Abstract
Background
HIV-infected patients have accelerated atherosclerosis. Abacavir has been associated with increased risk of cardiovascular events, for reasons that remain to be elucidated. As endothelial dysfunction is central to the pathogenesis of atherosclerosis, we tested the hypothesis that current treatment with abacavir is associated with impaired endothelial function.
Methods
We studied a cohort of 61 antiretroviral-treated patients who had undetectable plasma HIV RNA levels. Endothelial function was assessed by measuring flow-mediated vasodilation (FMD) of the brachial artery. We compared FMD in patients treated with or without abacavir, while adjusting for traditional risk factors and HIV-specific characteristics.
Results
The median age was 50 years (IQR 45–57). The median duration of HIV infection was 18 years, and the median CD4 cell count was 369 cells/mm3. Thirty subjects (49%) were receiving abacavir. Overall, the median FMD in the HIV-infected patients was low (3.5%; IQR 2.3–5.6%). The FMD was lower in the abacavir-treated patients than those not on abacavir (2.8% vs. 4.9%, p=0.01). After adjustment for traditional risk factors, HIV specific factors, and baseline brachial artery diameter, current abacavir use was independently associated with lower FMD (p=0.017). Duration of therapy and CD4 count were not associated with reduced FMD.
Conclusions
Endothelial function, a central mechanism in atherosclerosis and a marker of cardiovascular risk, is impaired among antiretroviral-treated patients with undetectable viral loads. Current use of abacavir was independently associated with impaired endothelial function. This finding suggests that abnormal endothelial function may underlie the clinically observed increased risk in myocardial infarction among abacavir-treated patients.
INTRODUCTION
A growing number of studies suggest that HIV-infected individuals are at increased risk for cardiovascular disease. The mechanism underlying this increased risk is unknown but may be related to traditional risk factors, inflammation, HIV infection itself, and/or antiretroviral therapy. Although most of the concern regarding drug toxicity initially focused on protease inhibitors[1], more recent data have emerged suggesting that abacavir use may also be associated with higher risk of cardiovascular events including the D:A:D study, the SMART study, and the French Hospital database on HIV[2–4]. The biological rationale for this increased risk associated with abacavir is not readily apparent as (in contrast to the protease inhibitors) abacavir is not associated with hyperlipidemia or insulin resistance[5–7]. Abacavir-associated inflammation has been proposed as one mechanism whereby this drug can cause premature cardiovascular morbidity.[3]
There are several well validated approaches to measuring cardiovascular risk and disease. Some approaches (including the measurement of carotid artery intima-media thickness) focus on development of subclinical atherosclerosis, but these measurements reflect long term exposure to cardiovascular risk factors and are not well suited to examining the short-term risk of changes in inflammation and other potential risk factors. For our current study, we chose to measure flow-mediated vasodilation (FMD) of the brachial artery, since this measurement is strongly predictive of future cardiovascular events [8– 11], changes rapidly in response to HIV medications[12], and is sensitive to the potential effect of chronic inflammation[13].
METHODS
Study Subjects
We recruited HIV-infected study participants who were enrolled in the SCOPE cohort at the University of California, San Francisco General Hospital. HIV-infected patients were required to be on at least 24 weeks of stable antiretroviral therapy with the two most recent HIV RNA levels < 75 copies/ml. Patients enrolled in the study underwent a detailed interview regarding HIV disease characteristics, health-related behaviors, and traditional cardiovascular risk factors. Chart review was performed on each patient in order to determine the duration of different types of antiretroviral medication. The FMD studies were performed in the context of studying vascular function in treated and suppressed HIV-infected individuals; we used these patients as a convenience sample to evaluate the effect of current abacavir use on endothelial function. Thus, while this objective was formulated post-hoc, it was the primary hypothesis tested. Importantly, subjects for this study were recruited independent of their antiretroviral drug regimen.
As FMD measurements are highly responsive to changes in medications, we excluded any individuals who had started or modified doses of any type of antihypertensive or lipid lowering medication in the 12 weeks prior to the study. As nitroglycerin is administered to assess endothelium-independent vasodilation, we also excluded patients who had taken sildenafil, vardenafil, or tadalafil within 72 hours, or who were hypotensive (systolic BP <100). The University of California, San Francisco Committee on Human Research approved the study, and all patients provided written informed consent.
Assessment of endothelial function
Subjects were asked to refrain from drinking alcohol and beverages containing caffeine for at least 12 hours prior to study. Patients were studied lying supine in a dark and quiet room. High-resolution ultrasound of the right brachial artery was performed just above the antecubital fossa using established guidelines[14] and methods.[15] We used a 10MHz linear array probe in conjunction with the GE VividSeven Imaging System (GE, Milwaukee, WI). To assess endothelium-dependent vasodilation, brachial artery diameter was measured under basal conditions and during reactive hyperemia following 5 minutes of an ischemic stimulus. A blood pressure cuff was placed on the forearm and inflated to suprasystolic pressures for 5 minutes to induce forearm ischemia. Following cuff deflation, the maximal increase in brachial artery diameter was measured at 1 minute of reactive hyperemia (endothelium-dependent vasodilation).[16] To assess endothelium-independent vasodilation, after 20 minutes of rest, brachial artery diameter was determined under basal conditions and following the administration of sublingual nitroglycerin (0.4 mg). Maximal brachial artery dilation was measured 3 minutes after the administration of sublingual nitroglycerin. Acquisition and analysis of the digitized images was performed using dedicated software (Information Integrity Inc., Iowa City, Iowa). The images were performed and analyzed by a single technician who was blinded to the subject’s HIV disease and treatment status. The vessel wall/lumen interface was determined by derivative based edge detection algorithm following identification of the region of the anterior and posterior wall by the investigator. The maximum diameter of the vessel was then determined.[17, 18] Three consecutive cardiac cycles were measured at end-diastole. We repeated measurements on 10 scans in a blinded manner and the correlations in reading were 0.998 with only small median differences of −0.18% to 0.08%. In addition, ten subjects underwent repeat scans within 14 days of study enrollment; the difference in FMD was 0.005% (−0.06% to +0.04%, p=0.99).
Statistical Analyses
For continuous variables, Kruskal-Wallis tests followed by pairwise Wilcoxon ranksum tests were used to perform unadjusted comparisons between groups while chi-square and Fisher’s exact tests were used for categorical variables. Spearman’s rank correlation coefficients was used to assess correlations between continuous variables. Linear regression was used to determine adjusted difference between groups; we included traditional cardiac risk factors such as age, gender, cigarette smoking, diabetes mellitus, hypertension, hyperlipidemia, and prior history of cardiovascular disease as potential confounders in multivariable models. In addition, we investigated the effect of HIV disease characteristics on FMD such as current CD4 count, nadir CD4 count, current use of abacavir, didanosine, tenofovir, or protease inhibitors. We also measured the duration of antiretroviral therapy and the duration of protease inhibitor therapy. All traditional risk factors as well as additional HIV factors associated with FMD in unadjusted analyses were initially included in multivariable models, then removed in a stepwise manner if their inclusion changed the beta coefficient of the primary predictor by <10%.
RESULTS
Participant Characteristics
We studied a total of 61 antiretroviral-treated HIV-infected patients with HIV RNA levels < 75 copies/ml; half (n=30, 49%) of these patients were currently on abacavir (Table 1). Three individuals were currently taking didanosine, and 33 (54%) were taking tenofovir. The median age of the patients was 50 years, and the majority (n=58, 95%) were male. The median duration of HIV diagnosis was 18 years (IQR 14–21), the median duration of antiretroviral therapy was 8.6 years (IQR 4–11), and the median duration of protease inhibitor therapy was 8.8 years (IQR 3.5 to 11.2). The median duration of abacavir therapy was 1.2 years (IQR 0 to 6.8). The individuals currently on abacavir were similar in age, gender, CD4 count, and traditional cardiovascular risk factors to the patients not on abacavir.
Table 1.
Characteristics of HIV-Infected Subjects
| HIV: no ABC (n=31) | HIV: +ABC (n=30) | P value | |
|---|---|---|---|
| Age (years)1 | 50.8 ± 1.8 | 49.6 ± 1.4 | 0.62 |
| Male/female | 28/3 | 30/0 | 0.08 |
| History of CAD | 3 (10%) | 3 (10%) | 0.97 |
| Coronary risk factors: | |||
| Cigarette smoking | 17 (55%) | 14 (47%) | 0.52 |
| Diabetes | 2 (7%) | 2 (7%) | 0.97 |
| Hypertension | 13 (42%) | 14 (47%) | 0.71 |
| Hyperlipidemia | 14 (45%) | 19 (63%) | 0.15 |
| Laboratory values: | |||
| Creatinine (mg/dL)1 | 0.97 ± 0.21 | 1.10 ± 0.31 | 0.25 |
| Hemodynamic values: | |||
| SBP (mm Hg)1 | 119 ± 13 | 121 ± 11 | 0.43 |
| DBP (mm Hg)1 | 74 ± 9 | 74 ± 8 | 0.96 |
| Heart rate (bpm)1 | 67 ± 10 | 64 ± 10 | 0.29 |
| HIV-Related Characteristics: | |||
| HIV Duration (years)2 | 18 (13 – 22) | 18 (15 – 20) | 0.89 |
| HAART duration (years) 2 | 6.3 (3.3 – 10.9) | 8.7 (5.5 – 10.9) | 0.32 |
| NRTI duration (years)2 | 10.9 (5.7 – 12.8) | 11.5 (8.3 – 15.5) | 0.20 |
| NNRTI duration (years)2 | 0.92 (0 – 6.2) | 3.63 (0 – 8.1) | 0.08 |
| PI duration (years)2 | 8.0 (3.4 – 11.4) | 9.0 (4.1 – 11.2) | 0.81 |
| CD4 count (cells/mm3)2 | 264 (164 – 675) | 481 (214 – 673) | 0.17 |
| Nadir CD4 count (cells/mm3)2 | 96 (22 – 203) | 145 (35 –221) | 0.42 |
| FMD Results: | |||
| Endothelium-dependent FMD (%)2 | 4.9 (2.9 – 6.4) | 2.8 (2.2 – 4.1) | 0.01 |
| Endothelium-independent FMD (%)2 | 13.3 (10.0 – 16.4) | 12.0 (9.5 –15.1) | 0.27 |
| Brachial artery diameter (mm)2 | 4.51(4.00 – 4.92) | 4.79 (4.36 – 5.04) | 0.03 |
average values shown ± standard deviation
median value (IQR)
SBP – systolic blood pressure
DBP – diastolic blood pressure
bpm – beats per minute
HAART – highly active antiretroviral therapy
NRTI – nucleoside reverse transcriptase inhibitors
NNRTI – non-nucleoside reverse transcriptase inhibitors
PI – protease inhibitors
FMD – flow-mediated vasodilation
Impaired endothelial function among individuals currently on abacavir treatment
The resting blood pressure and heart rates were similar between the current abacavir and no abacavir groups. The HIV-infected patients not on abacavir had a smaller baseline diameter of the brachial artery compared to those on abacavir (4.51 mm vs. 4.79 mm, p=0.03; see Table 1).
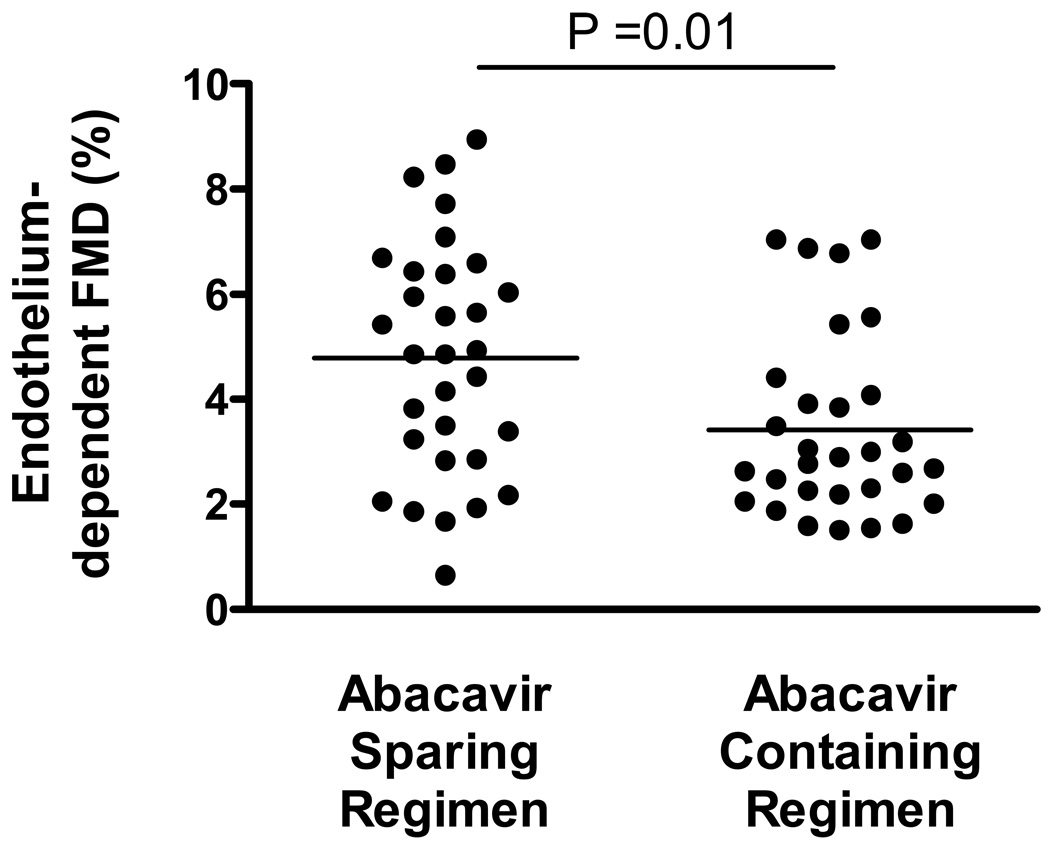
The HIV-infected participants maintaining treatment-mediated viral suppression in the current study had a median endothelium-dependent FMD of 3.5% (IQR 2.3–5.6%). Among those individuals currently on abacavir, the endothelium-dependent FMD was more impaired than those not on abacavir (2.8% vs. 4.9%, p=0.01; see Figure 1). Even after adjustment for baseline brachial artery diameter, participants currently receiving abacavir continued to have a mean 1.1% lower endothelium-dependent FMD than those receiving abacavir-sparing regimens. Among the 31 participants not currently receiving abacavir, those reporting past use of abacavir (n=5) also had lower endothelium-dependent FMD (p=0.04).
Figure 1.
The endothelium dependent FMD in the individuals currently on abacavir was 2.8% (IQR 2.2 to 4.1) which was significantly lower than those not on abacavir (4.9%, IQR 2.9 to 6.4, p=0.01). After adjustment for age, gender, and traditional risk factors as well as nadir CD4 count and current CD4 count, current abacavir use remained independently associated with lower FMD (p=0.02).
A similar proportion of current abacavir and non-abacavir users had a history of cardiovascular disease (CAD, 10% vs. 10%, p=0.68.) Current abacavir use continued to be associated with lower FMD even when restricting the analysis to those without a prior history of CAD (p=0.025). Abacavir users tended to be more likely to be taking statin medication than non-users (50% vs. 33%, p=0.20); however, there was no evidence for a relationship between statin medication use and FMD in unadjusted analyses (p=0.49), and current abacavir users continued to have lower FMD than non-users even when restricting the analysis to those not receiving statin therapy (p=0.058). Furthermore, as statins typically improve endothelial function, more frequent use of these agents among the abacavir users would have increased (i.e. normalized) the FMD and biased our hypothesis toward null. We found a significant reduction in FMD among abacavir users despite somewhat greater statin use. Current abacavir users were more likely to have a family history of CAD than non-users (43% vs. 19%, p=0.056). However, family history was not associated with endothelial function in unadjusted analyses (p=0.42) and current abacavir use continued to be associated with lower FMD even when restricting to those without a family history of CAD (p=0.023). Even when restricting the analysis to those without known CAD, diabetes mellitus, hyperlipidemia, hypertension, and family history of CAD, current abacavir used continued to be associated with lower FMD (p=0.05). In contrast, the median endothelium-independent FMD (as assessed after a dose of sublingual nitroglycerin) was similar between those currently receiving abacavir (12.0%) and those not on abacavir (13.3% p=0.27). Current didanosine or current tenofovir use were not associated with lower FMD.
Current abacavir therapy is independently associated with lower FMD
In unadjusted analyses, we found no evidence for a relationship between endothelium-dependent FMD and age, gender, diabetes mellitus, hypertension, hyperlipidemia, smoking (pack years or current use), prior coronary artery disease, family history of CAD, or serum creatinine (p>0.10 for all). We also found no evidence for a relationship between endothelium-dependent FMD and current CD4 count, nadir CD4 count, or duration of antiretroviral therapy. There was also no evidence of confounding by any of these factors in adjusted models as their inclusion failed to change the beta coefficient for current abacavir use by more than 10%.
DISCUSSION
Studies from our group and others have demonstrated that individuals with HIV infection have a higher rate of atherosclerosis compared to uninfected controls.[19, 20] HIV-infected patients also appear to have higher rates of myocardial infarction.[21] The underlying mechanism for these observations are still under active investigation but have been attributed to an excess of traditional risk factors, HIV-associated inflammation,[22] and direct antiretroviral therapy toxicity.[1, 23] With regard to the latter, among individuals enrolled in the D:A:D study, current treatment with abacavir (as well as didanosine) was associated with a higher rate of myocardial infarction. This effect was particularly evident among those with other risk factors for coronary artery disease.[2] This finding—which remains controversial—was also evident in 3 other studies (French Hospital Database, SMART, and STEAL)[24] while an effect of abacavir was not demonstrated in a retrospective analysis by GSK-sponsored studies [25] nor in a prospective ACTG cohort (the participants in these latter studies tended to be younger).[26] In our current study, we provide further evidence and a potential biological underpinning for a detrimental effect of abacavir on vascular function. In an unselected population of long-term antiretroviral treated patients, those on abacavir had a greater degree of endothelial dysfunction than those not on abacavir. These differences were not readily explained by differences in HIV disease-associated factors or by differences in traditional cardiovascular risk factors.
The endothelium serves to regulate vascular homeostasis by balancing the level of vasodilators and vasoconstrictors. An imbalance in these factors leads to endothelial dysfunction and eventually the development of atherosclerosis.[27] Endothelial dysfunction can occur in response to traditional risk factors and/or inflammation.[28, 29] Recent studies have shown that FMD is clearly impaired in antiretroviral untreated patients, and rapidly improves in response to combination therapy. A reduction in HIV-associated inflammation has been postulated as the major mechanism accounting for this improved FMD.[12] Our data also raise concerns about whether long-term effective antiretroviral therapy can “normalize” endothelial function, even with regimens that do not include abacavir. The median FMD across all patients was 3.5%, and the median among those not taking abacavir was 4.9%. These levels are generally lower than the levels which we have observed in healthy controls studied in our laboratory (average FMD of 6.2%, standard deviation of 1.8%, n=30). As multiple studies in a variety of patient populations and clinical settings have show that endothelial dysfunction both precedes and strongly predicts future cardiovascular events[10, 11, 30], our findings provide one potential mechanism for the abacavir risk noted in D:A:D and other studies.
Our data regarding abacavir and FMD also provide a potential explanation for the recent observation suggesting that abacavir promotes platelets aggregation. Using a study design comparable to ours, Satchell and colleagues observed that abacavir exposure was associated with enhanced platelet aggregation.[31] FMD measures the bioavailability of endothelium-derived nitric oxide (NO).[16] Endothelium-derived NO is a multifunctional molecule that, as well as promoting vasodilation, markedly inhibits platelet aggregation and adhesion.[32] Conversely, diseases associated with a loss of endothelium-derived NO are characterized by enhanced platelet aggregation and adhesion. In addition, platelets synthesize their own NO[33]; its bioavailability is diminished by some of the same mechanisms that impair endothelial function. Activated platelets produce factors such thromboxane, ADP, prostacyclin, and NO[34] which may shift the vascular homeostasis towards vasoconstriction. Thus, we propose that our finding of significantly diminished FMD in patients on abacavir is indicative of a state in which loss of NO derived from the endothelium or from platelets promotes a platelet pro-aggregatory, pro-thrombotic state.
In addition to reduced FMD, the subjects currently on abacavir also displayed larger brachial artery diameters. This may be yet another indication of increased cardiovascular risk. In that context, it is interesting that among the 2,792 elderly subjects in the Cardiovascular Health Study followed for 5 years, both reduced brachial artery FMD and increased brachial artery diameter predicted incident cardiovascular events, independently of traditional risk factors.[35] Increased brachial artery diameter may be a consequence of outward vascular remodeling (i.e. enlargement) described previously in an atherosclerotic milieu[36] and now potentially with abacavir.
Our study was observational and hence susceptible to many of the limitations associated with this approach. Patients who received abacavir in our clinics may have differered from those who did not receive in a number of unmeasured factors, each of which may have confounded our observations. For example, if abacavir was perceived to be a safer option than other drugs such as tenofovir for patients at risk for renal disease, then a potential “channeling” bias may have occurred. Although we have attempted to address this concern by controlling for traditional risk factors and we found no difference in traditional risk factors among the abacavir and non-abacavir treated patients, unmeasured confounders may still have been present. These same unmeasured confounders might also explain why past use of abacavir was associated with impaired FMD among participants not currently receiving abacavir. A prospective randomized study is needed to more definitively address the role of abacavir-based regimens compared to non-abacavir based regimens in causing impaired FMD. Future longitudinal studies may wish to consider carotid intima-media thickness and as well as biologic markers[37] to better understand the mechanism underlying the impairment in FMD associated with abacavir use. In addition, in vitro studies evaluating the effect of abacavir and others NRTIs on endothelial cells may be useful in understanding and supporting the clinical results of the present study.
Whether or not to use abacavir in HIV-infected individuals with known coronary artery disease or who are at high risk for future events remains unclear. Our study may help to provide a biologic mechanism for the association between myocardial infarction and recent or current abacavir use seen in observational studies. Future studies will be needed to confirm our findings and to help determine if abacavir-associated impairment in endothelial function contributes to the clinically observed relationship between current abacavir use and myocardial infarction.
Acknowledgments
Sources of Funding
Dr. Hsue is a recipient of grants from the NIH (K23A1066885, R01HL095130). This work was supported in part by the UCSF/Gladstone Center for AIDS Research (P30 AI27763), NIAID (AI055273, AI44595, K23AI065244, K24AI069994), and the UCSF Clinical and Translational Science Institute(UL1 RR024131-01).
Footnotes
Presented in part at the 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada February 8–11, 2009 and at the 58th Annual Scientific Session of the American College of Cardiology, Orlando, FL March 29–March 31, 2009
Role of Authors:
Dr. Hsue was responsible for the study design and oversight, supervising patient recruitment and FMD studies, assisting with data analysis, and writing the manuscript. Dr. Hunt assisted with data anlysis, manuscript preparation, and patient recruitment. Ms. Wu performed and analyzed all of the FMD studies. Ms. Schnell, Dr. Ho, Ms. Xie, and Dr. Hatano assisted with patient recruitment and patient studies. Dr. Ganz oversaw FMD studies, study design, and helped to write the manuscript. Dr. Martin and Dr. Deeks helped with study design, patient recruitment, and manuscript preparation.
REFERENCES
- 1.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 2.Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. Aids. 2008;22:F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Impact of Specific NRTI and PI Exposure on the Risk of Myocardial Infarction: A Case-Control Study Nested within FHDH ANRS CO4; Abstract #43LB. 16th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2009; Montreal, Canada. 2009. [Google Scholar]
- 5.Podzamczer D, Ferrer E, Sanchez P, Gatell JM, Crespo M, Fisac C, et al. Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96-week results of a randomized study. J Acquir Immune Defic Syndr. 2007;44:139–147. doi: 10.1097/QAI.0b013e31802bf122. [DOI] [PubMed] [Google Scholar]
- 6.Jones R, Sawleshwarkar S, Michailidis C, Jackson A, Mandalia S, Stebbing J, et al. Impact of antiretroviral choice on hypercholesterolaemia events: the role of the nucleoside reverse transcriptase inhibitor backbone. HIV Med. 2005;6:396–402. doi: 10.1111/j.1468-1293.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 7.Anastos K, Lu D, Shi Q, Tien PC, Kaplan RC, Hessol NA, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr. 2007;45:34–42. doi: 10.1097/QAI.0b013e318042d5fe. [DOI] [PubMed] [Google Scholar]
- 8.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 9.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 10.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 11.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 12.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation. 2002;106:184–190. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 15.Kinlay S, Behrendt D, Fang JC, Delagrange D, Morrow J, Witztum JL, et al. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol. 2004;43:629–634. doi: 10.1016/j.jacc.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 17.Stadler RW, Karl WC, Lees RS. New methods for arterial diameter measurement from B-mode images. Ultrasound Med Biol. 1996;(22):25–34. doi: 10.1016/0301-5629(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 18.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of Atherosclerosis as Assessed by Carotid Intima-Media Thickness in Patients With HIV Infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors Among Patients with HIV Disease. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. Aids. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 23.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 24.Reiss P. Abacavir and Cardiovascular Risk, abstract #152. 16th Conference on Retroviruses and Opportunistic Infections; February 8–11 2009; Montreal, Canada. [Google Scholar]
- 25.Brothers CH, Hernandez JE, Cutrell AG, Curtis L, Ait-Khaled M, Bowlin SJ, et al. Risk of myocardial infarction and abacavir therapy: no increased risk across 52 GlaxoSmithKline-sponsored clinical trials in adult subjects. J Acquir Immune Defic Syndr. 2009;51:20–28. doi: 10.1097/QAI.0b013e31819ff0e6. [DOI] [PubMed] [Google Scholar]
- 26.Benson C, Ribaudo H, Zheng E, Koletar S, Smurzynski M, Bosch RB, B Collier A, et al. No Association of Abacavir Use with Risk of Myocardial Infarction or Severe Cardiovascular Disease Events: Results from ACTG A5001; Abstract #721; February 8–11, 2009; Montreal, Canada. 2009. [Google Scholar]
- 27.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 28.Gokce N, Vita JA. Clinical manifestations of endothelial dysfunction. In: Loscalzo J, Schafer A, editors. Thrombosis and Hemmorrhage. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 685–706. [Google Scholar]
- 29.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 30.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 31.Satchell C, O'Connor E, Peace A, Cotter A, Sheehan G, Tedesco T, et al. Platelet Hyper-Reactivity in HIV-1-infected Patients on Abacavir-containing ART; Abstract 151LB; February 8–11, 2009; Montreal, Canada. 2009. [Google Scholar]
- 32.Jin RC, Voetsch B, Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 2005;12:247–258. doi: 10.1080/10739680590925493. [DOI] [PubMed] [Google Scholar]
- 33.Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest. 1997;100:350–356. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 35.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 36.Mohiaddin RH, Burman ED, Prasad SK, Varghese A, Tan RS, Collins SA, et al. Glagov remodeling of the atherosclerotic aorta demonstrated by cardiovascular magnetic resonance: the CORDA asymptomatic subject plaque assessment research (CASPAR) project. J Cardiovasc Magn Reson. 2004;6:517–525. doi: 10.1081/jcmr-120030576. [DOI] [PubMed] [Google Scholar]
- 37.van Vonderen MG, Hassink EA, van Agtmael MA, Stehouwer CD, Danner SA, Reiss P, Smulders Y. Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy. J Infect Dis. 2009;199:1186–1194. doi: 10.1086/597475. [DOI] [PubMed] [Google Scholar]