Abstract
ITK-SYK, a novel fusion tyrosine kinase (FTK) resulting from a recurrent t(5;9)(q33;q22), was recently identified in a poorly understood subset of peripheral T-cell lymphomas. However, the biochemical and functional properties of ITK-SYK are unknown. Here we demonstrate that ITK-SYK is a catalytically active tyrosine kinase that is sensitive to an established inhibitor of SYK. The expression of ITK-SYK, but not SYK, transformed NIH3T3 cells, inducing loss of contact inhibition and formation of anchorage-independent colonies in soft agar, in a kinase activity-dependent manner. ITK-SYK is unusual among FTKs in having an N-terminal phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology (PH) domain. Introduction of a well characterized loss-of-function mutation (R29C) into the PH domain of ITK-SYK inhibited its phosphorylation, markedly reduced its catalytic activity, and abrogated its ability to activate the ERK signaling pathway and to transform NIH3T3 cells. Although ITK-SYK was membrane-associated, ITK-SYK-R29C was not. However, each of these properties could be recovered by retargeting ITK-SYK-R29C back to the plasma membrane by the addition of an N-terminal myristylation sequence. Consistent with a model in which ITK-SYK requires PH domain-mediated binding to phosphatidylinositol 3,4,5-trisphosphate generated by phosphatidylinositol 3-kinase (PI3K), ITK-SYK activity was reduced by pharmacological inhibition of PI3K and increased by co-expression with a constitutively active form of PI3K. Together, these findings identify ITK-SYK as an active, transforming FTK dependent upon PH domain-mediated membrane localization, identify a novel mechanism for activation of an oncogenic FTK, and suggest ITK-SYK as a rational therapeutic target for t(5;9)(q33;q22)-positive lymphomas.
The generation of constitutively active fusion tyrosine kinases (FTKs)2 by chromosomal translocation is a recurrent mechanism of oncogenesis in hematological neoplasms (1, 2). In each case, FTKs comprise the C-terminal region of a tyrosine kinase, invariably including its catalytic domain, coupled to the N-terminal domains of a distinct “partner” protein. Well characterized examples include those involving the nonreceptor tyrosine kinase ABL and various N-terminal partners (most often BCR-ABL) in both chronic and acute leukemias (3) and those involving the receptor tyrosine kinase ALK and one of several N-terminal partners (most frequently NPM-ALK) in ALK-positive anaplastic large cell lymphoma (4). Understanding how such active FTKs are generated by chimerization of tyrosine kinases has shed considerable light on the molecular basis of these malignancies, and, as illustrated by the remarkable clinical activity of the ABL inhibitor imatinib, FTKs represent attractive targets for pharmacological intervention (3).
Peripheral T-cell lymphomas (PTCL) are clinically aggressive, often fatal, tumors arising from mature T-cells, for which current treatment strategies are inadequate (5, 6). This is the result, at least in part, of a very limited understanding of their genetic and biochemical pathogenesis and a consequent paucity of rational therapeutic targets. Recently, Streubel et al. (7, 8) described a novel, recurrent t(5;9)(q33;q22) in a subset of PTCL derived from follicular helper T-cells and showed this to involve ITK and SYK, genes encoding two nonreceptor tyrosine kinases. In this study, all PTCL carrying an ITK-SYK translocation contained identical ITK-SYK transcripts in which the 5′ coding sequence of ITK was joined to the 3′ coding sequence of SYK, predicting a chimeric protein in which the N-terminal pleckstrin homology (PH) and TEC homology domains of ITK (amino acids 1–165) are fused to much of interdomain B and the complete C-terminal kinase domain of SYK (amino acids 306–635) (see Fig. 1). Immunohistochemical staining for SYK was consistent with expression of this fusion protein in such cases; however, whether ITK-SYK is capable of cellular transformation, whether it is an active tyrosine kinase, and the biochemical mechanisms of such activity have not been established.
FIGURE 1.
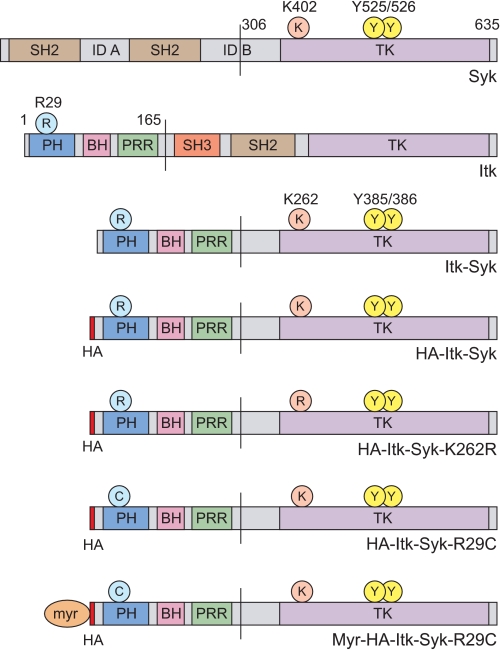
Domain structure of SYK, ITK, ITK-SYK, and mutants used in the present study. Amino acid numbers refer to the human proteins. SYK Lys402 (ITK-SYK Lys262) is an essential conserved residue within the ATP-binding site of the tyrosine kinase (TK) domain. The mutation of this residue in SYK or equivalent residues in other tyrosine kinases eliminates catalytic activity. SYK Tyr525/Tyr526 (ITK-SYK Tyr385/Tyr386) are conserved residues within the activation loop that are sites of autophosphorylation required for maximal activity. ITK Arg29 is a conserved residue within the lipid-binding pocket of the PH domain of TEC family kinases, the mutation of which prevents binding to PIP3 and subsequent membrane localization. myr represents the 12 N-terminal amino acids of LCK that contain the consensus sequence for both myristylation and palmitylation and are sufficient to localize several cytosolic proteins to the plasma membrane. SH2/3, SRC homology 2/3; ID A, interdomain A; ID B, interdomain B; BH, BTK homology; PRR, proline-rich region.
ITK and SYK both play key roles in lymphocyte antigen receptor signaling. ITK, a TEC family kinase, regulates several aspects of T-cell development, differentiation, and effector function (9, 10). Upon T-cell receptor ligation, ITK is recruited to and activated at the immunological synapse. This requires binding of its PH domain to membrane-associated phosphatidylinositol 3,4,5-trisphosphate (PIP3) generated by phosphatidylinositol 3-kinase (PI3K) (11–13), association with the SLP-76/LAT-nucleated adaptor complex (14), and phosphorylation by SRC family kinases such as LCK (15). ITK subsequently plays important roles in calcium signaling and actin reorganization, the former through phosphorylation and activation of phospholipase Cγ (9, 16). In lymphocytes, SYK is expressed at low levels in T-cells but at high levels in developing and mature B-cells, in which it is required for pre-BCR and BCR signaling (17, 18). Upon receptor ligation, SYK is activated by binding of its tandem N-terminal SH2 domains to diphosphorylated immunoreceptor tyrosine-based activation motifs, by subsequent phosphorylation (predominantly autophosphorylation) of tyrosine residues within the activation loop of the kinase domain, and by phosphorylation of additional tyrosine residues by SRC family kinases (19–21). SYK then binds and phosphorylates adaptor proteins and enzymes such as BLNK, VAV, phospholipase Cγ, and BTK to activate diverse downstream signaling pathways (22–25).
The discovery of an FTK involving SYK is not without precedent, because a TEL-SYK chimera resulting from a t(9;12)(q22;p12) in a patient with myelodysplastic syndrome/acute myeloid leukemia has been described (26). This FTK contains part of the C-terminal SH2 domain and the complete kinase domain of SYK fused to the N terminus of TEL/ETV6, an ets family transcription factor that is the N-terminal fusion partner in various FTKs found in several types of hematopoietic neoplasm (27). TEL-SYK is a constitutively active tyrosine kinase that induces cytokine independence when expressed in BaF3 cells and primary pre-B cells in vitro, the latter able to induce leukemia when subsequently injected into mice (26, 28).
Like other FTKs containing TEL, the tyrosine phosphorylation and transforming ability of TEL-SYK requires homodimerization mediated by the conserved N-terminal helix-loop-helix pointed domain of TEL (26). Indeed, the majority of oncogenic FTKs are activated by a common mechanism involving oligomerization via motifs in the N-terminal fusion partner and consequent autophosphorylation and constitutive activity, predominantly within the cytoplasm (1–3). Notably, however, ITK-SYK does not contain a recognized dimerization motif and instead is unusual among FTKs in containing a PH domain, presenting the possibility of a novel mechanism of FTK activation. In this study we have investigated the catalytic and transforming properties of ITK-SYK and examined the hypothesis that the PH domain might be an important determinant of the activation and downstream functions of this structurally unique FTK.
EXPERIMENTAL PROCEDURES
Cell Lines, Antibodies, and Reagents
Jurkat E6.1 T-cells and HEK-293T cells were grown in RPMI 1640, 10% fetal bovine serum. NIH3T3 cells were grown in DMEM, 10% NBCS. Anti-phospho-SYK (Tyr525/Tyr526) rabbit polyclonal, anti-phospho-p44/42 MAP kinase (Thr202/Tyr204) rabbit polyclonal, anti-p44/42 MAP kinase rabbit polyclonal, anti-phospho-AKT (Thr308) 244F9 rabbit monoclonal, and anti-AKT rabbit polyclonal antibodies were from Cell Signaling Technology/NEB (Hitchin, UK). Anti-SYK mouse monoclonal antibody 4D10 was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-HA mouse monoclonal antibody HA-7 was from Sigma-Aldrich (Gillingham, UK). Horseradish peroxidase-labeled rabbit anti-mouse and swine anti-rabbit monoclonal antibodies for Western blotting were from Dako (Ely, UK). Alexa Fluor 488-conjugated goat anti-mouse antibody for immunofluorescent staining was from Invitrogen. Sodium pervanadate was generated by adding 50 μl of 10 mm sodium orthovanadate to 450 μl of 10 mm H2O2. The PI3K inhibitor LY294002 and the SYK inhibitor Piceatannol (both from Calbiochem/Merck) were dissolved in Me2SO/phosphate-buffered saline as directed.
Plasmid Constructs
The ITK-SYK plasmid constructs used in this study are depicted in Fig. 1. The full ITK-SYK cDNA sequence was cloned into pIRESpuro2 (Clontech Laboratories/Takara Bio Europe, Saint-Germain-en-Laye, France) by reverse transcription-PCR of RNA from a PTCL carrying a t(5;9)(q33;q22)/ITK-SYK (the index case of Streubel et al. (7)) to create pIRESpuro2-ITK-SYK. pIRESpuro2-HA-ITK-SYK was generated by subcloning ITK-SYK into a pIRESpuro2 derivative containing an N-terminal HA tag (kindly provided by Dr. Alex Appert, University of Cambridge, Cambridge, UK). pIRESpuro2-HA-ITK-SYK-K262R and pIRESpuro2-HA-ITK-SYK-R29C were generated using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). pIRESpuro2-Myr-HA-ITK-SYK-R29C was generated by adding sequence encoding the first 12 amino acids (myristylation/palmitylation sequence, MGCVCSSNPEDDWMENIDVC) of chicken LCK to the 5′ end of HA-ITK-SYK-R29C by PCR. pMSCVpuro-HA-ITK-SYK, pMSCV-puro-HA-ITK-SYK-R29C, and pMSCVpuro-Myr-HA-ITK-SYK-R29C were generated by subcloning the respective inserts into pMSCVpuro (Clontech). Full-length human SYK cDNA (IMAGE clone 3542895) was obtained from Geneservice (Cambridge, UK) and subcloned into pMSCVpuro. pSG5-p110α-CAAX (29) was generated by Dr. Stefan Wennstrom and used with the kind permission of Dr. Julian Downward (CRUK-LRI, London, UK).
Transient Transfections and Stable Cell Lines
293T cells were transiently transfected with pIRESpuro2-derived or pMSCVpuro-derived plasmids using Lipofectamine 2000 (Invitrogen). Oligoclonal NIH3T3 cell lines stably expressing HA-ITK-SYK, HA-ITK-SYK-R29C, Myr-HA-ITK-SYK-R29C, or SYK (or carrying empty pMSCVpuro) were generated by retroviral transduction using the Phoenix ecotropic helper-free producer line followed by selection in puromycin. Jurkat E6.1 cells stably expressing ITK-SYK were generated by electroporation with pIRESpuro2-ITK-SYK using a Bio-Rad GenePulser II at 250 V, 950 microfarads, followed by puromycin selection and cloning by limiting dilution.
Immunoprecipitation, Western Blotting, and in Vitro Kinase Assay
Cell lysates were prepared using Triton X-100 lysis buffer (1% Triton X-100, 50 mm Tris, pH 7.4, 300 mm NaCl, 2 mm EDTA, 1 μg/ml each of aprotinin, leupeptin, and pepstatin, 1 mm phenylmethylsulfonyl fluoride, 2 mm Na3VO4, 20 mm NaFl). For immunoprecipitation, Triton X-100 lysates were incubated at 4 °C with antibody preadsorbed onto protein G-Sepharose beads (Amersham Biosciences). Western blotting of immunoprecipitated proteins or cell lysates resolved by SDS-PAGE and transferred to Immobilon P polyvinylidene difluoride membrane (Millipore, Billerica, MA) was performed using antibodies diluted in Tris-buffered saline, 0.1% Tween 20, 5% powdered nonfat milk and ECL (Amersham Biosciences). For in vitro kinase assays, post-immunoprecipitation Sepharose beads were used in a protein-tyrosine kinase assay kit (Sigma-Aldrich) in which phosphorylation of plate-bound poly-Glu-Tyr substrate was detected by horseradish peroxidase-conjugated anti-phosphotyrosine monoclonal antibody PT-66 using a PerkinElmer Victor plate reader at 490 nm.
Immunofluorescent Staining and Confocal Microscopy
Transfected 293T cells, grown on poly-l-lysine-coated coverslips (BD Laboratories) were fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, blocked with bovine serum albumin, sequentially incubated with anti-HA primary and goat anti-mouse Alexa Fluor 488 secondary antibodies, washed, and mounted with 4′,6-diamidino-2-phenylindole 1 mounting medium (Abbott, Maidenhead, UK). The cells were visualized using a Leica TCS SP laser scanning confocal microscope.
Focus Formation and Soft Agar Colony Formation Assays
For focus formation assays, stably transfected NIH3T3 cell lines were plated at 80% density and cultured for 2 weeks with fresh medium every 2–3 days. Subsequently, the cells were fixed in 1% glutaraldehyde and stained with 0.5% Crystal Violet, 25% methanol. For soft agar colony formation assays, stably transfected NIH3T3 cell lines were seeded at 50,000 cells/well in 2 ml of DMEM, 10% NBCS, 0.35% agarose over a base of 2 ml of DMEM, 10% NBCS, 0.6% agarose in 6-well plates. 2 ml of DMEM, 10% NBCS was added on top of the agar, and the cells were cultured for 3 weeks, with replacement of covering medium every 2–3 days.
RESULTS
ITK-SYK Is an Active Tyrosine Kinase
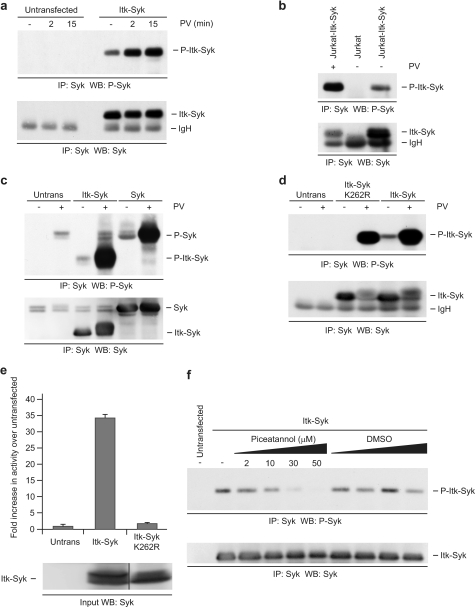
Phosphorylation of paired, conserved tyrosine residues (Tyr525/Tyr526) in the activation loop of the kinase domain of SYK occurs predominantly by autophosphorylation, is required for maximal catalytic activity and for downstream signaling activity, and is considered a good marker of active SYK (19, 21, 30–32). Therefore, to begin to determine whether ITK-SYK is a functional tyrosine kinase, we examined the phosphorylation of these residues (corresponding to Tyr385/Tyr386 of ITK-SYK) using a phosphospecific antibody. ITK-SYK was transiently transfected into 293T cells (Fig. 2a) and stably expressed in the Jurkat T-cell line (Fig. 2b) with similar results. ITK-SYK immunoprecipitated from either cell line was constitutively phosphorylated on Tyr385/Tyr386 in the absence of an exogenous stimulus, suggesting that it is present in an active state in these cells. Treatment of cells with the broad tyrosine phosphatase inhibitor pervanadate, known to activate many cellular tyrosine kinases, resulted in increased phosphorylation at these residues. Overexpression of SYK in 293T cells resulted in a basal level of phosphorylation at Tyr525/Tyr526, which could be enhanced by pervanadate treatment (Fig. 2c).
FIGURE 2.
ITK-SYK is an active tyrosine kinase. a, ITK-SYK is phosphorylated on Tyr385/Tyr386 in 293T cells. 293T cells, untransfected or transiently expressing ITK-SYK, were treated with pervanadate (PV) or left untreated as indicated and anti-SYK immunoprecipitates (IP) were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. b, ITK-SYK is phosphorylated on Tyr385/Tyr386 in Jurkat T-cells. Jurkat T-cells, untransfected or stably expressing ITK-SYK, were treated with PV or left untreated as indicated, and anti-SYK immunoprecipitates were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. c, overexpressed SYK is phosphorylated on Tyr525/Tyr526 in 293T cells. 293T cells, untransfected or transiently expressing ITK-SYK or SYK, were treated with pervanadate or left untreated as indicated, and anti-SYK immunoprecipitates were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. d, Kinase dead ITK-SYK is phosphorylated on Tyr385/Tyr386 in the presence but not absence of PV. 293T cells, untransfected or transiently expressing HA-ITK-SYK or HA-ITK-SYK-K262R, were treated for 15 min with PV or left untreated as indicated, and anti-SYK immunoprecipitates were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. e, ITK-SYK shows catalytic activity in vitro. 293T cells, untransfected or transiently expressing HA-ITK-SYK or HA-ITK-SYK-K262R, were treated for 15 min with PV, and anti-HA immunoprecipitates were subjected to in vitro kinase assay. The results are the means of duplicate wells from a representative experiment, and the error bars represent standard deviations. A portion of the input was probed with anti-SYK (the lanes shown were nonadjacent lanes in a single blot). f, ITK-SYK is inhibited by the SYK inhibitor Piceatannol. 293T cells, untransfected or expressing ITK-SYK, were treated for 30 min with Piceatannol or equivalent volumes of diluent control as indicated, and anti-SYK immunoprecipitates were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. WB, Western blotting.
To further examine whether the catalytic activity of ITK-SYK itself could mediate phosphorylation of Tyr385/Tyr386, cells were transfected with either ITK-SYK or a “kinase dead” mutant (ITK-SYK-K262R; Fig. 1) in which an essential, conserved lysine residue in the ATP-binding pocket of the SYK kinase domain was mutated to arginine (Fig. 2d) (31, 33). Unlike ITK-SYK, in the absence of pervanadate treatment ITK-SYK-K262R was not phosphorylated on Tyr385/Tyr386, suggesting that these residues represent target sites of autocatalytic activity in ITK-SYK as in SYK. Nevertheless, in the presence of pervanadate, ITK-SYK-K262R was phosphorylated at Tyr385/Tyr386, suggesting that these residues could be phosphorylated by other tyrosine kinases under these conditions.
To confirm that ITK-SYK is an active tyrosine kinase, its catalytic activity was examined using an in vitro kinase assay. As expected, ITK-SYK immunoprecipitated from pervanadate-treated cells (Fig. 2e) or from untreated cells (see Fig. 4c) showed readily detectable phosphorylation of an exogenous substrate, whereas ITK-SYK-K262R consistently showed no significant activity above control immunoprecipitates. In keeping with the catalytic activity of ITK-SYK, treatment of ITK-SYK-transfected cells with the well characterized, selective SYK inhibitor Piceatannol inhibited the autophosphorylation of Tyr385/Tyr386 in a concentration-dependent manner (Fig. 2f). Piceatannol reduced the phosphorylation of Tyr385/Tyr386 at a concentration of 10 μm, the approximate IC50 for inhibition of SYK, with maximal inhibition at 30–50 μm (34, 35).
FIGURE 4.
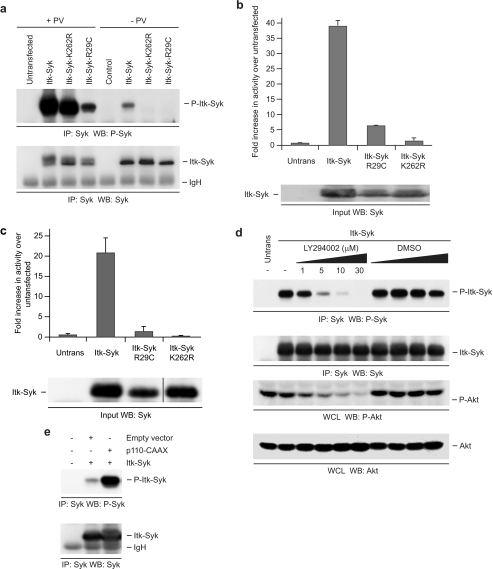
ITK-SYK activation requires a functional PH domain and active PI3K. a, PH domain mutation inhibits phosphorylation of ITK-SYK Tyr385/Tyr386. 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants, were treated for 15 min with PV or left untreated as indicated, and anti-SYK immunoprecipitates (IP) were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. b, PH domain mutation inhibits catalytic activity of ITK-SYK. 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants, were treated for 15 min with PV, and anti-HA immunoprecipitates were subjected to in vitro kinase assay. The results are the means of duplicate wells from a representative experiment, and the error bars represent standard deviations. A portion of the input was probed with anti-SYK. c, PH domain mutation inhibits catalytic activity of ITK-SYK. 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants, were left untreated, and anti-SYK immunoprecipitates were subjected to in vitro kinase assay. The results are the means of duplicate wells from a representative experiment, and the error bars represent standard deviations. A portion of the input was probed with anti-SYK (the lanes shown were nonadjacent lanes in a single blot). d, PI3K inhibition reduces phosphorylation of ITK-SYK Tyr385/Tyr386. 293T cells, untransfected or transiently expressing ITK-SYK, were treated for 30 min with LY294002 or equivalent volumes of diluent control as indicated, and anti-SYK immunoprecipitates were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. A portion of the same lysate was run separately and probed for phospho-AKT and AKT. e, Constitutively active PI3K increases phosphorylation of ITK-SYK Tyr385/Tyr386. 293T cells were left untransfected or transiently transfected with ITK-SYK and p110α-CAAX or ITK-SYK and an equal amount of empty control vector. Anti-SYK immunoprecipitates were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. WB, Western blotting; WCL, whole cells lysate.
ITK-SYK Induces Cellular Transformation in Vitro in a Kinase Activity-dependent Manner
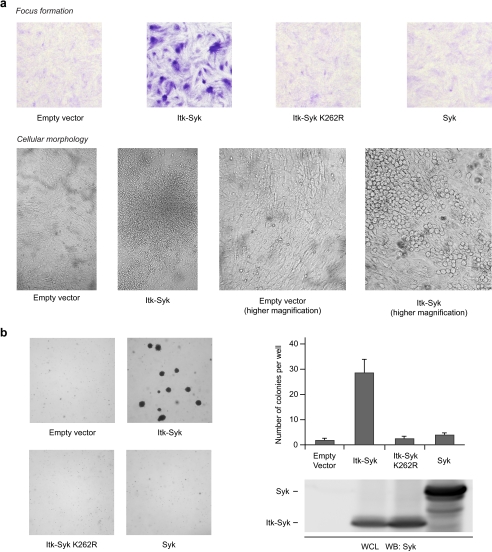
Although the recurrent detection of the ITK-SYK translocation in a subset of PTCL suggests a role for ITK-SYK in oncogenesis, whether ITK-SYK does indeed have transforming ability is unknown. To address this and to determine the role of the kinase activity of ITK-SYK in any transforming activity, we stably expressed ITK-SYK, ITK-SYK-K262R, or SYK in NIH3T3 cells and subjected these cell lines to standard in vitro transformation assays. Although cells carrying only empty vector grew to confluence and thereafter showed contact inhibition of growth, cells expressing ITK-SYK grew beyond confluence, without contact inhibition, forming numerous multilayered foci (Fig. 3a, top panels). The morphology of the transformed cells within these foci also became altered, the cells adopting a more rounded appearance (Fig. 3a, bottom panels). We also asked whether ITK-SYK could induce anchorage-independent growth in soft agar. Consistent with a transformed phenotype, cells expressing ITK-SYK were able to form large, anchorage-independent, multicellular colonies, whereas empty vector-transfected cells could not (Fig. 3b). Importantly, NIH3T3 cells expressing either SYK or ITK-SYK-K262R grew in a similar manner to empty vector-transfected cells, were unable to form foci in the focus formation assay (Fig. 3a), and did not form colonies in soft agar (Fig. 3b). These results show that ITK-SYK, but not SYK, has transforming activity in this in vitro system and indicate a requirement for catalytic activity in ITK-SYK-mediated transformation.
FIGURE 3.
Cellular transformation by ITK-SYK requires catalytic activity. a, focus formation assays. NIH3T3 cells stably transfected with HA-ITK-SYK grew beyond confluence and formed multilayered foci with altered cellular morphology, whereas cells transfected with empty vector, HA-ITK-SYK-K262R, or SYK did not. Upper panel, low power images of Crystal Violet stained cells; lower panel, high power images of cellular morphology and foci. The images are representative of several independent experiments. b, soft agar colony formation assays, NIH3T3 cells stably transfected with HA-ITK-SYK formed anchorage-independent colonies, whereas cells transfected with empty vector, HA-ITK-SYK-K262R or SYK did not. Left panels, low power images of representative wells. Right panels, quantification of macroscopically visible colonies. The results are the means of triplicate wells, and the error bars represent standard deviations. Whole cell lysates (WCL) of cell lines were probed with anti-SYK. The images and data are representative of several independent experiments. WB, Western blotting.
PH Domain-mediated Membrane Localization Is Required for ITK-SYK Activation
In the majority of FTKs the N-terminal fusion partner functions mainly to induce homo-oligomerization, enabling autophosphorylation and subsequent constitutive kinase activity (1–3). However, as noted above, ITK-SYK does not contain a recognized dimerization motif but does harbor an N-terminal PH domain that, in ITK and other TEC family kinases, mediates the binding to membrane-associated PIP3 required for kinase activation (11–13). To investigate whether a competent PH domain is required for the function of ITK-SYK, we introduced a point mutation, R29C, into the lipid-binding pocket of the PH domain (Fig. 1). This mutation abrogates both the binding of TEC family kinases to PIP3 and their associated membrane localization (36–38). As shown in Fig. 4a, whereas ITK-SYK was phosphorylated on Tyr385/Tyr386 in untreated cells, ITK-SYK-R29C, like the “kinase-dead” ITK-SYK-K262R, was not. Furthermore, the R29C mutation markedly reduced the phosphorylation of Tyr385/Tyr386 in cells treated with pervanadate, exerting a much greater effect than the K262R mutation under these conditions. Together, these results suggest that both the constitutive autophosphorylation of ITK-SYK and its maximal transphosphorylation at Tyr385/Tyr386 by other kinases require a PH domain able to bind PIP3. To confirm the requirement for a functionally intact PH domain in the activation of ITK-SYK, we compared ITK-SYK, ITK-SYK-R29C, and ITK-SYK-K262R in the in vitro kinase assay in both the presence (Fig. 4b) and absence (Fig. 4c) of pervanadate. Consistent with such a role, although ITK-SYK-R29C showed some enzymatic activity compared with untransfected cells or to ITK-SYK-K262R in the presence of pervanadate, there was a marked reduction in activity compared with ITK-SYK.
Because cellular PIP3 levels are controlled principally by PI3K and the activation of TEC family kinases requires PI3K activity (11, 38), we tested whether PI3K activity was required for the phosphorylation of ITK-SYK Tyr385/Tyr386. Cells transfected with ITK-SYK were treated with the PI3K inhibitor LY294002 prior to immunoprecipitation of ITK-SYK and assessment of Tyr385/Tyr386 phosphorylation (Fig. 4d). Treatment with LY294002 reduced the basal phosphorylation of the PI3K effector AKT, confirming effective inhibition of PI3K activity in these experiments. In the same experiments, LY294002 treatment produced a clear dose-dependent reduction in autophosphorylation of ITK-SYK Tyr385/Tyr386. Effective inhibition of ITK-SYK phosphorylation was also obtained using another PI3K inhibitor, wortmannin (data not shown). Treatment of the cells with pervanadate counteracted the inhibitory effects of LY294002 on ITK-SYK phosphorylation to a degree dependent on the relative lengths of exposure to pervanadate and LY294002 (data not shown). In complementary experiments, we assessed the effect of increasing cellular PI3K activity on the phosphorylation of ITK-SYK Tyr385/Tyr386 using a well characterized constitutively active form of PI3K, p110α-CAAX, composed of the catalytic p110α subunit fused to the membrane-targeting farnesylation/palmitylation signal of H-Ras (29). Co-expression of ITK-SYK with p110α-CAAX resulted in much higher levels of phosphorylation on Tyr385/Tyr386 than co-expression of ITK-SYK with an equal amount of control empty vector (Fig. 4e). Together, these data indicate that ITK-SYK is activated by a mechanism involving binding of its PH domain to PIP3 generated by PI3K.
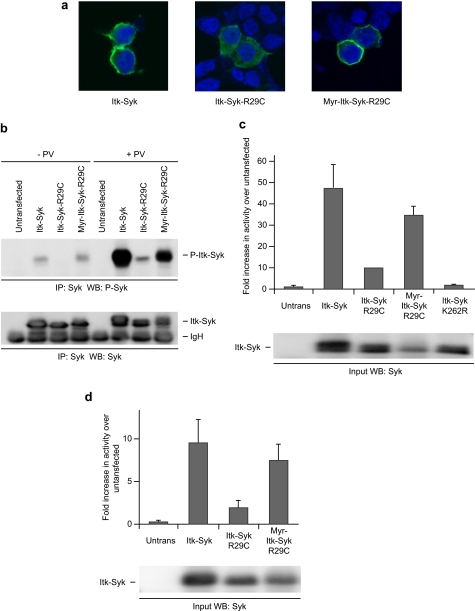
Activation of ITK and other TEC family kinases involves PIP3-mediated localization to the plasma membrane. We therefore began to assess the role of membrane localization in the activation of ITK-SYK by examining the subcellular localization of ITK-SYK and ITK-SYK-R29C by confocal microscopy. As shown in Fig. 5a, ITK-SYK was present predominantly at the plasma membrane, with staining also seen in the cytoplasm. By contrast, ITK-SYK-R29C was found in a purely cytoplasmic distribution, without evident membrane localization, indicating that ITK-SYK is targeted to the plasma membrane by binding to membrane-associated PIP3. The membrane localization of ITK-SYK but not its R29C mutant thus correlated with their phosphorylation and catalytic activity, suggesting that such localization might be necessary for the function of ITK-SYK. We therefore investigated whether retargeting ITK-SYK back to the plasma membrane, by adding an N-terminal myristylation/palmitylation sequence to create Myr-ITK-SYK-R29C (Fig. 1) (39, 40), could recover the loss of function caused by the R29C mutation. Confocal microscopy confirmed that Myr-ITK-SYK-R29C was almost exclusively associated with the membrane with little cytoplasmic staining (Fig. 5a). Analysis of ITK-SYK and its mutants immunoprecipitated from transfected cells showed that the addition of the myristylation sequence to ITK-SYK-R29C restored the phosphorylation of Tyr385/Tyr386 in unstimulated cells to the level seen on ITK-SYK itself (Fig. 5b). Similarly, the addition of the myristylation sequence to ITK-SYK-R29C recovered the phosphorylation of Tyr385/Tyr386 in cells stimulated with pervanadate. In keeping with the recovery of Tyr385/Tyr386 phosphorylation, the addition of the myristylation sequence was also able to rescue the ability of ITK-SYK-R29C to phosphorylate an exogenous substrate in vitro in the presence or absence of pervanadate (Fig. 5, c and d). Taking into account the tendency of Myr-ITK-SYK-R29C to be expressed at lower levels than ITK-SYK, this construct may in fact exhibit equivalent or higher activity compared with ITK-SYK itself. Together, these results suggest that membrane localization, rather than PIP3 binding per se, is an important determinant of the activation of ITK-SYK.
FIGURE 5.
Membrane relocalization of ITK-SYK substitutes for PH domain function. a, PH domain mutation prevents, and myristylation recovers, membrane association of ITK-SYK. 293T cells, transiently expressing HA-ITK-SYK or its mutants, were stained for HA and with 4′,6-diamidino-2-phenylindole and visualized by laser scanning confocal microscopy. Untransfected cells present demonstrate the specificity of staining for HA. b, membrane retargeting recovers phosphorylation of ITK-SYK-R29C. 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants, were treated for 15 min with PV or left untreated as indicated, and anti-SYK immunoprecipitates (IP) were probed sequentially for phospho-SYK (Tyr525/Tyr526) and SYK. c, membrane retargeting recovers the catalytic activity of ITK-SYK-R29C. 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants, were treated for 15 min with PV, and anti-SYK immunoprecipitates were subjected to in vitro kinase assay. The results are the means of duplicate wells from a representative experiment, and the error bars represent standard deviations. A portion of the input was probed with anti-SYK. d, membrane retargeting recovers the catalytic activity of ITK-SYK-R29C. 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants, were left untreated and anti-SYK immunoprecipitates were subjected to in vitro kinase assay. The results are the means of duplicate wells from a representative experiment, and the error bars represent standard deviations. A portion of the input was probed with anti-SYK. WB, Western blotting.
PH Domain-mediated Membrane Localization Is Required for ITK-SYK-mediated Downstream Signaling and Cellular Transformation
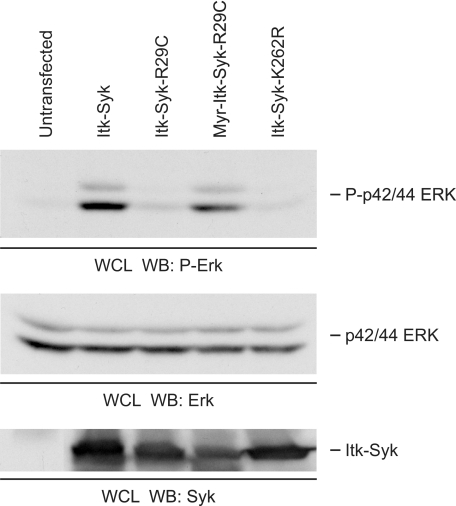
Because the above data demonstrate that PH domain-mediated membrane association is important for the phosphorylation and catalytic activity of ITK-SYK, we next examined its requirement in coupling to downstream signaling pathways. Transfection of ITK-SYK into 293T cells resulted in a substantial increase in the phosphorylation of p42/p44 ERK at activation loop residues associated with kinase activation (Fig. 6). This was dependent upon the catalytic activity of ITK-SYK because no increase in ERK phosphorylation was induced by the ITK-SYK-K262R mutant. Moreover, the ability of ITK-SYK to activate the ERK signaling pathway required PH domain-mediated membrane association as ITK-SYK-R29C was unable to induce phosphorylation of ERK, whereas the addition of the myristylation sequence completely recovered this phosphorylation. In the present system, therefore, the ability of ITK-SYK to regulate downstream signaling cascades requires catalytic activity dependent upon PH domain-mediated membrane localization.
FIGURE 6.
Requirement for kinase activity and PH domain function in downstream signaling by ITK-SYK. Whole cell lysates of 293T cells, untransfected or transiently expressing HA-ITK-SYK or its mutants were probed sequentially for phospho-ERK (Thr202/Tyr204) and ERK. A portion of the same lysate was run separately and probed with anti-SYK. WB, Western blotting; WCL, whole cells lysate.
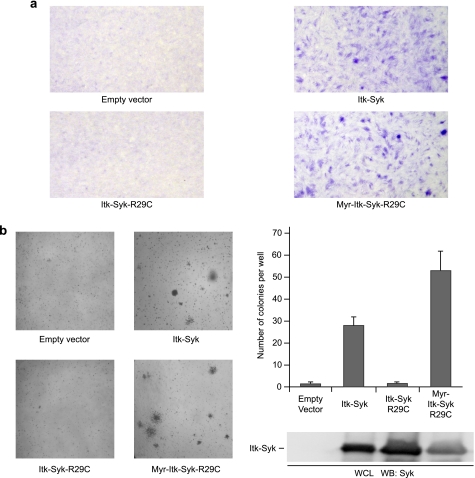
Finally, we assessed the requirement for a functional PH domain in ITK-SYK-mediated cellular transformation. NIH3T3 cells were stably transfected with ITK-SYK, ITK-SYK-R29C, or Myr-ITK-SYK-R29C and subjected to focus formation (Fig. 7a) and soft agar colony formation assays (Fig. 7b). Although cells expressing ITK-SYK grew as multilayered foci with altered cellular morphology in tissue culture and formed anchorage-independent colonies in soft agar (as described above; Fig. 3), those expressing ITK-SYK-R29C did not, instead growing in a similar manner to empty vector-transfected cells. However, in keeping with our data on ITK-SYK activation and signaling, retargeting this mutant back to the plasma membrane efficiently recovered the ability of transfected cells to grow as multilayered foci and to form colonies in soft agar. In fact, the expression of Myr-ITK-SYK-R29C resulted in an increased number of colonies compared with ITK-SYK, presumably as a result of a greater proportion of the total ITK-SYK pool being membrane-associated. Taken with the biochemical data, these results demonstrate that the transforming activity of ITK-SYK depends upon PH domain-mediated membrane localization and activation of the FTK.
FIGURE 7.
PH domain mutation inhibits, and membrane retargeting recovers, the transforming activity of ITK-SYK. a, focus formation assays. NIH3T3 cells stably transfected with HA-ITK-SYK or Myr-HA-ITK-SYK-R29C grew beyond confluence and formed multilayered foci, whereas cells transfected with empty vector or HA-ITK-SYK-R29C did not. Low power images of Crystal Violet stained cells. The images are representative of several independent experiments. b, soft agar colony formation assays. NIH3T3 cells stably transfected with HA-ITK-SYK or Myr-HA-ITK-SYK-R29C formed anchorage-independent colonies, whereas cells transfected with empty vector or HA-ITK-SYK-R29C did not. Left-hand panels, low power images of representative wells. Right-hand panels, quantification of macroscopically visible colonies. The results are the means of triplicate wells, and the error bars represent standard deviations. Whole cell lysates of cell lines were probed with anti-SYK. The images and data are representative of several independent experiments. WB, Western blotting; WCL, whole cells lysate.
DISCUSSION
The identification of the t(5;9)(q33;q22)/ITK-SYK (7) was an important step in understanding the pathogenesis of a subset of PTCL, but the mechanisms by which this translocation contributes to lymphoma development are unknown. We have now begun to address this issue by showing that ITK-SYK is an active tyrosine kinase with in vitro transforming properties and that ITK-SYK requires PH domain-mediated membrane association for activation, downstream signaling, and transformation.
Our observations permit consideration of some potential mechanisms by which t(5;9)(q33;q22)/ITK-SYK might, a priori, function at a molecular level. First, evidence is accumulating of an oncogenic role for SYK itself in lymphomagenesis. For example, the overexpression of SYK by gene amplification has been implicated in the pathogenesis of mantle cell lymphoma (41), and SYK is overexpressed by an unknown mechanism in at least some PTCL lacking an ITK-SYK translocation (42). However, the demonstration that the t(5;9)(q33;q22) generates a transforming fusion tyrosine kinase requiring the function of domains derived from both fusion partners indicates that this translocation is not simply a mechanism for the increased expression of the SYK kinase domain. The ability of ITK-SYK, but not SYK, to transform NIH3T3 cells in vitro adds further support to this interpretation. Second, although in almost all known FTKs the N-terminal fusion partners make a functional contribution to the kinase activity of the intact protein, there is at least one exception. In FIP1L1-platelet-derived growth factor receptor α, fusion to FIP1L1 appears to be only a mechanism to disrupt the auto-inhibitory juxtamembrane domain of platelet-derived growth factor receptor α, FIP1L1 itself being dispensable for transformation (43). Current evidence suggests that the kinase activity of SYK is regulated by its N-terminal region, the interdomain A of which is believed to complex with both interdomain B and the distal surface of the kinase domain to form an auto-inhibitory closed structure similar to that identified by structural studies of the SYK-homologue, ZAP-70 (44–46). The N terminus of ITK replaces that of SYK to generate ITK-SYK, and this inhibitory conformation of SYK is thus disrupted. However, our finding that ITK-SYK-R29C lacks signaling and transforming activity indicates that this disruption alone is insufficient to account for the properties of ITK-SYK.
Although the numerous FTKs identified are varied in their domain structure, ITK-SYK is unusual in containing an N-terminal PH domain. This raised the possibility that whereas most FTKs are activated in the cytoplasm, ITK-SYK might be activated by a mechanism requiring association with the plasma membrane. Indeed, in the system studied, we found that a substantial portion of the cellular pool of ITK-SYK was constitutively membrane-associated and showed that the R29C mutation, known to block the ability of TEC family PH domains to bind PIP3 and associate with the cell membrane (11–13), simultaneously abrogated the membrane association, signaling functions, and transforming activity of ITK-SYK. The demonstration that retargeting ITK-SYK-R29C back to the plasma membrane recovered and perhaps even enhanced these properties indicates that membrane localization is indeed key to the activation and function of ITK-SYK. In fact, although the requirement for membrane localization is unusual among FTKs, the necessity for some FTKs to localize to specific subcellular compartments is becoming increasingly recognized. For example, the ability of the N-terminal fusion partners to direct NUP214-ABL1 and FOP-FGFR1 to the nuclear pore complex and the centrosome, respectively, is thought to be critical for the activity of these FTKs (47, 48).
ITK-SYK is constitutively active in the cell types studied, in as much as its autophosphorylation, phosphorylation of an exogenous substrate, and activation of downstream signaling did not require an exogenous stimulus. Nevertheless, these functions required a PH domain able to bind PIP3, and the autophosphorylation of Tyr385/Tyr386 could be blocked or enhanced by respectively decreasing or increasing cellular PI3K activity, indicating regulation by upstream PI3K, which shows basal activity in both Jurkat and 293T cells (49). In this respect, the regulation of ITK-SYK shares similarities with the activation of ITK, which is also dependent upon PI3K for PH domain-mediated membrane association (11–13). Notably, however, studies have shown that the constitutive membrane association of ITK in Jurkat cells is insufficient for its activation (12, 49), suggesting that the basal activity of ITK-SYK in the cells studied here might reflect either differences in the regulation of the kinase domains of ITK and SYK or emergent properties arising from chimerization. In this regard it is interesting that the constitutive localization of SYK to the membrane, by fusion to a transmembrane domain or a myristylation sequence, results in phosphorylation and catalytic activation (50, 51). Our data raise the interesting possibility that during lymphomagenesis ITK-SYK might be activated via a PI3K-dependent mechanism. It will be important to examine whether the t(5;9)(q33;q22) might occur in PTCL together with genetic abnormalities or surface receptor signaling pathways that activate PI3K signaling.
Although the N-terminal portions of most FTKs contain established homo-oligomerization domains essential for constitutive kinase activity, such a recognized domain is not present in ITK-SYK. ITK itself dimerizes through complex intermolecular interactions requiring the SH2 and SH3 domains, but these domains are not retained in the ITK-SYK chimera (52). Nevertheless, the finding that, in unstimulated cells, phosphorylation of Tyr385/Tyr386 is prevented by both the R29C and K262R mutations suggests that this is an autophosphorylation event that occurs at the plasma membrane. Because this autophosphorylation is likely to occur in trans, our data raise the possibility that the PH domain-mediated membrane association of ITK-SYK may serve to bring ITK-SYK molecules into close proximity for cross-phosphorylation. Support for this hypothesis was recently provided by the finding that like ITK-SYK, ITK is constitutively membrane-associated when expressed in 293T cells and that in these cells ITK forms PH domain-dependent clusters, with an intermolecular distance of 80 Å or less in the region of activated PI3K (53).
Membrane association may also promote the activity of ITK-SYK in other ways. In the present experiments, treatment of cells with pervanadate increased the phosphorylation of both catalytically active and inactive ITK-SYK on Tyr385/Tyr386, suggesting that under these conditions ITK-SYK may be transphosphorylated by other tyrosine kinases. That this phosphorylation was markedly reduced by the R29C mutation and recovered by myristylation suggests that membrane association might serve to promote the phosphorylation of ITK-SYK by other kinases. In addition, membrane localization may also promote the association of ITK-SYK with other, as yet unidentified, signaling enzymes and adaptors, coupling it to the activation of downstream signaling pathways, such as the ERK pathway demonstrated herein. A final mechanism, in which PH domain-mediated membrane localization might contribute to the activation of ITK-SYK by inducing an activating conformational change, is suggested by experimentally derived models of other PH domain-containing tyrosine kinases. For example, binding of AKT to PIP3 is thought to induce a conformational change rendering the kinase susceptible to activating transphosphorylation by PDK1, whereas binding of BTK to PIP3 has been shown to allosterically enhance its autophosphorylation activity (54, 55). Further experiments to understand the ways in which membrane localization is required for the function of ITK-SYK are in progress.
In addition to elucidating an important aspect of the regulation of ITK-SYK, the present data are also significant for the demonstration that ITK-SYK is a biologically active tyrosine kinase with transforming properties. Expression of ITK-SYK in NIH3T3 cells resulted in altered morphology, loss of contact inhibition, and loss of anchorage dependence, well established characteristics of transformed cells, in a kinase activity-dependent manner. Similar phenotypes are produced in NIH3T3 cells by other FTKs identified in lymphoid leukemias and lymphomas including BCR-ABL, NPM-ALK, and TEL-FGFR3, an FTK identified in a patient with PTCL which progressed to acute myeloid leukemia (56–58). Together with the recurrent finding of t(5;9)(q33;q22)/ITK-SYK in primary PTCL samples, our data provide strong support for ITK-SYK as a rational therapeutic target in these lymphomas. We have shown that ITK-SYK can be inhibited by a selective inhibitor of the catalytic activity of SYK, and, because other SYK inhibitors are in development or are in early clinical trials for B-cell lymphomas, such compounds may be useful against t(5;9)(q33;q22)/ITK-SYK-positive PTCL. Importantly, the transforming activity of ITK-SYK was prevented by functional inactivation of the PH domain and rescued by constitutive membrane retargeting similarly to ITK-SYK signaling, indicating that transformation by ITK-SYK requires its activation at the plasma membrane. Our data thus suggest that the development of inhibitors able to block the PH domain-mediated association of ITK-SYK with the cell membrane represents an additional therapeutic approach for the treatment of these aggressive lymphomas.
Acknowledgments
We are grateful to Heike Laman, Fiona McDuff, and David Jones for help with retroviral transduction and transformation assays.
This work was supported by funds from the Medical Research Council (to S. R.), Leukaemia Research (to Y. H., M.-Q. D., and S. D. T.), and the Health Foundation, the Royal College of Pathologists, and the Pathological Society of Great Britain and Ireland (to C. M. B.).
- FTK
- fusion tyrosine kinase
- PH
- pleckstrin homology
- PIP3
- phosphatidylinositol 3,4,5-trisphosphate
- PI3K
- phosphatidylinositol 3-kinase
- PTCL
- peripheral T-cell lymphoma(s)
- BCR
- B-cell receptor
- DMEM
- Dulbecco's modified Eagle's medium
- MAP
- mitogen-activated protein
- HA
- hemagglutinin
- ERK
- extracellular signal-regulated kinase
- PV
- pervanadate
- NBCS
- newborn calf serum.
REFERENCES
- 1.Chalandon Y., Schwaller J. (2005) Haematologica 90, 949–968 [PubMed] [Google Scholar]
- 2.Turner S. D., Alexander D. R. (2006) Leukemia 20, 572–582 [DOI] [PubMed] [Google Scholar]
- 3.Wong S., Witte O. N. (2004) Annu. Rev. Immunol. 22, 247–306 [DOI] [PubMed] [Google Scholar]
- 4.Chiarle R., Voena C., Ambrogio C., Piva R., Inghirami G. (2008) Nat. Rev. Cancer 8, 11–23 [DOI] [PubMed] [Google Scholar]
- 5.Jaffe E. S., Harris N. L., Stein H., Vardiman J. W. (2001) World Health Organisation Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press, Lyon [Google Scholar]
- 6.Savage K. J. (2007) Blood Rev. 21, 201–216 [DOI] [PubMed] [Google Scholar]
- 7.Streubel B., Vinatzer U., Willheim M., Raderer M., Chott A. (2006) Leukemia 20, 313–318 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Moreau A., Dupuis J., Streubel B., Petit B., Le Gouill S., Martin-Garcia N., Copie-Bergman C., Gaillard F., Qubaja M., Fabiani B., Roncador G., Haioun C., Delfau-Larue M.-H., Marafioti T., Chott A., Gaulard P. (2009) Am. J. Surg. Pathol. 33, 682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg L. J., Finkelstein L. D., Lucas J. A., Schwartzberg P. L. (2005) Annu. Rev. Immunol. 23, 549–600 [DOI] [PubMed] [Google Scholar]
- 10.Felices M., Falk M., Kosaka Y., Berg L. J. (2007) Adv. Immunol. 93, 145–184 [DOI] [PubMed] [Google Scholar]
- 11.August A., Sadra A., Dupont B., Hanafusa H. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11227–11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching K. A., Kawakami Y., Kawakami T., Tsoukas C. D. (1999) J. Immunol. 163, 6006–6013 [PubMed] [Google Scholar]
- 13.Woods M. L., Kivens W. J., Adelsman M. A., Qiu Y., August A., Shimizu Y. (2001) EMBO J. 20, 1232–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogin Y., Ainey C., Beach D., Yablonski D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6638–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyeck S. D., Wilcox H. M., Bunnell S. C., Berg L. J. (1997) J. Biol. Chem. 272, 25401–25408 [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Rodriguez J., Readinger J. A., Viorritto I. C., Mueller K. L., Houghtling R. A., Schwartzberg P. L. (2007) Immunol. Rev. 218, 45–64 [DOI] [PubMed] [Google Scholar]
- 17.Chan A. C., van Oers N. S., Tran A., Turka L., Law C. L., Ryan J. C., Clark E. A., Weiss A. (1994) J. Immunol. 152, 4758–4766 [PubMed] [Google Scholar]
- 18.Turner M., Schweighoffer E., Colucci F., Di Santo J. P., Tybulewicz V. L. (2000) Immunol. Today 21, 148–154 [DOI] [PubMed] [Google Scholar]
- 19.Kurosaki T., Johnson S. A., Pao L., Sada K., Yamamura H., Cambier J. C. (1995) J. Exp. Med. 182, 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowley R. B., Burkhardt A. L., Chao H. G., Matsueda G. R., Bolen J. B. (1995) J. Biol. Chem. 270, 11590–11594 [DOI] [PubMed] [Google Scholar]
- 21.Keshvara L. M., Isaacson C. C., Yankee T. M., Sarac R., Harrison M. L., Geahlen R. L. (1998) J. Immunol. 161, 5276–5283 [PubMed] [Google Scholar]
- 22.Deckert M., Tartare-Deckert S., Couture C., Mustelin T., Altman A. (1996) Immunity 5, 591–604 [DOI] [PubMed] [Google Scholar]
- 23.Law C. L., Chandran K. A., Sidorenko S. P., Clark E. A. (1996) Mol. Cell. Biol. 16, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu C., Turck C. W., Kurosaki T., Chan A. C. (1998) Immunity 9, 93–103 [DOI] [PubMed] [Google Scholar]
- 25.Baba Y., Hashimoto S., Matsushita M., Watanabe D., Kishimoto T., Kurosaki T., Tsukada S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuno Y., Abe A., Emi N., Iida M., Yokozawa T., Towatari M., Tanimoto M., Saito H. (2001) Blood 97, 1050–1055 [DOI] [PubMed] [Google Scholar]
- 27.Bohlander S. K. (2005) Semin. Cancer Biol. 15, 162–174 [DOI] [PubMed] [Google Scholar]
- 28.Wossning T., Herzog S., Köhler F., Meixlsperger S., Kulathu Y., Mittler G., Abe A., Fuchs U., Borkhardt A., Jumaa H. (2006) J. Exp. Med. 203, 2829–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wennström S., Downward J. (1999) Mol. Cell. Biol. 19, 4279–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couture C., Williams S., Gauthier N., Tailor P., Mustelin T. (1997) Eur. J. Biochem. 246, 447–451 [DOI] [PubMed] [Google Scholar]
- 31.El-Hillal O., Kurosaki T., Yamamura H., Kinet J. P., Scharenberg A. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Billingsley M. L., Kincaid R. L., Siraganian R. P. (2000) J. Biol. Chem. 275, 35442–35447 [DOI] [PubMed] [Google Scholar]
- 33.Hanks S. K., Hunter T. (1995) FASEB J. 9, 576–596 [PubMed] [Google Scholar]
- 34.Oliver J. M., Burg D. L., Wilson B. S., McLaughlin J. L., Geahlen R. L. (1994) J. Biol. Chem. 269, 29697–29703 [PubMed] [Google Scholar]
- 35.Yamamoto N., Hasegawa H., Seki H., Ziegelbauer K., Yasuda T. (2003) Anal. Biochem. 315, 256–261 [DOI] [PubMed] [Google Scholar]
- 36.Salim K., Bottomley M. J., Querfurth E., Zvelebil M. J., Gout I., Scaife R., Margolis R. L., Gigg R., Smith C. I., Driscoll P. C., Waterfield M. D., Panayotou G. (1996) EMBO J. 15, 6241–6250 [PMC free article] [PubMed] [Google Scholar]
- 37.Bunnell S. C., Diehn M., Yaffe M. B., Findell P. R., Cantley L. C., Berg L. J. (2000) J. Biol. Chem. 275, 2219–2230 [DOI] [PubMed] [Google Scholar]
- 38.Várnai P., Rother K. I., Balla T. (1999) J. Biol. Chem. 274, 10983–10989 [DOI] [PubMed] [Google Scholar]
- 39.Resh M. D. (1994) Cell 76, 411–413 [DOI] [PubMed] [Google Scholar]
- 40.Zlatkine P., Mehul B., Magee A. I. (1997) J. Cell Sci. 110, 673–679 [DOI] [PubMed] [Google Scholar]
- 41.Rinaldi A., Kwee I., Taborelli M., Largo C., Uccella S., Martin V., Poretti G., Gaidano G., Calabrese G., Martinelli G., Baldini L., Pruneri G., Capella C., Zucca E., Cotter F. E., Cigudosa J. C., Catapano C. V., Tibiletti M. G., Bertoni F. (2006) Br. J. Haematol. 132, 303–316 [DOI] [PubMed] [Google Scholar]
- 42.Feldman A. L., Sun D. X., Law M. E., Novak A. J., Attygalle A. D., Thorland E. C., Fink S. R., Vrana J. A., Caron B. L., Morice W. G., Remstein E. D., Grogg K. L., Kurtin P. J., Macon W. R., Dogan A. (2008) Leukemia 22, 1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stover E. H., Chen J., Folens C., Lee B. H., Mentens N., Marynen P., Williams I. R., Gilliland D. G., Cools J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8078–8083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeitlmann L., Knorr T., Knoll M., Romeo C., Sirim P., Kolanus W. (1998) J. Biol. Chem. 273, 15445–15452 [DOI] [PubMed] [Google Scholar]
- 45.Brdicka T., Kadlecek T. A., Roose J. P., Pastuszak A. W., Weiss A. (2005) Mol. Cell. Biol. 25, 4924–4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deindl S., Kadlecek T. A., Brdicka T., Cao X., Weiss A., Kuriyan J. (2007) Cell 129, 735–746 [DOI] [PubMed] [Google Scholar]
- 47.Delaval B., Létard S., Lelièvre H., Chevrier V., Daviet L., Dubreuil P., Birnbaum D. (2005) Cancer Res. 65, 7231–7240 [DOI] [PubMed] [Google Scholar]
- 48.De Keersmaecker K., Rocnik J. L., Bernad R., Lee B. H., Leeman D., Gielen O., Verachtert H., Folens C., Munck S., Marynen P., Fornerod M., Gilliland D. G., Cools J. (2008) Mol. Cell 31, 134–142 [DOI] [PubMed] [Google Scholar]
- 49.Shan X., Czar M. J., Bunnell S. C., Liu P., Liu Y., Schwartzberg P. L., Wange R. L. (2000) Mol. Cell. Biol. 20, 6945–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolanus W., Romeo C., Seed B. (1993) Cell 74, 171–183 [DOI] [PubMed] [Google Scholar]
- 51.Kulathu Y., Hobeika E., Turchinovich G., Reth M. (2008) EMBO J. 27, 1333–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brazin K. N., Fulton D. B., Andreotti A. H. (2000) J. Mol. Biol. 302, 607–623 [DOI] [PubMed] [Google Scholar]
- 53.Qi Q., Sahu N., August A. (2006) J. Biol. Chem. 281, 38529–38534 [DOI] [PubMed] [Google Scholar]
- 54.Saito K., Scharenberg A. M., Kinet J. P. (2001) J. Biol. Chem. 276, 16201–16206 [DOI] [PubMed] [Google Scholar]
- 55.Calleja V., Alcor D., Laguerre M., Park J., Vojnovic B., Hemmings B. A., Downward J., Parker P. J., Larijani B. (2007) PLoS Biol. 5, e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Armstrong F., Duplantier M. M., Trempat P., Hieblot C., Lamant L., Espinos E., Racaud-Sultan C., Allouche M., Campo E., Delsol G., Touriol C. (2004) Oncogene 23, 6071–6082 [DOI] [PubMed] [Google Scholar]
- 57.Maeda T., Yagasaki F., Ishikawa M., Takahashi N., Bessho M. (2005) Blood 105, 2115–2123 [DOI] [PubMed] [Google Scholar]
- 58.Tao W. J., Lin H., Sun T., Samanta A. K., Arlinghaus R. (2008) Oncogene 27, 3194–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]