Abstract
Galectin-1 is a galactoside-binding lectin expressed in multiple tissues that has pleiotropic immunomodulatory functions. We previously showed that galectin-1 activates human monocyte-derived dendritic cells (MDDCs) and triggers a specific genetic program that up-regulates DC migration through the extracellular matrix, an integral property of mucosal DCs. Here, we identify the galectin-1 receptors on MDDCs and immediate downstream effectors of galectin-1-induced MDDC activation and migration. Galectin-1 binding to surface CD43 and CD45 on MDDCs induced an unusual unipolar co-clustering of these receptors and activates a dose-dependent calcium flux that is abrogated by lactose. Using a kinome screen and a systems biology approach, we identified Syk and protein kinase C tyrosine kinases as mediators of the DC activation effects of galectin-1. Galectin-1, but not lipopolysaccharide, stimulated Syk phosphorylation and recruitment of phosphorylated Syk to the CD43 and CD45 co-cluster on MDDCs. Inhibitors of Syk and protein kinase C signaling abrogated galectin-1-induced DC activation as monitored by interleukin-6 production; and MMP-1, -10, and -12 gene up-regulation; and enhanced migration through the extracellular matrix. The latter two are specific features of galectin-1-activated DCs. Interestingly, we also found that galectin-1 can prime DCs to respond more quickly to low dose lipopolysaccharide stimulation. Finally, we underscore the biological relevance of galectin-1-enhanced DC migration by showing that intradermal injection of galectin-1 in MRL-fas mice, which have a defect in skin DC emigration, increased the in vivo migration of dermal DCs to draining lymph nodes.
Dendritic cells (DCs)5 are critical regulators of immunity that sample and present antigen, initiate adaptive immune responses through T cell interactions, and maintain self-tolerance through T cell instruction (1, 2). To effectively mount an immune response, DCs must encounter antigen and receive a signal to initiate an activation program termed “maturation.” Both exogenous and endogenous signals can initiate DC maturation. Exogenous maturation signals include Toll-like receptor ligation via pathogen components such as bacterial proteins (e.g. LPS), bacterial DNA (through CpG-containing motifs), and viral double-stranded RNA (3, 4). In synergy with these pathogen signals, or alone, endogenous DC activators include inflammatory cytokines, prostaglandins, and other danger signals (5).
Recent work has also demonstrated that galectins, a family of endogenous β-galactoside binding lectins, can initiate DC maturation (6–8). The galectins have numerous known immunomodulatory activities involving T and B cells, but the role of these lectins in DC function is only beginning to be investigated. Galectin-9 matures DCs into IL-12-producing cells, which can elicit a Th1 response from T cells following co-culture (8). On the other hand, galectin-3 influences the type of adaptive immune response initiated by DCs but does not directly affect the maturation process (9). We and others have shown that galectin-1 matures DCs and further enhances the migratory capacity of these cells (6, 7). Furthermore, galectin-1 differentially regulates gene expression in maturing DCs, as compared with LPS stimulation, indicating that galectin-1 employs a distinct maturation pathway (7). In the current study we identify and characterize the immediate downstream effectors that preferentially mediate the effects of galectin-1 on DC maturation.
The downstream signaling events associated with classical DC maturation have been partially elucidated. For example, LPS induces activation of NF-κB and of MAPK pathways (particularly p38 and Erk1/2) (3). However, the persistence of the maturation signal and the nature of stimulus can have disparate effects on the functional outcomes of pathways leading to DC maturation (10). For example, Erk1/2 can exert both positive and negative effects on LPS-induced activation (10–12) and can synergize with p38 to increase cytokine production but exert inhibitory effects on migration. This highlights the remarkable ability of DCs to integrate the extracellular environment, which dictates the variety and persistent quality of signals, into distinct outcomes.
Less is known about immediate early activation events in DC maturation. In particular, what pathways or adaptor molecules link receptor engagement to these late activation events? Additionally, do early events differ between an endogenous stimulus (galectin-1) and exogenous stimulus (LPS), even though both stimuli result in DC maturation? Many immune cell signals employ adaptor proteins and kinases to integrate signals from different receptors into downstream events. Primarily, these upstream mediators include protein-tyrosine kinases (PTKs), such as Src family kinases. Phosphorylated PTKs in turn recruit and activate additional downstream effectors including MAPKs, small GTPases (Rac1 and Cdc42), and transcription factors (13). The recruitment of distinct PTKs and/or adaptor proteins could be one mechanism by which DCs coordinate and regulate distinct and multiple stimuli into a specific outcome.
Galectin-1 exists primarily as a noncovalent homodimer that recognizes the N-acetyl-lactosamine residues present on various glycoproteins and glycolipids. Thus, galectin-1 can cross-link multiple receptors on the cell surface, thereby eliciting different cell functions via formation of membrane microdomains (14). We have shown that dimeric galectin-1 is required for DC maturation (7), implying that cross-linking of receptors is involved in transducing the maturation signal. Here, we identify the cognate galectin-1 receptors on MDDCs and further characterize the immediate downstream effectors that mediate the novel effects of galectin-1 on these cells. Specifically, we show that galectin-1 binds to two DC surface glycoproteins, CD43 and CD45, and induces unipolar co-clustering of these two receptors on the DC surface. Additionally, using a kinome array and a systems biology approach, we identify multiple phosphorylated signaling mediators preferentially induced by galectin-1 over LPS and implicate Syk and PKC as unique PTKs that mediate galectin-1 specific effects on DCs. Furthermore, we show that galectin-1 can prime DCs to respond more quickly to low dose LPS stimulation, suggesting a synergism between endogenous and exogenous DC activation signals that has been heretofore unappreciated. Finally, we also show that galectin-1 can enhance the in vivo migration of dermal DCs to draining lymph nodes in lupus-prone MRL-fas mice, which have a defect in skin DC emigration, underscoring the biological relevance of our results.
EXPERIMENTAL PROCEDURES
Mice
MRL-fas mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were housed under guidelines set by the National Institutes of Health, and experiments were conducted in accordance with the University of California Los Angeles Chancellor's Animal Research Committee and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
MDDC Differentiation and Galectin-1 Treatment
MDDCs were differentiated with granulocyte macrophage-colony-stimulating factor and IL-4 from purified human monocytes as previously described (7). Mouse bone marrow-derived dendritic cells (BMDCs) were differentiated from mononuclear cells harvested from the marrow of femurs and tibias of 6–10-week-old mice as previously described (15). The indicated treatments were added to day 5 immature MDDCs and day 7 BMDCs, and the cells were cultured for an additional 48 h unless otherwise noted. To control for possible endotoxin contamination, all of the galectin-1 treated cells were preincubated with 10 μg/ml polymyxin B (Sigma) at 37 °C for 30 min.
Reagents
Recombinant human galectin-1 was produced as previously described (16). The following reagents were purchased from the indicated suppliers: lipopolysaccharide from Escherichia coli 0127:B8 (Sigma), bisindolylmaleimide I (Calbiochem), Syk inhibitor (Calbiochem 574711), U73122 (Calbiochem), LY294002 (Calbiochem), and polymyxin B and polymyxin B-agarose beads (Sigma).
Co-immunoprecipitation and Western Blots
5 × 106 MDDCs were used per condition for co-immunoprecipitation. The cells were allowed to bind with galectin-1 (20 μm) for 60 min at 4 °C, and then galectin-1 was cross-linked to cognate cell surface receptors using a non-membrane-permeable reversible cross-linker, 3,3′-dithiobis(sulfosuccinimidyl propionate) (Pierce). MDDCs were lysed in immunoprecipitation buffer (50 mm Tris-HCl, pH 7.4, 1% Nonidet P-40, 1 mm EDTA, 0.25% sodium deoxycholate, 1 mm NaF, 1 mm Na3VO4, 1× complete protease inhibitor mix; Roche Applied Science), and clarified lysate was then subjected to immunoprecipitation overnight at 4 °C using anti-galectin-1 rabbit polysera (1:100), which was cross-linked to protein G beads (Pierce) using dimethyl pimelimidate (Pierce). Immunoprecipitates were reduced in SDS buffer, separated on a 10% polyacrylamide gel, and then transferred to polyvinylidene difluoride membrane. Western blotting was performed using the antibodies indicated in the supplemental Methods.
Confocal Microscopy
MDDCs were differentiated on polylysine-coated coverslips (BD Biosciences) in 12-well plates. The MDDCs were treated with galectin-1 in the presence of polymyxin B (10 μg/ml) at the indicated concentrations for 1 h at 37 °C. All of the immunofluorescence staining was conducted on ice. The cells were washed twice with PBS and then fixed with 2% paraformaldehyde/PBS for 20 min. After fixing, the cells were washed again with Tris-buffered saline and then incubated with blocking solution (5% fetal bovine serum, 1% bovine serum albumin, PBS) for 20 min. The MDDCs were incubated in primary antibody in blocking solution for 1 h on ice, washed, incubated with secondary antibody in PBS for 1 h while protected from light, washed, and then mounted on slides using Prolong Gold (Invitrogen). Between each set of primary and secondary antibodies, the cells were fixed again with 2% paraformaldehyde/PBS. For intracellular pSyk staining, the cells were fixed with 2% paraformaldehyde/PBS after completion of cell surface staining, permeabilized with 0.1% Triton X-100 for 4 min, washed, and then blocked with blocking solution for an additional 20 min before staining with primary and secondary antibodies. The antibodies used are indicated in the supplemental Methods. The confocal fluorescence images were taken at 60× magnification on a Leica TCS-SP MP confocal and multiphoton-inverted microscope (Heidelberg, Germany) equipped with an argon, diode, and two-photon laser.
Calcium Flux
3 × 105 immature MDDCs were used per condition. The cells were incubated with 3 μm Indo-1AM and 0.02% w/v Pluronic F-127 (Invitrogen) in serum-free RPMI for 45 min at 37 °C, protected from light. After incubation, MDDCs were washed once with serum-free RPMI and then resuspended in RPMI containing 2% fetal bovine serum. MDDCs were then incubated at room temperature for 30 min protected from light prior to acquisition on the BD LSR flow cytometer. All galectin-1-treated cells were preincubated with 10 μg/ml polymyxin B (Sigma) for 30 min.
Kinex Antibody Microarray Preparation
MDDCs from three separate human donors were differentiated as described above for co-immunoprecipitation and Western blots. After differentiation, the cells were treated with galectin-1 (20 μm) in the presence of 10 μg/ml polymyxin B or buffer control (80 μm DTT and 10 μg/ml polymyxin B) for 5 min. The cells were washed twice in ice-cold PBS and then sonicated in Kinex lysis buffer (20 mm MOPS, 1% Triton X-100, 2 mm EGTA, 5 mm EDTA, 30 mm NaF, 1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 5 μm pepstatin A, 1× complete protease inhibitor mixture (Roche Applied Science), 1 mm DTT). 100 μg of cleared lysate protein combined from three donors were shipped to Kinexus Bioinformatics Corporation (Vancouver, Canada) for Kinex antibody microarray services.
Kinex Microarray Data Analysis
On receiving the Kinex protein microarray data, we created a corrected fold change to account for error in the replicates and stringently selected candidate proteins with a true increase that was outside the error limits of replicates. Using the data provided by Kinexus, we calculated our corrected fold change = [(average intensity of treated samples − % error of replicates)/(average intensity of control samples + % error of replicates)]. This corrected fold change was used to rank the candidate proteins, and those with a corrected fold change of >1.3 was used for pathway analysis with Ingenuity Pathway Analysis (Ingenuity Systems). See legend to supplemental Fig. S1 for details on further bioinformatic parsing.
Quantification of Cytokine Production
DC cytokine secretion was measured using cytokine-specific ELISA (eBioscience and BD). Human MDDCs and murine BMDCs were plated in 96-well plates (2 × 105 cells/well in 200 μl of RPMI). MDDCs were preincubated with the indicated lactose or chemical inhibitors for 30 min or 2 h, respectively, at 37 °C. Additionally, galectin-1-treated wells were preincubated with 10 μg/ml polymyxin B at 37 °C for 30 min. In conditions where MDDCs were co-stimulated with galectin-1 and LPS, galectin-1 was preincubated with polymyxin B-agarose beads (Sigma) for 30 min rotating at 4 °C to remove endotoxin contamination (17). The polymyxin B-agarose beads were subsequently removed to prevent inhibition of LPS activity. Cell culture supernatants were collected at the indicated times following treatment with the indicated doses of LPS, galectin-1, buffer control, or no treatment and stored at −80 °C until analysis.
Real Time Quantitative RT-PCR
Total RNA from MDDCs was extracted with the RNeasy mini kit (Qiagen) at 18 h after the indicated treatment. QuantiTect Probe RT-PCR kit was used for cDNA synthesis (Qiagen). Transcripts were quantified by real time quantitative PCR on an iQ5 system (Bio-Rad) with predesigned TaqMan gene expression assays and reagents according to the manufacturer's instructions (Applied Biosystems). For each sample, mRNA abundance was normalized to the amount of β-actin expressed.
MDDC Matrigel Migration Assays
MDDCs matured with LPS or galectin-1 were treated with the indicated chemical inhibitors or not and assayed for migration through Matrigel in a transwell assay as described (7). In all assays, the number of cells migrating through the Matrigel to the bottom membrane was counted at 40× magnification. Each experiment was counted by two independent counters, one blinded to the sample identity, and at least 50 fields were counted. Cells/field was normalized to 50 fields, and the data are expressed as relative migration compared with control cells (immature MDDCs).
In Vivo Skin DC Migration Assays
MRL-fas mice were treated with an 10-μl intradermal injection of galectin-1 (dose scale from 20–100 μm in injected bolus) or buffer control (equivalent volume of PBS/80 μm DTT) (n = 4 animals/group) into the dorsum of the ear. At 24 h post-injection, 25 μl of a skin sensitizing fluorochrome, 1% FITC (Sigma) in dibuytlphthalate:acetone (1:1), or vehicle alone (without FITC) was applied on the dorsal half of each ear. At 48 h after sensitization, the animals were sacrificed, and the draining cervical lymph nodes were harvested, separated into single cell suspensions, and analyzed by flow cytometry for the presence of FITC+ (migrated) cells. The migrant (FITC+) cells in the draining lymph nodes were further defined for different populations of DCs. Antibodies used include anti-mouse CD11c-biotin (clone N418), anti-mouse CD40-PE (clone 1C10), anti-mouse major histocompatibility complex II IA/IE-APC (clone M5), and anti-mouse CD207-APC (e-Bioscience).
RESULTS
Galectin-1 Specifically Binds to CD43 and CD45 and Co-clusters These Glycoconjugates on Human MDDC Surface
To identify galectin-1 receptors on MDDCs, total cell surface proteins were biotinylated prior to incubation with galectin-1. Following galectin-1 binding, MDDCs were lysed, and bound galectin-1 was immunoprecipitated with anti-galectin-1 sera, and co-immunoprecipitated proteins were separated by SDS-PAGE. Probing the Western blot with streptavidin-horseradish peroxidase revealed that galectin-1 bound to several cell surface ligands (Fig. 1A). Because CD43 and CD45 are known galectin-1 receptors on T cells (18), we probed for CD43 and CD45 in the co-immunoprecipitate using specific antibodies for each protein. Fig. 1B (lanes 3 and 7) shows that galectin-1 bound to both CD45 and CD43, corresponding to bands 1 and 2 in Fig. 1A, respectively. Interestingly, the endogenous levels of galectin-1 produced by MDDCs were sufficient to mediate co-immunoprecipitation of CD45 but not CD43 (Fig. 1B, compare lanes 1 and 5). Fig. 1B (lanes 2, 4, and 8) also shows that galectin-1 binding to CD43 and CD45 was completely abolished in the presence of 0.1 m lactose, indicating that galectin-1 specifically bound to glycans on both CD43 and CD45 on the MDDC surface.
FIGURE 1.
Galectin-1 specifically binds to CD43 and CD45 and co-cluster these glycoconjugates on human MDDC surface. A, MDDCs were cell surface biotinylated and then incubated with 20 μm of galectin-1, as described under “Experimental Procedures.” After cross-linking with cell-impermeant reversible cross-linker, anti-galectin-1 serum was used to immunoprecipitate (IP) galectin-1 and any bound cell surface receptors. Western blotting (WB) with streptavidin-horseradish peroxidase revealed putative galectin-1 counter receptors on MDDC surfaces. Note that band 1 and band 2 correspond to CD45 and CD43 identified as indicated below. B, CD45 and CD43 were identified as specific receptors in the co-immunoprecipitate by Western blotting with specific anti-CD45 or -CD43 antibodies (lanes 3 and 7, respectively). Galectin-1 binding to CD43 and CD45 was carbohydrate-dependent because it could be competed off with 0.1 m lactose (lanes 4 and 8). Note that the endogenous amount of galectin-1 produced by DCs was sufficient to mediate co-immunoprecipitation of CD45 (lanes 1 and 2) but not CD43 (lanes 5 and 6). C and E, MDDCs grown on polylysine-coated coverslips were exposed to galectin-1 (at indicated concentrations) for 1 h at 37 °C. The cells were then fixed and processed for confocal microscopy as detailed under “Experimental Procedures” using antibodies against CD43, CD45, and CCR5. 60× images are shown. D, the graph indicates the percentage of cells with CD43 and CD45 clustering (250 cells counted per condition). The buffer-, LPS-, and lactose-treated MDDCs served as additional specificity controls. Note that CD43 and CD45 co-clustering occurred in >50% of cells only above 10 μm concentration of galectin-1.
To determine whether galectin- 1 binding results in qualitative changes in membrane distribution of specific cell surface receptors, we used confocal microscopy to visualize the location of CD43 and CD45 before and after galectin-1 binding. Following 1 h of incubation with galectin-1, both CD43 and CD45 showed dose-dependent clustering on the MDDC surface (Fig. 1C). In contrast to the phenotype seen on T cells where CD43 and CD45 are clustered but segregated in different domains (14), both CD43 and CD45 co-clustered in a unipolar fashion on the cell membrane in MDDCs. LPS or a buffer control did not induce co-clustering of CD43 and CD45, and preincubation with 0.1 m lactose inhibited redistribution of these markers (Fig. 1C), underscoring the saccharide specificity of the effects of galectin-1. Furthermore, clustering was only seen in the majority of cells at galectin-1 concentrations at or above 10 μm (quantified in Fig. 1D). Because the Kd of dimerization for galectin-1 is in the 6–8 μm range (19), this suggested that cross-linking of receptors was involved in galectin-1 membrane reorganization. Additionally, galectin-1 did not cause global membrane reorganization, as evidenced by a lack of redistribution of CCR5, a highly expressed cell surface protein on MDDCs that is not known to bind galectin-1 (Fig. 1E).
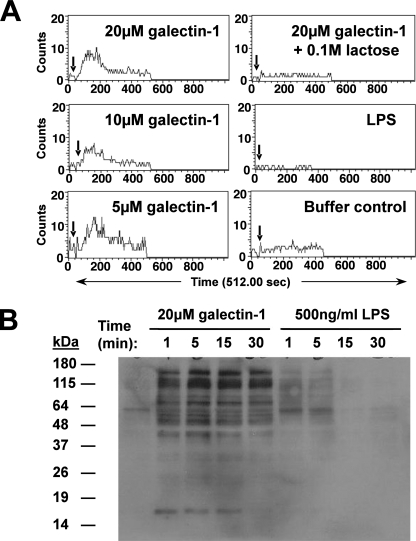
Galectin-1 Induces Calcium Flux and Differential Tyrosine Phosphorylation Patterns in MDDCs
Many intracellular signals involve calcium mobilization through release of intracellular calcium stores or an influx of extracellular calcium. Antibody cross-linking of CD43 causes calcium flux in DCs (20), but it is not known whether endogenous ligands can initiate calcium flux. We found that galectin-1, but not LPS or the buffer control, specifically induced calcium flux in MDDCs (Fig. 2A). Galectin-1-induced calcium flux was dose-dependent and inhibited by 0.1 m lactose, underscoring the specificity of the galectin-1 effects. Next, to determine whether galectin-1 ligation of DC cell surface receptors activates a differential signaling cascade compared with LPS, we immunoblotted MDDC lysate with the 4G10 monoclonal anti-phosphotyrosine antibody at the indicated time points following galectin-1 or LPS treatment (Fig. 2B), and monitored for changes in global tyrosine phosphorylation patterns. Significantly, galectin-1 and LPS treatment induced distinct phosphotyrosine patterns in MDDC lysate (Fig. 2B). Incubation with galectin-1 buffer components (DTT and polymyxin B) showed banding patterns similar to untreated cells (data not shown).
FIGURE 2.
Galectin-1 induces calcium flux and differential tyrosine-phosphorylation patterns in MDDCs. A, immature MDDCs were loaded with Indo-1AM (3 μm) + 0.02% pluronic and then stimulated with galectin-1 at indicated times (↓) during acquisition on a BD LSR flow cytometer. The data are shown as numbers of cells with calcium bound Indo-1/unbound Indo-1 (FL5/FL4) ratio above base line versus time. B, MDDCs were treated with galectin-1 (20 μm) or LPS (500 ng/ml) for the indicated times and then lysed, and equivalent amounts of cell lysate (30 μg/lane) were Western blotted using the 4G10 monoclonal anti-phosphotyrosine antibody. Galectin-1 was added to cells in the presence of polymyxin B (10 μg/ml) to eliminate any confounding effects of endotoxin contamination.
Galectin-1 Differentially Activates the Kinome in MDDCs
To identify and characterize the phosphorylated substrates and potential signaling pathways that were activated following galectin-1 treatment on MDDCs, we used a systems biology approach to screen the kinome and identify the key mediators of cognate signaling pathways.
First, we used an antibody-based kinome array screen (Kinex microarray; Kinexus) on MDDC lysate from galectin-1- or buffer control-treated cells, essentially to obtain a snapshot of the modified kinome upon galectin-1 treatment (supplemental Fig. S1B). We performed the screen at the 5-min time point, because that time point showed the greatest change in phosphorylation in our Western blot experiment (Fig. 2B).
Bioinformatic parsing of the kinome screen (see “Experimental Procedures”) resulted in identification of relevant proteins in Table 1 that were differentially phosphorylated with galectin-1 stimulation compared with buffer control treatment. These proteins were then examined for known signaling relations using Ingenuity Pathway Analysis (supplemental Fig. S1A). Included in supplemental Fig. S1 are our two primary candidate galectin-1 receptors, CD43 and CD45, and representations of the signaling families indicated in Table 1. We chose the highlighted signaling mediators (Syk/ZAP70, PKC, phosphatidylinositol 3-kinase (PI3K) and PLCγ) for further testing because they have been implicated as downstream effectors of CD43 (21–25) and CD45 (26) signaling in other systems or are known to be involved in calcium signaling pathways (23).
TABLE 1.
Phosphorylated proteins in galectin-1-stimulated MDDCs relative to buffer control using corrected fold change from Kinex array
| Protein name | Phosphorylation site(s) | Corrected fold change | Known signaling pathwaysa |
|---|---|---|---|
| Caveolin 2 | Ser23 | 14.73 | Scaffolding protein |
| Myosin regulatory light chain | Ser20 | 2.79 | Integrin signaling, Rho-dependent signaling |
| ζ-chain (TCR)b-associated kinase, 70 kDa (ZAP-70/spleen tyrosine kinase (Syk) | Tyr292 | 1.78 | MAPK signaling, TCR signaling |
| Protein kinase N1 (PKN1) | Thr774 | 1.62 | Inositol phosphate signaling, PKC signaling, Rho-dependent signaling |
| Phosphatase and tensin homolog (PTEN), pseudogene 1 (PTENP1) | Ser380, Ser382,and Ser385 | 1.55 | Inositol phosphate signaling, integrin signaling |
| Insulin receptor substrate 1 (IRS-1) | Tyr612 | 1.49 | Insulin receptor signaling, inositol phosphate signaling |
| LIM domain kinase 1/2 | Try508/Thr505 | 1.43 | Rho-dependent signaling, inositol phosphate signaling |
| Myristoylated alanine-rich protein kinase C substrate (Marcks) | Ser158 and Ser162 | 1.42 | PKC signaling |
| MAPK-interacting protein-serine kinase 1 (MNK1) | Thr209 and Thr214 | 1.38 | MAPK signaling |
| Retinoblastoma-associated protein 1 | Ser807 | 1.37 | Cell cycle |
| cAMP-responsive element binding protein 1 (CREB) | Ser133 | 1.32 | cAMP signaling, MAPK signaling, Calcium signaling |
| Ret receptor-tyrosine kinase | Ser696 | 1.31 | MAPK signaling, inositol phosphate signaling |
a Known signaling pathways obtained from ingenuity pathway analysis and EntrezGene data bases.
b TCR, T-cell receptor.
Syk and PKC Have Significant Roles in Galectin-1-induced MDDC Activation
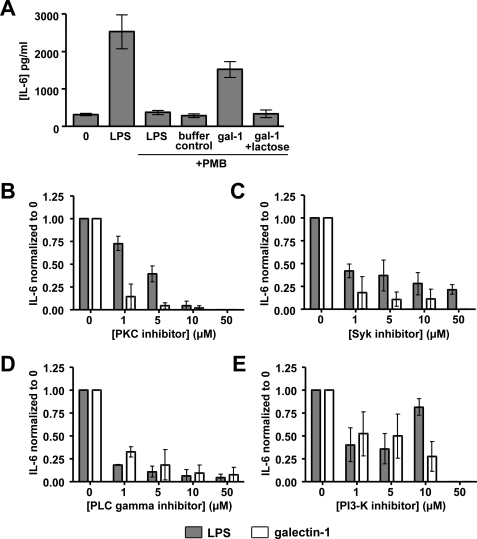
We first sought to establish the specificity of using IL-6 secretion as a functional downstream readout for MDDC activation (7, 27–29). Fig. 3A shows that LPS and galectin-1 both induced IL-6 secretion and that LPS-induced, but not galectin-1-induced, IL-6 production was completely inhibited by 10 μg/ml polymyxin B. The specificity of the effects of galectin-1 was further underscored by the ability of 0.1 m lactose to abrogate galectin-1-induced IL-6 secretion.
FIGURE 3.
Syk and PKC have significant roles in galectin-1-induced cytokine secretion from MDDCs. A, MDDCs were treated with LPS (250 ng/ml), buffer control (80 μm DTT), or galectin-1 (20 μm). 10 μg/ml of polymyxin B (PMB) and 0.1 m lactose were added 30 min prior to stimulation where indicated. Our galectin-1 stocks had minimal LPS contamination of 1–4 endotoxin units/ml as measured by the Limulus amebocyte lysate assay. 250 ng/ml of the LPS stock used had 750 endotoxin units/ml. Clearly, 10 μg/ml of polymyxin B was more than sufficient to eliminate any confounding effects of endotoxin contamination. B–E, MDDCs were preincubated with specific PTK inhibitors for PKC (bisindolylmaleimide I) (B), Syk (Calbiochem 574711) (C), PLCγ (U73122) (D), and PI3K (LY294002) (E) for 2 h prior to galectin-1 (20 μm) or LPS (250 ng/ml) stimulation. At 24 h, IL-6 secretion in the supernatant was assayed by ELISA. The data are shown as IL-6 levels normalized to the no inhibitor condition (0 μm) ± S.E. of five independent experiments in at least four human donors.
To evaluate the importance of each of the candidate pathways identified by the combination of our Kinex screen (Table 1) and Ingenuity Pathway Analysis (supplemental Fig. S1A), we preincubated MDDCs with chemical inhibitors of Syk/ZAP70, PKC, PI3K, and PLCγ. Although it is likely that all of these signaling mediators are involved in DC activation via various stimuli, our goal was to identify a pathway that is preferentially utilized by galectin-1.
Indeed, both PLCγ and PI3K appeared to be necessary for both galectin-1- and LPS-induced IL-6 secretion, because specific PLCγ and PI3K inhibitors equivalently abrogated IL-6 production regardless of stimulus (Fig. 3, D and E). A PKC inhibitor also inhibited both galectin-1 and LPS-induced IL-6 secretion at high concentrations (>10 μm), although galectin-1-induced IL-6 secretion appeared more sensitive to PKC inhibition at low doses (1 and 5 μm) of the inhibitor (Fig. 3B), suggesting that LPS and galectin-1 were differentially dependent on the PKC pathway at some level.
Galectin-1-stimulated IL-6 production was consistently more susceptible to Syk inhibition than LPS-induced IL-6 secretion (Fig. 3C). Treatment with high concentrations of Syk inhibitor (50 μm) completely blocked IL-6 production by galectin-1-activated MDDCs but only partially decreased IL-6 production by LPS activated MDDCs. This difference in sensitivity indicated that galectin-1-induced DC maturation may be more dependent on Syk than LPS-induced DC maturation.
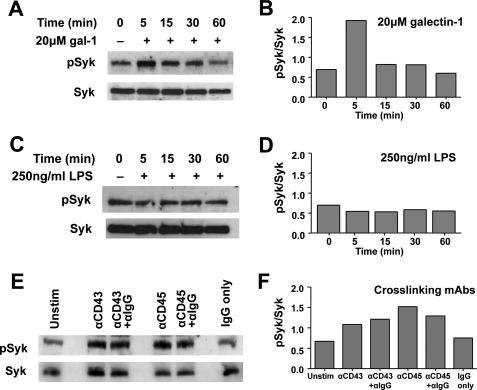
Galectin-1 but Not LPS Induces Increased Syk Phosphorylation in Human MDDCs
To confirm the specificity of our chemical inhibition experiments, we sought to determine whether galectin-1 physically activates Syk tyrosine kinase. We incubated MDDCs with galectin-1 for varying periods of time, collected whole cell lysate, and detected the levels of total and phosphorylated Syk by Western blot. Untreated MDDCs had a basal level of phosphorylated Syk (pSyk) expression. At 5 min post-galectin-1 stimulation, the pSyk/total Syk ratio increased by almost 3-fold (Fig. 4, A and B) and then declined back to basal levels by 60 min. Total Syk protein levels did not change throughout the time course (Fig. 4A). Notably, neither pSyk nor total Syk levels changed following LPS stimulation (Fig. 4, C and D), suggesting that Syk signaling was not an immediate effector of LPS signaling under the conditions examined. Cross-linking CD43 receptors with anti-CD43 monoclonal antibodies is known to trigger Syk phosphorylation (21), similar to the effects of galectin-1 stimulation (Fig. 4, E and F), and served as a positive control throughout this experiment. Intriguingly, cross-linking CD45 receptors on MDDCs with anti-CD45 monoclonal antibodies also induced Syk phosphorylation. Nonspecific IgGs did not affect Syk phosphorylation levels, underscoring the specificity of the CD43 and CD45 cross-linking effects (Fig. 4, E and F). Because galectin-1 binds to and clusters CD43 and CD45 on MDDCs (Fig. 1), this suggests that galectin-1 may cross-link CD43 and CD45 to induce Syk signaling.
FIGURE 4.
Galectin-1 induces increased Syk phosphorylation in human MDDCs. Western blots showing pSyk and total Syk protein in MDDC lysate (30 μg/lane) following treatment with galectin-1 (20 μm) (A) or LPS (250 ng/ml) (C) for the indicated times or 1 μg of mouse anti-CD43 monoclonal antibody or anti-CD45 monoclonal antibody (E) for 1 h. Where indicated, anti-mouse IgG was added during the last 30 min of incubation to super-cross-link the mouse monoclonal antibodies. Corresponding densitometric quantification of the pSyk/total Syk ratios is shown in B, D, and F, respectively. Galectin-1 was added to cells in the presence of polymyxin B (10 μg/ml) to eliminate any contaminating endotoxin effects.
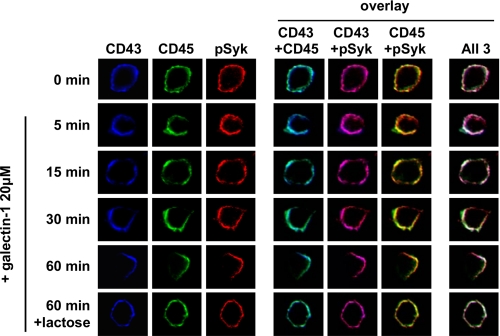
Galectin-1 Stimulates Redistribution of pSyk to Co-localize with the CD43 and CD45 Co-cluster on MDDCs
Many receptor-associated cell signaling events occur at the cell membrane because signaling molecules are recruited to form a complex termed a “signalosome.” To determine whether galectin-1 induces the formation of such a signaling complex, we used confocal microscopy to visualize the respective cellular locations of CD43, CD45, and pSyk following galectin-1 stimulation in human MDDCs. Before galectin-1 treatment, CD43 and CD45, and basal levels of pSyk were uniformly distributed around the cell membrane (Fig. 5, 0 min). At subsequent time points, galectin-1 began to cluster CD43 and CD45 until the 60-min time point where both CD43 and CD45 appeared unipolarly co-clustered. There was moderate base-line co-localization of pSyk with CD43 and CD45 (Fig. 5, CD43+pSyk (magenta) and CD45+pSyk (yellow) at time 0 min). However, with the increasing time of galectin-1 binding, pSyk appeared to follow the redistribution of these receptors because CD43 and CD45 began to show galectin-1-induced co-clustering on the cell membrane (Fig. 5). Galectin-1 binding was necessary to cluster these three molecules together in a putative “signalosome,” because the unipolar co-clustering was blocked by lactose, which inhibits galectin-1 binding and galectin-1-induced MDDC maturation (7). This suggests that the formation of the CD43-CD45-pSyk cluster may be critical for the galectin-1-induced effects of MDDCs.
FIGURE 5.
Galectin-1 stimulates redistribution of pSyk to co-localize with the CD43 and CD45 co-cluster on MDDCs. Confocal microscopy was performed on MDDCs treated with galectin-1 (20 μm) and prepared as in Fig. 1B except that the samples were processed at the indicated time points. In addition, intracellular staining for pSyk was performed in conjunction with anti-CD43 and anti-CD45 as described under “Experimental Procedures.” 60× images are shown. Fluorochromes were chosen for each antibody that minimized bleed-through for each of the filters used (Alexa 594 for anti-pSyk, Alexa 633 for anti-CD43, and Alexa 488 for anti-CD45). The Images are false-colored to represent CD43 (blue), CD45 (green), and pSyk (red). Note the co-localization of pSyk with CD43 and CD45 on the MDDC surface and their progressive unipolar clustering after galectin-1 binding. Treatment with 0.1 m lactose, a competitive inhibitor of galectin-1 binding, prevented the progressive co-clustering seen at 60 min. Blue (CD43) + green (CD45) = cyan; blue (CD43) + red (pSyk) = magenta; green (CD45) + red (pSyk) = yellow. Co-localization of all three primary colors is shown in white. Representative cells from each time point are shown (at least 100 cells were captured at each time point).
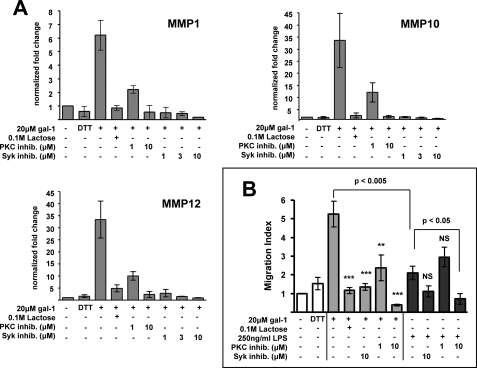
Syk and PKC Are Critical for Galectin-1-induced MMP Gene Expression and Enhanced Migration through Extracellular Matrix
One unique function of galectin-1-matured DCs is an enhanced migratory capacity through the extracellular matrix caused by up-regulation of multiple MMP genes (7). Because Syk and PKC inhibition prevented galectin-1-induced MDDC activation (Fig. 3), we wanted to determine the role of Syk and PKC in galectin-1-induced MMP gene up-regulation and human MDDC migration. Using quantitative RT-PCR, we determined the expression of MMP-1, MMP-10, and MMP-12 (the specific MMP genes that were up-regulated by galectin-1) (7) following galectin-1 treatment in the presence of increasing concentrations of Syk and PKC inhibitors. In at least five unique human donors, the presence of low dose Syk and PKC inhibitors resulted in nearly complete abolishment of galectin-1-induced MMP gene expression (Fig. 6A), indicating that signaling through Syk and PKC is required for the increased MMP gene expression that accompanies galectin-1-induced MDDC activation. In contrast, we observed no consistent inhibition of MMP expression with the Syk inhibitor in MDDCs stimulated with LPS (data not shown). These results further confirm that the galectin-1 and LPS activation pathways are distinct in MDDCs.
FIGURE 6.
Syk and PKC are critical for galectin-1-induced MMP gene expression and enhanced migration through the extracellular matrix. A, MMP gene expression was quantified by real time quantitative RT-PCR at 18 h following galectin-1 (20 μm) stimulation in the presence or absence of the indicated concentrations of Syk inhibitor (Calbiochem 574711), PKC inhibitor (bisindolylmaleimide I), or 0.1 m lactose. DTT refers to buffer control (80 μm DTT + 10 μg/ml polymyxin B). mRNA expression of each gene is normalized to the housekeeping gene β-actin. The data are shown as fold changes of normalized mRNA expression relative to the untreated condition. The graphs depict the means ± S.E. of three independent human donors. B, migration of MDDCs treated with 250 ng/ml LPS (black bars), 20 μm galectin-1 (gray bars), or DTT buffer control in the presence or absence of Syk inhibitor (10 μm), PKC inhibitor (1 or 10 μm) or 0.1 m lactose was measured using transwell assays with Matrigel-coated inserts (8.0-μm pore). The bottom chamber contained 200 ng/ml CCL19 (MIP-3β), which served as the chemoattractant. Each experiment consisted of at least two independent counts by different individuals, one blinded to the sample identity (see “Experimental Procedures”). The data are normalized as relative migration compared with immature DCs in which the average from all counts in each experiment is set at 1.0 (7). The data are shown as the means ± S.E. of four independent human donors. Significance was determined by two-tailed unpaired Student's t test. **, p < 0.005; ***, p < 0.0005 compared with galectin-1 stimulation alone. NS, not significant compared with LPS stimulation alone.
Next, we sought to determine whether Syk and PKC inhibition would also decrease galectin-1 enhancement of MDDC migration through extracellular matrix. This enhanced migratory activity is a novel function of galectin-1-activated MDDCs and requires MMPs, the expression of which was severely hampered by Syk and PKC inhibitors (Fig. 6A). To do this, we tested LPS or galectin-1-matured MDDCs in an in vitro migration transwell assay through Matrigel, a biologically active basement membrane matrix, in the presence of various doses of Syk and PKC inhibitors. As expected (7), galectin-1-matured MDDCs migrated better than LPS-matured or untreated MDDCs. However, treatment with low doses of Syk or PKC inhibitors completely eliminated this migratory enhancement, indicating that Syk and PKC were critically involved in mediating this unique function of galectin-1-matured DCs (Fig. 6B, p < 0.005). In contrast, neither 10 μm of Syk inhibitor nor 1 μm of PKC inhibitor affected the low level of LPS-induced migration. However, 10 μm of PKC inhibitor appeared to significantly affect LPS-induced migration, which was consistent with the results from the IL-6 ELISA assays (Fig. 3B) where 10 μm of PKC inhibitor also affected both LPS- and galectin-1-induced IL-6 secretion. In toto, these results implicate Syk and PKC as critical PTKs that preferentially mediate galectin-1-specific effects on DCs.
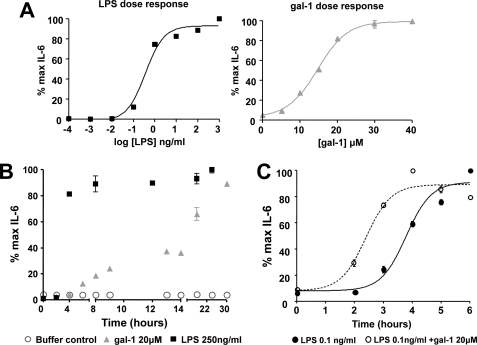
Galectin-1 Synergizes with LPS for Faster Activation of MDDCs
Because galectin-1 and LPS can activate DCs via overlapping by distinct pathways and galectin-1 is an endogenous lectin whose secretion by endothelium and immune cells is up-regulated during inflammation (30, 31), we asked whether galectin-1 can act as an inducible danger signal that functions to enhance the DC response to pathogenic stimuli. Thus, we tested whether galectin-1 can act cooperatively with LPS to stimulate DC activation. First, we established that MDDCs respond to LPS and galectin-1 stimulation in a dose-dependent manner (Fig. 7A). Interestingly, even at saturating doses, DCs responded more quickly to LPS stimulation, reaching 50% of maximal response by 3.4 h, whereas galectin-1 triggered a different pattern of IL-6 secretion, with the first wave occurring between 4 and 9 h and then a second, more significant, rise after 12 h (Fig. 7B). To show that galectin-1 can enhance the DC response to LPS stimulation, the DCs were first exposed to galectin-1 for 8 h prior to LPS stimulation. DCs primed with galectin-1 for 8 h prior to a suboptimal exposure to 0.1 ng/ml LPS responded more quickly, reaching 50% of maximal IL-6 production at 2.5 h as opposed to 3.8 h with 0.1 ng/ml LPS stimulation alone (Fig. 7C; p < 0.0001). These results imply that galectin-1 can act in conjunction with LPS to allow a faster, but not greater, activation response to suboptimal LPS stimulation.
FIGURE 7.
Galectin-1 enhances the inflammatory response of MDDCs to LPS. A, MDDCs were stimulated with increasing doses of LPS (10−4 to 103 ng/ml) or galectin-1 (0–40 μm). Supernatants were collected at 24 h, and IL-6 was measured by ELISA. Cells treated with galectin-1 were preincubated with polymyxin B (10 μg/ml) for 30 min to eliminate any contaminating endotoxin effects. B, MDDCs were treated with 250 ng/ml LPS (black squares), 20 μm galectin-1 (gray triangles), or buffer control (80 μm DTT + 10 μg/ml polymyxin B) (open circles), and IL-6 secretion was measured by ELISA of the culture supernatants at the indicated time points. The data are represented as percentages of maximal IL-6 production. C, MDDCs were stimulated with LPS (0.1 ng/ml) alone or were preincubated with galectin-1 (20 μm) for 8 h prior to LPS stimulation. Supernatant was collected after the indicated time periods, and IL-6 was measured by ELISA. The data are presented as percentages of maximal IL-6 secretion. The time to 50% maximal response is 3.8 h for LPS treatment alone (filled circle, solid line) and 2.5 h for LPS and galection-1 co-stimulus (open circle, dotted line) (p < 0.0001, two-way analysis of variance). Galectin-1 was preincubated with polymyxin B-agarose beads for 30 min to eliminate endotoxin contamination from the galectin-1 preparations.
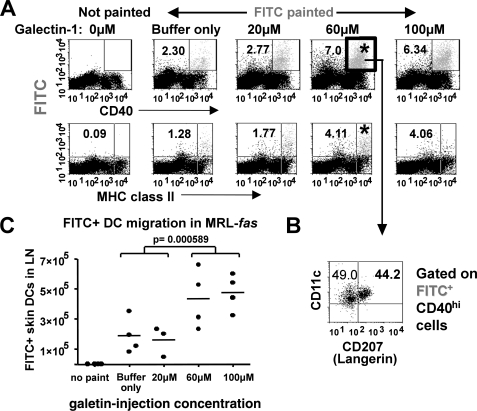
Galectin-1 Promotes the Migration of Skin DCs to Regional Lymph Nodes in Vivo
Because a cardinal feature of galectin-1-activated MDDCs is enhanced migration through the extracellular matrix, we sought to determine whether the effects of galectin-1 on MDDCs could be recapitulated in an in vivo model. We first established that recombinant galectin-1 could activate murine DCs to become phenotypically and functionally mature. BMDCs from 6–10-week-old mice treated with galectin-1 up-regulated cell surface maturation markers CD80 and CD86 and produced IL-6 comparable with BMDCs stimulated with LPS (supplemental Fig. S2), indicating that murine DCs were similarly responsive to galectin-1-induced maturation as human MDDCs.
To determine whether galectin-1 promotes in vivo migration of DCs, we studied the migratory activity of skin DCs to regional lymph nodes. Using MRL-fas mice, which have a defect in skin DC migration (32), we tracked skin DC emigrants to draining lymph nodes after treating the mice with a single intradermal injection of galectin-1 at the indicated concentrations (see “Experimental Procedures”) (Fig. 8A). The migrant (FITC+CD40+) cells in the draining lymph nodes were further defined for different populations of DCs, including epidermal DC (CD11chi-intLangerin+) and dermal DC (CD11chi-intLangerin−) (Fig. 8B) (33). The results show a dose-dependent increase in skin DC migrants in animals injected with galectin-1 (Fig. 8C), of which 35–45% of the migrant FITC-positive cells were also positive for the Langerin marker (Fig. 7B). Thus, in addition to our in vitro observations regarding the enhanced migratory activity of galectin-1-activated MDDCs, galectin-1 also enhanced the in vivo skin emigration that is defective in lupus-prone MRL-fas mice.
FIGURE 8.
Galectin-1 promotes the migration of skin DCs to regional lymph nodes in vivo. MRL-fas mice were intradermally injected with a single 10-μl bolus of 80 μm DTT buffer control or the indicated concentrations of galectin-1 (20 μm = 2.8 μg, 60 μm = 8.4 μg, and 100 μm = 14 μg) preincubated with 10 μg/ml polymyxin B and then painted with 25 μl of a FITC solution at the dorsum of the ear 24 h post-injection, as described under “Experimental Procedures.” A, representative dot plots of single cell suspensions of cervical lymph nodes harvested at 48 h post-FITC painting. Gated FSChiSSChi cells were analyzed for FITC and CD40 or major histocompatibility complex class II to identify putative skin DC emigrants. B, gated FITC+CD40+ cells were further analyzed for CD207 (Langerin) and CD11c. C, absolute numbers of migrant skin DCs (FITC+CD11c+CD40hi) in draining lymph nodes are shown from each mouse (n = 4/group). Galectin-1 significantly enhanced the trafficking of skin emigrants to draining lymph nodes (p < 0.0005, one-way analysis of variance for all groups; p = 0.00576 between the 0–20 μm groups and the 60–100 μm groups; unpaired Student's t test).
DISCUSSION
Although galectin-1 and LPS can both induce MDDC maturation, there are differences that distinguish the two stimuli. For example, galectin-1 specifically increases expression of genes related to cell motility and migration and enhances MDDC migration through the extracellular matrix (7).
In the current study, we identified galectin-1 receptors on MDDCs and characterized the immediate downstream effectors that mediate the effects of galectin-1 on MDDCs. Galectin-1 bound to glycans on CD43 and CD45 and co-clustered these glycoproteins on the DC surface (Fig. 1). Galectin-1 induced a calcium flux and activated overlapping as well as distinct signaling pathways compared with LPS (Figs. 2–4). Specifically, we identified Syk and PKC protein-tyrosine kinases as critical mediators of the effects of galectin-1 on MDDCs, because Syk and PKC inhibitors abrogated the effects of galectin-1 on MDDCs (Figs. 3 and 6). Galectin-1 stimulated Syk phosphorylation (Fig. 4), which then clustered with both galectin-1 receptors CD43 and CD45 on membranes of MDDCs (Fig. 5). Syk and PKC activities were also necessary for galectin-1-induced MMP gene expression and enhanced migration through Matrigel (Fig. 6). Finally, although galectin-1-induced maturation of DCs has been independently demonstrated in vivo using various murine models (6, 34), we now also provide in vivo evidence for a distinguishing feature of galectin-1-activated DCs: enhanced migration through the extracellular matrix. Fig. 8 shows that a single intradermal injection of recombinant galectin-1 could partially rectify the defect in skin DC emigration seen in lupus-prone MRL-fas mice and induced migration of various types of skin DCs into draining lymph nodes.
Although we have shown that galectin-1 clearly bound and clustered CD43 and CD45 on MDDCs, we acknowledge that galectin-1 may have additional counter-receptors on the DC plasma membrane, and the role of other receptors in galectin-1-mediated signaling on MDDCs remains to be determined. For example, Syk has essential roles in integrin signaling (35–37), and integrin β1 is another known galectin-1 receptor (18). Further, data from the kinome array shows that galectin-1 affected multiple phosphorylated proteins that have known roles in integrin signaling, and integrin β1 itself was also phosphorylated (Table 1). Galectin-1-mediated DC maturation likely involves coordinated binding of multiple receptors and utilization of unique upstream mediators to link common signaling pathways. Thus, future studies will test the contributory role of each galectin-1 receptor to fully delineate the complex mechanism of galectin-1 signaling in DCs.
The “danger signal model” proposes that immune responses can also be driven by self-signals that signal danger in the absence of a pathogen, i.e. by signals sent from dying or damaged cells (38–40). Because galectin-1 is present at high concentrations (up to 48 mg/kg) in extracellular matrix and in multiple anatomic sites (31) and its secretion by endothelium and immune cells is increased during inflammation (30) or can be concentrated in stromal sites to potentiate its activity (41), galectin-1 may function as an endogenous inducible danger signal (42) that enhances the inflammatory response to exogenous pathogen signals. Indeed, we found that priming the DCs with pre-exposure to galectin-1 enhances the inflammatory response time of DCs to suboptimal LPS stimulus (Fig. 7C). Thus, endogenous cues, such as galectin-1, which can be highly up-regulated in inflammatory situations, can potentially synergize with pathogen-derived signals (like LPS) and activate dendritic cells in a manner that has not been heretofore appreciated. Our results also suggest that, at some level, LPS and galectin-1 signaling pathways converge. Thus, although our data show that the Syk and PKC pathways can preferentially mediate galectin-1 effects on DCs, we do not imply that LPS does not activate these pathways at all. Indeed, LPS is known to activate the Syk pathway in neutrophils (43), and our inhibitor, time course, and phosphorylation studies indicate that LPS may simply activate the Syk pathway in DCs in a distinct but overlapping manner.
Our data also show that the role of galectin-1 as an endogenous activator of DCs can induce functionally different responses from a classic exogenous stimulus like LPS. Galectin-1 is well known as a fine-tuner of immune responses (44–46), and a multitude of studies have shown that exogenously administered galectin-1 can dampen a host of autoimmune and inflammatory responses in animal models (47). However, the vast majority of studies have focused on the ability of galectin-1 to modulate T cell responses. Galectin-1 clearly matures dendritic cells, as has been independently demonstrated by Morelli and co-workers (6), but even this work focused on the ability of transgenic galectin-1-matured DCs to modulate T cell responses in vivo. Here, we showed that our in vitro observation that galectin-1-activated DCs have enhanced migratory capacity through the extracellular matrix is also seen in vivo. Indeed, galectin-1 rescued the defect in skin DC emigration seen in lupus-prone MRL-fas mice. This raises the question of whether galectin-1 plays a role in the basal constitutive migration of tissue DCs from the periphery to central lymphoid organs, an event that is thought to be necessary for the maintenance of peripheral tolerance (48–50). Our results suggest that despite the unquestionable role that galectin-1 plays in T cell modulation, a more holistic view of how galectin-1 also affects DCs, a cell that is the nexus of innate and adaptive immunity, is called for.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of all members of the Lee laboratory, particularly Y. Wang and H. Nassanian, for technical assistance. We also acknowledge the support of D. Anisman-Posner from the UCLA AIDS Institute Virology Core (UCLA AIDS Institute AI26897) and T. Phung at the UCLA Flow Cytometry Core (UCLA Center for AIDS Research CA-16042).
This work was supported, in whole or in part, by National Institutes of Health Grant AI060694 (to B. L. and L. G. B.), Grants AI080778 and AR056465 (to R. R. S.), Training Grants AI07126-30 (to J. A. F.), Grant HL096392 (to M. H. C.), and Grant GM08042 (to J. A. F. and M. H. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and supplemental text.
- DC
- dendritic cell
- MDDC
- monocyte-derived dendritic cell
- PKC
- protein kinase C
- LPS
- lipopolysaccharide
- IL
- interleukin
- MAPK
- mitogen-activated kinase
- Erk
- extracellular signal-regulated kinase
- PTK
- protein-tyrosine kinase
- BMDC
- bone marrow-derived dendritic cells
- PBS
- phosphate-buffered saline
- DTT
- dithiothreitol
- MOPS
- 4-morpholinepropanesulfonic acid
- ELISA
- enzyme-linked immunosorbent assay
- RT
- reverse transcription
- FITC
- fluorescein isothiocyanate
- PLC
- phospholipase C
- MMP
- matrix metalloproteinase
- pSyk
- phosphorylated Syk
- PI3K
- phosphatidylinositol 3-kinase.
REFERENCES
- 1.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. (2000) Annu Rev. Immunol. 18, 767–811 [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J., Steinman R. M. (1998) Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 3.Akira S., Takeda K. (2004) Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A., Medzhitov R. (2004) Nat. Immunol. 5, 987–995 [DOI] [PubMed] [Google Scholar]
- 5.Gallucci S., Matzinger P. (2001) Curr. Opin. Immunol. 13, 114–119 [DOI] [PubMed] [Google Scholar]
- 6.Perone M. J., Larregina A. T., Shufesky W. J., Papworth G. D., Sullivan M. L., Zahorchak A. F., Stolz D. B., Baum L. G., Watkins S. C., Thomson A. W., Morelli A. E. (2006) J. Immunol. 176, 7207–7220 [DOI] [PubMed] [Google Scholar]
- 7.Fulcher J. A., Hashimi S. T., Levroney E. L., Pang M., Gurney K. B., Baum L. G., Lee B. (2006) J. Immunol. 177, 216–226 [DOI] [PubMed] [Google Scholar]
- 8.Dai S. Y., Nakagawa R., Itoh A., Murakami H., Kashio Y., Abe H., Katoh S., Kontani K., Kihara M., Zhang S. L., Hata T., Nakamura T., Yamauchi A., Hirashima M. (2005) J. Immunol. 175, 2974–2981 [DOI] [PubMed] [Google Scholar]
- 9.Breuilh L., Vanhoutte F., Fontaine J., van Stijn C. M., Tillie-Leblond I., Capron M., Faveeuw C., Jouault T., van Die I., Gosset P., Trottein F. (2007) Infect. Immun. 75, 5148–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luft T., Maraskovsky E., Schnurr M., Knebel K., Kirsch M., Görner M., Skoda R., Ho A. D., Nawroth P., Bierhaus A. (2004) Blood 104, 1066–1074 [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K., Yanagawa Y., Iwabuchi K., Onoé K. (2003) Immunol. Lett. 89, 149–154 [DOI] [PubMed] [Google Scholar]
- 12.Puig-Kröger A., Relloso M., Fernández-Capetillo O., Zubiaga A., Silva A., Bernabéu C., Corbí A. L. (2001) Blood 98, 2175–2182 [DOI] [PubMed] [Google Scholar]
- 13.Samelson L. E. (2002) Annu Rev. Immunol. 20, 371–394 [DOI] [PubMed] [Google Scholar]
- 14.Pace K. E., Lee C., Stewart P. L., Baum L. G. (1999) J. Immunol. 163, 3801–3811 [PubMed] [Google Scholar]
- 15.Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. (1999) J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 16.Pace K. E., Hahn H. P., Baum L. G. (2003) Methods Enzymol. 363, 499–518 [DOI] [PubMed] [Google Scholar]
- 17.Shimomura H., Matsuura M., Saito S., Hirai Y., Isshiki Y., Kawahara K. (2003) Infect. Immun. 71, 5225–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elola M. T., Chiesa M. E., Alberti A. F., Mordoh J., Fink N. E. (2005) J. Biomed. Sci. 12, 13–29 [DOI] [PubMed] [Google Scholar]
- 19.Cho M., Cummings R. D. (1995) J. Biol. Chem. 270, 5198–5206 [DOI] [PubMed] [Google Scholar]
- 20.Corinti S., Fanales-Belasio E., Albanesi C., Cavani A., Angelisova P., Girolomoni G. (1999) J. Immunol. 162, 6331–6336 [PubMed] [Google Scholar]
- 21.Miura Y., Mizutani C., Nishihara T., Hishita T., Yanagi S., Tohyama Y., Ichiyama S., Yamamura H., Uchiyama T., Tohyama K. (2001) Biochem. Biophys. Res. Commun. 288, 80–86 [DOI] [PubMed] [Google Scholar]
- 22.Wong R. C., Remold-O'Donnell E., Vercelli D., Sancho J., Terhorst C., Rosen F., Geha R., Chatila T. (1990) J. Immunol. 144, 1455–1460 [PubMed] [Google Scholar]
- 23.Silverman L. B., Wong R. C., Remold-O'Donnell E., Vercelli D., Sancho J., Terhorst C., Rosen F., Geha R., Chatila T. (1989) J. Immunol. 142, 4194–4200 [PubMed] [Google Scholar]
- 24.Pedraza-Alva G., Mérida L. B., Burakoff S. J., Rosenstein Y. (1996) J. Biol. Chem. 271, 27564–27568 [DOI] [PubMed] [Google Scholar]
- 25.Alvarado M., Klassen C., Cerny J., Horejsí V., Schmidt R. E. (1995) Eur. J. Immunol. 25, 1051–1055 [DOI] [PubMed] [Google Scholar]
- 26.Spertini F., Perret-Menoud V., Barbier N., Chatila T., Barbey C., Corthesy B. (2004) Immunology 113, 441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S. J., Nakagawa T., Kitamura H., Atsumi T., Kamon H., Sawa S., Kamimura D., Ueda N., Iwakura Y., Ishihara K., Murakami M., Hirano T. (2004) J. Immunol. 173, 3844–3854 [DOI] [PubMed] [Google Scholar]
- 28.Jonuleit H., Kühn U., Müller G., Steinbrink K., Paragnik L., Schmitt E., Knop J., Enk A. H. (1997) Eur. J. Immunol. 27, 3135–3142 [DOI] [PubMed] [Google Scholar]
- 29.Geisel J., Kahl F., Müller M., Wagner H., Kirschning C. J., Autenrieth I. B., Frick J. S. (2007) J. Immunol. 179, 5811–5818 [DOI] [PubMed] [Google Scholar]
- 30.Baum L. G., Seilhamer J. J., Pang M., Levine W. B., Beynon D., Berliner J. A. (1995) Glycoconj. J. 12, 63–68 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed H., Fink N. E., Pohl J., Vasta G. R. (1996) J. Biochem. 120, 1007–1019 [DOI] [PubMed] [Google Scholar]
- 32.Eriksson A. U., Singh R. R. (2008) J. Immunol. 181, 7468–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupasquier M., Stoitzner P., van Oudenaren A., Romani N., Leenen P. J. (2004) J. Invest. Dermatol. 123, 876–879 [DOI] [PubMed] [Google Scholar]
- 34.Blois S. M., Ilarregui J. M., Tometten M., Garcia M., Orsal A. S., Cordo-Russo R., Toscano M. A., Bianco G. A., Kobelt P., Handjiski B., Tirado I., Markert U. R., Klapp B. F., Poirier F., Szekeres-Bartho J., Rabinovich G. A., Arck P. C. (2007) Nat. Med. 13, 1450–1457 [DOI] [PubMed] [Google Scholar]
- 35.Mócsai A., Zhou M., Meng F., Tybulewicz V. L., Lowell C. A. (2002) Immunity 16, 547–558 [DOI] [PubMed] [Google Scholar]
- 36.Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. (2002) J. Cell Biol. 157, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vines C. M., Potter J. W., Xu Y., Geahlen R. L., Costello P. S., Tybulewicz V. L., Lowell C. A., Chang P. W., Gresham H. D., Willman C. L. (2001) Immunity 15, 507–519 [DOI] [PubMed] [Google Scholar]
- 38.Matzinger P. (2002) Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 39.Matzinger P. (1998) Semin Immunol. 10, 399–415 [DOI] [PubMed] [Google Scholar]
- 40.Matzinger P. (1994) Annu. Rev. Immunol. 12, 991–1045 [DOI] [PubMed] [Google Scholar]
- 41.He J., Baum L. G. (2004) J. Biol. Chem. 279, 4705–4712 [DOI] [PubMed] [Google Scholar]
- 42.Gallucci S., Lolkema M., Matzinger P. (1999) Nat. Med. 5, 1249–1255 [DOI] [PubMed] [Google Scholar]
- 43.Arndt P. G., Suzuki N., Avdi N. J., Malcolm K. C., Worthen G. S. (2004) J. Biol. Chem. 279, 10883–10891 [DOI] [PubMed] [Google Scholar]
- 44.Rabinovich G. A., Rubinstein N., Toscano M. A. (2002) Biochim. Biophys. Acta 1572, 274–284 [DOI] [PubMed] [Google Scholar]
- 45.Rabinovich G. A., Toscano M. A., Ilarregui J. M., Rubinstein N. (2004) Glycoconj. J. 19, 565–573 [DOI] [PubMed] [Google Scholar]
- 46.Rabinovich G. A., Baum L. G., Tinari N., Paganelli R., Natoli C., Liu F. T., Iacobelli S. (2002) Trends Immunol. 23, 313–320 [DOI] [PubMed] [Google Scholar]
- 47.Camby I., Le Mercier M., Lefranc F., Kiss R. (2006) Glycobiology 16, 137R–157R [DOI] [PubMed] [Google Scholar]
- 48.Steinman R. M., Hawiger D., Nussenzweig M. C. (2003) Annu. Rev. Immunol. 21, 685–711 [DOI] [PubMed] [Google Scholar]
- 49.Lutz M. B., Schuler G. (2002) Trends Immunol. 23, 445–449 [DOI] [PubMed] [Google Scholar]
- 50.Morelli A. E., Thomson A. W. (2007) Nat. Rev. Immunol. 7, 610–621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.