Abstract
Palmitoylation of tetraspanins affects protein-protein interactions, suggesting a key role in the assembly of the tetraspanin web. Since palmitoylation occurs on intracellular cysteine residues, we examined whether mutating these residues in the human tetraspanin CD81 would affect the association of CD81 with other surface membrane proteins. Mutation of at least six of the eight juxtamembrane cysteines was required to completely eliminate detectable CD81 palmitoylation, indicating that several sites can be palmitoylated. Interestingly, these mutated proteins exhibited reduced cell surface detection by antibody compared to wild-type CD81, but this was not due to differences in the level of protein expression, trafficking to the cell surface, protein stability, or anti-CD81 antibody binding affinity. Instead, the mutant CD81 proteins appeared to be partially hidden from detection by anti-CD81 antibody, presumably due to altered interactions with other proteins at the cell surface. Associations with the known CD81-interacting proteins CD9 and EWI-2 were also impaired with the mutant CD81 proteins. Taken together, these findings indicate that mutation of juxtamembrane cysteines alters the interaction of CD81 with other proteins, either because of reduced palmitoylation, structural alterations in the mutant proteins, or a combination of both factors, and this affects the CD81 microenvironment on the cell surface.
Keywords: tetraspanin, palmitoylation, CD81, CD9, EWI-2, proteolysis
INTRODUCTION
CD81 is a ubiquitously expressed member of the tetraspanin superfamily of proteins. Tetraspanins are defined by four transmembrane domains and by the presence of conserved cyteines in addition to a CCG motif in the large extracellular loop. Their most striking property is the ability to interact with multiple proteins (e.g., receptors, adhesion proteins, Ig superfamily members, and signaling molecules, as well as other tetraspanins). These associations are thought to occur mainly in membrane compartments such as lipid rafts and tetraspanin-enriched microdomains (TEMs), thereby forming a membrane network known as the “tetraspanin web”. Since they regulate the function of their binding partners, tetraspanins play roles in diverse cellular processes including adhesion, proliferation, fusion, and signaling [1, 2]. However, redundancy among the members of the superfamily and the multiplicity of associations make the understanding of their specific molecular mechanism a challenging task.
Research on CD81 has largely focused on its regulatory role in the immune system since its discovery in 1990 as a target of an antiproliferative antibody on lymphoma B cells [2, 3]. Through its direct association with CD19, CD81 participates in the B-cell coreceptor, a complex of integral membrane proteins (CD19/CD21/CD81) that lowers the threshold of activation of the B-cell receptor in response to opsonized antigens [4, 5]. CD81 is thought to facilitate trafficking of CD19 and stabilize the B-cell coreceptor [6, 7]. Accordingly, B cells from CD81-null mice express less CD19 on the cell surface and present a weaker antibody response to Th2-inducing antigens [8–11]. Lack of CD81 also results in a defect in allergen-induced airway hyper-reactivity, a Th2-dependent reaction commonly found in asthma patients [12]. T-cell function is also influenced by CD81 since T cells isolated from CD81-deficient mice proliferate more rapidly after T-cell receptor cross-linking in comparison with wild-type littermates [9]. Moreover, CD81 interacts with CD4 and CD8, and is able to induce a T-cell costimulatory signal [13–15]. CD81 has also been found to dynamically concentrate in the immune synapse, a cluster of proteins formed during the close contact between T cells and antigen-presenting cells essential for T-cell activation [16]. Thus, all these immunological CD81 functions depend on interactions and clustering with other proteins, but how CD81 is able to regulate these associations remains unclear.
It is likely that post-translational modifications have a role in these functions. Palmitoylation is a post-translational process that attaches palmitic acid to proteins via thioester linkages to cysteine residues. Since it is a reversible process, it can be considered an “on-off” switch similar to protein phosphorylation. Addition of fatty acids is thought to provide proteins more hydrophobic stability in cellular membranes, thus influencing their fate during trafficking and partitioning into membrane microdomains [17, 18]. Most tetraspanins possess cysteines in their intracellular domains that may be available for palmitoylation, and all tetraspanins tested so far have been shown to be acylated [19–23]. Palmitoylation-deficient constructs have been generated for several tetraspanins (CD9, CD81, CD82, and CD151), revealing a role for palmitoylation in protein association, cell motility, morphology, adhesion-dependent signaling, and oxidative stress [20–23]. In B cells, coligation of the B-cell receptor with the CD19/CD21/CD81 complex induces CD81 palmitoylation, a necessary event resulting in the localization of this cluster of proteins into lipid rafts and signaling [24]. In contrast, palmitoylation of CD9 and CD151 does not seem to be required for their association with TEMs [19–21].
In this study, we examined whether mutation of intracellular cysteine residues that are sites of palmitoylation would affect CD81 trafficking and/or interactions with other proteins. To address this issue, we characterized CD81 mutant proteins with substitutions in five, six, seven, or eight cysteines in the cytoplasmic and transmembrane domains. In particular, we assessed CD81 expression at the plasma membrane, palmitoylation levels, association with direct binding partners, and protein stability. Our results highlight the importance of intracellular and transmembrane cysteines for the interaction of CD81 with other proteins at the cell surface.
MATERIAL AND METHODS
Cell Lines, Antibodies and Plasmids
Baby Hamster Kidney (BHK-21), African green monkey COS-7, human embryonic kidney 293, and human fibrosarcoma HT1080 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). Anti-CD81 monoclonal antibody (5A6) was obtained from Scott C. Todd and its specificity for CD81 has been described previously [3]. Anti-hEWI-2 monoclonal antibody (8A12) was a generous gift from Eric Rubinstein (INSERM U268, France). Anti-V5-HRP antibody was obtained from Invitrogen (Carlsbad, CA), and anti-hCD9 monoclonal antibody (M-L13) was purchased from BD Biosciences (San Jose, CA). Human V5-tagged CD9 and EWI-2 constructs were generated by cloning the corresponding cDNAs into pcDNA™ 3.1/V5-His TOPO® vector (Invitrogen).
Site-Directed Mutagenesis and Transfection
The human CD81 mutant (CD81-5C) with mutations in the cysteines at positions 6, 9, 89, 227, and 228 in the intracellular domains has been described previously [23]. This earlier report, however, mistakenly labeled the residue in position 89 as being an alanine instead of a serine. The constructs CD81-6C, CD81-7C, and CD81-8C were obtained using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). All constructs were sequenced to verify the substitutions and to assure other nonspecific mutations did not occur.
BHK, COS-7, 293, and HT1080 cells were transfected with different plasmids as indicated using Lipofectamine™ 2000 (Invitrogen) according to the directions of the manufacturer.
Flow Cytometry
Transfected cells were harvested with 0.02% EDTA and stained with anti-CD81 antibody 5A6, anti-CD9 antibody, or anti-EWI-2 antibody (1 μg/1 × 106 cells) for 20–30 min on ice. Cells were washed with phosphate-buffered saline (PBS) before incubation with Alexa Fluor® 488 goat anti-mouse IgG (BD Biosciences) (1 μg/1 × 106 cells) for 20 min on ice. Samples were analyzed with a BD FACSCalibur™ flow cytometer (BD Biosciences) and WinMDI 2.9 software (The Scripps Research Institute).
Metabolic Labeling
To assess palmitic acid incorporation, COS-7 cells were transfected with a control vector expressing GFP or with vectors expressing wild-type or mutant CD81. At two days post-transfection, cells were serum starved for 3 hours and labeled overnight with 300 μCi/ml palmitic acid [9,10-3H(N)] (PerkinElmer Life and Analytical Sciences, Wellesley, MA) in serum-free medium supplemented with 5% delipidated Bovine Serum Albumin (Sigma-Aldrich, St. Louis, MO). The cells were harvested and washed three times with PBS followed by lysis and immunoprecipitation with anti-CD81 antibody 5A6 as described below. The samples were separated by non-reducing SDS-PAGE, transferred to PVDF membranes, and membranes were exposed to Kodak BioMax MS Film (Eastman Kodak Company, Rochester, NY) for one week at −80 °C.
To assess protein stability, BHK cells were transfected with vectors expressing wtCD81, CD81-6C, or CD81-8C. The next day, transfected cells were starved in DMEM Met/Cys-free medium (Mediatech, Inc., Herndon, VA) containing 5% dialyzed FBS (pulse-labeling medium) for one hour. TRAN35S Label (MP Biomedicals, Solon, OH) was then added to the cells at 0.25 mCi/ml in pulse-labeling medium for one hour. After removing the medium containing [35S]-methionine/cysteine, cells were incubated with pulse-labeling medium supplemented with 2 mM L-methionine and 2 mM L-cystine to start the chase. Cells were harvested at the indicated time points, incubated 1 hour at 4 °C in lysis buffer (150 mM NaCl, 20 mM Tris pH 7.5, 5 mM MgCl2, 10 units/ml aprotinin, 1mM PMSF, and 1 μg/ml pepstatin A) containing 1% Triton-X 100 (Sigma-Aldrich), and CD81 was immunoprecipitated with anti-CD81 antibody as described below. After separation by SDS-PAGE, gels were incubated in destain solution (10% acetic acid, 30% methanol) overnight, soaked twice in DMSO, and treated with 22.5% fluor 2,5-diphenyloxazole (Acros Organics, Morris Plains, NJ) to enhance the signal. Following several washes in water, gels were dried and exposed to X-ray film for 2–24 hours at −80 °C.
Immunoprecipitation
Cells were detached with 0.02% EDTA and lysed for one hour at 4 °C in lysis buffer containing 1% Brij-99 (Sigma-Aldrich). The insoluble material was removed by centrifugation at 21,000 g for 15 min at 4 °C. 5A6 antibody (10 μg per sample) was captured on Protein G-Sepharose® beads (Sigma-Aldrich) for one hour. After washing off the excess antibody, the lysates were added to the antibody-bead mixture and left rotating overnight at 4 °C. Suspensions were centrifuged at 2,300 g for 5 min at 4 °C, the supernatants were collected, and the pellets were washed twice with lysis buffer containing 1% Brij-99, separated by SDS-PAGE under non-reducing conditions, and transferred to an Immobilon-P membrane (Millipore, Billerica, MA).
Immunoblotting
Cells were lysed as described above and proteins immobilized on Immobilon-P membranes were blocked for one hour at 4 °C in Tris-buffered saline with 0.1% Tween-20 (TBS-T) containing 5% non-fat milk. To detect CD81, a solution of anti-CD81 antibody 5A6 (1.48 mg/ml stock solution) was diluted 1:10,000 in TBS-T containing 1% non-fat milk and was incubated with the membrane for one hour at room temperature. After washing, ImmunoPure® antibody goat anti-mouse HRP (diluted 1:20,000) (Pierce Biotechnology, Rockford, IL) was used as the secondary antibody for 30 min at room temperature. For V5 detection, anti-V5-HRP antibody (1 mg/ml stock solution) was used at 1:5,000 for 90 min at room temperature, while to detect 14-3-3β, antibody H-8 (Santa Cruz Biotechnology) was used at 0.2 μg/ml. All blots were visualized by applying SuperSignal® West Pico Chemiluminescent Substrate (Pierce Biotechnology) and exposing to X-ray film.
Surface Biotinylation Labeling
Cells were detached with 0.02% EDTA, washed three times with PBS (pH 8), and incubated with EZ-Link Sulfo-NHS-LC-Biotin (Pierce Biotechnology) or PBS alone for 30 min at 4 °C. Biotin was dissolved in PBS (pH 8) just before use. Following incubation, cells were washed three times with PBS (pH 7.2) containing 100 mM glycine to remove unbound biotin. Cells were lysed for one hour at 4 °C in lysis buffer containing 1% Triton X-100, insoluble material was removed by centrifugation at 21,000 g for 15 min at 4 °C, and biotinylated proteins were pulled down with Streptavidin-Agarose beads (Sigma-Aldrich) overnight at 4 °C. Samples were analyzed by immunoblotting as described above.
Saturation Binding Assay
BHK cells transfected with a control vector, or with vectors expressing wtCD81 or CD81-8C, were harvested with 0.02% EDTA, and incubated with increasing concentrations of 5A6-FITC (34, 67, 201, 402, 670, and 1072 nM) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for one hour on ice. Samples were analyzed with a BD FACSCalibur™ flow cytometer (BD Biosciences). Saturation isotherm curves and KD values were obtained using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA) and the following equation: Y=(Bmax.X)/(KD+X), where X is the antibody concentration and Y is the mean fluorescence intensity value. Mean Fluorescence Intensity (MFI) values from control vector samples (nonspecific binding) were subtracted from MFI values from wtCD81 and CD81-8C samples to obtain specific binding values. To confirm that the antibody concentrations were saturating under these conditions, supernatants were collected after antibody incubation to measure fluorescence from unbound 5A6-FITC molecules and compared to fluorescence from total number of 5A6-FITC molecules added to the cells using a VICTOR3 plate reader (PerkinElmer Life and Analytical Sciences). No difference in binding among samples was observed when an isotype control (normal mouse IgG1-FITC, Santa Cruz Biotechnology, Inc.) was used (data not shown).
Brefeldin A Treatment
The medium was removed from cells at one day post-transfection and replaced with fresh medium containing absolute ethanol (vehicle control wells) or brefeldin A (Sigma-Aldrich) at 10 μg/ml for 10 hours in the 37 °C incubator. Cells were then detached and assayed for CD81 expression by flow cytometry or immunoblotting as described above.
RESULTS
Mutation of six cysteines is necessary for complete inhibition of CD81 palmitoylation
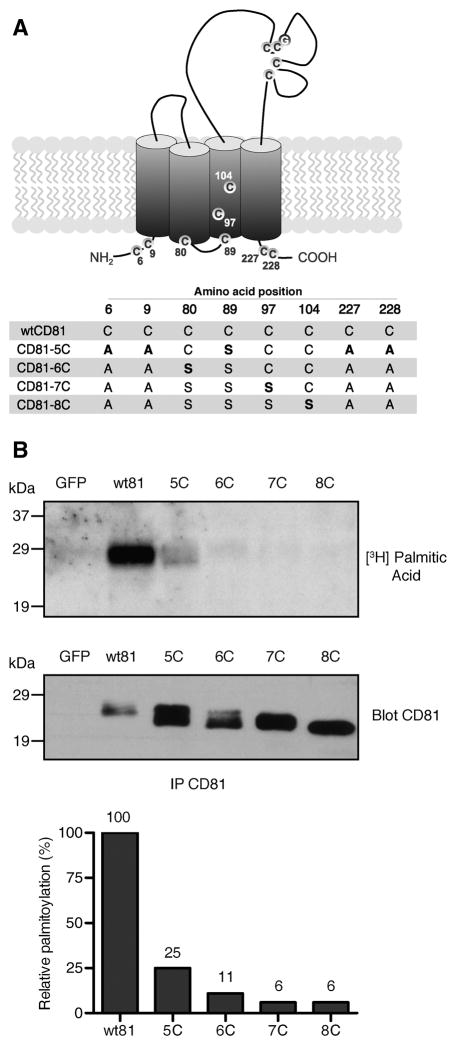
To block the palmitoylation of CD81, we generated a set of constructs with mutations in juxtamembrane cysteines, which are putative palmitoylation sites (Fig. 1A). A CD81 construct reported by Clark et al. [23] was used as a starting point (renamed CD81-5C herein). Predicted three-dimensional CD81 modeling [25] indicated that a cysteine at position 80 was an additional potential palmitoylation site. Therefore, we mutated this residue to a serine to generate CD81-6C. Further mutations in cysteines at positions 97 and 104 were also made to construct CD81-7C and -8C.
Fig. 1.
Palmitoylation levels of CD81 mutants. (A) The CD81 mutant constructs used in this study. Residues in bold type were altered in comparison to the construct immediately above. (B) COS-7 cells transfected with the CD81 constructs were labeled with [3H] palmitic acid. Samples were then lysed and immunoprecipitated with anti-CD81 mAb 5A6. Following SDS-PAGE and transfer to PVDF membranes, samples were exposed to autoradiography for one week (upper panel). The CD81 expression level was assayed by immunoblotting a duplicate membrane using 5A6 (middle panel). Relative palmitoylation values were obtained by normalizing the amount of [3H]-palmitic acid to the total amount of CD81 expressed, as assessed by densitometry (lower panel). The value of wtCD81 was set at 100%.
Measurements of palmitic acid incorporation in the mutant CD81 constructs confirmed the participation of cysteine at position 80 in CD81 palmitoylation (Fig. 1B). We observed that palmitoylation was reduced by 75% in CD81-5C, while additional mutation of the cysteine residue at position 80 reduced palmitoylation to 11% of wild-type CD81 (wtCD81). Further mutation of cysteines at positions 97 and 104 did not appreciably decrease palmitoylation further. Therefore, mutation of at least six juxtamembrane cysteines is necessary to fully inhibit CD81 palmitoylation. Interestingly, an apparent increase was consistently observed in the levels of expression of the mutant proteins compared to wtCD81 (Fig. 1B, lower panel), as discussed below.
CD81-8C proteins are localized normally to the plasma membrane and bind to anti-CD81 antibody 5A6 with normal affinity, but their detection by 5A6 on the cell surface is hindered
Since CD81 is predominantly localized at the plasma membrane, we asked whether disrupting CD81 palmitoylation would affect its intracellular distribution. Using flow cytometry, we observed a significant decrease in the surface detection of the mutant CD81 proteins compared to wild-type (Fig. 2A). This was despite an apparent increase in expression levels of the mutant proteins (Fig. 1B). The antibody used to detect CD81 was 5A6, a conformation-dependent monoclonal antibody which recognizes an epitope in the large extracellular loop of CD81 [3]. Since proper structure of the large extracellular loop is essential for 5A6 binding, it was possible that the decreased detection of mutant CD81 by flow cytometry could be due to misfolding of the mutant proteins, leading to a defect in antibody binding. We therefore sought to compare the 5A6 binding efficiency to wtCD81 and CD81-8C by performing a saturation binding assay. However, the results indicated that the KD values were similar for both proteins (74.45 nM and 75.11 nM), indicating that 5A6 binds equally well to wild-type and mutant CD81, and the epitope recognized by 5A6 was not disrupted when eight juxtamembrane cysteines were mutated (Fig. 2B). Consistent with the flow cytometry results, the saturation isotherm curve for wtCD81 (Bmax= 52.66) reached a plateau at a higher level than that of CD81-8C (Bmax= 22.66), indicating that cells expressing wtCD81 had approximately twice as many 5A6 binding sites as cells expressing CD81-8C. When flow cytometry was performed using a different anti-CD81 monoclonal antibody, JS-81, decreased expression levels of the CD81 mutants were also observed relative to wtCD81 (supplemental Fig. S1).
Fig. 2.
Surface detection of CD81 mutant proteins in BHK cells. (A) BHK cells were transfected with the indicated CD81 constructs and analyzed for surface CD81 expression by flow cytometry using 5A6. (B) 5A6 binding to CD81 was quantified by incubating BHK cells expressing wtCD81 or CD81-8C proteins with increasing concentrations of 5A6-FITC followed by flow cytometry analysis. (C) BHK cells expressing V5-tagged wtCD81 or CD81-8C were surface biotinylated, lysed, and biotinylated proteins were pulled down with Streptavidin beads. Immunoblots were probed with anti-V5 antibody. In panel A, the results shown are mean values +/− SE from 5 independent experiments normalized relative to wtCD81 levels (100%). In panel B, the results are mean +/− SE from two independent experiments.
This led us to hypothesize that the mutant CD81 proteins were not trafficking normally to the cell surface. To examine this, we surface-biotinylated cells expressing V5-tagged wtCD81 or CD81-8C. Following cell lysis, streptavidin was used to pull down biotinylated proteins and antibody against V5 was used to detect CD81 proteins (similar results were obtained when 5A6 was used; data not shown). These results showed an increase in the amount of biotinylated CD81-8C compared to wild-type (Fig. 2C). This increase correlated with the apparent increase in total protein levels seen with mutant CD81 proteins compared to wild-type (Fig. 1B and described below, Fig. 3A and Fig. 4). In addition, no difference in intracellular localization was observed when immunofluorescence was performed on BHK cells expressing wild-type or mutant CD81 (supplemental Fig. S2). Thus, mutation of juxtamembrane cysteines in CD81 did not appear to alter its localization to the plasma membrane.
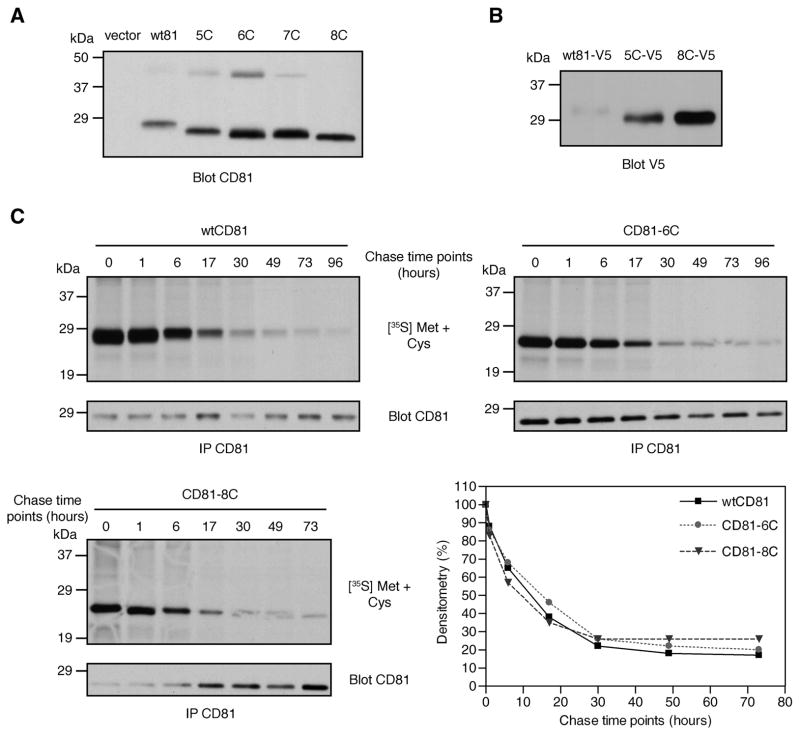
Fig. 3.
Expression and stability of CD81 mutant proteins. (A) BHK cells were transfected with the indicated CD81 constructs and analyzed for total CD81 levels by immunoblotting with 5A6. (B) V5-tagged wtCD81, CD81-5C, and CD81-8C were tested for expression by immunoblotting with anti-V5. (C) BHK cells expressing wtCD81, CD81-6C, or CD81-8C were pulse-labeled with [35S]-methionine/cysteine and chased for the indicated time periods. Following CD81 immunoprecipitation, samples were resolved by SDS-PAGE and gels were exposed to X-ray film. CD81 immunoblots were carried out using the same samples to show CD81 expression levels from each construct. Densitometry values were normalized to the amount of protein and the value obtained for 0 hours was set at 100% for each protein.
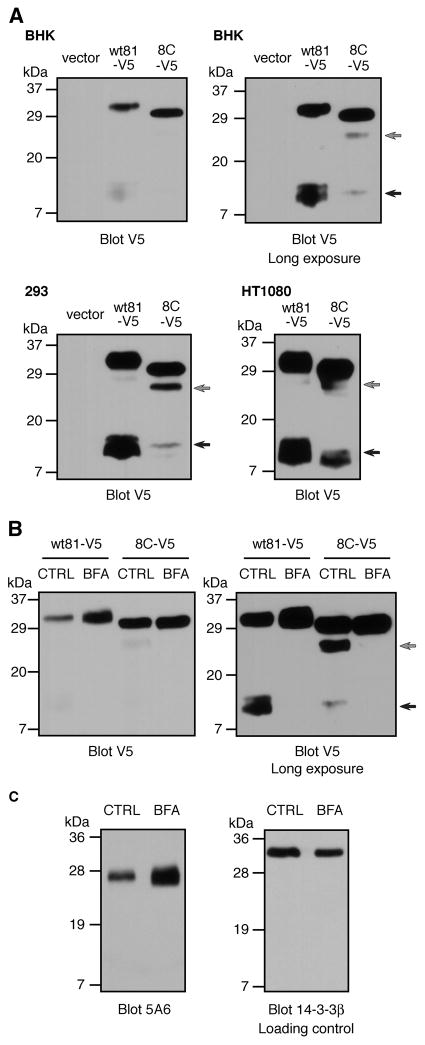
Fig. 4.
Processing of wtCD81 and CD81-8C proteins. (A) BHK, 293, or HT1080 cells transfected with V5-tagged wtCD81 or CD81-8C were immunoblotted using anti-V5 antibody. Faster migrating forms of CD81 are indicated by grey and black arrows. Two exposures of the same BHK immunoblot are presented to show the differences between wtCD81 and CD81-8C in the levels of full-length protein (upper left panel) and the faster migrating forms (upper right panel). (B) BHK cells transiently expressing V5-tagged wtCD81 or CD81-8C were treated with ethanol carrier (CTRL) or brefeldin A (BFA) and analyzed by anti-V5-antibody immunoblotting. A longer exposure of the same blot is presented (right panel) to show the CD81 processed forms (grey and black arrows). (C) Human HT1080 cells were treated with or without brefeldin A and endogenous human CD81 levels were analyzed by 5A6 immunoblotting. Levels of 14-3-3β were used as a loading control.
The apparent level of CD81 protein, but not protein stability, is increased when juxtamembrane cysteines are mutated
When total protein levels of the CD81 mutants were examined by immunoblotting using 5A6, an increase was consistently observed relative to wtCD81 (Figs. 1B and 3A). The mutant proteins also migrated faster on polyacrylamide gels, which was likely due to the absence of palmitic acid moieties. Similar results were observed when cells were lysed using a stronger detergent, Triton X-100 (supplemental Fig. S3), and when anti-V5 was used instead of 5A6 to detect V5-tagged CD81 proteins (Fig. 3B).
One possible explanation for the apparent increase in total expression of the mutant CD81 proteins is that the mutations affected the stability of CD81. To address this, we performed pulse-chase labeling on transfected BHK cells using [35S]-methionine/cysteine. Proteins were labeled for one hour and chased for various time intervals to measure the rates of protein degradation for wtCD81, CD81-6C, and -8C (Fig. 3C). Since CD81-6C and 8C had lower radiolabeling than wtCD81 (as expected, due to the absence of multiple cysteines), we normalized the densitometry values for each protein by taking time zero as 100%. No significant change in stability was observed among these proteins, with each having a half-life between 12 and 22 hours (values estimated from four independent experiments). Additionally, we observed a small upward shift in wtCD81 migration in the early time points that is not apparent in CD81-6C or -8C (Fig. 3C, compare 0 and 1 hour to 6 hours and later). We hypothesize that this decrease in migration is due to the addition of palmitic acid during CD81 maturation. These results indicate that palmitoylation does not affect the stability of CD81, and alterations in protein stability cannot explain the apparent increase in total protein expression of the mutants compared to wtCD81.
CD81 cleavage is inhibited by mutation of juxtamembrane cysteines or brefeldin A
The data presented thus far appeared contradictory: while our flow cytometry results indicated a reduced number of binding sites for anti-CD81 antibody on the surface of cells expressing the mutant CD81 proteins versus wild-type CD81, immunoblotting and surface biotinylation indicated that there were actually higher levels of the mutant CD81 proteins in the cells and at the cell surface. However, we then made an observation which explained this apparent paradox. When the expression of V5-tagged wtCD81 and CD81-8C proteins was examined by immunoblotting using anti-V5 antibody (Fig. 4A), we observed the appearance of additional faster migrating immunoreactive bands when blots were exposed longer: one band at 26–28 kDa (grey arrows) and additional bands at 10–12 kDa (black arrows). Interestingly, the amount of the 10–12 kDa bands in CD81-8C-V5 was greatly reduced compared to wtCD81-V5 (Fig. 4A). We did not observe the faster migrating 10–12 kDa bands on immunoblots using anti-CD81 antibody 5A6. This is consistent with these 10–12 kDa peptides arising due to proteolysis of CD81; since the 10–12 kDa fragments contain the C-terminal V5 tag, they would not be expected to include the 5A6 epitope, based on their size. On the other hand, the 26–28 kDa form probably does not arise from this same proteolysis event, since its levels correlated with those of full-length CD81 (Fig. 4A). Thus, CD81 appears to be proteolytically cleaved, and reduced cleavage of mutant CD81 explains the apparent increase in detection of full-length mutant CD81 by immunoblotting with either 5A6 or anti-V5 (Figs. 1, 3, and 4). Decreased amounts of the 10–12 kDa bands were also observed for CD81-8C compared to wtCD81 in other cell types (293 and HT1080 cells; Fig. 4A). In addition, similar bands were seen with C-terminally tagged CD9, indicating that this processing event may also occur with other tetraspanins (data not shown). These faster migrating bands were observed even though the cells were lysed in the presence of freshly prepared protease inhibitors, suggesting that they did not occur post-lysis.
We then used brefeldin A, an inhibitor of ER-Golgi transport, to examine whether CD81 targeting to the Golgi was required for the processing event observed in Fig. 4A. Surface levels of both wtCD81-V5 and CD81-8C-V5 (as measured by flow cytometry) were reduced by similar ratios after brefeldin A incubation (data not shown), verifying similar kinetics of transport to the cell surface. However, when cells expressing wtCD81-V5 or CD81-8C-V5 were assayed by immunoblotting with anti-V5, the levels of full-length wtCD81 were increased by brefeldin A treatment, while the levels of the 10–12 kDa bands decreased (Fig. 4B). Brefeldin A also had a similar effect on CD81-8C-V5 but it was less pronounced. The appearance of the 26–28 kDa CD81 band was also inhibited by brefeldin A (grey arrow).
Importantly, endogenous human full-length CD81 levels were similarly increased by brefeldin A treatment in human HT1080 cells as determined by immunoblotting with 5A6 (Fig. 4C), indicating that the observed processing event was not an artifact of CD81 transient overexpression (note that, as expected, the cleavage products were not detected by 5A6). These results suggest that the faster migrating forms of CD81 are due to posttranslational processing events that occur after CD81 enters the Golgi complex. Furthermore, the apparent increase in the levels of the full-length CD81 mutant proteins is because these proteins are not processed as readily as wild-type CD81. Regardless of whether this cleavage occurs in intact cells or post-lysis, it explains why we detect higher levels of full-length mutant proteins.
Association of EWI-2 or CD9 with palmitoylation-deficient CD81 is impaired
Since interactions with other molecules are critical for tetraspanin-mediated functions, we performed co-immunoprecipitations to examine the effect of cysteine mutagenesis on CD81 associations with other tetraspanin web members. We tested the ability of the CD81 mutants to bind to the tetraspanin CD9 and the Ig superfamily member EWI-2. Our results indicated that the amount of CD9 and EWI-2 co-immunoprecipitated by CD81 depended on the number of cysteines in the cytoplasmic and transmembrane domains (Fig. 5A and B). We observed a slight but consistent reduction in CD9 association to the palmitoylation-deficient proteins that was most pronounced when all eight cysteines were replaced (Fig. 5A). However, CD9 interaction with CD81-5C did not appreciably differ from interaction with wtCD81. When we assessed CD81 association with EWI-2, all mutated proteins co-immunoprecipitated EWI-2 at lower levels, with CD81-8C being the lowest (Fig. 5B). We also examined the surface levels of CD9 and EWI-2 when co-expressed with CD81-8C but did not detect any significant difference relative to co-expression with wtCD81 (Fig. 5C). In conclusion, the data suggest that mutating these cysteine residues affects the association of CD81 with CD9 and EWI-2 but does not affect the expression of CD9 or EWI-2 on the plasma membrane.
Fig. 5.
Association of CD81 mutants with CD9 and EWI-2. (A-B) BHK cells transfected with the indicated CD81 constructs and CD9-V5 or EWI-2-V5 were lysed with 1% Brij-99, and CD81 proteins were immunoprecipitated with 5A6. Immunoblots were probed with anti-V5 antibody to assess the co-immunoprecipitation of CD9-V5 (A) or EWI-2-V5 (B). In the right-hand panels, the relative amounts of CD9 and EWI-2 in the immunoprecipitated material or the supernatant were determined by densitometry. (C) Surface detection levels of CD9 (left panel) and EWI-2 (right panel) when co-expressed with wtCD81 or CD81-8C were assessed by flow cytometry using anti-EWI-2 or anti-CD9 (closed bars). CD81 levels were assessed using 5A6 (open bars). The transfected constructs are indicated below each panel.
DISCUSSION
This study presents novel findings concerning the effects of juxtamembrane cysteine mutagenesis on CD81 behavior in cells. Previous reports have used similar constructs to examine whether acylation had an effect on specific CD81 functions [23, 26, 27], but a general characterization of a palmitoylation-deficient CD81 has not been previously published. We found that complete inhibition of CD81 palmitoylation required mutagenesis of at least six juxtamembrane cysteine residues. In the course of studying the expression of these mutant proteins, we obtained data suggesting that the mutation of juxtamembrane cysteines in CD81 results in altered interactions between CD81 and other membrane proteins, which reduces access to CD81 by large molecules such as antibodies or proteases due to steric hindrance and/or localization in an altered membrane microenvironment.
Cysteine mutagenesis remains the most common method used to study the role of palmitoylation on a specific protein. To generate palmitoylation-deficient CD81 proteins, other groups have replaced five cysteines located in the intracellular domains at positions 6, 9, 89, 227, and 228 [23, 27]. However, the predicted three-dimensional conformation of the second transmembrane domain places another cysteine (position 80) at the plasma membrane-intracellular interface where it would be available for acylation [25]. Consistent with this model, we showed that CD81 proteins lacking only five cysteines still incorporate 25% of the wild-type levels of palmitic acid. However, the contribution of cysteines 97 and 104 to palmitoylation is unclear, since the sensitivity of the labeling assay used in this study cannot differentiate between the palmitoylation states of CD81-6C, -7C, and -8C. Since cysteines 97 and 104 are predicted to be embedded in the third transmembrane region [25], it is possible that their accessibility to palmitic acid is constrained by neighboring residues and phospholipids. Further studies using more sensitive methods such as mass spectrometry should provide more precise information on the number of cysteines that are available for palmitoylation.
Palmitoylation is known to regulate trafficking of a number of integral membrane proteins to the plasma membrane (e.g., CCR5, δ opioid receptor, thyrotropin receptor, and histamine H2 receptor) [18, 28–31]. Thus, the decrease in surface detection of the CD81 mutant proteins by flow cytometry initially led us to hypothesize that there was a defect in protein transport to the cell surface. However, this hypothesis was refuted when an increase in surface biotinylated CD81-8C compared to wtCD81 was observed, corresponding to the levels of total CD81 protein. An important difference between these assays is the use of biotin, a relatively small molecule (0.5 kDa), versus an antibody (150 kDa) to detect surface expression. Therefore, to explain these apparently contradictory results, we propose that these CD81 mutant proteins are expressed and transported to the cell surface normally, but once at the surface they become less accessible to bulky proteins such as antibodies. Either the lack of palmitoylation or structural alterations due to amino acid substitutions, or a combination of both factors, may cause CD81 to be displaced to a membrane compartment where it is surrounded by large neighboring proteins, thus reducing 5A6 binding by steric hindrance but still allowing normal binding by the smaller biotin molecule. A model illustrating this hypothesis is shown in Fig. 6. It has been shown that palmitoylation induces translocation of CD81 to lipid rafts [24]. Additionally, association of the CD81 mutant proteins with CD9 and EWI-2 is decreased compared to wtCD81 (Fig. 5), supporting the hypothesis that when these cysteines are mutated, the protein network surrounding CD81 is affected.
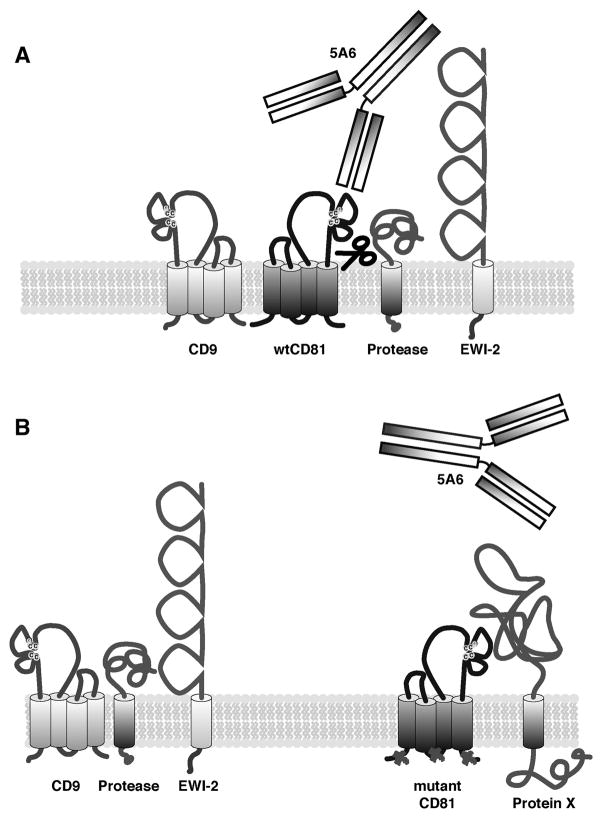
Fig. 6.
A model for how mutation of juxtamembrane cysteines affects the interactions of CD81 with other proteins in the membrane. (A) Wild-type CD81 resides in the plasma membrane, where it associates with many proteins as part of the tetraspanin web, including CD9 and EWI-2. A portion of CD81 undergoes cleavage by an unknown protease(s), and its large extracellular loop is accessible to anti-CD81 antibody 5A6. (B) Mutation of intracellular cysteines in CD81 affects the network of interactions surrounding CD81, resulting in reduced accessibility by 5A6 and proteases and reduced interactions with CD9 and EWI-2. This may be due to interactions with unknown protein(s) (indicated by Protein X) which “mask” or cover up CD81, or to localization of CD81 in different microenvironments in the membrane, or both.
While steric hindrance can explain the decreased surface detection of mutant CD81, another initially puzzling set of results was the apparent increase, using immunoblotting, in total CD81 protein levels when cysteines were mutated. We showed that protein stability was not affected in the CD81 mutants, and levels of mRNA expressed from the mutant and wtCD81 constructs were found to be similar (data not shown). Then, we observed additional faster migrating bands on immunoblots using an antibody specific for an epitope tag located at the C terminus of CD81, adding a new level of complexity to analyzing CD81 expression. An increase in full-length CD81 was observed when cells were treated with brefeldin A, which correlated with a decrease in the faster migrating CD81 bands. Thus, changes in full-length CD81 levels do not necessarily correspond to changes in total protein expressed, but may also reflect disparities in CD81 processing. The functional significance of these faster migrating CD81 forms remains to be understood. Other groups have used brefeldin A (at similar concentrations) and reported no difference in levels of endogenous tetraspanins including CD81 [20, 32]. However, when we tested the effect of brefeldin A on endogenous CD81 in a human cell line (HT1080), we observed an increase in full-length CD81 levels, implying that a similar type of processing occurs under physiological conditions. To our knowledge, the presence of similar faster migrating bands on tetraspanin immunoblots has not been previously reported. Most tetraspanin-specific antibodies would not detect these bands, since they bind at the large extracellular loop. There have been previous reports of shedding of soluble CD9-immunoreactive material into extracellular fluid by certain types of tumor cells [33]. It is not clear whether the CD81 processing that we have observed and this previously reported shedding of CD9 involve similar proteases or cleavage events. Given that brefeldin A inhibits protein transport to the Golgi complex, the most likely scenario is that the observed cleavage of CD81 occurs in the Golgi or plasma membrane. However, since brefeldin A also inhibits the maturation of certain proteases that traffic to the Golgi, we cannot completely rule out the possibility that the cleavage of CD81 occurs post-lysis, and that the inhibitory effect of brefeldin A is due to an effect on protease maturation.
Tetraspanins are relatively small proteins and have been described as “often hidden by a canopy of tall glycoprotein neighbors in the cell membrane” [1]. Thus, it is not difficult to envision that alterations in the organization of the tetraspanin web could result in CD81 becoming less accessible to large antibodies or proteases. At this time we cannot completely distinguish between the effects of inhibiting palmitoylation and possible conformational effects, but our results indicate that mutation of juxtamembrane cysteines in CD81 does alter the accessibility of the protein at the cell membrane, an observation which should be taken into account when assessing the role of palmitoylation in tetraspanin biology.
CONCLUSIONS
Mutagenesis of juxtamembrane cysteines is a commonly used strategy to study the effects of inhibiting palmitoylation on the function of proteins such as tetraspanins. Our results indicate that mutation of such residues can have unexpected effects on the accessibility of CD81 to antibodies and other proteins on the cell surface. The mutant proteins used in this study were expressed at normal levels, had normal stability, and trafficked normally to the cell surface. However, once at the cell surface they were less accessible to binding by antibody, less accessible to proteases, and bound less of the known CD81 binding partners CD9 and EWI-2 than wild-type CD81. These results suggest that the mutant CD81 proteins were localized in a different microenvironment in the plasma membrane than wild-type CD81. Such effects could have unexpected consequences regarding the interpretation of experiments designed to test the role of palmitoylation in tetraspanin function.
Supplementary Material
Acknowledgments
We thank Keith M. Woods for technical expertise on Real Time RT-PCR. This manuscript is Kansas Agricultural Experimental Station contribution #07-204-J.
ROLE OF THE FUNDING SOURCE: This work was supported by the National Institutes of Health grants AI052206, AI055052, RR16475, and RR17686, National Aeronautics and Space Administration grants NAG2-1274 and NCC-8-242, and the Kansas Agricultural Experimental Station. These funding sources had no role in the design or execution of this study, in writing this manuscript, or in the decision to publish.
References
- 1.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 2.Levy S, Shoham T. The tetraspanin web modulates immune-signaling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 3.Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- 5.Horvath G, Serru V, Clay D, Billard M, Boucheix C, Rubinstein E. CD19 is linked to the integrin-associated tetraspans CD9, CD81, and CD82. J Biol Chem. 1998;273:30537–30543. doi: 10.1074/jbc.273.46.30537. [DOI] [PubMed] [Google Scholar]
- 6.Shoham T, Rajapaksa R, Kuo CC, Haimovich J, Levy S. Building of the tetraspanin web: distinct structural domains of CD81 function in different cellular compartments. Mol Cell Biol. 2006;26:1373–1385. doi: 10.1128/MCB.26.4.1373-1385.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherukuri A, Shoham T, Sohn HW, Levy S, Brooks S, Carter R, Pierce SK. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J Immunol. 2004;172:370–380. doi: 10.4049/jimmunol.172.1.370. [DOI] [PubMed] [Google Scholar]
- 8.Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazaki T, Muller U, Campbell KS. Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 1997;16:4217–4225. doi: 10.1093/emboj/16.14.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsitsikov EN, Gutierrez-Ramos JC, Geha RS. Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc Natl Acad Sci U S A. 1997;94:10844–10849. doi: 10.1073/pnas.94.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maecker HT, Do MS, Levy S. CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune responses. Proc Natl Acad Sci U S A. 1998;95:2458–2462. doi: 10.1073/pnas.95.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, Yeung VP, Tsitoura D, DeKruyff RH, Umetsu DT, Levy S. Allergen-induced airway hyperreactivity is diminished in CD81-deficient mice. J Immunol. 2000;165:5054–5061. doi: 10.4049/jimmunol.165.9.5054. [DOI] [PubMed] [Google Scholar]
- 13.Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993;151:6470–6481. [PubMed] [Google Scholar]
- 14.Imai T, Kakizaki M, Nishimura M, Yoshie O. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J Immunol. 1995;155:1229–1239. [PubMed] [Google Scholar]
- 15.Witherden DA, Boismenu R, Havran WL. CD81 and CD28 costimulate T cells through distinct pathways. J Immunol. 2000;165:1902–1909. doi: 10.4049/jimmunol.165.4.1902. [DOI] [PubMed] [Google Scholar]
- 16.Mittelbrunn M, Yanez-Mo M, Sancho D, Ursa A, Sanchez-Madrid F. Cutting edge: dynamic redistribution of tetraspanin CD81 at the central zone of the immune synapse in both T lymphocytes and APC. J Immunol. 2002;169:6691–6695. doi: 10.4049/jimmunol.169.12.6691. [DOI] [PubMed] [Google Scholar]
- 17.Linder ME, Deschenes RJ. New insights into the mechanisms of protein palmitoylation. Biochemistry. 2003;42:4311–4320. doi: 10.1021/bi034159a. [DOI] [PubMed] [Google Scholar]
- 18.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 19.Charrin S, Manie S, Oualid M, Billard M, Boucheix C, Rubinstein E. Differential stability of tetraspanin/tetraspanin interactions: role of palmitoylation. FEBS Lett. 2002;516:139–144. doi: 10.1016/s0014-5793(02)02522-x. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Claas C, Kraeft SK, Chen LB, Wang Z, Kreidberg JA, Hemler ME. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol Biol Cell. 2002;13:767–781. doi: 10.1091/mbc.01-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berditchevski F, Odintsova E, Sawada S, Gilbert E. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J Biol Chem. 2002;277:36991–37000. doi: 10.1074/jbc.M205265200. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Liu L, Reddivari M, Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res. 2004;64:7455–7463. doi: 10.1158/0008-5472.CAN-04-1574. [DOI] [PubMed] [Google Scholar]
- 23.Clark KL, Oelke A, Johnson ME, Eilert KD, Simpson PC, Todd SC. CD81 associates with 14-3-3 in a redox-regulated palmitoylation-dependent manner. J Biol Chem. 2004;279:19401–19406. doi: 10.1074/jbc.M312626200. [DOI] [PubMed] [Google Scholar]
- 24.Cherukuri A, Carter RH, Brooks S, Bornmann W, Finn R, Dowd CS, Pierce SK. B cell signaling is regulated by induced palmitoylation of CD81. J Biol Chem. 2004;279:31973–31982. doi: 10.1074/jbc.M404410200. [DOI] [PubMed] [Google Scholar]
- 25.Seigneuret M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: conserved and variable structural domains in the tetraspanin superfamily. Biophys J. 2006;90:212–227. doi: 10.1529/biophysj.105.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvie O, Charrin S, Billard M, Franetich JF, Clark KL, van Gemert GJ, Saurwein RW, Dautry F, Boucheix C, Mazier D, Rubinstein E. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci. 2006;119:1992–2002. doi: 10.1242/jcs.02911. [DOI] [PubMed] [Google Scholar]
- 27.Bertaux C, Dragic T. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J Virol. 2006;80:4940–4948. doi: 10.1128/JVI.80.10.4940-4948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Percherancier Y, Planchenault T, Valenzuela-Fernandez A, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. Palmitoylation-dependent control of degradation, life span, and membrane expression of the CCR5 receptor. J Biol Chem. 2001;276:31936–31944. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- 29.Petaja-Repo UE, Hogue M, Leskela TT, Markkanen PM, Tuusa JT, Bouvier M. Distinct subcellular localization for constitutive and agonist-modulated palmitoylation of the human delta opioid receptor. J Biol Chem. 2006;281:15780–15789. doi: 10.1074/jbc.M602267200. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Nagayama Y, Nishihara E, Namba H, Yamashita S, Niwa M. Palmitoylation of human thyrotropin receptor: slower intracellular trafficking of the palmitoylation-defective mutant. Endocrinology. 1998;139:803–806. doi: 10.1210/endo.139.2.5911. [DOI] [PubMed] [Google Scholar]
- 31.Fukushima Y, Saitoh T, Anai M, Ogihara T, Inukai K, Funaki M, Sakoda H, Onishi Y, Ono H, Fujishiro M, Ishikawa T, Takata K, Nagai R, Omata M, Asano T. Palmitoylation of the canine histamine H2 receptor occurs at Cys(305) and is important for cell surface targeting. Biochim Biophys Acta. 2001;1539:181–191. doi: 10.1016/s0167-4889(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 32.Abache T, Le Naour F, Planchon S, Harper F, Boucheix C, Rubinstein E. The transferrin receptor and the tetraspanin web molecules CD9, CD81, and CD9P-1 are differentially sorted into exosomes after TPA treatment of K562 cells. J Cell Biochem. 2007;102:650–664. doi: 10.1002/jcb.21318. [DOI] [PubMed] [Google Scholar]
- 33.Komada Y, Sakurai M. Shedding of CD9 antigen in acute lymphoblastic leukemia. Leuk Lymphoma. 1994;12:365–372. doi: 10.3109/10428199409073777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.