Abstract
Host RNA-binding proteins are likely to play multiple, integral roles during replication of plus-strand RNA viruses. To identify host proteins that bind to viral RNAs, we took a global approach based on the yeast proteome microarray, which contains 4080 purified yeast proteins. The biotin-labeled RNA probes included two distantly related RNA viruses, namely Tomato bushy stunt virus (TBSV) and Brome mosaic virus (BMV). Altogether, we have identified 57 yeast proteins that bound to TBSV RNA and/or BMV RNA. Among the identified host proteins, eleven bound to TBSV RNA and seven bound to BMV RNA with high selectivity, whereas the remaining 39 host proteins bound to both viral RNAs. The interaction between the TBSV replicon RNA and five of the identified host proteins were confirmed via gel-mobility shift and co-purification experiments from yeast. Over-expression of the host proteins in yeast, a model host for TBSV, revealed that 4 host proteins that enhanced TBSV replication as well as 14 proteins that inhibited replication. Detailed analysis of one of the identified yeast proteins binding to TBSV RNA, namely translation elongation factor eEF1A, revealed that it is present in the highly purified tombusvirus replicase complex. We also demonstrate binding of eEF1A to the p33 replication protein and a known cis-acting element at the 3′ end of TBSV RNA. Using a functional mutant of eEF1A, we provide evidence on the involvement of eEF1A in TBSV replication.
INTRODUCTION
Plus-stranded (+)RNA viruses, the largest group among viruses, contain relatively small genomes and thus greatly depend on the infected hosts in many steps during their infectio cycles. Indeed, viruses are known to recruit numerous host proteins to facilitate their replication and spread (Ahlquist et al., 2003; Nagy, 2008; Noueiry and Ahlquist, 2003). Several host RNA-binding proteins have been implicated in replication of (+)RNA viruses, including ribosomal proteins, translation factors and RNA-modifying enzymes (Ahlquist et al., 2003; Buck, 1996; Buck, 1999; Nagy, 2008; Noueiry and Ahlquist, 2003; Strauss and Strauss, 1999; Wang and Nagy, 2008). In addition, recent genome-wide screens of yeast genes conducted with two distantly related viruses, Brome mosaic virus (BMV) (Kushner et al., 2003) and Tomato bushy stunt virus (TBSV) (Jiang et al., 2006; Panavas et al., 2005b) revealed that their replication is affected by ~100 different host genes. The genome-wide screens with TBSV also identified ~30 host genes affecting TBSV RNA recombination (Cheng, Serviene, and Nagy, 2006; Serviene et al., 2006; Serviene et al., 2005). The identified host genes code for proteins involved in various cellular processes, such as translation, RNA metabolism, protein modifications and intracellular transport or membrane modifications (Jiang et al., 2006; Kushner et al., 2003; Panavas et al., 2005b). Additional genome-wide screens with Drosophila virus C and West Nile virus have also identified over 100 host genes (Cherry et al., 2005; Krishnan et al., 2008). However, these genome-wide screens likely missed the identification of host genes with overlapping functions. Therefore, additional screens are needed, which are less affected by gene redundancy, to identify the total number of host genes affecting virus replication.
One of the major groups of host factors that likely affect RNA virus replication is RNA-binding proteins that play essential roles in many cellular processes, such as transcription, splicing, translation, mRNA turnover, and antiviral mechanisms. These protein-RNA interactions affect the structures and functions of abundant ribonucleoprotein complexes, which contain many different proteins and RNAs. Thus, subverting some RNA-binding proteins could be beneficial for facilitating replication of RNA viruses.
TBSV and other tombusviruses are useful model viruses that infect a wide range of plants. The 4.8 kb TBSV genomic (g)RNA codes for two replication proteins, termed p33 and p92pol, and three proteins involved in encapsidation, cell-to-cell movement and suppression of gene silencing (Nagy and Pogany, 2008; White and Nagy, 2004). Interestingly, yeast cells expressing p33 and p92pol replication proteins can efficiently replicate a short TBSV-derived replicon (rep)RNA, termed defective interfering (DI) RNA (Panavas and Nagy, 2003; Panaviene et al., 2004). The tombusviral RNA plays several functions during infection, including serving as a template for replication and as an assembly platform for the viral replicase complex (Nagy and Pogany, 2008; Panaviene, Panavas, and Nagy, 2005; Pogany, White, and Nagy, 2005). The viral or DI RNA also participate in RNA recombination (Serviene et al., 2005; White and Morris, 1994b; White and Nagy, 2004), which likely plays major role in virus evolution.
To identify host proteins that interact with viral RNA, we took a global approach based on the yeast proteome microarray (protein array) (Zhu et al., 2001; Zhu, Bilgin, and Snyder, 2003). Previous studies using the yeast protein array have identified numerous yeast proteins involved in protein-protein interactions, lipid binding, DNA binding and small substrate binding, thus demonstrating the usefulness of the global analysis approach (Hall et al., 2004; Huang et al., 2004; Smith et al., 2005; Zhu et al., 2001; Zhu, Bilgin, and Snyder, 2003). Also, the yeast protein array identified 58 yeast proteins interacting with the TBSV p33 and an additional 11 yeast proteins interacting with the readthrough portion of p92pol replication protein (Li et al., 2008). In addition, a yeast protein array approach was used to identify many host proteins interacting with a 3′ fragment of the BMV RNA (Zhu et al., 2007). Two of those host proteins were found to affect BMV infection in plants.
In the present work, we expanded the versatility of the yeast protein array by screening for host proteins that bind to viral RNA. Altogether, this work identified 57 host proteins that bound to either TBSV or to BMV RNA or both RNAs. Eleven of the identified host proteins that bound to the TBSV RNA included known helicases, translation factors, and RNA modifying enzymes. Host proteins binding to BMV RNA included tRNA binding proteins, and proteins that are part of large mRNP complexes. More detailed work with a TBSV RNA binding protein, namely eukaryotic translation elongation factor 1A (eEF1A), has revealed that eEF1A is a component of the purified replicase and binds to the 3′ end of the TBSV RNA as well as to TBSV p33 replication co-factor. An eEF1A mutant has provided evidence that eEF1A is important for TBSV replication by stabilizing the p33 replication protein.
RESULTS
Yeast protein array-based identification of host proteins binding to TBSV and BMV RNAs
To probe the yeast protein array for RNA-binding proteins, we used biotinylated RNAs of two different RNA viruses, TBSV and BMV, which can replicate in yeast (Ishikawa et al., 1997; Panavas and Nagy, 2003). Because TBSV and BMV are only distantly related, they provided good substrates to identify virus-specific and nonspecific RNA-binding proteins. For example, the TBSV RNA is uncapped and nonpolyadenylated (White and Nagy, 2004), whereas the BMV RNA is capped and carries a tRNA-like structure at the 3′ end (Noueiry and Ahlquist, 2003). In the experiments involving TBSV, we used either the full-length genomic (g)RNA or a defective interfering (DI) RNA, whereas we chose RNA1 of the three-component BMV for comparison. A nonspecific unlabeled competitor, in the form of poly(dC) - poly(dG), was used in the RNA binding solution to reduce signals derived from nonspecific binding to the chip and to general DNA-binding proteins (Hall et al., 2004). The biotinylated viral RNA probes bound to the purified host proteins on the protein array (Fig. 1) were detected by using Streptavidin-Alexa Fluor647 and a microchip scanner (see Materials and Methods).
Fig. 1.
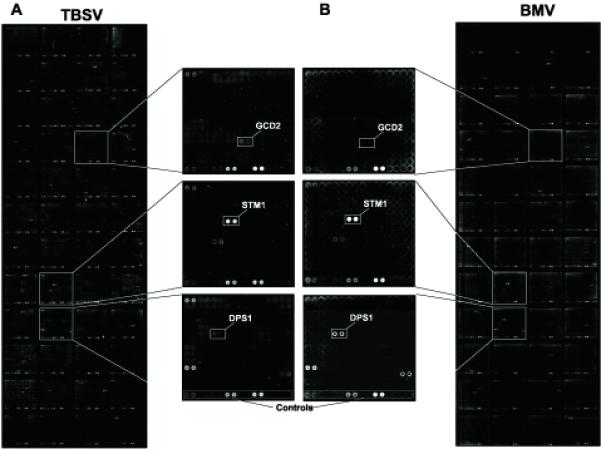
Identification of viral RNA-binding proteins by the yeast proteome microarray. (A) Biotinylated TBSV gRNA (noncapped) or (B) BMV RNA1 (capped) probes were used as indicated. Three subarrays are shown at higher magnification to illustrate the binding of host proteins to TBSV (top), to BMV (bottom) or to both RNAs (middle). The array contains variable amounts of yeast proteins (supplied by Invitrogen) which were used to calculate the binding for each protein (see Table 1).
In total, we have identified 57 RNA-binding proteins among the 4,080 yeast proteins present on the protein array (Table 1). Eleven of these proteins bound to TBSV (+)RNA with at least 3-fold higher efficiency than to BMV (+)RNA, whereas seven proteins bound to the BMV RNA more selectively than to the TBSV RNA (Table 1). The remaining 39 proteins bound to both viral RNAs to similar extents (i.e., showing less than a 3-fold difference in binding to either RNA). All the identified host proteins that bound to the full-length TBSV gRNA also bound to DI-72 (+)RNA (not shown). Fig. 1 represents data obtained with selected host proteins that bound strongly to TBSV, BMV or both RNAs.
Table 1. The name and functions of yeast proteins bound to viral RNAs.
| Gene1 | TBSV2 | BMV2 | Ratio3 | Gene function | RPB | |
|---|---|---|---|---|---|---|
| GCD2 | ++++ | + | 79 | Delta subunit of the translation initiation factor eIF2B | yes 5 | |
| YNL196C | ++++ | + | 16 | Unknown | no | |
| YFR042W | + | − | 7.7 | Protein required for cell viability | no | |
| SEC62 | ++ | + | 7 | SRP-dependent cotranslational protein-membrane targeting | no | |
| YFR038W | ++ | + | 6 | helicase activity | no | |
| DEG1 | ++ | + | 5.8 | Non-essential tRNA:pseudouridine synthase | yes | |
| SRP40 | ++++ | ++ | 4.1 | Nucleolar protein | yes | |
| YPR174C | ++ | + | 4 | Unknown, localizes to the nuclear periphery | no | |
| UTP7 | ++ | + | 3.8 | Component of the small subunit (SSU) processosome | binds to p92 4 | yes |
| DBP2 | ++ | + | 3.3 | Essential ATP-dependent RNA helicase of the DEAD-box protein family | yes | |
| MAP1 | ++ | + | 3.2 | Methionine aminopeptidase | binds to p92 | no |
| HAA1 | ++++ | ++ | 2.3 | Transcriptional activator | no | |
| MSE1 | ++ | + | 2.1 | Glutamate-tRNA ligase activity | yes | |
| DIM1 | + | + | 2.1 | Essential 18S rRNA dimethylase | yes | |
| RPL8A | ++++ | ++++ | 2.1 | Ribosomal protein L4 of the large (60S) ribosomal subunit | binds to p33, p92 | yes |
| TEF2 | + | + | 2 | Translational elongation factor eEF1A | binds p92/BMVRNA | yes |
| RPL26B | ++++ | ++ | 1.8 | Protein component of the large (60S) ribosomal subunit | yes | |
| JJJ1 | ++++ | ++ | 1.8 | Protein that may function as a cochaperone | binds to p33, p92 | no |
| TRM1 | ++++ | ++++ | 1.7 | tRNA methyltransferase | binds to p33, p92 | yes |
| YOR309C | ++++ | ++++ | 1.7 | unknown | binds to p92 | no |
| MDM38 | ++ | ++ | 1.6 | Mitochondrial inner membrane protein | no | |
| YKL023W | ++++ | ++++ | 1.4 | unknown | binds to p92 | no |
| PUS4 | + | + | 1.3 | Pseudouridine synthase | binds BMV RNA | yes |
| CWC25 | ++ | ++ | 1.3 | Component of a complex involved in pre-mRNA splicing | yes | |
| CKA1 | + | + | 1.3 | Alpha subunit of protein kinase CK2 | no | |
| VPS66 | ++ | ++ | 1.3 | Protein-vacuolar targeting | binds to p92 | no |
| RPL4A | ++++ | ++++ | 1.3 | Component of the large (60S) ribosomal subunit | yes | |
| FOX2 | + | + | 1.3 | Multifunctional enzyme of the peroxisomal fatty acid beta-oxidation pathway | binds to p33 | no |
| NOB1 | ++++ | ++++ | 1.2 | Required for cleavage of 20S pre-rRNA to generate the mature 18S rRNA | binds to p92 | yes |
| TOM71 | ++++ | ++++ | 1.2 | Translocase of the outer mitochondrial membrane, | binds to p33, p92 | no |
| RNY1 | ++ | ++ | 1.2 | RNAse; member of the T(2) family of endoribonucleases | yes | |
| YJL218W | + | + | 1.2 | acetyltransferase activity | no | |
| YGR026W | + | + | 1.1 | Unknown, localizes to the cell periphery | no | |
| GLO3 | + | + | 1.1 | GTPase activating protein, involved in ER-Golgi transport; | no | |
| NOP53 | ++++ | ++++ | 1 | Processing of 27S pre-rRNA | yes | |
| APM2 | ++ | ++ | 1 | Vesicle-mediated transport | no | |
| HAS1 | ++ | ++ | 1 | Putative ATP-dependent RNA helicase | yes | |
| MDH3 | + | + | 1 | Cytoplasmic malate dehydrogenase, peroxisomal matrix, | no | |
| YCR016W | ++++ | ++++ | 1 | Unknown | binds to p33, p92 | no |
| STM1 | ++++ | ++++ | 0.9 | Protein that binds quadruplex nucleic acids | binds to p33, p92 | yes |
| YBL055C | ++ | ++ | 0.9 | Unknown | no | |
| SAS10 | ++++ | ++++ | 0.9 | Part of small (ribosomal) subunit (SSU) processosome | binds to p33, p92 | yes |
| BUD21 | ++ | ++ | 0.9 | Component of small ribosomal subunit (SSU) processosome | binds to p33/ repl. | yes |
| SUB1 | ++ | ++ | 0.8 | Transcriptional coactivator | yes | |
| SSF2 | + | ++ | 0.8 | rRNA binding | yes | |
| NUP53 | + | + | 0.8 | Subunit of the nuclear pore complex | yes | |
| LHP1 | ++ | ++++ | 0.7 | RNA binding protein required for maturation of tRNA and snRNA precursors | yes | |
| UBP10 | ++ | ++++ | 0.7 | Ubiquitin-specific protease | binds to p33, p92 | no |
| SNL1 | ++ | ++ | 0.6 | Nuclear pore organization and biogenesis | no | |
| PCS60 | + | ++ | 0.6 | Peroxisomal AMP-binding protein | no | |
| NPL3 | ++ | ++++ | 0.3 | RNA-binding protein that carries poly(A)+ mRNA from the nucleus | binds to p92 | yes |
| YHL013C | + | ++ | 0.3 | Unknown | binds to p33, p92 | no |
| LRP1 | + | ++ | 0.3 | Substrate-specific nuclear cofactor for exosome activity | yes | |
| BFR1 | + | ++ | 0.3 | Component of mRNP complexes associated with polyribosomes | binds to p33 | yes |
| EGD1 | − | + | 0.2 | Subunit of the nascent polypeptide-associated complex/ protein targeting | no | |
| DPS1 | − | ++ | 0.1 | Aspartate-tRNA ligase activity | yes | |
| ARC1 | − | + | 0 | Protein that binds tRNA and methionyl- and glutamyl-tRNA synthetases | yes |
Genes binding specifically to either TBSV gRNA (lines at the top) or the BMV RNA 1 (lines at the bottom) are shown in gray boxes
Binding of the yeast protein to TBSV RNA (second column) or BMV RNA (third column) was calculated based on the following formula: (signal - background signal/ amount of the selected protein on the array) x10,000. The binding values are indicated as follows: ++++, above 1,000; ++, between 300 and 1000; +, between 50–300; −, below 50. The values were derived from repeated experiments.
Relative binding to the viral RNAs was calculated by the dividing the unit value in the TBSV column with that in the BMV column.
Documented effect on TBSV replication (repl) or protein-protein interaction based on previous genome-wide and proteomics screens (Jiang et al., 2006; Li et al., 2008; Panavas et al., 2005b; Serva and Nagy, 2006; Serviene et al., 2006; Serviene et al., 2005; Zhu et al., 2007).
RBP, RNA binding protein, which has documented RNA binding function based on Saccharomyces Genome Database (http://www.yeastgenome.org/).
In vitro binding of host proteins to TBSV repRNA
To confirm the viral RNA-binding ability of the identified yeast proteins (Table 1), we expressed 10 proteins, namely Gcd2p, Utp7p, Deg1p, Dbp2p, Tef2p (eEF1A), Rpl8Ap, Pus4p, Has1p, YFR038W and Sec62p, tagged with maltose binding protein (MBP) in E. coli, followed by affinity-purification. The obtained recombinant proteins were used in standard gel mobility shift experiments with 32P-labeled DI-72(+) RNA probe. All these yeast proteins bound to DI-72(+) RNA probe in vitro (Fig. 2 and data not shown), confirming that the identified yeast proteins can bind to TBSV RNA. Note that Deg1p and Pus4p bound to the (+)repRNA much better in the standard replicase buffer (Nagy and Pogany, 2000) based on similar gel mobility shift assay (not shown).
Fig. 2.
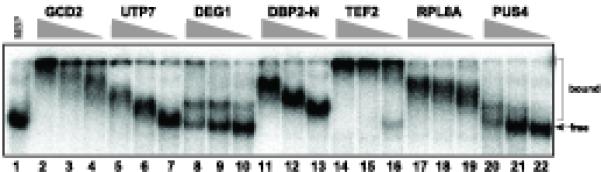
Binding of recombinant host proteins to the TBSV (+)repRNA in vitro. Gel mobility shift assay was performed with the 32P-labeled DI-72 (+)repRNA as the probe. The recombinant host proteins purified from E. coli as MBP fusion proteins were tested in three different dilutions: 2.0, 0.6 and 0.2 μg). The purified recombinant MBP (2.0 μg) was used as a negative control.
Co-purification of selected yeast proteins with TBSV RNA from yeast
To demonstrate that some of the above identified host RNA-binding proteins can also bind to the tombusviral RNA in yeast cells, we selected five yeast proteins for follow-up experiments. These proteins included Deg1p pseudouridine synthase, Dbp2p RNA helicase, Gcd2p translation initiation factor, Tef2p, called translation elongation factor 1A (eEF1A), and Utp7p processome component (Table 1). While the Tef2p form of eEF1A was utilized in these studies, eEF1A is encoded by two genes with identical coding sequences, TEF1 and TEF2 and the protein is henceforth referred to as eEF1A. Four of these proteins were expressed separately from their natural promoter and chromosomal location in yeast. These proteins carried a TAP-tag (Puig et al., 2001) to facilitate co-purification experiments with the expressed TBSV repRNA. We observed that Gcd2p was not expressed in high enough level for these experiments (not shown).
After the two-step TAP-affinity purification, we found that the TBSV (+)repRNA co-purified with three of the yeast RNA-binding proteins (5-to-124-fold enrichment of TBSV repRNA) (Fig. 3A). The highest extent of enrichment for TBSV repRNA was obtained with Deg1p and Dbp2p, whereas Utp7p preparations showed ~5-fold repRNA enrichment (Fig. 3A). However, this difference in repRNA enrichment might partially be due to the different levels of host proteins recovered in these preparations. For example, since Deg1p was present in greater abundance than Dbp2p or Utp7p in the purified preparations (Fig. 3B).
Fig. 3.
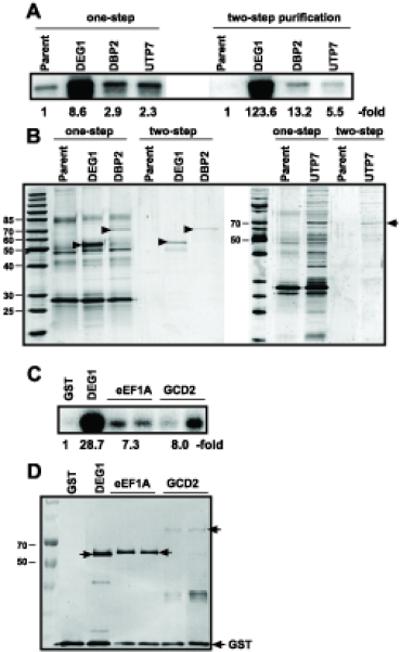
Co-purification of TBSV (+)repRNA with selected yeast proteins from yeast cells. (A) Three TAP-tagged host proteins (expressed from their native promoters and chromosomal locations) were co-expressed with TBSV (+)repRNA in yeast, followed by two-step TAP-affinity purification. Northern blot analysis was used to estimate the amount of co-purified TBSV (+)repRNA present in the purified protein samples. The left side of the panel shows (+)repRNA present after one-step purification, whereas the right side of the panel shows the (+)repRNA present after two-step purification. The ratio of the repRNA present was compared to the control sample obtained from yeast lacking any TAP-tagged protein. (B) The purified proteins were analyzed by SDS-PAGE and silver-staining. The expected full-length proteins are marked with arrowheads. (C) Three GST-tagged host proteins (expressed from GAL1 promoter from expression plasmids) were co-expressed with TBSV (+)repRNA in yeast, followed by one-step GST-affinity purification. Northern blot analysis was used to estimate the amount of co-purified TBSV (+)repRNA present in the purified protein samples. (D) The purified proteins were analyzed by Western blotting using an anti-His antibody. The expected-size proteins are marked with arrowheads.
To test if eEF1A and Gcd2p can bind to the TBSV repRNA in yeast, we expressed them as GST-tagged fusion proteins from an expression plasmid. This approach led to detectable expression of eEF1A and Gcd2p as well as Deg1p control protein in yeast (Fig. 3D). Northern blot analysis of the co-purified RNAs revealed enrichment for TBSV (+)repRNA in the GST affinity-purified eEF1A, Gcd2p and Deg1p preparations when compared to the GST control preparation (Fig. 3C). Altogether, the co-purification of the selected host proteins and the TBSV repRNA suggests that these yeast proteins can bind to the TBSV repRNA in yeast, verifying the usefulness of our global analysis approach.
Effect of over-expression of selected host proteins on TBSV repRNA replication in yeast
To test if the above identified viral RNA binding proteins of yeast could affect tombusvirus RNA replication, we over-expressed 45 host proteins individually in yeast cells from the galactose inducible GAL1 promoter (Gelperin et al., 2005). After 20 h expression of the particular host protein, we launched TBSV repRNA replication from CUP1 promoter as described in the Materials and Methods. Yeast cells were harvested 24 h later, at which point the amount of viral repRNA was assessed by Northern blotting and compared with yeast ribosomal RNA for normalization of TBSV repRNA accumulation. These experiments revealed that over-expression of four host proteins, Dbp2p, Gcd2p, Mdm38p and Stm1p, increased repRNA accumulation by, 35, 59, 55 and 43%, respectively, when compared to yeast carrying the empty expression vector (Fig. 4A). In contrast, over-expression of 14 viral RNA-binding proteins highlighted in gray reduced TBSV repRNA accumulation by 2.5-to-10-fold in yeast (Fig. 4A-B). Over-expression of the remaining 28 host proteins inhibited TBSV repRNA accumulation by two-fold or less (Fig. 4A). This level of inhibition of TBSV repRNA accumulation might not be significant, because over-expression of a pseudogene (APT2), which has no enzymatic activity when expressed (Alfonzo et al., 1999), also led to reduced TBSV repRNA accumulation (63.7 ± 15.9%), suggesting that two-fold or less reduction might be due to the reduced ability of yeast cells to support repRNA accumulation under the protein over-expression condition. Together, the above experiments indicated that over-expression of ~40% of the 45 RNA-binding host proteins could affect TBSV repRNA accumulation in yeast.
Fig. 4.
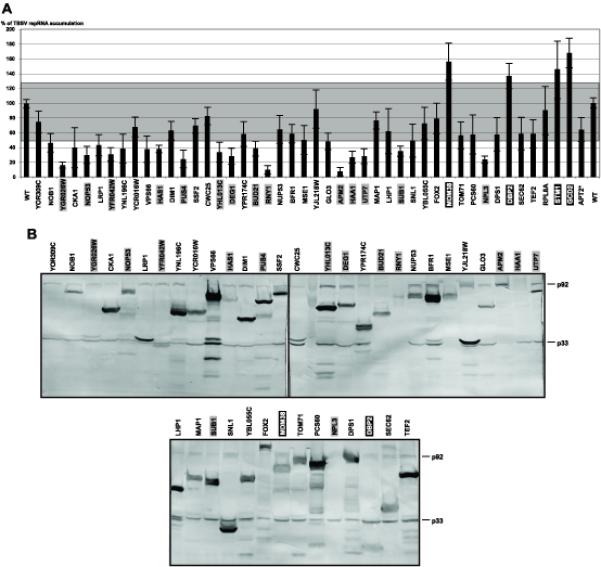
The effect of over-expression of selected yeast proteins on TBSV repRNA accumulation in yeast. (A) The given host protein was over-expressed from the GAL1 promoter prior to launching TBSV repRNA replication from the CUP1 promoter. The accumulation level of the plus-stranded TBSV repRNA was estimated by using Northern blotting with a TBSV-specific probe. The data were normalized based on 18S ribosomal RNA levels. The average effect of each host protein on repRNA accumulation is shown based on 6–8 separate samples. Host proteins in black and gray boxes stimulated and inhibited, respectively, TBSV repRNA accumulation more significantly than over-expression of the APT2 pseudogene (marked with asterisk). The average accumulation of TBSV repRNA in yeast carrying an empty expression vector, shown as wt, was taken as 100% (based on two sets of experiments with total of 12 samples). (B) Western blotting analysis of over-expressed host proteins and p33 and p92 replication proteins in yeast using anti-His antibody. Note that each host proteins carry a 19KDa C-terminal tag.
eEF1A is a component of the purified tombusvirus replicase
To further test the functional relevance of the identified yeast proteins interacting with the TBSV repRNA, we selected eEF1A, which functions as a translation elongation factor and has been shown to be part of replicase complexes of several RNA viruses (Blackwell and Brinton, 1997; Blumenthal, Young, and Brown, 1976; Brinton, 2001; Nishikiori et al., 2006) and its ability to bind to the tombusvirus p92 RdRp protein (Li et al., 2008). In addition, we performed preliminary pull-down experiments with biotin-labeled DI-72 (+)repRNA that was immobilized onto the streptavidin coated magnetic beads, followed by binding assay using a yeast cytosolic extract (Pogany and Nagy, 2008), which have led to the identification of eEF1A by mass-spectrometry (not shown). This finding suggested that eEF1A is likely one of the major TBSV RNA binding proteins in the yeast cytosol. To test if eEF1A is a component of the tombusvirus replicase complex, we used a eEF1A-specific antibody in Western blotting of a highly purified replicase preparation that was obtained via two-step Nickel/FLAG-affinity purification of the solubilized tombusviral p33HF and p92HF proteins tagged with 6xHis- and FLAG affinity tags from enriched membranes derived from yeast (Li et al., 2008). eEF1A was easily detectable in the purified replicase complex, regardless of whether or not crosslinking was applied (Fig. 5, lanes 2 and 4). Similar preparations obtained via two-step affinity purification from yeast expressing only single 6xHis-tagged p33H and p92H proteins contained only small amount of eEF1A, suggesting that the presence of eEF1A in the highly purified tombusvirus replicase preparation is not due to contamination of the affinity-resin with the abundant eEF1A (Fig. 5, lanes 1 and 3). Since eEF1A binds to the TBSV repRNA, it is possible that these proteins are part of the solubilized/purified replicase complex via binding to the TBSV repRNA. This possibility was tested by treating the solubilized/purified replicase complex with an RNase during the first affinity purification step to destroy the RNA components, followed by FLAG-affinity purification. Interestingly, the amount of co-purified eEF1A was unchanged in the highly purified replicase (Fig. 5, lane 6), suggesting that eEF1A not only binds to the repRNA, but to a protein component of the viral replicase as well.
Fig. 5.
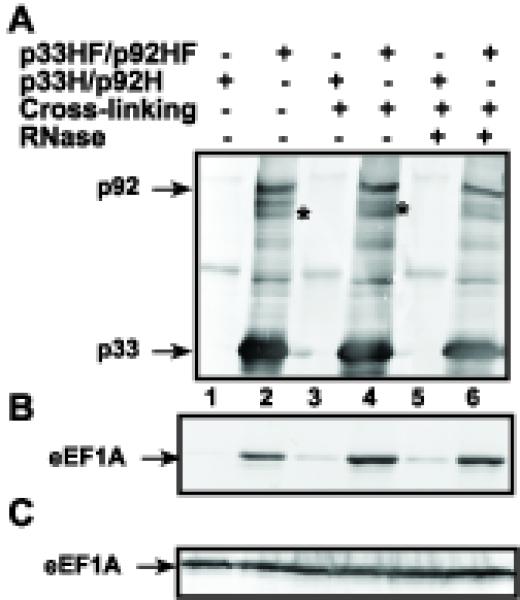
Co-purification of eEF1A with the tombusvirus replicase from yeast. (A) The membrane-enriched fraction of yeast expressing the FLAG/6xHis-tagged p33HF/p92HF (+) or the 6xHis-tagged p33H/p92H (−, lanes 1, 3 and 5) was solubilized and was sequentially purified on Ni-affinity and FLAG-affinity columns. Western-blotting with anti-6xHis antibody detected the presence of p33 and p92pol in the purified replicase complex that is active in an in vitro replication assay (not shown) as demonstrated earlier (Serva and Nagy, 2006). The asterisks indicate p33 homodimers that are partly resistant to denaturing conditions. Yeast cells were subjected to formaldehyde cross-linking (lanes 3–6) prior to lysis, purification and digestion with RNase A (lanes 5–6) (B) Western blotting of the same samples as in panel A with anti-eEF1A antibody. Note that the native eEF1A was expressed from its original promoter and original location on the chromosome. (C) The original amount of eEF1A in solubilized membrane preparations from yeast was detected with anti-eEF1A antibody in total protein samples.
To test if eEF1A can indeed interact with p33 replication cofactor, we used the split-ubiquitin yeast two-hybrid assay, which, unlike the original yeast two-hybrid system, allows the analysis of protein interactions on the cytosolic surfaces of membranes where p33 protein is localized (McCartney et al., 2005; Panavas et al., 2005a). Briefly, the split-ubiquitin assay is based on the ability of N-terminal (NubG) and C-terminal (Cub) halves of ubiquitin to reconstitute a functional protein (Fetchko, Auerbach, and Stagljar, 2003; Fetchko and Stagljar, 2004). When NubG and Cub, which are fused separately to interacting proteins, are brought to close proximity and reconstitute a functional ubiquitin protein, then cleavage by endogenous ubiquitin specific proteases leads to the release of the transcription factor, resulting in growth on selective media. The split ubiquitin assay revealed that eEF1A interacted with the membrane-bound p33 when used as N-terminal fusion with NubG (Fig. 6) indicating that eEF1A interacts with both the TBSV repRNA and the membrane-bound p33 in yeast.
Fig. 6.
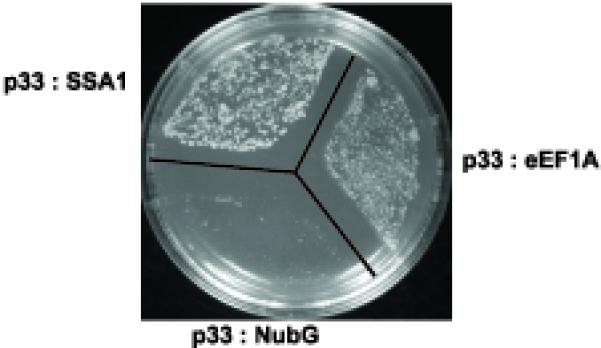
Interaction between eEF1A and p33 in the split-ubiquitin two-hybrid assay. The full-length TEF2 sequence was fused to NubG as N-terminal fusion, and the p33 sequence was fused to Cub. The heat shock protein 70 (SSA1) was used as a positive control because it is known to interact with p33 (Serva and Nagy, 2006).
eEF1A binds to the silencer sequence in the 3′ UTR of the TBSV repRNA
Human and plant eEF1A is known to bind to the 3′ UTR of several RNA viruses in vitro (Blackwell and Brinton, 1997; Dreher, 1999). To test if the 3′ UTR of the TBSV RNA is also bound to eEF1A, we made a 32P-labeled RNA probe containing the conserved three 3′ terminal stem-loops in the TBSV RNA, termed SL1, SL2 and SL3 (Na and White, 2006). Gel mobility shift assay with purified recombinant eEF1A revealed binding to the WT SL1/2/3 sequence (Fig. 7A, lanes 2–4). A G-to-C modification at the 3′ terminus in construct SL1/2/3 that weakens SL1 (termed mutant gPR-C) and the middle-range interaction between gPR and SL3, which is critical for the assembly of the replicase (Panaviene, Panavas, and Nagy, 2005), did not change the binding to eEF1A in vitro (Fig. 7A, lanes 6–8). On the contrary, deletion of 5 nts within a known cis-acting element, termed replication silencer element (RSE) (Pogany et al., 2003), inhibited binding to eEF1A (Fig. 7A, lanes 9–12). This deletion is within an 8 nt long internal loop and is not expected to change the structure of SL3 dramatically, albeit it destroys the middle-range interaction between SL3 and gPR, which includes SL1 (see the WT construct in Fig. 7A) (Pogany et al., 2003). Template competition experiments with unlabeled competitors confirmed the above results, since construct SL1/2/3ΔGGGCU(+) lacking 5nt of the RSE element was a poor competitor when compared with the wt and other RNAs carrying mutations within the gPR sequence (Fig. 7B). Altogether, these data indicate that eEF1A binds to RSE, an important cis-acting element involved in the assembly of the tombusvirus replicase (Panaviene, Panavas, and Nagy, 2005).
Fig. 7.
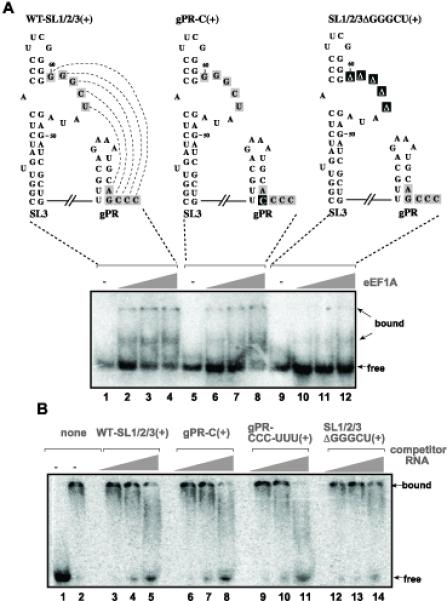
Binding of eEF1A to the replication silencer sequence of the TBSV (+)repRNA in vitro. (A) Gel mobility shift assay was performed with the 32P-labeled WT-SL1/SL2/SL3(+) RNA probe. The SL1/SL2/SL3 sequence represents the 86 nt 3′ terminal, highly structured sequence of the TBSV (+)RNA with two known cis-acting elements: the replication silencer element required for the assembly of the viral replicase and gPR that is an essential promoter for initiation of minus-strand synthesis and it is also required for replicase assembly. The mutated/deleted bases are indicated with black boxes. The recombinant eEF1A purified from yeast as GST-fusion protein was tested in three different dilutions: 2.0, 0.8 and 0.4 μg. (B) Template competition assay to test the binding of eEF1A to the viral template. Gel mobility shift assay was performed with the 32P-labeled WT-SL1/SL2/SL3(+) RNA probe and purified eEF1A (as in panel A), whereas the unlabeled competitor RNA was used in 5x, 20x and 100x excess over the labeled one as shown. Template gPR-CCC-UUU(+) has the 3′ terminal three Cs changed to three Us (not shown).
A mutation affecting guanine exchange factor (GEF) requirement of eEF1A inhibits TBSV repRNA accumulation in yeast
To obtain additional functional relevance for eEF1A in tombusvirus replication, we utilized a strain where the only form of eEF1A contains a 6xHis tag and is expressed from a copper repressible promoter (TKY616) (Anand et al., 2006). Indeed, the level of eEF1A decreased by ~40% in TKY616 strain 12 h after addition of copper to the growth media (Fig. 8C, lanes 4–6 versus 1–3). Northern blot analysis revealed that the accumulation of TBSV repRNA decreased to 39% of that present in the WT strain (Fig. 8A). The expression level of p33 replication protein also decreased (Fig. 8B), suggesting that either the translation or the stability of p33 was reduced in the presence of lower level of eEF1A.
Fig. 8.
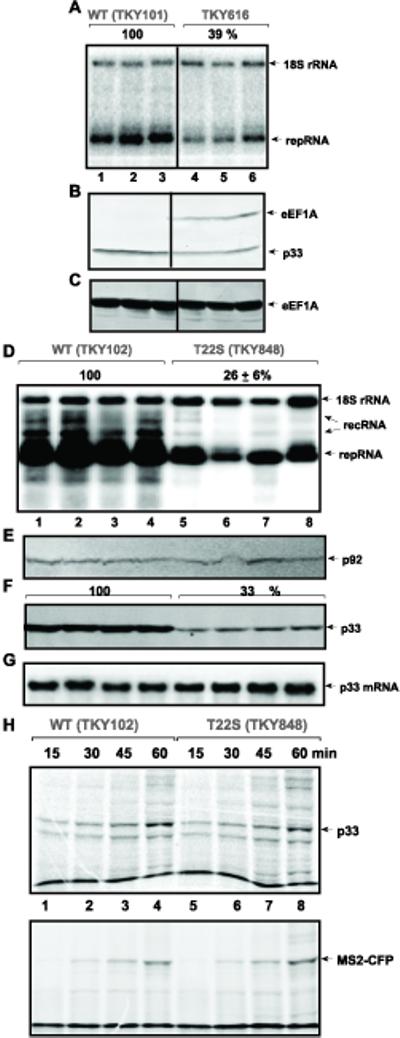
The effect of eEF1A on TBSV repRNA accumulation and on the replication proteins. (A) The expression of eEF1A protein was down-regulated from a copper-repressible promoter in TKY616. Replication of the TBSV repRNA was measured by Northern blotting 12 h after initiation of TBSV replication and suppression of eEF1A expression. (B) Accumulation of p33 and (C) eEF1A was estimated by Western blotting using anti-His and anti-eEF1A antibodies, respectively. (D) Reduced accumulation of TBSV repRNA in TKY848 yeast expressing eEF1A(T22S) as the only form of eEF1A 24 h after induction of TBSV repRNA replication. The TBSV repRNA and 18S ribosomal RNA levels were estimated by Northern blotting. RecRNA shows novel recombinant RNAs derived from TBSV repRNA and characterized earlier (Cheng, Serviene, and Nagy, 2006). (E) Accumulation of p92pol and (F) p33 was estimated by Western blotting using anti-His antibody in TKY848 and TKY102 yeast strains. (G) The production of p33 mRNA from an expression plasmid was estimated by Northern blotting using p33 mRNA-specific probe. (H) Translation of p33 in extracts prepared from TKY848 and TKY102 yeast strains. The in vitro translation assay of p33 from an exogenously added mRNA in the presence of 35S methionine was performed with extracts containing comparable amounts of yeast proteins. Samples were taken at various time points as shown. Bottom panel: Translation of MS2-CFP from an exogenously added mRNA was used as a control. The band at the bottom of both gels represents the dye front.
To further analyze the role of eEF1A in TBSV replication, we assayed a bank of eEF1A mutants (Carr-Schmid et al., 1999; Dinman and Kinzy, 1997; Gross and Kinzy, 2005; Gross and Kinzy, 2007; Ozturk et al., 2006). The 22 eEF1A mutants belong to four groups based on their mapped functions as specified in Table 2. The mutations decrease translation fidelity, have altered requirement for the guanine nucleotide exchange factor eEF1Bα encoded by the TEF5 gene, or show reduced binding to actin (Table 2). These strains lack the chromosomal TEF1 and TEF2 genes and eEF1A is expressed from a centromeric plasmid under the control of the native TEF promoter.
Table 2. The effect of eEF1A mutations on TBSV repRNA accumulation.
| strain | mutation | Effect on eEf1A function | repRNA accumulation5 |
p33 level6 |
|---|---|---|---|---|
| TKY102 1 | WT | One copy of TEF (tef1Δ) | 100.0 ± 21.8 | 100 |
| TKY111 1 | E317K | Translational misreading: Increased +1 ribosomal frameshifting | 59.4 ± 12.5 | 93 |
| TKY112 1 | E40K | Translational misreading: Increased +1 ribosomal frameshifting | 60.1 ± 11.0 | 82 |
| TKY113 1 | E122K | Translational misreading: Increased −1 ribosomal frameshifting | 53.3 ± 11.2 | 78 |
| TKY114 1 | T142I | Translational misreading: Increased +1 ribosomal frameshifting | 69.4 ± 23.0 | 78 |
| TKY115 1 | E295K | Translational misreading: Increased −1 ribosomal frameshifting | 117.6 + 26.8 | 68 |
| TKY116 1 | E122Q | Translational misreading: Increased −1 ribosomal frameshifting | 59.6 ± 15.8 | 79 |
| TKY117 1 | D130N | Translational misreading: Increased −1 ribosomal frameshifting | 64.0 ± 16.3 | 81 |
| TKY278 2 | D156N | Decreased translation fidelity/ GTP-binding site mutant | 64.5 ± 16.0 | 72 |
| TKY280 2 | N153T | Decreased translation fidelity/ GTP-binding site mutant | 117.9 + 74.0 | 89 |
| TKY282 2 | N153T D156E |
Decreased translation fidelity/ GTP-binding site mutant | 126.6 + 38.5 | 85 |
| TKY789 3 | R164K | GEF independent growth, mutation in nucleotide binding domain | 106.0 + 25.0 | 85 |
| TKY791 3 | A117V T172A |
GEF independent growth, mutation in nucleotide binding domain | 120.2 + 38.8 | 73 |
| TKY846 3 | D156E | GEF independent growth, mutation in nucleotide binding domain | 90.1 ± 34.4 | 91 |
| TKY847 3 | D156N | GEF independent growth, mutation in nucleotide binding domain | 71.9 ± 5.2 | 86 |
| TKY848 3 | T22S | Increased nonsense suppression/GEF independent growth | 26.6 ± 6.3 | 33 |
| TKY849 3 | A112T | Increased nonsense suppression/GEF independent growth | 85.5 ± 18.5 | 85 |
| TKY850 3 | E122K | GEF independent growth, mutation in nucleotide binding domain | 98.2 ± 13.3 | 82 |
| TKY851 3 | A117T | GEF independent growth, mutation in nucleotide binding domain | 103.9 ± 15.1 | 84 |
| TKY852 3 | A117V | GEF independent growth, mutation in nucleotide binding domain | 142.7 ± 22.7 | 84 |
| TKY896 4 | WT | One copy of TEF | 100.0 ± 18.0 | 100 |
| TKY898 4 | K333E | Reduced binding to actin | 120.0 ± 31.0 | 113 |
| TKY901 4 | F308L | Reduced binding to actin, reduced translation initiation | 104.0 ± 20.0 | 111 |
| TKY903 4 | S405P | Reduced binding to actin, reduced translation initiation | 104.0 ± 29.0 | 147 |
Out of 22 eEF1A mutant strains tested for the accumulation of TBSV repRNA, we found that only TKY848 (T22S) reduced TBSV RNA level significantly (down to 26%, Fig. 8D, lanes 5–8) and Table 2. Western blot analysis of replication protein levels in the TKY848 (T22S) strain revealed that the p92pol was expressed at a level comparable to the WT eEF1A strain (Fig. 8E), while p33 level was reduced to 33% (Fig. 8F). These data suggested that eEF1A (T22S) selectively reduced p33 levels, but not that of p92pol. Northern blotting of p33 mRNA transcribed from an expression plasmid revealed comparable levels in yeast strains expressing either eEF1A (T22S) or WT eEF1A (Fig. 8G), excluding the possibility that RNA transcription is the reason for reduced level of p33 in eEF1A(T22S) yeast.
Because eEF1A is a translation factor, it is possible that translation of p33 from the p33 mRNA is selectively inhibited by T22S mutation. To test this possibility, we have adapted a yeast cell-free translation assay that can be programmed with externally added mRNA transcripts (Materials and Methods). The in vitro translation assay prepared from yeast strains expressing either WT eEF1A or eEF1A(T22S) supported the production of comparable levels of p33 and the control MS2-CFP (Fig. 8H) from the added mRNAs, suggesting that the T22S mutation in eEF1A did not affect the translation of p33.
eEF1A(T22S) reduces the half-life of p33 replication co-factor in yeast
Since production of p33 is not affected by eEF1A(T22S), we also tested the half-life of p33 in yeast expressing either eEF1A(T22S) or WT eEF1A after the shut down of protein synthesis by cyclohexamide. The steady-state level of p33 was measured with Western blotting in samples obtained at various time points from yeast strains expressing either eEF1A(T22S) or WT eEF1A. These experiments have demonstrated that the half-life of p33 was reduced to ~130 min in yeast expressing eEF1A(T22S) (Fig. 9, lanes 1–6) from over 330 min in yeast expressing WT eEF1A (Fig. 9, lanes 7–12). Thus, eEF1A is likely involved in stabilization of p33 replication cofactor in yeast.
Fig. 9.
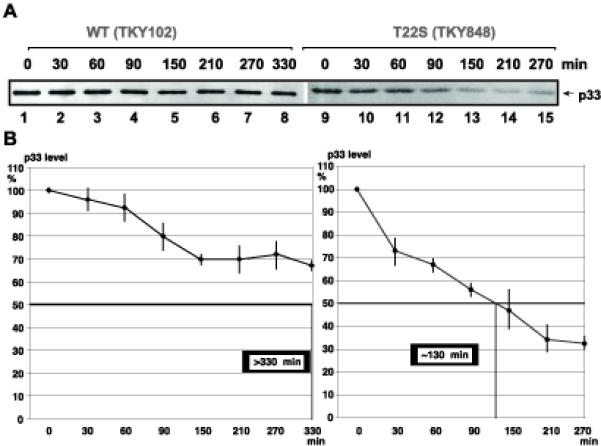
Reduced half-life of p33 in TKY848 yeast strain. (A) Translation of mRNAs was stopped in yeast with cyclohexamide, followed by Western blot analysis of p33 levels with anti-His antibody at various time points. Strain TKY102 expressing WT eEF1A was used as a control. (B) Calculation of the half-life of p33 in TKY848 and TKY102. The experiments were repeated and the standard deviation is shown.
DISCUSSION
RNA-binding proteins of the host likely play multiple roles during the replication of RNA viruses. They might affect (i) translation of the viral RNA, (ii) RNA template selection by viral replication proteins, (iii) recruitment of the viral RNA for replication, (iv) RNA synthesis, and/or (v) RNA stability (Ahlquist et al., 2003; Brinton, 2001; Cristea et al., 2006; Nagy, 2008; Nagy and Pogany, 2006; Shi and Lai, 2005). A proteome-wide approach using the yeast protein array allowed us to test ~70% of all yeast proteins for their abilities to bind to viral RNAs (TBSV or BMV). Among the 57 host proteins that bound significantly to viral RNAs (either TBSV or BMV), thirty are known RNA-binding proteins, whereas the other 27 had not previously been shown to bind to RNA (Table 1). We found eleven host proteins that bound to TBSV RNA more selectively than to the BMV RNA, including two known helicases (encoded by DBP2 and YFR038W), a translation initiation factor (GCD2), and two RNA modifying proteins, such as a pseudourine synthase (DEG1) and one of the components of the small subunit processome (UTP7). The remaining six proteins include an RNA-binding protein (SRP40), a membrane-targeting protein (SEC62), an aminopeptidase (MAP1) and three proteins of unknown function (YNL196C, YFR042W, YPR174C).
Among the seven identified host proteins that bound to BMV RNA more efficiently than to the TBSV RNA, there are two proteins known to bind to tRNAs (DPS1 and ARC1) (Table 1). The 3′ ends of the BMV RNAs form tRNA-like structures, and the BMV RNA has been shown to bind to tRNA-specific proteins (Dreher, 1999). Moreover, the 3′ end of the BMV RNA can be aminoacylated (Dreher, 1999), confirming the functional interaction between tRNA-modifying enzymes and the BMV RNA. Additional genes coding for known RNA binding proteins, which also bound to BMV RNA, include NPL3, LRP1 and BFR1 (Table 2). The RNA binding activities of the remaining two proteins coded by EGD1 and YHL013C genes have not been documented before.
The remaining 39 host proteins identified with both TBSV and BMV RNA probes are involved in a variety of cellular processes, such as translation initiation or elongation, transcription activation, ribosomal RNA processing/binding, mRNA transport, protein membrane targeting or are predicted to have various biochemical activities, such as helicase, tRNA ligase, tRNA methyltransferase, rRNA dimethylase, ribonuclease, co-chaperone, and protein kinase (Table 1). One of these proteins, translation elongation factor eEF1A, is the homolog of the plant eEF1A, which has been shown to bind to the BMV RNA (Bastin and Hall, 1976). In addition, the identified pseudouridine synthase Pus4p (Table 1) could be involved in pseudouridynylation--based modification of BMV RNA, which has been shown to occur in vivo (Baumstark and Ahlquist, 2001). Interestingly, Pus4p has also been identified in a similar screen with a unique yeast protein array using a 3′ end cis-acting element from BMV RNA (Zhu et al., 2007).
To validate the protein array approach for identification of RNA binding proteins, we demonstrated that several recombinant yeast proteins bound to TBSV RNA in a gel mobility shift assay (Fig. 2 and not shown) and via co-purification from yeast cells (Fig. 3). The data obtained support the idea that a number of RNA-binding proteins from yeast likely interact with the TBSV (+)RNA. We found that many of the identified host RNA binding proteins affected TBSV repRNA replication, confirming their relevance. For example, Dbp2p, Gcd2p, Stm1p and Mdm38 enhanced TBSV repRNA accumulation, while 14 proteins out of 45 yeast proteins tested decreased TBSV repRNA accumulation by 2.5–10-fold in a protein over-expression assay (Fig. 4A-B). Based on these data, it is likely that 18 of the tested host RNA binding proteins (Figs. 4-5) interact with the TBSV repRNA in vivo, whereas the functional relevance of the remaining 27 host proteins require further analysis.
Two yeast proteins among the stimulators of replication when over-expressed are involved in translation or degradation of mRNAs. Gcd2p is the delta subunit of the translation initiation factor 2B (eIF2B), the guanine nucleotide exchange factor for eIF2. Dbp2p is a homolog of the human p68 (Iggo et al., 1991) and the Arabidopsis AtDrh1p (Okanami, Meshi, and Iwabuchi, 1998), known RNA helicases that could be involved in nonsense-mediated mRNA decay, rRNA processing and replication of Hepatitis C virus (Bond et al., 2001; Goh et al., 2004). We propose that Gcd2p and Dbp2p could facilitate the recruitment of the viral RNA into replication (via inhibiting translation of the genomic RNA) or promoting the assembly of the viral replicase complex prior to replication. Also, Dbp2p might enhance replication via interacting and inhibiting Nog1p, a putative GTPase, whose down-regulation led to increased TBSV repRNA accumulation in yeast (Jiang et al., 2006). It is less straightforward to propose a model for the activity of Mdm38p, which is a mitochondrial membrane protein with no known RNA-binding activity (Table 2). Mdm38p might facilitate TBSV RNA accumulation via a novel function.
The identified 14 yeast proteins inhibiting tombusvirus accumulation when over-expressed might interfere with the recruitment of the viral RNA into replication, or the assembly of the viral replicase complex prior to replication. Alternatively, they could also increase the degradation of the viral RNA (e.g., Rny1p ribonuclease, and Utp7p, which is part of the processosome).
eEF1A is a newly identified component of the tombusvirus replicase complex
Eukaryotic viral RNA replicases contain viral- and host-coded components. Previous proteomic approaches with highly purified tombusvirus replicase or using the yeast protein array have led to the identification of four host proteins that are integral components of the tombusvirus replicase complex: the heat shock protein 70 chaperones (Ssa1/2p in yeast), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, encoded by TDH2 and TDH3 in yeast), pyruvate decarboxylase (Pdc1p) and Cdc34p ubiquitin ligase (Li et al., 2008; Serva and Nagy, 2006; Wang and Nagy, 2008). The current work establishes that eEF1A is also an integral part of the replicase complex. Replicase purification experiments with or without protein cross-linking confirmed the presence of eEF1A (Fig. 5). In addition, we have shown that eEF1A can bind to the TBSV repRNA in vitro as well as the TBSV repRNA can be co-purified with eEF1A from yeast (Fig. 3). Moreover, eEF1A has been shown to interact with the p33 replication co-factor based on the yeast split-ubiquitin two-hybrid assay (Fig. 6). Interestingly, eEF1A has also been shown to bind to the non-overlapping portion of the p92pol RdRp protein in vitro based on yeast protein array experiments (Li et al., 2008). Thus, the accumulating evidence shows that eEF1A interacts with components of the tombusvirus replicase, including repRNA, p33 and p92pol replication proteins. It is also interesting that eEF1A has been shown to interact with the yeast Tdh2p (GAPDH) (Gavin et al., 2006), which is also a component of the tombusvirus replicase. Overall, the multiple interactions of eEF1A with various components of the tombusvirus replicase could be important for eEF1A to regulate the functions of the viral replicase complex.
eEF1A is a highly abundant protein with the canonical role of delivering aminoacyl-tRNA to the elongating ribosome in a GTP dependent manner. Many additional functions have been ascribed to eEF1A including quality control of newly produced proteins, ubiquitin-dependent protein degradation, and organization of the actin cytoskeleton (Chuang et al., 2005; Gross and Kinzy, 2005). The first evidence that a translation elongation factor plays a role in (+)RNA virus replication was obtained with bacteriophage Qbeta (Blumenthal, Young, and Brown, 1976). Subsequently, eEF1A was found to bind to many viral RNAs (Dreher, 1999). The list includes the 3′UTR of (+)RNA of West Nile virus (WNV) based on nitrocellulose filter binding assays and RNase footprinting (Blackwell and Brinton, 1997; Brinton, 2001). This is supported by functional data, since mutations in the eEF1A binding site of WNV RNA decreased minus-strand synthesis and eEF1A is co-localized with the WNV replicase in the infected cells (Davis et al., 2007). Similarly, eEF1A was shown to bind to the 3′ end of Turnip yellow mosaic virus (+)RNA leading to enhanced translation but repressed minus-strand synthesis (Dreher, 1999; Dreher, Uhlenbeck, and Browning, 1999; Matsuda, Yoshinari, and Dreher, 2004). In addition, eEF1A was reported to bind to Dengue virus, Tobacco mosaic virus and Turnip mosaic virus (+)RNA (De Nova-Ocampo, Villegas-Sepulveda, and del Angel, 2002; Nishikiori et al., 2006; Thivierge et al., 2008; Zeenko et al., 2002). eEF1A has also been shown to interact with various viral replication proteins or the replicase as shown for the NS5A replication protein of BVDV (Johnson et al., 2001), NS4A of HCV (Kou et al., 2006), the TMV replicase (Yamaji et al., 2006), and Gag polyprotein of HIV-1 (Cimarelli and Luban, 1999). It is also part of the replicase complex of vesicular stomatitis virus, a negative-stranded RNA virus (Qanungo et al., 2004). Overall, eEF1A likely plays a role in the replication of several RNA viruses via its interactions with viral RNAs and viral replication proteins. The high abundance of eEF1A in cells might facilitate its recruitment into virus replication.
eEF1A affects TBSV repRNA accumulation by stabilizing p33 replication cofactor in yeast
Since eEF1A is an essential G protein affecting translation elongation, it is very difficult to obtain evidence for its direct involvement in virus replication in living cell. Accordingly, down-regulation of eEF1A has led to not only decreased TBSV repRNA accumulation, but also reduced p33 levels (Fig. 8A-C). However, using functional eEF1A mutants defective in various functions and domains could potentially lead to identification of functions provided by eEF1A during TBSV repRNA replication in yeast. In this work, we have used four different groups of eEF1A mutants (Table 2). The most interesting mutant was eEF1A(T22S), which has mutation within the P-loop leading to decreased binding of eEF1A to GDP (Ozturk et al., 2006). This mutation renders eEF1A functionally independent of its guanine nucleotide exchange factor eEF1Bα. We found that eEF1A(T22S) supported TBSV repRNA replication only at ~26% level when compared with the WT eEF1A (Fig. 8D). Interestingly, yeast expressing eEF1A (T22S) displayed normal levels of p92pol, but reduced levels of p33 replication protein (Fig. 8E-F). Since eEF1A (T22S) is known to reduce total translation level by 35% and decrease translational fidelity (Ozturk et al., 2006), we tested if eEF1A (T22S) affected p33 translation using an in vitro assay (Fig. 8H). However, the translation level of p33 was comparable in extracts prepared from yeast expressing either WT eEF1A or eEF1A(T22S). Thus, it is unlikely that eEF1A (T22S) would affect p33 level through its effect on general translation. More importantly, the half-life of p33 was reduced by more than 2-fold in yeast expressing eEF1A(T22S), suggesting that eEF1A is involved in stabilization of p33 in yeast.
The eEF1A mutants deficient in interaction with actin had no major effect on TBSV repRNA accumulation, suggesting that the ability of eEF1A to interact with actin filaments/patches might not be critical for TBSV replication in yeast. Several mutations in the GTP-binding domain similar or that decreased translational fidelity had 30–50% inhibition of TBSV repRNA accumulation (Table 2). However, other mutants with similar characteristics have not inhibited TBSV repRNA accumulation. Altogether, the use of eEF1A mutants revealed that eEF1A is involved in stabilization of p33 replication protein in yeast. Future work will dissect the regions of eEF1A and the interaction with nucleotide to identify domains or functions required for TBSV replication.
Usefulness and limitation of the protein array approach in identification of host proteins binding to viral RNAs
One of the surprising observations is that most of the yeast RNA-binding proteins identified in this work were not identified in previous screens with the YKO and yTHC libraries (Jiang et al., 2006; Kushner et al., 2003; Panavas et al., 2005b; Serviene et al., 2006; Serviene et al., 2005). The only exception is BUD21(UTP16), whose deletion increased replication of TBSV repRNA (Panavas et al., 2005b). It is possible that many genes involved in TBSV repRNA replication and recombination are functionally redundant. Indeed, the host proteins that were identified in a highly purified functional tombusvirus replicase complex (such as Ssa1/2p and Tdh2/3p), are coded by two or more genes in the yeast genome (Serva and Nagy, 2006) and eEF1A (this work) are coded by two or more genes in the yeast genome. This functional redundancy in host genes justifies the need for multiple genome- or proteome-wide approaches in the identification of host genes affecting TBSV replication and recombination.
The above protein array-based approach led to the identification of two known BMV RNA binding host proteins (i.e., eEF1A and Pus4p) (Bastin and Hall, 1976; Zhu et al., 2007). However, this approach failed to identify Ded1p and Lsm1p, two known RNA-binding proteins that affected BMV replication in yeast (Diez et al., 2000; Noueiry and Ahlquist, 2003). Ded1p selectively inhibits the translation of 2a protein from RNA2, whereas we used RNA1 in our experiments, which might bind inefficiently to Ded1p. It is also possible that some proteins are not functional in RNA-binding when fixed on the chip. Alternatively, the interaction between the host protein and the viral RNA could be facilitated by additional factors, such as the 1a replication protein of BMV, which were not present in our in vitro analysis. The Lsm1p-like proteins are known to form multimeric, doughnut-shaped complexes (Noueiry and Ahlquist, 2003), and this screen would likely miss any protein that acts in a heteromeric complex. In addition, the protein array contains only ~70% of the known/predicted number of proteins expressed in yeast. The above examples highlight the possibility that our current work is likely an under estimation of the number of host proteins that bind to viral RNAs. Regardless of these limitations, the protein array approach could provide a rapid tool for identification or confirmation of RNA binding host proteins.
MATERIALS AND METHODS
Yeast strains and expression plasmids
Saccharomyces cerevisiae strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) was obtained from Open Biosystems (Huntsville, AL, USA). Plasmid-borne TEF1/2 TKY strains (MATα ura3–52 leu2–3, 112 trp1-Δ1 lys2–20 met2–1 his4–713 tef1::LEU2 tef2Δ pTEF2 URA3) were published before (Carr-Schmid et al., 1999; Dinman and Kinzy, 1997; Gross and Kinzy, 2007; Ozturk et al., 2006).
To study the effect of over-expression of selected yeast proteins on viral RNA replication and recombination, we used the yeast ORF collection from Open Biosystems (Huntsville, AL, USA), in which each yeast gene ORF is expressed from 2μ plasmid BG1805 under the control of GAL1 promoter and fused to a tandem affinity tag that includes an HA epitope tag and the ZZ domain of Protein A at the C-terminus (Gelperin et al., 2005).
The LEU2 expression plasmid pGAD-His92-CUP1, carrying Cucumber necrosis virus (CNV) p92 gene behind the CUP1 promoter has been described (Li et al., 2008). Construction of the HIS3 dual expression plasmid pHisGBK-His33/DI-72-CUP1 (co-expressing CNV p33 from the ADH1 promoter and DI-72 RNA from the CUP1 promoter) was done by inserting DI-72 sequence including the ribozyme at the 3′ end of pGBK-His33. Sequence of the CUP1 promoter and DI-72 plus the ribozyme were amplified by PCR with primer pairs #1779/#1780 and #471/#1069, respectively, followed by digestion with Xho I and ligation of these PCR products with each other. Then, the ligation mixture was used as template to amplify the expression cassette CUP1-DI-72 with primers #1779/#1069, and then digested with EcoR I and Sac I. The PCR products were recovered and ligated with pHisGBK-His33/DI-72 treated with EcoR I and Sac I (Jiang et al., 2006), resulting in pHisGBK-His33/DI-72-CUP1. pHisGBK-His33-CUP1/DI-AU-FP-Gal1 (co-expressing CNV p33 from the CUP1 promoter and DI-AU-FP RNA from the GAL1 promoter and HIS3 marker) has been described (Li et al., 2008).
To study viral replication and protein stability in the TKY102 and TKY848 strains, we used plasmid pCM185-TET-His92 (TRP) or pCM189-TET-His92 (URA3), both expressing 6xHis-tagged CNV p92 under the control of tetracycline/doxycycline regulatable promoter, and pGBK-CUP1-His33, expressing 6xHis-tagged CNV p33 driven by CuSO4 inducible promoter CUP1 (Jaag et al., 2007). We also generated pESCHIS4-ADH-His33/CUP1-DI-72 (HIS4) that co-expressed 6xHis-tagged CNV p33 and DI-72 repRNA under the control of ADH1 and CUP1 promoters, respectively. To obtain this plasmid, the HIS4 sequence was amplified by PCR from yeast genomic DNA with primers #2548 and #2549 and digested by Xma I and Sph I. Primers #2544 and #2545 were used to amplify sequences from plasmid pESC-p33-DI72 (Pathak, Sasvari, and Nagy, 2008), co-expressing 6xHis-tagged CNV p33 of from the GAL1 promoter and TBSV DI-72 repRNA from the GAL10 promoter, without the HIS3 coding region. The resulting PCR product was digested with Xma I and Sph I and with the HIS4 fragment to make plasmid pESCHIS4-DI72-p33. To generate pESCHIS4-ADH-His33/CUP1-DI-72, plasmid pESCHIS4-DI72-p33 was digested with Nco I and Sac I and then the DNA fragment containing the plasmid backbone sequence was recovered. The recovered DNA was then ligated with the ADH-His33/CUP-DI-72 expressing cassette obtained with NcoI and XhoI digestion of plasmid pHisGBK-His33/DI72-CUP1.
To study the p33-eEF1A interaction in yeast by split-Ub assay, the TEF2 sequence was amplified with PCR using primers #1786 and #2088, followed by digestion with BamHI and BglII and cloned into pPR-C-RE to produce pPR-C-TEF2 (NubG) as described previously (Li et al., 2008).
To produce recombinant yeast proteins in E. coli as maltose binding protein (MBP) fusions (Rajendran and Nagy, 2004), the yeast genes were amplified from the yeast genome by PCR using gene-specific primers as shown in Table 1. The PCR products were digested with the restriction enzymes shown in the name of the primers, followed by gel isolation and ligation into gel-isolated pMalc-2X plasmid digested with BamHI and SalI. In order to remove the intron from the DBP2 gene, primers #1998 and #1999 were kinased and used in combination with primers #1874 and #1875 to amplify the DBP2 sequence upstream and downstream of the intron, respectively. The PCR products were gel-isolated and ligated. The full-length cDNA of the DBP2 was amplified from the above ligation reaction using primers #1874 and #1875 and cloned into pMalc-2X plasmid as described above. MBP fusion proteins were expressed and purified according to (Rajendran and Nagy, 2004), except that after the last washing step, the columns were spinned at 600 g for 2 min at 4 °C. Then 0.5 ml elution buffer was added and after 5 min incubation at 4 °C, the columns were centrifuged for 2 min at 4 °C. The eluted purified proteins were stored at 4 °C until use.
RNA-labeling for the protein array experiments
TBSV gRNA, TBSV DI-72 RNA (uncapped) and BMV RNA1 (capped with m7GpppG), respectively, were labeled with biotin by in vitro T7 RNA polymerase (Takara) transcription with linearized plasmids using biotin-UTP as described (Cheng and Nagy, 2003). The plasmids carrying TBSV and BMV sequences, respectively, with T7 promoter at the 5′ end were linearized with SmaI or EcoRI (Nagy and Bujarski, 1993; White and Morris, 1994a). In addition, the BMV RNA was capped with m7GpppG (Nagy and Bujarski, 1992), whereas the TBSV RNA was uncapped, similar to natural virus infections. After the T7 transcription, the unincorporated biotin-UTP was removed on a Qiagen mini gel filtration column. The final RNA concentration was 200 μg/ml and RNA quality was determined to be majority full-length products on a 1 % agarose gel. The efficiency of biotinylation was tested using Streptavidin MagneSphere Paramagnetic Particles (SA-PMPs). Briefly, a 20 μl suspension of SA-PMPs (Promega) was washed three times with 300 μl of 0.5x SSC buffer and finally re-suspended in 50 μl 0.5x SSC buffer. Biotinylated RNA (5 μl) was then added followed by 10 min incubation at room temperature with occasional gentle mixing (Cheng and Nagy, 2003). The SA-PMPs were collected on the side of the tube with a magnetic stand. The amount of unbound RNA in the solution was analyzed on a 1% agarose gel showing that 90–95 % of the RNA was bound to SA-PMPs.
Yeast protein array analysis
The Protein Array slide (Invitrogen) was blocked for 1h at 4°C in the blocking buffer (1xPBS, 1.0% BSA and 0.1% Tween 20). Then, the RNA probe for each protein array was added in 120 μl volume, containing 20 μg of biotinylated RNA, 25 μg/ml poly dG-dC (Sigma), 1.0% BSA, 8 units of RNasin (Fermentas) in 50 mM Tris pH-8.0, 10 mM MgCl2, 10 mM DTT, and 25 mM KCl. The slide was then covered and incubated at room temperature for 1h. After three 5-min washes with a buffer (1xPBS, 5mM MgCl2, 0.5 mM DTT, 5% glycerol, 1.0% BSA and 0.05% Tween 20), the protein array was incubated on ice with 0.1 μg/ml Streptavidin-Alexa Fluor 647 in the binding buffer. After centrifugation for 4 min at 800 g, the protein array slide was left in the slide holder to air dry. Scanning and quantification were performed with ScanArray 4000 (Packard Bioscience, Billerica, MA) scanner and QuantArray V3.0 software.
TAP-based affinity purification of protein-RNA complexes
For the protein-TBSV RNA co-purification experiments, we used the TAP library of yeast strains, which express selected gene products from their native promoters and in their natural chromosomal locations (Open Biosystems, Huntsville, AL) tagged at their C-terminus with the TAP-tag (Puig et al., 2001). The TAP tag consists of Protein A sequence, a TEV protease cleavage site and the calmodulin binding protein domain and affinity purification was performed as described (Puig et al., 2001). Selected yeast strains from the TAP-library were transformed with pYC-DI-72 to express TBSV DI-72(+) RNA from GAL1 promoter (Panavas and Nagy, 2003). The transformed strains were grown in 3L cultures under inducing conditions (~1.0 OD), followed by pelleting and homogenization in liquid nitrogen. Lysates were resuspended in 10 ml of ice-cold IPP-150 buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.1% NP-40), followed by centrifugation for 15 min at 30,000g at 4°C. The supernatant was loaded on a 0.2 ml IgG agarose containing column (Sigma) followed by incubation and washing with 30 ml of ice-cold IPP-150 buffer. Then, the column was equilibrated with 1 ml of TEV protease cleavage buffer, followed by addition of 10 μl of TEV protease (AcTEV protease from Invitrogen) and incubation for 1 h at room temperature. The TAP-tagged protein eluted in 1.2 ml TEV protease cleavage buffer was mixed with 3.5 μl of 1M CaCl2 and added to a column containing 0.2 ml Calmodulin beads (Stratagene). After incubation at 4°C for 2 h, the column was washed with 30 ml of Calmodulin Binding Buffer (10 mM Tris-HCl pH 8.0, 1mM magnesium acetate, 150 mM NaCl, 0.1% NP-40, 1mM imidazole, 2 mM CaCl2, and 10 mM β-mercaptoethanol) and the TAP-tagged protein was eluted in 800 μl of Calmodulin elution buffer (10 mM Tris-HCl pH 8.0, 1mM magnesium acetate, 150 mM NaCl, 0.1% NP-40, 1 mM imidazole, 2 mM EGTA, and 10 mM β-mercaptoethanol) (Puig et al., 2001). Half of the protein samples were used for RNA extraction with phenol/chlorophorm 1:1 followed by ethanol/sodium acetate precipitation (Panavas and Nagy, 2003). The co-purified TBSV DI-72(+) RNA was detected by Northern blot using 32P-labeled DI-72(−) RNA probe (Panavas and Nagy, 2003; Panavas et al., 2005b). The other half of the purified samples was used for 10% SDS-PAGE (after concentration of samples via TCA precipitation). The gels were stained with silver and scanned (Rajendran and Nagy, 2004).
GST-affinity purification of protein-RNA complexes from yeast
For protein-RNA co-purification experiments, we used pHisGBK-His33/DI-72-CUP1 (Jiang et al., 2006), which is a dual construct expressing a p33 protein with 6xHis tag from the ADH1 promoter and the DI-72 repRNA from the CUP1 promoter, and pGAD-His92 (Panaviene et al., 2004), which has p92 with 6xHis tag at the N-terminus driven by the ADH1 promoter. BY4741 yeast cells were also co-transformed with expression plasmids selected from a GST-6×His ORF library (a generous gift from Dr. Brenda Andrews) (Sopko et al., 2006). Transformed yeast cells were cultured in a 1L flask containing 100 ml of synthetic complete dropout medium lacking uracil, leucine and histidine (SC-ULH−) starting at an OD600 of 0.1.. The yeast cells at an OD600 of 0.9 were then pelleted (100 mg) and re-suspended in 150 μl GST lysis buffer (14 mM NaCl, 0.27 mM KCl, 0.8 mM Na2HPHO4, 0.18 mM KH2PO4, 10% glycerol, 2 mM EDTA, 0.1% NP- 40, and 10 mM beta-mercaptoethanol) containing 1% Yeast Protease inhibitor cocktail (YPic, Sigma) in a deep well plate on ice, containing 0.25 ml volume of glass beads. The cells were broken by a genogrinder for 2 min at 1,500 rpm. The homogenized sample was re-suspended in 0.6 ml GST lysis buffer containing 1% YPic, and was centrifuged at 100 × g for 5 min at 4 °C. The supernatant was transferred to a microcentrifuge tube, followed by centrifugation at 21,000 × g for 10 min at 4°C. The supernatant was incubated with GST-affinity resin for 2 h at 4°C, followed by 5 washes with lysis buffer and elution with 50 μl Glutathione elution buffer (10 ml buffer containing 0.03g glutathione and 0.5 ml 1M Tris, pH 8.0).
A two-step co-purification method to identify eEF1A in the viral replicase
Yeast strain SC1 (Invitrogen) was transformed with plasmids pGBK-33HF and pGAD-92HF or pGBK-His33 and pGAD-His92 (Serva and Nagy, 2006) to express 6xHis/FLAG-tagged p33 and p92 or only 6xHis-tagged p33 and p92 together with pYC2-DI-72 expressing DI-72 repRNA. Transformed yeast cells were cultured overnight in 15-ml culture tubes containing 3 ml SC-UTL− with 2% glucose as carbon source at 29 °C. Yeast cells were pelleted, washed and then inoculated into 200 ml of SC-UTL− containing 2% galatose. Next, yeast cells were cultured at 23 °C until reaching an OD600 of 0.8–1.0 at which cells were pelleted, and washed once with 1 X Phosphate Buffer Saline (PBS) buffer, then suspended in 200 ml pre-chilled 1X PBS buffer, and cooled on ice. The yeast cells were then treated with 5.5 ml of 37% formaldehyde (1% final concentration) and incubated for 1 h on ice with gentle shaking. The cross-linking reaction with formaldehyde was quenched by addition of 10 ml 2.5 M glycine (final concentration 0.125 M). Yeast were then pelleted, washed twice with 1X PBS buffer, and stored at −80 °C until use.
Co-purification was done according to previously described method with minor modification (Serva and Nagy, 2006). Briefly, 200 mg of yeast cells were re-suspended and homogenized in TG buffer (50 mM Tris-HCl [pH 7.5], 10% glycerol, 15 mM MgCl2, and 10 mM KCl) supplemented with 0.5 M NaCl, 0.1% Nonidet P-40 (NP-40), 10 mM β-mercaptoethanol and 1% [V/V] yeast protease inhibitor cocktail (Sigma) by glass beads using Tallboys™ high throughput homogenizer (Thorofare, NJ, USA) (Li et al., 2008). Unbroken cells and debris were removed by centrifugation for 5 min at 100 ×g at 4 °C, while the membrane fraction containing the viral replicase complex was collected by centrifugation for 15 min at 21,000 ×g at 4 °C. Solubilization of the membrane-bound replicase was performed in 1 ml TG buffer with 0.5 M NaCl, 1% NP-40, 5% SB3–10 [caprylyl sulfobetaine] (Sigma), 10 mM β-mercaptoethanol, 1% [V/V] yeast protease inhibitor cocktail and 5 mM imidazole via gentle rotation for 30 min at room temperature. The solubilized membrane fraction was centrifuged at 21,000 ×g at 4 °C for 15 min and the supernatant was added to 1 ml of ProBond™ Nickel-resin (Invitrogen) pre-equilibrated with TG buffer supplemented with 0.5 M NaCl and 1% NP-40, followed by gentle rotation for 1 h at 4 °C. The unbound proteins were removed by gravity flow, and the resin was washed three times with TG buffer supplemented with 0.5 M NaCl, 1% NP-40, and 10 mM imidazole. The bound proteins were eluted 3 times with 330 μl TG buffer supplemented with 0.5 M NaCl, 0.5% NP-40, 500 mM imidazole and 1% yeast protease inhibitor cocktail. For RNase treatment, 2.5 μg RNase A was added to the eluates. The eluates were pooled and loaded onto 50 μl anti-FLAG M2-agarose affinity resin (Sigma) pre-equilibrated with 0.7 ml TG buffer supplemented with 0.5 M NaCl and 0.5% NP-40. After 2 h gentle rotation at 4 °C, the unbound materials were removed by gravity flow and the affinity resin in the column was washed 5 times with 1 ml TG buffer with 0.5 M NaCl and 0.5% NP-40, and twice with 1 ml TG buffer with 50 mM NaCl and 0.1% NP-40. Proteins bound to affinity resin were eluted by incubation in 50 μl SDS-PAGE sample buffer at 85 °C for 10 min and then subjected to SDS-PAGE and western blotting with anti-His and anti-eEF1A antibodies In order to prevent cross-linking, protein eluates were heated at 100 °C for 20 min to reverse the cross-linking prior to SDS-PAGE.
RepRNA replication assay in yeast
To study the effect of over-expression of selected yeast proteins on viral RNA replication and recombination, we used the S. cerevisiae ORF collection from Open Biosystems (BY4741 background). We performed the replication and recombination assays with six to eight independent samples for each strain over-expressing a given yeast ORF as described previously (Li et al., 2008). The parental strain (BY4741) was co-transformed with three separate plasmids: (i) pHisGBK-His33/DI-72-CUP1, (ii) pGAD-His92-CUP1, and (iii) one of the individual yeast ORF clones (Open Biosystems) or pYES-NT-C (Invitrogen) as a control using the standard lithium acetate method (Gietz and Woods, 2002). Transformants were selected by complementation of auxotrophic markers. Each transformed strain was inoculated into SC-ULH− medium with 2% galactose and cultured for 20 h at 29°C to pre-express the given yeast ORF, and then copper sulfate (50 μM, final concentration) was added into media to induce the expression of p92 and DI-72 RNA from the copper-inducible CUP1 promoter. The cells were grown further for 24 h at 29°C after adding copper sulfate and were harvested by centrifugation.
To study replication of the TBSV repRNA in the presence of mutant forms of eEF1A in the TKY strains (Table 2), p92 was expressed from pCM185-TET-His92, while p33 and the DI-72(+) repRNA were launched from a dual-expression construct, pESCHIS4-ADH-His33/CUP1-DI-72 from ADH1 and CUP1, respectively. Yeast cells were grown in SC-ULTH− containing 2% glucose. CuSO4 was added to yeast culture to final concentration of 50 μM to induce TBSV repRNA replication. After 40 h incubation/shaking at 29 °C, the cultures were collected and analyzed by Northern and Western blotting as described below.
TBSV RNA analysis with Northern blotting
Total RNA isolation and Northern blot analysis were done as described previously (Panaviene et al., 2004). Briefly, yeast cells were resuspended in RNA extraction buffer (50 mM sodium acetate, pH 5.2, 10 mM EDTA, 1% SDS) and water-saturated phenol. Cells were shaken for 1 to 2 min at room temperature, followed by incubation for 4 min at 65°C and then on ice for 5 min. After removal of phenol, the RNA was recovered by precipitation with ethanol. The RNA samples were separated by 1.5% agarose gel electrophoresis and were transferred to a Hybond-XL membrane (Amersham). Northern blotting was done as described (Panavas and Nagy, 2003). For the replication assay, RNA hybridization was done with a mixture of two probes to detect DI-72 (+)RNA and 18S yeast ribosomal RNA (rRNA) as described previously (Panavas et al., 2005b). For the recombination assay, we used 32P-labeled DI-72 RIII/IV (−) probe [specific to DI-72 (+)], which was obtained by in vitro transcription with T7 RNA polymerase on PCR template. PCR was performed with primers #1165 and #22 on DI-72 template. The 18S rRNA probes were prepared by T7 transcription from PCR products amplified from the yeast genomic DNA with primers #1251 and #1252. Hybridization signals were detected using a Typhoon 9400 imaging scanner (Amersham) and quantified by ImageQuant software.
Split-Ub yeast two-hybrid assay
The split-Ub assay was done on the basis of Dualmembrane kit 3 (Dualsystems Biotech) with modification (Li et al., 2008). Briefly, yeast strain NMY51 [MATa his3□Δ200 trp1–901 leu2–3,112 ade2 LYS2::(lexAop)4-HIS3 ura3::(lexAop)8-lacZ ade2::(lexAop)8-ADE2 GAL4; Dualsystems] was transformed with pGAD-BT2N-His33, expressing 6xHis-tagged p33 with LexA-VP16-Cub fusion at the N terminus, and pPR-C-TEF2 (NubG), expressing eEF1A with C-terminus NubG fusion (Li et al., 2008). The yeast transformation mixture was plated onto SC media without tryptophan and leucine (SC-TL−) to select the transformed plasmid. After 2–3 days, colonies were resuspended in 100 μl water, spread onto SC media lacking tryptophan, leucine, histidine and adenine (SC-TLHA−) plates and grown at 29 °C for 3–5 days.
Gel mobility shift assay (EMSA)
EMSA was performed as described previously (Pogany, White, and Nagy, 2005), except that the binding reaction was done in the presence of 10 mM Tris pH 7.5, 50 mM NaCl, 1 mM DTT, 1 mM EDTA 5% glycerol, 6 U of RNasin and 0.1 μg tRNA in a 10 μl reaction. We used 5 ng 32P-labeled probe of DI-72(+) repRNA, whereas the purified recombinant yeast proteins were used in three different dilutions (2.0, 0.6 and 0.2 μg).
To study the binding of eEF1A to the 3′ end of TBSV repRNA, we made the cDNA template of the 3′ terminal 86-bp sequence from DI-72 (+) repRNA by PCR using DI-72XP as a template (White and Morris, 1994a). For amplification of the WT-SL1/2/3 sequence, we used primers #1662 and #1190. For amplification of gPR-G4-C sequence, primer pair of #1662 and #343 was used to introduce a G to C mutation in the genomic promoter sequences. Construction of the SL1/2/3ΔGGGCU was done by using a DI-72XP derivative plasmid carrying GGGCU deletion in SL3 (Pogany et al., 2003) as a template for PCR with primers #1662 and #1190. The #1662 primer also included the T7 promoter sequences (underlined in Table 3) to facilitate synthesis of RNA probes. The RNA transcripts were quantified by UV spectrophotometry (Beckman). EMSA was performed in a 10 μl-reaction containing 20 mM HEPES [pH 7.6], 50 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 10% [vol/vol] glycerol, 0.1 μg tRNA, 10 U of RNase inhibitor, 10 nM RNA probe (Rajendran and Nagy, 2003) and three different dilutions of purified GST-eEF1A (Tef2p) protein (0.4, 0.8 and 2.0 μg). GST-eEF1A was purified from yeast as described above. Reactions were incubated at room temperature for 20 min and then resolved in 4% nondenaturing polyacrylamide gel as described previously (Rajendran and Nagy, 2003).
TABLE 3. The names and sequences of primers used to make recombinant protein expression vectors for E. coli.
| Name | Sequence (5′-to-3′) |
|---|---|
| 1868/GCD2/BglII/F | ccgcAGATCTATGAGCGAATCGGAAGCCA 1 |
| 1869/GCD2/Xho/R | ccgcCTCGAGTTATGCGGAACCTTTGTAC |
| 1870/UTP7/BamHI/F | ccgcGGATCCATGGGTCACAAGAAGAACGGTC |
| 1871/UTP7/Xho/R | ccgcCTCGAGTTAGCCGAATCTGCTCAATG |
| 1872/DEG1/BglII/F | ccgcAGATCTATGAGTAATTTCATTAGAAGGCT |
| 1873/DEG1/Xho/R | ccgcCTCGAGTTACTTATTTTTGTTGTTCTTTTTCTTGGAG |
| 1874/DBP2/BamHI/F | ccgcGGATCCATGACTTACGGTGGTAGAG |
| 1875/DBP2/Xho/R | ccgcCTCGAGTCAATAGTTTGAACGACCTC |
| 1998/Dbp2/1273/R | CGATACCTCTGGCGGCCAC |
| 1999/Dbp2/1274/F | ATGTCAAAGGTATCAATTAC |
| 1876/TEF2/BamHI/F | ccgcGGATCCATGGGTAAAGAGAAGTCTCAC |
| 1877/TEF2/Xho/R | ccgcCTCGAGTTATTTCTTAGCAGCCTTTTGAGCAGC |
| 1878/RPL8A/BamHI/F | ccgcGGATCCATGGCCCCAGGTAAGAAAGTC |
| 1879/RPL8A/Xho/R | ccgcCTCGAGTTAAGCGGAGTCGGAGTTC |
| 1880/PUS4/BglII/F | ccgcAGATCTATGAATGGAATATTTGCTATTGAG |
| 1881/PUS4/SalI/R | ccgcGTCGACTTACACCTGTTCGATTTTTCTTTTTAGTG |
| List of additional primers used: | |
| #22 | GTAATACGACTCACTATAGGGCTGCATTTCTGCAATGTTCC |
| #343 | GGGGTGCATTTCTGCAATGTTC |
| #471 | CCCGCTCGAGGGAAATTCTCCAGGATTTCTC |
| #1069 | CCGGTCGAGCTCTACCAGGTAATATACCACAACGTGTGT |
| #1165 | AGCGAGTAAGACAGACTCTTCA |
| #1190 | GGGCTGCATTTCTGCAATG |
| #1251 | TGGTGGAGTGATTTGTCTGCTT |
| #1252 | TAATACGACTCACTATAGGTTTGTCCAAATTCTCCGCTCT |
| #1662 | TAATACGACTCACTATAGGACACGGTTGATCTCACCCTTC 2 |
| #1779 | CGCGGAATTCGACATTTGGGCGCTATACGTGCATATGT |
| #1780 | CGCGCTCGAGTACAGTTTGTTTTTCTTAATATCTATTTCGA |
| #1786 | CCGCGGATCCATGGGTAAAGAGAAGTCTCAC |
| #2088 | CCGCGGATCCCTCGAGTTTCTTAGCAGCCTTTTGAGCAG |
| #2544 | CCGCGCATGCTGACACCGATTATTTAAAGC |
| #2545 | ACAGCCCGGGCTTTGCCTTCGTTTATCTTG |
| #2548 | ACAGCCCGGGATGGTTTTGCCGATTCTACC |
| #2549 | CCGCGCATGCCTACTGGAAATCCTTTGGGA |
Restriction enzyme sites are shown bold-faced
T7 promoter sequence is underlined
In vitro translation using a yeast soluble cell extract
Preparation of the yeast extract supporting translation in vitro was performed as described (Iizuka and Sarnow, 1997). Yeast cells were cultured in 350 ml YPD media at 29 °C for 15–17 h with shaking. Cells were harvested when they reached an OD600 of 1.0, followed by centrifugation at 5000 rpm for 4 min at 4°C. The pelleted cells were resuspended in 10 ml ice-cold sterile dH2O by vortexing, followed by centrifugation at 4000 rpm at 4°C for 4 min in a swinging bucket rotor several times. The cells were resuspended in ice cold breaking buffer using 2.5 ml breaking buffer per 3g of yeast (Iizuka and Sarnow, 1997) plus 7 μl 1M DTT and 10 g ice cold glass beads. Yeast cells were broken manually by shaking the tube 10 times for 30 sec, followed by centrifugation at 5,000 rpm at 4 °C for 5 min in a swinging bucket rotor. The supernatant (~2.5 ml) was transferred to microcentrifuge tubes, followed by centrifugation at 21,000 × g at 4 °C for 5 min. The supernatant was loaded onto a prechilled Sephadex column. The active fractions (500 μl) were collected and 80 μl of the solubile extract was treated with Micrococcal nuclease (0.5 μl of 100 mM CaCl2 and 0.75 μl microccoccal nuclease) at room temperature for 5 min. Then, 1.5 μl of 100 mM EGTA pH 8.0 and 0.8 μl RNase inhibitor were added.
The in vitro translation reactions used 4 μl micrococcal nuclease treated yeast soluble cell extract in 8 μl total volume also containing 1.36 μl translation mix, 0.8 μl sterile dH2O, 0.25 μl amino acid mix without methionine (1 mM, Promega), 0.08 μl 35S methionine (10 mCi/ml), 0.2 μl RNasin, and 0.3 μl creatine kinase (10 mg/ml). The assay also contained 1 μl m7GpppG-capped RNA transcript. After 1 h incubation at room temperature, 4 μl of 3x SDS-PAGE loading dye was added, followed by treatment at a 100 °C for 2 min and analysis on an SDS-PAGE gel.
Protein stability assay in yeast
Yeast strains TKY102 and TKY848 were transformed with plasmid pGBK-CUP1-His33 expressing 6xHis-tagged CNV p33 from the inducible CUP1 promoter. Yeast transformants were cultured in SC media lacking tryptophan with 2% glucose at 29 °C until reaching OD600 0.8–1.0. To induce the expression of p33, 50 μM CuSO4 was added to the yeast cultures for 1 h at 29 °C, followed by the addition of Cycloheximide to a final concentration of 100 μg/ml to inhibit protein synthesis. Equal amounts of yeast cells were collected at 0, 20, 40, 80, 120 and 180 min time points, and cell lysates were prepared by NaOH method as described previously (Panavas and Nagy, 2003). The total protein samples were analyzed by SDS/PAGE and Western blotting with anti-His antibody (Amersham).
ACKNOWLEDGEMENTS
The authors thank Drs. David Smith and Daniel Barajas for valuable comments. This work was supported by the National Science Foundation (IOB-0517218), NIH-NIAID and the Kentucky Tobacco Research and Development Center to PDN, NIH T32AI007403 (AME) and NIH RO1GM57483 (TGK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlquist P, Noueiry AO, Lee WM, Kushner DB, Dye BT. Host factors in positive-strand RNA virus genome replication. J Virol. 2003;77(15):8181–6. doi: 10.1128/JVI.77.15.8181-8186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonzo JD, Crother TR, Guetsova ML, Daignan-Fornier B, Taylor MW. APT1, but not APT2, codes for a functional adenine phosphoribosyltransferase in Saccharomyces cerevisiae. J Bacteriol. 1999;181(1):347–52. doi: 10.1128/jb.181.1.347-352.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand M, Balar B, Ulloque R, Gross SR, Kinzy TG. Domain and nucleotide dependence of the interaction between Saccharomyces cerevisiae translation elongation factors 3 and 1A. J Biol Chem. 2006;281(43):32318–26. doi: 10.1074/jbc.M601899200. [DOI] [PubMed] [Google Scholar]
- Bastin M, Hall TC. Interaction of elongation factor 1 with aminoacylated brome mosaic virus and tRNA’s. J Virol. 1976;20(1):117–22. doi: 10.1128/jvi.20.1.117-122.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumstark T, Ahlquist P. The brome mosaic virus RNA3 intergenic replication enhancer folds to mimic a tRNA TpsiC-stem loop and is modified in vivo. Rna. 2001;7(11):1652–70. [PMC free article] [PubMed] [Google Scholar]
- Blackwell JL, Brinton MA. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J Virol. 1997;71(9):6433–44. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T, Young RA, Brown S. Function and structure in phage Qbeta RNA replicase. Association of EF-Tu-Ts with the other enzyme subunits. J Biol Chem. 1976;251(9):2740–3. [PubMed] [Google Scholar]
- Bond AT, Mangus DA, He F, Jacobson A. Absence of Dbp2p alters both nonsense-mediated mRNA decay and rRNA processing. Mol Cell Biol. 2001;21(21):7366–79. doi: 10.1128/MCB.21.21.7366-7379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton MA. Host factors involved in West Nile virus replication. Ann N Y Acad Sci. 2001;951:207–19. doi: 10.1111/j.1749-6632.2001.tb02698.x. [DOI] [PubMed] [Google Scholar]
- Buck KW. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv Virus Res. 1996;47:159–251. doi: 10.1016/S0065-3527(08)60736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KW. Replication of tobacco mosaic virus RNA. Philos Trans R Soc Lond B Biol Sci. 1999;354(1383):613–27. doi: 10.1098/rstb.1999.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr-Schmid A, Durko N, Cavallius J, Merrick WC, Kinzy TG. Mutations in a GTP-binding motif of eukaryotic elongation factor 1A reduce both translational fidelity and the requirement for nucleotide exchange. J Biol Chem. 1999;274(42):30297–302. doi: 10.1074/jbc.274.42.30297. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Nagy PD. Mechanism of RNA recombination in carmo- and tombusviruses: evidence for template switching by the RNA-dependent RNA polymerase in vitro. J Virol. 2003;77(22):12033–47. doi: 10.1128/JVI.77.22.12033-12047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CP, Serviene E, Nagy PD. Suppression of viral RNA recombination by a host exoribonuclease. J Virol. 2006;80(6):2631–40. doi: 10.1128/JVI.80.6.2631-2640.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, Perrimon N. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 2005;19(4):445–52. doi: 10.1101/gad.1267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SM, Chen L, Lambertson D, Anand M, Kinzy TG, Madura K. Proteasome-mediated degradation of cotranslationally damaged proteins involves translation elongation factor 1A. Mol Cell Biol. 2005;25(1):403–13. doi: 10.1128/MCB.25.1.403-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimarelli A, Luban J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J Virol. 1999;73(7):5388–401. doi: 10.1128/jvi.73.7.5388-5401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IM, Carroll JW, Rout MP, Rice CM, Chait BT, MacDonald MR. Tracking and elucidating alphavirus-host protein interactions. J Biol Chem. 2006;281(40):30269–78. doi: 10.1074/jbc.M603980200. [DOI] [PubMed] [Google Scholar]
- Davis WG, Blackwell JL, Shi PY, Brinton MA. Interaction between the Cellular Protein eEF1A and the 3′-Terminal Stem-Loop of West Nile Virus Genomic RNA Facilitates Viral Minus-Strand RNA Synthesis. J Virol. 2007;81(18):10172–87. doi: 10.1128/JVI.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nova-Ocampo M, Villegas-Sepulveda N, del Angel RM. Translation elongation factor-1alpha, La, and PTB interact with the 3′ untranslated region of dengue 4 virus RNA. Virology. 2002;295(2):337–47. doi: 10.1006/viro.2002.1407. [DOI] [PubMed] [Google Scholar]
- Diez J, Ishikawa M, Kaido M, Ahlquist P. Identification and characterization of a host protein required for efficient template selection in viral RNA replication. Proc Natl Acad Sci U S A. 2000;97(8):3913–8. doi: 10.1073/pnas.080072997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman JD, Kinzy TG. Translational misreading: mutations in translation elongation factor 1alpha differentially affect programmed ribosomal frameshifting and drug sensitivity. Rna. 1997;3(8):870–81. [PMC free article] [PubMed] [Google Scholar]
- Dreher TW. Functions of the 3′-Untranslated Regions of Positive Strand Rna Viral Genomes. Annu Rev Phytopathol. 1999;37:151–174. doi: 10.1146/annurev.phyto.37.1.151. [DOI] [PubMed] [Google Scholar]
- Dreher TW, Uhlenbeck OC, Browning KS. Quantitative assessment of EF-1alpha.GTP binding to aminoacyl-tRNAs, aminoacyl-viral RNA, and tRNA shows close correspondence to the RNA binding properties of EF-Tu. J Biol Chem. 1999;274(2):666–72. doi: 10.1074/jbc.274.2.666. [DOI] [PubMed] [Google Scholar]
- Fetchko M, Auerbach D, Stagljar I. Yeast genetic methods for the detection of membrane protein interactions: potential use in drug discovery. BioDrugs. 2003;17(6):413–24. doi: 10.2165/00063030-200317060-00004. [DOI] [PubMed] [Google Scholar]
- Fetchko M, Stagljar I. Application of the split-ubiquitin membrane yeast two-hybrid system to investigate membrane protein interactions. Methods. 2004;32(4):349–62. doi: 10.1016/j.ymeth.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Dumpelfeld B, Edelmann A, Heurtier MA, Hoffman V, Hoefert C, Klein K, Hudak M, Michon AM, Schelder M, Schirle M, Remor M, Rudi T, Hooper S, Bauer A, Bouwmeester T, Casari G, Drewes G, Neubauer G, Rick JM, Kuster B, Bork P, Russell RB, Superti-Furga G. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440(7084):631–6. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Gelperin DM, White MA, Wilkinson ML, Kon Y, Kung LA, Wise KJ, Lopez-Hoyo N, Jiang L, Piccirillo S, Yu H, Gerstein M, Dumont ME, Phizicky EM, Snyder M, Grayhack EJ. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 2005;19(23):2816–26. doi: 10.1101/gad.1362105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Goh PY, Tan YJ, Lim SP, Tan YH, Lim SG, Fuller-Pace F, Hong W. Cellular RNA helicase p68 relocalization and interaction with the hepatitis C virus (HCV) NS5B protein and the potential role of p68 in HCV RNA replication. J Virol. 2004;78(10):5288–98. doi: 10.1128/JVI.78.10.5288-5298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SR, Kinzy TG. Translation elongation factor 1A is essential for regulation of the actin cytoskeleton and cell morphology. Nat Struct Mol Biol. 2005;12(9):772–8. doi: 10.1038/nsmb979. [DOI] [PubMed] [Google Scholar]
- Gross SR, Kinzy TG. Improper organization of the actin cytoskeleton affects protein synthesis at initiation. Mol Cell Biol. 2007;27(5):1974–89. doi: 10.1128/MCB.00832-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Zhu H, Zhu X, Royce T, Gerstein M, Snyder M. Regulation of gene expression by a metabolic enzyme. Science. 2004;306(5695):482–4. doi: 10.1126/science.1096773. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhu H, Haggarty SJ, Spring DR, Hwang H, Jin F, Snyder M, Schreiber SL. Finding new components of the target of rapamycin (TOR) signaling network through chemical genetics and proteome chips. Proc Natl Acad Sci U S A. 2004;101(47):16594–9. doi: 10.1073/pnas.0407117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo RD, Jamieson DJ, MacNeill SA, Southgate J, McPheat J, Lane DP. p68 RNA helicase: identification of a nucleolar form and cloning of related genes containing a conserved intron in yeasts. Mol Cell Biol. 1991;11(3):1326–33. doi: 10.1128/mcb.11.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka N, Sarnow P. Translation-competent extracts from Saccharomyces cerevisiae: effects of L-A RNA, 5′ cap, and 3′ poly(A) tail on translational efficiency of mRNAs. Methods. 1997;11(4):353–60. doi: 10.1006/meth.1996.0433. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Janda M, Krol MA, Ahlquist P. In vivo DNA expression of functional brome mosaic virus RNA replicons in Saccharomyces cerevisiae. J Virol. 1997;71(10):7781–90. doi: 10.1128/jvi.71.10.7781-7790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Serviene E, Gal J, Panavas T, Nagy PD. Identification of essential host factors affecting tombusvirus RNA replication based on the yeast Tet promoters Hughes Collection. J Virol. 2006;80(15):7394–404. doi: 10.1128/JVI.02686-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Perez DR, French R, Merrick WC, Donis RO. The NS5A protein of bovine viral diarrhoea virus interacts with the alpha subunit of translation elongation factor-1. J Gen Virol. 2001;82(Pt 12):2935–43. doi: 10.1099/0022-1317-82-12-2935. [DOI] [PubMed] [Google Scholar]
- Kou YH, Chou SM, Wang YM, Chang YT, Huang SY, Jung MY, Huang YH, Chen MR, Chang MF, Chang SC. Hepatitis C virus NS4A inhibits cap-dependent and the viral IRES-mediated translation through interacting with eukaryotic elongation factor 1A. J Biomed Sci. 2006;13(6):861–74. doi: 10.1007/s11373-006-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F, Montgomery RR, Lev S, Mason PW, Koski RA, Elledge SJ, Xavier RJ, Agaisse H, Fikrig E. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455(7210):242–5. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner DB, Lindenbach BD, Grdzelishvili VZ, Noueiry AO, Paul SM, Ahlquist P. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc Natl Acad Sci U S A. 2003;100(26):15764–9. doi: 10.1073/pnas.2536857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Barajas D, Panavas T, Herbst DA, Nagy PD. Cdc34p Ubiquitin-Conjugating Enzyme Is a Component of the Tombusvirus Replicase Complex and Ubiquitinates p33 Replication Protein. J Virol. 2008;82(14):6911–26. doi: 10.1128/JVI.00702-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda D, Yoshinari S, Dreher TW. eEF1A binding to aminoacylated viral RNA represses minus strand synthesis by TYMV RNA-dependent RNA polymerase. Virology. 2004;321(1):47–56. doi: 10.1016/j.virol.2003.10.028. [DOI] [PubMed] [Google Scholar]
- McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell. 2005;17(12):3513–31. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H, White KA. Structure and prevalence of replication silencer-3′ terminus RNA interactions in Tombusviridae. Virology. 2006;345(2):305–16. doi: 10.1016/j.virol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nagy PD. Yeast as a model host to explore plant virus-host interactions. Annu Rev Phytopathol. 2008;46:217–42. doi: 10.1146/annurev.phyto.121407.093958. [DOI] [PubMed] [Google Scholar]
- Nagy PD, Bujarski JJ. Genetic recombination in brome mosaic virus: effect of sequence and replication of RNA on accumulation of recombinants. J Virol. 1992;66(11):6824–8. doi: 10.1128/jvi.66.11.6824-6828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy PD, Bujarski JJ. Targeting the site of RNA-RNA recombination in brome mosaic virus with antisense sequences. Proc Natl Acad Sci U S A. 1993;90(14):6390–4. doi: 10.1073/pnas.90.14.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy PD, Pogany J. Partial purification and characterization of Cucumber necrosis virus and Tomato bushy stunt virus RNA-dependent RNA polymerases: similarities and differences in template usage between tombusvirus and carmovirus RNA-dependent RNA polymerases. Virology. 2000;276(2):279–88. doi: 10.1006/viro.2000.0577. [DOI] [PubMed] [Google Scholar]
- Nagy PD, Pogany J. Yeast as a model host to dissect functions of viral and host factors in tombusvirus replication. Virology. 2006;344(1):211–20. doi: 10.1016/j.virol.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Nagy PD, Pogany J. Multiple roles of viral replication proteins in plant RNA virus replication. Methods Mol Biol. 2008;451:55–68. doi: 10.1007/978-1-59745-102-4_4. [DOI] [PubMed] [Google Scholar]
- Nishikiori M, Dohi K, Mori M, Meshi T, Naito S, Ishikawa M. Membrane-bound tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J Virol. 2006;80(17):8459–68. doi: 10.1128/JVI.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noueiry AO, Ahlquist P. Brome Mosaic Virus RNA Replication: Revealing the Role of the Host in RNA Virus Replication. Annu Rev Phytopathol. 2003 doi: 10.1146/annurev.phyto.41.052002.095717. [DOI] [PubMed] [Google Scholar]
- Okanami M, Meshi T, Iwabuchi M. Characterization of a DEAD box ATPase/RNA helicase protein of Arabidopsis thaliana. Nucleic Acids Res. 1998;26(11):2638–43. doi: 10.1093/nar/26.11.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk SB, Vishnu MR, Olarewaju O, Starita LM, Masison DC, Kinzy TG. Unique classes of mutations in the Saccharomyces cerevisiae G-protein translation elongation factor 1A suppress the requirement for guanine nucleotide exchange. Genetics. 2006;174(2):651–63. doi: 10.1534/genetics.106.059899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panavas T, Hawkins CM, Panaviene Z, Nagy PD. The role of the p33:p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of Cucumber necrosis tombusvirus. Virology. 2005a;338(1):81–95. doi: 10.1016/j.virol.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Panavas T, Nagy PD. Yeast as a model host to study replication and recombination of defective interfering RNA of Tomato bushy stunt virus. Virology. 2003;314(1):315–25. doi: 10.1016/s0042-6822(03)00436-7. [DOI] [PubMed] [Google Scholar]
- Panavas T, Serviene E, Brasher J, Nagy PD. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc Natl Acad Sci U S A. 2005b;102(20):7326–31. doi: 10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaviene Z, Panavas T, Nagy PD. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J Virol. 2005;79(16):10608–18. doi: 10.1128/JVI.79.16.10608-10618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaviene Z, Panavas T, Serva S, Nagy PD. Purification of the cucumber necrosis virus replicase from yeast cells: role of coexpressed viral RNA in stimulation of replicase activity. J Virol. 2004;78(15):8254–63. doi: 10.1128/JVI.78.15.8254-8263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak KB, Sasvari Z, Nagy PD. The host Pex19p plays a role in peroxisomal localization of tombusvirus replication proteins. Virology. 2008;379(2):294–305. doi: 10.1016/j.virol.2008.06.044. [DOI] [PubMed] [Google Scholar]
- Pogany J, Fabian MR, White KA, Nagy PD. A replication silencer element in a plus-strand RNA virus. Embo J. 2003;22(20):5602–11. doi: 10.1093/emboj/cdg523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany J, Nagy PD. Authentic replication and recombination of Tomato bushy stunt virus RNA in a cell-free extract from yeast. J Virol. 2008;82(12):5967–80. doi: 10.1128/JVI.02737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany J, White KA, Nagy PD. Specific Binding of Tombusvirus Replication Protein p33 to an Internal Replication Element in the Viral RNA Is Essential for Replication. J Virol. 2005;79(8):4859–69. doi: 10.1128/JVI.79.8.4859-4869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24(3):218–29. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- Qanungo KR, Shaji D, Mathur M, Banerjee AK. Two RNA polymerase complexes from vesicular stomatitis virus-infected cells that carry out transcription and replication of genome RNA. Proc Natl Acad Sci U S A. 2004;101(16):5952–7. doi: 10.1073/pnas.0401449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran KS, Nagy PD. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J Virol. 2003;77(17):9244–58. doi: 10.1128/JVI.77.17.9244-9258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran KS, Nagy PD. Interaction between the replicase proteins of Tomato bushy stunt virus in vitro and in vivo. Virology. 2004;326(2):250–61. doi: 10.1016/j.virol.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Serva S, Nagy PD. Proteomics analysis of the tombusvirus replicase: Hsp70 molecular chaperone is associated with the replicase and enhances viral RNA replication. J Virol. 2006;80(5):2162–9. doi: 10.1128/JVI.80.5.2162-2169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviene E, Jiang Y, Cheng CP, Baker J, Nagy PD. Screening of the yeast yTHC collection identifies essential host factors affecting tombusvirus RNA recombination. J Virol. 2006;80(3):1231–41. doi: 10.1128/JVI.80.3.1231-1241.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviene E, Shapka N, Cheng CP, Panavas T, Phuangrat B, Baker J, Nagy PD. Genome-wide screen identifies host genes affecting viral RNA recombination. Proc Natl Acad Sci U S A. 2005;102(30):10545–50. doi: 10.1073/pnas.0504844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ST, Lai MM. Viral and cellular proteins involved in coronavirus replication. Curr Top Microbiol Immunol. 2005;287:95–131. doi: 10.1007/3-540-26765-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Jona G, Ptacek J, Devgan G, Zhu H, Zhu X, Snyder M. Global analysis of protein function using protein microarrays. Mech Ageing Dev. 2005;126(1):171–5. doi: 10.1016/j.mad.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Sopko R, Papp B, Oliver SG, Andrews BJ. Phenotypic activation to discover biological pathways and kinase substrates. Cell Cycle. 2006;5(13):1397–402. doi: 10.4161/cc.5.13.2922. [DOI] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. Viral RNA replication. With a little help from the host. Science. 1999;283(5403):802–4. doi: 10.1126/science.283.5403.802. [DOI] [PubMed] [Google Scholar]
- Thivierge K, Cotton S, Dufresne PJ, Mathieu I, Beauchemin C, Ide C, Fortin MG, Laliberte JF. Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology. 2008;377(1):216–25. doi: 10.1016/j.virol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Wang RY, Nagy PD. Tomato bushy stunt virus Co-Opts the RNA-Binding Function of a Host Metabolic Enzyme for Viral Genomic RNA Synthesis. Cell Host Microbe. 2008;3(3):178–87. doi: 10.1016/j.chom.2008.02.005. [DOI] [PubMed] [Google Scholar]
- White KA, Morris TJ. Nonhomologous RNA recombination in tombusviruses: generation and evolution of defective interfering RNAs by stepwise deletions. J Virol. 1994a;68(1):14–24. doi: 10.1128/jvi.68.1.14-24.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KA, Morris TJ. Recombination between defective tombusvirus RNAs generates functional hybrid genomes. Proc Natl Acad Sci U S A. 1994b;91(9):3642–6. doi: 10.1073/pnas.91.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KA, Nagy PD. Advances in the molecular biology of tombusviruses: gene expression, genome replication, and recombination. Prog Nucleic Acid Res Mol Biol. 2004;78:187–226. doi: 10.1016/S0079-6603(04)78005-8. [DOI] [PubMed] [Google Scholar]
- Yamaji Y, Kobayashi T, Hamada K, Sakurai K, Yoshii A, Suzuki M, Namba S, Hibi T. In vivo interaction between Tobacco mosaic virus RNA-dependent RNA polymerase and host translation elongation factor 1A. Virology. 2006;347(1):100–8. doi: 10.1016/j.virol.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Zeenko VV, Ryabova LA, Spirin AS, Rothnie HM, Hess D, Browning KS, Hohn T. Eukaryotic elongation factor 1A interacts with the upstream pseudoknot domain in the 3′ untranslated region of tobacco mosaic virus RNA. J Virol. 2002;76(11):5678–91. doi: 10.1128/JVI.76.11.5678-5691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M. Global analysis of protein activities using proteome chips. Science. 2001;293(5537):2101–5. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- Zhu H, Bilgin M, Snyder M. Proteomics. Annu Rev Biochem. 2003;72:783–812. doi: 10.1146/annurev.biochem.72.121801.161511. [DOI] [PubMed] [Google Scholar]
- Zhu J, Gopinath K, Murali A, Yi G, Hayward SD, Zhu H, Kao C. RNA-binding proteins that inhibit RNA virus infection. Proc Natl Acad Sci U S A. 2007;104(9):3129–34. doi: 10.1073/pnas.0611617104. [DOI] [PMC free article] [PubMed] [Google Scholar]


