Abstract
The protected transport of nitric oxide (NO) by hemoglobin (Hb) links the metabolic activity of working tissue to the regulation of its local blood supply through hypoxic vasodilation. This physiologic mechanism is allosterically coupled to the O2 saturation of Hb and involves the covalent binding of NO to a cysteine residue in the β-chain of Hb (Cys β93) to form S-nitrosohemoglobin (SNO-Hb). Subsequent S-transnitrosation, the transfer of NO groups to thiols on the RBC membrane and then in the plasma, preserves NO vasodilator activity for delivery to the vascular endothelium. This SNO-Hb paradigm provides insight into the respiratory cycle and a new therapeutic focus for diseases involving abnormal microcirculatory perfusion. In addition, the formation of S-nitrosothiols in other proteins may regulate an array of physiological functions.
Introduction
The discovery in the 1980s that nitric oxide (NO) is a biological signaling molecule, followed by nearly three decades of research in which a multiplicity of functions was identified for this ubiquitous molecule, has reawakened the search for mechanisms underlying a number of physiological processes and provided new ways of interpreting old observations. One of the novel concepts to emerge is that the ability of hemoglobin (Hb) to combine with NO—known long before NO was identified as an endogenous biomolecule—performs a vital physiological function. And this function, hypoxic vasodilation, has recently been the subject of intense interest, especially because it involves more than the release of dilator substances from the vessels themselves and does not depend directly on the presence of endothelial nitric oxide synthase (eNOS).
Matching perfusion with metabolism
In living organisms, the dynamic relationship between central control and peripheral autonomy of organ function is elegantly reflected in the physiological regulation of blood flow. When the full resources of an organism must be mobilized, for example during strenuous exercise, both central and local mechanisms provide a coordinated response in which cardiac output and systemic perfusion adjust to metabolic demand. However, when increased demand is confined to specific tissues, for example to a region of the brain or to the muscles of a limb, local vasodilation alone can enable sufficient increases in blood flow and O2 extraction to meet the requirements of the working tissues. This process, the dilation of microvascular beds to supply more oxygen to specific groups of cells, is called hypoxic vasodilation (sometimes considered part of the autoregulation of blood flow) and excludes changes due to altered arterial pressure, overriding control by the central nervous system or the effect of circulating hormones.
Definitions, disparities and dichotomies
The recent history of this field has been characterized by a rapidly proliferating literature and conflicting interpretations. Some confusion, however, can be dispelled by the careful definition of physiological events. Accordingly, hypoxic vasodilation is a prompt vascular response to increased local demand for oxygen due to a change in metabolic activity in the absence of injury or disease. By contrast, post-ischemic or reactive hyperemia is a pathological response to an abnormal condition, for example transient oligemia or ischemia, that is manifest because local circulation is restored and often accompanied by tissue injury through the temporary deprivation of necessary metabolic substrates, tissue hypoxia and metabolite accumulation. And unlike hypoxic vasodilation, reactive hyperemia can decrease vascular resistance at a considerable distance from the ischemic site [1]. Conflation of hypoxic vasodilation with post-ischemic hyperemia was frequent in the earlier literature [2] and still occasionally occurs [3]. Also, hypoxic vasodilation should be distinguished from hypoxic potentiation of vasoactivity; for example, the vascular effects of some agents, including NO itself, are dependent on the partial pressure of oxygen (PO2) and are not coupled to changes in Hb oxygen saturation. Yet another misinterpretation results from confusing hypoxic vasodilation with systemic vasodilation in alveolar hypoxia [4,5] because the former is a limited response to local metabolic conditions, whereas the latter is a response to a general limitation in O2 availability—global hypoxemia. Although the fall in tissue PO2 that occurs in hypoxemia recruits vasodilator mechanisms that are similar or related to those caused by an increased local metabolic demand for oxygen, the decrease in arterial O2 saturation of Hb is not coupled to an increase in the rate of O2 uptake. Furthermore, prolonged changes in metabolic rate, such as those caused by vigorous exercise, can also elicit multiple mechanisms of vasodilation.
Attempts to understand the role of NO in hypoxic vasodilation have been hindered by disparities in experimental results reported by different laboratories. For example, in a recent attempt to investigate hypoxic vasodilation, mice were genetically altered to prevent a reversible interaction between NO and Hb [6]. The paper reported that such an interaction was not required for hypoxic vasodilation because systemic blood pressure and time-to-fatigue in exercise had not significantly changed [6]. But these two physiological parameters are not related to hypoxic vasodilation. In other cases, misinterpretations occur when physiological differences in experimental preparations are not taken into consideration. For example, since the late 19th century it has been known that pulmonary vessels, unlike the systemic vasculature, do not dilate in response to local hypoxia—they constrict to prevent the gas in poorly ventilated alveoli from equilibrating with the pulmonary capillary blood and lowering the arterial PO2 [7]. Nevertheless, isolated pulmonary vessels have occasionally been used to investigate hypoxic vasodilation [8], on which they shed no light.
Other difficulties arise from analytical procedures that alter or destroy evanescent biochemical species [9,10] or create conditions not found in living organisms. In addition, if endogenous substances are administered at supra-physiological levels, effects are elicited that are pharmacological rather than physiological [11]. This interpretation is corroborated when the effects of lower levels of these agents are too weak or too slow to account for authentic physiological responses [12]. Moreover, some cellular or molecular events fall outside the detection capabilities of certain techniques (for example, the venerable Griess reaction or tri-iodide chemiluminescence [9,13]) and require other, more appropriate, analytical methods [14–18]. Finally, multiple complementary mechanisms might be involved in hypoxic vasodilation, yet some authors claim that evidence for one pathway excludes others, thereby creating false dichotomies [19].
The objectives of this review are to lay out a conceptual framework within which data about the involvement of Hb and NO in hypoxic vasodilation can be evaluated, distinguish what is known from what is unknown, consider the clinical implications of this new knowledge and briefly look beyond this physiological mechanism to others that might employ similar chemistries for a range of different functions. (Please see Box 3, Outstanding Questions, at the end of this review.)
Concepts
Stimulus, sensor, signal and effector
Physiological signaling mechanisms evoke corrective responses to changes in homeostatic variables. Most such systems comprise, at a minimum, a stimulus, a sensor, a signal and an effector. However, any putative ordering of components and events may only offer a first-order understanding of what actually takes place at cellular and molecular levels because signaling pathways, like a matryoshka doll, often contain entities within entities. As for hypoxic vasodilation, it has long been known that the stimulus is hypoxia and the effector is vascular smooth muscle—but the sensor and the signal have been matters of speculation.
The stimulus
The hallmark of hypoxic vasodilation is local reduction in vascular resistance correlated with decreased oxygen saturation of Hb. This effect was reported in the late 1950s and early 1960s in the seminal studies of Arthur Guyton and colleagues, who concluded that local oxygen lack causes vasodilation by preventing the energy production needed to maintain basal vascular tone [20]. In other words, the postulated sensor is in the red blood cell (RBC) and the signal is decreased ATP production.
Guyton's idea was bolstered by the observation that mixed venous blood (hypoxic blood) caused prompt vasodilation when infused into normoxic tissues, but the same blood after passage through the lungs (normoxic blood) restored normal vascular caliber. This and other work clearly demonstrated that the vascular response is linked to HbO2 saturation and is independent of tissue PO2. However, it was thought that if a vasodilator in the infused venous blood were involved, it would have to be cleared or inactivated in the lungs. Because no endogenous agent was known to behave in this way, this explanation was rejected. This intriguing possibility—that an endogenous vasodilator could be inactivated in the lung—was conceived decades before it could be investigated rigorously.
The sensor
Recent data support the RBC—more specifically the Hb molecule—as the sensor that transduces the hypoxic stimulus into a signal as it undergoes allosteric transformation from the R-state (relaxed) to the T-state (tense), by releasing the dilator molecule NO or, more correctly, a functional equivalent termed nitric oxide bioactivity. In the original proposal [21,22], NO bioactivity bound to a cysteine in the beta chain (Cys β93) of the Hb tetramer is released and subsequently carried in protected form as low molecular weight S-nitrosothiols (SNOs), which have substantially longer biological residence times than free NO and are not scavenged by Hb hemes [23,24]. For example, S-nitrosocysteine (CysNO) and S-nitrosoglutathione (GSNO) have biological activity in blood that unprotected NO lacks, and which they then convey to appropriate cellular targets.
In 2003, a different idea was proposed in which Hb would function as a `reductase' to transform nitrite (NO2−), which is not vasoactive at physiological concentrations, into NO [11]. More recently, others have proposed that eNOS and xanthine oxidoreductase (XOR) also reduce NO2− to NO in the RBCs. However, these proposals are problematic because XOR reduces NO2− only when PO2 is extremely low [25] and NO2− levels are very high. And the hemes in Hb itself are well recognized and powerful scavengers of free NO [26]. In addition, the NO2− reductase activity is reportedly maximal at the P50 of Hb—when Hb is 50% saturated with O2 [3]. However, this would be incompatible with optimal O2 delivery because it would divert blood away from the most hypoxic tissues to those with higher PO2. Nonetheless, for the SNO mechanism—the prototype for the involvement of Hb in the delivery of NO activity to hypoxic vascular beds—as well as for the NO2− reductase proposals, a common sensor (Hb) transduces similar signals from the hypoxic stimulus (Box 1).
Other hypotheses have been put forward that involve neither NO bioactivity, nor Hb nor the RBC [27]. For example, important work has shown that ATP, adenosine, vasoactive peptides, CO2, H+, K+ and other endogenous substances are significant factors in the local regulation of perfusion [28–31], some of which might act in concert with NO-mediated pathways, whereas others might oppose or operate independently of NO. One or more of these other factors likely plays a part in hypoxic vasodilation; however, none alone explains the autoregulation of blood flow. The task of placing the assumed roles of these other dilators and their interrelationships into a unified mechanism is beyond the scope of this review; however, a recent survey of many of these control mechanisms is available [32]. There are also many vasoregulatory mechanisms that act on longer times scales than needed to accommodate moment-to-moment changes in metabolic demand; some of these more chronic adaptations involve remodeling of the vascular wall [33].
Box 1. Proposed mechanisms for hypoxic vasodilation.
(1996 [21]) SNO-Hemoglobin Mechanism
(2003 [11]) Hemoglobin as a Nitrite Reductase
(2008 [25]) eNOS and XOR as Nitrite Reductases
The signal
It is widely agreed that NO is the principal effector of local relaxation of vascular smooth muscle under hypoxic conditions and has the potential to act in the form of the free radical (NO) or as chemical species with the characteristics of nitrosyl (NO+) or nitroxyl (NO−) ions [34], or as SNOs [35–37]. However, two additional points must be made. First, the entry and egress of necessary chemical species across the red cell membrane must be compatible with the chemistries of Hb and NO. Second, because free NO has a short half-life in blood, perhaps only milliseconds [38], due to scavenging molecules in RBCs and plasma, some way to protect or preserve its vasodilator activity must be part of any cogent scheme.
The SNO mechanism avoids both these difficulties because free NO is not required. Instead, nitric oxide, as NO+, is transferred from one low molecular weight thiol (R-SH) to another by S-transnitrosation (Box 2).
There are suitably distributed cytosolic, trans-membrane and plasma thiols to justify postulating a pool of donor and acceptor molecules, including the Hb β93 Cys, the highly conserved amino acid at position 93 in the beta chain of human Hb and that of all other mammals, birds and many other vertebrates [39]. Also, there are thiol-bearing transporters in the RBC membrane and low molecular weight thiols in the blood plasma that can be taken up, for instance, by amino acid transporters in the plasma membrane of the endothelial cell [40]. These molecules offer protected routes for NO bioactivity to reach the heme moiety of endothelial soluble guanylate cyclase (sGC) or calcium-dependent potassium channels in vascular smooth muscle, which can similarly be directly activated [41]. It has long been known that SNOs `are potent, bioactive compounds with NO-like effects', whose activity is independent of NO itself, as demonstrated by Mathews and Kerr in 1993 (cited in [42]) and confirmed more recently [43]. Furthermore, the kinetics of S-transnitrosation is much faster than the NO release from low molecular weight nitrosothiols such as GSNO and CysNO [44].
By contrast, the original NO2− reductase hypothesis leaves unspecified how NO bioactivity would escape the Hb molecule or the RBC, or survive long enough to reach its target in the endothelium. Physiological concentrations of NO2− do not in fact elicit prompt vasodilation when added to blood vessels in vitro [45,46] or infused into the forearm [11,47], but rather require biotransformation within the circulation to an active species [48]. In other words, NO2− does not meet the requirement of vasodilation at timescales commensurate with transit from arteries to veins. Moreover, some theoreticians have used mathematical modeling to explain how free NO could—like a broken-field runner—avoid rapid re-conversion to NO2− without the protection of a molecular carrier. Such conjectures include diffusion barriers interposed between NO and scavenger molecules, zones near vessel walls that for hydrodynamic reasons are free of RBCs and a favorable balance between NO production and consumption. Many of these proposals, some complex, are described in a recent review [38]. However, the challenge for all such model systems, no matter how comprehensive or plausible, is that the parameters needed to anchor calculations to actual physiological conditions are often unknown or difficult to quantify. Recently, the idea that physiological reduction of NO2− is one of the sources for SNO-Hb has been adapted to the concept of protected NO transport by SNOs in the revised hypothesis of van Faassen and colleagues: `Low molecular weight nitrosothiols may contribute to exportable vasodilatory activity' [49]. However, it is important to understand that in this model the slow reduction of NO2− by deoxygenated hemes in Hb would occur in venous blood and could not be a direct effector of hypoxic vasodilation, which occurs in the arterioles.
Box 2. S-transnitrosation.
Nitric Oxide, as NO+, is transferred from one low-moleculer-weight thiol (R-SH) to another by S-transnitrosation.
Box 3. Outstanding questions.
• How does NO bioactivity enter the erythrocyte? The extent to which NO itself can diffuse across the RBC membrane is not clear. However, SNOs may be imported directly by mechanisms not yet identified. Alternatively, NO may be protectively escorted across the RBC membrane, for example by protein disulphide isomerase, or captured by hemoglobin associated with the RBC membrane, which then relocates to the cytosol. This is an area of active investigation.
• What is the role of nitrite (NO2−) or other higher oxides of nitrogen in the storage of nitric oxide bioactivity? It is clear that nitrite, even at pharmacological concentrations, does not induce RBC-mediated vasodilation rapidly enough to fulfill the physiological requirements of hypoxic vasodilation. Yet, it is possible that nitrite can, through slower processes, add to the store of intra- and extracellular SNOs and thus contribute indirectly to hypoxic vasodilation. However, any such biochemical pathways still need to be identified.
• How will the SNO-Hb paradigm be applied to the treatment of disease? There is no simple answer to this question, because the defects of NO transport and release by hemoglobin associated with different diseases have several different causes. For example in diabetes, the glycosylation of Hb may be the interfering circumstance, whereas in sickle-cell disease it is the aberrant structure of Hb itself-resulting from a genetic mutation, as well as a membrane processing defect resulting from membrane damage. However, it is clear that the paradigm opens new mechanistic fronts from which to attack these pathologies.
• How pervasive is the mechanism of S-nitrosylation in the regulation of protein function? Several such mechanisms have been identified involving proteins other than Hb, but this is a new field of inquiry and it remains to be seen if this process is as ubiquitous as, say, phosphorylation.
Integrating what we know
Physiological context
Many chemistries involving biological molecules can be implemented in the laboratory that are never found in living organisms. Thus, physiology has rules that are set by the organism under study, and include specific chemical, thermodynamic and temporal requirements. For any investigation of a physiological process such as hypoxic vasodilation, one must evaluate experimental conditions against authentic physiological criteria and exclude trauma, disease or pharmacological effects.
So what do we know that is relevant to this process? We know that NO interacts with metalloproteins—especially those containing hemes—and with thiols. Hemes either transform NO into nitrate or promote its transformation into SNOs [50,51]. Nitrate lacks physiological vasodilator activity, whereas low molecular weight SNOs, such as CysNO, transduce NO-based signals largely by means of S-nitrosation [52]. Furthermore, SNOs retain vasodilatory activity in the blood, where newly developed assays have confirmed their ample presence [9,16,18], whereas in animals S-nitrosation coordinates a multi-component physiological response to hypoxia [53–55]. We also know that RBCs act in hypoxic vasodilation through an NO-based mechanism that is independent of eNOS in the vasculature [29,45,56–58].
S-nitrosothiol-mediated vasodilation
Thiols have been identified as necessary for the action of nitrodilators for nearly three decades. For example, the potent and well known organic nitrodilator nitroglycerin requires thiol [59]. Plasma thiols do convey NO bioactivity in the mammalian circulation [60], and cytoplasmic entry of NO bioactivity may involve facilitation by protein disulphide isomerase (PDI) [61] on cell surfaces [62]. Finally, thiol modification, for example by N-ethylmalei-mide or diamide, inhibits guanylate cyclase as well as ion channels that mediate vasodilation [41,63].
Recently, additional confirmatory evidence was provided for S-nitrosothiol-mediated vasodilation by genetically-altered mice in which the β93 Cys on Hb was replaced by the amino acid alanine so that SNO-Hb could not be formed [6]. The investigators concluded that SNO Hb was not required for hypoxic vasodilation, but their findings of elevated levels of non-Hb SNOs and a nearly 10% increase in Hb concentrations, compensatory mechanisms for impaired O2 delivery to tissues, are consistent with defective SNO-mediated hypoxic vasodilation [58].
How might SNO be transferred out of the RBCs, for example, from SNO-Hb? Three sets of observations provide clues. First, vasodilation evoked by native RBCs is dependent on the transfer of NO activity into the cell membrane and is potentiated by plasma thiols, such as Cys and N-acetylcysteine (NAC) [45,52,64]. Second, fresh RBCs export SNO, whereas ATP-depleted cells do not [21]. Third, deoxygenation of RBCs depletes SNO-Hb, resulting in the commensurate accumulation of both membrane and extra-cellular SNOs [15,55]. From these facts, two mechanisms can be inferred.
First, SNO-Hb binds Anion Exchanger 1 (AE1). By transnitrosation, the NO group then transfers to Cys in the N-cytoplasmic tail of AE1, leaving the free thiolate HbS−. AE1 is the most abundant protein in the red cell membrane where it facilitates electroneutral anion exchange [65]. Thiol-coupled NO activity might also exit the RBC as GSNO, or related species, in an ATP-dependent mechanism [21]. In this case, the transporter seems to be an ATP-binding cassette (ABC) protein. This family of proteins uses energy derived from ATP to move various substrates across cell membranes. RBCs export glutathione disulfide and glutathione conjugates, as shown by Kondo and Beutler and others, by means of both high- and low-Km transporters [66]. These mechanisms are also potentiated by thiols [67]. It is plausible that these two transport pathways are linked; for example, SNO-AE1 in the membrane may be converted to GSNO since S-nitrosoglutathione reductase (GSNOR) knockout mice accumulate SNO-Hb at high levels [53]. What is unknown, however, is how NO bioactivity enters the RBC. Either SNOs are imported or the NO group is transferred across the membrane (for example, by PDI). It also possible that free NO is captured by membrane-associated Hb, which then relocates it to the cytosol. In the 1950s, Roughton and Comroe demonstrated that NO access by diffusion is hindered by the erythrocyte membrane [68,69] and similar results have been reported more recently [70].
Nitrite reduction
The idea that NO2− is an abundant source of NO in the blood and that some blood components are capable of reducing it to NO, which then accounts for hypoxic vasodilation [71,72], was initially proposed as an alternative to the SNO paradigm because investigators using tri-iodide chemiluminescence did not detect circulating SNOs in quantities thought sufficient to account for hypoxic vasodilation. This led to the 2003 proposal that the NO2− reductase is Hb (Box 1)and that it releases NO under allosteric control as it makes the transition from the R- to the T-state [11,73].
Indeed, NO2− reduction does occur in vivo, especially at low pH, as in the digestive tract and perhaps in ischemic vessels, and a recent compilation of such reports provides a convenient entry into this literature [49]. For example, NO2−-dependent vasodilation has been reported in the mouse, rat, dog, sheep, primate and human [11,74]. According to these studies, NO2− is consumed in A-V transit, consistent with conversion to NO or to nitrate [72]; NO2− at 40 μM promptly dilates isolated aortic rings in cell-free buffer at pH 6.6 [75]; vasodilation by inhaled NO is associated with increased plasma NO2− [76]; NO2− infused into the human forearm at 0.2 and 2.5 mM produces significant vasodilation associated with partial deoxygenation of Hb. Moreover, during exercise, vasodilation has been observed after five minutes at systemic NO2− concentrations of 16 μM [11]. That is, only high pharmacological concentrations of NO2− dilate vessels quickly (in seconds), whereas lower pharmacological concentrations (still many times physiological plasma levels) require biotransformation over minutes [77], thereby precluding direct involvement in hypoxic vasodilation. In addition, other investigators have found that SNOs are consumed in A-V transit, whereas NO2− levels actually increase [78]. These issues have yet to be resolved. In any case, the low pH levels that can occur in ischemic vascular beds in vivo might explain the observed NO2− depletion.
When Hb is exposed to NO2−, ferrous nitrosyl-hemoglobin (NO-Fe2+Hb) is formed [79] and, hypothetically, free NO can be produced according to the following overall reaction [11]:
Moreover, because the formation of HbNO increases as Hb oxygen saturation drops, it was conjectured that circulating deoxyHb acts as a NO2− reductase in hypoxia. However, this reaction requires NO2− concentrations greater than that of Hb, which would be much higher than are found in vivo. Although NO can dissociate from NO-Fe2+Hb, physiologically meaningful levels of free NO would be unsustainable in the face of the Hb concentrations present in the RBC. Indeed, massive infusions of NO2− are required to overcome the associated increases in NO-Fe2+Hb and induce vasodilation in humans in hypoxia [4]. At physiological levels of NO2− (300 nM in humans), NO-Fe2+Hb formation constitutes NO complexation [46,80], which must be overcome for NO bioactivity to increase.
A careful review of these measurements leaves nothing that demonstrates that NO2− reduction by Hb is necessary or sufficient for hypoxic vasodilation at physiological levels of NO2− (approximately 300 nM in humans, 200 nM in rats and 400 nM in mice [81]) or at the short timescales, seconds or less, required of a physiological response to increased metabolic demand [82]. More recent studies also support no role for either Fe2+Hb or met-Hb(FeIII) (methemoglobin) in the generation of free NO and further question the ability of NO2− reduction mediated by deoxy-Hb(FeII) to mediate physiological vasodilation. After mixing NO2− with deoxy-Hb(FeII) in solution, free NO does not accumulate in amounts significant for vasoregulatory control, even after minutes at supra-physiological concentrations of NO2−. Under these conditions, NO2− reduction seems to be pre-empted by the autocapture of NO by deoxyHb, preventing the accumulation of free, unbound NO [83].
This insuperable difficulty has been acknowledged by some proponents of the NO2− hypothesis, who have noted that it is the `conversion of NO2− into longer-lived NO metabolites that accounts for most of the vasodilation' [49]. Infact, Hb(FeIII) serves as a route to SNO-Hb production, and NO-Fe2+Hb might also undergo oxidation-promoted transfer of NO to Cys β93 [84]. It is important to note that NO-Fe2+Hb and metHb constitute micropopulations (99% of the Hb is found as HbO2 and Fe2+Hb) that can co-exist within the Hb tetramer to favor SNO formation [50].
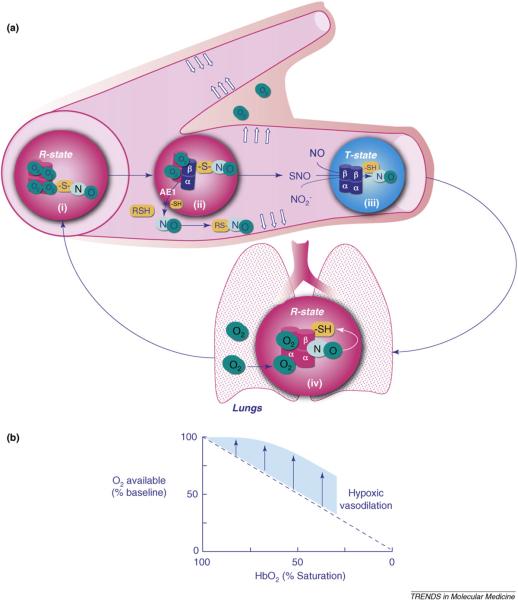
SNO-Hb offers the more parsimonious explanation for what we know about Hb, NO and hypoxic vasodilation: (i) that unprotected NO is highly susceptible to degradation by Hb and other biochemical scavengers; (ii) that the conversion of free NO to nitrate is much faster than NO2− reduction to NO; and (iii) that the presence of thiols, both in the RBC membrane and the plasma, is important for hypoxic vasodilation. Careful measurements have confirmed the existence of heme-bound NO on deoxyhemoglobin, of SNO on oxygenated Hb and the release of vasodilator activity on transition from the R- to T-states [43,64]. This model, in which vasodilation is progressive across a physiological O2 gradient and tracks the O2 desaturation of Hb rather than tissue PO2, replicates the signature feature of blood flow autoregulation described by early investigators and corroborated by more recent studies [43,45,85]. Finally, when Hb is reoxygenated in the pulmonary circulation, the vasoactive SNO on the Cys93 residue in the β-chains is no longer released—behaving just as the dilator that Guyton and colleagues had envisioned in 1962 (see Figure 1).
Figure 1.
Hypoxic vasodilation. (a) RBCs containing oxygenated Hb (R-state) approaching a microvascular branch point (i) are preferentially directed to a dilated capillary that supplies actively metabolizing tissue because desaturation of preceding RBCs (ii) has caused the allosteric release of NO bioactivity in the form of SNOs. RBCs containing deoxygenated Hb (T-state) enter the venous system where NO from several sources in the plasma as well as from SNO-Hb (thiol to heme transfer) recharge the HbFe- NO stores (iii) by binding hemes in the β-chains, forming metHb-containing hybrids (FeIII/NO). Re-oxygenation in the lungs (inset, iv) displaces NO from the hemes to β93 cysteines, restoring the dilator capacity of the RBCs. AE1, Anion Exchanger 1. (b) The contribution of RBC-transported NO bioactivity is shown in the graph, in which the straight descending line reflects the availability of O2 without vasodilation. The shaded area represents the increased O2 extraction enabled by hypoxic vasodilation (data from [15,20,22,85]).
Implications for clinical medicine
The SNO-Hb paradigm expands our understanding of the respiratory cycle by identifying NO as a third respiratory gas and provides new insights into the pathophysiology of the many diseases in which microcirculatory blood flow is abnormal. Preliminary animal studies using the gas ethyl nitrite (ENO) [86,87], which when inhaled forms SNOs in the blood more readily than NO itself, might lead to methods for replenishing SNO-Hb. In a 2009 clinical trial, SNO-Hb is being measured before and after transfusion of RBCs [88], and the data are not yet available. In the future, this knowledge will be tested clinically as the handling of NO in the blood becomes a therapeutic target in the management of microcirculatory diseases and measurement of erythrocyte NO content becomes standard clinical practice.
Most vascular diseases involve abnormal regulation of blood vessel tone, and recent investigations of several important clinical conditions in which vascular function is impaired have implicated disordered delivery or release of NO bioactivity from the RBCs to microvessels in specific tissues. Diseases involving abnormalities in S-nitrosation include diabetes [89], sickle-cell anemia [90], congestive heart failure [91], sepsis [92], preeclampsia [93] and pulmonary hypertension [57]. In many of these states, circulating levels of SNO-Hb are low or undetectable, which implies that local control of the microcirculation is impaired. In other disorders, it is the presence of S-nitrosation that can be a factor in the disease processes, for example inducing pathological fission in cerebral mitochondria in Alzheimer's disease [94]. In addition, spontaneous SNO depletion in banked blood can increase the chance for complications following blood transfusions [95,96]. Therefore, an important goal of current research into the mechanisms by which Hb and SNOs mediate hypoxic vasodilation is to identify effective diagnostic strategies and therapeutic interventions for these pathological conditions.
S-nitrosylation: beyond hypoxic vasodilation
Both NO and thiols are ubiquitous constituents of mammalian cellular biology, and the roles of SNO proteins are not confined to hypoxic vasodilation. It has become increasingly apparent that the allosterically regulated covalent bonding of NO to thiols on other large molecules besides Hb is a versatile regulatory device that is vital to the functioning of many proteins, including ion channels, enzymes, transport and storage biomolecules, subcellular structural components, signaling molecules, clotting factors, proteases and kinases [97]. Because this posttranslational modification of proteins by SNO can be compared with phosphorylation and other similar processes—for example glycosylation, acylation, methylation and sumoylation—it has proven useful to identify this general process as S-nitrosylation [98]. Therefore, SNO-Hb-mediated hypoxic vasodilation is but the prototypical example of a rich biochemistry that has now been opened to investigation and awaits translation to clinical practice.
Acknowledgements
This work was supported by Office of Naval Research Grant N00014-04-1-0171 (to CAP and BWA), by NIH grants RO1-AI064789 (to CAP) and R01-HL091876 (to JSS).
Glossary
- β93 cysteine (Cys βb93)
the thiol-containing amino acid occupying position 93 in the structure in the beta chain of normal human Hb and that of all other mammals and birds, as well as many other vertebrates [39]. The fact that this structural feature is so highly conserved across many species indicates that it has an important function.
- Hypoxic vasodilation
the rapid dilation of microvascular beds in response to increased local metabolic demand, which is critically coupled to decreased Hb O2 saturation and occurs in the time that blood transits a capillary bed from the arteries to the veins. This mechanism excludes changes because of altered arterial pressure, overriding control by the central nervous system, the effect of circulating hormones, prolonged hypoxia or ischemia.
- Iron nitrosohemoglobin (HbFe2+-NO)
Hb that has a nitroso group (NO) coordinated to a heme and which has no vasodilator activity.
- Microcirculation
the smallest functional unit of the circulation, consisting of an arteriole, the capillaries it supplies and the venule that drains them. The contractile state of smooth muscle fibers fine tunes the perfusion of individual microcirculatory units to match local metabolic demand.
- Nitric oxide (NO)
the endogenously synthesized free radical NO· that at low concentrations is an intercellular signaling molecule.
- Nitric oxide synthase (NOS)
the group of enzymes that synthesize NO from O2 and the amino acid arginine, converting the latter into citrulline. The three known forms (isozymes) of this enzyme were named for the context in which they were originally discovered, neuronal NOS (nNOS or NOS I) in neuronal tissue, inducible NOS (iNOS or NOS II) in macrophages after a triggering event such as infection and endothelial NOS (eNOS or NOS III) in the vascular endothelium. However, all isoforms are widely distributed and their functions are diverse. Thus, for example, NO generated by any of the three isozymes can affect vascular function.
- S-nitrosation and S-nitrosylation
these two terms refer to the covalent binding of a nitroso group (NO) to a thiol; however, the second is used more specifically in the context of posttranslational modification of proteins, cell-to-cell communication and intracellular signaling. The composite term, S-nitros(yl)ation, has come into use to more broadly refer to both sets of concepts.
- S-nitrosothiols
organic compounds, ranging from low molecular weight species such as Cys or glutathione up to proteins such as Hb, which have a nitroso group covalently bound to the sulfur atom of a thiol; all have the general formula RSNO and are commonly referred to as SNOs.
- S-nitrosohemoglobin (SNO-Hb)
Hb that has a nitroso group bound to Cys in position 93 in a β-chain and which can transfer protected NO vasodilator activity to other thiols by S-transnitrosation.
- Soluble guanylate cyclase (sGC)
a heme-containing intracellular enzyme that is a receptor for NO that is present, for example, in endothelial cells. The binding of NO to sGC greatly increases the activity of the enzyme, which leads to cyclic guanosine monophosphate (cGMP) formation and the subsequent biochemical cascade that relaxes smooth muscle.
- S-transnitrosation
the transfer of a nitroso group from one thiol to another, without the release of free NO.
Footnotes
Disclosure statement JSS holds equity in LifeHealth, N30 Pharma and Vindica, companies developing assays and uses for NO-based molecules.
References
- 1.Zelis R, Mason DT. Mechanism of systemic hemodynamic response during limb reactive hyperemia. Am J Physiol. 1969;217:1742–1746. doi: 10.1152/ajplegacy.1969.217.6.1742. [DOI] [PubMed] [Google Scholar]
- 2.Crawford DG, et al. Oxygen lack as a possible cause of reactive hyperemia. Am J Physiol. 1959;197:613–616. doi: 10.1152/ajplegacy.1959.197.3.613. [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation. 2008;117:594–597. doi: 10.1161/CIRCULATIONAHA.107.753897. [DOI] [PubMed] [Google Scholar]
- 4.Maher AR, et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 5.Hunter CJ, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 6.Isbell TS, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14:773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 8.Deem S, et al. Pulmonary vascular effects of red blood cells containing S-nitrosated hemoglobin. Am J Physiol Heart Circ Physiol. 2004;287:H2561–2568. doi: 10.1152/ajpheart.00310.2004. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, et al. Measurements of nitric oxide on the heme iron and β93 thiol of human hemoglobin during cycles of oxygenation and deoxygenation. PNAS. 2003;100:11303–11308. doi: 10.1073/pnas.2033883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladwin MT, et al. Relative role of heme nitrosylation and β-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. PNAS. 2000;97:9943–9948. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 12.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausladen A, et al. Assessment of nitric oxide signals by triiodide chemiluminescence. PNAS. 2007;104:2157–2162. doi: 10.1073/pnas.0611191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamler JS. S-nitrosothiols in the blood: Roles, amounts and methods of analysis. Circulation Research. 2004;94:414–417. doi: 10.1161/01.RES.0000122071.55721.BC. [DOI] [PubMed] [Google Scholar]
- 15.Doctor A, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. PNAS. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha W, et al. Amperometric S-nitrosothiol sensor with enhanced sensitivity based on organoselenium catalysts. Biosensors and Bioelectronics. 2009;24:2441–2446. doi: 10.1016/j.bios.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia HY, et al. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. Journal of the American Chemical Society. 2009;131:40–41. doi: 10.1021/ja808033w. [DOI] [PubMed] [Google Scholar]
- 18.Bramanti E, et al. Determination of S-nitrosoglutathione and other nitrosothiols by p-hydroxymercurybenzoate derivatization and reverse phase chromatography coupled with chemical vapor generation atomic fluorescence detection. Talanta. 2008;77:684–694. doi: 10.1016/j.talanta.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Allen BW, Piantadosi CA. How do red blood cells dilate blood vessels? Circ Res. 2004;94:e105. [PubMed] [Google Scholar]
- 20.Ross JM, et al. Autoregulation of blood flow by oxygen lack. Am. J. Physiol. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Jia L, et al. S-nitrosohemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 22.Stamler JS, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 23.Noble DR, et al. Nitric oxide release from S-nitrosoglutathione (GSNO) Chem. Commun. 1999:2317–2318. [Google Scholar]
- 24.Myers PR, et al. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990;345:161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- 25.Webb AJ, et al. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia. Role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008;108:175810. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter CD, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 27.Wiley KE, Davenport AP. Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol. 2002;136:1146–1152. doi: 10.1038/sj.bjp.0704815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellsworth ML, et al. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Alonso J, et al. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 30.Harrington LS, et al. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol. 2007;72:1132–1136. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- 31.Lange T, et al. Reduced number of CFTR molecules in erythrocyte plasma membrane of cystic fibrosis patients. Molecular Membrane Biology. 2006;23:317–323. doi: 10.1080/09687860600738304. [DOI] [PubMed] [Google Scholar]
- 32.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Lemus LA, et al. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology. 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 34.Stamler JS, et al. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 35.Sandmann J, et al. Specific transport of S-nitrosocysteine in human red blood cells: implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Letters. 2005;579:4119–4124. doi: 10.1016/j.febslet.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Angelo M, et al. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. PNAS. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gow A, et al. S-nitrosothiol measurements in biological systems. Journal of Chromatography B. 2007;851:140–151. doi: 10.1016/j.jchromb.2007.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsoukias NM. Nitric oxide bioavailability in the microcirculation: Insights from mathematical models. Microcirculation. 2008;15:813–834. doi: 10.1080/10739680802010070. [DOI] [PubMed] [Google Scholar]
- 39.Bonaventura J, Lance VP. Nitric oxide, invertebrates and hemoglobin. Amer. Zool. 2001;41:346–359. [Google Scholar]
- 40.Li S, Whorton AR. Identification of stereoselective transporters for S-nitroso-l-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J. Biol. Chem. 2005;280:20102–20110. doi: 10.1074/jbc.M413164200. [DOI] [PubMed] [Google Scholar]
- 41.Bolotina VM, et al. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, et al. S-transnitrosation reactions are involved in the metabolic fate and biological actions of nitric oxide. J Pharmacol Exp Ther. 1998;284:526–534. [PubMed] [Google Scholar]
- 43.McMahon TJ, et al. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J. Biol. Chem. 2000;275:16738–16745. doi: 10.1074/jbc.M000532200. [DOI] [PubMed] [Google Scholar]
- 44.Tsikas D, et al. Investigations of S-transnitrosylation reactions between low and high molecular weight S-nitroso compounds and their thiols by high-performance liquid chromatography and gas chromatography-mass spectrometry. Analytical Biochemistry. 1999;270:231–241. doi: 10.1006/abio.1999.4084. [DOI] [PubMed] [Google Scholar]
- 45.Diesen DL, et al. Hypoxic vasodilation by red blood cells: evidence for an S-nitrosothiol based signal. Circulation Research. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalsgaard T, et al. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01298.2006. 01298.02006. [DOI] [PubMed] [Google Scholar]
- 47.Lauer T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci. 2001;98:2814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gladwin MT, Schechter AN. NO contest: nitrite versus S-nitrosohemoglobin. Circ Res. 2004;94:851–855. doi: 10.1161/01.RES.0000126697.64381.37. [DOI] [PubMed] [Google Scholar]
- 49.van Faassen EE, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Medicinal Research Reviews. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells. Annual Review of Physiology. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 51.Foster MW, et al. A genetic analysis of nitrosative stress. Biochemistry. 2009;48:792–799. doi: 10.1021/bi801813n. [DOI] [PubMed] [Google Scholar]
- 52.Pawloski JR, et al. Impaired vasodilation by red blood cells in sickle-cell disease. PNAS. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 54.Lima B, et al. Endogenous S-nitrosothiols protect against myocardial injury. PNAS. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer LA, et al. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J. Clin. Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roach R, et al. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol. 1999;276:H438–445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- 57.McMahon TJ, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. PNAS. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamler JS, et al. SNO-hemoglobin and hypoxic vasodilation. Nat Med. 2008;14:1008–1009. doi: 10.1038/nm1008-1008. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends in Cardiovascular Medicine. 2006;16:259–265. doi: 10.1016/j.tcm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Lauer T, et al. Indexes of NO bioavailability in human blood. News Physiol Sci. 2002;17:251–255. doi: 10.1152/nips.01405.2002. [DOI] [PubMed] [Google Scholar]
- 61.Shah CM, et al. Interactions between cell surface protein disulphide isomerase and S-nitrosoglutathione during nitric oxide delivery. Nitric Oxide. 2007;16:135–142. doi: 10.1016/j.niox.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO reports. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang A, et al. Contrasting effects of thiol-modulating agents on endothelial NO bioactivity. Am J Physiol Cell Physiol. 2001;281:C719–725. doi: 10.1152/ajpcell.2001.281.2.C719. [DOI] [PubMed] [Google Scholar]
- 64.Pawloski JR, et al. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 65.Walsh S, et al. Cation transport activity of Anion Exchanger 1 mutations found in inherited distal renal tubular acidosis. Am J Physiol Renal Physiol. 2008;295:F343–350. doi: 10.1152/ajprenal.00587.2007. [DOI] [PubMed] [Google Scholar]
- 66.Köck K, et al. Expression of adenosine triphosphate-binding cassette (ABC) drug transporters in peripheral blood cells: relevance for physiology and pharmacotherapy. Clinical Pharmacokinetics. 2007;46:449–470. doi: 10.2165/00003088-200746060-00001. [DOI] [PubMed] [Google Scholar]
- 67.Ignarro LJ, Gruetter CS. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochimica et Biophysica Acta (BBA) - General Subjects. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- 68.Carlsen E, Comroe JH., Jr The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. J. Gen. Physiol. 1958;42:83–107. doi: 10.1085/jgp.42.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gibson QH, Roughton FJW. The kinetics and equilibria of the reaction of nitric oxide with sheep hemoglobin. Journal of Physiology. 1957;136:507–526. doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joshi MS, et al. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. PNAS. 2002;99:10341–10346. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gladwin MT, et al. Inhaled nitric oxide augments nitric oxide transport on sickle-cell hemoglobin without affecting oxygen affinity. J. Clin. Invest. 1999;104:937–945. doi: 10.1172/JCI7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gladwin MT, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. PNAS. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagababu E, et al. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 74.Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 75.Modin A, et al. Nitrite-derived nitric oxide: a possible mediator of `acidic-metabolic' vasodilation. Acta Physiologica Scandinavica. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 76.Cannon RO, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J. Clin. Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dejam A, et al. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 78.Ng ESM, et al. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circ Res. 2004;94:559–565. doi: 10.1161/01.RES.0000117771.63140.D6. [DOI] [PubMed] [Google Scholar]
- 79.Doyle M, et al. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 80.Isbell TS, et al. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:H2565–2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 81.Kleinbongard P, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biology and Medicine. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 82.Honig CR, et al. Capillary recruitment in exercise: rate, extent, uniformity and relation to blood flow. Am J Physiol Heart Circ Physiol. 1980;238:H31–42. doi: 10.1152/ajpheart.1980.238.1.H31. [DOI] [PubMed] [Google Scholar]
- 83.Tu C, et al. Reactions of nitrite with hemoglobin measured by membrane inlet mass spectrometry. Free Radical Biology and Medicine. 2009;46:14–19. doi: 10.1016/j.freeradbiomed.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luchsinger BP, et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. PNAS. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nature Medicine. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 86.Auten RL, et al. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am. J. Respir. Crit. Care Med. 2007;176:291–299. doi: 10.1164/rccm.200605-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moya MP, et al. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. The Lancet. 2002;360:141–143. doi: 10.1016/S0140-6736(02)09385-6. [DOI] [PubMed] [Google Scholar]
- 88.NIH . Clinical trial ( NCT00787124): transfusions and nitric oxide level in preterm infants. 2009. PB Smith, principal investigator. [Google Scholar]
- 89.James PE, et al. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 90.Bonaventura C, et al. Heme redox properties of S-nitrosated hemoglobin A0 and hemoglobin S: Implications for interactions of nitric oxide with normal and sickle red blood cells. J. Biol. Chem. 2002;277:14557–14563. doi: 10.1074/jbc.M107658200. [DOI] [PubMed] [Google Scholar]
- 91.Datta B, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 92.Crawford JH, et al. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 93.Gandley RE, et al. S-nitroso-albumin-mediated relaxation is enhanced by ascorbate and copper: effects in pregnancy and preeclampsia plasma. Hypertension. 2005;45:21–27. doi: 10.1161/01.HYP.0000150158.42620.3e. [DOI] [PubMed] [Google Scholar]
- 94.Cho D-H, et al. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bennett-Guerrero E, et al. Evolution of adverse changes in stored RBCs. PNAS. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reynolds JD, et al. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. PNAS. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stamler JS, et al. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 98.Foster MW, et al. Protein S-nitrosylation in health and disease: A current perspective. Trends in Molecular Medicine. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]