Abstract
Little is known about the regulation of eicosanoid synthesis proximal to the activation of cytosolic phospholipase A2α (cPLA2α), the initial rate-limiting step. The current view is that cPLA2α associates with intracellular/phosphatidylcholine-rich membranes strictly via hydrophobic interactions in response to an increase of intracellular calcium. In opposition to this accepted mechanism of two decades, ceramide 1-phosphate (C1P) has been shown to increase the membrane association of cPLA2α in vitro via a novel site in the cationic β-groove of the C2 domain (Stahelin, R. V., Subramanian, P., Vora, M., Cho, W., and Chalfant, C. E. (2007) J. Biol. Chem. 282, 20467–204741). In this study we demonstrate that C1P is a proximal and required bioactive lipid for the translocation of cPLA2α to intracellular membranes in response to inflammatory agonists (e.g. calcium ionophore and ATP). Last, the absolute requirement of the C1P/cPLA2α interaction was demonstrated for the production of eicosanoids using murine embryonic fibroblasts (cPLA2α−/−) coupled to “rescue” studies. Therefore, this study provides a paradigm shift in how cPLA2α is activated during inflammation.
Eicosanoids are a class of bioactive lipids derived from the 20-carbon fatty acid, arachidonic acid (AA),2 including prostaglandins, prostacyclins, thromboxanes, and leukotrienes. The production of AA is the initial rate-limiting step in the production of eicosanoids, and the major phospholipase that regulates eicosanoids synthesis in response to agonists is group IVA cytosolic phospholipase A2 (cPLA2α) (2, 3). Activation of cPLA2 in cells requires the association of the enzyme with intracellular membranes in a Ca2+-dependent manner. This translocation of cPLA2α from the cytosol to intracellular membranes is mediated by a Ca2+-dependent lipid binding domain (CaLB domain) located at the N terminus of the enzyme (4–7). The CaLB domain is ∼60 amino acids and binds phosphatidylcholine (PC) in a Ca2+-dependent manner (3, 8–10). However, it is not known if physiologic calcium is sufficient to activate and translocate cPLA2α to membranes in cells or if activation also requires the generation of other activating lipids, such as the focus of this study, ceramide 1-phosphate (C1P).
One possible activating lipid, phosphatidylinositol 4,5-diphosphate, was ruled out by Balboa and co-workers (11) as a lipid co-factor required for the translocation of the enzyme. This group showed that the interaction with this lipid (via its catalytic domain) was required for full activity of cPLA2α after the enzyme translocated to the membrane (11). Another recent report by Leslie and co-workers (12) confirmed these findings, and a recent study by our laboratory corroborated these findings utilizing biophysical approaches (1). Specifically, we showed that C1P induced a dramatic increase of cPLA2α activity strictly by increasing the residence time of cPLA2α to membranes, whereas phosphatidylinositol 4,5-diphosphate enhanced the enzymes catalytic activity and membrane penetration (13, 14).
Recent studies from our laboratory have also demonstrated that C1P enhances the association of cPLA2α with membranes in vitro via a novel interactions site adjacent to the calcium binding region II of the C2 domain. Mutations of specific amino acids of this region significantly reduced the affinity for C1P (>65%) without an effect on basal enzyme activity, calcium-dependent PC affinity (supplemental Table 1), and phosphatidylinositol 4,5-diphosphate activation/affinity (1, 14). The identification and characterization of the C1P interaction site in cPLA2α allowed our laboratory to determine whether C1P played a role in regulating cPLA2α translocation and, thus, eicosanoid synthesis in response to inflammatory agonists.
EXPERIMENTAL PROCEDURES
Cells Culture
A549 lung adenocarcinoma cells were obtained from American Type Culture Collection. The cells were grown in 50% Dulbecco's modified Eagle's medium (Invitrogen) and 50% RPMI (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 2% penicillin/streptomycin (BioWhittaker) at standard incubation conditions and passaged every 3 days. Mouse embryonic fibroblasts (MEFs) were isolated from 13 or 14 days pregnant mice as previously described (15). MEFs were grown in high glucose Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 20% fetal bovine serum (Invitrogen) and 2% penicillin/streptomycin (BioWhittaker) at standard incubation conditions and passage every 3 days. MEFs were only utilized at passage 2 or 3. Human embryonic kidney 293 (HEK293), for packaging adenoviruses, were cultured in Dulbecco's modified Eagle's medium (Invitrogen) that was supplemented with 10% fetal bovine serum (Invitrogen) and 2% penicillin/streptomycin (BioWhittaker) at standard incubation conditions.
Construction of Fluorescent Tagged cPLA2α
For GFP-tagged cPLA2α(WT), adeno-X expression system 1 (Clontech Laboratories) was used to construct recombinant adenoviruses. The procedure used conventional in vitro ligation to incorporate a mammalian expression cassette into a replication incompetent human adenoviral type 5 genome. GFP-cPLA2α(WT) (a gracious gift from Dr. Christina Leslie, National Jewish Health, Denver, CO) was cloned into the pTREshuttle2 vector via the NheI and SalI sites. The three basic amino acids in the C2 domain of cPLA2α were mutated to generate the mutant cPLA2α R57A/K58A/R59A using the QuikChange site-directed mutagenesis kit (Stratagene) as previously described by our laboratory (1). For enhanced cyan fluorescent protein (CFP)-tagged cPLA2α(WT) and enhanced yellow fluorescent protein (YFP)-tagged cPLA2α(R57A/K58A/R59A), the GFP tag was replaced with ECFP or EYFP using NheI and BsrGI. The fluorescently tagged cPLA2α was then excised from pTREshuttle2 by the double digestion PI-SceI/I-CeuI and ligated into the adeno-X-viral DNA. In the last stage, the recombinant adeno-X vector was packaged into infectious adenovirus by transfecting HEK293 cells to allow the propagation of the virus. The recombinant adenovirus was harvested by lysing the cells by three freeze/thaw cycles. All constructs and mutations were confirmed by DNA sequencing.
Confocal Microscopy
A549 cells or MEFs cPLA2α−/− (0.5 × 105 and 0.2 × 105 cells per well, respectively) were seeded onto 22 × 22-mm coverslips in 35-mm plates in the appropriate media and incubated under standard incubator conditions. The following day the cells were infected with adenovirus containing either the combination GFP-cPLA2α(WT) or GFP-cPLA2α(R57A/K58A/R59A) and Tet-Off adenovirus (10, 10, and 50 multiplicity of infection, respectively) or the combination CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A) with Tet-Off adenovirus (10, 10, and 50 multiplicity of infection, respectively). After 48 h of incubation, the cells were rested in 2% serum media for 2 h and treated with inflammatory agonists, TNFα (50 ng/ml for 1–4 h), Il-1β (100 ng/ml for 1–4 h), ATP (100 μm for 1–4 h), or calcium ionophore such as A23187 (10 μm for 5 min) or ionomycin (10 μm for 5 min). Cells were washed twice with cold PBS to remove excess serum and fixed with 100% cold methanol for 10 min at −20 °C. After washing with PBS, the coverslips were mounted in 10 mm n-propyl gallate in glycerol and viewed using a Leica confocal microscope (TCS-SP2 (AOBS)). All experiments were de-identified and under taken in a double-blind manner. The excitation/emission spectrums were 488/509 for GFP, 436/480 for CFP, and 500/535 for YFP.
Live Cell Confocal Microscopy
A549 cells (0.5 × 105 cells) were seeded in 35-mm plates with glass bottoms in their appropriate media and incubated under standard incubator conditions. The following day the cells were infected with adenovirus containing the combination CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A) with Tet-Off adenovirus (10, 10, and 50 multiplicity of infection, respectively). After 48 h of incubation, the cells were rested in 2% serum media for 2 h and treated with calcium ionophore such as A23187 (10 μm) or ionomycin (10 μm) and viewed using a Leica confocal microscope over 9–10 min. Data were collected every minute during this time frame.
Subcellular Fractionation of Endogenous and Ectopically Expressed cPLA2α
For ectopic expression of cPLA2α, mouse embryonic fibroblasts, cPLA2α−/− (0.2 × 105 cells per 35-mm plate) were infected with adenovirus containing GFP-cPLA2α(WT) or GFP-cPLA2α(R57A/K58A/R59A). After 48 h the cells were used for the fractionation procedure. For endogenous cPLA2α, A549 cells were plated into a 10-cm dish at the concentration of 1 × 106 cells per dish in their appropriate media and incubated under standard incubator conditions overnight. The following day the cells were rested for 2 h in 2% serum containing media and then treated with calcium ionophore (10 μm A23187 for 5 min or 10 μm ionomycin for 5 min). For fractionation, cells were placed on ice for a few minutes and washed with cold PBS. 600 μl of lysis buffer (20 mm HEPES, 1 mm EGTA, and 0.34 m sucrose) containing protease inhibitors were added to the plate, and cells were scraped. After sonication (3 × 15s) on ice, the cell extract was centrifuged at 100,000 × g for 1 h. The supernatant was separated and contained the proteins from the cytosol. The pellet was resuspended into 450 μl of lysis buffer and constituted the membrane fraction.
Immunoblotting
Western blot analysis was performed as described previously (16, 17) using 10 μg of protein from each extract. Mouse anti-cPLA2α (1:1,000) (Santa Cruz) was used to identify the protein of interest. Anti β-actin antibody (1:5000) (Sigma) and anti-protein-disulfide isomerase antibody (1:1000) (Stressgen) were used as normalizing factors.
RNA Interference
Silencing of ceramide kinase (CERK) was performed using sequence-specific siRNA purchased from Dharmacon as described previously (18). The human CERK RNA interference sequence starts at 142 nucleotides after the start codon (UGCCUGCUCUGUGCCUGUAdTdT and UACAGGCACAGAGCAGGCAdTdT) as detailed previously (19). siRNA was transfected into A549 cells using Dharmafect1 (Dharmacon) according to the manufacturer's instructions. Briefly, A549 cells (0.5 × 105 cells) were seeded onto 22 × 22-mm coverslips in 35-mm plates in their appropriate media and incubated at 37 °C under standard incubator conditions. After overnight incubation, siRNA was transfected into the cells. After 36 h of incubation, the slides were subjected to analysis by confocal microscopy.
RNA Isolation, Reverse Transcription-PCR, and Quantitative PCR
To evaluate the down-regulation of CERK, total RNA was isolated using RNeasy kit (Qiagen) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed using Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's protocol. The level of CERK transcript was monitored using quantitative PCR and TaqMan technology (Applied Biosystems) specific to CERK with 18 S as a control. Premixed primer-probe sets and Taqman Universal PCR master mix were purchased from Applied Biosystems, and the cDNA was amplified using ABI 7900HT.
Mixed-micelle Assay for cPLA2α
cPLA2α activity was measured in a PC mixed-micelle assay in a standard buffer composed of 80 mm HEPES (pH 7.5), 150 mm NaCl, 10 μm free Ca2+, and 1 mm dithiothreitol. The assay also contained 0.3 mm PAPC with 250,000 dpm [14C]PAPC, 2 mm Triton X-100, 26% glycerol, and 10 μg of the cell extract or 1 μg of purified cPLA2 protein in a total volume of 200 μl. To prepare the substrate, an appropriate volume of cold PAPC in chloroform and [14C]PAPC in toluene-ethanol (1:1) solution was evaporated under nitrogen. Triton X-100 was added to the dried lipid to give a 4-fold concentrated substrate solution (1.2 mm PAPC). The solution was probe-sonicated on ice (1 min on, 1 min off for 3 min). The reaction was initiated by adding the enzyme and was stopped by the addition of 2.5 ml of Dole reagent (2-propanol, heptane, and 0.5 m H2SO4, 400:100:20, v/v/v). The amount of [14C]AA produced was determined using the Dole procedure as described previously (1, 14). All assays were conducted for 45 min at 37 °C.
PGE2 Rescue Experiment
MEFs, cPLA2α−/−, or cPLA2α+/+ were plated on a 35-mm plate and grown under standard incubator conditions in their appropriate media. The following day the cPLA2α−/− cells were transfected with viruses containing cPLA2α(WT) or cPLA2α(R57A/K58A/R59A) and/or Tet-Off viruses (1, 1, and 5 multiplicity of infection, respectively). After 48 h the cells were rested for 2 h in 2% serum, treated with various inflammatory agonists, TNFα (50 ng/ml for 0.5 to 8 h), Il-1β (100 ng/ml for 0.5 to 8 h), or ATP (100 μm for 0.5 to 8 h), and the culture medium was collected at 0.5, 2, 4, 6, and 8 h for PGE2 measurement. PGE2 levels were measured using the PGE2 Express EIA kit (Cayman Chemical) according to the manufacturer's instructions as previously described (20, 21).
C1P Detection by Mass Spectrometry and Liquid Chromatography/Electrospray Ionization/Tandem Mass Spectroscopy Protocol
A549 cells (1 × 106) were plated on 10-cm plates in the appropriate medium and grown under standard incubator conditions overnight. The next day cells were subjected to the relevant treatment. After treatment the plates were placed on ice, and the cells were washed twice with ice-cold PBS and harvested by scraping in 200 μl of PBS followed by sonication to obtain a homogenous mixture. At this time an aliquot of cells was taken before sonication for total cell count. Lipids were extracted from the remaining cells using a modified Bligh and Dyer method and analyzed as described by Merrill et al. (22) with slight modifications. Briefly, to 250 μl of the cells in PBS, 1.5 ml of methanol/chloroform 2:1 was added. The samples were spiked with 500 pmol of d18:1/12:0 C1P as the internal standard (Avanti). The mixture was sonicated to disperse the cell clumps and incubated overnight at 48 °C. The following day the extracts were subjected to base hydrolysis for 2 h at 37 °C and neutralized afterward. The neutralization was confirmed with pH paper. The extracts thus obtained were dried down and resolubilized in the mobile phase (60% A, 40% B) and used for analysis of C1P. The lipids were separated using a Discovery C18 column on a Shimadzu high performance liquid chromatography and eluted using a linear gradient (solvent A, 58:41:1 CH3OH/water/HCOOH 5 mm ammonium formate; solvent B, 99:1 CH3OH/HCOOH 5 mm ammonium formate, 60–100% B in 4 min and at 100% B for 6 min at a flow rate of 1 ml/min at 60 °C). Electrospray ionization with tandem mass spectroscopy using an API 4000 QTRAP instrument (Applied Biosystems, MDS Sciex) was used to detect C1P under positive ionization. Multiple reaction monitoring transitions monitored for C1P were 562.4/264.4 (d18:1/12:0), 590.4/264.4 (d18:1/14:0), 618.4/264.4 (d18:1/16:0), 620.4/264.4 (d18:0/16:0), 644.4/264.4 (d18:1/18:1), 646.4/264.4 (d18:1/18:0), 674.4/264.4 (d18:1/20:0), 702.4/264.4 (d18:1/22:0), 728.4/264.4 (d18:1/24:1), 730.4/264.4 (d18:1/24:0), 756.4/264.4 (d18:1/26:1), 758.4/264.4 (d18:1/26:0) used at the optimum collision energies of +41, +43.5, +43.5, +46.0, +46.0, +48.5, +51.0, +53.5, +56.0, +56.0, +58.5, and +58.5 V, respectively.
C1P Analysis by 32P Pulse and Steady State Labeling
A549 cells (1 × 106 per plate) were seeded overnight followed by the addition of [32P]orthophosphate (PerkinElmer Life Sciences) (30 μCi/ml) for 4 h (pulse) or 24 h (steady state). The cells were then treated with A23187 (10 μm), the plates were then placed on ice, and the lipids were extracted using the Bligh and Dyer method (23) followed by a base hydrolysis with 0.4 m methanolic NaOH for 2 h at 37 °C (24). The samples were dried under N2 and stored at −80 °C. For detection of C1P, the samples were resuspended in chloroform-methanol (75:25) and spotted onto a 10 × 10 cm TLC plate (silica gel) (VWR International). The lipids were separated using a chloroform-acetone-methanol-acetic acid-water (10:4:3:2:1) solvent mixture. The 32P-labeled lipids were detected by autoradiography and quantified by scintillation counting.
Lipid Vesicle Binding
Surface plasmon resonance was performed at 25 °C to test the Ca2+ dependence of binding of cPLA2α(WT) and the (R57A/K58A/R59A) mutant to 1-palmitoyl-2-oleoylphosphatidylcholine vesicles. 6500 resonance units of 1-palmitoyl-2-oleoylphosphatidylcholine were coated to flow cell 2 in a Biacore X instrument, and flow cell 1, left blank, was a control to monitor nonspecific binding as previously described (1). Equilibrium (steady state) surface plasmon resonance measurements were then performed at a flow rate of 5 μl/min, and the saturating response at each respective protein concentration was recorded. The saturating responses, termed Req, were then plotted versus protein concentrations (C), and the Kd value was determined by a nonlinear least-squares analysis of the binding isotherm using an equation Req = Rmax/(1 + Kd/C). Each data set was repeated three times to calculate the S.D.
RESULTS
Endogenous C1P Is Required for Translocation of cPLA2α in Response to Increases in Intracellular Calcium
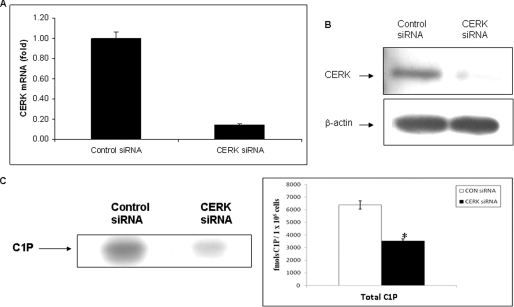
To assess the role of C1P in the translocation of cPLA2α, we first characterized formation of C1P in A549 human lung adenocarcinoma cells, a model of lung epithelial cells. CERK, the only verified enzyme to produce C1P in mammalian cells, was down-regulated utilizing siRNA as previously described (19, 21), and C1P levels were analyzed by radioactive labeling and quantitative mass spectrometry (21, 25). CERK siRNA down-regulated the enzyme >80% after 36 h (Fig. 1, panel A and B), which led to a >40% decrease in the total mass of both [32P]C1P and d-erytho-C16 C1P (Fig. 1, panels C and D). The finding for specific loss of d-erytho-C16 C1P is in accord with the recent findings of Bornancin and co-workers (26, 27). CERK siRNA also inhibited the increase in the total mass of d-erytho-C16 C1P when stimulated by calcium ionophore (Fig. 1, panel E).
FIGURE 1.
Endogenous C1P is required for the translocation of cPLA2α in response to calcium ionophore. A, A549 cells (0.5 × 105 cells/35-mm plate) were transfected with siRNA specific to CERK using Dharmafect1 reagent as previously described (19, 21). After 36 h, the total RNA was isolated and analyzed by quantitative PCR. Data are representative of n = 4 from 2 separate occasions. B, A549 cells (0.5 × 106 cells/10-cm plate) were simultaneously transfected with CERK siRNA as described in panel A. Protein extracts were produced, and the level of CERK was examined by Western blot analysis using a monoclonal antibody specific to CERK (21). β-Actin was used as a loading control. C, A549 cells (0.5 × 106 cells/10-cm plate) were again transfected with CERK siRNA as described in panel A. C1P levels were then analyzed by steady state labeling with [32P]orthophosphate as described under “Experimental Procedures.” The lipids were harvested and subjected to lipid analysis using TLC and autoradiography. The spots on the TLC plate corresponding to C1P as denoted by the standards were scraped and counted by scintillation counting. The graph represents the amount of C1P detected and is representative of n = 6 from 2 separate occasions. Statistical significance was evaluated with Student's t test (*, p < 0.001). D, using the same methodology as panel B; the cells were processed for mass spectrometry as described under “Experimental Procedures.” The graph represents the level of the different C1P subspecies in fmol of C1P/1 × 106 cells. Data are representative of n = 4 from 2 separate occasions. Statistical significance was evaluated with Student's t test (*, p < 0.05). E, A549 cells (0.5 × 106 cells per 10-cm plate) were transfected with control siRNA or siRNA-specific to CERK using Dharmafect reagent as described under “Experimental Procedures.” After 36 h of incubation, the cells were treated with DMSO or A23187 for 5 min, and C1P levels were then analyzed by mass spectrometry as described previously. The graph represents the % control of the level of d-erytho-C16 C1P. Data are representative of n = 6 from 3 separate occasions. Statistical significance was evaluated with Student's t test (*, p < 0.001; **, no statistical difference compared with untreated samples). F, A549 cells (0.5 × 105 cells) were seeded overnight onto 22 × 22-mm coverslips in 35-mm plates. The cells were infected with GFP-cPLA2α(WT) adenovirus. After 24 h, siRNA specific to CERK was transfected into the cells using Dharmafect1 reagent. After 36 h, slides were analyzed by confocal microscopy. The figure depicts translocation of GFP-cPLA2α(WT) treated with CERK siRNA or control siRNA upon activation with calcium ionophore (A23187 (10 μm, 5 min) or ionomycin (10 μm, 5 min)). DIC, differential interference contrast. G, a graph representing the % of translocation from the data depicted in panel D. Data are the mean ± S.E. and are representative of at least three different experiments. Statistical significance was evaluated with Student's t test (*, p < 0.05).
Once CERK was confirmed as the anabolic enzyme for C1P formation in response to A23187 in our cell model, we examined the role of endogenous C1P on the translocation of cPLA2α. A549 cells were transfected with GFP-cPLA2α followed by down-regulation of CERK by siRNA technology. Down-regulation of CERK blocked the translocation of wild type (WT) cPLA2α by 60% (Fig. 1, panels F and G) in a relevant time frame for the formation of newly synthesized C1P (supplemental Fig. 1). Thus, endogenous generation of C1P is necessary for the translocation of cPLA2α in cells.
The C1P/cPLA2α Interaction Is Required for the Translocation of cPLA2α in Responses to Increases in Intracellular Calcium
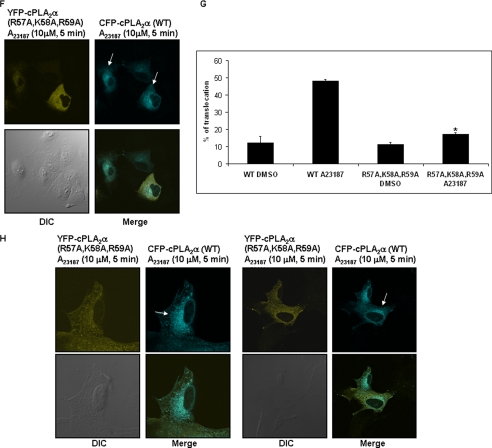
To specifically examine the role of C1P/cPLA2α interaction in the activation of cPLA2α, the previously characterized mutant of the C1P binding site (R57A/K58A/R59A) of cPLA2α was utilized (1). This mutant of cPLA2α has normal basal activity, calcium-dependent PC-affinity (supplemental Table 1), phosphatidylinositol 4,5-diphosphate responsiveness, and intracellular localization but dramatically reduced affinity for C1P. Translocation of GFP-cPLA2α(WT) and GFP-cPLA2α(R57A/K58A/R59A) was monitored by confocal microscopy upon activation with calcium ionophore (Fig. 2, panels A–C). In this double-blind study, translocation of the wild type enzyme was again observed in 58% of cells in response to calcium ionophore, in contrast to 19% for the mutant enzyme, corresponding to a 72% reduction of cPLA2α translocation.
FIGURE 2.
The C1P/cPLA2α interaction is required for translocation of the enzyme in response to calcium ionophore. A549 cells (0.5 × 105 cells) were seeded overnight onto 22 × 22-m coverslips in 35-m plates. The cells were infected with adenovirus containing GFP-cPLA2α(WT) (panel A), GFP-cPLA2α(R57A/K58A/R59A) (panel B), or CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A) (panel F). After 48 h, the cells were treated with calcium ionophore (A23187 (10 μm, 5 min)) and viewed using a Leica confocal microscope. A, effect of calcium ionophore on GFP-cPLA2α(WT). DIC, differential interference contrast. B, effect of calcium ionophore on GFP-cPLA2α(R57A/K58A/R59A). C, a graph representing the % of translocation from the data depicted in panels A and B. Data are the mean ± S.E. and are representative of at least six different experiments. Statistical significance was evaluated with Student's t test (*, p < 0.05). D, A549 cells were plated into a 10-cm dish at the concentration of 1 × 106 cells per dish in their appropriate media and incubated under standard incubator conditions overnight. The following day the cells were rested for 2 h in 2% serum-containing media and then treated with calcium ionophore (10 μm A23187 for 5 min). The cells were fractionated into cytosol and membrane fractions. Protein samples were analyzed by Western immunoblotting analysis using anti-cPLA2α antibody (1:1000) (Santa Cruz), anti-protein-disulfide isomerase antibody (1:1000) (Stressgen) to normalize the membrane fractions, and anti β-actin (1:5000) (Sigma) to normalize the cytosolic fractions. E, mouse embryonic fibroblasts, cPLA2α−/− (0.2 × 105 cells/35-mm plate), were infected with adenovirus containing GFP-cPLA2α(WT) or GFP-cPLA2α(R57A/K58A/R59A). After 48 h the cells were rested for 2 h in 2% serum-containing media and then treated with calcium ionophore (10 μm A23187 for 5 min). The cells were fractionated as detailed under “Experimental Procedures” into cytosol fraction and membrane fraction. Protein samples were analyzed by Western immunoblotting analysis using anti-cPLA2α antibody (1:1000) (Santa Cruz), anti-protein-disulfide isomerase antibody (1:1000) (Stressgen) to normalize the membrane fractions and anti-β-actin (1:5000) (sigma) to normalize the cytosolic fractions. F, effect of calcium ionophore on co-transfected CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A). G, a graph representing the % of translocation from the data depicted in panel F. Data are the mean ± S.E. and are representative of at least six different experiments. Statistical significance was evaluated with Student's t test. (*, p < 0.05). H, cPLA2α−/− MEFs (0.2 × 105 cells) were seeded overnight onto 22 × 22-mm coverslips in 35-mm plates. The cells were then co-infected with adenovirus containing CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A). After 48 h of incubation, the cells were treated with of calcium ionophore (A23187, 10 μm, 5 min) and analyzed by confocal microscopy. Data are representative of at least n = 3 on 3 separate occasions.
The 58% perinuclear translocation observed by the GFP-cPLA2α in response to A23187 correlated with the association of endogenous cPLA2α with membranes to the same percentage (Fig. 2, panel D). Furthermore, cell fractionation studies corroborated the inability of the cPLA2α(R57A/K58A/R59A) to translocate to membranes in response to A23187 (Fig. 2, panel E).
To further analyze the requirement of the C1P/cPLA2α interaction for the translocation of the enzyme, we co-transfected the cPLA2α(WT) (CFP-tagged) and the cPLA2α(R57A/K58A/R59A) (YFP-tagged) into A549 cells. As demonstrated for GFP-cPLA2α, CFP-cPLA2α(WT) translocated in 58% of A549 cells upon stimulation with calcium ionophore (Fig. 2, panels F and G; supplemental Fig. 2). In contrast, the YFP-cPLA2α(R57A/K58A/R59A) did not demonstrate any observable translocation (Fig. 2, panels F and G). Live cells were also examined over 9–10 min without observable translocation of YFP-cPLA2α(R57A/K58A/R59A) in complete contrast to CFP-cPLA2α(WT) (supplemental Fig. 2 and Animation 1).
To determine whether this phenomenon translated to another cell type, murine embryonic fibroblasts obtained from cPLA2α−/− mice were also co-transfected with CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A). As with A549 cells, only the translocation of CFP-cPLA2α(WT) was observed in these cells upon stimulation with calcium ionophore (Fig. 2, panel H). The culmination of these data demonstrates that both down-regulation of endogenous C1P levels or mutation of the C1P binding site in cPLA2α blocked the translocation of the enzyme in response to an increase of intracellular calcium. Therefore, these data demonstrate that the C1P/cPLA2α interaction is required for the activation of cPLA2α in response to increasing levels of intracellular calcium.
The C1P/cPLA2α Interaction Is Required for the Translocation of cPLA2α in Responses to Inflammatory Agonists
Although the indication of cPLA2α translocation by calcium ionophore is the “gold standard” assay for the activation of this enzyme in cells, we also desired to examine more physiological agonists for the activation/translocation of cPLA2α in cells. ATP is a well established inflammatory agonist for the activation of cPLA2α (via the purinergic receptors) and has roles in inflammatory disorders and cancers (28–30). To examine ATP-induced activation of cPLA2α, cPLA2α−/− MEFs were again co-transfected with CFP-cPLA2α(WT) and YFP-cPLA2α(R57A/K58A/R59A). Treatment of these cells with ATP (100 μm for 4 h) induced substantial translocation for the CFP-cPLA2α(WT). In contrast, the YFP-cPLA2α(R57A/K58A/R59A) did not demonstrate any significant translocation (Fig. 3 and supplemental Fig. 3). Similar data were also found for TNFα stimulation (supplemental Fig. 4). Therefore, the C1P/cPLA2α interaction is also required for the activation of cPLA2α in response to a physiologic inflammatory agonist.
FIGURE 3.
The C1P/cPLA2α interaction is required for translocation of the enzyme in response to ATP. cPLA2α−/− MEFs (0.2 × 105 cells) were seeded overnight onto 22 × 22-mm coverslips in 35-mm plates. The cells were infected with adenovirus containing CFP-cPLA2α(WT) or YFP-cPLA2α(R57A/K58A/R59A). After 48 h incubation, the cells were treated with DMSO (A) or ATP 100 μm (B) for 4 h and then analyzed by confocal microscopy as detailed under “Experimental Procedures.” Data are representative of at least n = 3 on 3 separate occasions. DIC, differential interference contrast.
The C1P/cPLA2α Interaction Is a Required Proximal Event in the Induction of Eicosanoid Synthesis
Thus far, the presented data have shown that the lipid cofactor, C1P, is essential for the translocation of cPLA2α, the initial rate-limiting step in eicosanoid synthesis. Based on these data, we therefore hypothesized that C1P/cPLA2α interaction is also essential for the production of eicosanoids in response to inflammatory agonists. To explore our hypothesis, cPLA2α+/+ and −/− MEFs were utilized. In response to ATP, IL-1β, and TNFα, cPLA2α+/+ MEFs increased the levels of the prostaglandin, PGE2, but the levels of other analyzed eicosanoids (thromboxane B2, prostaglandin D2, and leukotriene B4) were unaffected (data not shown and Fig. 4). Compared with cPLA2α+/+ MEFs, cPLA2α−/− MEFs did not respond to ATP, TNFα, or IL-1β for the induction of PGE2 synthesis (Fig. 4).
FIGURE 4.
The C1P/cPLA2α interaction is required for production PGE2 in response to inflammatory agonists. Mouse embryonic fibroblasts, cPLA2α+/+ and cPLA2α−/− (0.2 × 105 cells/35-mm plate) were infected with adenovirus containing GFP-cPLA2α(WT) or GFP-cPLA2α(R57A/K58A/R59A). After 48 h, the cells were treated with Il-1β (100 ng/ml), ATP (100 μm) or TNFα (50 ng/ml), and the media were collected at 0.5, 2, 4, 6, and 8 h. Simultaneously, the cells were collected and proteins extracted. A, effect of ATP on the PGE2 production of MEFs transfected by cPLA2α(WT) viruses or cPLA2α(R57A/K58A/R59A) viruses (multiplicity of infection = 1). Mut, mutant. B, Western immunoblot showing the expression of cPLA2α(WT) and cPLA2α(R57A/K58A/R59A) in MEFs from the depicted experiment. C, effect of Il-1β on the PGE2 production of MEFs transfected by either cPLA2α(WT) virus or cPLA2α(R57A/K58A/R59A) viruses (multiplicity of infection = 1). D, Western immunoblot showing the expression of cPLA2α(WT) and cPLA2α(R57A/K58A/R59A) in MEFs from the panel C experiment. E, effect of TNFα on the PGE2 production of MEFs transfected by either cPLA2α(WT) virus or cPLA2α(R57A/K58A/R59A) viruses (multiplicity of infection = 1). F, Western immunoblot showing the expression of cPLA2α(WT) and cPLA2α(R57A/K58A/R59A) in MEFs from the panel E experiment. Data are representative of at least n = 3 on 3 separate occasions. Statistical significance was evaluated with Student's t test (*, p < 0.05). G, mouse embryonic fibroblasts, cPLA2α−/− (0.2 × 105 cells/35-mm plate) were infected with adenovirus containing GFP-cPLA2α(WT) or GFP-cPLA2α(R57A/K58A/R59A). After 48 h the cells were collected in buffer containing 50 mm Tris (pH 7.4), 0.1 m KCl, and 30% glycerol. The cells were sonicated 3 times for 10 s, and they were analyzed for cPLA2α activity as detailed under “Experimental Procedures.” Data are representative of n = 4 for 2 separate occasions.
To examine the role of the C1P/cPLA2α interaction in PGE2 synthesis, wild type cPLA2α was again re-introduced in cPLA2α−/− MEFs by adenovirus-mediated transfection. Fig. 4 shows that adenovirus transfection with cPLA2α(WT) efficiently rescued the ATP (100 μm) (panels A and B), IL-1β (100 ng/ml) (panels C and D), and TNFα (50 ng/ml) (panels E and F) induction of PGE2 synthesis. In complete contrast, the re-introduction of mutant cPLA2α(R57A/K58A/R59A) was unable to rescue PGE2 synthesis in response to these agonists (Fig. 4). The effect on ATP, IL-1β, and TNFα responsiveness by re-expression of cPLA2α(WT) was also dose-dependent (data not shown). The inability of cPLA2α(R57A/K58A/R59A) to rescue eicosanoid synthesis also could not be attributed to effects on enzyme activity, as the activity of the ectopically expressed cPLA2α mutant was the same as wild type cPLA2α in accord with our previous reports (1, 14) (Fig. 4, panel G). Therefore, the C1P/cPLA2α interaction is intrinsically required for the production of PGE2 in response to inflammatory agonists (e.g. ATP, TNFα, and IL-1β).
DISCUSSION
In this study we demonstrate that C1P is a proximal and required bioactive lipid for the activation of cPLA2α. Specifically, the interaction of C1P with cPLA2α was necessary for the translocation of the enzyme to internal membranes and for the production of the eicosanoid subspecies, PGE2, in response to various inflammatory agonists (e.g. calcium ionophore, ATP, TNFα, and IL-1β). Previously activation/translocation of cPLA2α in cells was shown to require the association of cPLA2α with membranes via the Ca2+-dependent lipid binding domain (CaLB domain) (3, 5, 8–10). For two decades, the theory of cPLA2α activation/translocation held that the enzyme interacted with PC-rich membranes in response to an intracellular calcium increase via charge-negation and hydrophobic interactions. Whereas this theory could not explain the micromolar kca for the interaction of the enzyme with PC vesicles, the CaLB domain was indeed required for the enzyme to bind substrate PC in a Ca2+-dependent manner as well as required for agonist-induced activation of the enzyme in cells (3, 5, 6, 8, 9, 31). However, this study explicitly demonstrates that Ca2+ alone is not sufficient to activate and translocate the enzyme to internal membranes in cells (3, 32). We demonstrate that the interaction of C1P with β-groove of the CaLB domain of cPLA2α is a requirement for efficient recruitment of the enzyme to internal PC-rich membranes in response to calcium and inflammatory agonists, dispelling the dogmatic theory of two decades while corroborating the requirement of the CaLB domain. Furthermore, substrate PC is not sufficient to effect the translocation of cPLA2α under physiologic conditions as the enzyme is cytosolic before activation even though PC is present in membranes in great abundance (33). Therefore, this study demonstrates the absolute requirement of the C1P/cPLA2α interaction for the activation of cPLA2α during the inflammatory response. Because C1P has been shown to be essential in the translocation of cPLA2α, we can also now deduce a link between the production of C1P and inflammatory responses.
The requirement of C1P in the translocation/activation of cPLA2α adds another level of complexity to the activation of this enzyme. Multiple levels of regulation are not uncommon for many rate-limiting steps in biosynthetic pathways, and both lipid co-factors and post-translational modifications such as phosphorylation at serine 505, 515, and 727 have been reported in regulating the activation of cPLA2α. Currently, the role of phosphorylation of cPLA2α in the induction of translocation is controversial due to conflicting reports, but the requirement of the phosphorylation of cPLA2α at serine 505, 515, and 727 for full activity of the enzyme in vitro and in vivo is not questioned based on studies from a number of laboratories (34–36). Specifically, recent findings suggest that in response to an inflammatory agonist, cPLA2α is phosphorylated on serine 515 by CAMKII followed by serines 505 and 727 by serine/threonine kinases of the mitogen-activated protein kinase pathway (34–36). As with the lipid co-factor, phosphatidylinositol 4,5-diphosphate, the bulk of the data suggests that phosphorylation plays more of a role in enzyme activity once the enzyme is associated with endogenous membranes. In regard to the translocation of cPLA2α, the only other report as to a required mechanism for the translocation of cPLA2α suggested that ceramide played a role in the association of cPLA2α to membranes via the CaLB domain (37). These findings have met with skepticism in recent years because of the similarity of ceramide effects to diacylglycerol on cPLA2α activity in vitro and the inability of other laboratories to repeat the findings on the ceramide/cPLA2α interaction at near physiologic salt concentrations. Thus, ceramide 1-phosphate is currently the only lipid known to strongly interact with cPLA2α and dramatically regulate the association of enzyme with PC-rich vesicles and, now, because of this study, internal membranes in “live” cells.
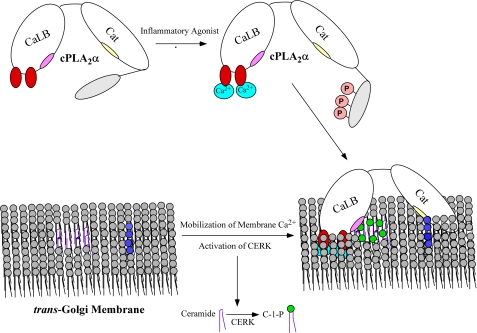
Based on the findings in this study and the reports in the literature, one can now theorize the scenario of this complex activation of cPLA2α. Essentially, most inflammatory agonists induce an increase in intracellular calcium. Upon the increase in intracellular calcium, CAMKII becomes activated, leading to the phosphorylation of serine 515 followed by phosphorylation of serine 505 and serine 727. Simultaneously, calcium and possibly CAMKII (there is a consensus CAMKII phospho-site in ceramide kinase) lead to the induction of ceramide 1-phosphate in PC-rich membranes. A fully active cPLA2α interacts with C1P increasing residence time at internal membranes followed by phosphoinositide (PIP) association and membrane penetration of the catalytic domain of cPLA2α (Fig. 5). In support of this theory of cPLA2α activation by PIPs after C1P association, PIPs would be localized near “pools” of C1P in the trans-Golgi due to 1) ceramide kinase localizing to PIPs via its PH-domain and 2) ceramide transport protein, the protein that delivers substrate ceramide to the trans-Golgi for ceramide kinase, which requires PIP association to insert ceramide into membranes. Thus, future studies are warranted to explore this hypothesis in detail.
FIGURE 5.
The hypothetical mechanism of cPLA2α activation in response to inflammatory agonists and the generation of ceramide 1-phosphate. The role of C1P in cPLA2α activation and eicosanoid synthesis in response to inflammatory agonists begins with the activation of CERK by increases in intracellular calcium (light blue) and other possibly other unknown mechanisms (e.g. CAMKII phosphorylation) after activation of a receptor by an inflammatory agonists (e.g. ATP via the purinergic receptor). CERK utilizes ceramide (dark purple) provided by CERT (ceramide transport protein) to produce C1P membrane structures (dark green) localized near phosphoinositides (dark blue). Simultaneously, cPLA2α is phosphorylated by CAMKII and enzymes of the mitogen-activated protein kinase pathway in the C terminus leading to a fully active enzyme. cPLA2α is then recruited to the trans-Golgi via C1P association with the C1P interaction site (light pink) in the CaLB domain, which penetrates the membrane via calcium binding subdomains I and III (dark red). Membrane penetration of the catalytic (cat)domain follows, which is dramatically enhanced by association with PIPs via the specific PIP interaction site (yellow). AA is then produced via the action of this enzyme and utilized by cyclooxygenases or lipoxygenases (e.g. COX-2 or 5-LO) beginning the eicosanoid biosynthetic pathway.
One inconsistency with our findings of a role for CERK-derived C1P as a major player in cPLA2α activation and eicosanoid synthesis in cells and in vivo are recent studies by Bornancin and co-workers (26, 27). These researchers found that cells derived from mice with the genetic ablation of CERK had no effect on AA release and eicosanoid synthesis in response to inflammatory agonists. There are two plausible explanations for the incongruent findings of this study and the Bornancin study. First, at least one other biosynthetic pathway for the generation of C1P exists (see Fig. 1, panel D), which may provide the C1P for activation of cPLA2α in the cell models used by the Bornancin group (e.g. peritoneal macrophages) (26). Our presented study utilizes a mutant cPLA2α, which specifically focuses on the interaction of C1P and cPLA2α, negating the unknown specificity of CERK for eicosanoid generation in other cell models. A second explanation could simply be adaptation in the CERK knock-out mouse. To date, only one chain length of C1P has been examined in the CERK knock-out mouse. It is plausible that increases (or changes in subcellular localization) in other chain lengths of C1P via up-regulation of separate anabolic pathways could lead to adaptation, making conclusions for a non-role of CERK in eicosanoid synthesis premature at this time. Regardless, the presented study demonstrates an absolute requirement for the C1P/cPLA2α interaction for the induction of cPLA2α translocation and eicosanoid synthesis in response to inflammatory agonists. Further studies will hopefully determine whether these unknown pathways of C1P generation can “cross”-signal or whether they play roles in different cellular mechanisms.
This plausible link between C1P generation and inflammation coupled with this study demonstrating that C1P is upstream of cPLA2α activation may lead to a much-needed and new generation of therapeutics for inflammatory disorders and cancer. For example, a chemical inhibitor of cPLA2α as well as genetic ablation of cPLA2α was also shown to prevent the development of airway inflammation in the AHR mouse model of asthma (38). Ablation of the cPLA2α gene also significantly reduced pulmonary edema, and cPLA2α is also found to be a mediator of acute lung injury induced by sepsis via production of inflammatory mediators, such as eicosanoids (38). In regard to cancer phenotypes, COX-2-derived eicosanoids, such as PGE2, contribute to tumorigenesis (39). Indeed, COX-2 has been shown to be up-regulated in colon cancer, breast, and lung cancers (40–42), and research studies have shown that the levels of PGE2 is higher in cancer tissue and than in normal tissue (43, 44). Recent studies by Nemenoff and co-workers (45) demonstrated that increased PGE2 levels are necessary for the production of lung tumors in mice. Furthermore, breeding the knock-out mice for cPLA2α or COX-2 with a mouse model of adenomatous polyposis dramatically reduced the multiplicity and size of colon tumors (46, 47) as well as in the urethane mouse model of lung tumorigenesis (45). Taken together, these observations suggested that ablation of this pathway regulated by the direct target of C1P, cPLA2α, has potential as a drug target for lung inflammation, osteoarthritis, and cancer.
For specific targeting of eicosanoid synthesis, inhibitors of the cPLA2α/C1P interaction would provide high specificity for this mechanism while keeping the potential therapeutic value for treating the mentioned disorders. The production of this type of inhibitor may not even be a challenging undertaking because C1P interacts with cPLA2α with a stoichiometry of >4.8 molecules of C1P (13, 20). Furthermore, published findings from Goñi and Alonso suggest that a multimeric lipid structure of ≥6 molecules of C1P is formed in cellular membranes (48). Based on the calcium dependence of the C1P/cPLA2α interaction, it is not inconceivable that calcium may regulate the conformation of this lipid structure modulating the specificity of protein/lipid interactions. Compounds synthesized to specifically block the formation of this structure may indeed provide a new generation of specific anti-inflammatory therapeutics while limiting unwanted side effects.
In conclusion, this study shows that C1P fulfills all five requirements of a regulated and bioactive lipid. First, both this study and previous studies have shown that C1P levels are regulated in response to inflammatory agonists. Second, our previous work has also shown that exogenous C1P induces a specific biochemical and cellular response (release of AA), and this action of C1P demonstrated high lipid specificity in the induction of cPLA2α interaction/activation, AA release, and eicosanoid synthesis (1, 13, 14, 20, 49). Third, endogenous C1P generated in cells by sphingomyelinase D reproduced these effects (19). Fourth, a direct target (cPLA2α) that binds/interacts with and is activated by C1P has been identified and validated (1, 14, 20, 49). Last, the interaction of C1P and cPLA2α is explicitly required for activation and translocation of the enzyme as well as subsequent prostanoid synthesis. Therefore, the presented study provides the last “missing link” in cPLA2α translocation and activation, validating C1P as a new lipid messenger in biological systems. This study should generate significant insight into novel aspects of signal transduction by lipids, activation of cPLA2α, and the regulation of prostaglandin synthesis. It also suggests C1P/cPLA2α interaction as a novel target for the development of anti-inflammatory therapeutics for inflammatory disorders, cancer, and sepsis.
Supplementary Material
Acknowledgments
Microscopy was performed at the Virginia Commonwealth University Department of Neurobiology and Anatomy Microscopy Facility, supported in part by funding from NINDS, National Institutes of Health Center Core Grant 5P30 NS-047463 and by 1C06 RR-17393 (to Virginia Commonwealth University for laboratory renovation).
This work was supported, in whole or in part, by National Institutes of Health Grants HL-072925 and CA-117990 (to C. E. C.) and DK54741 and DK39773 (to J. V. B.). This work was also supported by grants from the Veterans Administration (VA Merit Review I and a Research Career Scientist award (to C. E. C.)), Virginia Commonwealth University funds (to C. E. C.), and American Heart Association Postdoctoral Fellowship AHA0625502U (to N. F. L.) and Research Grant SDG 0735350N (to R. V. S.)).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1, Figs. 1–4, and Animation 1.
- AA
- arachidonic acid
- C1P
- ceramide 1-phosphate
- CERK
- ceramide kinase
- IL-1β
- interleukin-1β
- PC
- phosphatidylcholine
- PGE2
- prostaglandin E2
- PLA2
- phospholipase A2
- cPLA2
- cytosolic PLA2
- siRNA
- small interfering RNA
- TNFα
- tumor necrosis factor α
- WT
- wild type
- GFP
- green fluorescent protein
- CFP
- cyan fluorescent protein
- YFP
- yellow fluorescent protein
- CaLB domain
- Ca2+-dependent lipid binding domain
- PIP
- phosphoinositide
- MEF
- mouse embryonic fibroblast
- PBS
- phosphate-buffered saline
- PAPC
- 1-palmitoyl-2-arachidonyl-sn-phosphatidylcholine
- CAMKII
- calcium/calmodulin-dependent protein kinase.
REFERENCES
- 1.Stahelin R. V., Subramanian P., Vora M., Cho W., Chalfant C. E. (2007) J. Biol. Chem. 282, 20467–20474 [DOI] [PubMed] [Google Scholar]
- 2.Clark J. D., Schievella A. R., Nalefski E. A., Lin L. L. (1995) J. Lipid Mediat. Cell Signal. 12, 83–117 [DOI] [PubMed] [Google Scholar]
- 3.Leslie C. C. (1997) J. Biol. Chem. 272, 16709–16712 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds L. J., Hughes L. L., Louis A. I., Kramer R. M., Dennis E. A. (1993) Biochim. Biophys. Acta 1167, 272–280 [DOI] [PubMed] [Google Scholar]
- 5.Nalefski E. A., Sultzman L. A., Martin D. M., Kriz R. W., Towler P. S., Knopf J. L., Clark J. D. (1994) J. Biol. Chem. 269, 18239–18249 [PubMed] [Google Scholar]
- 6.Kramer R. M., Sharp J. D. (1997) FEBS Lett. 410, 49–53 [DOI] [PubMed] [Google Scholar]
- 7.Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. (1991) Cell 65, 1043–1051 [DOI] [PubMed] [Google Scholar]
- 8.Tay A., Simon J. S., Squire J., Hamel K., Jacob H. J., Skorecki K. (1995) Genomics 26, 138–141 [DOI] [PubMed] [Google Scholar]
- 9.Sharp J. D., White D. L., Chiou X. G., Goodson T., Gamboa G. C., McClure D., Burgett S., Hoskins J., Skatrud P. L., Sportsman J. R., et al. (1991) J. Biol. Chem. 266, 14850–14853 [PubMed] [Google Scholar]
- 10.Channon J. Y., Leslie C. C. (1990) J. Biol. Chem. 265, 5409–5413 [PubMed] [Google Scholar]
- 11.Casas J., Gijón M. A., Vigo A. G., Crespo M. S., Balsinde J., Balboa M. A. (2006) Mol. Biol. Cell 17, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker D. E., Ghosh M., Ghomashchi F., Loper R., Suram S., St. John B., Girotti M., Bollinger J. G., Gelb M. H., Leslie C. C. (2009) J. Biol. Chem. 284, 9596–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian P., Stahelin R. V., Szulc Z., Bielawska A., Cho W., Chalfant C. E. (2005) J. Biol. Chem. 280, 17601–17607 [DOI] [PubMed] [Google Scholar]
- 14.Subramanian P., Vora M., Gentile L. B., Stahelin R. V., Chalfant C. E. (2007) J. Lipid Res. 48, 2701–2708 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Zhao L., Wang C., Lei B. (2003) Sichuan Da Xue Xue Bao Yi Xue Ban 34, 344–346 [PubMed] [Google Scholar]
- 16.Towbin H., Staehelin T., Gordon J. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 18.Lassus P., Opitz-Araya X., Lazebnik Y. (2002) Science 297, 1352–1354 [DOI] [PubMed] [Google Scholar]
- 19.Pettus B. J., Bielawska A., Spiegel S., Roddy P., Hannun Y. A., Chalfant C. E. (2003) J. Biol. Chem. 278, 38206–38213 [DOI] [PubMed] [Google Scholar]
- 20.Pettus B. J., Bielawska A., Subramanian P., Wijesinghe D. S., Maceyka M., Leslie C. C., Evans J. H., Freiberg J., Roddy P., Hannun Y. A., Chalfant C. E. (2004) J. Biol. Chem. 279, 11320–11326 [DOI] [PubMed] [Google Scholar]
- 21.Lamour N. F., Stahelin R. V., Wijesinghe D. S., Maceyka M., Wang E., Allegood J. C., Merrill A. H., Jr., Cho W., Chalfant C. E. (2007) J. Lipid Res. 48, 1293–1304 [DOI] [PubMed] [Google Scholar]
- 22.Merrill A. H., Jr., Sullards M. C., Allegood J. C., Kelly S., Wang E. (2005) Methods 36, 207–224 [DOI] [PubMed] [Google Scholar]
- 23.Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 24.Perry D. K., Bielawska A., Hannun Y. A. (2000) Methods Enzymol. 312, 22–31 [DOI] [PubMed] [Google Scholar]
- 25.Sullards M. C., Allegood J. C., Kelly S., Wang E., Haynes C. A., Park H., Chen Y., Merrill A. H., Jr. (2007) Methods Enzymol. 432, 83–115 [DOI] [PubMed] [Google Scholar]
- 26.Graf C., Niwa S., Müller M., Kinzel B., Bornancin F. (2008) Biochem. Biophys. Res. Commun. 373, 159–163 [DOI] [PubMed] [Google Scholar]
- 27.Boath A., Graf C., Lidome E., Ullrich T., Nussbaumer P., Bornancin F. (2008) J. Biol. Chem. 283, 8517–8526 [DOI] [PubMed] [Google Scholar]
- 28.Wirkner K., Sperlagh B., Illes P. (2007) Mol. Neurobiol. 36, 165–183 [DOI] [PubMed] [Google Scholar]
- 29.Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., Rubartelli A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Virgilio F., Baricordi O. R., Romagnoli R., Baraldi P. G. (2005) Curr. Drug Targets Cardiovasc. Haematol. Disord. 5, 85–99 [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee S. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1523–1533 [DOI] [PubMed] [Google Scholar]
- 32.Balsinde J., Balboa M. A., Li W. H., Llopis J., Dennis E. A. (2000) J. Immunol. 164, 5398–5402 [DOI] [PubMed] [Google Scholar]
- 33.Evans J. H., Spencer D. M., Zweifach A., Leslie C. C. (2001) J. Biol. Chem. 276, 30150–30160 [DOI] [PubMed] [Google Scholar]
- 34.Thomas W., Coen N., Faherty S., Flatharta C. O., Harvey B. J. (2006) Steroids 71, 256–265 [DOI] [PubMed] [Google Scholar]
- 35.Pavicevic Z., Leslie C. C., Malik K. U. (2008) J. Lipid Res. 49, 724–737 [DOI] [PubMed] [Google Scholar]
- 36.Hefner Y., Borsch-Haubold A. G., Murakami M., Wilde J. I., Pasquet S., Schieltz D., Ghomashchi F., Yates J. R., 3rd, Armstrong C. G., Paterson A., Cohen P., Fukunaga R., Hunter T., Kudo I., Watson S. P., Gelb M. H. (2000) J. Biol. Chem. 275, 37542–37551 [DOI] [PubMed] [Google Scholar]
- 37.Huwiler A., Johansen B., Skarstad A., Pfeilschifter J. (2001) FASEB J. 15, 7–9 [DOI] [PubMed] [Google Scholar]
- 38.Malaviya R., Ansell J., Hall L., Fahmy M., Argentieri R. L., Olini G. C., Jr., Pereira D. W., Sur R., Cavender D. (2006) Eur. J. Pharmacol. 539, 195–204 [DOI] [PubMed] [Google Scholar]
- 39.Jakobsson P. J., Thorén S., Morgenstern R., Samuelsson B. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7220–7225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutchera W., Jones D. A., Matsunami N., Groden J., McIntyre T. M., Zimmerman G. A., White R. L., Prescott S. M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 4816–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hida T., Yatabe Y., Achiwa H., Muramatsu H., Kozaki K., Nakamura S., Ogawa M., Mitsudomi T., Sugiura T., Takahashi T. (1998) Cancer Res. 58, 3761–3764 [PubMed] [Google Scholar]
- 42.Wolff H., Saukkonen K., Anttila S., Karjalainen A., Vainio H., Ristimäki A. (1998) Cancer Res. 58, 4997–5001 [PubMed] [Google Scholar]
- 43.McLemore T. L., Hubbard W. C., Litterst C. L., Liu M. C., Miller S., McMahon N. A., Eggleston J. C., Boyd M. R. (1988) Cancer Res. 48, 3140–3147 [PubMed] [Google Scholar]
- 44.Hubbard W. C., Alley M. C., Gray G. N., Green K. C., McLemore T. L., Boyd M. R. (1989) Cancer Res. 49, 826–832 [PubMed] [Google Scholar]
- 45.Blaine S. A., Meyer A. M., Hurteau G., Wick M., Hankin J. A., Murphy R. C., Dannenberg A. J., Geraci M. W., Subbaramaiah K., Nemenoff R. A. (2005) Carcinogenesis 26, 209–217 [DOI] [PubMed] [Google Scholar]
- 46.Fosslien E. (2000) Crit. Rev. Clin. Lab. Sci. 37, 431–502 [DOI] [PubMed] [Google Scholar]
- 47.Williams J. L., Borgo S., Hasan I., Castillo E., Traganos F., Rigas B. (2001) Cancer Res. 61, 3285–3289 [PubMed] [Google Scholar]
- 48.Goñi F. M., Alonso A. (2009) Biochim. Biophys. Acta 1788, 169–177 [DOI] [PubMed] [Google Scholar]
- 49.Wijesinghe D. S., Subramanian P., Lamour N. F., Gentile L. B., Granado M. H., Szulc Z., Bielawska A., Gomez-Munoz A., Chalfant C. E. (2009) J. Lipid Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.