Abstract
We have previously shown that when a stimulus consisting of two concentric rings moves, saccade latencies are much longer (by 150 ms) when attention is directed to the larger ring than to the smaller ring. Here, we investigated whether this effect can be explained by a deferral of the “cost” of making a saccade while the target remains inside the attentional field, or by purely visual factors (eccentricity or contrast). We found 1) latencies were shorter when attention was directed to small features irrespective of retinal eccentricity; 2) saccade latency distributions were systematically determined by the ratio between the amplitude of the stimulus step and the diameter of the attended ring: stimulus steps that were larger than the attended ring resulted in short latencies, whereas steps smaller than the attended ring resulted in proportionally longer and more variable latencies; 3) this effect was not seen in manual reaction times to the same target movement; and 4) suprathreshold changes in the contrast of targets, mimicking possible attentional effects on perceived contrast and saliency, had little effect on latency. We argue that the spatial scale of attention determines the urgency of saccade motor preparation processes by changing the rate and rate variability of the underlying decision signal, to defer the cost of saccades that result in little visual benefit.
INTRODUCTION
The classic experimental situation for studying saccades is to have a person or monkey fixating a target, which unexpectedly jumps to a new location, thereby eliciting a saccade. The latency of the resulting saccade has been much studied both as an exemplar of simple decisions and in terms of the temporal processes that make up the latency. With respect to the former, the latency distribution has been modeled either as a random-walk or diffusion process in which the decision variable is subject to noise at each moment (Ratcliff 1978) or as a process in which the noise is manifested by trial-to-trial changes in the rate of accumulation of information indicating that the target has moved [“LATER” (Linear Approach to Threshold with Ergodic Rate) model; Carpenter and Williams 1995]. These models have been reviewed by Glimcher (2003), Schall (2003), and Smith and Ratcliff (2004). With respect to the components of the saccadic latency, in addition to the influence of purely visual characteristics of the target and the constraints imposed by the time required to program and execute a saccade, attention can shorten the saccade latency either by being drawn to the target location by a cue or by being withdrawn from the previous location by having a gap before the target appears (Posner 1980; Weber and Fischer 1995).
Implicit in most latency models is the notion that, because vision is most acute at the fovea, it is desirable to make the saccade as soon as one can reliably decide where the target has moved to. The accumulating visual information has been seen as driving the decision variable (but see Ludwig et al. 2005). Here we will argue that saccades have costs as well as benefits, and thus in some circumstances it might be best to delay saccades.
Consider the situation in which one is fixating a frog hidden in the grass and one is trying to decide whether it is of an edible species (if one is a predator) or whether it is one’s pet frog (if one is a herpetologically inclined child). If the frog hops a few centimeters so that its image is now outside one’s fovea, the rate of visual information acquisition would be drastically reduced until one refixated it with another saccade—the cost of not making a saccade. However, during the saccade, the rate of information acquisition falls substantially and, if the frog jumps again during one’s saccade, one may lose track of its location entirely, so there are costs of making the saccade as well. In this case, the cost of not making the saccade would be greater than that of making a saccade and thus the saccade latency would be short.
Consider now that the frog is replaced by a rabbit (again either prey or pet). In this case, the hop of a few centimeters, much less than the size of the animal, would not impair one’s inspection of the rabbit by much, and so the cost of not making a saccade would be minimal, but the cost of making the saccade would remain. In this situation, it might be best to withhold the saccade. We posit that the higher brain centers involved in the discrimination need not inform the motor system of the details of the discrimination nor of the acuity required for it, but only of the spatial extent of the attended area.
This line of speculation is based on our recent finding (Madelain et al. 2005) that a sudden small movement of a large target produces shorter latencies when subjects are attending to a small feature of the target than when they are attending to a large feature. To be specific, when a stimulus consisting of two rotating concentric rings (Fig. 1A) stepped by an amount larger than the small ring but smaller than the large ring, saccadic latencies were much longer (by 150 ms, with essentially no overlap in the latency distributions) if subjects attended to the large outer ring than to the small inner one. Herein, we present results that show that the parameter that best predicts the latency is the ratio of the step amplitude to the diameter of the attended object. From this finding, we conjecture that the motor system may solve the cost/benefit ratio of making a saccade in the situation described in the preceding paragraphs by normalizing the effect of the target’s jump by the spatial scale of attention (that is, by dividing the amplitude of the jump by the diameter of the attentional focus). In this way, the oculomotor system could make the optimal decision of a short-latency saccade in the case of the frog (ratio >1) and a long-latency saccade (or no saccade) in the case of the rabbit (ratio <1).
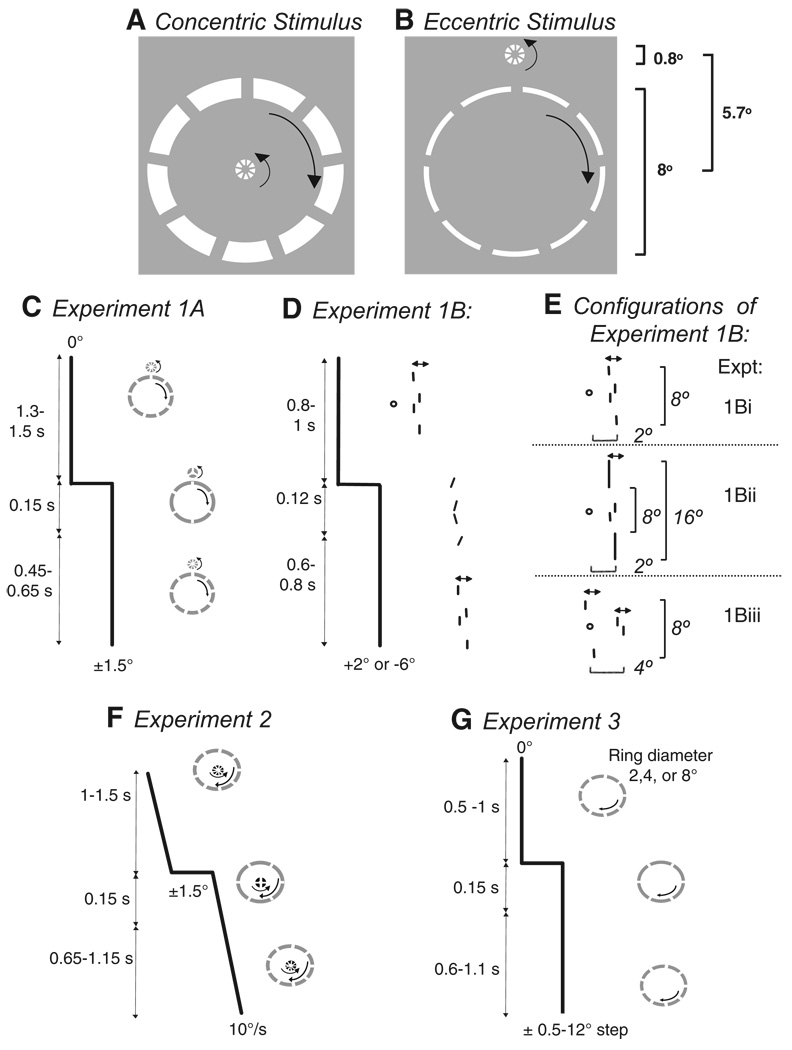
FIG. 1.
Stimuli and trial design. A: stimulus used both in the previous study (Madelain et al. 2005) and in experiment 2. A compound stimulus consisting of concentric 0.8 and 8° rings, rotating in opposite directions, stepped together by 1.5° and simultaneously the number of breaks in each ring changed briefly (150 ms). Subjects were required to report the number of breaks in the attended ring during the transient change. The task difficulty was adjusted for each ring size to yield 70–80% accuracy. B: stimulus used in experiment 1A. Subjects fixated the center of the large ring while attending to the rotating breaks in either the large (8°) or the small (0.8°) ring. The rings stepped together by 1.5° at which time the number of breaks changed for 150 ms. C: temporal sequence of a trial in experiment 1A. D: temporal sequence of a trial in experiment 1B. After the fixation point faded out, the oscillating line segments stepped and each line segment briefly stopped oscillating and changed its orientation. E: the 3 configurations of oscillating line pairs used in experiment 1B. F: temporal sequence of a trial in experiment 2. A compound ring stimulus (inner ring diameter 0.8°; outer ring diameter 8°) moves from −13° at 10°/s and, after a delay of 1–1.5 s, steps forward or backward by 1.5°. Three contrasts of the inner ring were used (0.2, 0.4, 0.8). G: temporal sequence of a trial in experiment 3. A single rotating ring of 2, 4, or 8° diameter steps by 0.5–12°.
In addition, we examine several nonmotor alternative explanations. First, the longer latencies while attending to the larger ring could have been unrelated to the scale of attention per se and simply have been due to retinal eccentricity, or to attention being divided between the two rings when one is attending to the outer one. We now test the latency to single rotating rings and dissociate the effects of eccentricity from those of attention field size by having the small stimulus be the more eccentric one. Second, because attention increases perceived contrast (Carrasco et al. 2004), perhaps broader attention fields lead to a smaller increase in the relative saliency of the compound ring stimuli steps, resulting in slower detection and reaction. We test this by assessing whether the latency difference is reduced by increasing the contrast of the inner ring. Third, saccades may require focal attention (McPeek et al. 1999) and thus may react generally more slowly and erratically if attention is required to be beyond a certain spatial extent; consequently, latency may be a function of the size of the attended ring. We assess this possibility by measuring the latencies to a wide range of step and ring sizes. Finally, we test whether this effect of the spatial scale of attention is specific to saccades by measuring the reaction time to a manual button-press to the same stimulus.
METHODS
Seven subjects (20–45 yr old, four male and three female) were involved over the course of the experiments. Four of the participants were naïve to the experimental design and hypotheses. Experiments were approved by the City College Institutional Review Board and all subjects gave their written informed consent.
In general, each trial required both a perceptual discrimination and a saccade. In most experiments, the discrimination required attending to a rotating segmented ring and reporting with a key-press the number of segments during a transient change that lasted 150 ms (see Supplemental video for a slowed simulation of the discrimination task).1 All ring stimuli had nine equally spaced breaks in their perimeter; rings <2° changed transiently to three or four breaks, larger rings changed to five or seven (two-alternative forced choice). To prevent the discrimination from being made by inspecting a single break, we kept the break sizes constant before, during, and after the transient change. In experiment 1B, the discrimination task consisted of two pairs of vertical lines, oscillating horizontally, that tilted transiently either in parallel or in opposite directions. Auditory feedback of the response accuracy was given after each trial. Task difficulty was adjusted in advance for each subject at each spatial scale to maintain an equal performance of about 70–80% correct responses in both the attend-small and attend-large conditions, by adjusting rotation speeds between 30 and 60 rpm or line tilts between 10 and 40°. The percentage of correct responses in each attention condition was well matched (overall averages of 73 vs. 74%), with only small differences across experiments and subjects. The task constrains spatial attention to the extent that detection of the number of breaks in the unattended ring is at chance level (Madelain et al. 2005). In contrast to the earlier experiments, we now made the oculomotor task explicit: Subjects were instructed to start at the screen center and to saccade to the center of the attended stimulus when it moved. Each attention condition was presented in alternating blocks of either 24, 30, or 64 trials, with the direction of the stimulus step varied randomly trial by trial. Each experiment was run on at least three subjects and consisted of ≥112 trials at each attention scale in each direction (between 480 and 3,024 trials per subject).
Experiment 1: does saccade latency depend on the size of the attended object or on its eccentricity?
In the stimulus used in our published experiments (Fig. 1A), the smaller central ring was at the fovea, thereby leaving unresolved whether the shorter saccade latencies when this ring was attended were due to the ring’s foveal location or its smaller diameter. The aim of this experiment was to distinguish between the effects of retinal eccentricity and attention scale. We carried out several variants of this experiment with different stimulus arrangements. In experiment 1A, we placed the small ring outside of the fixated large ring (Fig. 1B). The small (0.8° diameter) ring was offset by 5.7° from the center of the large (8°) ring. The width of the lines making up the small ring was the same as that in our previous study (0.2°) because it was not feasible to increase the line width in accordance with the cortical magnification factor (Rovamo and Virsu 1979) without changing the gross appearance of the stimulus; thus to achieve the same end, we reduced the width of the lines making up the large ring to 0.15°. Background and stimulus luminances were 6.4 and 23 cd/m2, respectively. Following a randomized delay (1,300–1,500 ms), the rings stepped to the left or right by 1.5° (Fig. 1C). Each subject was presented with 480 trials, divided equally between the attention-small and attention-large conditions.
In experiment 1B (Fig. 1, D and E), we replaced the two rings by two pairs of vertically aligned lines that spanned 1° (attend-small condition) and either 8 or 16° (attend-large condition). We instructed subjects to attend to the orientations of one line pair while maintaining fixation on a central fixation point. The lines oscillated by 0.5° around their mean horizontal position to help maintain attention at the correct scale. After a variable presentation period (800–1,000 ms), the fixation point faded out over 50 ms, whereupon the lines stepped 2° more eccentric or 6° across the midline. The lines simultaneously assumed a stationary tilt for 120 ms, before returning to vertical and oscillating around the new horizontal position. Subjects were asked to report via a keypad whether the attended pair of lines had been parallel during this interval.
We used three different configurations in three separate versions of experiment 1B (Fig. 1E). In the first (experiment 1Bi), the four vertical line segments were identical (0.4° long, 0.1° wide; inner lines spanning 1°, outer lines spanning 8°), vertically aligned and offset horizontally from the central fixation point by 2°, such that the centers of gravity of the inner and outer pairs were both at 2° of eccentricity. The second configuration (experiment 1Bii) also had the center of gravity of the line pairs at 2° to the left or right, but the outer line pairs were longer (4°) to compensate for their greater distance from the fovea and to encourage attention to span across a large area. The minimum separation of these outer lines was 8° (total span of 16°). Following displacement, these configurations were at 4° to the left or right of center. In the third configuration (experiment 1Biii), we equalized the eccentricity of the middle of each line, rather than of the center of gravity of the line pair, by having the centers of each line segment (0.4° long, 0.1° wide) lie on an invisible circle 4° in diameter, centered on the fixation point. Thus the center of gravity of the attend-small pair of line segments was 4° eccentric, whereas the center of gravity of the attend-large pair lay on the fovea. Both targets stepped together, either stepping out by 2° with the small line pair landing at 6° (large pair at 2°) or stepping 6° across the midline with the small line pair landing at 2° (with the large pair now 6° eccentric). Each configuration was tested over 1,024 trials (four sessions), with the side of initial and final target position randomized.
Experiment 2: effect of contrast on latency
Because focal attention can increase the apparent contrast of a stimulus (Carrasco et al. 2004), we considered the possibility that, when two rings were present, the smaller, inner ring might appear higher in contrast than the outer one and this might account for the shorter latency when it moved. If so, one would expect that the latency should be made shorter by increasing the actual contrast of the inner ring, even if attention were directed toward the outer ring. To test this conjectured explanation for the size-latency effect, we used a compound stimulus, consisting of two concentric rings, one 0.8° in diameter, the other 8° in diameter, like the stimulus used in the previous study (Madelain et al. 2005), but we varied the contrast of the inner ring over a fourfold range.
Specifically, the contrast [(Imax − Imin)/(Imax + Imin)] of the outer ring was kept constant at 0.2, whereas the inner ring contrast was varied between 0.2, 0.4, and 0.8 [background luminance (Imin) was 6.4 cd/m2; the ring luminances (Imax) were either 9.6, 14.8, or 56.1 cd/m2]. Subjects were instructed to attend to either the small or large rings in alternating blocks of 24 trials. The compound stimulus started 13° to the left of the screen center. Following a randomized delay (500–1,000 ms) the rings moved smoothly at 10°/s to the right and then, after a randomized interval (1–1.5 s), the rings stepped forward or backward by 1.5°, thereafter continuing to the right at 10°/s (Fig. 1F). The contrast and direction of perturbation were interleaved randomly. The number of breaks in the rings changed for 150 ms following the target step and subjects were asked to type on a keypad the number of breaks in the ring. Three subjects were run on 1,344 trials (112 trials per condition) spread over four sessions.
Experiment 3A: parametric study of effect of step amplitude and ring diameter
To understand the relationship between the size of the attended object and the amplitude of the step it makes, we systematically explored both parameters. Preliminary studies suggested that the latency depended on the ratio of stimulus step amplitude to the diameter of the attended ring, both for position steps during pursuit (Harwood et al. 2003) and during fixation (Madelain et al. 2004). We used these data to select a range of step amplitudes and ring diameters for a more thorough evaluation. Stationary rotating rings (contrast 0.2; background: 14.1 cd/m2; target: 21.1 cd/m2) with diameters of 2, 4, or 8° stepped by 0.5 to 12°, giving step-amplitude/ring-diameter ratios from 0.125 to 3 (Fig. 1G). We used 24 different step-amplitude/ring-diameter conditions, concentrating on the step/ring ratios up to one; the midsized ring was used at all 11 step amplitudes, but the largest step/ring ratios for the 2 and 8° rings were 2 and 1.5, respectively. Each condition was presented 112 times, except the smallest step at each ring diameter was presented twice as often because we expected fewer saccades to be elicited. The use of single rotating rings also tested whether the longer latencies observed when the larger ring in the compound stimulus was attended were related to attention being divided between the two rings. Three subjects (AK, MH, XZ) each performed 3,024 trials spread over seven sessions. To compare our new data with our previous findings, we also include data from one subject (LM) who had participated both in the previous experiments and in the preliminary studies (Madelain et al. 2004, 2005), but not in the final version of experiment 3. These data differed in that a 6° ring was included and the step amplitudes were slightly different.
Experiment 3B: manual reaction times to same stimulus steps
Our hypothesis that saccades are delayed when the visual benefit to be gained is low compared with the cost of impaired vision resulting from the saccade predicts that the size-latency effect should be restricted to the latencies of saccades, rather than response latencies in general. Therefore we tested two subjects with six amplitudes of steps (1–12°) of a 4° ring, with instructions to track the target with their eyes and to press a button as soon as they detected the target step. To make the attentional demands identical to those of experiment 3A, they were also instructed to report whether there were five or seven breaks in the ring during a 150-ms transient change in the rings by responding on a separate keypad at the end of the trial. Thus for each trial we collected the saccadic and manual reaction times, as well as the discrimination.
Data acquisition and analysis
Visual stimuli were generated by Vision Works software (Vision Research Graphics, Durham, NH) and displayed on a 200-Hz monochrome cathode-ray tube monitor at a viewing distance of 57 cm. The head was stabilized by use of a bite bar. Eye movements of the right eye were recorded by infrared video-oculography (ISCAN, Burlington, MA), at a sampling rate of 240 Hz, and calibrated before each experimental session by measuring seven fixations at each of seven equally spaced positions along the horizontal meridian (−15 to +15°).
Eye velocity was obtained from position traces using a two-point central difference algorithm (−3 dB at 27 Hz), which gives minimal bias in locating saccade start- and endpoints at our recording noise level (SD ~0.1°; Harwood 2003). Saccades were identified by the velocity being >10°/s for at least four consecutive samples and in the same direction as the target step. All records were reviewed off-line through a custom-written (Matlab, The MathWorks, Natick, MA) interactive graphics interface and trials contaminated with blinks were excluded.
Saccades with latencies <80 or >600 ms were excluded as being anticipatory or potentially unconnected to the movement of the visual stimulus. In experiments 1 and 2, we calculated median latencies based only on trials containing saccades. In experiment 3, we calculated median and cumulative probability distributions of saccade latencies in two ways: 1) only on trials containing saccades and 2) on all trials, considering trials lacking saccades as saccades with infinite latency (since otherwise the slope of the cumulative probability distribution would change depending on the proportion of trials containing saccades, e.g., double the slope if only 50% of the trials were considered). Both sets of analyses gave very similar results. Those presented are based on all trials. We excluded the 6 of 92 data sets that had <75% of trials containing saccades. The median and cumulative latencies of the remaining data sets were barely affected by including or excluding the no-saccade trials.
Decision model (LATER) analysis
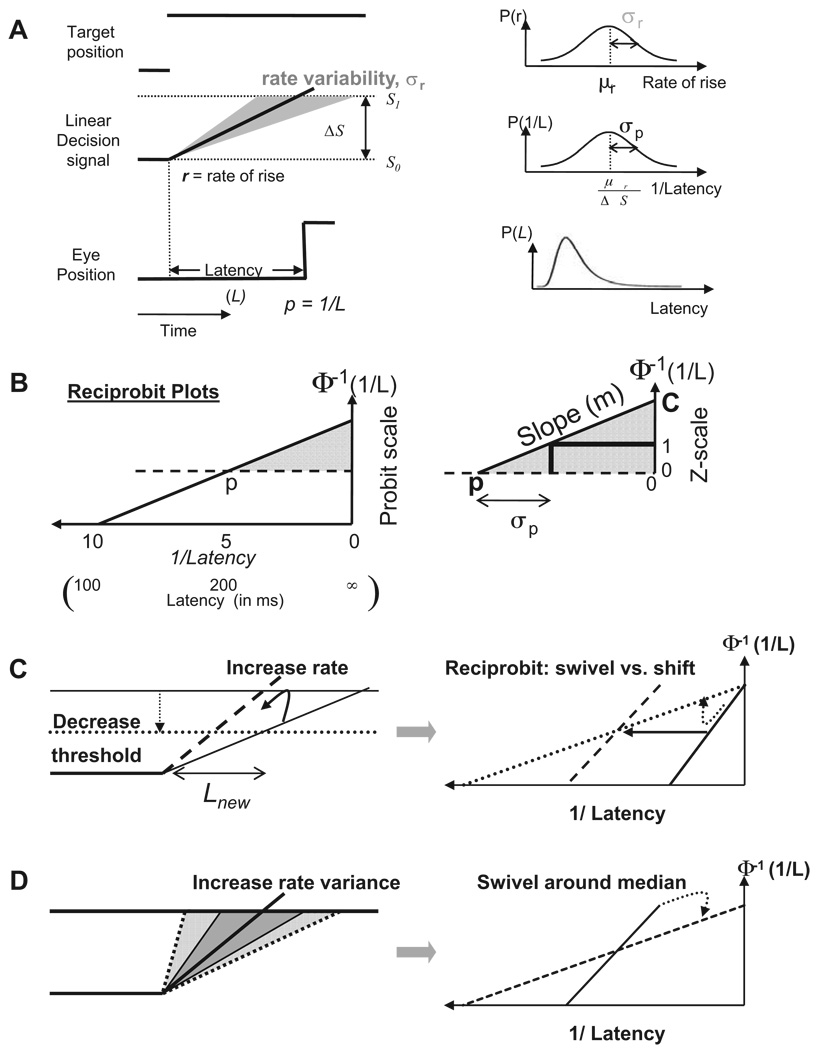
One simple model of decision processes has a decision signal rising linearly at a rate r from a baseline level of S0 to a threshold level of S1, at which point the decision (e.g., to move) is made (Fig. 2A, LATER model; Carpenter and Williams 1995). The ergodic rate of signal rise (r varying randomly from decision to decision, forming a Gaussian distribution of rates with mean μr) captures the skewed frequency distributions with long tails found in saccade latencies (Carpenter 1981). A Gaussian distribution of r produces a Gaussian distribution of reciprocal latency: the recinormal distribution. The reciprocal latency (or “promptness”) with mean p is directly related to the mean rate μr by the distance to threshold ΔS (Eq. 1). The mean promptness equals the median latency Lmed, rather than the mean latency, which is biased by the long tail of the latency distribution
| (1) |
In contrast to the variable threshold model of Grice (1968), the LATER model assumes that there is no stochastic variation in ΔS, and thus the SD of the rate signal σr is
| (2) |
Plotting the latency distributions as reciprocals on the x-axis versus an inverse Gaussian probability scale (Φ−1) (e.g., either a probit scale or a cumulative Z-score scale) on the y-axis gives straight reciprobit lines (Fig. 2B, left). On the right in Fig. 2B, we copy the top half of the reciprobit plot to an enlarged version from the median (Z = 0) to infinite-time intercept (c). By definition, each unit increase in Z corresponds to 1SD of the reciprocal latency (σp) and the slope of the reciprobit (m) is
| (3) |
and
| (4) |
By combining Eq. 1, Eq. 2, and Eq. 4, we also have
| (5) |
See Carpenter (1981) for a similar set of equations derived for probit scales (using the Gaussian error function and thus including ascale factor).
FIG. 2.
Linear decision signals. A: stimulus position, LATER model (see text) and saccadic response are shown in the left panel. The probability distributions of the rate (r), the reciprocal latency (“promptness”), and the latency (L) are plotted in the right panel. B: the cumulative distribution is plotted as a straight line on a reciprobit plot (left panel). An equivalent Z-scale (i.e., in SDs from the mean) plot is shown on the right for the top half of the distribution from the median to the infinite-time intercept (c). C: the same decrease in median latency can result either from a reduction in the action threshold, causing a swiveling of the reciprobit distribution around the infinite-time intercept (dotted lines), or by an increase in the mean decision signal rate, producing parallel shifts in the reciprobit lines (dashed lines). D: an increase in decision rate variance causes the distribution to swivel around the median latency.
These relationships are manifested in three simple modes of change in the distribution of saccades. First, if changing between experimental conditions results in the threshold being reduced but with the decision rate being unaffected (μr and σr constant), then the mean promptness (p) increases proportionately (Eq. 1), as does its SD (Eq. 2), resulting in no change to the infinite-time intercept (Eq. 4): the reciprobit curve swivels clockwise around its intercept (Fig. 2C, right dotted line). Alternatively, the same reduction in median latency can be accomplished by increasing the mean rate μr by an equivalent proportion, leaving σr and ΔS constant, which increases p but leaves σp constant, resulting in a proportionate increase in c: the reciprobit line shifts leftward in parallel (dashed line). A third possibility (Fig. 2D), usually excluded from conventional LATER models, is that the SD of the rate signal σr increases, thereby decreasing the intercept for a fixed mean rate and threshold (Eq. 5), resulting in a swivel around the median, which remains constant (Eq. 4). Evidence in favor of this possibility comes from the finding that one can train subjects to alter the variability of their latencies without altering the median latency (Madelain et al. 2007).
Carpenter and Williams argued that the decision signal represents a log-likelihood computation. If a variable is a major determinant of the decision signal, then the median latency should be modulated by the log of this variable. In particular, to test whether the decision rate is modulated by the ratio of step amplitude to ring diameter, we argue that this variable augments a LATER process of rate r
| (6) |
By applying the relationship among median latency, distance to decision threshold (ΔS), and rate as in Eq. 1, and adding proportionality constant A, we have
| (7) |
which we fitted to our median latency data.
We used reciprobit plots to characterize the shape of the complete latency distributions and to relate these to decision models. Any two of the median latency, reciprobit slope, and infinite-time intercept variables completely characterize straight reciprobit distributions. The most appropriate choices for our data were the reciprobit slope, which provides a more useful measure of the distribution dispersion than the latency SD (since the latency distribution is positively skewed) and the median. We measured reciprobit slopes by linear regression on the central portion of the distribution (median ± 1SD, i.e., 16th to 84th percentiles) and used cumulative Z-score ordinates to emphasize the connection between reciprobit slope and the variance of the distribution.
RESULTS
Across all experiments, we found that saccadic latencies to steps of a stimulus were remarkably shorter when attention was directed to a spatial scale that was small relative to the size of the step. This size-latency effect did not rely on retinal eccentricity or visual salience. In experiment 3 we provide strong quantitative support for our hypothesis that the effects of the spatial scale of attention affect saccade initiation at a motor decision stage.
Effect of retinal eccentricity
Because our previous experiments left it uncertain whether the short latencies when attending to the small central ring were due to its size or its foveal location, we manipulated the relative eccentricity of attend-small and attend-large stimuli in four ways, all of which resulted in shorter latencies for the attend-small condition.
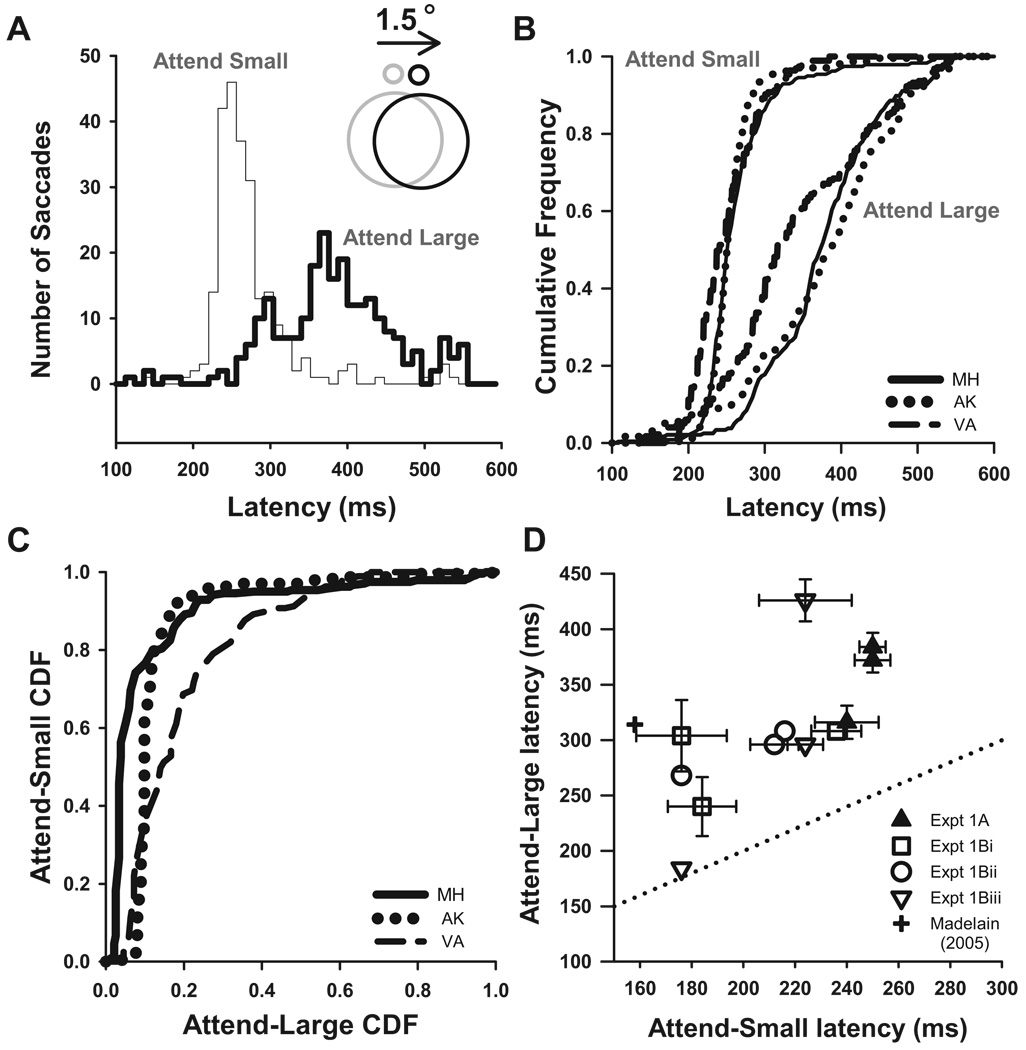
When the subject fixated a large ring and a small ring was positioned outside of it, and both rings moved together (experiment 1A), saccade latencies were 110 ms shorter if subjects were attending to the small ring (Fig. 3A; mean of 247 ms for attend-small vs. 357 ms for attend-large). The medians were significantly different between attention conditions in each subject (Wilcoxon rank-sum tests: P < 0.001), as were the cumulative distributions (Fig. 3B; Kolmogorov–Smirnov statistics: 0.38, 0.44, 0.46; P < 0.001), although the differences in latency were less than those in our previous experiments with the small ring centered at zero eccentricity (158 ms for attend-small vs. 314 ms for attend-large). The area under the receiver-operating-characteristic (ROC) curves (Fig. 3C) showed that the distributions were sufficiently separated to identify the attention condition based on the latency of a single saccade with accuracies of 81, 87, and 90% in each of the three subjects, values similar to the 94% accuracy we had observed when the small ring was in the center of the large ring (Madelain et al. 2005).
FIG. 3.
Effect of varying the eccentricity of the smaller stimulus. A–C: saccadic latencies in experiment 1A, when attention is directed to the large central ring (attend-large) vs. to the eccentric small ring (attend-small). A: latency histograms for a typical subject. B: cumulative frequency distributions for 3 subjects. C: receiver-operating-characteristic (ROC) curves for the 3 subjects, plotted as attend-small cumulative distribution functions (CDFs) against attend-large CDFs. D: summary of median latencies from all the stimulus configurations in experiment 1. Plus symbol indicates the average latency from the previous paper (Madelain et al. 2005). Each experiment was run on 3 subjects. Error bars are 95% confidence intervals, and a line of equality (dotted) is plotted. Error bars smaller than the symbol are not shown.
To test whether the size-latency effect holds more generally than for rings, we tested three configurations of line segments. First, when the centers of gravity of the attend-small line pairs (1° separation) and the attend-large line pairs (8° separation) were equated at 2° eccentricity (Fig. 1E, experiment 1Bi), the size-latency effect was still manifest (199 vs. 284 ms; squares in Fig. 3D; Wilcoxon rank-sum tests, P < 0.001). Second, when we used longer lines, more separated from each other, in the attend-large condition to ensure that a large attention field encompassed the whole line-pair span (Fig. 1E, experiment 1Bii), this configuration gave similar latency differences (circles; 202 vs. 291 ms; Wilcoxon rank-sum tests, P < 0.001). Again, the important result is that attending to the closely separated lines gave much shorter latencies than attending to the more separated pair. Third, when we equalized the radial eccentricity of each line segment around an imaginary 4° circle, rather than equalizing their center of gravity (Fig. 1E, experiment 1Biii), we again found shorter latencies for the small attentional scale, despite the fact that the line segments relevant to the attend-large condition were now centered at the fovea and the attend-small line segments were 4° laterally offset (open triangles; 208 vs. 302 ms). In this case, all subjects had significant median latency differences (Wilcoxon rank-sum tests, P < 0.05), which were highly significant in two of the three subjects (Wilcoxon rank-sum tests, P < 0.001).
In summary, in all four stimulus configurations, the latency was primarily determined by the size, not the eccentricity, of the attended part of the stimulus, so that saccadic latencies were shorter when attention was on small eccentric targets than when it was on large central ones (Fig. 3D), although the saccadic latency is even shorter for steps of an attended foveal stimulus (plus in Fig. 3D; Madelain et al. 2005).
Varying the contrast of the inner ring
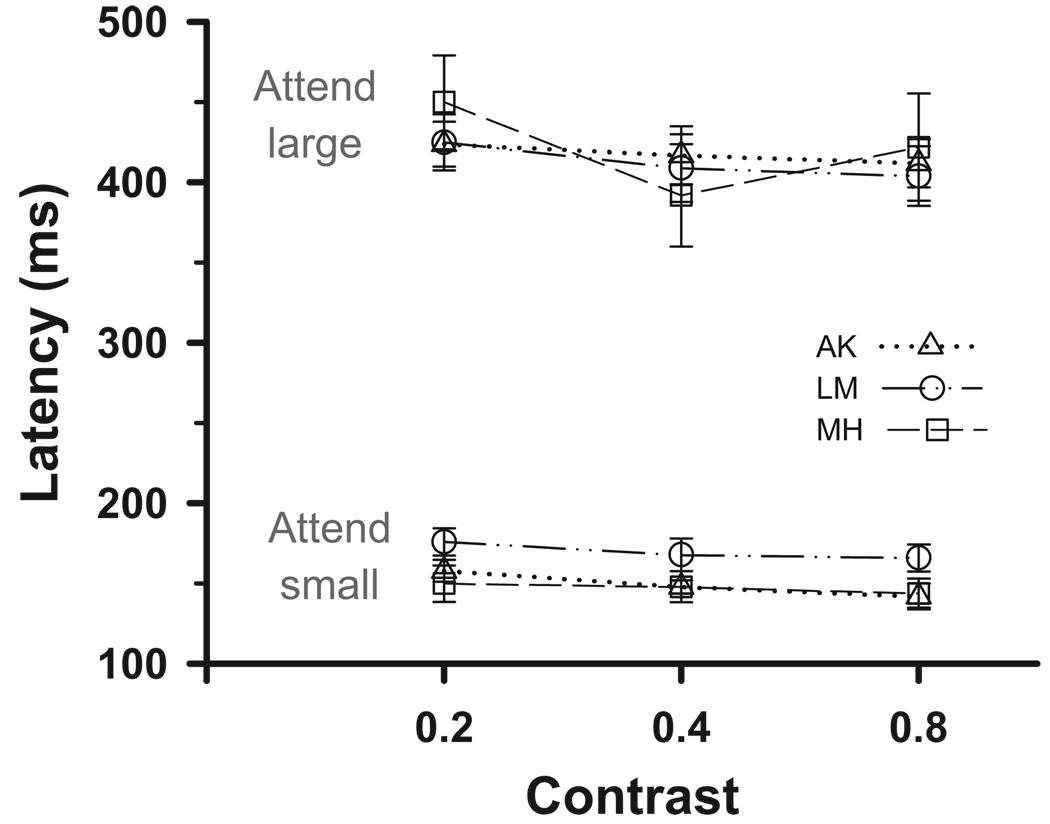
To test whether the size-latency effect was related to changes in the perceived contrast of the small ring, we used compound stimuli consisting of two concentric rings, one 0.8° and the other 8°, and raised the contrast of the inner ring. The rationale was that if attending to the small ring gave shorter latencies because attention increased the apparent contrast of the inner ring step, then increasing the actual contrast of the inner ring should mimic this process and therefore reduce the latency while attending to the large ring. Figure 4 shows this not to be the case. Across the fourfold increase in contrast, decreases in latencies were small and similar in both attention conditions (11 and 20 ms for small vs. large, averaged across subjects and directions). There was a main effect of contrast on latencies (ANOVA, F = 7.18, P < 0.01), but there was no interaction of attention condition with contrast (ANOVA, F = 1.91, P > 0.05). In other words, the magnitude of the size-latency effect was unchanged by the contrast, even though there were small latency changes that were related to contrast. Furthermore, when the contrast of the outer ring was increased to match the inner ring, the same size-latency effect was present. For example, subject LM had latencies of 158 and 392 ms for attend-small and attend-large when both rings were at their highest (0.8) contrasts (note these data are not plotted in Fig. 4).
FIG. 4.
Effect of varying the contrast of the inner ring. Median saccade latency in response to 1.5° steps during pursuit of a compound ring target, for each of the 3 subjects attending either to the 0.8° inner ring or to the 8° outer ring. The outer ring contrast was constant (0.2). Error bars represent 95% confidence intervals.
Single rings of varying diameter and step amplitude
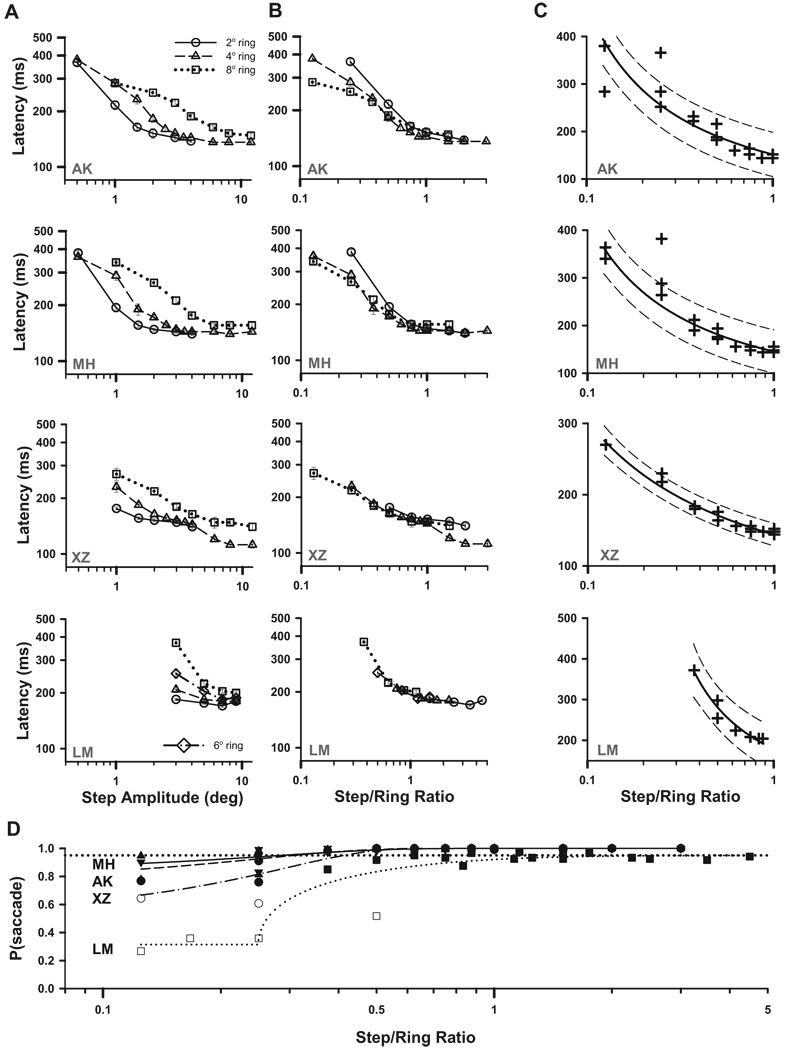
To test our hypothesis that saccades are delayed while the target remains within the attentional field, we parametrically examined combinations of target steps and ring sizes to give step amplitudes that were specific proportions of attended-ring size. The midsized ring was the only ring tested at all 11 ratios of step amplitude to ring diameter.
MEDIAN LATENCY
For a given attended ring diameter, saccade latency depended on the step amplitude (Fig. 5A). Equally, for a given step amplitude, latency depended on ring diameter. Our most striking finding was that the saccadic latencies were best described by the ratio of step amplitude to ring diameter. When plotted this way, the several curves of Fig. 5A tend to collapse into a single curve, as shown in Fig. 5B. The general pattern was that as the ratio increases the latency decreases until, when the ring was stepped by more than its diameter (i.e., no overlap between prestep and poststep target area, step/ring ratios >1), the latency reaches a minimum asymptote. When the ring is stepped by less than the ring diameter (ratios <1), the latencies are well described by a power law as indicated by the approximately straight lines on log-log axes (Fig. 5B). A line fitted over this range of ratios, according to Latency = a(step/ring)b, accounted for 87% of the variance in the medians on average (r2 range: 0.83–0.90) and 57% of the variance of individual latencies (r2 range: 0.52–0.66). (Best fits of latency as a function of step amplitude alone explained less variance, 57 and 38%, respectively, for medians and individual latencies.) The exponent b had a mean of −0.51.
FIG. 5.
Effect of varying the diameter and step amplitude of single rings. A: median latency plotted on a log-log scale against step amplitude for rings of 2, 4, and 8° diameter, for each subject. Note that the midsized ring was the only ring tested at all step amplitudes, yielding 11 ratios of step amplitude to ring diameter. The data for subject LM included an additional ring diameter (6°) and different step amplitudes (1–9°). Error bars are 95% confidence intervals. B: median latency plotted against the ratio of step amplitude to ring diameter. C: same data plotted on a linear ordinate scale for each subject up to a step/ring ratio of one. The solid curve fits the median latencies according to: Lmed = ΔS/{r + A × [log (step/ring)]}, with ΔS set to 1. The dashed curves are 95% prediction bounds for the fit. D: the probability of each subject making a saccade at each step/ring ratio condition, with Weibull fits overlaid. The horizontal dotted line marks the 95% saccade probability. Open symbols indicate the 6/92 conditions excluded both from further analysis and from A–C.
When the data are plotted in terms of the log of the step/ring ratio against linear latency (Fig. 5C), as will be required to evaluate whether they conform to the expectations of an optimal decision process based on log-likelihood (see METHODS and DISCUSSION), weighted regressions described the median latencies well, explaining 93% of the variation in the medians (higher r2 than linear fits), despite the poorer fit of the data points from the smallest step/ring ratio.
Longer latencies at low step/ring ratios were also associated with a reduced probability of making a saccade (Fig. 5D). We excluded the 6 of 92 conditions with <75% of trials containing saccades from the analysis (open symbols). We found that irrespective of whether we included the trials in which no saccades were made had little consequence for any of the parameters of the remaining data sets.
Latencies were similar whether the stimulus was a single ring or two rings (e.g., the median of these four subjects was 340 ms for a 1° step to an 8° ring, compared with 314 ms in the previous study for a 1.5° step of an 8° compound ring). Thus the size-latency effect was not caused by a division of attention between the two rings of the compound stimulus.
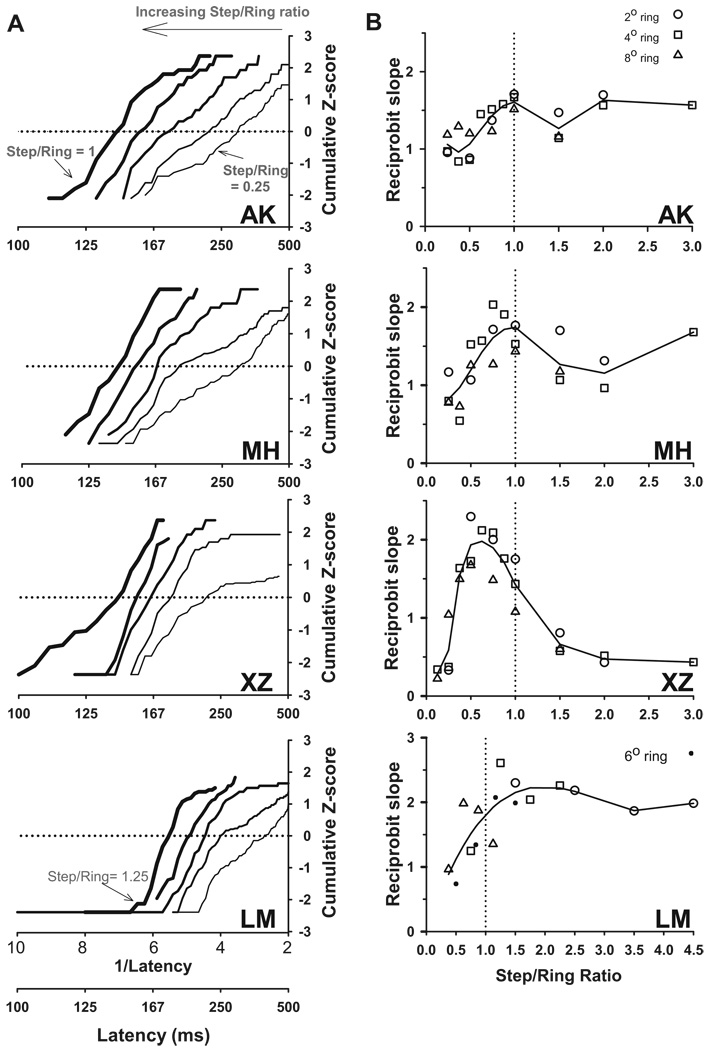
CUMULATIVE DISTRIBUTIONS
Like the medians, the slopes of the cumulative saccade latency distributions (i.e., the variability of the reciprocal latencies) collapsed across ring size as a function of step/ring ratio. To describe the shape of the latency distribution with respect to the step/ring ratio, we plotted the data on reciprobit axes (see METHODS). Figure 6A shows typical distributions for five step/ring ratios. The latency distributions for progressively larger ratios shifted leftward (shorter latencies), staying predominantly parallel to each other, as opposed to swiveling around the infinite-time intercept. This implies that the rate of the decision variable increases with increasing step/ring ratios, as opposed to the threshold decreasing. To quantify these shifts, we performed linear regressions on the middle 68% of the distribution (i.e., median ± 1SD), over which range the slopes were highly linear (goodness-of-fit, r2: 0.85–0.99). The reciprobit slopes for different ring diameters formed a single curve when plotted as a function of the step/ring ratio (Fig. 6B). Regressions over the central 95% had similar slopes and goodness-of-fits (r2: 0.73–0.99), but with more variable slopes because of the paucity of points in each tail and because different decision strategies could have been used at either end of the distribution, as discussed in the following text.
FIG. 6.
Reciprobit plots of latency distributions. A: cumulative latency distributions for each subject on reciprobit axes (i.e., cumulative Z-score vs. reciprocal latency). The ratios shown are 0.25, 0.375, 0.5, 0.625, and 1.0 for subjects AK, MH, and XZ for the 4° ring; and 0.375, 0.5, 0.625, 0.875, and 1.25 for LM over 4, 6, and 8° ring sizes. Larger target steps shift the reciprobit lines to the left, forming an approximately parallel family of curves (bins were 5 ms wide). B: regression slopes for all conditions (excluding ratio = 0.125 for AK, MH) plotted as a function of step/ring ratio. Fitted curves are 5th-order polynomials (9thorder for XZ). Note the different y-axes and x-axis for LM panel. Trials that did not contain saccades were included as saccades with infinite latency (see METHODS).
The key observation from the cumulative distributions is that all subjects showed the trend of increasing reciprobit slopes up to a step/ring ratio of approximately 1, and generally decreasing thereafter. We subsequently interpret the implications of this finding for the decision process.
In contrast to the similarity of all subjects in the range of ratios from 0.25 to 1.0, individual differences were evident at ratios outside this range. In one subject, XZ, bimodal latency distributions emerged at ratios >1, with an express saccade latency peak around 115 ms, which mostly accounted for her lower slopes (~0.5) at the highest ratios. At low ratios, we found that some subjects had long latencies and high slopes when plotted on reciprobit axes. To illustrate this pattern, we have plotted in Supplemental Fig. S1 the data from the lowest two ratios (0.125, 0.25) from subjects AK and MH of the present study and two similar subjects from our previous study. The low slope case (AK at 0.25, offset by 2SD for clarity) was most typical of distributions at low ratios. The cumulative distributions of the long-latency cases are as straight (unimodal) as that of the short-latency case, but with higher slope. The intermediate case (MH at 0.25) has a normal slope (<1) for his shorter-latency saccades, but a higher slope (>1) for the longer-latency ones. Perhaps when the normal decision processes were not leading to a saccade, subjects may have usurped these by a higher-level command (e.g., “enough already, just go”), resulting in “impatient saccades,” an explanation similar to that Reddi and Carpenter (2000) used to explain anticipatory saccades or to that of Ditterich (2006) who posited an increasing decision rate signal within a trial to increase responses to weak perceptual stimuli. These differences at the highest and lowest ratios tested explain the finding in our previous paper of two distinct patterns among our subjects. On the basis of our much finer-grained analysis in the present study, we see that all subjects were similar in the region of ratios between 0.25 and 1.0.
In addition to slope measurements, the infinite-time intercept is often used to characterize reciprobit distributions (e.g., Reddi et al. 2003). Over the latency-changing range of our data (ratios <1), these intercepts increased both as a function of the leftward shift of the median with increasing step/ring ratio and as a function of the increased reciprobit slopes. Thus these distributions formed a diverging family of curves with respect to the infinite-time intercept.
SACCADE AMPLITUDES
The size-latency effect cannot be explained by subjects progressively trading off saccade accuracy for faster reaction times as the step/ring ratio increases. Saccade amplitudes were highly correlated with target displacement (r = 0.95). Saccade gain was approximately flat (mean: 0.91) for ratios between 0.25 and 2, but exhibited a small range effect (Kapoula and Robinson 1986), overshooting to the smallest ratio and undershooting to the largest. Saccade variability showed the typical increase of SD with saccade amplitude (van Opstal and van Gisbergen 1989), although variability was high at the smallest step/ring ratio. Saccade amplitude accounted for only 4.3 ms of latency in a multiple regression of latency with saccade amplitude and step/ring ratio. Thus variations in saccade amplitude could not explain the size-latency effect.
We are also confident that subjects were responding to the movement of the whole ring, rather than targeting the nearest ring edge, or simply responding arbitrarily to the instruction to make a saccade. First, the reader can see from the simulation of the stimulus (Supplemental video) that it is much easier to do the discrimination by keeping one’s eyes and attention centered on the ring. Second, subjects did not make fewer saccades to step/ring ratios of around 0.5, for which one side of the ring would land near the fovea (Fig. 5D, with the exception of LM in one condition). If subjects targeted one portion of the ring, one would expect fewer saccades if the attended portion landed on the fovea. Third, as just mentioned, the saccade gains were high and the saccade amplitudes were highly correlated with target displacement. Although the instruction to make a saccade may have altered behavior at the lowest step/ring ratio resulting in larger and more variable saccades (perhaps reflecting an endogenous attempt to initiate a saccade faster than would normally be generated by the weak exogenous shift in the stimulus, as suggested for the preceding cumulative distributions), saccade amplitudes and variability displayed no other abnormal features.
TASK EFFECTS
Varying difficulties of the discrimination task also cannot explain the effect of attention scale on latency. In experiments 1 and 2, psychophysical accuracy was the same for attend-small and attend-large (75 and 72%, respectively; paired t-test: P > 0.1) and yet latencies were very different. In experiment 3, although accuracy differed across ring diameters (61, 72, and 78% for 2, 4, and 8° rings) and decreased with increasing step/ring ratio, task accuracy accounted for only 1.4 ms of latency (P > 0.1; multiple regression of latency against step/ring ratio and accuracy). The partial correlation of accuracy with latency, holding the step/ring ratio constant, was r = 0.01. Although the task difficulties were not as well matched across ring sizes as they had been in experiments 1 and 2, latency was clearly determined by the step/ring ratio and not by task accuracy. Therefore subjects did not trade off task accuracy for faster reaction times.
However, to further demonstrate that the size-latency effect does not originate from trade-offs between the discrimination and oculomotor tasks, two subjects each repeated three sessions of experiment 3 (1,296 trials) without the requirement to report the transient change in the stimuli. Latency curves were similar to those recorded in the main experiment and collapsed onto the function of step/ring ratio (Supplemental Fig. S2A). Latencies from the main experiment were mostly shorter (dashed curves) than those without the discrimination task and the average difference over the asymptotic latency region was 29 ms. This task-specific reduction in latencies could not strategically improve task performance because even the shortest latency saccades were almost all counterproductive since the discrimination target had disappeared by the time the saccade landed. Even the fastest subject at her fastest step/ring ratio (XZ at step/ring ratio = 2) landed on average 10 ms after the discriminandum disappeared and never got even 20 ms of postsaccadic viewing time.
The most prominent task-specific effect was that, without the discrimination task, the slopes of the cumulative distributions did not increase with the step/ring ratio (Supplemental Fig. S2B). The reciprobit slopes were constant for step/ring ratios ≤1 (i.e., shifting in parallel with increasing ratio) and were near the peak value seen in the main experiment. At higher ratios, the reciprobit slopes were approximately the same with or without the task requirement.
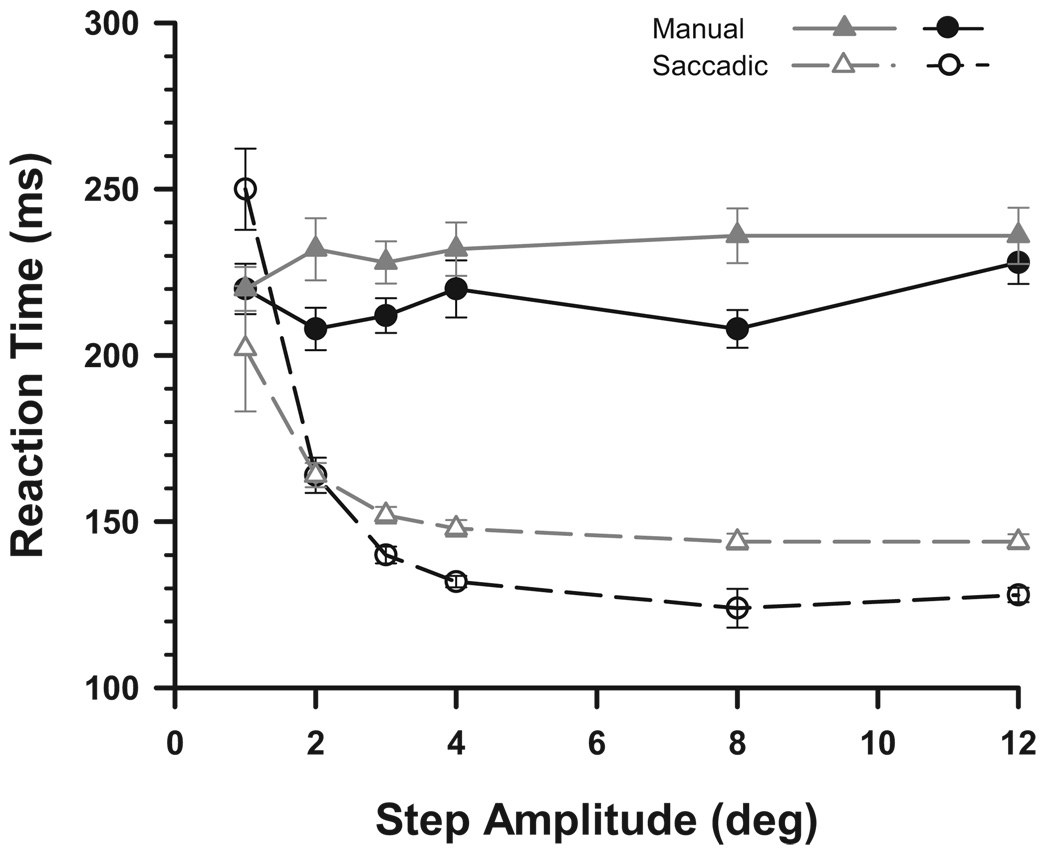
Manual reaction times
When subjects were instructed to press a button as soon as they detected the target step and also to track the target with their eyes, no effect of step amplitude on manual reaction time was observed (Fig. 7). This confirms that the size-latency effect is due to saccade motor planning. The flat function of manual reaction times implies that all step amplitudes are equally detectable. In contrast, when the ring stepped by less than its 4° ring diameter, saccades to the same stimulus step were substantially delayed, compared with the minimum latency.
FIG. 7.
No size-latency effect for manual reaction times. Two subjects did a control experiment using the 4° ring of experiment 3 with 6 step sizes. Subjects were instructed to press a button as soon as they detected the target step and to also track the target with their eyes. They reported their ring discrimination response at the end of a trial on a separate keypad. The target step sizes were equally detectable, as indicated by the flat function of manual reaction time. Error bars represent 95% confidence intervals.
Decision model analysis
Stochastic accumulator decision models, such as the LATER (see METHODS) or diffusion models (Ratcliff 1978; Ratcliff and Rouder 1998), can change median latency by altering either the rate of accumulation of the decision variable or the distance of the action threshold from the starting level of the decision variable (biases based on prior information).
WHICH DECISION PARAMETERS DETERMINE THE EFFECT OF THE STEP/RING RATIO ON LATENCY?
We infer that latency falls with increasing step/ring ratio (Fig. 5 and Supplemental Fig. S2) because the decision rate increases, rather than because the decision threshold is lowered. This is evident in that the slopes of the reciprobit plots are largely parallel to one another (Fig. 6). If the shorter latencies had resulted from a decrease in threshold, the LATER model predicts that the slope of the cumulative distribution would decrease as the step/ring ratio increased (swiveling clockwise around the infinite-time intercept; Fig. 2B, dotted line). Instead, the slopes were constant (experiment without a discrimination task) or increased rather linearly with the step/ring ratio, reaching a peak near a ratio of 1 (Fig. 6B).
This interpretation is consistent with the fact that, in a wide variety of sensory tasks, there is an asymptotic power relationship between reaction time and stimulus intensity (Pieron 1914; Pins and Bonnet 1996). Thus Fig. 5B can be interpreted as a motor version of Pieron’s law with step/ring ratio acting like stimulus intensity. Pieron’s law can be reproduced by a diffusion model in which accumulation rate is proportional to stimulus strength (Palmer et al. 2005). Similarly in the LATER model, increasing stimulus strength causes a directly proportional increase in mean decision rate signal, which reduces median latency.
If the slopes of all our cumulative latency distributions had been constant, the conventional LATER explanation would be unambiguous. However, when we required our subjects to make a discrimination, in addition to an eye movement, all of them showed increasing slopes as the step/ring ratios increased up to a value of 1, and decreasing slopes thereafter. Because the conventional LATER model explanation attributes slope changes to threshold changes, we must consider whether our data should be explained by a combination of rate and threshold changes.
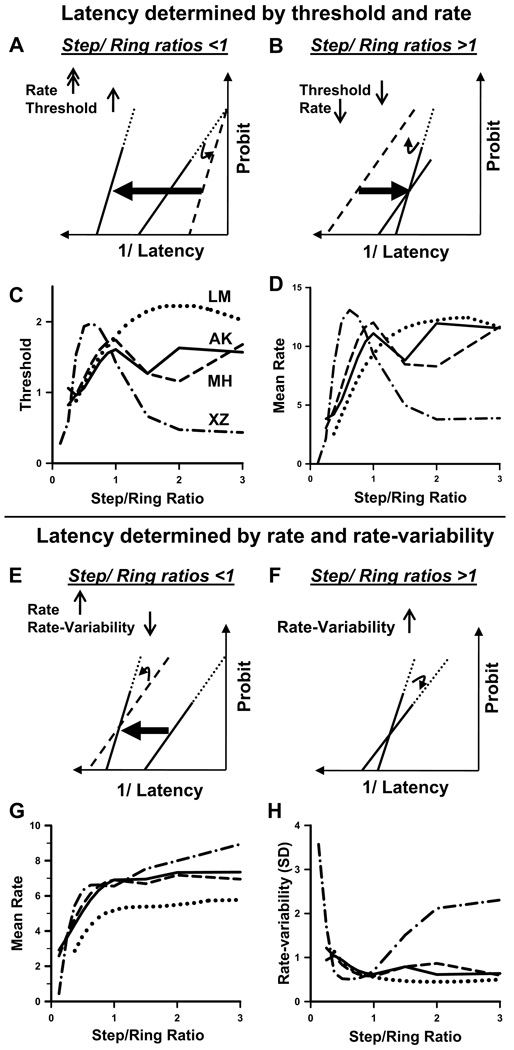
To explain the steeper slopes as ratios increase toward 1, the conventional LATER explanation would necessitate postulating a higher threshold for larger target steps (dashed line in Fig. 8A)—a rather counterintuitive deduction. Because a higher threshold would cause longer latencies, one would also need to postulate a much larger increase in the rate signal (leftward arrow) to compensate for this effect of the increased threshold.
FIG. 8.
Alternative inferences of decision signals from the behavioral data. A–D: variable threshold and rate explanation (fixed rate variability). A: at ratios <1, two changes would need to occur: an increase in threshold causes the saccade latency distribution to swivel anticlockwise about the infinite-time intercept with the probit axis (resulting in the dashed line) and a large increase in decision rate shifts the distribution to the left. B: at ratios >1, a decrease in threshold causes the saccade latency distribution to swivel clockwise about the infinite-time intercept with the probit axis (resulting in the dashed line) and a decrease in decision rate shifts the distribution back to the median asymptote. C: threshold changes inferred using σr = 1 (see text) and fits to the data in Fig. 6B. D: mean rates inferred from the infinite-time intercepts. E–H: variable rate and rate variability explanation (fixed threshold). E: at ratios <1, a small increase in decision rate shifts the distribution to the left (resulting in the dashed line), and a reduction in rate variance causes the line to rotate anticlockwise about the median. F: at ratios >1, the decision rate and median remain fixed and a small increase in variance causes the line to rotate clockwise about the median. G: mean rates inferred from reciprocal latencies (ΔS = 1). H: SD of rates inferred from the reciprocal of reciprobit slopes. All the fits are 5th-order polynomials.
To explain the shallower slopes as the ring steps by more than its diameter (ratios >1; Fig. 6B and Supplemental Fig. S2), where the latencies have already reached a minimum asymptote and are not changing, one would need to postulate that the threshold decreases (dashed line in Fig. 8B) and the decision rate now also reversed its relationship with step/ring ratio and decreased by exactly enough to compensate for the decreased threshold (rightward arrow in Fig. 8B).
To put our data in this form, involving changes only in threshold and rate, but not in rate variability (σr = 1), we can estimate the threshold from the reciprobit slope (Eq. 2 and Eq. 3, ΔS = 1/σp = slope) and plot this in Fig. 8C. Similarly, if the rate variability is constant, we can estimate the mean rate from the reciprobit intercept (Eq. 5, Fig. 8D). Notice that both parameters show oscillations and substantial inter-subject variability.
Alternatively, we propose that the reciprobit slope changes can be attributed to changes in decision rate variability. Because changing the rate variability alone would be manifested as a rotation of the cumulative distribution around its median, a small increase in rate variability could explain the decreased slopes without an effect on latency, as we observed at step/ring ratios >1 (Fig. 8F). Furthermore, the increased slope as the step/ring ratio increases up to a ratio of 1 could be explained by the decision rate increasing (leftward arrow in Fig. 8E) and the variability decreasing (counterclockwise tilt in Fig. 8E).
To reexpress our data in these terms, we make use of the fact that, if changes in rate variability cause the changes in reciprobit slope, there is no need for the threshold to change between conditions and, if the threshold is fixed (ΔS = 1), the mean decision rate is simply the reciprocal median latency (Eq. 1; Fig. 8G). Similarly, the rate variability (SD) can be inferred from the reciprocal of the reciprobit slopes (Eq. 2 and Eq. 3; Fig. 8H). It seems more biologically plausible that the decision rate and its variability should saturate at step/ring ratios >1 (at which point there is no longer any overlap between prestep and poststep target area), rather than the decision rate and threshold oscillating wildly, as occurs if the rate variability is not allowed to change independently. The mean rates are also more consistent across subjects in this alternative model.
Therefore it seems more parsimonious to describe the effects of the step/ring ratio on latency as due to changes in decision rate and rate variability, rather than rate and threshold. In keeping with other studies in which the stimulus strength increases the decision rate variable, we view the step/ring ratio as a measure of stimulus strength for a motor-preparatory decision process.
Beyond the issue of how to explain the latency and variability changes observed, these analyses illustrate another important point. Because the ratio of rate to rate variability defines the infinite-time intercept (Eq. 5), if one fixes the rate variability as in the conventional LATER model (e.g., σr = 1), the intercept also becomes a measure of the rate and is so-named in Fig. 8D. In the more general case of rate variability being unconstrained, the intercept becomes a useful reflection of the signal-to-noise ratio (SNR). We find it significant that as the step/ring ratio increases, the SNR increases along with the rate of the decision variable and the decreasing latency, peaking around a step/ring ratio of 1 (Fig. 8D). This peak may suggest that the decision process underlying saccades may be especially efficient when the saccade amplitude approximates the size of the attentional field.
DISCUSSION
We have extended our previous finding that saccadic reaction times are much shorter when attention is directed to a small part of a stimulus (Madelain et al. 2005), by demonstrating that it is not the size of the target that is important, but the ratio of the target step to the size of the attended region. Before discussing this finding further in terms of motor decision processes, we first examine alternative explanations.
Visual and task explanations
Because our stimuli were concentric rings, one might attribute shorter latencies when the inner ring is attended to its being closer to the fovea. Therefore in experiment 1A we placed the small ring outside the large ring and found that attending to the small ring still resulted in saccades with latencies 90 ms shorter than when attending to the large ring (Fig. 3A). Experiment 1B also revealed that the size-latency effect is not restricted to ring stimuli (Fig. 3D).
One might also suppose that when stimuli of multiple parts are present, as in experiments 1 and 2, the longer latencies when attending to the larger stimulus component might be due to the subjects dividing attention between the parts. However, in experiment 3 we found the same size-latency effect with single rings as with concentric double rings.
Because attention can alter perceived contrast (Carrasco et al. 2004), we considered the possibility that attending to the smaller of two rings might increase its perceived contrast, causing the shorter latency when the rings moved. We found, however, that simulating this hypothesized increase in perceived contrast by explicitly increasing the contrast of the small ring, or of both rings, had very little effect on the latencies.
If saccades require focal attention to be effectively elicited (McPeek et al. 1999), perhaps saccade latencies are more variable and longer when attention is distributed more broadly. Experiment 3 shows that it is not the size of the target but the ratio of step amplitude to the diameter of the attended ring that is important.
One might also argue that if the psychophysical discrimination were easier with the small ring, more attentional resources might be available to the oculomotor task, thereby shortening latencies. In experiments 1 and 2 we adjusted the rotation speed to yield 70–80% accuracy and, even when we removed the discrimination task, a strong size-latency effect was still present. Therefore trade-offs between task accuracy and speed of reaction cannot explain our results.
Speed–accuracy trade-offs within the oculomotor task between saccade amplitude and latency also cannot explain our data because saccade amplitudes were highly correlated with target displacement, as we also found previously (Madelain et al. 2005), and saccade gains were close to one across a wide range of conditions that strongly affected latencies.
Thus experiments 1 and 2 show that neither stimulus eccentricity, task difficulty, nor contrast can account for the size-latency effect. The results of experiment 3 imply that a motor decision underlies our latency differences, by showing that the latency is consistently related to the size of the attended ring and the amount of its movement.
Motor decision explanations
Our key results are that saccade latency is well predicted by the ratio of step amplitude to the diameter of the attended ring, with latencies declining as the ratio increases to about 1 (i.e., the target steps by the same distance as the size of the attended object), and being relatively constant at higher ratios. The collapse of latency curves for different ring diameters onto a single function of latency against step-size/ring-diameter ratio shows that, in our experiments, saccades are postponed if the target in its new location overlaps the field of attention of the previous location.
We postulate that this regularity may be explicable by the cost versus benefit of deferring a saccade. If the attended object moves by an amount that is small relative to its size (as when the rabbit of the INTRODUCTION makes a small hop), the relevant visual information has hardly changed and so the benefit of a saccade is low compared with its cost in disrupted vision both during and after the saccade. Thus the urgency to make a saccade is low and the saccade is postponed. In contrast, if a smaller attended object moves by the same amount (like the frog of the INTRODUCTION), the visual benefit outweighs the cost of the saccade and so the latency is short. A corollary of this postulate is that the size-latency effect should be specific to saccades—manual responses have no visual costs or benefits. We found that manual latencies did not change with the spatial scale of attention (Fig. 7), indicating that the effect must arise at an oculomotor planning stage. Thus the scale of attention appears to convey to the motor system all it needs to know about what the visual system is up to, enabling it to make a saccade of optimal latency.
We view the orderly decrease in latency with increasing step/ring ratio as a manifestation of an underlying motor urgency rule, which does not depend on the immediate task-specific cost/benefit ratio of the saccade. Indeed, because we displayed the discriminandum only briefly, subjects could not improve their discrimination performance by adjusting saccade latency. Consequently, our size-latency effect is different from that of Montagnini and Chelazzi (2005) who showed that latencies can be reduced by 30 ms by briefly presenting a discriminandum at a time when the subject could foveate it longer by making a short-latency saccade (“perceptual urgency”). We find that our discrimination task shortens saccade latency by a similar amount relative to experiments without a discrimination task, perhaps because of a nonspecific volitional urgency, i.e., a speed–accuracy trade-off (Reddi and Carpenter 2000). This urgency may have common currency with perceptual and motor urgencies if it involves a modulation of the subject’s internal sense of the saccade’s benefit.
The general validity of the size-latency effect is attested to not only by our finding that it occurs without a discrimination task, but also because Kalesnykas and Hallett (1996) have shown that saccade latencies are progressively longer to two light-emitting diodes (LEDs) 2–4° apart than to a single LED. Moreover, latencies increase by as much as 150 ms when very small targets step by distances smaller than the width of the foveola (Kalesnykas and Hallett 1994; Wyman and Steinman 1973), as though attention is set to the width of the foveola, resulting in the target steps being within the attentional field. Consistent with this view, in most studies the target steps are large relative to the small spot stimuli (Becker 1991; Darrien et al. 2001) or even to spatially extended targets (Dick et al. 2004; Kowler and Blaser 1995; Ploner et al. 2004), and thus would fall in our asymptotic fast latency region, where the latencies do not change with step size.
These findings suggest that the size-latency effect may occur in the absence of a task that explicitly sets the spatial scale of attention because attention is automatically set to the scale of the objects present (Hopf et al. 2006). The tightly constrained nature of our task (restricting the location, scale, and load of attention, as well as the task timing) probably makes the motor urgency especially apparent. We have the impression that attending to the rotation of a ring prevents attention from wandering elsewhere: if another ring is present, it probably attracts few attentional resources, as shown by accuracy of reporting the number of breaks in the unattended ring being at chance levels. In addition, when one’s attention is locked onto the ring’s rotation, subjects reported that the attended ring appears to slow, thereby providing perceptual feedback that helps the subject maintain attention. The sparseness of our stimuli might also contribute to the tight size–latency relationship. Had there been more features within each ring area, a larger transient of motion energy across spatial scales might have increased the rate signal for a given step-size/ring-diameter ratio.
To put these operations into neuronal terms, consider two neuronal populations in a retinotopically organized map, such as the superior colliculus. If the attentional field corresponded to the area of activated neurons, a target step outside the field might be more easily discriminated because the activity would shift from one neuronal population to the other, whereas a step within the attentional field would involve two overlapping groups of neurons.
Rate modulation versus distance-to-threshold changes
In diffusion or LATER models, latencies can be shortened by increases in the mean rate of rise of a decision signal, as implicated in perceptual urgency experiments, or by a lowering of the threshold, as implicated in volitional urgency experiments. On reciprobit plots, increased rates cause leftward parallel shifts of latency cumulative distributions, whereas lower thresholds cause decreased slopes brought about by clockwise swiveling around the infinite-time intercept (see Fig. 2C).
Simple inferences of decision mechanisms from latency distributions are complicated if the decision rate variability is considered a free parameter (Madelain et al. 2005; Montagnini and Chelazzi 2005). Changing rate variability leads to swiveling of latency distributions around the median on reciprobit plots, which has been demonstrated in subjects trained to alter their sensorimotor variability (Madelain et al. 2007). Once the possibility of the rate variability changing is introduced, there is no unique combination of changes in rate, rate variability, and threshold to cause a given distribution. Indeed, even a strict swiveling about the infinite-time intercept can be achieved without change in threshold if the rate and the rate variability change by exactly the same proportion (see Eq. 5). Common sense cannot always resolve such ambiguities. Consider these two findings: if the probability that the target will step right versus left is changed, latency distributions suggested a threshold change (Carpenter and Williams 1995), whereas changing the probability that dots will move left or right produced a rate signal change in parietal cortex (Mazurek et al. 2005). What might explain these divergent results? Gold and Shadlen (2001) have shown how one can combine sensory evidence, prior probability, and anticipated costs and benefits into one likelihood ratio (LR), which is the optimal decision signal between competing hypotheses. Thus
This ratio, updated at each sample of sensory evidence, computes the likelihood that the sensory evidence arose, for example, from the hypothesis that the target has stepped right or the alternative hypothesis that it stepped left. Carpenter and Williams provided compelling evidence that saccade latencies are based on this log-likelihood ratio by showing that the median latency varies precisely with the log of the prior probability that the target will step left versus right. Thus changes in prior probability of the saccade direction would be expected to alter the starting level bias, thus altering the distance to threshold, as was found. However, because accumulator models integrate successive new samples of sensory evidence, if the prior probability of a sensory event added a bias to each of these, the net effect would be multiplicative and thus would be equivalent to increasing the rate signal, as Mazurek et al. (2005) found. Volitional urgency (e.g., Palmer et al. 2005; Reddi and Carpenter 2000) might arise from an increase in rate and proportional increase in rate variability, as opposed to the more usually presumed change in threshold.
We have argued that the step/ring ratio alters the cost/benefit ratio of the choice between making a saccade and maintaining fixation and that it operates via decision rate modulation. We proposed that the step/ring ratio is monotonically related to the cost/benefit ratio and so can be substituted into the preceding equation with appropriate scaling constants (an “equivalent decision rule”; Gold and Shadlen 2001). In our case, increasing the step/ring ratio, like prior probability in the Mazurek study, clearly modulates the rate, as shown by the reciprobit curves shifting leftward with their slopes increasing over a broad parameter space of 24 conditions. The good fit of the median latency to the log of the step/ring ratio (Fig. 5C) is what would be expected from an optimal decision process based on log-likelihood ratios and operating via rate modulation. This extends the inference of Carpenter and Williams, but in our case with an increased rate and a decreased rate variability or, less likely, an increased threshold.
Conclusions
Our results provide evidence in support of three largely overlooked features of saccade reaction time: that the spatial scale of attention can have a profound effect on saccadic—but not manual—reaction times; that the rate variability of the decision variable may be of as great importance as the decision threshold; and that saccade latencies may reflect the cost/benefit ratio of each saccade.
Supplementary Material
Acknowledgments
GRANTS
This research was supported by National Institutes of Health Grants EY-12212 to R. J. Krauzlis and RR-03060 to J. Wallman and M. R. Harwood, by Wellcome Trust Grant GR065025M to M. R. Harwood and by French Agence Nationale de la Recherche Grant JC-1ACA to L. Madelain.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Becker W. Saccades. In: Carpenter RHS, editor. Vision and Visual Dysfunction. London: Macmillan; 1991. pp. 95–137. [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. Eye Movements: Cognition and Visual Perception. Hillsdale, NJ; Erlbaum; 1981. Oculomotor procrastination; pp. 237–246. [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrien JH, Herd K, Starling LJ, Rosenberg JR, Morrison JD. An analysis of the dependence of saccadic latency on target position and target characteristics in human subjects. BMC Neurosci. 2001;2:13–20. doi: 10.1186/1471-2202-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick S, Ostendorf F, Kraft A, Ploner CJ. Saccades to spatially extended targets: the role of eccentricity. Neuroreport. 2004;15:453–456. doi: 10.1097/00001756-200403010-00014. [DOI] [PubMed] [Google Scholar]
- Ditterich J. Evidence for time-variant decision making. Eur J Neurosci. 2006;24:3628–3641. doi: 10.1111/j.1460-9568.2006.05221.x. [DOI] [PubMed] [Google Scholar]
- Glimcher PW. The neurobiology of visual-saccadic decision making. Annu Rev Neurosci. 2003;26:133–179. doi: 10.1146/annurev.neuro.26.010302.081134. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci. 2001;5:10–16. doi: 10.1016/s1364-6613(00)01567-9. [DOI] [PubMed] [Google Scholar]
- Grice GR. Stimulus intensity and response evocation. Psychol Rev. 1968;75:359–373. doi: 10.1037/h0026287. [DOI] [PubMed] [Google Scholar]
- Harwood MR. The Fourier Analysis of Saccadic Eye Movements (PhD dissertation) London: University College London; 2003. [Google Scholar]
- Harwood MR, Madelain L, Krauzlis RJ, Wallman J. Spatial scale of attention strongly modulates saccade latency, but not by modulating stimulus saliency (Abstract) J Vis. 2003;3:685. [Google Scholar]
- Hopf JM, Luck SJ, Boelmans K, Schoenfeld MA, Boehler CN, Rieger J, Heinze HJ. The neural site of attention matches the spatial scale of perception. J Neurosci. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnykas RP, Hallett PE. Retinal eccentricity and the latency of eye saccades. Vision Res. 1994;34:517–531. doi: 10.1016/0042-6989(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Kalesnykas RP, Hallett PE. Fixation conditions, the foveola and saccadic latency. Vision Res. 1996;36:3195–3203. doi: 10.1016/0042-6989(96)00029-6. [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Robinson DA. Saccadic undershoot is not inevitable: saccades can be accurate. Vision Res. 1986;26:735–743. doi: 10.1016/0042-6989(86)90087-8. [DOI] [PubMed] [Google Scholar]
- Kowler E, Blaser E. The accuracy and precision of saccades to small and large targets. Vision Res. 1995;35:1741–1754. doi: 10.1016/0042-6989(94)00255-k. [DOI] [PubMed] [Google Scholar]
- Ludwig CJ, Gilchrist ID, McSorley E, Baddeley RJ. The temporal impulse response underlying saccadic decisions. J Neurosci. 2005;25:9907–9912. doi: 10.1523/JNEUROSCI.2197-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelain L, Champrenaut L, Chauvin A. Control of sensorimotor variability by consequences. J Neurophysiol. 2007;98:2255–2265. doi: 10.1152/jn.01286.2006. [DOI] [PubMed] [Google Scholar]
- Madelain L, Harwood MR, Krauzlis RJ, Wallman J. Spatial scale of attention influences saccade latencies (Abstract) J Vis. 2004;4:644. doi: 10.1152/jn.00589.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelain L, Krauzlis RJ, Wallman J. Spatial deployment of attention influences both saccadic and pursuit tracking. Vision Res. 2005;45:2685–2703. doi: 10.1016/j.visres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Hanks T, Yang T, Shadlen MN. 2005 Abstract Viewer and Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Prior probability changes the rate of evidence accumulation in a motion discrimination task: behavior and LIP physiology. Program No. 621.3. Online. [Google Scholar]
- McPeek RM, Maljkovic V, Nakayama K. Saccades require focal attention and are facilitated by a short-term memory system. Vision Res. 1999;39:1555–1566. doi: 10.1016/s0042-6989(98)00228-4. [DOI] [PubMed] [Google Scholar]
- Montagnini A, Chelazzi L. The urgency to look: prompt saccades to the benefit of perception. Vision Res. 2005;45:3391–3401. doi: 10.1016/j.visres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis. 2005;5:376–404. doi: 10.1167/5.5.1. [DOI] [PubMed] [Google Scholar]
- Pieron H. Recherches sur les lois de variation des temps de latence sensorielle en fonction des intensités excitatrices [On the laws of variation of sensory processing time as a function of the excitatory intensity] L’Année Psychologique. 1914;20:17–96. [Google Scholar]
- Pins D, Bonnet C. On the relation between stimulus intensity and processing time: Pieron’s law and choice reaction time. Percept Psychophys. 1996;58:390–400. doi: 10.3758/bf03206815. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Ostendorf F, Dick S. Target size modulates saccadic eye movements in humans. Behav Neurosci. 2004;118:237–242. doi: 10.1037/0735-7044.118.1.237. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychol Rev. 1978;85:59–108. [Google Scholar]
- Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychol Sci. 1998;9:347–356. [Google Scholar]
- Reddi BA, Asrress KN, Carpenter RH. Accuracy, information, and response time in a saccadic decision task. J Neurophysiol. 2003;90:3538–3546. doi: 10.1152/jn.00689.2002. [DOI] [PubMed] [Google Scholar]
- Reddi BA, Carpenter RH. The influence of urgency on decision time. Nat Neurosci. 2000;3:827–830. doi: 10.1038/77739. [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Exp Brain Res. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural correlates of decision processes: neural and mental chronometry. Curr Opin Neurobiol. 2003;13:182–186. doi: 10.1016/s0959-4388(03)00039-4. [DOI] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- van Opstal AJ, van Gisbergen JA. Scatter in the metrics of saccades and properties of the collicular motor map. Vision Res. 1989;29:1183–1196. doi: 10.1016/0042-6989(89)90064-3. [DOI] [PubMed] [Google Scholar]
- Weber H, Fischer B. Gap duration and location of attention focus modulate the occurrence of left/right asymmetries in the saccadic reaction times of human subjects. Vision Res. 1995;35:987–998. doi: 10.1016/0042-6989(94)00186-p. [DOI] [PubMed] [Google Scholar]
- Wyman D, Steinman RM. Latency characteristics of small saccades (Letter) Vision Res. 1973;13:2173–2175. doi: 10.1016/0042-6989(73)90195-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.