Abstract
Glioblastoma, the most malignant type of primary brain tumor, is one of the solid cancers where cancer stem cells have been isolated, and studies have suggested resistance of those cells to chemotherapy and radiotherapy. Here, we report the establishment of CSC-enriched cultures derived from human glioblastoma specimens. They grew as neurospheres in serum-free medium with epidermal growth factor and fibroblast growth factor 2, varied in the level of CD133 expression and very efficiently formed highly invasive and/or vascular tumors upon intracerebral implantation into immunodeficient mice. As a novel therapeutic strategy for glioblastoma-derived cancer stem-like cells (GBM-SC), we have tested oncolytic herpes simplex virus (oHSV) vectors. We show that although ICP6 (UL39)-deleted mutants kill GBM-SCs as efficiently as wild-type HSV, the deletion of γ34.5 significantly attenuated the vectors due to poor replication. However, this was significantly reversed by the additional deletion of α47. Infection with oHSV G47△ (ICP6-, γ34.5-, α47-) not only killed GBMSCs but also inhibited their self-renewal as evidenced by the inability of viable cells to form secondary tumor spheres. Importantly, despite the highly invasive nature of the intracerebral tumors generated by GBM-SCs, intratumoral injection of G47Δ significantly prolonged survival. These results for the first time show the efficacy of oHSV against human GBM-SCs, and correlate this cytotoxic property with specific oHSV mutations. This is important for designing new oHSV vectors and clinical trials. Moreover, the new glioma models described in this study provide powerful tools for testing experimental therapeutics and studying invasion and angiogenesis.
Introduction
Glioblastoma (GBM), the most common type of primary brain tumor in adults, is fatal despite currently available multimodal treatments such as surgery, radiation, and chemotherapy (1). Although the introduction of temozolomide has achieved a minimal survival benefit, tumors almost inevitably recur locally. This treatment-refractory nature and its invasive phenotype pose an urgent needfor novel therapeutics with substantial efficacy.
From a variety of malignancies, investigators have identified a subpopulation of cells capable of initiating and sustaining tumor growth in vivo (2). These cells, which have been called “tumor-initiating,” “cancer stem,” or “cancer stem-like” cells, were found to possess characteristics of adult organ stem cells such as selfrenewal and multilineage differentiation, implicating a transformed normal stem cell as the cell of tumor origin. Hereafter, we refer to these cells as cancer stem-like cells or CSCs; or when specifically speaking of GBM as GBM stem-like cells or GBM-SCs. GBM is among several cancers where CSCs have been isolated (3-5). The CD133 cell surface antigen, which is also expressed on normal neural and hematopoietic stem cells, has been considered a useful, although not definitive, marker for the identification of GBM-SCs, and has been associatedwith highly tumorigenic phenotypes (4). Importantly, tumors initiated by GBM-SCs often histopathologically recapitulate the tumor of patients from which the cells were derived, indicating the ability to reproduce the cellular heterogeneity found in human GBM (4). This suggests that GBM-SCs might be useful for generating clinically relevant orthotopic GBM models in mice because commonly used human glioma cell lines typically form a well-delineated mass composed of homogeneous cells, a pathology distinct to that of human GBM.
Recent publications have provided insights into the biology of GBM-SCs with relevance to therapeutic approaches. CD133-positive glioma stem cells have been shown to display enhanced resistance to ionizing radiation due to their increased activation of the DNA damage checkpoint (6). Additionally, the CD133-positive human GBM cells have been shown to mediate enhanced resistance to a variety of chemotherapeutic agents (7). These observations suggest that the CSC fraction, resistant to radiotherapy and chemotherapy and more likely to survive those modalities, may be the source of cancer recurrence, and hence novel therapies may need to target GBM-SCs.
Oncolytic viruses are natural or genetically modified viruses that, upon infection, selectively replicate in and kill neoplastic cells while sparing normal cells (8-11). Among them, oncolytic herpes simplex virus (oHSV) type 1 is one of the most extensively studied and considered a promising agent for treating brain cancers as well as other types of cancer (8, 12). In a direct comparison between oncolytic adenovirus and oHSV in glioma cell lines, oHSV was shown to be more efficacious (13). Mutations of specific viral genes, namely γ34.5 (responsible for neurovirulence and inhibition of host protein synthesis shut-off) and UL39 (encoding the large subunit of viral ribonucleotide reductase), have been shown to confer selectivity to cancer cells, which has enabled translational studies to humans (8, 12). For example, G207, with deletions of both copies of γ34.5 gene and mutation of UL39, has been safely tested in phase 1 clinical trials for recurrent malignant gliomas (14). Another γ34.5 deletion mutant, 1716, was also shown to be safe after intratumoral injection (15).
Recent studies have explored the activity of oncolytic adenoviruses against CSCs derived from breast cancer (16) and brain tumors (17), demonstrating their efficacy. However, there have been no reports studying the effect of oHSV on CSCs including GBM-SCs. Because oHSV relies primarily on oncolysis, which is different from killing mechanisms of other cytocidal agents, we hypothesized that oHSV may represent an efficacious agent against CSCs. From a clinical perspective, it is crucial to determine the effect of γ34.5 deletions on therapeutic efficacy for GBM-SCs, as to date, all the clinical trials testing oHSV in the brain have used γ34.5- mutants (18). To address this, we isolated and established expandable CSC cultures from human GBM specimens, and sought to elucidate their susceptibilities to oHSV. Here, we show evidence that oHSVs have a significant antitumor activity against GBM-SCs and that this activity correlates with specific oHSV mutations. Furthermore, we show that the established GBM-SC cultures are highly tumorigenic and generate novel orthotopic glioma models with histopathological hallmarks of GBM. An extremely invasive behavior exhibited by certain xenografts offers unique tools applicable to a variety of brain tumor research fields.
Materials and Methods
Isolation and culture of GBM-SCs
Surgical specimens of GBM were collected at Massachusetts General Hospital (GBM series) with approval by the Institutional Review Board. BT74, originally obtained from Dr. C. David James (University of California, San Francisco, San Francisco, CA; as GBM6; ref. 19), was maintained as s.c. xenografts in mice at Brigham and Women's Hospital. Mechanically minced tissues were digested with 0.1% Trypsin and 10 U/mL of DNaseI at 37°C for 45 min. After washes, tissues were triturated and passed through a 100-μm cell strainer. Cells were plated in EF medium composed of Neurobasal medium (Invitrogen) supplemented with 3 mmol/L L-Glutamine (Mediatech), 1× B27 supplement (Life Technologies), 0.5 × N2 supplement (Life Technologies), 2 μg/mL heparin (Sigma), 20 ng/mL recombinant human EGF (R & D systems), 20 ng/mL recombinant human FGF2 (Peprotech), and 0.5 × penicillin G/streptomycin sulfate/amphotericin B complex (Mediatech). Part of the digested tissue was also grown in DMEM supplemented with 10% FCS to generate standard primary adherent cultures. The cultures for CSCs were fed every third day with 1/3 volume of fresh medium. Passaging of the cultures was performed by dissociating neurospheres using NeuroCult Chemical Dissociation kit (StemCell Technologies).
Human neural stem/progenitor cells
Human embryonic stem cell- derived neural progenitor cells were generated from WA07 using stromal feeder-based induction in combination with Noggin (20), and expanded with fibroblast growth factor (FGF)2 and epidermal growth factor (EGF; ref. 21). V-myc-immortalized human neural stem cells were as described(22).
Virus and infection studies
Wild-type strain F and its derivatives R3616 (both obtained from Dr. B. Roizman, The University of Chicago, Chicago, IL), G207 and G47Δ, have been described. R3616 contains 1-kb deletions of both copies of γ34.5 (23). G207 (γ34.5-, ICP6-) was derived from R3616 by an inactivating insertion of Escherichia coli lacZ into UL39 (ICP6; ref. 24). G47Δ (γ34.5-, ICP6-, ICP47-) was derived from G207 by deleting α47 and the US11 promoter (placing the late US11 gene under control of an immediate-early α47 promoter; ref. 25). FΔ6 is a strain F-derived recombinant with an ICP6-inactivating lacZ insertion, created by cotransfection of strain F DNA with the XbaI-HindIII fragment of pKX2-βG3 (24). G47ΔBAC virus was generated by homologous recombination between G47Δ DNA and pBAC-ICP6EF, and contains a cytomegalovirus promoter-driven enhanced green fluorescent protein (EGFP) in place of lacZ in G47Δ. bG47Δ-empty has the BAC and EGFP sequences removed from G47ΔBAC as described by Fukuhara and colleagues (26). d120BAC virus was generated from d120, which contains a deletion in both copies of ICP4, by insertion of EGFP into the ICP6 locus (27).
Differentiation induction and immunocytochemistry
Dissociated cells were plated onto fibronectin/poly-l-ornithine-coated coverslips. To induce differentiation, the cells were grown in 1%FCS-containing medium devoid of EGF and FGF2 for 10 to 14 d. The cells were then fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X, washed, and blocked with 10% goat serum, before incubation with primary antibodies at 4°C overnight. The antibodies used were rabbit anti-GFAP (Sigma, 1:200), monoclonal anti-βIII tubulin (Chemicon, 1:150), and rabbit anti-nestin 130 (1:50; a gift from Dr. Ron McKay, National Institute of Neurological Disorders and Stroke, Bethesda, MD; ref. 28) that recognizes human and mouse nestin. FITC- or Cy3-conjugated secondary antibodies (1:400; Jackson ImmunoResearch) were applied at 4°C for 4 h to visualize immunoreactivity before microscopic observation.
Flow cytometric analysis
GBM-SC cultures were dissociated and stained with phycoerythrin (PE)-conjugated anti-CD133/2 (Miltenyi) according to manufacturer's instructions before analysis with FACScalibur (BD Biosciences). OHSV infection, cell death, and CD133 expression status were examined simultaneously by three-color flow cytometric analysis. G47ΔBAC- or mock-infected cells were collected on days 1 and 3, stained with phycoerythrin-conjugated anti-CD133/2, washed, and nonviable cells labeled with 7-AAD (BD) before the samples were subjected to flow cytometric analysis. Data were analyzed by FlowJo software (Tree Star).
Tumorigenicity studies and immunohistochemistry
Dissociated GBMSCs or trypsinized FCS-grown cells were stereotactically implanted into the brains (right striatum, 2.5-mm lateral from Bregma and 2.5-mm deep) of athymic or severe combined immunodeficient mice under anesthesia with pentobarbital. Mice were monitored and sacrificed when they developed significant neurologic symptoms. Formalin-fixed paraffin-embedded sections were stained with H&E. Immunohistochemistry was performed using anti-GFAP or anti-Ki67 [MIB-1; Dako; with antigen retrieval by microwave treatment in 10 mmol/L sodium citrate buffer (pH 6.0)] antibodies and Vectastain Elite ABC kit (Vector Laboratories) with 3,3′-diaminobenzidine (Dako) as chromogen. Immunopositivity for GFAP and MIB-1 was evaluated at four randomly selected high power fields within tumor tissues.
Infection spread assay
Cells were infected with EGFP-expressing vectors, d120BAC or G47ΔBAC, 1 d before they were mixed with uninfected cells labeled with orange dye, CellTracker CMTMR (Invitrogen). Cells were grown in EF medium and observed 24 h later by microscopy.
Viral cytotoxicity assay
GBM-SC spheres were dissociated, cells resuspended at 5 to 10 × 106 cells/mL, and infected at multiplicity of infection (MOI) of 0.2 for 45 min at 37°C. After centrifugation to remove unadsorbed virus, cells were seeded in 24-well plates at 2 × 104 cells per well in EF medium. The cells were harvested on days 3 and 7, dissociated with trypsin/EDTA, and viable trypan blue-excluding cells counted on a hemocytometer.
Viral growth assay
After infection as described above, cells were grown in EF medium and harvested with supernatant at the indicated time points. After three freeze/thaw cycles and sonication, the titers of infectious virus were determined by plaque assay on Vero cells (American Type Culture Collection).
Secondary neurosphere formation assay
Seven days after virus infection, cell counts were determined on a hemocytometer of trypan blue-stained cells. Viable cells were resuspended in fresh EF medium and seeded into 96-well plates at 1 or 10 cells per well. Sixteen days later, the number of wells containing neurospheres (diameter, >60 μm) was recorded.
In vivo treatment studies
Dissociated GBM-SCs in 3 μL were stereotactically implanted to generate orthotopic xenografts as described above. Six or 7 days later, 5 μL of G47Δ [2 × 106 plaque-forming unit (pfu)] or PBS was injected intratumorally using the same coordinates as for tumor cell implantation. Animals were followed for survival as described above. The brains were fixed in 4% paraformaldehyde and frozen sections obtained. 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) staining was performed to identify lacZ-expressing infected cells. Fluorescence immunohistochemistry was conducted using anti-human specific nuclei (Chemicon) and anti-β-galactosidase (Millipore) antibodies followed by secondary antibodies. All in vivo procedures were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital.
Statistics
Comparisons of data in cell survival and viral yield assays were performed using a two-tailed Student's t test (unpaired). Survival analysis was conducted by Kaplan-Meier curves, and their comparison was determined by logrank test. P values of <0.05 were considered significant.
Results
Isolation and characterization of GBM-SC cultures from human GBM specimens
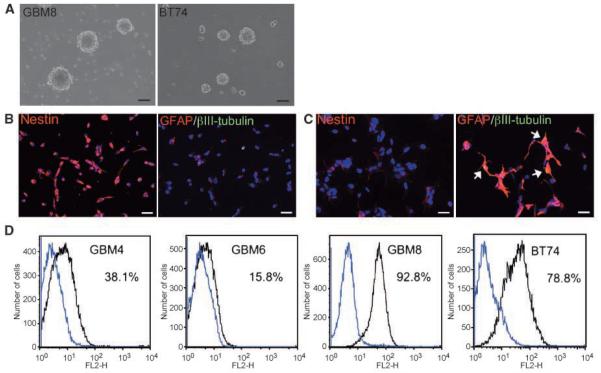
We obtained surgical specimens of human GBM to isolate and grow CSCs. Under the culture conditions originally designed for selective expansion of neural stem cells, most tissue cultures gave rise to typical neurosphere-like structures within 10 days (Fig. 1A). Of 17 specimens collected, we were able to establish 7 stable cultures that could be passaged for >3 months and, here, present 4 cultures with which we have performed extensive studies. Importantly, single dissociated neurosphere cells were able to form secondary spheres in 2% to 19% of plated wells (Table 1). Immunocytochemical studies revealed that the majority of cells from these cultures are immunoreactive for nestin, a marker for neural stem/progenitor cells (Fig. 1B, left). These cells were also weakly positive for astrocytic marker GFAP, whereas expression of βIII-tubulin, a marker for immature neuronal cells, was not detected (Fig. 1B, right). To examine the capability of the cells to give rise to different neural cell lineages, we induced the cells to differentiate by withdrawing mitogens and supplementing with serum. Along with apparent morphologic changes, a large reduction in nestin expression (Fig. 1C, left) and concomitant up-regulation of GFAP (Fig. 1C, right) were observed. Upon differentiation, some cells were found to be double positive for GFAP and βIII-tubulin (Fig. 1C, right), suggesting aberrant differentiation signaling in the cells. Similar staining patterns were observed in FCS-grown adherent cells (Supplementary Fig. S1). We next determined the expression of the CD133 antigen, a putative brain tumor stem-like cell marker (4), on dissociated neurosphere cells. Flow cytometric analysis revealed that the levels of its expression varied significantly between cultures; with the positive population ranging from 15.8% (GBM6) to 92.8% (GBM8; Fig. 1D). Despite this variation in CD133 status, nestin expression in the cultures was consistently high (>80%). Of note, the efficiency of neurosphere formation after each dissociation process had no correlation with the level of CD133 (Table 1).
Figure 1.
In vitro characterization of human GBM-derived cancer stem cells. A, typical appearance of “neurosphere” structures grown in the serum-free specific culture conditions (GBM8, left; BT74, right). Scale bars, 100 μm. B and C, immunocytochemical analysis of human GBM-SCs and their differentiated progeny. B, undifferentiated BT74 cells; C, differentiated BT74 cells. Left, staining for neural stem/progenitor marker nestin (Cy3, red); right, merged images of staining for astrocytic marker GFAP (Cy3, red) and neuronal marker βIII tubulin (FITC, green). A significant reduction in nestin expression and concomitant up-regulation of GFAP expression were observed after induction of differentiation. There are cells double positive for GFAP and βIII tubulin in the differentiated population (yellow, white arrows). Nuclei were visualized by staining with 4′,6-diamidino-2-phenylindole. Scale bars, 20 μm. D, flow cytometric analysis for neuronal stem cell marker CD133 on GBM-SC cultures. Dissociated neurospheres were stained either with isotype control (blue line) or anti-CD133/2 antibody (black line) conjugated with phycoerythrin before the analysis. The percentage of CD133-positive cells is indicated.
Table 1.
GBM-SCs surviving infection with G47ΔBAC are unable to form secondary neurospheres
| Cell | MOI | Seeded cell number per well | No. of well with sphere(s)/96 wells |
|---|---|---|---|
| GBM4EF | 0 | 100 | 96 |
| 10 | 81 | ||
| 1 | 18 | ||
| 0.2 | 10 | 0 | |
| 1 | 10 | 0 | |
| GBM6EF | 0 | 10 | 65 |
| 1 | 12 | ||
| 1 | 10 | 0 | |
| 1 | 0 | ||
| GBM8EF (exp. 1) | 0 | 10 | 21 |
| 1 | 2 | ||
| 0.2 | 10 | 0 | |
| 1 | 0 | ||
| 1 | 10 | 0 | |
| 1 | 0 | ||
| GBM8EF (exp. 2) | 0 | 10 | 23 |
| 1 | 6 | ||
| 0.2 | 10 | 0 | |
| 1 | 10 | 0 |
NOTE: GBM-SCs infected with G47ΔBAC at MOI of 0, 0.2, or 1 were grown for 7 d, when viable cells (excluding trypan blue dye) were enumerated. The cells were then subjected to a limiting dilution assay in 96-well plates, and the number of wells containing neurospheres was counted 16 d later to quantify the clonogenic potential of the cells.
Hence, we found that the obtained cultures are enriched for cells with undifferentiated stem-like characteristics, and their differentiation seems to be preferentially directed toward an astrocytic lineage.
Tumor-initiating capability of isolated cultures
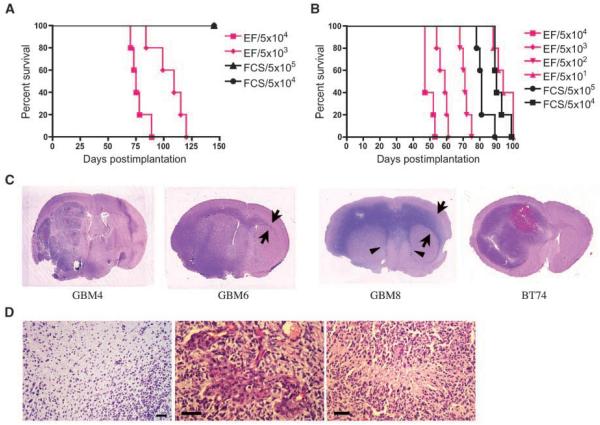
Because in vivo tumorigenicity is a hallmark of CSCs, we next determined the ability of the cultures to form tumors in mice. Intracerebral injection of 5 × 103 GBM4 cells grown in EF medium was sufficient to consistently form tumors (Fig. 2A). In contrast, the same patient-derived adherent cells grown in FCS were unable to do so even with 5 × 105 cells (Fig. 2A). The difference in tumorigenic potential was also evident with GBM8-derived cells. Strikingly, implantation of only 5 × 101 cells grown in EF medium was sufficient to generate fatal tumors within 100 days in 5 of 5 mice (Fig. 2B). Unlike GBM4 cells, FCS-grown GBM8 cells were able to drive tumor formation with 5 × 104 cell implants and led to animal sacrifice at around 80 days (Fig. 2B). The presence of a minor (<20%) subpopulation of CD133-positive cells may account for the tumorigenicity of FCS-grown GBM8 cells. Comparable survival periods caused by implantation of 5 × 104 FCS cells and5 ×10 1 EF cells, however, indicated an approximate 1,000-fold difference in tumorigenicity between EF-grown and FCS-grown GBM8 cells. GBM6EF cells, with the lowest proportion of CD133+ cells, were less tumorigenic and took considerably longer for tumors to form (Supplementary Table S1). Nevertheless, these data show the highly efficient capacity of the EF medium-grown cultures to initiate/drive tumor growth in vivo. Together with the in vitro characterization, this indicates that we have obtained GBM-derived cultures enriched for CSCs.
Figure 2.
In vivo tumorigenicity of GBM-SCs and histopathology of the xenografts. A and B, Kaplan-Meier survival curves of mice implanted with GBM4 cells (A) or GBM8 cells (B). Immunodeficient mice were orthotopically implanted with cells grown in EF medium (pink lines), or in 10% FCS medium (FCS, black lines). Numbers, cell number implanted. Mice were followed for survival. n = 5 mice per group. Median survival times were statistically different between groups that received 5 × 104 EF cells and FCS cells (in both GBM4 and GBM8 cells; logrank test, P < 0.0001). C, photographs of magnified coronal sections of xenografts stained with H&E. GBM4 tumor was characterized by a massive, multilobulated, and hypervascular mass with intratumoral bleeding. GBM6 tumor and GBM8 tumor extended through the corpus callosum to the contralateral brain (arrows). GBM8 tumor mimicked the butterfly-like growth pattern characteristic of human GBM, and tended to grow along subventricular areas, compressing the lateral ventricles (arrowheads). BT74 cells formed a massive tumor with intermediate invasiveness and hemorrhage resulting in an enlarged hemisphere. D, GBM-SC-derived intracerebral xenografts recapitulate GBM histopathology. Left, showing tumor cell infiltration into surrounding brain without distinctive border (GBM8). Endovascular proliferation (middle) and pseudopalisading necrosis (right), hallmarks of the GBM histopathology, were observed (BT74). Scale bars, 50 μm.
Histopathological investigation of xenografts showed that GBM4EF tumors displayed relatively clear borders with surrounding brain (Supplementary Fig. S2A) and intratumoral bleeding (Fig. 2C), whereas GBM6EF and GBM8EF produced tumors with no distinctive border (Fig. 2D, left), and extended through the corpus callosum to the contralateral brain, suggesting their highly motile and invasive nature (Supplementary Fig. S2B; Fig. 2C). GBM8EF tumors also tended to grow along subventricular areas (Fig. 2C). BT74 tumors exhibited a large mass with moderate invasiveness and occasional intratumoral hemorrhage (Fig. 2C). Microscopic investigation revealed proliferation of cells with cellular heterogeneity and increased vascularity, findings compatible with malignant astrocytic tumors (Supplementary Fig. S3). Endovascular proliferation and pseudopalisading necrosis, hallmarks of GBM histopathology, were found in BT74 tumors (Fig. 2D, middle, right). Immunohistochemistry for GFAP revealed variable positivity among xenografts (Supplementary Table S1; Supplementary Fig. S3); GBM8EF tumors consisted of neoplastic cells mostly immunonegative for GFAP, whereas a significant proportion of tumor cells were GFAP-positive in GBM4EF, GBM6EF, and BT74 tumors. Ki67 (MIB-1) expression showed the highly proliferative nature of all the xenografts (Supplementary Table S1; Supplementary Fig. S3).
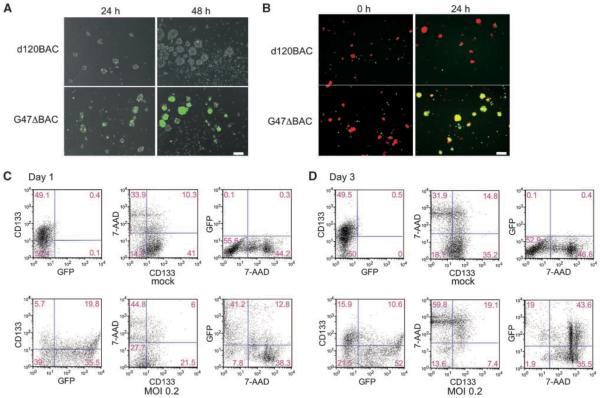
oHSVs infect, replicate, and spread in GBM stem cell cultures
Next, we sought to determine whether oHSV is able to infect GBM-SCs. Infection with EGFP-expressing replication-deficient and replication-competent vectors revealed that GBM-SCs are efficiently infected (Fig. 3A). Infection with replication-competent G47ΔBAC, but not replication-defective d120BAC, led to an increase of EGFP-positive cells from 24- to 48-hours postinfection (Fig. 3A), indicating viral replication. To show virus spread, orange dye-labeled uninfected cells were cocultured with EGFP+ d120BAC- or G47ΔBAC-infected cells. G47ΔBAC, but not d120BAC, produced cells double-positive for EGFP and orange dye the following day (Fig. 3B), indicating cell-to-cell spread of virus. We further examined whether G47ΔBAC infects and kills CD133+ cells by performing flow cytometric analysis for CD133 and EGFP expression as well as cell death on days 1 (Fig. 3C) and 3 (Fig. 3D) postinfection. On day 1, most EGFP-positive-infected cells were viable as shown by negative 7-AAD staining (Fig. 3C, bottom). On day 3, the EGFP-positive population increased from 55.3% to 62.6% (Fig. 3D, bottom). Notably, the ratio of the EGFP+/7-AAD+ double positive compartment rose from 12.8% to 43.6%, indicating ongoing cell death in the infected cells. Among viable 7-AAD-negative cells on day 3, the percentage of CD133-positive in the G47ΔBAC-infected sample was 35.3% (7.4 of 21) compared with 66.0% (35.2 of 53.3) in the mock-infected, displaying a decline in CD133 status after oHSV infection and the susceptibility of CD133-positive cells to oHSV (Fig. 3D, middle). Together, these results show that oHSVs can infect, replicate, spread in, and kill GBM-SCs.
Figure 3.
oHSV is able to infect, replicate, and spread in GBM-SC cultures, killing both CD133-positive and -negative cells. A, growing neurospheres (GBM8) were dissociated to single-cell suspension to ensure interaction of virus with individual cells. Cells were infected with either replication-defective vector d120BAC (top) or replication-competent vector G47ΔBAC (bottom) at MOI of 0.2, and the expression of EGFP (green) was observed serially. At 24 h postinfection, infection with both viral vectors resulted in comparable degrees of EGFP+ cells, whereas at 48 h postinfection, G47ΔBAC, but not d120BAC, showed a large increase in the proportion of EGFP-expressing cells, indicating viral replication and spread. Overlaid images of fluorescence and phase contrast are shown. Scale bar, 100 μm. B, oncolytic viral infection spreads cell-to-cell among GBM-SCs. GBM8 cells were infected with either d120BAC (top) or G47ΔBAC (bottom) at MOI of 1, washed, and plated with uninfected GBM-SCs labeled with orange dye (0 h). The merged images of green and orange fluorescence were recorded 24 h later. G47ΔBAC generated cells double-positive for EGFP and orange fluorescence (yellow) indicative of cell-to-cell infectious spread, whereas d120BAC did not. Scale bar, 100 μm. C and D, flow cytometric analysis of oncolytic viral infection, CD133 status, and cell death. GBM8 cells were either mock-infected (top) or infected with EGFP-expressing G47ΔBAC at MOI of 0.2 (bottom). On days 1 (C) and 3 (D) postinfection, the cells were stained with phycoerythrin-conjugated anti-CD133 antibody, and nonviable cells labeled with 7-ADD, before being subjected to 3-color fluorescence-activated cell sorting analysis. Numbers, percent of cells in each quadrant.
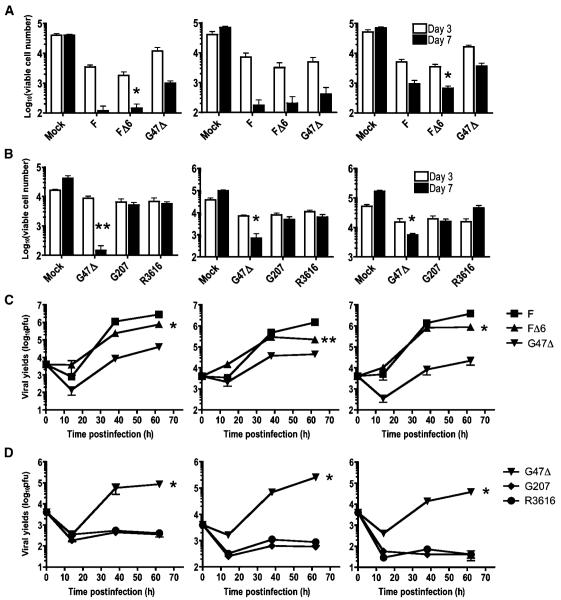
Effect of different oHSV mutations on GBM-SCs cytotoxicity
Next, we tested the efficiency of cell killing and viral replication by viruses with different genetic mutations. G47Δ (ICP6-, γ34.5-, ICP47-), F△6 (ICP6-), and wild-type strain F, all exhibited significant cytotoxicity against GBM4, GBM8, and BT74 cells (Fig. 4A). F△6 killed all the GBM-SCs tested as efficiently as strain F, whereas G47Δ was less potent than F△6 in two of three cultures. To examine the effect of the γ34.5 deletion on the attenuated phenotype of oHSV, we compared R3616 (γ34.5-) with other γ34.5 mutants, G207 and G47Δ (which have additional deletions or mutations). Whereas both R3616 and G207 exhibited only a marginal cytotoxicity at day 3, G47Δ consistently displayed enhanced cell killing over G207 and R3616 (Fig. 4B). These results indicate that the deletion of γ34.5 significantly attenuates the potency of oHSV against GBM-SCs, whereas the deletion of ICP6 has minimal effect. Importantly, deletion of α47, present in G47Δ, partly but significantly reverses the attenuation caused by the γ34.5 deletion.
Figure 4.
GBM-SCs are susceptible to killing and replication by oHSVs. A and B, oHSVs mediate killing of GBM-SCs, GBM4 (left), GBM8 (middle), and BT74 (right). A, dissociated GBM-SCs were infected with mock, G47Δ, FΔ6, or strain F at MOI of 0.2, and 2 × 104 cells were plated in EF medium in 24-well plates. After 3 and 7 d in culture, trypan blue-excluding viable cells were counted for each well. All the viruses exerted significant cell killing activity against the three tested GBM-SCs, albeit with different potency. FΔ6 kills cells as efficiently as wild-type strain F, whereas G47Δ is less potent than FΔ6 in 2 of 3 GBM-SCs (*, P < 0.05; FΔ6 versus G47Δ). B, comparison of cytotoxicity among three different γ34.5 mutants. G47Δ displays greater cell killing at day 7 compared with G207 and R3616. *, P < 0.05; **, P < 0.01 compared with the values of G207. C and D, replication of oHSVs in GBM-SCs in vitro. GBM4 (left), GBM8 (middle), and BT74 (right) cells were infected as above, and harvested with medium at the indicated time points for virus yield to be determined. C, strain F, FΔ6, and G47Δ all displayed significant viral replication in the tested GBM-SCs, whereas the magnitude of FΔ6 replication was greater than that of G47Δ in all 3 cells (*, P < 0.0001; **, P < 0.005). D, G47Δ displays reasonable viral replication, whereas G207 and R3616 do not, with virus yields much less than input (4,000 pfu per well) over the 62-h time course. *, P < 0.0001, G47Δ compared with G207 or R3616. All experiments were performed in triplicate and data are presented as mean with SD.
We next quantified viral replication in GBM-SCs after virus infection at MOI of 0.2 (Fig. 4C and D). The virus yield of FΔ6 was only 1.6-6.7-fold less than wild-type strain F in all three cultures (Fig. 4C). In contrast, neither G207 nor R3616 replicated in any of the cultures, as shown by viral yields much less than input (Fig. 4D). G47Δ displayed substantial but significantly less replication than FΔ6. Collectively, these results indicate a close correlation between the efficiency of cell killing and the magnitude of viral replication. Because GBM-SCs possess some neural stem cell-like properties, we tested proliferating human embryonic stem cell-derived neural progenitor cells and v-myc-immortalized neural stem cells in the same viral growth assay. The virus yields in both neural stem/progenitor cells were comparable with those in GBM-SCs for all four viruses (Supplementary Fig. S4). This might implicate the presence of common pathways in GBM-SCs and proliferating neural stem/progenitor cells that oHSVs rely on to replicate.
Impaired self-renewal of GBM-SCs after oHSV infection
Because self-renewal is a critical property of CSCs, we investigated the effect of oHSV infection on self-renewal of GBM-SCs. We used the formation of neurospheres from dissociated cells (clonogenicity) as a measure of self-renewal. Although the viruses cause extensive oncolytic cell death in GBM-SCs, we observed a minor population of viable cells remaining 7 days postinfection. These cells were then subjected to a limiting dilution assay to determine the ability to generate secondary neurospheres. After 16 days of culture, none of the cells that initially survived viral infection were able to generate neurosphere structures even when plated at 10 cells per well (Table 1). This suggests that oHSV infection may be able to suppress self-renewal of GBM-SCs that are not infected and/or do not undergo oncolysis.
Intratumoral injection of oHSV prolongs survival of mice bearing GBM-SC-derived xenografts
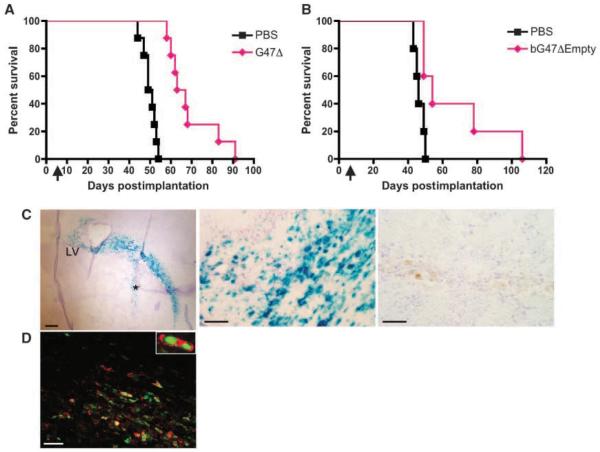
Although FΔ6 was more active in vitro than G47Δ, there are safety concerns with the use of such a singly mutated oHSV in patients' brains. Therefore, we evaluated the efficacy of G47Δ in vivo, using two GBM-SC orthotopic xenograft models (GBM8EF and BT74) because their aggressive behaviors are representative of human GBM. The highly invasive histopathology of GBM8EF (Fig. 2C) poses a particularly challenging model for therapeutics. G47Δ, its BAC-derived equivalent, bG47Δ-Empty, or PBS was administered intratumorally after intracerebral implantation of GBM-SCs into mice. A single injection of G47Δ exerted considerable anti-tumoral activity in both models, with a significant prolongation of survival over PBS treatment in GBM8 (Fig. 5A) and BT74 (Fig. 5B) tumor-bearing mice. In a separate experiment using the GBM8 model, brains were collected 24 hours after G47Δ injection. X-gal staining of the sections showed an extensive distribution of β-galactosidase positivity, which seemed to overlap the tumor area, reaching close to the midline, a substantial distance (~1.2 mm) from the vector injection site (Fig. 5C, left). Observation under higher power magnification revealed highly efficient infection of tumor cells by G47Δ (Fig. 5C, middle). Immunofluorescent colocalization of β-galactosidase and human-specific nuclei antigen showed that the majority of LacZ-expressing cells are of human origin (Fig. 5D). These results reveal that G47Δ can efficiently target human GBM-SCs and inhibit tumor growth in vivo.
Figure 5.
Intratumoral injection of G47Δ prolongs survival of mice bearing GBM-SC xenografts. A and B, Kaplan-Meier survival curves of the mice bearing GBM-SCs xenografts treated with oHSV vectors. Forty thousand GBM8EF cells (A) or 2 × 104 BT74 cells (B) were implanted into the brains of athymic (A) or severe combined immunodeficient (B) mice. Six (A) or 7 (B) d later, G47Δ (A) or bG47Δ-Empty (B; 2 × 106 pfu, pink diamond) or PBS (black square) was stereotactically injected at the same coordinates as the tumor cells. Treatment with G47Δ resulted in significantly prolonged survival compared with mock treatment (median survival time, 62 versus 50 d, P < 0.0001 for A; median survival time 54 versus 46 d, P < 0.03 for B). n = 8 (A) or 5 (B) per group. Arrows, time of virus injection. C and D, G47Δ infects GBM-SC tumors in vivo. Twenty-four hours after injection of G47Δ (left and middle in C and D) or PBS (C, right) into GBM8 xenografts, the brains were collected. X-gal staining of the sections (C) revealed an extensive infection of tumor tissue that displays a progression along white matter tracts (left). LV, lateral ventricle. *, injection track. Scale bar, 200 μm. Efficient in vivo infection by G47Δ is shown at higher power magnification (middle), whereas no lacZ positivity is seen in a PBS-treated section (right). Scale bars, 50 μm. D, immunofluorescent staining showing colocalized detection of β-galactosidase (Cy3, red) and human nuclei (FITC, green) on a G47Δ-infected brain section. Scale bar, 50 μm.
Discussion
In this article, we have isolated stem cell-like cells from human GBM and showed efficient tumorigenicity in an orthotopic mouse model, with as few as 50 cells. We report four key findings: (a) we provide the first evidence that human GBM-SCs are susceptible to oncolysis by oHSV; (b) we show the requirement for HSV γ34.5, or complementing genes, on the ability of oHSV to kill GBM-SCs; (c) we show that the small population of GBM-SCs surviving oncolysis can no longer propagate as secondary neurospheres; and (d) in vitro oncolysis of GBM-SCs translates into in vivo efficacy.
Our approach to obtaining GBM-SCs was to select and expand the cells as stable cultures using defined culture medium, instead of selecting cells using a specific marker such as CD133. The cultures could be maintained by repeated passaging for at least 3 mo without apparent changes in in vitro growth rates or in neurosphere-forming efficiencies (data not shown). The establishment of stable cultures allowed us to perform in vitro investigations to test their susceptibilities to oHSV as well as in vivo therapeutic studies. It is important to note that each of our cultures contained both CD133+ and CD133- cells, whereas cells with tumor-initiating potential were clearly enriched. Investigators have used cell sorting of CD133+ cells to obtain as pure a CSC population as possible. Recent publications, however, provide evidence that GBM-SCs can exist in the CD133-negative population (29, 30). Hence, we reasoned that using cultures based on the neurosphere phenotype as the source of GBM-SCs would avoid the uncertainty of the CD133+ phenotype.
The GBM-SC cultures displayed a variety of histopathologic features upon intracerebral implantation in mice, e.g., from a highly invasive nature to well-delineated tumor masses with increased vascularity, suggesting a large diversity in the characteristics of GBM-SC-derived tumors. The markedly invasive growth pattern shown by GBM6EF and GBM8EF tumors is of great value for glioma research because currently available mouse models of human glioma, such as U87MG xenograft, do not display invasiveness, an important hallmark of human GBM (31) and, thus, might not faithfully represent the human disease. CD133 expression by GBM-SCs has been reported to correlate with the invasive phenotype of tumor growth (32). Our findings that both CD133-high (GBM8) and CD133-low (GBM6) cultures display a similar tumor phenotype implicate other factors in determining tumor invasiveness. Rather, the correlation of CD133 expression levels with tumor-forming efficiency and aggressive behavior is suggested because the CD133-high cell lines (GBM8, BT74) displayed highly efficient tumor-forming ability and an aggressive course.
Use of oHSV with different mutations allowed us to analyze and compare the effects of those alterations on viral replication and oncolytic potency. The ICP6- virus, FΔ6, displayed comparable replication and cell killing activity as parental wild-type strain F, suggesting the presence of sufficient deoxynucleotide triphosphate pools in GBM-SCs under the growth conditions used. In contrast, deletion of γ34.5 negatively affected both viral replication and cell killing. The lack of viral replication in GBM-SCs with γ34.5 mutants is an important finding because thus far, all clinical trials with oHSV for glioma have used γ34.5 mutants (G207 and 1716; refs. 10, 15, 16, 19). It is possible that the premature shutoff of protein synthesis in GBM-SCs induced by the lack of γ34.5 protein causes the observed attenuated phenotype of the viruses. Importantly, G47Δ, G207 with an additional deletion of α47 gene, showed significantly increased viral yield and associated killing of GBM-SCs compared with G207 and R3616. The increased potency of G47Δ over G207 is explained by the immediate-early expression of the late US11 gene product, which can compensate for the loss of γ34.5 by inhibiting the activation of double-stranded RNA-dependent protein kinase R (33, 34).
Using two different orthotopic models of GBM-SCs, we showed the therapeutic efficacy of a single intratumoral inoculation of oHSV. Intratumoral treatment with G47Δ resulted in extensive infection of migrating tumor cells and subsequent survival prolongation. Experimental treatment of human GBM spheroid-derived tumors with G207 was recently reported (35), where significant spread of the vector was observed without significant survival benefit. Although the model used in this study is different from ours, the superior potency of G47Δ over G207 against GBM-SCs might be a factor leading to different outcomes. Given that G47Δ was shown to be as safe as G207 upon inoculation into HSV-susceptible mice brains (25), our finding is relevant to the clinical application of oHSV because the use of G47Δ might potentially overcome the inability of G207 to efficiently destroy GBM-SCs.
Although a single inoculation of oHSV significantly increased survival of tumor-bearing mice, we did not achieve cures. This suggests the need for further improvement of the treatment strategy. Because the tumor cells exhibit extensive infiltration to surrounding normal brain separate from the tumor mass, multiple treatments or convection-enhanced delivery of the viruses might enhance the infection of the migrating front-line cells (36). Maximizing viral spread may be achieved by pharmacologic suppression of host immune responses to oHSV (37-39). Gene therapeutic strategy using prodrug-converting enzymes may be promising because of their “bystander effect” (40). From the standpoint of stem cell biology, studies suggest the preferential presence of glioma stem cells within a microvascular niche environment (41), which represents a potential therapeutic target. In this regard, one should be aware that some oHSV mutants induce angiogenic responses in vivo (42, 43), rather than exerting antiangiogenic activity (44). Use of oHSV armed with antiangiogenic transgenes might augment depletion of GBM-SC subpopulation through efficient destruction of the neovasculature (45, 46). Finally, the concept of combining virotherapeutics with chemotherapeutics is important. Histone deacetylase inhibitors have been shown to enhance antitumor activity of oHSV (47, 48). As another example, we reported a synergistic interaction of G207 and temozolomide due to increased viral replication caused by chemotherapy-induced activation of DNA repair pathways (49). Because sublethal doses of temozolomide was shown to decrease the GBM-SC subpopulation (50), this multimodal strategy should be particularly relevant to the clinic.
In conclusion, we have established expandable cultures enriched for GBM-SCs. The new glioma models described in this study provide powerful tools for testing experimental therapeutics and studying invasion and angiogenesis. We have shown the efficacy of oHSV against human GBM-SCs and correlated this cytotoxic property with specific oHSV mutations. This is important for designing new oHSV vectors and future clinical trials.
Supplementary Material
Acknowledgments
Grant support: NIH grant NS-032677 (R.L. Martuza), Department of Defense grant W81XWH-07-1-0359 (S.D. Rabkin), and NIH grant K08 CA124804 and a Sontag Foundation Distinguished Scientist Grant (S. Kesari).
We thank Dr. Jeffrey Macklis and Dr. Noriyuki Kishi at Massachusetts General Hospital for providing technical assistance for growing GBM-SCs.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest R.L. Martuza and S.D. Rabkin are consultants to MediGene Inc, which has a license from Georgetown University for G207. The other authors disclosed no potential conflicts of interest.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 3.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 9.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 10.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 11.Selznick LA, Shamji MF, Fecci P, Gromeier M, Friedman AH, Sampson J. Molecular strategies for the treatment of malignant glioma-genes, viruses, and vaccines. Neurosurg Rev. 2008;31:141–55. doi: 10.1007/s10143-008-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–78. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann D, Wildner O. Comparison of herpes simplex virus - and conditionally replicative adenovirus-based vectors for glioblastoma treatment. Cancer Gene Ther. 2007;14:627–39. doi: 10.1038/sj.cgt.7701055. [DOI] [PubMed] [Google Scholar]
- 14.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 15.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–66. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson M, Guse K, Bauerschmitz G, et al. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24-/low cells. Mol Ther. 2007;15:2088–93. doi: 10.1038/sj.mt.6300300. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of Δ-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–4. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 18.Aghi M, Rabkin S. Viral vectors as therapeutic agents for glioblastoma. Curr Opin Mol Ther. 2005;7:419–30. [PubMed] [Google Scholar]
- 19.Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplifiedEGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 20.Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–8. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S, Kang UJ, Isacson O, Kim KS. Neural precursors derived from human embryonic stem cells maintain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008;104:316–24. doi: 10.1111/j.1471-4159.2007.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villa A, Snyder EY, Vescovi A, Martinez-Serrano A. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS. Exp Neurol. 2000;161:67–84. doi: 10.1006/exnr.1999.7237. [DOI] [PubMed] [Google Scholar]
- 23.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to γ 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–6. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 24.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutatedherpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–43. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 25.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–8. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 27.Kuroda T, Martuza RL, Todo T, Rabkin SD. Flip-Flop HSV-BAC: bacterial artificial chromosome basedsystem for rapid generation of recombinant herpes simplex virus vectors using two independent site-specific recombinases. BMC Biotechnol. 2006;6:40. doi: 10.1186/1472-6750-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab invest. 1992;66:303–13. [PubMed] [Google Scholar]
- 29.Beier D, Hau P, Proescholdt M, et al. CD133+ and CD133- glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 30.Ogden AT, Waziri AE, Lochhead RA, et al. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–14. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- 31.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–48. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunther HS, Schmidt NO, Phillips HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27:2897–909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 33.Cassady KA, Gross M. The herpes simplex virus type 1 Us11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acidsequence adjacent to a kinase substrate domain. J Virol. 2002;76:2029–35. doi: 10.1128/jvi.76.5.2029-2035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey M, Poppers J, Ladd A, Mohr I. A herpesvirus ribosome-associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-relatedfunction. J Virol. 1999;73:3375–85. doi: 10.1128/jvi.73.4.3375-3385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huszthy PC, Goplen D, Thorsen F, et al. Oncolytic herpes simplex virus type-1 therapy in a highly infiltrative animal model of human glioblastoma. Clin Cancer Res. 2008;14:1571–80. doi: 10.1158/1078-0432.CCR-07-2000. [DOI] [PubMed] [Google Scholar]
- 36.Szerlip NJ, Walbridge S, Yang L, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sizedparticles. J Neurosurg. 2007;107:560–7. doi: 10.3171/JNS-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 37.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl AcadSci U S A. 2006;103:12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 39.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis. Gene Ther. 2004;11:214–23. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guffey MB, Parker JN, Luckett WS, Jr., et al. Engineered herpes simplex virus expressing bacterial cytosine deaminase for experimental therapy of brain tumors. Cancer Gene Ther. 2007;14:45–56. doi: 10.1038/sj.cgt.7700978. [DOI] [PubMed] [Google Scholar]
- 41.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Aghi M, Rabkin SD, Martuza RL. Angiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptide. Cancer Res. 2007;67:440–4. doi: 10.1158/0008-5472.CAN-06-3145. [DOI] [PubMed] [Google Scholar]
- 43.Kurozumi K, Hardcastle J, Thakur R, et al. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther. 2008;16:1382–91. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cinatl J, Jr., Michaelis M, Driever PH, et al. Multi-mutatedherpes simplex virus g207 is a potent inhibitor of angiogenesis. Neoplasia. 2004;6:725–35. doi: 10.1593/neo.04265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu TC, Zhang T, Fukuhara H, et al. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin Cancer Res. 2006;12:6791–9. doi: 10.1158/1078-0432.CCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 46.Liu TC, Zhang T, Fukuhara H, et al. Oncolytic HSV armedwith platelet factor 4, an antiangiogenic agent, shows enhancedefficacy. Mol Ther. 2006;14:789–97. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Liu TC, Castelo-Branco P, Rabkin SD, Martuza RL. Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol Ther. 2008;16:1041–7. doi: 10.1038/mt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otsuki A, Patel A, Kasai K, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–55. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 49.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 50.Beier D, Rohrl S, Pillai DR, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–15. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.