurotoxins types A and B (BoNT/A and BoNT/B), which bind a single molecule of ganglioside in conjunction with a protein co-receptor (31–33), displayed only 40- and 24-fold reductions in toxicity, respectively, relative to wild type littermates (34). Together, these observations support a model in which TeNT toxicity requires simultaneous interaction with two molecules of ganglioside. The observation that high affinity binding of TeNT to PC12 cells was insensitive to treatment of PC12 cells with proteinase K also supported the conclusion that gangliosides were necessary and sufficient for high affinity binding of TeNT.
Glycosphingolipids including gangliosides act as cellular receptors for several bacterial toxins and viruses. GM1a mediates the entry of cholera toxin and Escherichia coli heat-labile toxin type I (LT I), whereas GD1b and GD1a are the receptors for E. coli LT type IIa and LT IIb, respectively (35). Globoside Gb3 is the cellular receptor for Shiga toxin and Shiga-like toxins (36). GM1a is also the receptor for simian virus 40 (37), whereas GD1a and GT1b are the receptors for Polyoma virus. A shared feature of these toxins and viruses is the ability to cross-link multiple ganglioside molecules to drive the entry of the toxin into cells (38). The observation that TeNT requires two functional ganglioside-binding pockets suggests that although cross-linking gangliosides may be a general mechanism for toxin and virus entry, TeNT is unique in directly binding two gangliosides through a single protein domain.
Although several proteins have been identified to interact with TeNT in vitro, thus far a requirement for these proteins in vivo has not been demonstrated. Schiavo and co-workers (39) showed that TeNT interacts with the GPI-anchored protein Thy-1 in PC12 cells and spinal cord neurons. However, spinal cord cells isolated from Thy-1 knock-out mice showed similar sensitivity to TeNT as cells isolated from wild type animals, suggesting that Thy-1 was not an essential receptor for TeNT (39). Because Thy-1 is a highly expressed glycoprotein, it is possible that the sugar motif of the protein interacts with TeNT to mimic ganglioside binding. The same group also showed that HCR/T is retrograde trafficked within motor neurons in Rab7-positive structures that are shared with the neurotrophin receptors p75NTR and TrkB (40). In addition, Swaminathan and co-workers (28) showed that the tri-peptide, Tyr-Glu-Trp, bound to the HCR domain with interactions to Arg1226, which suggests that a protein as well as a ganglioside can bind to the R region. The functional significance of these observations remains to be fully determined but may be related to the need for tetanus toxin to utilize unique receptors to bind and enter neurons of the peripheral and central nervous systems to elicit spastic pathology. A role for proteins in tetanus toxin binding to neuronal cell membranes was previously reported (8).
In the current study, gangliosides are shown to be necessary and sufficient for high affinity binding of TeNT to cultured cells. Although protein(s) may contribute to TeNT entry and trafficking in cells, the affinity for TeNT was too low to detect by the far Western and cell binding assays used in the current study. The interaction between TeNT and a potential protein receptor could be either direct or may be bridged by the sialic acid moieties of gangliosides. One model is that gangliosides are necessary and sufficient for high affinity binding and entry into cells and that a host protein contributes to TeNT-specific intracellular trafficking events. Future studies will address the cellular basis for the intracellular trafficking of TeNT in neurons.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Award 1-U54-AI-057153 (Region V Great Lakes Regional Center of Excellence), the NIAID, National Institutes of Health Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research Program, and NIGMS, National Institutes of Health Grant GM62116 to the Consortium for Functional Glycomics.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
The atomic coordinates and structure factors (codes 3HMY and 3HN1) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- TeNT
- tetanus neurotoxin
- HCR/T
- C-terminal receptor-binding domain of tetanus toxin
- VAMP2
- vesicle-associated membrane protein2
- sialic acids
- N-acetylneuraminic acids
- PPMP
- glucosyl ceramide synthase inhibitor d,l-threo-l-phenyl-2-hexadecanoylamino-3-morpholino-propanol
- SNARE
- soluble NSF attachment protein receptors
- HRP
- horseradish peroxidase.
REFERENCES
- 1.Pappas G., Kiriaze I. J., Falagas M. E. (2008) Int. J. Infect. Dis. 12, 347–350 [DOI] [PubMed] [Google Scholar]
- 2.Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. (1992) Nature 359, 832–835 [DOI] [PubMed] [Google Scholar]
- 3.Bilous J., Eggers R., Gasse F., Jarrett S., Lydon P., Magan A., Okwo-Bele J. M., Salama P., Vandelaer J., Villeneuve P., Wolfson L. (2006) Lancet 367, 1464–1466 [DOI] [PubMed] [Google Scholar]
- 4.Schiavo G., Papini E., Genna G., Montecucco C. (1990) Infect. Immun. 58, 4136–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton R. M., Balfour Y. M. (1962) Biochem. Pharmacol. 11, 974–976 [DOI] [PubMed] [Google Scholar]
- 6.van Heyningen S. (1976) FEBS Lett. 68, 5–7 [DOI] [PubMed] [Google Scholar]
- 7.Fotinou C., Emsley P., Black I., Ando H., Ishida H., Kiso M., Sinha K. A., Fairweather N. F., Isaacs N. W. (2001) J. Biol. Chem. 276, 32274–32281 [DOI] [PubMed] [Google Scholar]
- 8.Critchley D. R., Habig W. H., Fishman P. H. (1986) J. Neurochem. 47, 213–222 [DOI] [PubMed] [Google Scholar]
- 9.Williamson L. C., Bateman K. E., Clifford J. C., Neale E. A. (1999) J. Biol. Chem. 274, 25173–25180 [DOI] [PubMed] [Google Scholar]
- 10.Montecucco C. (1986) Trends Biochem. Sci. 11, 314–317 [Google Scholar]
- 11.Karlsson K. A. (1998) Acta Biochim. Pol. 45, 429–438 [PubMed] [Google Scholar]
- 12.Mocchetti I. (2005) Cell Mol. Life Sci. 62, 2283–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emsley P., Fotinou C., Black I., Fairweather N. F., Charles I. G., Watts C., Hewitt E., Isaacs N. W. (2000) J. Biol. Chem. 275, 8889–8894 [DOI] [PubMed] [Google Scholar]
- 14.Rummel A., Bade S., Alves J., Bigalke H., Binz T. (2003) J. Mol. Biol. 326, 835–847 [DOI] [PubMed] [Google Scholar]
- 15.Chen C., Baldwin M. R., Barbieri J. T. (2008) Biochemistry 47, 7179–7186 [DOI] [PubMed] [Google Scholar]
- 16.Bochner B. S., Alvarez R. A., Mehta P., Bovin N. V., Blixt O., White J. R., Schnaar R. L. (2005) J. Biol. Chem. 280, 4307–4312 [DOI] [PubMed] [Google Scholar]
- 17.Minor W., Tomchick D., Otwinowski Z. (2000) Structure 8, R105–R110 [DOI] [PubMed] [Google Scholar]
- 18.CCP4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 19.Jin R., Rummel A., Binz T., Brunger A. T. (2006) Nature 444, 1092–1095 [DOI] [PubMed] [Google Scholar]
- 20.Swaminathan S., Eswaramoorthy S. (2000) Nat. Struct. Biol. 7, 693–699 [DOI] [PubMed] [Google Scholar]
- 21.Roussel A., Inisan A. G., Knoop-Mouthuy A., Cambillau C. (1999) TURBO-FRODO CNRS/Universitite, Marseille, France [Google Scholar]
- 22.Walton K. M., Sandberg K., Rogers T. B., Schnaar R. L. (1988) J. Biol. Chem. 263, 2055–2063 [PubMed] [Google Scholar]
- 23.Margolis R. U., Mazzulla M., Greene L. A., Margolis R. K. (1984) FEBS Lett. 172, 339–342 [DOI] [PubMed] [Google Scholar]
- 24.Yowler B. C., Kensinger R. D., Schengrund C. L. (2002) J. Biol. Chem. 277, 32815–32819 [DOI] [PubMed] [Google Scholar]
- 25.Basu M., Girzadas M., Dastgheib S., Baker J., Rossi F., Radin N. S., Basu S. (1997) Indian J. Biochem. Biophys. 34, 142–149 [PubMed] [Google Scholar]
- 26.van Heyningen S. (1974) Science 183, 656–6574810267 [Google Scholar]
- 27.Nakayama J., Fukuda M. N., Hirabayashi Y., Kanamori A., Sasaki K., Nishi T., Fukuda M. (1996) J. Biol. Chem. 271, 3684–3691 [PubMed] [Google Scholar]
- 28.Jayaraman S., Eswaramoorthy S., Kumaran D., Swaminathan S. (2005) Proteins 61, 288–295 [DOI] [PubMed] [Google Scholar]
- 29.Sinha K., Box M., Lalli G., Schiavo G., Schneider H., Groves M., Siligardi G., Fairweather N. (2000) Mol. Microbiol. 37, 1041–1051 [DOI] [PubMed] [Google Scholar]
- 30.Kitamura M., Igimi S., Furukawa K., Furukawa K. (2005) Biochim. Biophys. Acta 1741, 1–3 [DOI] [PubMed] [Google Scholar]
- 31.Dong M., Richards D. A., Goodnough M. C., Tepp W. H., Johnson E. A., Chapman E. R. (2003) J. Cell Biol. 162, 1293–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong M., Yeh F., Tepp W. H., Dean C., Johnson E. A., Janz R., Chapman E. R. (2006) Science 312, 592–596 [DOI] [PubMed] [Google Scholar]
- 33.Rummel A., Mahrhold S., Bigalke H., Binz T. (2004) Mol. Microbiol. 51, 631–643 [DOI] [PubMed] [Google Scholar]
- 34.Kitamura M., Takamiya K., Aizawa S., Furukawa K., Furukawa K. (1999) Biochim. Biophys. Acta 1441, 1–3 [DOI] [PubMed] [Google Scholar]
- 35.Chinnapen D. J., Chinnapen H., Saslowsky D., Lencer W. I. (2007) FEMS Microbiol. Lett. 266, 129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G. T. (1986) J. Exp. Med. 163, 1391–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai B., Gilbert J. M., Stehle T., Lencer W., Benjamin T. L., Rapoport T. A. (2003) EMBO J. 22, 4346–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf A. A., Jobling M. G., Saslowsky D. E., Kern E., Drake K. R., Kenworthy A. K., Holmes R. K., Lencer W. I. (2008) Infect. Immun. 76, 1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herreros J., Ng T., Schiavo G. (2001) Mol. Biol. Cell 12, 2947–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. (2006) Neuron 52, 293–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.