Abstract
In four experiments, we assessed the generality of previous findings (Swithers & Davidson, 2008) that increased caloric intake, body weight gain, and reduced caloric compensation are exhibited by rats that consume a diet containing a nonnutritive, high intensity sweetener. In this earlier work, rats consumed a diet in which saccharin was mixed in low-fat yogurt, and animals were provided with a fixed amount of the yogurt. The present experiments showed that the effects of saccharin on energy intake and body weight gain are also obtained when rats were given Acesulfame Potassium (AceK), a nonnutritive high intensity sweetener that is chemically distinct from saccharin. Increased energy intake and body weight gain and impaired caloric compensation were also obtained with a saccharin-sweetened base diet (refried beans) that was calorically similar, but nutritionally distinct from low-fat yogurt. The present studies also extended earlier findings by showing that body weight differences persist after saccharin-sweetened diets are discontinued and following a shift to a diet sweetened with glucose. In addition, rats first exposed to a diet sweetened with glucose still gain additional weight when subsequently exposed to a saccharin sweetened diet. The effect of saccharin on caloric compensation was more complex in that it appeared to depend on the type of diet (yogurt or beans) in which saccharin was consumed prior to testing. The results of these experiments add support to the hypothesis that exposure to weak or non-predictive relationships between sweet tastes and caloric consequences may lead to positive energy balance.
Keywords: learning, energy balance, obesity, food intake
Several indices related to energy regulation in rats appear to be sensitive to the relationship between the sweet taste and the caloric content of food. For example, after being maintained on a diet in which sweet tastes are not associated with increased calories, rats exhibit increased food intake, body weight gain, and adiposity, while caloric compensation and the thermic effect of food are decreased, relative to rats maintained on a diet where sweet tastes are better predictors of caloric outcomes (Swithers & Davidson, 2008). These data suggest that interfering with or weakening the normally predictive relationship between sweet taste and calories can impair energy balance. However, the importance of these findings is difficult to assess because they have been obtained under a limited set of conditions. Namely, to weaken the predictive relationship between sweet taste and calories, we gave rats repeated opportunities to consume saccharin mixed in a fixed amount (30g) of a plain, low-fat yogurt, base diet. No other nonnutritive, high intensity sweetener, base diet, or quantity of diet was studied. The purpose of the present experiments was to examine the generality of the previously reported effects of exposure to non-predictive sweet taste – calorie relations on energy balance.
Thus, the following experiments investigated the effects of consuming yogurt diets sweetened with an alternative high-intensity sweetener (Acesulfame Potassium; AceK). In all experiments, animals were provided with an unsweetened version of a supplemental diet, as well as with a version of the supplemental diet that was sweetened with either a caloric sweetener (glucose) that roughly doubled its caloric density or a high-intensity sweetener that did not affect the caloric density. Therefore, in each experiment, animals were provided with diets in which the addition of a sweet taste either explicitly did (glucose) or did not (saccharin or AceK) predict the addition of calories. In addition, the consequences of sweetening a base diet other than yogurt (pureed refried beans) using a caloric versus a non-caloric sweetener were examined along with the effects of more restricted access to smaller quantities of the diets. We also assessed the persistence of body weight differences following the discontinuation of the sweetened diets, and the consequences of reversing the predictive and non-predictive relationships on weight gain and energy intake. If the pattern of results obtained in the present studies is consistent with the results reported previously, this would show that the effects of exposure to non-predictive sweet taste-calorie relationships are not confined to a specific combination of sweet taste or dietary stimuli.
Methods
Experiment 1
Subjects were progeny of male and female Sprague-Dawley rats (Harlan, Indianapolis) bred in the laboratory. Litters were housed together undisturbed except for routine cage maintenance until weaning on day 23. Litters (n=7) were then separated into same-sex groups, and same-sex littermates were housed together until testing. At the start of testing, animals were separated into individual cages and one or two rats from each litter were assigned to one of three diet groups. In all diet groups, animals received 30 g of low-fat, unflavored yogurt (~0.6 kcal/g) daily in addition to ad lib chow (Lab Diets 5001) for 14 days; on seven days, plain yogurt was provided while sweetened yogurt was provided on the other seven days. For one group, the yogurt was sweetened with 20% glucose (w/w; ~1.2 kcal/g). For the second group, yogurt was sweetened with 0.3% saccharin. For the third group, yogurt was sweetened with 0.3% AceK. Groups were matched for body weight at the start of testing (Means ± SEM = 274 ± 10, 276 ± 12 and 274 ± 14 for glucose, saccharin, and AceK groups, respectively, n=8 animals per group). In each group, half of the animals received sweetened yogurt on the first presentation, and the other half received unsweetened yogurt. The order of the presentation on the following days was randomized, with the constraint that each yogurt type could be presented no more than three consecutive days. Yogurts were available for 23 hr per day; yogurt intake and body weights were collected daily.
Statistical analyses: Body weight gain was assessed using a 2-way (Diet × Days) repeated measures ANOVA with Days as a within-subjects factor, Diet as a between subjects factor and Starting body weight as a covariate; a p value < 0.05 was taken as significant. Post-hoc testing was done using Fisher's LSD test, with p < 0.01 taken as significant to control for multiple comparisons.
Experiment 2
Thirty-three naïve, adult male Sprague-Dawley rats (purchased from Harlan, Indianapolis) were matched for body weight and assigned to one of three conditions as in Experiment 1 (Starting body weights = 337 ± 3; 338 ± 3 and 337 ± 4 g for glucose, saccharin and AceK groups, respectively). Yogurt was prepared as in Experiment 1; 20 g of yogurt was given to animals for one hr per day ending 30 min prior to lights off; chow was not available during the hour that yogurt was provided. Yogurt was presented six days per week for two weeks, with one day of chow and water intervening between the first and second weeks. In each group, half of the animals received sweetened yogurt on the first presentation, and the other half received unsweetened yogurt. The order of the presentation on the following days was randomized, with the constraint that each yogurt type could be presented no more than three consecutive days. Body weight gain was analyzed with separate 2-way (Diet × Days) repeated measures ANOVAs with Days as a within-subjects factor, Diet as a between subjects factor and Starting body weight as a covariate. Yogurt intake was analyzed with a two-way (Day × Diet) repeated measures ANOVA. A p <0.05 was taken as significant for ANOVA. Post-hoc testing was done using Fisher's LSD tests, with p < 0.01 taken as significant to control for multiple comparisons.
Experiment 3
Twenty-six naïve, adult male Sprague-Dawley rats (purchased from Harlan, Indianapolis) were matched for body weight and assigned to one of two conditions, Saccharin (n = 13; starting BW= 425 ± 3 g) and Glucose (n = 13; starting BW = 428 ± 4 g). Twenty grams of sweetened or plain yogurt diets were provided for 1 hr per day as in Experiment 2; at the end of two weeks, yogurts were no longer provided, and body weight gain was measured for an additional two weeks. In each group, half of the animals received sweetened yogurt on the first presentation, and the other half received unsweetened yogurt. The order of the presentation on the following days was randomized, with the constraint that each yogurt type could be presented no more than three consecutive days. Yogurt intake was analyzed using a two-way, repeated measures ANOVA (Day × Diet); intake data from the fifth day of yogurt presentation were lost due to experimenter error, therefore these data were not included in the analyses. Body weight gain during yogurt presentation was compared with body weight gain following yogurt discontinuation and analyzed with a 3-way (Diet × Testing Period [during versus after yogurt] × Days) repeated measures ANOVAs with Days as a within-subjects factor and Diet as a between subjects factor. Because Starting body weights differed at the beginning of yogurt presentation compared with the end of yogurt presentation, initial body weight was used as a covariate for the “during yogurt” Testing period and body weight at the end of yogurt presentation was used as a covariate for the “after yogurt” Testing period. A p value < 0.05 was taken as significant for ANOVAs. Post-hoc testing was done using LSD tests, with p < 0.01 taken as significant to control for multiple comparisons in the post-hoc tests.
Experiment 4
Sixty-three naïve, adult male Sprague-Dawley rats (purchased from Harlan, Indianapolis) were assigned to one of eight groups; all groups received 30 g of a plain, unsweetened diet and 30 g of a sweetened diet for 23 hr per day, six days per week (three days sweetened and three days plain). During Phase 1 (two weeks) of training, two of the groups received saccharin-sweetened yogurt and two groups received glucose-sweetened yogurt. The remaining four groups received plain and sweetened pureed refried beans (Gordon Food Service, plain beans = ~0.7 kcal/g), with two groups receiving saccharin-sweetened beans (0.3% w/w) and two groups receiving glucose-sweetened beans (20% w/w; ~ 1.4 kcal/g); pilot testing had indicated that rats would readily consume the beans, and could discriminate the plain from the sweetened beans. All animals were also provided ad lib access to chow (Harlan 2018) and water. At the end of two weeks of diet presentation, animals received a single day of chow presentation, and then began Phase 2 of training (Table 1). In each group, half of the animals received sweetened diet on the first presentation, and the other half received unsweetened diet. The order of the presentation on the following days was randomized, with the constraint that each diet type could be presented no more than three consecutive days.
Table 1.
Experiment 3 Design
| Phase 1 |
Phase 2 |
|||
|---|---|---|---|---|
| Group | Base Diet | Sweetener | Base Diet | Sweetener |
| BY | Beans | Glucose | Yogurt | Glucose |
| Beans | Glucose | Yogurt | Saccharin | |
| Beans | Saccharin | Yogurt | Glucose | |
| Beans | Saccharin | Yogurt | Saccharin | |
| YB | Yogurt | Glucose | Beans | Glucose |
| Yogurt | Glucose | Beans | Saccharin | |
| Yogurt | Saccharin | Beans | Glucose | |
| Yogurt | Saccharin | Beans | Saccharin | |
During Phase 2 (two weeks), all animals that had received yogurt during Phase 1 were given sweetened and unsweetened beans, while all animals that had received beans during Phase 1 were given sweetened and unsweetened yogurt during Phase 2. For half of the animals in each group, the Phase 2 diet was sweetened with the same sweetener they received during Phase 1. For the remaining animals, the Phase 2 sweetener was the opposite from the Phase 1 sweetener. This created a total of eight groups; four groups that received beans followed by yogurt (Saccharin-Saccharin, Saccharin-Glucose, Glucose-Saccharin and Glucose-Glucose) and four that received yogurt followed by beans. Phase 2 comprised 2 weeks of presentation of the prescribed diet. Following Phase 2 training, rats were food deprived overnight, then were tested for caloric compensation for calories provided by a novel, sweet tasting diet (5 g Chocolate Ensure Plus, thickened with 2.5% guar to approximate the thickness of yogurt). Half of the animals in each group were given the 5 g premeal for one hr following overnight food deprivation, while the remaining animals received no premeal. Chow was returned immediately following the premeal, and chow intake was measured after one, two, and four hr. Three days later, animals were again food deprived overnight, and tested with the premeal conditions reversed.
During Phases 1 and 2, most animals given yogurt ate all 30 g that were offered; animals that failed to consume at least 70% of the yogurt in either Phase 1 or 2 were removed from analysis (n=3). In contrast, animals rarely consumed all 30 g of beans, and a cutoff of 70% would have eliminated most of the animals. To control for animals that consumed significantly fewer beans than average, the overall average total bean consumption was calculated across the 12 days of presentation for each phase, and animals that consumed less than 70% of this average were removed from analysis (n=9). Final sample size in each of the eight groups was 5 – 7. Body weight gain was analyzed separately for Phase 1 and Phase 2 using 3-way (Base diet X Sweetener group X Days) ANOVAs with body weight at the start of each phase used as a covariate. Body weight across both phases was analyzed with a 3-way (Base diet order X Sweetener group X Days) ANOVA with body weight at the start of Phase 1 as a covariate. Caloric compensation for premeals was analyzed using by separate 4-way (First Sweetener X Second Sweetener X Premeal X Time) ANOVAs for each base diet. Post-hoc tests were conducted using Fisher's LSD test as in previous experiments.
Results and Discussion
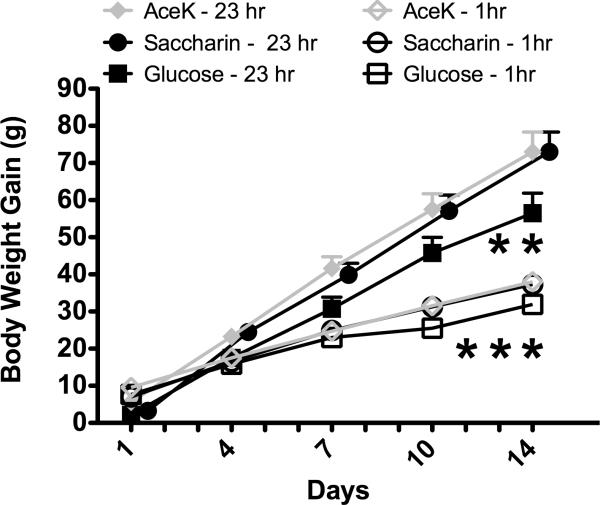
In Experiment 1, when 30 g of plain yogurt or yogurt sweetened with saccharin, AceK, or glucose were available for 23 h per day over the course of 14 days, body weight gain was significantly affected by starting body weight, time and the diet offered (Figure 1; main effect of Starting body weight; F [1, 20] = 19.5, p = 0.00026; main effect of Days, F [13, 260] = 43.8, p < 0.000001, Days X Starting body weight interaction, F [13, 260] = 17.9, p < 0.000001; Days X Diet interaction, F [26, 260] = 2.02, p = 0.0031). Post-hoc analysis revealed that on days 13 and 14 of testing, cumulative weight gain was significantly greater for rats given the saccharin or AceK sweetened yogurt compared with rats given the yogurt sweetened with glucose. Differences in weight gain between the Saccharin and AceK groups were not significant on any test day.
Figure 1.
Body weight gain is significantly higher in male rats given either 23-hr access or 1-hr access to yogurt diets sweetened with Saccharin or AceK compared with rats given yogurt diets sweetened with glucose. Body weight was recorded daily - data points are omitted for clarity.
* p < 0.01 compared with Saccharin and AceK animals given the same duration (1 or 23 hr) of access to yogurt
These data confirm previous work indicating that rats consuming yogurt diets sweetened with non-caloric saccharin gain more weight than animals consuming yogurt diets sweetened with glucose (Swithers & Davidson, 2008). Of special interest, in this study, like rats given yogurt sweetened with saccharin, rats given yogurt diets sweetened with noncaloric AceK also gained significantly more weight than animals given glucose-sweetened yogurt. This result was obtained despite the caloric content of both the saccharin and AceK-sweetened yogurts being lower than the yogurt sweetened with glucose. The similarity in body weight gain for the saccharin and AceK groups suggests that increased weight gain is not attributable to the chemical properties of saccharin per se, but instead reflects differences related to the caloric versus non-caloric nature of the sweeteners.
In Experiment 2, when 20 g of plain or sweetened yogurt was available for 1 hr per day, body weight gain was also significantly affected by the starting by weight of the animals (Days X Starting body weight interaction, F [12, 348] = 2.8, p = 0.0013) as well as by the diet offered (Days X Diet interaction, F [24, 348] = 1.7, p = 0.017). Post-hoc tests revealed that body weight gain was significantly lower in animals given glucose-sweetened yogurts compared with AceK or saccharin-sweetened yogurts on days 12, 13 and 14 of testing (Figure 1). Intake of the yogurt diets was affected by the day of testing, as well as by the diet offered (main effect of Diet, F [2, 30] = 13.3, p = 0.000073; main effect of Day, F [11, 330] = 37.6, p < 0.000001; Diet X Day interaction, F [22, 330] = 5.0, p < 0.000001), with animals in the glucose-sweetened group consuming significantly less of the yogurt (7.9 ± 0.5g daily) compared with animals in the saccharin- (10.3 ± 0.5g daily) or AceK- (11.7 ± 0.5g daily) sweetened groups. Animals in all groups consumed smaller quantities of the diets on the first two days compared with subsequent days, consistent with neophobia for the novel yogurt diets. In addition, animals in the glucose group consumed smaller quantities of both sweetened and unsweetened yogurt compared with animals in the AceK and saccharin groups beginning on the fourth day of exposure.
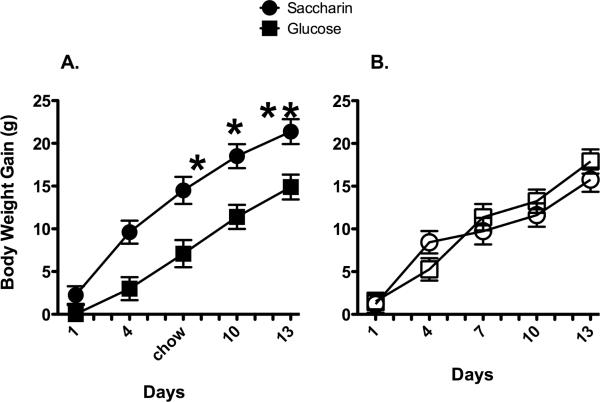
In Experiment 3, when 20 g of plain or sweetened yogurt was available during two weeks of training, body weight gain was affected by the Testing period, Starting body weight, Day, and Diet offered (main effect of Starting body weight, F [1, 47] = 4.6, p = 0.038; Diet X Testing period interaction, F [1, 47] = 8.8, p = 0.0047; Day X Testing period interaction, F [12, 564] = 2.5, p =0.0034; Day X Testing period X Diet interaction, F [12, 564] = 1.92, p = 0.030). Post-hoc analyses revealed that during the period that yogurt was available, animals receiving saccharin-sweetened yogurt gained significantly more weight than animals given glucose-sweetened yogurt (Figure 2, left). However, once yogurt presentation was discontinued, body weight gain was similar across the two groups of animals (Figure 2, right). While yogurt intake was significantly affected by the day and by the diet (main effect of Day, F [10, 240] = 18.8, p < 0.000001; Day X Diet interaction, F [10, 240] = 4.2, p = 0.00002), post-hoc tests revealed no significant differences between groups on any individual day (mean daily yogurt intake = 11.8 ± 0.7g for glucose-sweetened animals and 11.9 ± 0.7 for saccharin-sweetened animals).
Figure 2.
Body weight gain was significantly greater during presentation of saccharin-sweetened yogurt compared with glucose-sweetened yogurt (A). Following termination of yogurt presentation (B), no differences in body weight gain were observed.
* p < 0.01 compared with Glucose
Taken together, Experiments 2 and 3 demonstrate that animals given restricted access to the non-predictive diets continued to show increased body weight gain during the 2 weeks of sweetened diet availability. Further, while animals in Experiment 2 consumed fewer grams of yogurt in the glucose-sweetened group, their caloric intake from yogurt was still higher than the caloric intake of the animals in the AceK and saccharin groups due to the higher caloric density of the glucose-sweetened yogurt. Despite the greater caloric intake of glucose-sweetened yogurt, the rats in the glucose-sweetened group gained less weight. In addition, in Experiment 3, yogurt consumption was not significantly lower in the glucose-sweetened group, and these animals continued to show lower body weight gain compared with the saccharin-sweetened group. Thus, differences in yogurt consumption are unlikely to explain the differences in body weight gain observed. Further, the data from Experiment 3 indicate that once the diets were discontinued, body weight gain was similar across the two diet groups. These results suggest that following experience with a non-predictive sweet taste-calorie relationship, rats do not appear to adjust their subsequent intake or physiological response to compensate for the excess weight gain that occurred during the non-predictive exposure. Thus, excess weight gain that occurs during exposure to non-predictive sweet taste-calorie relations does not appear to be easily reversed when exposure to that non-predictive relationship has been terminated.
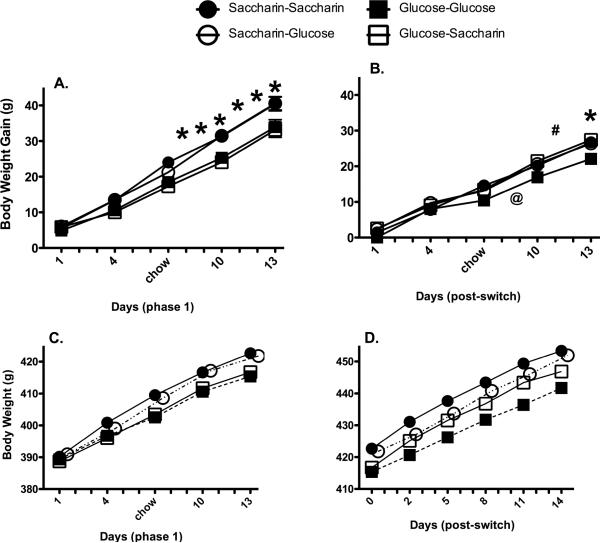
In Phase 1 of Experiment 4, when animals received 30 g of yogurt or refried beans sweetened with saccharin or glucose, body weight gain was significantly affected by the sweetener (main effect of sweetener group, F [3, 42] = 4.8, p = 0.0060; sweetener group X time interaction, F [36, 504] = 3.5, p < 0.000001). Post-hoc analyses indicated that animals in the saccharin groups gained significantly more weight during phase 1 compared with animals in the glucose groups (Figure 3). During Phase 1, while there was a main effect of the Base diet (yogurt versus beans) on body weight gain (F [1, 42] =10.8, p = 0.0020), with animals receiving beans gaining significantly more weight (22.9 − 0.3 g) compared with animals receiving yogurt (18.9 ± 0.9g), there were no significant interactions of base diet with sweetener group. This result was somewhat surprising, since the caloric density of the yogurt and beans diets were similar; at present the mechanisms underlying differences in body weight gain that resulted from the base diet independent of the sweetener remain unknown. Nevertheless, these results demonstrate that the effects of predictive versus non-predictive sweet taste – calorie relationship on body weight gain do not depend on the base diet in which the sweet taste occurs.
Figure 3.
Body weight gain (A) and body weight (C) during Phase 1 of exposure to yogurt or bean diets sweetened with Saccharin (Circles) or Glucose (Squares). During phase 2, base diets were switched and body weight gain (B) and body weight (D) were affected by the sweeteners employed. In all panels, sweetener groups are collapsed across both beans and yogurt base diets.
* p < 0.05 compared with Glucose-Saccharin, Saccharin-Glucose and Saccharin-Saccharin
# p < 0.05 compared with Glucose-Saccharin and Saccharin-Saccharin
@ p < 0.05 compared with Glucose-Saccharin
During Phase 2, base diets were switched and animals continued to receive the same sweetener as in Phase 1, or were switched to the alternative sweetener. Body weight gain during this Phase was also significantly affected by the sweetener group (main effect of Group; F [3, 42 = 4.03, p = 0.013; Time X Group interaction; F [36, 504] = 1.6, p = 0.021). Animals that received glucose-sweetened diets during Phases 1 and 2 (glucose-glucose) gained significantly less weight during Phase 2 compared with animals in all other groups (glucose-saccharin, saccharin-glucose, and saccharin-saccharin), which did not differ from each other at any time (Figure 3). Thus, animals that had experience in which sweet taste always predicted increased calories showed the lowest body weight gain compared with animals that received experience in which sweet taste had sometimes predicted increased calories (saccharin-glucose and glucose-saccharin) or in which sweet taste consistently did not predict increased calories (saccharin-saccharin). The effects of the base diet in Phase 2 were modest; animals that received beans during the second phase gained significantly more weight on the first day of reversal only (3.0 ± 0.5 vs. 0.2 ± 0.4 g) compared with animals that received yogurt during the second phase (Base diet X Time interaction; F [12, 504] = 3.7, p = 0.000021). Similar to the effects observed in Phase 1, there were no interactions involving base diet with sweetener group. In addition to the differences in body weight gain, when analyzed across both phases, body weight was significantly affected by the sweetener group, but not the base diet (Figure 3; main effect of Group; F [3, 42] = 4.3, p = 0.0010; Time X Group interaction; F [84, 1176] = 2.6, p < 0.000001). Post-hoc analyses indicated that animals in the Glucose-Glucose group weighed significantly less than rats in the other three groups.
These data suggest that the effects of exposure to non-predictive sweet taste-calorie relationships are not strongly influenced by prior experience with sweet tastes and calories, at least within the present testing context. In other words, upon consuming saccharin-sweetened diets, animals that had previously received glucose-sweetened diets showed increased body weight gain, similar to the increases displayed by animals accustomed to consuming saccharin-sweetened diets. In addition, animals that had previously received saccharin-sweetened diets continued to show increased body weight and body weight gain even when switched to glucose-sweetened diets. These results suggest that the consequences of non-predictive relationships between sweet tastes and calories on body weight gain are not rapidly reversed, even when sweet tastes do come to predict calories.
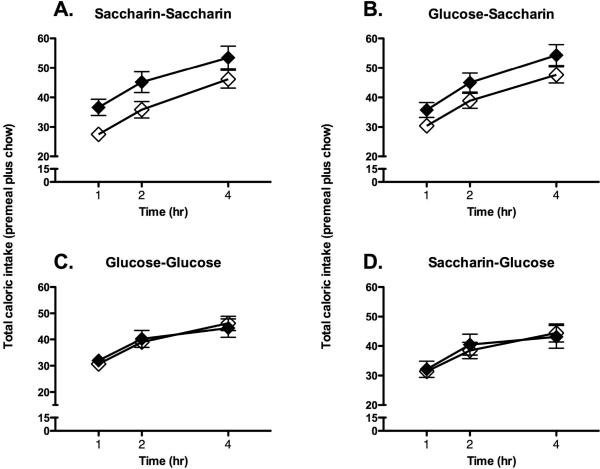
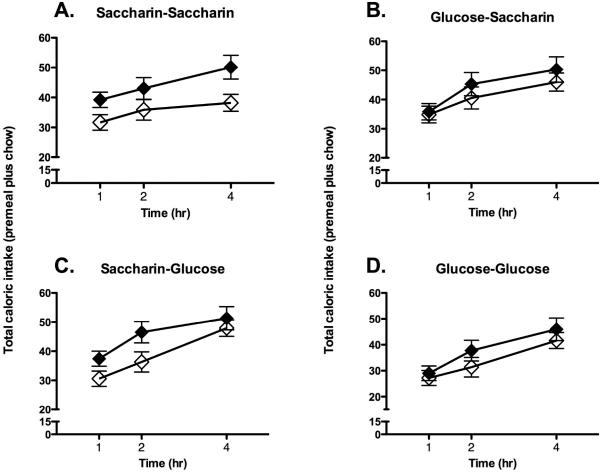
While body weight gain appeared to be persistently influenced by exposure to saccharin-sweetened diets, the effects of experience with the sweetened diets on caloric compensation tests were more complex, and depended on the order in which the base diets and sweeteners were presented. During caloric compensation tests in rats that had received beans in Phase 1 and yogurt in Phase 2 (Table 1; Group BY), chow intake was affected by the premeal and the sweetener received during Phase 2, but not by the sweetener received during Phase 1 (Figure 4; main effect of Premeal, F [1, 18] = 8.6, p = 0.0091; Premeal X Second sweetener interaction, F [1, 18] = 7.1, p = 0.016; Time X Second sweetener interaction, F [2, 36] = 5.1, p = 0.012). Post-hoc analysis revealed that animals that received saccharin-sweetened yogurt during Phase 2 consumed significantly more calories during the test in which a novel premeal was provided compared with the test when no premeal was provided. Total caloric intake in animals that received glucose-sweetened yogurt did not differ on premeal vs. no premeal days, suggesting more complete caloric compensation for the sweet premeal in these groups (Figure 4).
Figure 4.
Chow intake following a novel sweetened diet was affected by the sweetener provided during phase 2 when sweetened beans were provided during phase 1 and sweetened yogurt was provided during phase 2. Animals given saccharin-sweetened yogurt during phase 2 of training showed significantly weaker compensation for calories provided in a novel, sweet-tasting premeal (A and B; closed symbols represent intake on premeal days, open symbols represent intake on no premeal days) compared with animals given glucose-sweetened yogurt during phase 2 (C and D; symbols as in A and B).
In contrast, in animals that had received yogurt during phase 1 and beans during phase 2 (Table 1; Group YB), there was a significant effect of premeal on total caloric intake (main effect of premeal, F [1, 18] = 16.2, p = 0.00079), but there were no significant effects of the first or second sweetener on total caloric intake (Figure 5). In other words, animals in all groups appeared impaired in the ability to compensate for the calories provided in the novel, sweetened premeal. Visual inspection of the data suggested that animals that had received glucose-sweetened yogurt during Phase 1 (glucose-glucose and glucose-saccharin groups) had smaller differences between premeal and no premeal tests compared with animals that had received saccharin-sweetened yogurt during Phase 1, however no statistically significant interaction was obtained, perhaps due to small sample sizes.
Figure 5.
Chow intake following a novel sweetened diet was not affected by the sweetener provided during phase 2 in when sweetened yogurt was provided during phase 1 and sweetened beans were provided during phase 2. Both animals given saccharin-sweetened beans during phase 2 of training (A and B; closed symbols represent intake on premeal days, open symbols represent intake on no premeal days) and animals given glucose-sweetened beans during phase 2 (C and D; symbols as in A and B) showed greater intake on premeal days compared with no premeal days.
The results of this experiment demonstrate that the consequences of predictive versus non-predictive sweet-taste experience on compensation for the calories provided by the Chocolate Ensure Plus premeal were influenced by the order in which the diets were provided, perhaps due to differences in generalization from the base diets to the test premeal. In particular, the data are consistent with the hypothesis that there was greater generalization from the sweetened yogurt to the sweetened premeal than from the sweetened beans to the sweetened premeal. For example, when animals were given sweetened beans diet followed by sweetened yogurt diet, compensation for the sweet-tasting premeal appeared to be influenced by the more recent experience with the yogurt sweetener, as animals in both the Glucose-Saccharin and Saccharin-Saccharin groups failed to compensate for the novel premeal calories, with animals in the Saccharin-Glucose and Glucose-Glucose groups compensating more precisely. In contrast, animals given the sweetened yogurt followed by the sweetened beans showed a different pattern of results, with no significant evidence of compensation for the premeal in any group. However, there was an indication that the sweetener delivered during the yogurt phase (phase 1 in this group) may have had some influence since Glucose-Saccharin and Glucose-Glucose animals showed moderate evidence of better compensation for the calories, particularly during the first hour.
Because these effects were not significant, at present, it is not possible to determine whether the influence of the presentation order of the sweetened beans and yogurts on the ability to compensate for the calories in the sweetened chocolate premeal was related to generalization across the training and test meal, or due to some other consequence of providing animals with beans and yogurt diets. Nevertheless, the data from the compensation tests indicate that unlike with body weight gain, rats do make adjustments in short-term caloric compensation based on recent experience with the predictive relationship between sweet taste and calories. Thus, the mechanisms that underlie caloric compensation in the short term may differ from those affecting long-term body weight gain, with caloric compensation more sensitive to recent experience and body weight gain being more persistently altered, perhaps due to increased adiposity in animals consuming the non-predictive diets, as previously demonstrated (Swithers & Davidson, 2008). General Discussion
The results of these four experiments are consistent with the hypothesis that animals exposed to dietary experiences in which sweet tastes are not predictive of increased calories gain more weight and are impaired in the ability to compensate for sweet-tasting calories relative to animals given dietary experiences in which sweet tastes do predict calories. These effects do not appear to depend on the particular non-predictive sweetener employed, nor on the base diet in which the sweet tastes are provided. The present results are consistent with other work which shows that sensory experiences that do not predict caloric consequences can interfere with the ability to control food intake and body weight in rats as well as humans (e.g., (Fowler et al., 2008; Pierce, Heth, Owczarczyk, Russell, & Proctor, 2007; Ramirez, 1990; Rogers, Carlyle, Hill, & Blundell, 1988; Swithers & Davidson, 2008; Swithers, Doerflinger, & Davidson, 2006; Viskaal-van Dongen, de Graaf, Siebelink, & Kok, 2009; Warwick & Schiffman, 1991)
It does not seem likely that the differences observed in the present studies are based on differences in preference for or the palatability of the different types of sweeteners that were used. For example, although the animals in our experiments were required to differentiate between sweetened and unsweetened versions of a diet, at no point were they exposed to both the calorically and non-calorically sweetened versions of the same diet. Further, even if the diets differed in sweetness per se, it is not clear on what basis such differences in sweetness would lead to changes in body weight gain, or result in impaired caloric compensation for a novel diet. Thus, it is difficult to conceive how differences in intensity, preference, or palatability among the sweeteners per se could have produced the pattern of results we obtained.
The behavioral and physiological mechanisms underlying differences in body weight gain and caloric compensation following consumption of high-intensity sweeteners compared with caloric sweeteners remain to be specified. Our previous work documented that caloric intake (yogurt and chow combined) can be increased in animals given access to saccharin-sweetened yogurt (Swithers & Davidson, 2008). The pattern of weight gain we observed in the present experiments suggests that consumption of saccharin-sweetened supplements may have had effects on total intake like those we reported previously. However, future analyses that integrate measures of chow and yogurt intake patterns, quantities consumed, and energy expenditure, will provide a more complete description of the effects of exposure to caloric and non-caloric sweeteners on energy regulation.
Several recent studies have documented circumstances under which administration of a variety of high-intensity sweeteners can produce physiological responses that are similar to those produced by administration of caloric sweeteners, as well as circumstances under which high-intensity sweeteners produce responses distinct from caloric sweeteners. For example, recent findings show that administration of both caloric sweeteners and high-intensity sweeteners in rats results in increased expression of intestinal transporters implicated in glucose absorption (SGLT1) and induction of translocation of the glucose transporters (GLUT2) to the brush border membrane; such changes may serve to facilitate the absorption and metabolism of ingested sugars (Dyer et al., 2007; Egan & Margolskee, 2008; Jang et al., 2007; Margolskee et al., 2007). However, in the case of high-intensity sweeteners, because these gut changes are not accompanied by the presence of ingested sugars, one consequence may be increased energy intake. In addition, recent work has documented that the relative to glucose, the high-intensity sweetener sucralose can alter gut microflora and increase body weight gain when delivered to rats (Abou-Donia, El-Masry, Abdel-Rahman, McLendon, & Schiffman, 2008). Further, work from our laboratories has documented diminished thermic responses to novel sweet-tasting caloric diets in animals exposed to saccharin-sweetened yogurt relative to rats exposed to glucose-sweetened yogurt, suggesting that experience with non-predictive sweet taste - calorie relationships may impaired experience-based metabolic responses to sweet tasting diets (Swithers & Davidson, 2008).
Further evidence indicates that in animals, including humans, sweet tastes can produce physiological effects distinct from those produced by caloric consequences. For example, mice in which a cellular mechanism for sweet taste transduction has been impaired through knockout of the trpm5 pathway fail to learn preferences for non-caloric sweet solutions, but remain able to learn preferences for caloric sweet solutions, suggesting that mechanisms that underlie the detection of calories typically provided by sweet tastes are not identical to those that underlie detection of sweet tastes (de Araujo et al., 2008). In addition, work in humans has documented differential neural activation in the hypothalamus during consumption of caloric versus non-caloric sweeteners, again suggesting differences in the consequences of consumption of such sweeteners can be detected (Smeets, de Graaf, Stafleu, van Osch, & van der Grond, 2005). Taken together, these data support the hypothesis that consumption of sweet tastes in the absence of calories produces significantly different effects compared with consumption of sweet tastes associated with calories, and over time these effects can contribute to positive energy balance and increased body weight gain.
Although our current findings expand what is known about the conditions under which saccharin consumption is associated with increased intake and weight gain in rats, the effects of many other parametric variables remain to be investigated. For example, it seems likely that factors such as the concentration of saccharin and the type and concentration of caloric sweeteners employed could influence the outcome of these types of studies. Furthermore, a variety of evidence shows that animals, including humans, do not compensate as well for calories delivered in liquid, compared with solid or semi-solid forms (Davidson & Swithers, 2005; DiMeglio & Mattes, 2000; Mattes & Campbell, 2009). Thus, increased body weight gain in some earlier experiments (e.g., Kanarek, Mathes, Heisler, Lima, & Monfared, 1997; Yeomans & Clifton, 1997) may also have reflected poor compensation for liquid calories. The relative roles of providing calories in liquid form versus providing sweet tastes that do not predict calories in promoting increased intake and body weight gain merit additional examination. In addition, there is some possibility that the use of glucose as the caloric sweetener may influence the outcome. For example, consumption of glucose, compared with other sweet-tasting mono- or disaccharides, such as fructose or sucrose, has been demonstrated to produce differences in a variety of physiological responses (e.g., Blaak & Saris, 1996; Elliott, Keim, Stern, Teff, & Havel, 2002; Kanarek & Orthen-Gambill, 1982; Tappy & Jequier, 1993). The consequences of employing other caloric sweeteners also merit further investigation.
Similarly, the role of changes in the ability of sweet tastes to signal calories in regulation of food intake and energy balance in human populations remains controversial. Presently, there are increasing opportunities for exposure to such relationships as the availability and consumption of diets in which tastes do not predict calories has expanded through the use of high-intensity sweeteners and fat substitutes which mimic the sensory properties of sweet and fat with fewer or no calories. Thus, to the extent that exposure to diets in which tastes do not predict caloric consequences can disrupt energy balance, consumption of such diets may contribute to excess body weight gain, overweight and/or obesity. Data in humans that address this question directly are lacking. However, a variety of differences in BMI, appetite and food intake patterns have been reported to differ in people who are high consumers of artificially sweetened beverages (e.g., (Appleton & Blundell, 2007) and recent prospective studies have suggested a link between individuals who consume foods (such as diet sodas) manufactured with high-intensity sweeteners increased risk for obesity, metabolic syndrome and high blood pressure (Dhingra et al., 2007; Fowler et al., 2008; Lutsey, Steffen, & Stevens, 2008). The work from animal models suggests that this association may result from the consequences of the disrupting the relationship between sweet tastes and calories, and that these consequences may be somewhat resistant to reversal.
Acknowledgements
Supported by NIH grants R01 DK076078 and P01 HD052112 to S.E.S and T.L.D. We thank Melissa McCurley, Anna Barajas, Erica Hamilton and Matt Siuba for technical assistance.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J Toxicol Environ Health A. 2008;71(21):1415–1429. doi: 10.1080/15287390802328630. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Blundell JE. Habitual high and low consumers of artificially-sweetened beverages: effects of sweet taste and energy on short-term appetite. Physiol Behav. 2007;92(3):479–486. doi: 10.1016/j.physbeh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Blaak EE, Saris WH. Postprandial thermogenesis and substrate utilization after ingestion of different dietary carbohydrates. Metabolism. 1996;45(10):1235–1242. doi: 10.1016/s0026-0495(96)90241-3. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE. Food viscosity influences caloric intake compensation and body weight in rats. Obes Res. 2005;13(3):537–544. doi: 10.1038/oby.2005.57. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- Dyer J, Daly K, Salmon KS, Arora DK, Kokrashvili Z, Margolskee RF, Shirazi-Beechey SP. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans. 2007;35(Pt 5):1191–1194. doi: 10.1042/BST0351191. [DOI] [PubMed] [Google Scholar]
- Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8(2):78–81. doi: 10.1124/mi.8.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek RB, Mathes WF, Heisler LK, Lima RP, Monfared LS. Prior exposure to palatable solutions enhances the effects of naltrexone on food intake in rats. Pharmacol Biochem Behav. 1997;57(1–2):377–381. doi: 10.1016/s0091-3057(96)00337-1. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr. 1982;112(8):1546–1554. doi: 10.1093/jn/112.8.1546. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc. 2009;109(3):430–437. doi: 10.1016/j.jada.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce WD, Heth CD, Owczarczyk JC, Russell JC, Proctor SD. Overeating by young obesity-prone and lean rats caused by tastes associated with low energy foods. Obesity (Silver Spring) 2007;15(8):1969–1979. doi: 10.1038/oby.2007.235. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Stimulation of energy intake and growth by saccharin in rats. J Nutr. 1990;120(1):123–133. doi: 10.1093/jn/120.1.123. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Carlyle JA, Hill AJ, Blundell JE. Uncoupling sweet taste and calories: comparison of the effects of glucose and three intense sweeteners on hunger and food intake. Physiol Behav. 1988;43(5):547–552. doi: 10.1016/0031-9384(88)90207-7. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005;82(5):1011–1016. doi: 10.1093/ajcn/82.5.1011. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122(1):161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Doerflinger A, Davidson TL. Consistent relationships between sensory properties of savory snack foods and calories influence food intake in rats. Int J Obes (Lond) 2006;30(11):1685–1692. doi: 10.1038/sj.ijo.0803329. [DOI] [PubMed] [Google Scholar]
- Tappy L, Jequier E. Fructose and dietary thermogenesis. Am J Clin Nutr. 1993;58(5 Suppl):766S–770S. doi: 10.1093/ajcn/58.5.766S. [DOI] [PubMed] [Google Scholar]
- Viskaal-van Dongen M, de Graaf C, Siebelink E, Kok FJ. Hidden fat facilitates passive overconsumption. J Nutr. 2009;139(2):394–399. doi: 10.3945/jn.108.096123. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Schiffman SS. Flavor-calorie relationships: effect on weight gain in rats. Physiol Behav. 1991;50(3):465–470. doi: 10.1016/0031-9384(91)90531-r. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Clifton PG. Exposure to sweetened solutions enhances the anorectic effect of naloxone but not d-fenfluramine. Physiol Behav. 1997;62(2):255–262. doi: 10.1016/s0031-9384(97)00112-1. [DOI] [PubMed] [Google Scholar]