Abstract
Background
Bovine tuberculosis (BTB) today primarily affects developing countries. In Africa, the disease is present essentially on the whole continent; however, little accurate information on its distribution and prevalence is available. Also, attempts to evaluate diagnostic tests for BTB in naturally infected cattle are scarce and mostly complicated by the absence of knowledge of the true disease status of the tested animals. However, diagnostic test evaluation in a given setting is a prerequisite for the implementation of local surveillance schemes and control measures.
Methodology/Principal Findings
We subjected a slaughterhouse population of 954 Chadian cattle to single intra-dermal comparative cervical tuberculin (SICCT) testing and two recently developed fluorescence polarization assays (FPA). Using a Bayesian modeling approach we computed the receiver operating characteristic (ROC) curve of each diagnostic test, the true disease prevalence in the sampled population and the disease status of all sampled animals in the absence of knowledge of the true disease status of the sampled animals. In our Chadian setting, SICCT performed better if the cut-off for positive test interpretation was lowered from >4 mm (OIE standard cut-off) to >2 mm. Using this cut-off, SICCT showed a sensitivity and specificity of 66% and 89%, respectively. Both FPA tests showed sensitivities below 50% but specificities above 90%. The true disease prevalence was estimated at 8%. Altogether, 11% of the sampled animals showed gross visible tuberculous lesions. However, modeling of the BTB disease status of the sampled animals indicated that 72% of the suspected tuberculosis lesions detected during standard meat inspections were due to other pathogens than Mycobacterium bovis.
Conclusions/Significance
Our results have important implications for BTB diagnosis in a high incidence sub-Saharan African setting and demonstrate the practicability of our Bayesian approach for diagnostic test evaluation.
Introduction
Mycobacterium bovis is the causative agent of bovine tuberculosis (BTB) and belongs to the Mycobacterium tuberculosis complex (MTBC) of bacteria [1]. BTB is a major problem in developing countries, which bear the largest part of the world-wide disease burden and where millions of people are affected by neglected zoonotic diseases such as BTB [2]–[5]. The disease causes economic loss by its effects on animal health and productivity and by international trade restrictions [6]. It can also affect health of wildlife [7] and infected wildlife populations serve as reservoirs and hamper disease eradication programs in several countries [8]. Moreover, M. bovis infections are of public health concern due to the pathogen's zoonotic potential [2], [3].
BTB control and surveillance is scarce in sub-Saharan Africa and mostly limited to abattoir meat inspections. However, the performance of meat inspection is rather poor and depends on the disease stage in which infected animals reside, the accuracy of the carcass examination and the presence of other lesion causing pathogens [9]–[13]. Recent studies have detected a high proportion of non-tuberculous mycobacteria (NTM) in lesions from Chadian, Ugandan, Ethiopian and Sudanese cattle, suggesting that a considerable amount of lesions detected during abattoir meat inspection of African cattle might be due to other bacteria than M. bovis [14]–[17].
Current ante mortem diagnosis of BTB mainly relies on the single intra-dermal comparative cervical tuberculin (SICCT) test, which, although imperfect, could not yet be replaced by any other more accurate diagnostic method [13]. SICCT is based on the cell mediated immune (CMI) response against tuberculosis infection. TB in cattle is characterized by an early Th1 type CMI response, whilst humoral immune responses develop as disease progresses. At late disease stages, the CMI response can decrease and SICCT anergic animals can show false negative test results [13], [18], [19]. Moreover, SICCT performance is influenced by animal exposure to NTM strains as their antigens can cross-react with tuberculin [13]. Serological tests detecting humoral immune responses may be more useful to detect late stage diseased animals. Fluorescence polarization assays (FPA) constitute a technique for antibody detection with a shown potential for diagnostic purposes [20]. An assay for the detection of M. bovis antibodies has been described recently [21]–[25].
Attempts to evaluate diagnostic tests for BTB in naturally infected cattle in Africa are scarce but a prerequisite for the implementation of surveillance schemes and control measures. Gobena et al. have used detailed post mortem examination to define the BTB disease status of Ethiopian cattle for the evaluation of SICCT in this setting [26]. However, due to the generally low sensitivity and specificity of post mortem meat inspection, its use as a gold standard test is not ideal [12]. We have recently assessed three different tests for the diagnosis of BTB (SICCT and two newly developed FPA methods) in Chadian cattle. Our previous evaluation was also based on a gold standard approach using PCR confirmed MTBC infected and lesion negative animals as the positive and negative population, respectively [25]. Drawbacks of this study were the small number of positive animals and the unknown true disease status of the lesion negative cattle.
Choi et al. [27] developed a Bayesian model for the receiver operating characteristic (ROC) estimation of two diagnostic tests in the absence of a gold standard test. In the present study, we have further extended this model and applied it to evaluate the performance of the diagnostic tests previously assessed by the gold standard approach [25]. Our Bayesian model integrated information from three different diagnostic methods and was independent of a gold standard test; moreover, it allowed us to estimate the true BTB prevalence in the sampled population and the true disease status of each tested animal. Using this information, we could in addition calculate the diagnostic errors of four post-mortem tests (meat inspection, microscopic examination of BTB-like lesions, microscopic examination of derived bacterial cultures and PCR on microscopy positive cultures).
Results
Test results
A total number of 954 sequentially selected slaughter animals from Southern Chad were subjected to multiple tests for the diagnosis of BTB. Three ante-mortem tests with continuous numerical outcome values (continuous outcome) were used, namely, SICCT and two recently developed FPA tests termed SENTRY 100 and GENios Pro [25]. Also, four post-mortem tests giving either a positive or negative test result (binary outcome) were applied. These tests were the post-mortem meat inspection, direct microscopy, culture and microscopy and PCR (see materials and methods for details on the applied tests).
Before slaughter, blood samples were collected and animals underwent SICCT testing. Altogether, 8% (CI: 6%–10%) of the animals tested, reacted positively to SICCT when the official OIE cut-off (>4 mm; [28]) was used (Table 1). Serum extracted from the blood samples was subjected to the FPA tests SENTRY 100 and GENios Pro, for which we have determined most appropriate cut-off values within this study (results shown below; Table 1). After slaughter, cattle carcasses underwent meat inspection; lesions suggestive of tuberculosis were isolated from 108 animals (lesion prevalence: 11%; CI: 9%–14%; Table 1). In lesions of 51 animals (47% of animals with lesions; CI: 38%–57%), acid-fast bacilli (AFB) were observed by direct microscopy (Table 1). Culture of lesions and subsequent microscopic examination detected AFB in samples from 50 animals (49% of the animals tested; CI: 39%–59%; Table 1). The microscopy results obtained before and after culture agreed by 86%. In AFB containing cultures of 20 animals MTBC strains could be detected by real-time PCR (Table 1). In cultures of 13 animals, NTM strains were detected; three of which showed a mixed infection with MTBC strains.
Table 1. Tests applied for the diagnosis of BTB in Chadian cattle.
| Test | No. of animals tested | Outcome | Ante-/post mortem | No. of animals tested pos. | % pos. |
| SICCT (OIE cut-off>4 mm)* | 930 | continuous | ante-mortem | 72 | 7.7% |
| SICCT (cut-off>2 mm)* | 930 | continuous | ante-mortem | 144 | 15.5% |
| SENTRY 100 (cut-off≥15 ΔmP)* | 953 | continuous | ante-mortem | 62 | 6.5% |
| GENios Pro (cut-off≥38 ΔmP)* | 954 | continuous | ante-mortem | 119 | 12.5% |
| Meat inspection | 954 | binary | post-mortem | 108 | 11.3% |
| Direct microscopy | 108 | binary | post-mortem | 51 | 47.2% |
| Culture and microscopy | 102 | binary | post-mortem | 50 | 49.0% |
| PCR | 50 | binary | post-mortem | 20 | 40.0% |
% pos.: Number of animals tested positive divided by the total number of animals subjected to the respective test.
SICCT, SENTRY 100 and GENios Pro results without missing data were available for 929 animals.
Model selection
Based on the same data, we have previously reported the evaluation of SICCT, SENTRY 100 and GENios Pro using a subset of animals with either PCR confirmed MTBC infections or no visible lesions [25]. Drawbacks of this approach were the small number of positive animals and the uncertainty about the true disease status of lesion negative animals [25]. The latter is due to the fact that no gross lesions may be observed at early stages of BTB. Here, we describe a Bayesian method for the estimation of the true disease prevalence in the sampled population and the means and variance-covariances of SICCT, SENTRY 100 and GENios Pro test outcomes for the diseased and non-diseased animals. In an initial model we have included data from the post-mortem tests with binary outcomes and attempted to directly estimate their sensitivities and specificities. Prior assumptions and model estimates are indicated in Table S1 (models 1A and 1B).
Model estimates for tests with binary outcome were highly sensitive to the priors. We therefore decided to consider solely tests with continuous outcome for Bayesian modeling (model 2A and 2B; see Table S1). Parameter estimations for these tests did not appear to be sensitive to the prior assumptions and were only marginally different in models 1A, 1B, 2A and 2B (see Table S1).
Diagnostic test performances
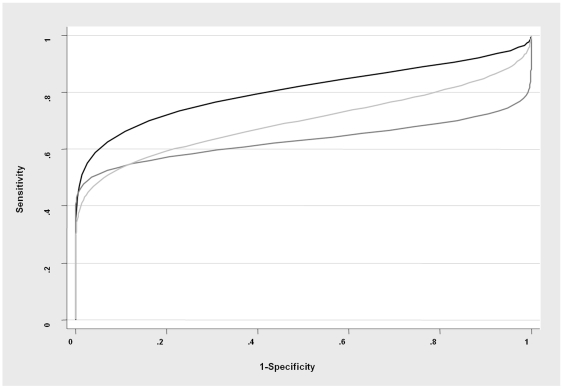
Based on the estimates for the means and variance-covariances of SICCT, SENTRY 100 and GENios Pro test results for the diseased and non-diseased animals in model 2A (see Table S1), ROC curves were calculated for each test (Fig. 1) and the most appropriate cut-off for positive test interpretation was defined as the point from the ROC curve with the largest distance from the diagonal line (sensitivity = 1−specificity). For SICCT, a cut-off greater than 2 mm (>2 mm) appeared to be most appropriate for our setting. For SENTRY 100 and GENios Pro the best cut-off values were determined at 15 ΔmP (≥15 ΔmP) and 38 ΔmP (≥38 ΔmP), respectively. Using these values, the sensitivities and specificities of the tests were calculated (Table 2). The prevalence of M. bovis infection in the sampled population was estimated at 8% (CI: 6%–11%).
Figure 1. Calculated ROC curves for SICCT (black), SENTRY 100 (dark gray) and GENios Pro (light gray).
Table 2. Parameter estimates for different diagnostic tests based on results from model 2A (see Table S1).
| Test | AUC | 95% CI | S | 95% CI | C | 95% CI |
| SICCT (OIE cut-off>4 mm) | 0.80 | 0.73–0.87 | 51.1% | 42.1–60.1% | 98.6% | 97.9–99.2% |
| SICCT (cut-off>2 mm) | 0.80 | 0.73–0.87 | 66.3% | 57.5–74.6% | 89.2% | 86.6–91.5% |
| SENTRY 100 (cut-off≥15 ΔmP) | 0.57 | 0.51–0.65 | 45.5% | 39.3–52.9% | 96.4% | 95.4–97.4% |
| GENios Pro (cut-off≥38 ΔmP) | 0.64 | 0.57–0.72 | 47.2% | 39.9–54.7% | 92.4% | 90.7–93.9% |
| Meat inspection* | - | - | 36.1% | 26.6–46.9% | 90.8% | 88.6–92.5% |
| Direct microscopy* | - | - | 90.0% | 74.4–96.5% | 66.7% | 55.2–76.5% |
| Culture and microscopy* | - | - | 93.3% | 78.6–98.2% | 69.4% | 58.0–78.8% |
| PCR* | - | - | 71.4% | 52.9–84.7% | 100.0% | 85.1–100% |
| True prevalence | 8.4% | 6.1–11.0% |
AUC: area under the ROC curve; CI: confidence interval; S: sensitivity; C: specificity.
Estimates are based on modeled latent disease state of the animals and refer to the sample; 95% CI are Wilson confidence intervals.
In addition to the parameters described above, Bayesian modeling allowed us to compute the latent disease status of the sampled animals. We have used this information from model 2A (see Table S1) to calculate the sensitivities and specificities of the post-mortem tests with binary outcome to detect modeled M. bovis infected animals (Table 2). It must be noted, that these estimates refer to the diagnostic performance for our sample, whereas the Bayesian model estimates consider the fact that our sample was a sub-population of the general slaughterhouse population.
Surprisingly, 72% of the animals with gross visible tuberculous lesions detected during standard meat inspection showed a negative result for modeled M. bovis infection. Thus, our analysis suggested that 72% of the animals exhibiting tuberculosis-like lesions were infected with other pathogens than M. bovis.
Risk factors
We performed logistic regression to identify risk factors for modeled M. bovis infection. Univariate logistic regression with modeled M. bovis infection as outcome variable and age, sex, animal breed and body condition as explanatory variables identified age and a very bad body condition as risk factors for modeled M. bovis infection (Table 3). However, in the multiple model, only age turned out to be significantly associated with modeled M. bovis infection (Table 3). Interestingly, only the presence of organ lesions in general and in particular the presence of lung and liver lesions was significantly associated with modeled M. bovis infection (Table 4). The presence of lymph node lesions was not associated with modeled M. bovis infection (Table 4).
Table 3. Logistic regression with modeled M. bovis infection as outcome variable and age, sex, breed and body condition as explanatory variables.
| Explanatory variable | Univariate model | Multiple model* | |||||
| Category | Subcategory | OR | 95% CI | p | OR | 95% CI | p |
| Age | 1.15 | 1.05–1.26 | <0.01 | 1.14 | 1.02–1.29 | <0.05 | |
| Sex | 1.59 | 0.96–2.64 | 0.07 | 1.11 | 0.61–2.01 | 0.74 | |
| Breed | 1.28 | 0.79–2.06 | 0.31 | 1.54 | 0.94–2.54 | 0.09 | |
| Body condition | |||||||
| good | 1.00 | - | - | 1.00 | - | - | |
| bad | 1.07 | 0.66–1.73 | 0.79 | 0.96 | 0.58–1.58 | 0.86 | |
| very bad | 2.81 | 1.33–5.95 | <0.01 | 1.96 | 0.88–4.38 | 0.10 | |
OR: odds ration; CI: confidence interval; p: p-value.
The multiple model was adjusted for age, sex, breed and body condition.
Table 4. Lesion distribution and association between lesion location and modeled M. bovis infection.
| N | % | RR | Fisher | |
| Animals with lesions | 108 | 100% | N/A | N/A |
| Lymph node lesions | 98 | 91% | 0.66 | 0.46 |
| Pre-scapular lymph nodes | 64 | 59% | 1.19 | 0.67 |
| Mammary lymph nodes | 37 | 34% | 1.11 | 0.82 |
| Head associated | 8 | 7% | 0.43 | 0.44 |
| Popliteal lymph nodes | 1 | 1% | 0.00 | 1.00 |
| Organ lesions | 22 | 20% | 2.99 | <0.01 |
| Lung | 17 | 16% | 3.10 | <0.01 |
| Liver | 8 | 7% | 2.50 | <0.04 |
| Others | 3 | 3% | 2.50 | 0.19 |
N: Number of animals with lesions at the specified location. %: Percentage of animals with lesions at the specified location. RR: Risk ratio for modeled M. bovis infection. Fisher: Fisher's exact test p-value.
Discussion
Practicability and significance of Bayesian ROC estimation
The performance of diagnostic tests is often setting dependent [29]. Thus, evaluations of diagnostic tests for a given region are a prerequisite for the implementation of local disease surveillance schemes and control measures [29]. However, to date, only few studies have assessed the performance of tests for the diagnosis of BTB in high incidence countries in Africa. Furthermore, test evaluation is hampered by the absence of a gold standard method for the identification of the animal's true disease status. Here, we applied a Bayesian approach for the evaluation of multiple tests for the diagnosis of BTB in a naturally infected slaughterhouse population of cattle in Southern Chad. Our approach did not require knowledge of the true disease status of the tested animals. Moreover, it allowed the estimation of the true disease prevalence in the sampled population, the calculation of the BTB disease status of all sampled animals and the evaluation of four post-mortem tests for the diagnosis of BTB.
We have previously reported the evaluation of SICCT, SENTRY 100 and GENios Pro using a subset of the same data [25]. In a gold standard approach, PCR confirmed MTBC infected animals were defined as the positive population and lesion negative animals as the negative population and used for the construction of ROC curves for each test. Drawbacks of this approach were the relatively small amount of confirmed infections and the unknown true disease status of lesion negative animals. Table 5 compares the results from the present and our previously published study [25]. The accordance of our results using the two different approaches further supports the accuracy of our estimates and the practicability of our Bayesian method. Noteworthy, Bayesian modeling gave rise to parameter estimates with in many cases considerably smaller confidence intervals compared to the gold standard approach (Table 5).
Table 5. Comparison of parameter estimates derived from the herein described Bayesian model and from a previously applied gold standard approach [25].
| Cut-off | SICCT | SENTRY 100 | GENios Pro | |
| >4mm | >2mm | ≥15 | ≥38 | |
| Bayesian method: | ||||
| Sensitivity | 51.1% (42.1–60.1%) | 66.3% (57.5–74.6%) | 45.5% (39.3–52.9%) | 47.2% (39.9–54.7%) |
| Specificity | 98.6% (97.9–99.2%) | 89.2% (86.6–91.5%) | 96.4% (95.4–97.4%) | 92.4% (90.7–93.9%) |
| AUC | 0.80 (0.73–0.87) | 0.80 (0.73–0.87) | 0.57 (0.51–0.65) | 0.64 (0.57–0.72) |
| Gold standard approach: | ||||
| Sensitivity | 20.0% (5.7–43.7%)* | 65.0% (43.3–81.9%) | 30.0% (14.5–51.9%) | 50.0% (29.9–70.1%) |
| Specificity | 93.1% (91.1–94.6%) | 86.7% (84.2–88.9%) | 94.4% (92.7–95.8%) | 88.4% (86.1–90.4%) |
| AUC | 0.80 (0.71–0.88) | 0.80 (0.71–0.88) | 0.70 (0.58–0.82) | 0.67 (0.52–0.82) |
The previously conducted diagnostic test evaluation considered animals with PCR confirmed infections and animals not showing lesions during post mortem meat inspection as disease positive and negative animals, respectively.
95% binomial exact confidence intervals are indicated because (estimated value)×(sample size)≤5; for all other parameter estimates in the gold standard approach, Wilson confidence intervals are shown.
SICCT
Our results indicated that the most appropriate cut-off for positive SICCT test interpretation was significantly lower then the OIE suggested standard cut-off (>2 mm versus >4 mm). However, our criteria for cut-off selection attributed equal weights to sensitivity and specificity and did not consider the disease prevalence and the cost of misclassifications. As an alternative approach for cut-off selection, the misclassification-cost term (MCT) can be calculated for each point of the ROC curve. The point with the lowest MCT value would then be most appropriate for positive test interpretation [30]. This method requires to quantify the cost of false negative (CFN) and false positive (CFP) diagnosis, which we were not able to accurately do. However, the cost of a false negative diagnosis is likely to exceed the cost of a false positive result by several folds as disease transmission amplifies the total economical losses due to BTB. We found that, assuming a disease prevalence of 8.4% (10.0%), a cut-off >2 mm would be ideal if CFN/CFP lies between 8 and 16 (7 and 13). This suggests that our chosen cut-off values may be acceptable for a broad range of reasonable CFN/CFP ratios.
A cut-off >2 mm was also found to be most appropriate for positive SICCT test interpretation in a recent study in Ethiopia [26] and in SICCT reactor prevalence studies in Uganda and Tanzania, lower cut-offs than the OIE standard cut-off have been used, however without detailed justification [31], [32]. Accordingly, our results are likely to apply for many other countries in sub-Saharan Africa with similar environmental and economic conditions.
SICCT showed a relatively low sensitivity irrespective of whether our suggested or the OIE cut-off was used (Table 2). Comparable results were obtained in previous studies in Ireland and Madagascar [13], [33]. This relatively weak performance may be explained by several factors. A high proportion of pre-allergic animals at an early stage of BTB infection or a high amount of SICCT anergic animals at a very late disease stage could have accounted for this observation [13]. Antigens of co-infecting NTM strains, cross reacting with PPD-A could also cause false negative test results as well as nutritional stress or concurrent infections with pathogens leading to immuno-depression [13]. For SICCT anergy due to generalized BTB, one would expect the presence of gross visible lesions. Amongst all animals with a modeled M. bovis infection and visible lesions (N = 30), 9 or 19 (30% or 63%) did not show a positive reaction to SICCT depending on whether a cut-off >2 mm or >4 mm was applied, respectively. This indicates a considerable proportion of SICCT anergic animals (9 or 19 of altogether 83 animals with modeled M. bovis infection). Unfortunately, our sample size was too small to conclusively assess the ability of the FPA tests to detect such animals.
Cause of lesions
Our data suggests that a surprisingly high proportion of lesions detected during standard meat inspection at the Sarh abattoir in Southern Chad was caused by other bacteria than M. bovis. For 72% of the animals in which lesions have been detected, no M. bovis infection was modeled. This finding was in line with the relatively low amount of MTBC strains detected in animals with lesions (20 of altogether 108 animals with lesions; Table 1). Interestingly, modeled M. bovis infection was only significantly associated with organ lesions in general and the presence of lung and liver lesions in particular (Table 4). The presence of lymph node lesions was not associated with modeled M. bovis infection (Table 4). Altogether, this suggests that a significant amount of gross visible lesions detected during standard meat inspection at the Sarh abattoir has been caused by other pathogens than M. bovis and that especially a large proportion of the detected lymph node lesions may have been caused by these pathogens.
NTM infections without concomitant M. bovis infections have been isolated from 10 out of 50 animals tested by PCR. This could indicate that some of the lesions may have been associated with NTMs. This is also supported by the comparatively low specificity of Ziehl-Neelsen staining and microscopic examination of extracted lesions or bacterial cultures in our setting compared to previous studies (Table 2) [34]–[38]. Nevertheless, the low amount of cultures in which AFB have been detected (50 of 108 animals with lesions) suggests that in addition, other pathogens may have been responsible for the detected lesions.
Altogether, our data indicates that the amount of gross visible granulomatous lesions caused by other pathogens than M. bovis may be greatly underestimated in this setting. Low recovery of M. bovis from cultures of granulomatous lesions have been reported in several studies on BTB in sub-Saharan Africa [16], [17], [39], [40]. It is conceivable that in many of these cases, lesions may have been caused by other pathogens and that these bacteria may have remained undetected e.g. due to the decontamination procedure or different culture growth requirements.
However, it has to be noted that the proportion of lesions due to other pathogens than M. bovis is dependent on the accuracy of the meat inspection. Inaccurate meat inspection e.g. biased toward superficial lymph nodes could have distorted the relative proportion of lesions found in different organs. In particular, it is surprising that no lesions were detected in the bronchial or mediastinal lymph nodes, as these are usually the most often affected tissues in bovine tuberculosis [9], [10], [41], [42]. Also, the sensitivity of meat inspection to detect M. bovis infected cattle was lower in our setting compared to the results of previous studies [9]–[12]. Therefore, the proportion of lesions caused by other pathogens than M. bovis may have to be interpreted with caution.
Risk factors
In a previous study on BTB in Chadian cattle we have reported that the prevalence of BTB was significantly higher in Mbororo zebus than in Arab zebus [40]. Our results from the logistic regression analysis could not show any evidence that M. bovis infection was significantly associated with breed (Table 3). Nevertheless, the presence of lesions was still significantly associated with Mbororo zebus (N = 944, χ2 = 5.23, p = 0.02). This observation could suggest that Mbororo breeds in fact, are not more likely to be infected with M. bovis but more often develop advanced stages of the disease. Host genetic factors as well as environmental factors or animal husbandry could account for this observation.
Conclusions
In summary, the present study shows the practicability of a Bayesian method for the evaluation of multiple tests for the diagnosis of BTB in naturally infected cattle and in absence of knowledge of the true disease status of the animals. Our model allowed us to compute the disease status of each sampled animal and the modeling results supported our previous observation that the cut-off for positive SICCT interpretation should be lowered to >2 mm in many countries of sub-Saharan Africa. Moreover, we provide evidence that an unexpectedly high proportion of BTB suspect lesions detected during slaughterhouse meat inspection was due to other pathogens than M. bovis.
Materials and Methods
Animals
The animal population subjected to this study has previously been described, in detail [25]. A total of 954 slaughter animals were sampled during three intervals of approximately one month between July and November 2005 at abattoirs in Southern Chad. We can assume that the tested animals constitute a representative sample of slaughter cattle from a large number of different herds and a big area in Southern Chad [25]. Presumably, none of the animals has ever undergone tuberculin skin testing. Four types of phenotypic zebu breeds were encountered: Arab (N = 658), Mbororo (N = 286), Bogolodjé (N = 7) and cross breeds (N = 3).
Physical examination of animals
All 954 animals were physically examined before slaughter. Body condition was assessed by assigning one of the following three scores: 1 – good body condition, 2 – bad body condition, 3 – very bad body condition [25].
Test procedures
SICCT
Valid SICCT testing results were available for 930 animals. SICCT testing and reading was carried out as explained previously and according to standard protocols [25], [28].
Fluorescence polarization assays
Valid SENTRY 100 and GENios Pro FPA results were available for 953 and 954 animals, respectively. The methods have been previously described in detail [21], [25].
Meat inspection
After slaughter, all 954 animals underwent meat inspection, which included organ and lymph node palpation, visual inspection and incision of organs and lymph nodes according to standard procedures [43]. However, we were not able to fully exclude potential irregularities during the carcass examinations. Meat inspection was done by local meat inspectors at the abattoirs in Southern Chad. Gross visible lesions were detected in altogether 108 of the 954 sampled animals. Lesion containing tissue specimens from all visibly affected organs and lymph nodes were collected and transported on ice to the Chadian National Veterinary and Animal Husbandry Laboratory (Laboratoire de Recherches Vétérinaries et Zootéchniques de Farcha) in N'Djaména and stored at −20°C.
Direct microscopy
Specimens from all 108 animals with lesions were subjected to direct microscopy and processed as previously described [40]. After homogenisation, specimens were colorized by Ziehl-Neelsen staining and examined under the light-microscope for the presence of Acid-Fast Bacilli (AFB). The samples were decontaminated with N-acetyl-L-cysteine sodium hydroxide (0.5% NALC 2% NaOH) and again examined for the presence of AFB under the microscope. If either of the two microscopic examinations revealed presence of AFB the result was considered to be positive.
Culture and microscopy
Specimens of lesions from altogether 102 animals were subjected to culture and microscopy. Decontaminated samples were inoculated into two Middlebrook 7H9 medium flasks containing OADC and PANTA and either glycerol (0.75%) or pyruvate (0.6%) [25]. Samples were put into culture until growth was detected or for a minimum of 8 weeks. Presence of AFB in cultures was examined by Ziehl-Neelsen staining and microscopy [40]. Bacterial growth was detected in cultures of 102 animals; cultures of 50 animals showed presence of AFB by Ziehl-Neelsen staining.
Real-time PCR
AFB containing cultures from 50 animals were subjected to molecular characterization. Heat inactivation of the cultures was carried out as previously explained [25]. Thermolysates were shipped to the Swiss Reference Centre for Mycobacteria, DNA was extracted by means of the InstaGene™ Matrix (Bio-Rad) and identification of MTBC and NTM strains was carried out by means of Light Cycler® PCR as previously described by Lachnik et al. [44].
Statistical analyses
A Bayesian model was developed to estimate the true M. bovis infection status of all sampled animals. The model combined the results of the continuous as well as the binary diagnostic tests for BTB (SICCT, SENTRY 100, GENios Pro, meat inspection, direct microscopy, culture and microscopy and PCR), applied to the same animal population without considering a gold standard. It also allowed the estimation of the true disease prevalence in the sampled population as well as the sensitivities and specificities of the diagnostic tests. A mathematical description of the model and the WinBUGS code are provided in Text S1 and Text S2, respectively.
Risk factors for the modeled M. bovis infection status were identified by univariate and multiple logistic regression analysis in Stata (Stata/IC v10.0). Association between lesion localisation and modeled M. bovis infection was assessed by the Fisher's exact test in Stata.
Bayesian modeling of disease status
We assumed that the distribution of the test values of SICCT, SENTRY 100 and GENios Pro was trivarite normal with means and variance-covariances separately estimated for the diseased and the non-diseased animals. The normality assumption was verified via the shape of the histogram of the test values. In an initial model we have included the data from the multiple post mortem tests for the detection of M. bovis infected animals (meat inspection, direct microscopy, culture and microscopy and PCR) and tried to directly model their sensitivities and specificities (models 1A and 1B, see Table S1). Because the parameter estimations for the binary post-mortem tests were highly sensitive to the prior assumptions [9]–[12], [34]–[38], [45], [46], we eventually excluded the respective data from the Bayesian model formulation (models 2A and 2B, Table S1). In order to estimate the performance of the binary tests, we used the modeled latent M. bovis infection status of each animal to calculate sensitivities and specificities of the respective tests. Model fit was done in the statistical package WinBUGS (Imperial College and Medical Research Council, UK). The mathematical description of the model and the WinBUGS code are shown in Text S1 and Text S2. Real-time PCR was assumed to be 100% specific and animals with a positive PCR test outcome were therefore defined as MTBC infected in all Bayesian models.
ROC curve and cut-off selection
From the estimates of the means and variance-covariances of the multivariate normally distributed continuous test values of SICCT, SENTRY 100 and GENios Pro for the diseased and non-diseased animals, a ROC curve was calculated in Stata. Pairs of 1-specificity and sensitivity were calculated and plotted for all possible cut-off points according to the following formula:
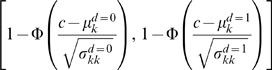 |
Φ is the cumulative distribution of a standard normal variable; c is the cut-off value;  and
and 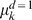 are the means of the multivariate normal distribution of the test values for the non-diseased (d = 0) and the diseased (d = 1) animal population subjected to test k (k = 1 for SICCT, k = 2 for SENTRY 100, k = 3 for GENios Pro), respectively;
are the means of the multivariate normal distribution of the test values for the non-diseased (d = 0) and the diseased (d = 1) animal population subjected to test k (k = 1 for SICCT, k = 2 for SENTRY 100, k = 3 for GENios Pro), respectively; 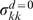 and
and  are the variance of the non-diseased and the diseased animal population subjected to test k, respectively.
are the variance of the non-diseased and the diseased animal population subjected to test k, respectively.
We considered the point of the ROC plot with the greatest distance from the diagonal line (sensitivity = 1−specificity) as the best cut-off; this corresponds to the point with the largest Youden index (J = sensitivity+specificity−1) [30], [47]. In cases where several points showed the same distance, the point with the highest sensitivity was chosen. For cut-off selection using the misclassification-cost term (MCT), the point with the smallest MCT value [MCT = (CFN/CFP) P (1−Se)+(1−P) (1−Sp)] was chosen, with CFN and CFP being the cost of false negative and false positive diagnosis, respectively and P being the disease prevalence in the target population [30]. We were unable to accurately estimate CFN/CFP but the cost of false-negative diagnosis is likely to exceed the cost of false positive diagnosis. Therefore, MCT values for each possible cut-off point and different ratios of CFN/CFP were calculated and compared, assuming a disease prevalence of 8.4%, as estimated by our model. In addition, MCT values for different CFN/CFP ratios were calculated for a 10.0% disease prevalence.
Supporting Information
Priors and model estimates for different parameters. μd: Mean diagnostic value for non-diseased (d = 0) and diseased (d = 1) animals, respectively. τd: precision of the diagnostic values for non-diseased (d = 0) and diseased (d = 1) animals, respectively. mS/σS, mC/σC, mπ/σπ : Mean and standard deviation of the test sensitivity, specificity and true disease prevalence, respectively. The normal distribution is parametrized in terms of mean and variance. The Gamma distribution is parametrized in a non-conventional way in terms of mean and variance instead of the shape and scale parameters. Model-based estimates correspond to the posterior mean and standard deviation in brackets.
(0.04 MB XLS)
Mathematical model description
(0.12 MB DOC)
WinBUGS code
(0.03 MB DOC)
Acknowledgments
We would like to thank Prof. Erik C. Böttger, Dr. Boris Böddinghaus, Dr. Burkhard Springer and the technicians of the Swiss National Centre for Mycobacteria in Zurich for providing technical support and laboratory facilities. We are indebted to the cattle holders, traders and butchers who were collaborating with us within this project.
Footnotes
Competing Interests: Irene Schiller, Beatrice Marg-Haufe and Bruno Oesch were employees of Prionics AG, at the time of this study; Prionics AG has developed the FPA tests herein described.
Funding: Our work has received financial support from the Swiss National Science Foundation (http://www.snf.ch/E/Pages/default.aspx; project no. 107559) and from Prionics AG, Schlieren-Zurich, Switzerland (www.prionics.com). The Swiss National Science Foundation had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Prionics AG had no role in study design, data collection and analysis and decision to publish but employees of Prionics AG were involved in manuscript writing.
References
- 1.Smith NH, Gordon SV, de la Rua-Domenech R, Clifton-Hadley RS, Hewinson RG. Bottlenecks and broomsticks: the molecular evolution of Mycobacterium bovis. Nature Reviews Microbiology. 2006;4:670–681. doi: 10.1038/nrmicro1472. [DOI] [PubMed] [Google Scholar]
- 2.Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, de K I, Meslin FX. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayele WY, Neill SD, Zinsstag J, Weiss MG, Pavlik I. Bovine tuberculosis: an old disease but a new threat to Africa. Int J Tuberc Lung Dis. 2004;8:924–937. [PubMed] [Google Scholar]
- 4.WHO. The Control of Neglected Zoonotic Diseases. A route to poverty allevation 2006 [Google Scholar]
- 5.Thornton PK, Kruska RL, Henninger N, Kristjanson PM, Reid RS, Atieno F, Odero AN, Ndegwa T. Mapping Poverty and Livestock in the Developing World. Nairobi, Kenya: International Livestock Research Institute (ILRI); 2002. [Google Scholar]
- 6.Zinsstag J, Schelling E, Roth F, Kazwala RR. Economics of bovine tuberculosis. In: Thoen CO, Steele JH, Gilsdorf MJ, editors. Mycobacterium bovis Infection in Animals and Humans. Blackwell Publishing; 2006. [Google Scholar]
- 7.Renwick AR, White PC, Bengis RG. Bovine tuberculosis in southern African wildlife: a multi-species host-pathogen system. Epidemiol Infect. 2006:1–12. doi: 10.1017/S0950268806007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corner LA. The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: how to assess the risk. Vet Microbiol. 2006;112:303–312. doi: 10.1016/j.vetmic.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Teklul A, Asseged B, Yimer E, Gebeyehu M, Woldesenbet Z. Tuberculous lesions not detected by routine abattoir inspection: the experience of the Hossana municipal abattoir, southern Ethiopia. Rev Sci Tech. 2004;23:957–964. doi: 10.20506/rst.23.3.1534. [DOI] [PubMed] [Google Scholar]
- 10.Asseged B, Woldesenbet Z, Yimer E, Lemma E. Evaluation of abattoir inspection for the diagnosis of Mycobacterium bovis infection in cattle at Addis Ababa abattoir. Trop Anim Health Prod. 2004;36:537–546. doi: 10.1023/b:trop.0000040934.32330.44. [DOI] [PubMed] [Google Scholar]
- 11.Corner L, Melville L, McCubbin K, Small KJ, McCormick BS, Wood PR, Rothel JS. Efficiency of inspection procedures for the detection of tuberculous lesions in cattle. Aust Vet J. 1990;67:389–392. doi: 10.1111/j.1751-0813.1990.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadorn DC, Stark KD. Evaluation and optimization of surveillance systems for rare and emerging infectious diseases. Vet Res. 2008;39:57. doi: 10.1051/vetres:2008033. [DOI] [PubMed] [Google Scholar]
- 13.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006;81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Diguimbaye-Djaïbe C, Vincent V, Schelling E, Hilty M, Ngandolo R, Mahamat HH, Pfyffer G, Baggi F, Tanner M, Zinsstag J. Species identification of non-tuberculous mycobacteria from humans and cattle of Chad. Schweiz Arch Tierheilkd. 2006;148:251–256. doi: 10.1024/0036-7281.148.5.251. [DOI] [PubMed] [Google Scholar]
- 15.Oloya J, Kazwala R, Lund A, Opuda-Asibo J, Demelash B, Skjerve E, Johansen TB, Djonne B. Characterisation of mycobacteria isolated from slaughter cattle in pastoral regions of Uganda. BMC Microbiol. 2007;7:95. doi: 10.1186/1471-2180-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulieman MS, Hamid ME. Identification of acid fast bacteria from caseous lesions in cattle in Sudan. J Vet Med B Infect Dis Vet Public Health. 2002;49:415–418. doi: 10.1046/j.1439-0450.2002.00565.x. [DOI] [PubMed] [Google Scholar]
- 17.Berg S, Firdessa R, Habtamu M, Gadisa E, Mengistu A, Yamuah L, Ameni G, Vordermeier M, Robertson BD, Smith NH, Engers H, Young D, Hewinson RG, Aseffa A, Gordon SV. The burden of mycobacterial disease in ethiopian cattle: implications for public health. PLoS ONE. 2009;4:e5068. doi: 10.1371/journal.pone.0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollock JM, Welsh MD, McNair J. Immune responses in bovine tuberculosis: towards new strategies for the diagnosis and control of disease. Vet Immunol Immunopathol. 2005;108:37–43. doi: 10.1016/j.vetimm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Welsh MD, Cunningham RT, Corbett DM, Girvin RM, McNair J, Skuce RA, Bryson DG, Pollock JM. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology. 2005;114:101–111. doi: 10.1111/j.1365-2567.2004.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolley ME, Nasir MS. The use of fluorescence polarization assays for the detection of infectious diseases. Comb Chem High Throughput Screen. 2003;6:235–244. doi: 10.2174/138620703106298419. [DOI] [PubMed] [Google Scholar]
- 21.Jolley ME, Nasir MS, Surujballi OP, Romanowska A, Renteria TB, De la Mora A, Lim A, Bolin SR, Michel AL, Kostovic M, Corrigan EC. Fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in bovine sera. Vet Microbiol. 2007;120:113–121. doi: 10.1016/j.vetmic.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Lin M, Sugden EA, Jolley ME, Stilwell K. Modification of the Mycobacterium bovis extracellular protein MPB70 with fluorescein for rapid detection of specific serum antibodies by fluorescence polarization. Clin Diagn Lab Immunol. 1996;3:438–443. doi: 10.1128/cdli.3.4.438-443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surujballi OP, Romanowska A, Sugden EA, Turcotte C, Jolley ME. A fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in cattle sera. Vet Microbiol. 2002;87:149–157. doi: 10.1016/s0378-1135(02)00044-5. [DOI] [PubMed] [Google Scholar]
- 24.Waters WR, Palmer MV, Thacker TC, Bannantine JP, Vordermeier HM, Hewinson RG, Greenwald R, Esfandiari J, McNair J, Pollock JM, Andersen P, Lyashchenko KP. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin Vaccine Immunol. 2006;13:648–654. doi: 10.1128/CVI.00061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngandolo BNR, Müller B, Diguimbaye-Djaïbe C, Schiller I, Marg-Haufe B, Cagiola M, Jolley M, Surujballi O, Akakpo AJ, Oesch B, Zinsstag J. Comparative assessment of fluorescence polarization and tuberculin skin testing for the diagnosis of bovine tuberculosis in Chadian cattle. Prev Vet Med. 2009;89:81–89. doi: 10.1016/j.prevetmed.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Ameni G, Hewinson G, Aseffa A, Young D, Vordermeier M. Appraisal of interpretation criteria for the comparative intradermal tuberculin test for the diagnosis of bovine tuberculosis in central Ethiopia. Clin Vaccine Immunol. 2008 doi: 10.1128/CVI.00114-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YK, Johnson WO, Collins MT, Gardner IA. Bayesian inferences for receiver operating characteristic curves in the absence of a gold standard. Journal of Agricultural Biological and Environmental Statistics. 2006;11:210–229. [Google Scholar]
- 28.International Office of Epizootics (OIE) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2004. Paris.: 11-21-2006. [Google Scholar]
- 29.Greiner M, Gardner IA. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med. 2000;45:3–22. doi: 10.1016/s0167-5877(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 30.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 31.Cleaveland S, Shaw DJ, Mfinanga SG, Shirima G, Kazwala RR, Eblate E, Sharp M. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis (Edinb ) 2007;87:30–43. doi: 10.1016/j.tube.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Oloya J, Opuda-Asibo J, Djonne B, Muma JB, Matope G, Kazwala R, Skjerve E. Responses to tuberculin among Zebu cattle in the transhumance regions of Karamoja and Nakasongola district of Uganda. Trop Anim Health Prod. 2006;38:275–283. doi: 10.1007/s11250-006-4322-7. [DOI] [PubMed] [Google Scholar]
- 33.Quirin R, Rasolofo V, Andriambololona R, Ramboasolo A, Rasolonavalona T, Raharisolo C, Rakotoaritahina H, Chanteau S, Boisier P. Validity of intradermal tuberculin testing for the screening of bovine tuberculosis in Madagascar. Onderstepoort Journal of Veterinary Research. 2001;68:231–238. [PubMed] [Google Scholar]
- 34.Varello K, Pezzolato M, Mascarino D, Ingravalle F, Caramelli M, Bozzetta E. Comparison of histologic techniques for the diagnosis of bovine tuberculosis in the framework of eradication programs. J Vet Diagn Invest. 2008;20:164–169. doi: 10.1177/104063870802000204. [DOI] [PubMed] [Google Scholar]
- 35.Watrelot-Virieux D, Drevon-Gaillot E, Toussaint Y, Belli P. Comparison of three diagnostic detection methods for tuberculosis in French cattle. J Vet Med B Infect Dis Vet Public Health. 2006;53:321–325. doi: 10.1111/j.1439-0450.2006.00957.x. [DOI] [PubMed] [Google Scholar]
- 36.Estrada-Chavez C, Diaz Otero F, Arriaga-Diaz C, Villegas-Sepulveda N, Perez Gonzalez R, Gonzalez Salazar D. Agreement between PCR and conventional methods for diagnosis of bovine tuberculosis. Veterinaria Mexico. 2004;35:225–236. [Google Scholar]
- 37.Moore DF, Curry JI. Detection and Identification of Mycobacterium-Tuberculosis Directly from Sputum Sediments by Amplicor Pcr. Journal of Clinical Microbiology. 1995;33:2686–2691. doi: 10.1128/jcm.33.10.2686-2691.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nutrition and Health in Developing Countries. Totowa, , NJ, USA: Humana Press Inc; 2001. [Google Scholar]
- 39.Müller B, Steiner B, Bonfoh B, Fane A, Smith NH, Zinsstag J. Molecular characterisation of Mycobacterium bovis isolated from cattle slaughtered at the Bamako abattoir in Mali. BMC Vet Res. 2008;4:26. doi: 10.1186/1746-6148-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diguimbaye-Djaïbe C, Hilty M, Ngandolo R, Mahamat HH, Pfyffer GE, Baggi F, Hewinson G, Tanner M, Zinsstag J, Schelling E. Mycobacterium bovis isolates from tuberculous lesions in Chadian zebu carcasses. Emerg Infect Dis. 2006;12:769–771. doi: 10.3201/eid1205.050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whipple DL, Bolin CA, Miller JM. Distribution of lesions in cattle infected with Mycobacterium bovis. Journal of Veterinary Diagnostic Investigation. 1996;8:351–354. doi: 10.1177/104063879600800312. [DOI] [PubMed] [Google Scholar]
- 42.Liebana E, Johnson L, Gough J, Durr P, Jahans K, Clifton-Hadley R, Spencer Y, Hewinson RG, Downs SH. Pathology of naturally occurring bovine tuberculosis in England and Wales. Veterinary Journal. 2008;176:354–360. doi: 10.1016/j.tvjl.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Herenda D, Chambers PG, Ettriqui A, Seneviratna P, da Silva TJP. Manual on meat inspection for developing countries. 1994 [Google Scholar]
- 44.Lachnik J, Ackermann B, Bohrssen A, Maass S, Diephaus C, Puncken A, Stermann M, Bange FC. Rapid-cycle PCR and fluorimetry for detection of mycobacteria. J Clin Microbiol. 2002;40:3364–3373. doi: 10.1128/JCM.40.9.3364-3373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor GM, Worth DR, Palmer S, Jahans K, Hewinson RG. Rapid detection of Mycobacterium bovis DNA in cattle lymph nodes with visible lesions using PCR. BMC Vet Res. 2007;3:12. doi: 10.1186/1746-6148-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez Cancela MM, Garcia Marin JF. Comparison of Ziehl-Neelsen staining and immunohistochemistry for the detection of Mycobacterium bovis in bovine and caprine tuberculous lesions. J Comp Pathol. 1993;109:361–370. doi: 10.1016/s0021-9975(08)80299-x. [DOI] [PubMed] [Google Scholar]
- 47.Gardner IA, Greiner M. Receiver-operating characteristic curves and likelihood ratios: improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Veterinary Clinical Pathology. 2006;35:8–17. doi: 10.1111/j.1939-165x.2006.tb00082.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Priors and model estimates for different parameters. μd: Mean diagnostic value for non-diseased (d = 0) and diseased (d = 1) animals, respectively. τd: precision of the diagnostic values for non-diseased (d = 0) and diseased (d = 1) animals, respectively. mS/σS, mC/σC, mπ/σπ : Mean and standard deviation of the test sensitivity, specificity and true disease prevalence, respectively. The normal distribution is parametrized in terms of mean and variance. The Gamma distribution is parametrized in a non-conventional way in terms of mean and variance instead of the shape and scale parameters. Model-based estimates correspond to the posterior mean and standard deviation in brackets.
(0.04 MB XLS)
Mathematical model description
(0.12 MB DOC)
WinBUGS code
(0.03 MB DOC)