Abstract
In healthy cells, glutathione disulfide (GSSG) is rapidly reduced back to glutathione (GSH) by glutathione reductase to maintain redox status. The ratio of GSH/GSSG has been used as an indicator of oxidative stress. However, hypochlorous acid (HOCl) generated by the myeloperoxidase-H2O2-Cl− system of neutrophils converts GSH to irreversible oxidation products. Although several such products have been identified, yields of these compounds are very low in biological systems, and they cannot account quantitatively for thiol loss. In the current studies, we use liquid chromatography-mass spectrometry (LC-MS) to demonstrate that HOCl and chloramines oxidize GSSG to two irreversible products in high yield. The products, termed M-45 and M-90, are, respectively, 45 or 90 atomic mass units lighter than GSSG. The reaction pathway involves chloramine and aldehyde intermediates, and converts the γ-glutamyl residues of GSSG to 5-hydroxybutyrolactam. Importantly, M-45 and M-90 were resistant to reduction by glutathione reductase. Moreover, the monohydroxylbutyrolactam M-45 accounted for >90% of the endogenous GSH oxidation products generated by activated neutrophils. Because the reaction pathway involves chlorinating intermediates, hydroxylbutyrolactams are likely to be specific products of HOCl, which is generated only by myeloperoxidase. Therefore, our observations implicate M-45 as a potential biomarker for myeloperoxidase activity in vivo.
Glutathione (GSH), a tripeptide synthesized in the cytosol from glutamate, cysteine, and glycine, is the predominant antioxidant in mammalian cells. Its concentration ranges from millimolar inside cells to micromolar in plasma (1, 2). In many cells, GSH accounts for >90% of total nonprotein thiol (3, 4). The free thiol group in GSH is responsible for biological activity. As a nucleophilic scavenger, GSH can directly react with electrophilic substances, such as reactive oxygen/nitrogen species, or be oxidized by GSH peroxidase to glutathione disulfide (GSSG). Therefore, it is essential for maintaining intracellular redox status and defending against oxidative injury. Under normal circumstances, GSSG is rapidly reduced back to GSH by glutathione reductase and NADPH. Thus, most of the GSH remains in the reduced form. Under oxidative stress, however, GSH is converted to GSSG, which potentially accumulates (2, 5). Indeed, the GSH/GSSG ratio has been used to evaluate oxidative stress in biological systems. Alterations of this ratio associate with a variety of diseases, including atherosclerosis, cancer, and human immunodeficiency virus infection (6–10).
One important source of oxidative stress in humans is myeloperoxidase (MPO),2 a heme protein expressed by neutrophils, monocytes, and certain populations of macrophages (11–13). Activation of these inflammatory white blood cells results in the secretion of MPO, which uses hydrogen peroxide (H2O2, produced by NADPH oxidase) and chloride anion to generate hypochlorous acid (HOCl) (14). HOCl rapidly reacts with a wide range of functional groups (15–19). At physiological pH, thiol groups and free amino groups are its main targets, and the initial products are oxidized thiols and chloramines.
HOCl generates other products in addition to GSSG when it reacts with GSH. Chesney et al. (20) suggested that it oxidizes GSH to a higher oxidation state than the disulfide form because the molar ratio of HOCl consumed to GSH oxidized was 4:1 instead of 1:1 in Escherichia coli. Winterbourn (21) reported that approximately half of the GSH oxidized by HOCl could not be regenerated. These researchers have identified glutathione sulfonamide (GSA), glutathione thiosulfonate, and dehydroglutathione as irreversible higher oxidation products (22, 23). Their observations suggest that the formation of higher order GSSG oxidation products might account in part for the irreversible loss of GSH induced by HOCl. However, activated neutrophils (the source of MPO and therefore of HOCl) generate only low yields of these higher oxidation products, suggesting that the major products of GSH oxidation by MPO remain to be identified.
The disulfide and α-amino groups of GSSG are also potential targets of HOCl (17). Disulfides can be oxidized to sulfonic acid via a sulfenyl chloride intermediate (16). α-Amino groups yield chloramines, which undergo decarboxylation, intramolecular H-abstraction, or other reaction pathways to form various products, such as aldehydes and carboxymethyllysine (16, 24). These reactions may be biologically relevant, because carboxymethyllysine production is impaired in mice deficient in the phagocyte NADPH oxidase (25). These observations suggest that GSSG is a potential scavenger of HOCl. Indeed, GSSG reportedly competes for HOCl with its rate constant expected to be 2 × 105 m−1 s−1 (26, 27). Studies from Bast et al. (28) demonstrated that GSSG protects acetylcholinesterase from oxidative inactivation by HOCl. Nagy and Ashby (29) studied the kinetics and mechanism of GSSG oxidation by HOCl. They proposed that HOCl generates the bis-N-chloro-γ-l-glutamyl derivative of GSSG. These studies suggest that GSSG itself may function as an antioxidant.
In the current study, we investigated the reaction of GSSG with HOCl and other oxidants. Using liquid chromatography in concert with mass spectrometry (LC-MS), we identified two groups of novel oxidation products, which we termed M-45 and M-90. We characterized their structures and potential reaction pathways. Our results indicate that HOCl and chloramines oxidize the γ-glutamyl moiety of GSSG to 5-hydroxybutyrolactam in high yield.
EXPERIMENTAL PROCEDURES
Materials
Sodium hypochlorite (NaOCl), hydrogen peroxide (H2O2), trifluoroacetic acid, and HPLC grade acetonitrile (CH3CN) were purchased from Fisher Scientific. Oxidized glutathione (GSSG), glutathione (GSH), dithiothreitol, sodium borohydride (NaBH4), phorbol myristate acetate (PMA), formic acid, N-α-acetyl-l-lysine, NADPH, glutathione reductase from bakers' yeast, and 5,5′-dithiobis(2-nitrobenzoic acid) were obtained from Sigma. Hanks' balanced salt solution (HBSS) was purchased from Invitrogen. 2,4-Dinitrophenylhydrazine (DNPH) was purchased from Spectrum Chemical Manufacturing Corp. (Gardena, CA). Concentrations of NaOCl (ϵ292 = 350 m−1 cm−1) (30) and H2O2 (ϵ240 = 39.4 m−1 cm−1) (31) were determined spectrophotometrically.
Preparation of N-Chloramine
N-Chloramine was freshly prepared by chlorinating N-α-acetyl-lysine. Briefly, N-α-acetyl-lysine (5.5 mm) was incubated with sodium hypochloride (5 mm) on ice in phosphate buffer (25 mm, pH 7.4) for 10 min. The formation of N-α-acetyl-lysine chloramine was determined by measuring absorption at 252 nm (ϵ252 = 429 m−1 cm−1) (32).
Preparation of Peroxynitrite
Peroxynitrite was synthesized by incubating acidified hydrogen peroxide with a nitrite solution and stabilizing the resulting peroxynitrous acid by rapidly quenching the reaction with excess sodium hydroxide (33). The concentration of peroxynitrite was determined by measuring absorption at 302 nm (ϵ252 = 1670 m−1 cm−1) (34).
Oxidation of GSSG
GSSG (20 μm) was incubated with different concentrations of HOCl or other oxidants in phosphate buffer (25 mm, pH 7.4) at 37 °C for 1 h. Methionine (1:10, mol/mol, oxidant/Met) was added to terminate the oxidation reactions.
Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC-ESI-MS)
LC-MS analyses were performed in positive ion mode with a Finnigan Mat LCQ XP Plus ion trap mass spectrometer (San Jose, CA) coupled to an Agilent 1100 HPLC (Palo Alto, CA). Reaction mixtures were separated on a C18 small pore column (2.1 × 250 mm, Vydac, Deerfield, IL) at a flow rate of 0.2 ml/min, using 0.1% (v/v) formic acid in H2O as solvent A and 0.1% (v/v) formic acid in acetonitrile as solvent B. GSSG and its oxidation products were eluted with a linear gradient of 0–30% solvent B over 30 min. The collision energy for MS/MS was 35%. The temperature of the heated capillary was 220 °C.
D/H Exchange MS Analysis of GSSG Oxidation Products
The collected oxidation products of GSSG were reconstituted in deuterium oxide, incubated at 37 °C for 30 min, and then diluted 1:1 with 50% CH3CN (v/v) in deuterium oxide containing 0.5% (v/v) formic acid (35). They were analyzed by MS.
NMR Spectrometry
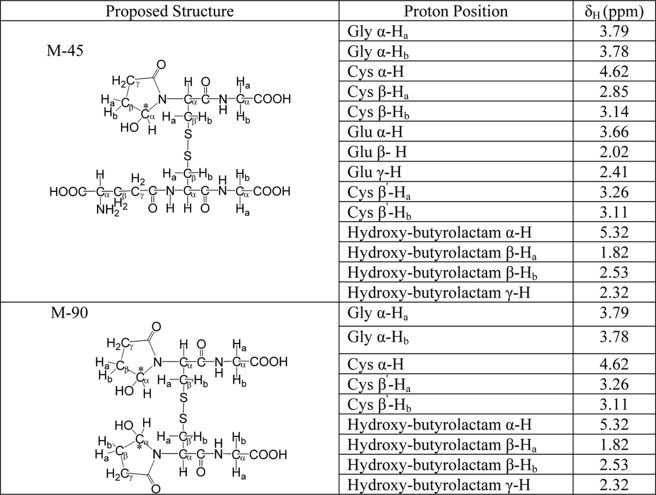
M-45 and M-90 were purified by HPLC. Their 1H NMR spectra were taken with an AV-300 Brucker NMR spectrometer (Brucker Optics, Inc., Fremont, CA) at 300 MHz, using deuterium oxide (D2O) as the solvent. The 1H chemical shifts (ppm) were referenced to solvent (D2O, δ = 4.811 ppm).
Reduction of GSSG, M-45, and M-90 by Glutathione Reductase
GSSG, M-45, or M-90 (20 μm) were added to 22 nm glutathione reductase in phosphate buffer (100 mm, pH 7.5) containing 1 mm EDTA. After a 5-min incubation, the reaction was initiated by adding 500 μm NADPH. After 10 min, the reaction was stopped by adding 20% (v/v) trifluoroacetic acid to reach pH 2. The reaction mixture was then filtered through a 3-kDa cutoff spin filter (Millipore, Bedford, MA) to remove the macromolecular components. The resulting mixtures were collected for LC-MS analysis. Mixtures without NADPH were used as controls.
Kinetic Studies of Glutathione Reductase-catalyzed GSSG, M-45, and M-90 Reduction
Twenty micromolar GSSG, M-45, or M-90 were added to a reaction mixture containing 22 nm glutathione reductase, 570 μm 5,5′-dithiobis(2-nitrobenzoic acid), and 1 mm EDTA in phosphate buffer (100 mm, pH 7.5). After a 5-min incubation, the reaction was initiated by adding 500 μm NADPH. Substrate reduction was determined indirectly by monitoring the increase in absorbance of 5-thio(2-nitrobenzoic acid) at 412 nm. The absorbance readings were recorded in a kinetic mode every 15 s for 10 min by Spectramax 190 (Molecular Devices, Sunnyvale, CA).
Isolation of Human Neutrophils
Neutrophils were isolated from EDTA-anticoagulated blood by buoyant density centrifugation, using Polymorph-Prep (Nycomed, Sunnyvale, CA). The neutrophils were washed twice by centrifugation with HBSS buffer (pH 7.4, magnesium-, calcium-, phenol-, and bicarbonate-free) containing 100 μm diethylenetriamine pentaacetic acid to inhibit metal-catalyzed reactions. After centrifugation, the neutrophils were resuspended in HBSS buffer and used immediately for reactions. They were activated with 200 nm PMA in the presence or absence of catalase (50 μg/ml), methionine (2 mm), or sodium azide (2 mm). Reactions were terminated by adding methionine (final, 1 mm). Cells were pelleted by centrifugation. Supernatants were filtered through a 3-kDa cutoff spin filter (Millipore, Bedford, MA) to remove the macromolecular components. The resulting supernatant was collected for LC-MS analysis.
RESULTS
HOCl Converts GSSG to Multiple Products
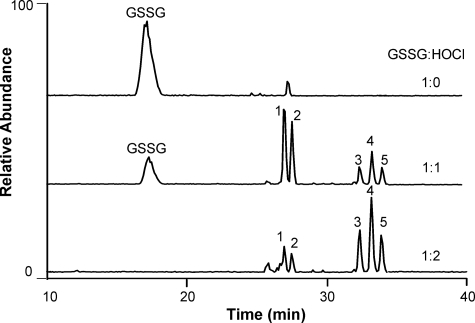
To search for higher oxidation products of GSSG, we exposed the disulfide to different concentrations of HOCl at pH 7.4, and analyzed the reaction mixture with LC-ESI-MS. At a 1:1 molar ratio of GSSG/HOCl, GSSG was almost quantitatively converted to two major groups of products (Fig. 1, peaks 1 and 2 and peaks 3–5). When the molar ratio was 2:1, the GSSG peak disappeared, peaks 1 and 2 dramatically decreased, and peaks 3–5 increased markedly. These observations suggest that peaks 3–5 represent higher oxidation products of peaks 1 and 2. The retention times of all oxidation products were longer than that of GSSG, indicating greater hydrophobicity. Thus, oxidation may have deleted one or more hydrophilic groups from GSSG.
FIGURE 1.
Total ion current chromatograms of GSSG oxidized by HOCl. GSSG (20 μm) was incubated with HOCl at 0, 20, or 40 μm in phosphate buffer (25 mm, pH 7.4) at 37 °C for 1 h. The reaction mixtures were analyzed by LC-MS.
HOCl Modifies the γ-Glutamic Acid Residue of GSSG
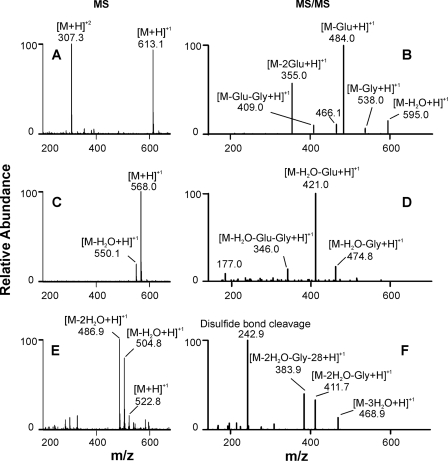
LC-MS demonstrated that peaks 1 and 2 had the same molecular mass (Fig. 2C, m/z 568.0, singly charged), suggesting they were isomers. Detection of an ion of m/z 550.1 indicated that both compounds could easily lose one molecule of water. MS/MS analysis of the m/z 550.1 ion revealed that peaks 1 and 2 had identical mass spectra, with major fragment ions of m/z 421.0, 346.0, and 177.0 (Fig. 2D), again suggesting the presence of isomers. Because identical masses were 45 atomic mass units less than that of GSSG, we called the isomers M-45. Similar results were obtained from MS and MS/MS analyses of peaks 3–5. All three peaks shared the same molecular mass (Fig. 2E, m/z 522.8) and identical MS/MS spectra, with major fragment ions of m/z 411.7, 383.9, and 242.9 (Fig. 2F). Detection of m/z 504.8 ([M-H2O + H]+) and 486.9 ([M-2H2O + H]+) (Fig. 2E) indicated that these products readily lost water during mass analysis. These observations suggest that peaks 3, 4, and 5 were isomers with a molecular mass of 90 atomic mass units less than that of the precursor, GSSG. We named these isomeric species M-90.
FIGURE 2.
MS and MS/MS analysis of GSSG and its oxidation products. The experimental conditions were the same as described in the legend to Fig. 1. A, C, and E, mass spectrometric analysis (MS) of GSSG, peaks 1 and 2 (M-45) and peaks 3–5 (M-90), respectively; B, D, and F, tandem MS (MS/MS) analysis of m/z 613.1, 550.0, and 487.0, respectively.
GSSG exhibited both singly and doubly charged ions of m/z 613.1 and 307.3, respectively (Fig. 2A). However, only singly charged ions were detected from the oxidation products, suggesting the loss of an amino group. MS/MS analysis of M-45 revealed a major fragment ion of m/z 421.0, which had a mass shift of 129 atomic mass units from the dehydrated product of M-45 (m/z 550.1). The material with this 129-atomic mass unit loss is a well known fragment of GSH and its conjugates; it corresponds to the loss of pyroglutamic acid from the γ-glutamyl residue. Further loss of the glycine residue formed a fragment ion of m/z 346.0. A fragment ion of m/z 177.0 represented the remaining cysteine and glycine residues. These results indicated that M-45 had retained one GSH moiety and that the missing 45 atomic mass units was lost from the carboxyl group of one γ-glutamyl residue of GSSG. Hence, we hypothesize that HOCl attacks the amino group of glutamic acid to form a chloramine, which further undergoes decarboxylation and imine hydrolysis to become the aldehyde via the Grob fragmentation pathway (16, 23, 36). Thus, M-45 is possibly an aldehyde that is formed by converting the -CH(NH2)COOH group of one of the GSSG glutamyl residues into -CHO. Because GSSG has a symmetric structure and oxidation product M-90 differed from M-45 by 45 atomic mass units, we propose that M-90 resulted from decarboxylation of the remaining glutamyl residue in M-45.
M-45 and M-90 Are Aldehydes
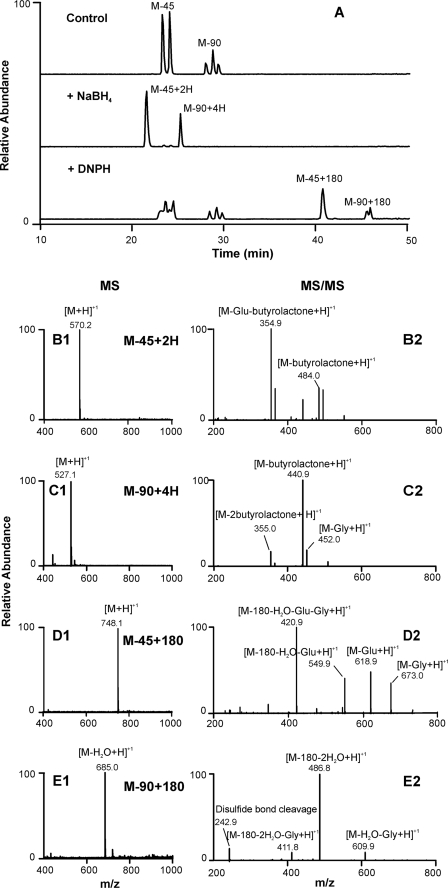
To determine whether M-45 and M-90 might contain aldehyde groups, we exposed each product to sodium borohydride, which selectively converts ketones and aldehydes to alcohols (Fig. 3). Using LC-MS, we detected two peaks of m/z 570.2 (M-45 + 2H) and 527.1 (M-90 + 4H), suggesting that the aldehyde groups in M-45 and M-90 had been reduced to alcohol, increasing the mass by 2 or 4 units, respectively (Fig. 3, B1 and C1). MS/MS analyses of m/z 570.2 and 527.1 revealed the loss of γ-butyrolactone from the alcohol ends of M-45 and M-90 (Fig. 3, B2 and C2). These observations demonstrated that sodium borohydride reduced M-45 and M-90 to their corresponding alcohols, which is consistent with our hypothesis that M-45 and M-90 are aldehydes.
FIGURE 3.
Characterization of GSSG oxidation products M-45 and M-90. GSSG (50 μm) was incubated with HOCl (50 μm) in phosphate buffer (50 mm, pH 7.4) at 37 °C for 1 h (Control). The reaction mixture was then reduced by sodium borohydride (NaBH4, 10 mm) at room temperature for 1 h or reacted with dinitrophenylhydrazine (DNPH, 1.5 mm) at 37 °C for 1 h at pH 2. The resulting reaction mixtures were analyzed with LC-MS and MS/MS. A, reconstructed ion chromatograms of M-45, M-90, and its reduction or derivatization products; B1–E1, mass spectrometric (MS) analysis, and B2–E2, tandem MS (MS/MS) analysis.
To further characterize the structures of M-45 and M-90, we treated each product with DNPH, a reagent widely used to selectively derivatize aldehydes or ketones (37) (Fig. 3A). LC-MS detected two additional peaks, with m/z 748.1 (M-45 + 180) and 685.0 (M-90 + 180-H2O), after DNPH treatment, indicating the formation of DNPH adducts in each case (Fig. 3, D1 and E1). MS/MS analysis of m/z 748.1 and 685.0 confirmed that DNPH formed adducts with both products through their aldehyde groups (Fig. 3, D2 and E2). Collectively, our results provide strong evidence that both products are aldehydes.
1H NMR Analysis of M-45 and M-90
To confirm our observations, we analyzed purified M-45 and M-90 with 1H NMR. Surprisingly, we were unable to detect any aldehydic protons (chemical shift between 9–10 ppm), suggesting that both products assume a ring conformation after an amide nitrogen is added to the Cys residue adjacent to the carbonyl group (Scheme 1). Based on the spectra of similar compounds, the 1H NMR chemical shifts (Table 1) were consistent with the proposed 5-hydroxybutyrolactam structure (29, 38–40). Disappearance of Glu α, β, and γ protons in the NMR spectrum of M-90 indicated that both Glu groups in GSSG had been modified. The chemical shifts of two β protons in the 5-hydroxybutyrolactam moiety differed from each other due to the chiral center that was created. One was shifted downfield to around 2.5 ppm, and the other was shifted upfield to 1.8 ppm. The chemical shift of the α proton of 5-hydroxybutyrolactam was shifted further downfield to 5.3 ppm due to the deshielding effect of the hydroxyl group that is attached to the same carbon.
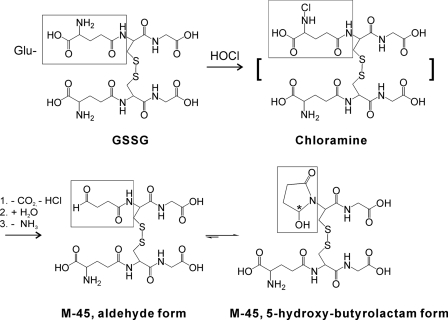
SCHEME 1.
Proposed pathway for the formation of M-45.
TABLE 1.
1H NMR chemical shifts (ppm) of M-45 and M-90 at 300 MHz
Deuterium/Hydrogen (D/H) Exchange Analysis of M-45 and M-90
We used D/H exchange to determine the number of exchangeable hydrogens in M-45 and M-90. MS analysis indicated that M-45 contained 9 exchangeable hydrogens compared with 12 exchangeable hydrogens in GSSG (4 amide bond hydrogens, 4 amino hydrogens, and 4 hydrogens on the carboxyl groups). The absence of 2 amino hydrogens and 1 carboxyl hydrogen on the glutamyl residue explains why M-45 incorporated 3 fewer deuterium atoms than GSSG (Scheme 1). Dehydrated M-45 had 8 exchangeable hydrogens. Dehydrated M-90 incorporated 4 deuterium atoms, including 2 amide bond hydrogens and 2 hydrogens on the carboxyl groups of glycine residues. These observations confirmed the proposed structures of M-45 and M-90.
M-45 and M-90 Are Specific Products of HOCl
To determine whether other oxidants convert GSSG to M-45 or M-90, we incubated GSSG with different concentrations of peroxynitrite (ONOO−), H2O2, or chloramine (generated by the reaction of N-α-acetyl-lysine with HOCl). Neither M-45 nor M-90 were detected when GSSG was treated with up to a 50-fold molar excess of peroxynitrite or H2O2 (data not shown). In contrast, we detected M-45 after exposing GSSG to a 50-fold molar excess of chloramine. Thus, only reactive chlorinating intermediates were able to generate M-45 and M-90.
GSSG Reacts with HOCl and Chloramines to Generate a High Yield of M-45
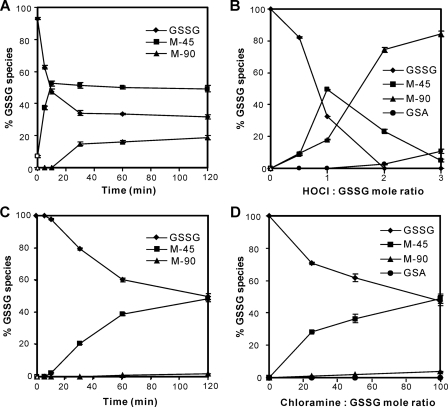
We used LC-MS to derive a progress curve and product yields of GSSG oxidation by HOCl or chloramine. All reactions were carried out at 37 °C in phosphate buffer (25 mm, pH 7.4). As shown in Fig. 4A, GSSG reacted rapidly with HOCl. A 10-min exposure of 10 μm GSSG to a 1:1 molar ratio of HOCl converted ∼50% of GSSG to M-45. A 30-min incubation produced M-90 with a 15% yield. We then exposed GSSG to different amounts of HOCl (Fig. 4B). The yield of each product was dependent on the HOCl/GSSG molar ratio. M-45 production peaked at a molar ratio of 1:1. As the ratio increased, the yield of M-45 decreased significantly, whereas the percentage of M-90 increased dramatically, indicating that M-45 may be further oxidized to M-90 when the concentration of HOCl is high. We also detected a low level of GSA (Fig. 4B), which has been reported as an oxidation product of GSH by HOCl (22, 23, 41).
FIGURE 4.
Time course and concentration dependence of GSSG oxidation by HOCl or chloramine. GSSG (10 μm) was incubated with increasing concentrations of oxidants in phosphate buffer (50 mm, pH 7.4) for 1 h (B and D) or with 10 μm HOCl (500 μm of chloramines) at different time points (A and C) at 37 °C. The percentages of GSSG species were determined by dividing the peak area of the compound of interest by the sum of all the detected peaks. The peak area was determined from reconstructed ion chromatograms of GSSG, m/z 613.1 (singly charged) and 307.1 (doubly charged); M-45, m/z 550.1 and 568.1; M-90, m/z 487.1, 505.1 and 523.1; GSA, m/z 338.1. The results are expressed as mean ± S.D. from 3 experiments.
Next, we measured the levels of GSSG oxidation products generated by chloramine, using a chloramine/GSSG molar ratio up to 100. High chloramine concentrations generated M-45 as the major product and M-90 as a minor product (Fig. 4D). However, chloramine reacted much more slowly than HOCl (Fig. 4C). Collectively, these observations indicate that HOCl rapidly converts the γ-glutamyl(s) in GSSG to a high yield of mono- or di-5-hydroxybutyrolactam (M-45 or M-90) and chloramine derived from HOCl generates the same products, although in lower yields, suggesting that these reactions could occur in vivo at sites of inflammation.
M-45 and M-90 Resist Reduction by Glutathione Reductase
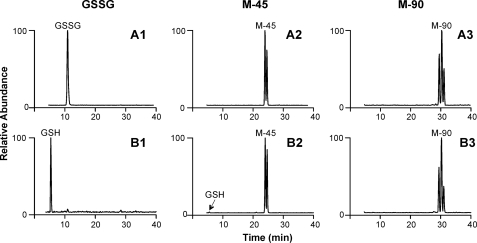
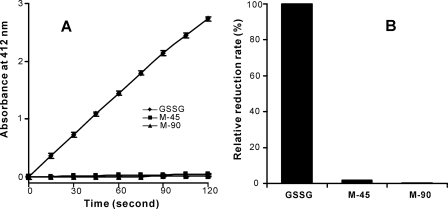
Under normal physiological conditions, glutathione reductase rapidly reduces GSSG back to GSH to maintain the normal redox status. To test if glutathione reductase can reduce M-45 or M-90, we incubated 20 μm of each substrate with the enzyme for 10 min in the presence of NADPH. After macromolecules were removed, the reaction mixture was analyzed by LC-MS. As shown in Fig. 5, GSSG was completely reduced to GSH by 10 min. However, M-45 and M-90 remained almost unchanged. These observations indicate that M-45 and M-90 are very poor substrates for glutathione reductase. We further compared the kinetics of M-45, M-90, and GSSG reduction by glutathione reductase, using the 5,5′-dithiobis(2-nitrobenzoic acid) assay (Fig. 6A). When the initial concentration of each substrate was 20 μm, GSSG was reduced very rapidly. In contrast, the reduction rates of M-45 and M-90 were reduced to 1.7 and 0.4% of that of GSSG (Fig. 6B), indicating that the products are resistant to glutathione reductase reduction. This would be expected to permanently deplete the store of GSSG and disrupt GSH recycling, thereby exacerbating oxidative stress in vivo.
FIGURE 5.
Reduction of GSSG, M-45, and M-90 by glutathione reductase. 20 μm GSSG, M-45, or M-90 were added to 22 nm glutathione reductase in phosphate buffer (100 mm, pH 7.5) containing 1 mm EDTA. After a 5-min incubation, the reaction was initiated by adding 500 μm NADPH (B1–B3, glutathione reductase system) or buffer (A1–A3, control). The reactions were terminated at 10 min by adding 20% trifluoroacetic acid to reach pH 2. The reaction mixtures were then filtered through a 3-kDa cutoff spin filter to remove macromolecules. The resulting reaction mixtures were analyzed by LC/MS.
FIGURE 6.
Time course of GSSG, M-45, and M-90 reduction catalyzed by glutathione reductase. 20 μm GSSG, M-45, or M-90 were added to a reaction mixture containing 22 nm glutathione reductase and 570 μm 5,5′-dithiobis(2-nitrobenzoic acid) in phosphate buffer (100 mm, pH 7.5) containing 1 mm EDTA. After a 5-min incubation, the reaction was initiated by adding 500 μm NADPH. Absorbance at 412 nm was recorded for 5-thio(2-nitrobenzoic acid) in a kinetic mode every 15 s for 2 min, using a plate reader. The results are expressed as mean ± S.D. from 3 experiments.
Activated Neutrophils Convert GSSG to M-45 and M-90
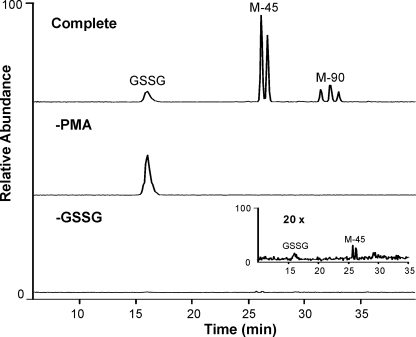
To determine whether the HOCl that is generated by human neutrophils can oxidize GSSG, we incubated the disulfide (5 μm) with human neutrophils (1 × 106/ml) at 37 °C in HBSS, activated the cells with PMA, and analyzed the supernatant with LC-MS (Fig. 7). Under these conditions, nearly 70% of the GSSG was converted to M-45 and M-90. Generation of M-45 and M-90 required cellular activation. Interestingly, we were able to detect a small amount of M-45 in the supernatant from activated human neutrophils that had not received exogenous GSSG, suggesting that activated neutrophils oxidize endogenous GSSG.
FIGURE 7.
Activated human neutrophils oxidize GSSG. GSSG (5 μm) was incubated with phorbol ester-activated human neutrophils (1 × 106/ml) in HBSS buffer (pH 7.4) at 37 °C for 1 h (Complete). After the cells were pelleted, the supernatant was collected and macromolecules were removed with a 3,000-Da cutoff spin filter. Reaction conditions were varied as indicated. The resulting supernatant was analyzed by LC-MS.
Activated Neutrophils Generate M-45 from Endogenous GSH
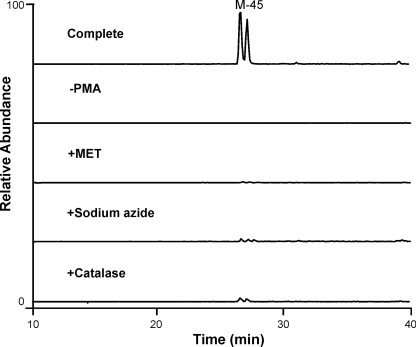
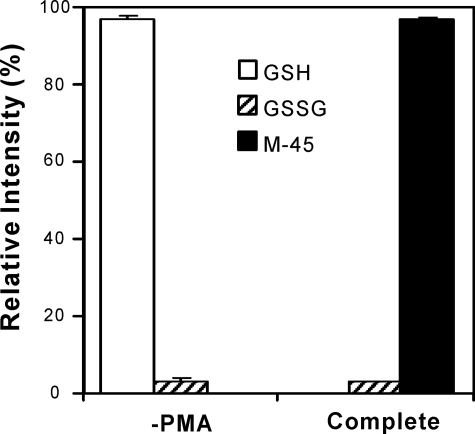
Neutrophils secrete GSH and GSSG, and activated neutrophils also release MPO, the only enzyme known to generate HOCl in humans. Thus, M-45 generated by endogenous GSSG and MPO might be an indicator of neutrophil activation. To determine whether HOCl produced by MPO oxidizes endogenous GSSG to M-45 or M-90, we exposed human neutrophils (5 × 106/ml) to phorbol ester in the absence or presence of catalase, methionine, or sodium azide. LC-MS/MS with selective reaction monitoring demonstrated that M-45 formation required cell activation and was inhibited by the H2O2 scavenger catalase, the thiol-containing antioxidant methionine, or the heme poison sodium azide (Fig. 8). We used selective reaction monitoring to quantify the relative abundance of GSH, GSSG, M-45, and M-90 in human neutrophils with or without PMA treatment (Fig. 9). As expected, most of the GSH secreted from inactivated neutrophils remained in its reduced form. However, GSH was not detectable in supernatant from activated neutrophils, whereas we detected M-45 as a major oxidation product, which accounted for >90% of the total products quantified by peak area. These results suggest that HOCl generated by the MPO system of activated human neutrophils initially oxidizes the GSH that is secreted from neutrophils to GSSG. The disulfide is then further oxidized to M-45. We failed to detect M-90 in the supernatant of activated human neutrophils. Collectively, these results indicate that activated neutrophils use MPO to convert endogenous secreted GSH and GSSG to M-45 at high yield, suggesting M-45 could be a useful indicator of neutrophil activation or a biomarker for HOCl oxidation in vivo.
FIGURE 8.
Selective reaction monitoring of M-45 generated by activated human neutrophils. Neutrophils (5 × 106/ml) in HBSS buffer (pH 7.4) were stimulated with 200 nm PMA (Complete) in the presence or absence of catalase (50 μg/ml), methionine (2 mm), or sodium azide (2 mm). The incubation was performed at 37 °C for 1 h. Supernatant was collected, and macromolecules were removed with a 3,000 Da cutoff spin filter. The filtered supernatant was analyzed by LC-MS/MS selective reaction monitoring of M-45 (m/z 568.1 → 550.1).
FIGURE 9.
Quantification of GSH, GSSG, and M-45 produced by activated human neutrophils. The experimental conditions were the same as described in the legend to Fig. 6. Relative intensity of GSH, GSSG, and M-45 were determined by dividing the peak area of the compound of interest by the sum of all the detected peaks. The peak area was determined by LC-MS/MS, using the selective reaction monitoring mode (GSH, m/z 308.1 → 179.0; GSSG, m/z 307.5 → 484.0; M-45, m/z 568.1 → 550.1). Results are mean ± S.D. from 3 independent experiments.
DISCUSSION
We demonstrated that HOCl specifically converts the γ-glutamyl groups of GSSG to two sets of isomeric oxidation products, M-45 and M-90, in high yield. At a 1:2 molar ratio of GSSG/HOCl, GSSG was completely converted to M-45 and M-90. Analysis of the reaction kinetics and the product yields at various molar ratios of oxidant strongly suggested that M-45 was the initial oxidation product. This isomer mixture was in turn oxidized to M-90. Reduction with borohydride and derivatization with DNPH provided strong evidence that both M-45 and M-90 contain aldehyde groups. Thus, we hypothesized that M-45 and M-90 were generated after HOCl converted the γ-glutamyl residue of GSSG into chloramines, which then initiated the Grob fragmentation pathway to generate aldehyde (16, 36). However, no aldehyde H was detected by NMR, suggesting that the aldehyde groups in M-45 or M-90 had formed the cyclic carbinolamine derivative.
Detection of multiple isomers (relative ratios were 1:1 for M-45 and 1:2:1 for M-90) indicated the formation of diastereoisomers resulting from the new chiral centers in the products. Furthermore, when we isolated each isomer of M-45, incubated it at pH 7.4, and analyzed the reaction mixture with LC-MS, we detected two isomers of M-45, indicating that they are likely to be in equilibrium.
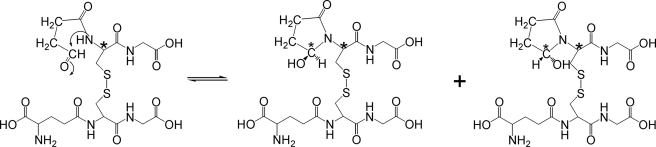
Based on these observations, we propose that HOCl oxidizes GSSG by the pathway shown in Scheme 1. The amino group in the Glu residue is first chlorinated by HOCl. Due to the electron withdrawing effect of Cl, the terminal -COOH on Glu is decarboxylated to the imine (42). The unstable imine is further hydrolyzed to an aldehyde, which is then converted to 5-hydroxybutyrolactam through nucleophilic addition to the amide nitrogen of the adjacent Cys residue.
The proposed reaction pathway implies that M-45 exists as an equilibrium mixture of the aldehyde and butyrolactam ring. As with glucose, however, in which 99% of the molecules are closed rings, the predominant species of M-45 is 5-hydroxybutyrolactam. This explains the failure to detect an aldehydic proton in M-45 by NMR. Our NMR results are also consistent with the proposed ring structures of M-45 and M-90.
Nucleophilic attack at the carbonyl group in M-45 creates a chiral center during the formation of the butyrolactam ring, resulting in a 1:1 ratio of two diastereoisomers (Scheme 2). Because both Glu residues of GSSG become 5-hydroxybutyrolactam in M-90, we detected three isomeric peaks with a ratio of 1:2:1. When M-90 reacted with DNPH, one aldehyde group was conjugated with DNPH. Because only the remaining aldehyde group could be converted to 5-hydroxybutyrolactam, we detected two isomeric peaks for DNPH adducts of M-90.
SCHEME 2.
Proposed mechanism of the isomerization of M-45.
Irreversible oxidation of GSSG to butyrolactam (M-45) and bis-butyrolactam (M-90) derivatives would explain the lack of detectable GSSG formation observed in previous studies of GSH oxidation by neutrophils (43–45). Our observations indicate that M-45 and M-90 are resistant to reduction by glutathione reductase. The formation of these irreversible oxidation products would disrupt GSH recycling, lowering its antioxidant capacity. Therefore, oxidation of GSSG may contribute to thiol loss and local GSH depletion.
The GSH/GSSG ratio has been used as an indicator of oxidative stress in vivo. However, HOCl generated by activated phagocytes during inflammation may further oxidize GSSG to M-45, increasing the GSH/GSSG ratio. Thus the observed GSH/GSSG ratio may not reflect the actual level of oxidative stress in vivo. In contrast, M-45, which appears stable under our experimental conditions, could be a specific biomarker of oxidative stress during inflammation.
We also examined whether HOCl converts GSSG to GSA, which has been reported to be a significant oxidation product of GSH by HOCl (23, 38). We found that GSA was only a minor product at high molar ratios of HOCl, accounting for ∼10% of the total products at a 3:1 ratio of HOCl/GSSG. However, our studies of oxidation of endogenous GSH/GSSG by activated neutrophils indicated that the yield of GSA in biological systems could be much lower (<4%, data not shown) than that of M-45 (>90%, Fig. 9). Moreover, long-lived chloramines derived from HOCl oxidized GSH to GSSG and M-45 but not to GSA (data not shown). M-45 therefore could be a sensitive and specific biomarker of HOCl oxidation in vivo.
In conclusion, we demonstrated that HOCl, which is generated only by MPO in humans, oxidized the γ-glutamyl group in GSSG to a novel irreversible product, 5-hydroxybutyrolactam, in high yield, hampering thiol regeneration during oxidative stress. Most importantly, we detected this product from activated neutrophils, suggesting that the HOCl generated by these cells may oxidize GSSG at sites of inflammation. This in turn suggests that M-45, the 5-hydroxybutyrolactam derivative of GSSG, may be a potential marker for neutrophil activation in vivo.
Acknowledgments
We thank Dr. Henry Rosen (University of Washington) for help preparing human neutrophils and helpful discussions. Mass spectrometry experiments were performed by the Mass Spectrometry Resource, Department of Medicine, University of Washington, and the Mass Spectrometry Laboratory, Research Division, Puget Sound Blood Center. NMR experiments were performed in the Chemistry Department, University of Washington.
This work was supported, in whole or in part, by National Institutes of Health Grant HL075381.
- MPO
- myeloperoxidase
- HOCl
- hypochlorous acid
- PMA
- phorbol myristate acetate
- GSA
- glutathione sulfonamide
- DNPH
- 2,4-dinitrophenylhydrazine
- LC
- liquid chromatography
- MS
- mass spectrometry
- MS/MS
- tandem mass spectrometry
- HBSS
- Hanks' balanced salt solution
- HPLC
- high performance liquid chromatography
- ESI
- electrospray ionization.
REFERENCES
- 1.Jones D. P. (2002) Methods Enzymol. 348, 93–112 [DOI] [PubMed] [Google Scholar]
- 2.Wu G., Fang Y. Z., Yang S., Lupton J. R., Turner N. D. (2004) J. Nutr. 134, 489–492 [DOI] [PubMed] [Google Scholar]
- 3.Meister A. (1988) J. Biol. Chem. 263, 17205–17208 [PubMed] [Google Scholar]
- 4.Rodes J., Rizzetto M., Reichen J., Benhamou J. (2007) Textbook of Hepatology: From Basic Science to Clinical Practice, 3rd Ed., Blackwell Publishing Limited, Malden, MA [Google Scholar]
- 5.Griffith O. W. (1999) Free Radic. Biol. Med. 27, 922–935 [DOI] [PubMed] [Google Scholar]
- 6.Ashfaq S., Abramson J. L., Jones D. P., Rhodes S. D., Weintraub W. S., Hooper W. C., Vaccarino V., Harrison D. G., Quyyumi A. A. (2006) J. Am. Coll. Cardiol. 47, 1005–1011 [DOI] [PubMed] [Google Scholar]
- 7.Serru V., Baudin B., Ziegler F., David J. P., Cals M. J., Vaubourdolle M., Mario N. (2001) Clin. Chem. 47, 1321–1324 [PubMed] [Google Scholar]
- 8.Herzenberg L. A., De Rosa S. C., Dubs J. G., Roederer M., Anderson M. T., Ela S. W., Deresinski S. C., Herzenberg L. A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1967–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinman W. A., Richie J. P., Jr. (2000) Biochem. Pharmacol. 60, 19–29 [DOI] [PubMed] [Google Scholar]
- 10.Lang C. A., Mills B. J., Mastropaolo W., Liu M. C. (2000) J. Lab. Clin. Med. 135, 402–405 [DOI] [PubMed] [Google Scholar]
- 11.Vaisar T., Shao B., Green P. S., Oda M. N., Oram J. F., Heinecke J. W. (2007) Curr. Atheroscler. Rep. 9, 417–424 [DOI] [PubMed] [Google Scholar]
- 12.Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. (1994) J. Clin. Invest. 94, 437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinecke J. W. (1999) J. Lab. Clin. Med. 133, 321–325 [DOI] [PubMed] [Google Scholar]
- 14.Ogino T., Packer L., Maguire J. J. (1997) Free Radic. Biol. Med. 23, 445–452 [DOI] [PubMed] [Google Scholar]
- 15.Peskin A. V., Winterbourn C. C. (2001) Free Radic. Biol. Med. 30, 572–579 [DOI] [PubMed] [Google Scholar]
- 16.Hawkins C. L., Pattison D. I., Davies M. J. (2003) Amino Acids 25, 259–274 [DOI] [PubMed] [Google Scholar]
- 17.Pattison D. I., Davies M. J. (2001) Chem. Res. Toxicol. 14, 1453–1464 [DOI] [PubMed] [Google Scholar]
- 18.Fu X., Kassim S. Y., Parks W. C., Heinecke J. W. (2003) J. Biol. Chem. 278, 28403–28409 [DOI] [PubMed] [Google Scholar]
- 19.Fu X., Mueller D. M., Heinecke J. W. (2002) Biochemistry 41, 1293–1301 [DOI] [PubMed] [Google Scholar]
- 20.Chesney J. A., Eaton J. W., Mahoney J. R., Jr. (1996) J. Bacteriol. 178, 2131–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winterbourn C. C. (1985) Biochim. Biophys. Acta 840, 204–210 [DOI] [PubMed] [Google Scholar]
- 22.Winterbourn C. C., Brennan S. O. (1997) Biochem. J. 326, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwood D. T., Kettle A. J., Winterbourn C. C. (2006) Biochem. J. 399, 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson M. M., Hazen S. L., Hsu F. F., Heinecke J. W. (1997) J. Clin. Invest. 99, 424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson M. M., Heinecke J. W. (2003) Diabetes 52, 2137–2143 [DOI] [PubMed] [Google Scholar]
- 26.Prütz W. A. (1996) Arch. Biochem. Biophys. 332, 110–120 [DOI] [PubMed] [Google Scholar]
- 27.Pattison D. I., Davies M. J. (2006) Curr. Med. Chem. 13, 3271–3290 [DOI] [PubMed] [Google Scholar]
- 28.den Hartog G. J., Haenen G. R., Vegt E., van der Vijgh W. J., Bast A. (2002) Biol. Chem. 383, 709–713 [DOI] [PubMed] [Google Scholar]
- 29.Nagy P., Ashby M. T. (2007) Chem. Res. Toxicol. 20, 79–87 [DOI] [PubMed] [Google Scholar]
- 30.Morris J. C. (1966) J. Phys. Chem. 70, 3798–3805 [Google Scholar]
- 31.Nelson D. P., Kiesow L. A. (1972) Anal. Biochem. 49, 474–478 [DOI] [PubMed] [Google Scholar]
- 32.Thomas E. L., Grisham M. B., Jefferson M. M. (1986) Methods Enzymol. 132, 569–585 [DOI] [PubMed] [Google Scholar]
- 33.Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes M. N., Nicklin H. G. (1968) J. Chem. Soc. A. 2, 450–452 [Google Scholar]
- 35.Fu X., Kao J. L., Bergt C., Kassim S. Y., Huq N. P., d'Avignon A., Parks W. C., Mecham R. P., Heinecke J. W. (2004) J. Biol. Chem. 279, 6209–6212 [DOI] [PubMed] [Google Scholar]
- 36.Hazen S. L., d'Avignon A., Anderson M. M., Hsu F. F., Heinecke J. W. (1998) J. Biol. Chem. 273, 4997–5005 [DOI] [PubMed] [Google Scholar]
- 37.Andreoli R., Manini P., Corradi M., Mutti A., Niessen W. M. (2003) Rapid Commun. Mass Spectrom. 17, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harwood D. T., Nimmo S. L., Kettle A. J., Winterbourn C. C., Ashby M. T. (2008) Chem. Res. Toxicol. 21, 1011–1016 [DOI] [PubMed] [Google Scholar]
- 39.Rabensterin D. L., Keire D. A. (1989) in Coenzymes and Cofactors: Glutathione ( Dolphin D., Poulson R., Avramovic O. eds) Vol. 3, Part A, pp. 67–101, John Wiley & Sons, New York [Google Scholar]
- 40.Staubmann R., Schubert-Zsilavecz M., Hiermann A., Kartnig T. (1999) Phytochemistry 50, 337–338 [Google Scholar]
- 41.Pullar J. M., Vissers M. C., Winterbourn C. C. (2001) J. Biol. Chem. 276, 22120–22125 [DOI] [PubMed] [Google Scholar]
- 42.Queralt J. J., Andres J., Canle L. M., Cobas J. H., Santaballa J. A., Sambrano J. R. (2002) Chem. Phys. 280, 1–14 [Google Scholar]
- 43.Roum J. H., Buhl R., McElvaney N. G., Borok Z., Crystal R. G. (1993) J. Appl. Physiol. 75, 2419–2424 [DOI] [PubMed] [Google Scholar]
- 44.Carr A. C., Winterbourn C. C. (1997) Biochem. J. 327, 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chai Y. C., Ashraf S. S., Rokutan K., Johnston R. B., Jr., Thomas J. A. (1994) Arch. Biochem. Biophys. 310, 273–281 [DOI] [PubMed] [Google Scholar]