Abstract
Protein ubiquitination regulates numerous cellular functions in eukaryotes. The prevailing view about the role of RING or U-box ubiquitin ligases (E3) is to provide precise positioning between the attached substrate and the ubiquitin-conjugating enzyme (E2). However, the mechanism of ubiquitin transfer remains obscure. Using the carboxyl terminus of Hsc70-interacting protein as a model E3, we show herein that although U-box binding is required, it is not sufficient to trigger the transfer of ubiquitin onto target substrates. Furthermore, additional regions of the E3 protein that have no direct contact with E2 play critical roles in mediating ubiquitin transfer from E2 to attached substrates. By combining computational structure modeling and protein engineering approaches, we uncovered a conformational flexibility of E3 that is required for substrate ubiquitination. Using an engineered version of the carboxyl terminus of Hsc70-interacting protein ubiquitin ligase as a research tool, we demonstrate a striking flexibility of ubiquitin conjugation that does not affect substrate specificity. Our results not only reveal conformational changes of E3 during ubiquitin transfer but also provide a promising approach to custom-made E3 for targeted proteolysis.
Protein modification by ubiquitin and ubiquitin-like proteins is a common mechanism through which numerous cellular pathways are regulated (1). The canonical cascade of ubiquitination involves the action of three enzymes, termed E1, E2, and E3, which activate and then conjugate ubiquitin to its substrates (2, 3). The E3 ligase catalyzes the final step in ubiquitin transfer in a substrate-specific manner. Despite advances in understanding the enzymatic cascade of ubiquitination, the mechanism of ubiquitin transfer to the substrate remains an outstanding issue (4). In particular, the role of E3 ubiquitin ligases and how they adapt to progressively modified substrates to maintain specific ubiquitin chain topology is still a mystery.
The known E3s belong to three protein families: HECT, RING, and U-box. HECT domain enzymes form a covalent intermediate with ubiquitin before the final transfer of ubiquitin to substrates. In contrast, RING and U-box E3s have been suggested to function as adaptors that position the substrate in close proximity to the E2-ubiquitin thioester (E2-Ub) (5). It has become common “wisdom” that the substrate has to be precisely positioned to get ubiquitinated (6). The positioning hypothesis originally predicted that E3 substrates would have a specific ubiquitination site. However, the absence of “consensus” ubiquitination sites has become apparent in an increasing list of E3 substrates (7–9). In addition, the crystal structures of several ubiquitination machinery components have revealed a puzzling gap (∼50 Å) between the substrate binding sites and the E2 active sites (10, 11). This raises a fundamental question in ubiquitin transfer. How does the ubiquitin molecule shuttle from the E2 to substrates? Though several interesting models for ubiquitin transfer have been proposed, only limited explicit experimental evidence support these models (4).
We used carboxyl terminus of Hsc70-interacting protein (CHIP)3 as a model E3 system to investigate the role of substrate positioning in its ubiquitination. CHIP is a protein quality control E3 that consists of an NH2-terminal tetratricopeptide repeat (TPR) domain, a helical linker domain, and a COOH-terminal U-box domain (12, 13). The TPR domain of CHIP binds directly to EEVD motifs located at the COOH termini of Hsc/Hsp70 and Hsp90, whereas the U-box domains possess ubiquitin ligase activity. CHIP recruits E2 enzymes of the Ubc4/5 family to ubiquitinate misfolded proteins that occupy the chaperone substrate-binding sites, thus remodeling the chaperones from protein-refolding complexes to complexes that promote degradation (14). Using the chaperone as an adaptor, CHIP targets a variety of substrates for ubiquitination (15). In the absence of substrates, CHIP is also able to ubiquitinate the bound chaperones (16). Thus, there is apparent substrate diversity for CHIP-mediated ubiquitination. Insights into the mechanism of action of CHIP have been provided by an x-ray crystal structure which reveals a remarkable, highly asymmetric dimer (25). Here, we demonstrate the existence of intrinsic structural flexibility in the CHIP homodimer that is required for substrate polyubiquitination. The flexible orientation allows CHIP to accommodate substrates with different sizes and structures. Mutations that restrict the flexibility of CHIP markedly decrease substrate ubiquitination, whereas maintaining flexibility enables us to rebuild a functional ubiquitin ligase with altered substrate specificity. Our results provide evidence for the importance of structural flexibility in E3 ligases, which we propose is of general importance to orchestrate progressive ubiquitin conjugation on substrates.
EXPERIMENTAL PROCEDURES
Plasmids
Prokaryotic constructs expressing glutathione S-transferase or His6-tagged CHIP, CHIP(K30A), CHIP(H260Q), CHIP(ΔTPR), UbcH5a, UbcH5a(C85A) were described previously. FK506 binding protein (FKBP) and FKBP12-rapamycin binding domain (FRB) domains were amplified by PCR from constructs pC4EN-F1 and pC4-RHE, respectively (provided by ARIAD Pharmaceuticals, Inc). Green fluorescent protein (GFP) and U-box fusion constructs were made using standard cloning techniques and were subcloned into pET vector (Novagen).
Antibodies and Reagents
Monoclonal anti-Hsp70 (SPA810) and Hsp70 purified protein were purchased from Stressgen. Polyclonal anti-UbcH5 antibody was from Boston Biochem. Monoclonal anti-FLAG was from Sigma, anti-hemagglutinin from Roche Applied Science, anti-His6 from BD Biosciences. Rabbit E1 and Ub(K0) were purchased from Boston Biochem.
Expression and Purification of Proteins
All proteins were expressed in Escherichia coli BL21(DE3) (Stratagene). His-tagged proteins were purified via nickel-nitrilotriacetic acid agarose (BD Biosciences) and glutathione S-transferase-tagged proteins were purified using glutathione-Sepharose (Amersham Biosciences). For untagged proteins, the purified proteins were subject to cleavage by thrombin (Novagen). Proteins were dialyzed against Dulbecco's phosphate-buffered saline (pH 7.4).
In Vitro Reconstitution Assay
In vitro ubiquitination assays were performed in the presence of 0.1 μm purified rabbit E1 (BioMol), 2 μm UbcH5a, 3 μm CHIP, 50 μm ubiquitin (Sigma), 1 mm dithiothreitol (DTT), 2 mm MgCl2, and 4 mm ATP. Reactions were carried out at 37 °C, and samples were analyzed by SDS-PAGE and immunoblotting using appropriate antibodies.
RESULTS
Variations on Ubiquitin Conjugation Within a Single Reaction System
A typical ubiquitination reaction involves E1, E2, E3, and substrates, with the products of ubiquitin conjugation varying from free ubiquitin chains, ubiquitin conjugated substrates, and autoubiquitination products of E2 and E3 (1). It is thus important to differentiate the substrate ubiquitination from the other ubiquitin conjugates to fully analyze the functionality of E3. E3 autoubiquitination has been treated as a hallmark activity for single subunit E3s (2). How this process is coordinated with substrate ubiquitination remains unexplored. To evaluate CHIP-mediated ubiquitin conjugation, we performed a reconstituted in vitro ubiquitination assay using UbcH5 as E2 and Hsp70 as the substrate of CHIP. Consistent with our previous report (3), we observed nearly complete polyubiquitination of Hsp70 in the presence of wild-type CHIP (Fig. 1A, top panel). Notably, CHIP autoubiquitination was restricted predominantly to monoubiquitination under these circumstances (Fig. 1A, middle panel). This was not due to the competition with Hsp70, as we observed a similar ubiquitination pattern with CHIP(K30A), a TPR mutant that does not bind chaperones (Fig. 1A). In fact, the pattern of CHIP autoubiquitination was unchanged in the absence or presence of Hsp70 (supplemental Fig. S1).
FIGURE 1.
Ubiquitin transfer in a CHIP-mediated reaction system. A, in vitro ubiquitination of Hsp70 in the presence of wild-type (WT) CHIP, CHIP(K30A), or CHIP(H260Q). At the indicated times, reaction aliquots were removed and subjected to immunoblotting analysis with indicated antibodies. For UbcH5a detection, reaction aliquots were treated with 1 mm DTT before immunoblot analysis. B, wild-type UbcH5a (nontagged) was mixed with UbcH5a(C86A) (His6-tagged) in the presence (+) or absence (−) of CHIP. At the indicated times, reaction aliquots were removed and treated with 1 mm DTT before immunoblot analysis. The above results are representatives of at least three experiments. UbcH5∼Ub, UbcH5-Ub thioester.
Unexpectedly, UbcH5a underwent autoubiquitination in a substrate-independent, E3-dependent manner (Fig. 1A, bottom panel). To separate isopeptide conjugates (UbcH5a-Ub) from UbcH5a-Ub thioesters, we treated samples with DTT and found that the DTT-sensitive thioesters migrate faster on SDS-PAGE (17) (supplemental Fig. S2). By using a low concentration of DTT, we were able to detect both UbcH5a-Ub and UbcH5a-Ub thioester species on the same gel. Unlike some E2s that are capable of assembling ubiquitin chains without E3 (1, 18), UbcH5a requires the presence of E3 to mediate ubiquitin transfer. A U-box mutant of CHIP, H260Q, failed to generate any ubiquitin conjugates, resulting in accumulation of UbcH5a-Ub thioesters (Fig. 1A, bottom panel). Therefore, the ratio of UbcH5a-Ub and UbcH5a-Ub thioester species can be used as an indicator of the relative efficiency of ubiquitin transfer.
E2 autoubiquitination could be due to an intramolecular transfer of ubiquitin from its own thioester bond, or to an intermolecular transfer. To distinguish these possibilities, we mixed nontagged wild-type UbcH5a and His6-tagged UbcH5a(C85A), an active site mutant that cannot load ubiquitin via the thioester bond. In the absence of CHIP, no ubiquitin transfer occurred, confirming that ubiquitin transfer by UbcH5a is E3-dependent (Fig. 1B). In the presence of CHIP, both wild-type UbcH5a and the C85A mutant were ubiquitinated (Fig. 1B). These results are consistent with an intermolecular transfer from one activated E2-Ub thioester to another molecule of UbcH5a, which do not need to be in the form of an E2-Ub thioester. Therefore, the E3 not only recruits an E2-Ub thioester but also plays a critical role in the activation of E2.
U-box Binding Is Required for Catalytic Activity of UbcH5
The variations of ubiquitin conjugates in CHIP-mediated reactions share one important feature. The formation of ubiquitin isopeptide conjugates requires the presence of a functional U-box. In the presence of CHIP(H260Q), a U-box mutant that abolishes E2 binding, we did not detect any ubiquitin conjugates (Fig. 1A). Thus, the labile thioesters of UbcH5a-Ub do not automatically lead to ubiquitin transfer. Further supporting this notion, we failed to observe any ubiquitin transfer from UbcH5-Ub to C85A mutant in the absence of CHIP (Fig. 1B). In contrast, CHIP(K30A), a TPR domain mutant that abolishes chaperone binding, maintains the capacity to mediate the formation of ubiquitin isopeptide conjugates of UbcH5 (Fig. 1A). Our results are in line with the recent report that the RING E3 triggers subtle conformation changes in the bound E2, stimulating ubiquitin release from the E2 cysteine and transfer to the substrate (4).
The E2 is at the center of a cascade of ubiquitin transfer, linking activation of the ubiquitin by E1 to its eventual E3-catalyzed attachment to substrates (5). In the CHIP-mediated ubiquitination reaction, we considered UbcH5 autoubiquitination as the by-product of molecular collision, similar to Cdc34 in a SCF-mediated ubiquitination reaction (6). Because the catalytic activity of UbcH5-Ub strictly requires functional binding with the U-box domain, the formation of ubiquitin isopeptide conjugates of UbcH5 provides an independent assessment of E3 ligase activity. This is especially useful to evaluate the ligase activity of E3 mutants with altered or no substrate binding, because no direct measurement of the thioester bond reactivity in E2-Ub is currently available. Therefore, the nonprocessive UbcH5 autoubiquitination is a substrate-independent index of E3 ligase activity.
U-box Binding Is Not Sufficient for the Catalytic Activity of UbcH5
Based on the requisite role of U- box binding in triggering the catalytic activity of UbcH5, we asked whether the U-box alone is sufficient to trigger ubiquitin transfer from E2. In contrast to full-length CHIP, the presence of the U-box domain alone did not lead to any conversion from UbcH5a-Ub thioesters to the UbcH5a-Ub species (Fig. 2A). Because E2 autoubiquitination is substrate-independent, we asked whether the substrate-binding domain TPR is dispensable for E2 activation. However, deletion of the TPR domain completely abolished the ubiquitin transfer from E2 (Fig. 2B). Interestingly, removal of the helical linker region between the TPR and U-box also significantly reduced the ligase activity of CHIP (Fig. 2B). These results indicated that regions of the E3 other than the U-box alone play critical roles in mediating ubiquitin transfer from the bound E2.
FIGURE 2.
Regions of CHIP other than the U-box contribute to the catalytic activity of UbcH5a. A, in vitro ubiquitination in the presence of full-length CHIP or U-box (U) alone. At the indicated times, reaction aliquots were removed and immunoblotted with indicated antibodies. B, in vitro ubiquitination by deletion mutants of CHIP, ΔTPR, and ΔLinker. At the indicated times, reaction aliquots were removed and immunoblotted with indicated antibodies. The above results are representatives of at least three experiments. UbcH5∼Ub, UbcH5-Ub thioester.
Dynamic Asymmetry of CHIP Homodimer
For many single subunit RING or U-box E3s, there is accumulating evidence indicating that oligomerization is required for function (21–23). CHIP forms a homodimer, and the helical linker that connects the TPR and U-box domains is required for dimer assembly (24). A striking feature of the available atomic structure of full-length CHIP homodimer is a radical asymmetry that arises because the helical linker adopts two different conformations (25). The asymmetric arrangement of TPR domains leaves only one U-box of a dimer accessible to the E2 (Ref. 25 and Fig. 3). Because neither the TPR domain nor the helical linker appears to make direct contact with the bound E2 (25), we speculated that a concerted and cooperative mechanism underlies the activation of E2 by CHIP homodimer. We propose a dynamic asymmetry model in which a CHIP homodimer is capable of converting between symmetric and asymmetric conformations without violating structural constraints. We animated the putative conformational transition of the CHIP homodimer using the three-dimensional morphing software Blender (see supplemental Movie S1). Importantly, the modeled conformational switch involving the two helical linkers does not disturb their dimerization interface (Fig. 3). Interestingly, the model passes through a symmetric conformation in the middle of the transition that is strikingly similar to the symmetric structure of CHIP lacking the TPR domain (Fig. 3, middle panel, and Ref. 26). The conformation at the end point of the transition is identical to the starting point but rotated 180°, consistent with the observation that only one conformation for the full-length CHIP was found in the x-ray crystal structure (25). An immediate consequence of this dynamic asymmetry is that the E2 binding site of each U-box cycles between “on” and “off” due to the recurring unmasking/masking by the TPR domain. This molecular switch would provide an alternating access mechanism for TPR-attached substrates to approach the U-box-bound E2, followed by refilling with subsequent E2 molecules.
FIGURE 3.
Computational modeling of the dynamic asymmetry of CHIP homodimer. The top panels show crystal structures of the CHIP homodimer exhibiting asymmetric (A and C) and symmetric (B) conformations, adopted from Refs. 25 and 26. Schematics were generated using PyMOL software. The bottom panels show three-dimensional views of CHIP simulated using Blender 3D software based on matching locations of different domains. Both the asymmetric (D and F) and symmetric (E) conformations are illustrated during the cycle of conformational change. The TPR domain is shown as a blue cube with a dent as the chaperone-binding site. The U-box is shown as an orange ball with a flat surface as the E2 binding site. The helical linker is shown as a green bar. The flexible region in the helical linker is shown in gray in top panels.
Rebuilding a Functional Ubiquitin Ligase
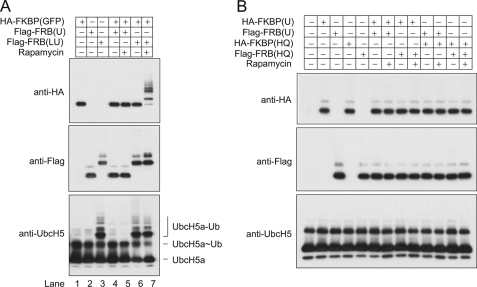
The dynamic asymmetry model of the CHIP homodimer predicts that the TPR domain plays dual roles in ubiquitination: recruiting substrates and regulating E2 binding and activation. As the substrate-binding-defective CHIP mutant K30A retains its ability to activate E2 (Fig. 1A), the two putative roles of the TPR domain appear to be uncoupled. We therefore asked whether the TPR domain could be replaced by other domains without affecting E2 activation. To this end, we replaced the TPR domain with FKBP. Remarkably, this chimeric protein FKBP(LU) retained the same capacity as wild-type CHIP to activate E2 (Fig. 4A). In addition, we observed similar results with a different domain swap using FRB.
FIGURE 4.
Engineering ubiquitin ligases. A, the TPR domain of CHIP was replaced with FKBP or FRB domains, generating recombinant ubiquitin ligases FKBP(LU) and FRB(LU). Using a rapamycin-mediated dimerization approach, GFP proteins fused to FKBP or FRB were used as substrates. In vitro ubiquitination was performed in the absence (−) or presence (+) of 1 μm rapamycin (Rap). At the indicated times, reaction aliquots were removed and immunoblotted with indicated antibodies. B, a schematic representation of different U-box fusion proteins is shown. The CHIP U-box domain was fused to the carboxyl terminus of either FKBP or FRB in the absence or presence of the helical linker. Fusion proteins containing the helical linker (i.e. FKBP(LU) and FRB(LU)) form tight homodimers inherently. For fusion proteins without a helical linker (such as FKBP(U) and FRB(U)), dimerization was induced using rapamycin. The functionality of chimeric U-box fusion proteins was evaluated by either UbcH5a autoubiquitination or GFP substrates ubiquitin conjugation. ×, does not activate UbcH5a-Ub; √, activates UbcH5a-Ub; Ø, not done; HA, hemagglutinin. UbcH5∼Ub, UbcH5-Ub thioester.
FKBP and FRB domains dimerize with high affinity in the presence of rapamycin (27). This property allowed us to test whether the ligase activity of the chimeric E3 can be redirected toward new substrates. We constructed FRB-fused GFP, which can be recruited to FKBP(LU) by rapamycin (supplemental Fig. S3). Remarkably, addition of rapamycin induced efficient ubiquitin conjugation on FRB(GFP) (Fig. 3A). Reciprocal experiments that swapped FKBP and FRB domains showed similar patterns of ubiquitin conjugation. Notably, the chimeric proteins faithfully recapitulate the E3 properties of the full-length CHIP. For example, the autoubiquitination of FKBP(LU) and FRB(LU) was independent of the ubiquitination of GFP substrates (Fig. 4A).
An Engineered Ubiquitin Ligase Mimics the CHIP Homodimer
The successful redesign of a ubiquitin ligase allowed us to further substantiate the finding that structural flexibility is required for CHIP-mediated ubiquitination. Consistent with the results using the deletion mutant of CHIP lacking the helical domain, which eradicates CHIP structural flexibility, the corresponding deletion mutation completely abolished the ligase activity of recombinant E3 (FKBP-U and FRB-U) (Fig. 4B and supplemental Fig. S4). For these experiments, we used the FKBP-FRB dimerization approach, positioning the GFP proteins bearing FRB domain (FRB-GFP) in the vicinity of recombinant ligase FKBP-U by adding rapamycin. No ubiquitin conjugation on FRB-GFP was detected (Fig. 4B and supplemental Fig. S4). Using a similar approach, we further tested the functionality of an artificial U-box dimer FKBP(U)/FRB(U) and again found no evidence of E2 activation. To mimic the asymmetry of the CHIP dimer that exhibits half-of-sites E2-binding activity but without structural flexibility, we prepared a U-box heterodimer by dimerizing a wild-type U-box and H260Q mutant within the FKBP/FRB system. Once again, neither FKBP(U)/FRB(HQ) nor FKBP(HQ)/FRB(U) triggered ubiquitin transfer (Fig. 3B and supplemental Fig. S4). Taken together, these results indicate that the full functionality of the U-box in the context of full-length of CHIP cannot be replicated in the form of U-box monomer, U-box dimer, or half-of-sites heterodimer. Instead, it relies on the presence of both the helical linker and the TPR domain, and the function of the TPR domain can be substituted by similarly positioned but unrelated structures.
Flexible Orientation Accommodates Substrate Plasticity
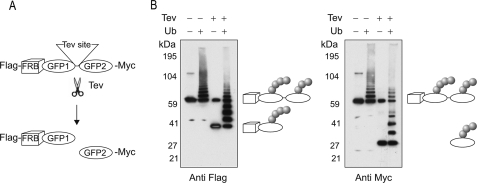
Several lines of evidence suggest that transient E2/E3 interactions are required for repeated ubiquitin conjugation to attached substrates. For example, the mutually exclusive binding of E1s and E3s to E2s necessitates a recycling of E2s for addition of successive ubiquitins to substrates (28). Dynamic E2/E3 interactions also provide a mechanism for E3 enzymes to accomplish a challenging task: targeting a substrate of continuously increasing size (due to the growing ubiquitin chain) and of different character, particularly at the initial stages. The dynamic model of CHIP we propose here coordinates substrate approach, E2 activation, and subsequent reloading. The intrinsic structural flexibility of CHIP enables E2 molecules to target acceptor lysines on multiple substrates with varied distances. To rigorously test the structural flexibility of CHIP in ubiquitin transfer, we constructed a fusion protein comprised of tandem GFPs with FRB at the NH2 terminus to permit its recruitment to the recombinant ligase FKBP-LU (Fig. 5A). To differentiate the ubiquitination of GFP1 from that of GFP2, we inserted a tobacco etch virus (TEV) cleavage site between GFP1 and GFP2. After FKBP(LU)-mediated ubiquitination in the presence of rapamycin, the reaction products were treated with the AcTEV enzyme. The separated GFP proteins were resolved on SDS-PAGE followed by immunoblotting using antibodies recognizing distinct epitope tags located at GFP1 or GFP2 respectively. Remarkably, both GFP proteins were ubiquitinated by the recombinant ubiquitin ligase (Fig. 5B). GFP forms a cylindrical structure that has a diameter of 30 Å and a length of 40 Å. Thus, even a distance of about 100 Å (for tandem GFPs plus FRB) does not prevent the substrate from accepting ubiquitin. Our results demonstrate a striking flexibility within the CHIP dimer that facilitates ubiquitin transfer and provides a mechanism to accommodate substrates of diverse size and structure for ubiquitination.
FIGURE 5.
Spatial flexibility of ubiquitin transfer allows ubiquitination of distant substrates. A, a schematic diagram depicting the double GFP fusion protein bearing FRB. A Tev cleavage site was introduced between GFP proteins to separate them after ubiquitination (Ub). B, in vitro ubiquitination of the double GFP fusion protein by FKBP-LU in the presence of 1 μm rapamycin. Reaction products were treated with AcTEV enzyme and immunoblotted with indicated antibodies. For simplicity and clarity, only the linear ubiquitin chain was illustrated, although we do not exclude the possibility of multiple ubiquitin chains or monoubiquitination. The above results are representatives of at least three experiments. +, presence of indicated molecule; −, absence of indicated molecule.
DISCUSSION
Although multiple E2 and E3 enzyme structures have been determined, including several E2-E3 complexes, it is becoming increasingly apparent that static structural snapshots are not sufficient to explain E3 catalysis (4). The prevailing view of the function of RING finger (or U-box) E3s is that they provide a scaffold that brings ubiquitin-charged E2 and substrate into close proximity. However the hypothesis of “catalysis by proximity” is not readily aligned with ubiquitination of substrates of varied sizes. In addition, it remains unanswered how ubiquitin shuttles from E2 to substrates. Our results reveal structural flexibility of the E3 CHIP is required for the activation of a bound E2 and the subsequent ubiquitin transfer. There is growing evidence that structural flexibility is an intrinsic property of E3s. Conformational flexibility has been described in a HECT E3 WWP1/AIP5 (29) and, via cryo-electron microscopy, in the multi-subunit RING E3 APC/C (30), although a causal link of flexibility to E3 activity has until now been elusive. Analogous to the ribosome, where structural flexibility is required for elongation factor delivery and GTPase activation in the synthesis of polypeptide (31), it may be that E3 cycling through different conformational states is a common feature when ubiquitin is assembled on a substrate molecule in a progressive manner.
In addition to conformational flexibility, ligase asymmetry is also a common theme. In the BRCA1/BARD1 heterodimer, only the RING domain in BRCA1 is responsible for recruiting E2 (21). MDM2, the E3 designated for p53 ubiquitination, has a close partner, MDMX, whose RING domain is not functional in E2 binding (22). Intriguingly, the central acidic domain of MDM2 plays a critical role in p53 ubiquitination (32, 33). The observation that the RING domains of MDM2 and MDMX are swappable only in the presence of the acidic domain not only suggests asymmetric features similar to those described here for CHIP but also indicates the importance of structural flexibility.
The successful engineering of ubiquitin ligases implies a novel approach to achieving specific protein knock-out. Researchers have explored the possibility of modifying the ubiquitin pathway to accelerate degradation of specific cellular proteins (34). However, the approaches based on the substrate-binding protein F-box require endogenous Skp1-Cullin1-F-box-protein core machinery to achieve ubiquitination. High levels of the F-box chimera may squelch the core Skp1-Cullin1-F-box-protein and thus cripple the degradation of both the intended and native substrates. Here, we demonstrate activity for an engineered single-chain ubiquitin ligase, which is cell friendly because it does not rely on other subunits for its functionality. Moreover, we showed the novel E3 ligase can be readily engineered into a small molecule-inducible version. This strategy suggests a powerful and versatile tool to control protein ubiquitination and degradation.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM61728, HL65619, and AG024282 (to C. P.) and GM75156 (to W. J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental references, Figs. S1–S4, and Movie S1.
- CHIP
- carboxyl terminus of Hsc70-interacting protein
- TPR
- tetratricopeptide repeat
- Ub
- ubiquitin
- E2-Ub
- ubiquitin-conjugated E2
- GFP
- green fluorescent protein
- DTT
- dithiothreitol
- FKBP
- FK506 binding protein
- FRB
- FKBP12-rapamycin binding domain
- TEV
- tobacco etch virus.
REFERENCES
- 1.Pickart C. M., Eddins M. J. (2004) Biochim. Biophys. Acta 1695, 55–72 [DOI] [PubMed] [Google Scholar]
- 2.Glickman M. H., Ciechanover A. (2002) Physiol. Rev. 82, 373–428 [DOI] [PubMed] [Google Scholar]
- 3.Kerscher O., Felberbaum R., Hochstrasser M. (2006) Annu. Rev. Cell Dev. Biol. 22, 159–180 [DOI] [PubMed] [Google Scholar]
- 4.Hochstrasser M. (2006) Cell 124, 27–34 [DOI] [PubMed] [Google Scholar]
- 5.Pickart C. M. (2001) Annu. Rev. Biochem. 70, 503–533 [DOI] [PubMed] [Google Scholar]
- 6.Passmore L. A., Barford D. (2004) Biochem. J. 379, (Pt 3) 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardozo T., Pagano M. (2004) Nat. Rev. Mol. Cell Biol. 5, 739–751 [DOI] [PubMed] [Google Scholar]
- 8.Paltoglou S., Roberts B. J. (2007) Oncogene 26, 604–609 [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Herr R. A., Chua W. J., Lybarger L., Wiertz E. J., Hansen T. H. (2007) J. Cell Biol. 177, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang P., Chu C., Koepp D. M., Elledge S. J., Pagano M., Conaway R. C., Conaway J. W., Harper J. W., Pavletich N. P. (2002) Nature 416, 703–709 [DOI] [PubMed] [Google Scholar]
- 11.Zheng N., Wang P., Jeffrey P. D., Pavletich N. P. (2000) Cell 102, 533–539 [DOI] [PubMed] [Google Scholar]
- 12.Ballinger C. A., Connell P., Wu Y., Hu Z., Thompson L. J., Yin L. Y., Patterson C. (1999) Mol. Cell Biol. 19, 4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson C. (2002) Sci. STKE 2002, PE4. [DOI] [PubMed] [Google Scholar]
- 14.McDonough H., Patterson C. (2003) Cell Stress Chaperones 8, 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyr D. M., Hohfeld J., Patterson C. (2002) Trends Biochem. Sci. 27, 368–375 [DOI] [PubMed] [Google Scholar]
- 16.Qian S. B., McDonough H., Boellmann F., Cyr D. M., Patterson C. (2006) Nature 440, 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozkan E., Yu H., Deisenhofer J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18890–18895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z. J., Niles E. G., Pickart C. M. (1991) J. Biol. Chem. 266, 15698–15704 [PubMed] [Google Scholar]
- 19.Deleted in proof
- 20.Deleted in proof
- 21.Brzovic P. S., Keeffe J. R., Nishikawa H., Miyamoto K., Fox D., 3rd, Fukuda M., Ohta T., Klevit R. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5646–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marine J. C., Jochemsen A. G. (2005) Biochem. Biophys. Res. Commun. 331, 750–760 [DOI] [PubMed] [Google Scholar]
- 23.Ohi M. D., Vander , Kooi C. W., Rosenberg J. A., Ren L., Hirsch J. P., Chazin W. J., Walz T., Gould K. L. (2005) Mol. Cell Biol. 25, 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolay R., Wiederkehr T., Rist W., Kramer G., Mayer M. P., Bukau B. (2004) J. Biol. Chem. 279, 2673–2678 [DOI] [PubMed] [Google Scholar]
- 25.Zhang M., Windheim M., Roe S. M., Peggie M., Cohen P., Prodomou C., Pearl L. H. (2005) Mol. Cell 20, 525–538 [DOI] [PubMed] [Google Scholar]
- 26.Xu Z., Devlin K. I., Ford M. G., Nix J. C., Qin J., Misra S. (2006) Biochemistry 45, 4749–4759 [DOI] [PubMed] [Google Scholar]
- 27.Clackson T., Yang W., Rozamus L. W., Hatada M., Amara J. F., Rollins C. T., Stevenson L. F., Magari S. R., Wood S. A., Courage N. L., Lu X., Cerasoli F., Jr, Gilman M., Holt D. A.(1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10437–10442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eletr Z. M., Huang D. T., Duda D. M., Schulman B. A., Kuhlman B. (2005) Nat. Struct. Mol. Biol. 12, 933–934 [DOI] [PubMed] [Google Scholar]
- 29.Verdecia M. A., Joazeiro C. A., Wells N. J., Ferrer J. L., Bowman M. E., Hunter T., Noel J. P. (2003) Mol. Cell 11, 249–259 [DOI] [PubMed] [Google Scholar]
- 30.Dube P., Herzog F., Gieffers C., Sander B., Riedel D., Müller S. A., Engel A., Peters J. M., Stark H. (2005) Mol. Cell 20, 867–879 [DOI] [PubMed] [Google Scholar]
- 31.Diaconu M., Kothe U., Schlünzen F., Fischer N., Harms J. M., Tonevitsky A. G., Stark H., Rodnina M. V., Wahl M. C. (2005) Cell 121, 991–1004 [DOI] [PubMed] [Google Scholar]
- 32.Kawai H., Wiederschain D., Yuan Z. M. (2003) Mol. Cell Biol. 23, 4939–4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meulmeester E., Frenk R., Stad R., de Graaf P., Marine J. C., Vousden K. H., Jochemsen A. G. (2003) Mol. Cell Biol. 23, 4929–4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou P. (2005) Curr. Opin. Chem. Biol. 9, 51–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.