Abstract
In dementia research, animal models have become indispensable tools. They not only model aspects of the human condition, but also simulate processes that occur in humans and hence provide insight into how disease is initiated and propagated. The present review discusses two prominent human neurodegenerative disorders, Alzheimer's disease and frontotemporal dementia. It discusses what we would like to model in animals and highlights some of the more recent achievements using species as diverse as mice, fish, flies and worms. Advances in imaging and therapy are explored. We also discuss some anticipated new models and developments. These will reveal how key players in the pathogenesis of Alzheimer's disease and frontotemporal dementia, such as the peptide Aβ (amyloid β) and the protein tau, cause neuronal dysfunction and eventually, neuronal demise. Understanding these processes fully will lead to early diagnosis and therapy.
Keywords: Alzheimer's disease, amyloid, Caenorhabditis elegans, Drosophila, frontotemporal dementia, mouse, rat, tau, TAR DNA-binding protein 43 (TDP-43), transgenic, zebrafish
Abbreviations: Aβ, amyloid β; AD, Alzheimer's disease; AGE, advanced glycation end-product; APP, amyloid precursor protein; BAC, bacterial artificial chromosome; CaMKII, Ca2+/calmodulin-dependent protein kinase II; FAD, familial AD; FTD, frontotemporal dementia; FTDP-17, familial FTD with parkinsonism linked to chromosome 17; GFP, green fluorescent protein; JIP1, c-Jun N-terminal kinase-interacting protein 1; MND, motor neuron disease; MRI, magnetic resonance imaging; NFT, neurofibrillary tangle; PET, positron emission tomography; PIB, Pittsburgh Compound-B; PSEN, presenilin; SOD1, superoxide dismutase 1; TDP-43, TAR DNA-binding protein 43; TH, tyrosine hydroxylase
INTRODUCTION
The present review is based on a Plenary Lecture given by J.G. at the 12th ICAD (International Conference on Alzheimer's Disease) in Vienna in July 2009.
Animal models have become indispensable in basic and biomedical research (Götz and Ittner, 2008). The following definitions found on the web underscore two important attributes of a model: the open-source platform Wikipedia states that an animal model is “a non-human animal that has a disease or injury that is similar to a human condition” (http://en.wikipedia.org/wiki/Animal_model; accessed 7 July 2009)”. Another site highlights a second important aspect by defining an animal model as “a laboratory animal used in research that simulates processes comparable to those that occur in humans” (http://science.education.nih.gov/supplements/nih4/self/other/glossary.htm; accessed 7 July 2009). Applying these definitions, e.g. to neurodegenerative disorders, illustrates that models are valuable because they represent a certain stage of disease and because processes that lead to this stage can be monitored longitudinally.
In modelling AD (Alzheimer's disease), the most important form of dementia, and FTD (frontotemporal dementia), which in prevalence ratings comes second (Ballatore et al., 2007), familial forms of these diseases, as well as histopathological and clinical features in the human patient, provide guidance.
In the present review, first, we discuss the genes that cause FAD (familial AD) and FTD as remarkably enough a subset of these genes also encode the proteins within the major lesions that define the two diseases. In FAD, which accounts for less than 1% of all cases, autosomal-dominant mutations have been identified in three genes: APP (amyloid precursor protein), PSEN1 (presenilin 1) and PSEN2 (presenilin 2) (Bertram and Tanzi, 2008). APP is a membrane-associated protein from which the peptide Aβ (amyloid β) is derived by proteolytic cleavage. The presenilins are components of the γ-secretase complex that, together with β-secretase, generates Aβ, while α-secretase activity precludes Aβ formation. In addition to the three FAD genes, a series of susceptibility genes have been identified in SAD (sporadic AD); these include APOE (apolipoprotein E) as the most established risk gene (Bertram and Tanzi, 2008). Very recently, three additional risk factor genes have been found, CLU encoding clusterin, PICALM encoding the phosphatidylinositol-binding clathrin assembly protein and CR1, the complement component (3b/4b) receptor 1 (Harold et al., 2009; Lambert et al., 2009). Compared with AD, FTD is a much more heterogeneous group of related dementias, which is reflected both by the types of genes that are mutated and by the proteins that are deposited. The first mutations identified in FTD were in FTDP-17 (familial FTD with parkinsonism linked to chromosome 17), where they were found in the MAPT gene that encodes the microtubule-associated protein tau (Cruts and Van Broeckhoven, 2008). This subset of FTD cases is characterized by tau inclusions (see below). Another subset of familial FTD is characterized by mutations in the PGRN gene that encodes the pleiotropic protein progranulin, and in the VCP gene that encodes valosin-containing protein; these cases are characterized by inclusions of the TDP-43 (TAR DNA-binding protein 43), a highly conserved hnRNP (heteronuclear ribonucleoprotein) (Neumann et al., 2006). Finally, mutations in CHMP2B, encoding chromatin-modifying protein 2B, lead to FTD in the absence of either tau or TDP-43 inclusions (Cruts and Van Broeckhoven, 2008). For detailed information, we refer to http://www.molgen.ua.ac.be/ADMutations and http://www.molgen.ua.ac.be/FTDMutations as a constantly updated source of mutations in FAD and FTD, as well as of the families in which they occur.
Histopathologically, AD is characterized by Aβ plaques and neurofibrillary lesions. Aβ in the plaques is fibrillar. Neurofibrillary lesions contain hyperphosphorylated, fibrillar aggregates of tau that are found in cell bodies and apical dendrites as NFTs (neurofibrillary tangles), in distal dendrites as neuropil threads, and in the abnormal neurites that are associated with some Aβ plaques. Truncation of tau, in addition to hyperphosphorylation (Chen et al., 2004a), has been linked to pathogenesis (Horowitz et al., 2004). Tau is generally perceived as a neuronal protein, with a mainly axonal localization, but this concept needs to be revisited as discussed below. In addition to plaques and neurofibrillary lesions, the AD brain is also characterized by massive neuronal cell and synapse loss at specific predilection sites (Selkoe, 2002). With regard to the formation of plaques and neurofibrillary lesions, sporadic cases of AD are not different from familial cases, whether they carry mutations in APP, PSEN1 or PSEN2. Hence, this led to the notion that understanding the pathogenesis of familial cases would also provide insight into the sporadic cases.
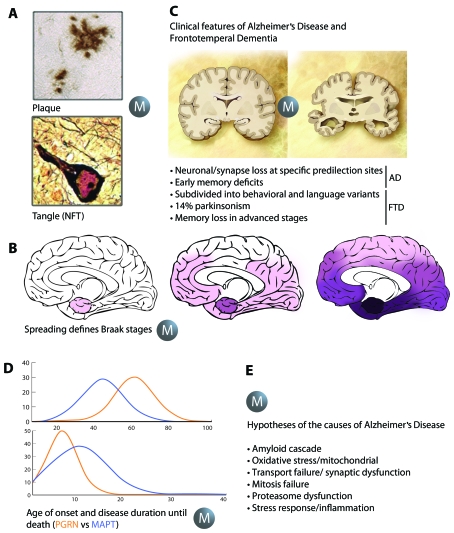
In addition to AD, NFTs are also abundantly present in a significant subset of FTD such as FTDP-17, in which there is an absence of overt plaques. Another subset of FTD with tau-negative and ubiquitin-positive lesions, also in the absence of plaques, has been termed FTLD-U or FTDU-17 (Cruts and Van Broeckhoven, 2008). Here, TDP-43 has been identified in ubiquitin-positive inclusions; these have also been found in sporadic ALS (amyotrophic lateral sclerosis). Similar to tau, TDP-43 in the aggregates is hyperphosphorylated, ubiquitinated and C-terminally truncated (Neumann et al., 2006). Both types of protein aggregates can be visualized under the light microscope either by immunohistochemistry, thioflavin S or silver impregnation methods such as those developed by Gallyas and Bielschowsky [as described previously (Ittner et al., 2008)]. Massive protein aggregation and, in particular, Gallyas reactivity was one of the defining hallmark aspects of the human pathology that the early animal models tried to achieve, but in which they failed: although tau formed aggregates as the mice aged, NFTs did not develop (Figure 1A) (Götz and Ittner, 2008).
Figure 1. Aspects of AD and FTD one would like to model in animals.
Animal models have become indispensable in basic and biomedical research. They reproduce aspects of AD and FTD (A–D), but also allow for testing the many hypotheses that have been put forward to determine what exactly causes these diseases (E). In both AD and FTD, protein aggregation leads to lesions that can be visualized under the light microscope either by immunohistochemistry, by dyes such as thioflavin S, or by silver impregnation methods such as those developed by Gallyas and Bielschowsky, an aspect to be modelled (as indicated by the ‘M’) in animals (A). A second aspect is that of the spreading of the key histopathological hallmarks of AD, the Aβ plaques and the tau tangles (NFTs) that has led to the definition of the Braak stages (B). A third is the distinct clinical features such as memory impairment in AD or parkinsonism that characterizes a subset of FTD cases (C). The fourth is the distinct age of onset and disease duration that discriminates, e.g. carriers of mutations in the tau-encoding MAPT gene compared with those in the progranulin-encoding PGRN gene (D) based on data in (Cruts and Van Broeckhoven, 2008). The list of hypotheses in the field is led by the amyloid cascade hypothesis, but animal models provide support for all proposed hypotheses (E).
Another defining feature of the human pathology is the characteristic spreading of the hallmark lesions in the AD brain that, in the case of tau, is subject to little inter-individual variation and provides a basis for distinguishing six stages: the transentorhinal stages I and II representing silent cases; the limbic stages III and IV; and the neocortical stages V and VI (Braak and Braak, 1991). A similar type of staging has subsequently been defined for the Aβ pathology (Thal et al., 2002) and is now also available for the Lewy body pathology in PD (Parkinson's disease) (Braak et al., 2003). The spreading of the major histopathological hallmarks of AD, associated with selective neuronal cell loss, is not well understood at the cellular and molecular level. The Braak staging implies selective vulnerability of distinct neuronal populations (Götz et al., 2009). This staging is a feature one definitely would like to recapitulate in animals, as neuronal and synapse loss at specific predilection sites causes the clinical features that discriminate AD from FTD (Figure 1B). In the AD brain, atrophy is initiated in the hippocampus and entorhinal cortex, followed by the association cortices and subcortical structures, including the amygdala and NBM (nucleus basalis of Meynert). The histopathology is reflected by the clinical features that characterize AD: early memory deficits that are followed by a gradual erosion of other cognitive functions (Arnold et al., 1991). Atrophy in the FTD brain is mainly found in frontal and/or temporal cortices. Hence, in contrast with AD, FTD is associated predominantly with behavioural and/or language impairment, with memory impairment mostly found only late in disease (Cruts and Van Broeckhoven, 2008). A significant subset of FTD is characterized by parkinsonism (Figure 1C), an aspect that has been modelled in animals as outlined below.
A further remarkable aspect one would like to model in animals is that of the distinct age of onset and disease duration until death that, e.g. discriminates carriers of PGRN mutations from those of MAPT mutations (Cruts and Van Broeckhoven, 2008) (Figure 1D).
In understanding the aetiology of the sporadic cases of AD and FTD, a lot of hypotheses have been put forward (addressing an important aspect highlighted by the second definition we have presented above). These include the amyloid cascade hypothesis, oxidative stress/mitochondrial dysfunction, transport/synaptic dysfunction, mitosis failure, proteasome dysfunction, and stress response and inflammation hypotheses. They have been tackled by a series of ‘omics’ approaches which encompass lipidomics, proteomics, transcriptomics and genomics (Noorbakhsh et al., 2009).
VERTEBRATE AND INVERTEBRATE ANIMAL MODELS
The mouse is the major species used in AD and FTD research, but significant additional insight has been provided from species such as the roundworm Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and two types of fish, the sea lamprey and the zebrafish (Götz et al., 2007).
As far as rodents are concerned, there are non-transgenic models available such as the SAM (senescence-accelerated mice), or chemically induced lesion models, but these lack the characteristic hallmark lesions of AD (Van Dam and De Deyn, 2006). These have been modelled by transgenesis (Ittner and Götz, 2007), almost exclusively in mice, as reviewed recently (Götz and Ittner, 2008), although there are a few transgenic rat models available (Zilka et al., 2006; Koson et al., 2008). Although the list of strains that were discussed in Götz and Ittner (2008) is long, it is still incomplete, since a myriad of AD and FTD mouse strains has been generated since the early 1990s and it is impossible to present them all.
However, a few select models may provide a flavour of what has been achieved so far. Of the many APP mutant mice that have been generated in the past, strains such as the PDAPP, J20, APP23 or Tg2576 mice are good representatives as they are widely used owing to their robust APP/Aβ pathology (Games et al., 1995; Hsiao et al., 1996; Sturchler-Pierrat et al., 1997; Mucke et al., 2000). Representative models with NFT formation are strains such as the JNPL3 or pR5 mice, both of which express P301L mutant tau that previously has been identified in FTDP-17 (Lewis et al., 2000; Götz et al., 2001a). Aβ in the above plaque mice is fibrillar, as is tau in the NFTs of the P301L mutant strains; in both instances, the histopathology is associated with behavioural impairment, following assessment of hippocampus- and amygdala-dependent tasks (Götz and Ittner, 2008). In conclusion, the aggregation of both Aβ and tau has been faithfully reproduced in vivo, with the hallmark lesions of AD and FTD being visible at a light microscopic level. Similarly, aspects of memory impairment have been reproduced.
The microtubule-associated protein tau is generally perceived as a neuronal protein, however, tauopathies such as CBD (corticobasal degeneration) and PSP (progressive supranuclear palsy) are characterized by a pronounced glial tau pathology that is often even more pronounced than the concomitant neuronal pathology (Götz, 2001; Kurosinski and Götz, 2002). The glial tau pathology has been modelled, e.g. in mice that express wild-type human tau in astrocytes (Forman et al., 2005). Not only do these mice develop glial lesions that are remarkably similar to the human pathology, with tufted astrocytes and astrocytic plaques, but the authors also report on a focal neuronal degeneration. In other words, a glial pathology causes a neuronal pathology. The glial lesions were Gallyas-positive, as in the human patients. Similar inclusions were obtained by expressing P301L mutant tau in astrocytes; this again was associated with a neuronal pathology as evidenced by impaired axonal transport shown for the optic nerve (Higuchi et al., 2005).
While these studies model tau aggregation and NFT or GFT (glial fibrillary tangle) formation, other models addressed the question of how, if at all, the tau aggregates are related to disease (Götz et al., 2008a). To this end, a CaMKII (Ca2+/calmodulin-dependent protein kinase II) promoter-driven transactivator system was employed, achieving a 15-fold overexpression of P301L mutant tau (Ramsden et al., 2005; Santacruz et al., 2005). This resulted in NFT formation, neuronal loss and memory impairment. NFT formation was linked to the appearance of a 64 kDa ‘toxic’ tau species termed tau*. When tau expression was suppressed after supplementing the drinking water with doxycycline, this resulted in a lower, 2.5-fold overexpression. Interestingly, memory functions recovered, but NFTs continued to accumulate. In more recent work it was shown that, in the young brain, elevation of the levels of phosphorylated tau species per se was not sufficient to cause a lasting tau pathology (Dickey et al., 2009). A CaMKII promoter-driven transactivator system was also employed to express truncated forms of tau comprising mainly the C-terminal microtubule-binding domain. Both a pro- and an anti-aggregation mutant were generated, with the former resulting in neuronal loss (Mocanu et al., 2008). An interesting observation was that human ‘pro-aggregation’ tau co-aggregated with mouse tau, the possibility of tau forming mixed aggregates being an issue of discrepancy in the field for quite some time. For comparison, previous work including a BAC (bacterial artificial chromosome) transgenic approach combined with a tau knockout had suggested that endogenous mouse tau is inhibitory to the aggregation of transgenic human tau (Andorfer et al., 2003). In the pro-aggregation mouse model, again, when the mice were fed with doxycycline, tau levels went down, while NFTs persisted (Mocanu et al., 2008). In related work in flies, overexpression of both wild-type and R406W mutant tau was shown to cause premature death, with mutant tau showing an earlier age of onset (Wittmann et al., 2001). Cholinergic neurons (a major target of the cholinergic therapy in AD) were found to be degenerating in the flies. Interestingly, the tau-associated pathology occurred in the absence of NFT formation, suggesting that their formation may not be required for tau to execute its toxic effects.
Coming back to NFT formation, this has been linked by several groups to phosphorylation of tau at distinct epitopes including AT100 (Thr212/Ser214) and pS422 (Ser422) (Götz and Nitsch, 2001; Götz et al., 2001b; Allen et al., 2002; Ferrari et al., 2003; Guillozet-Bongaarts et al., 2006; Le Corre et al., 2006; Deters et al., 2008, 2009). The notion that phosphorylation of tau at specific sites is both necessary and sufficient for tau to form filaments has been recently challenged, again by work in flies. First it was shown that tau simultaneously pseudo-phosphorylated at 14 sites (E14 tau) was more toxic than wild-type tau. Next, these sites were mutated to alanine residues, both in combination and alone. The analysis of individual alanine point mutations suggested that tau phosphorylation sites rather than working ‘in isolation’ act ‘in concert’ in order to promote toxicity (Steinhilb et al., 2007). However, the final word is not yet spoken, especially as toxicity may depend on species and cell types. From a therapeutic perspective, whether general rather than site-specific phosphorylation needs to be impaired to prevent tau from aggregating has important implications, as boosting the activity of more promiscuous phosphatases may be a preferred strategy compared with reducing the activity of phospho-site-specific kinases (Iqbal and Grundke-Iqbal, 2008).
Similar to the tau field, with a hunt for what is thought to be the ‘toxic species’, there is also an ongoing search for the toxic species in the Aβ field [reviewed in (Götz et al., 2008b; Yankner and Lu, 2009)]. Here, a dodecameric form of Aβ termed Aβ*56 has been identified in mutant APP transgenic Tg2576 mice that appeared when memory impairment was initiated and that disappeared when memory decline was stabilized. When a preparation enriched for Aβ*56 was injected into young recipient rat brains this was shown to disrupt memory (Lesne et al., 2006). However, in more recent work, fibrillar Aβ was identified as a pathogenic entity that altered neuronal membrane properties such that there was hyperexcitability of pyramidal cells, culminating in epileptiform activity (Minkeviciene et al., 2009). Moreover, an analysis of mitochondrial impairment suggests that both oligomeric and fibrillar species of Aβ exert a similar degree of toxicity (Eckert et al., 2008).
While this addresses memory impairment as the major clinical feature characterizing AD, another clinical feature, parkinsonism, that characterizes a significant subset of FTD cases, has also been modelled in mice. Several mini-gene tau constructs have been used to establish a series of transgenic mouse strains, of which one particular strain reproduced tau accumulation in astrocytes and neurons including these of the nigro-striatal pathway (Dawson et al., 2007). The rate-limiting enzyme in dopamine synthesis, TH (tyrosine hydroxylase), was found to accumulate in varicosities (axonal swellings). Furthermore, the mice showed memory and motor impairment (Dawson et al., 2007). A second model was based on the identification of the K369I mutation of tau in a patient with Pick's disease, an extreme form of FTD (Neumann et al., 2001). The tau lesions in Pick's disease (and also in the brain of this particular patient) show a remarkable feature: they are Bielschowsky-positive and Gallyas-negative; in addition tau is phosphorylated at many epitopes but 12E8 (Ser262/Ser356). This distinct Pick pathology was reproduced in the K3 mouse strain that expresses K369I mutant tau (Ittner et al., 2008). The transgenic mice also model early-onset parkinsonism (resting tremor, bradykinesia, postural instability and gait anomalies). They show an increased cataleptic response to haloperidol and an early, but not late, response to L-Dopa, with similarities to the efficacy of the treatment in FTD patients using this form of medication.
In the K3 mice, axonal transport is impaired. Indeed in recent years, impaired axonal transport emerged as a central pathomechanism in AD (Stokin et al., 2005; Götz et al., 2006; Magnani et al., 2007; Thies and Mandelkow, 2007; Dixit et al., 2008; Pigino et al., 2009). Impaired axonal transport is conceptually linked to oxidative stress and mitochondrial dysfunction: mitochondria are a major organelle that needs to be efficiently transported along the long processes for neurons to function and they are a major source of ROS (reactive oxygen species) (Su et al., 2008; Tatsuta and Langer, 2008). In the K3 mice, there is a selectively impaired axonal transport of distinct cargos, such as mitochondria and TH-containing vesicles (Ittner et al., 2008). This is because a component of the kinesin motor machinery, the scaffold/adapter protein JIP1 (c-Jun N-terminal kinase-interacting protein 1), is trapped by elevated tau, preventing it from executing its physiological function. In a follow-up study a pathological interaction between tau and JIP1 was also revealed in AD and not control brain (Ittner et al., 2009). Interestingly, this pathological interaction requires phosphorylation of tau. Since JIP1 is involved in regulating cargo binding to kinesin motors, these findings may, at least in part, explain how hyperphosphorylated tau mediates impaired axonal transport in AD and FTD, before tau starts to aggregate (Ittner et al., 2009). This presents inhibition of abnormal hyperphosphorylation of tau as a promising therapeutic target for the development of disease-modifying drugs (Iqbal and Grundke-Iqbal, 2008).
Another interesting aspect of tau that has recently emerged is that of the transmission, secretion and spreading of tau. Using a stereotaxic injection approach (Clavaguera et al., 2009), a donor brain extract was derived from 6-month-old NFT-forming P301S mice (Allen et al., 2002) and injected into brains of 3-month-old ALZ17 mice (Probst et al., 2000; Götz and Nitsch, 2001), a (wild-type) tau transgenic strain, that develops a tau-associated amyotrophy, but fails to develop NFTs (Clavaguera et al., 2009). The study found that the donor extract induced NFTs in the recipient brain, a feature termed ‘transmission’. Secondly, NFT induction was not confined to the injection site, a feature referred to by the investigators as ‘spreading’. Finally, insoluble rather than soluble tau was found to be responsible for these effects (Clavaguera et al., 2009). In related work, it has been shown that extracellular tau aggregates can transmit a misfolded state from the outside to the inside of a cell, similar to prions (Frost et al., 2009). These features of tau are supported by work in the sea lamprey, a marine fish characterized by so-called giant neurons, which can be injected with plasmids encoding tau expression constructs. Work spanning one and a half decades support the notion that tau is secreted and reproduces aspects of the staging that characterizes the tau pathology (Hall and Cohen, 1983; Hall et al., 2001; Kim et al., 2009). Similar aspects have been reported for α-synuclein, a protein with a structure very much like tau, that induced inclusion formation and neuronal cell death through interneuronal transmission (Desplats et al., 2009). Taken together, these data contribute to an understanding of how, in human disease, Aβ and tau impair cellular functions, which eventually leads to neuronal loss.
Several aspects of the human AD and FTD pathology have been modelled in the nematode C. elegans (Morcos and Hutter, 2009). This roundworm has a number of features that make it a powerful research tool: (i) it is easy to culture as it feeds on bacteria grown on agar plates; (ii) it reproduces and develops rapidly: within 3 days it develops from an egg to an adult worm, with approx. 300 progenies originating from one self-fertilized hermaphrodite; (iii) its small size allows assays in microtitre format, studying hundreds of animals in a single well; (iv) the worm is transparent, which is ideal for the use of fluorescence markers in vivo; (v) it is a complex multicellular animal: an adult hermaphrodite has exactly 959 somatic cells that form different organs, including 302 neurons forming the nervous system; (vi) it has a short life span of 2–3 weeks, allowing studies in aged animals within a reasonable time frame (mice, in comparison, often need to age for 1 year or even longer before they develop a phenotype); and (vii) genetic modifications, such as transgenic expression or RNAi (RNA inteference)-mediated gene knockdown are quite easy compared with other in vivo systems. Furthermore, most human disease genes and pathways are present in C. elegans (Kaletta and Hengartner, 2006). This includes the APP homologue APL-1, a mutation of which results in early larval lethality (Hornsten et al., 2007). C. elegans has two PSEN homologues, SEL-12 and HOP-1 (Levitan and Greenwald, 1995; Li and Greenwald, 1997), and a single tau-like protein called PTL-1 (protein with tau-like repeats) (Goedert et al., 1996; McDermott et al., 1996)
C. elegans has been successfully used as model organism to study pathomechanisms in MND (motor neuron disease) and FTD; e.g. expression of SOD1 (superoxide dismutase 1) carrying a human pathogenic mutation identified in familial MND results in severe locomotor deficits in transgenic C. elegans (Wang et al., 2009). Expression of wild-type SOD1, however, does not affect motor behaviour. Similarly, expression of human tau carrying FTD mutations, but not wild-type tau, in C. elegans results in neurodegeneration with an accumulation of hyperphosphorylated tau and associated uncoordinated locomotion (Unc) (Kraemer et al., 2003; Miyasaka et al., 2005; Brandt et al., 2009). C. elegans is not capable of producing endogenous Aβ. The first published transgenic model targeted Aβ to the body wall of muscle cells, causing progressive paralysis (Link, 1995). Aβ deposition in another model was associated with oxidative stress (Drake et al., 2003). A further aspect linked to oxidative stress that is evident from studies in C. elegans is the similarity between diabetes mellitus and AD, in particular with regards to the formation of AGEs (advanced glycation end-products) (Morcos and Hutter, 2009). Together this demonstrates the potential of C. elegans as a model organism in AD and FTD research. The findings obtained in the worm are complemented by studies in Drosophila, that in addition has shown its potential as a model and a powerful system to screen for modifiers that either enhance or suppress an AD-associated pathology (Shulman and Feany, 2003; van de Hoef et al., 2009).
That farm animals are also attractive as AD models is illustrated by the recent establishment of a porcine model: while the transgene was found to be expressed in brain, the authors speculate that it may take until the age of 1–2 years until Aβ may accumulate in the porcine brain (Kragh et al., 2009).
THE Aβ–TAU AXIS
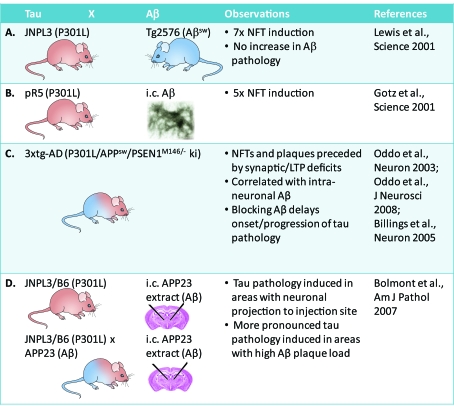
A central hypothesis in AD research is the amyloid cascade hypothesis as revisited by Hardy (2006). Animal models were central in providing support for this hypothesis that claims, in simplified terms, that an Aβ pathology causes a tau pathology (Figure 2). While initially plaques and NFTs were thought to be the key players, in recent years the focus has shifted from these end-stage lesions to the precursors, oligomeric forms of Aβ and soluble forms of tau, as the major (but not exclusive) culprit. When P301L mutant tau transgenic JNPL3 mice were crossed with APP mutant Tg2576 mice, this caused a 7-fold NFT induction, but no increased Aβ pathology (Lewis et al., 2001) (Figure 2A). Similarly, stereotaxic injections of fibrillar preparations of Aβ42 into the hippocampus and somatosensory cortex of P301L tau mutant pR5 mice caused a 5-fold induction of NFTs in the amygdala, a site which projects to the CA1 neurons in the hippocampus (Götz et al., 2001b) (Figure 2B). The pathology in the amygdala is reflected by an impairment in amygdala-dependent tasks (Pennanen et al., 2004, 2006). Injections of Aβ42 into wild-type tau transgenic ALZ17 mice that lack an NFT pathology (Probst et al., 2000) failed to induce NFTs, suggesting that the toxic effect of Aβ is dependent on a pre-existing tau pathology (Götz et al., 2001b).
Figure 2. Transgenesis supports the amyloid cascade hypothesis in mice.
Crossing P301L tau mutant JNPL3 mice with APP mutant Tg2576 mice causes a 7-fold increased NFT induction, but no increased Aβ pathology (A). Similarly, intracerebral (i.c.) injections of synthetic Aβ42 preparations cause a 5-fold induction of NFTs in P301L tau mutant pR5 mice (B). 3xtg-AD mice (P301L tau/APPsw/PSENM146/− knockin), that combine an NFT and plaque pathology, show a prominent role for Aβ (C). A stereotaxic approach was used to inject extracts from Aβ plaque-forming APP23 mice [and not synthetic Aβ as in (B)] into P301L tau mutant JNPL3/B6 mice, again showing a role for Aβ in inducing a tau pathology (D).
Subsequently, the so-called 3xtg-AD mice (P301L tau/APPsw/PSENM146/− knock-in) were generated that combine an NFT and plaque pathology (Figure 2C). Their formation was found to be preceded by synaptic and LTP (long-term potentiation) deficits (Oddo et al., 2003). The clinical features were correlated with intraneuronal Aβ formation (Billings et al., 2005), and blocking of Aβ was shown to delay onset and progression of the tau pathology (Oddo et al., 2008). A stereotaxic approach was used, not by injecting synthetic Aβ preparations (Götz et al., 2001b), but by injecting extracts from Aβ plaque-forming APP23 mice into P301L tau mutant JNPL3/B6 mice (Figure 2D). This induced a tau pathology in areas with a neuronal projection to the injection site (Bolmont et al., 2007). When the injections were performed in APP23/JNPL3/B6 mice with both a plaque and NFT pathology, a more pronounced tau pathology was induced in areas with a high Aβ plaque load (Bolmont et al., 2007) (Figure 2D).
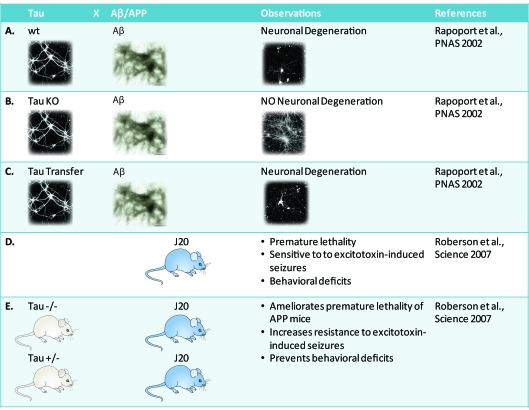
While this supports the amyloid cascade hypothesis in mice, there is also a role for tau (Figure 3). Several years ago, Aβ toxicity in primary neuronal cultures was shown to depend on the presence of tau: wild-type neurons were found to degenerate when incubated with Aβ42 as did tau-transfected neurons (Figures 3A and 3B). However, primary neurons derived from tau knockout mice turned out to be resistant to the toxic effects of Aβ (Rapoport et al., 2002) (Figure 3C). This was translated in vivo by breeding APP mutant J20 mice on to a tau-knockout background (Roberson et al., 2007). Most, if not all, APP mutant strains with a robust Aβ plaque pathology are characterized by premature death of unknown cause (Palop et al., 2007; Minkeviciene et al., 2009; Palop and Mucke, 2009) (Figure 3D). Roberson and colleagues reported the remarkable finding that tau reduction ameliorates the premature lethality of the J20 mice, increases the resistance to excitotoxin-induced seizures and prevents behavioural deficits in the Morris water maze (Roberson et al., 2007) (Figure 3E).
Figure 3. Critical role for tau in Aβ toxicity.
That tau is critical for Aβ-mediated toxicity, has been shown in primary neuronal cultures: wild-type (wt) neurons (A) degenerated when incubated with Aβ42, as did tau-transfected neurons (C). Primary neurons derived from tau knockout (KO) mice, however, were resistant to the toxic effects of Aβ (B). This has been translated in vivo. Most, if not all, APP mutant strains with a robust Aβ plaque pathology are characterized by premature death of unknown cause. This includes APP mutant J20 mice (D). By crossing these on to a hetero- or homo-zygous tau knockout background many clinical features could be ameliorated (E).
Taken together, these studies suggest that Aβ and tau contribute synergistically to neurodegeneration in AD (Götz et al., 2007). The findings further indicate that a combinatorial therapy targeting both Aβ and tau may be useful (Golde et al., 2009).
ADVANCES IN ANIMAL IMAGING AND THERAPY
The preclinical diagnosis of AD depends on neuropsychological testing and, increasingly, the use of imaging techniques and biomarkers in CSF (cerebrospinal fluid) and blood. The major imaging techniques are PET (positron emission tomography), CT (computed tomography), MRI (magnetic resonance imaging) and multiphoton imaging. The field has been boosted significantly with the development of the PET tracer PIB (Pittsburgh Compound-B), a thioflavin T derivative. Using four-dimensional multiphoton imaging of transgenic mouse brain, PIB was shown to enter the brain extremely rapidly, Aβ was targeted within 1 min, and unbound florescence cleared within several minutes (Bacskai et al., 2003). This was soon followed by the first study using PIB in humans (Klunk et al., 2004). While PET has its undisputed beauty, there are, however, some major drawbacks, especially when PET is compared with MRI: it takes around 45 min for an entire scan (a situation with which healthy people are not comfortable let alone patients suffering from dementia); a cyclotron needs to be close to the imaging facility as the t1/2 (half-life) of carbon-20 is only 20 min, and finally, PET is approx. 50 times as expensive as MRI. Multiphoton imaging has also been used to determine that plaque formation in mice can be a surprisingly rapid process, with an Aβ plaque forming within 24 h (Meyer-Luehmann et al., 2008).
Imaging assists in diagnosis, but has its place also in monitoring the efficacy of therapies. The current treatment rests on the use of AChE (acetylcholine esterase) inhibitors and memantine, an NMDA (N-methyl-d-aspartate) receptor antagonist. The AD major advocacy forum, AlzForum, at the time of writing this article lists 39 ongoing clinical trials which include NGF (nerve growth factor), DHA (docosahexanoic acid), vitamin A, anti-Aβ antibodies, γ-secretase inhibitors, α-secretase activators, oestrogen, natural IgG, melatonin, a calcium channel agonist, anti-inflammatory substances [NSAIDs (non-steroidal anti-inflammatory drugs)], a nicotinic α7 agonist, statins and the chelator PBT2 (http://www.alzforum.org).
Many of these therapies have their foundation in animal work. This includes the vaccination approach, which has been pioneered by researchers of the biotech company Elan. Using both an active and passive immunization approach, existing plaques/Aβ pathology could be removed, the formation of plaques/Aβ pathology prevented, and age-dependent learning deficits reduced or prevented (Schenk et al., 1999; Bard et al., 2000). In follow-up work in 3xtg-AD mice, which present with a combined Aβ plaque and NFT pathology, these improvements were correlated with reductions in soluble Aβ and tau (Oddo et al., 2006). The last years have seen many modifications of the initial approach that has been tested in mice, using different routes of administration, changes to the peptide, alterations to the adjuvans, a DNA vaccination strategy, or as has been done recently, by coupling of Aβ to retro-particles (Bach et al., 2009). As the brain is generally conceived to be immune-privileged, the efficacy of the Aβ-targeted immunization approach came as a surprise, most likely also for those who had initiated the first vaccinations. In the case of tau, one would think that efficacy would be even more difficult to achieve as, different from Aβ, tau is intracellular and hence, at least at first sight, not seen by the immune system. However, it seems as if tau is presented as an antigen or released into the extracellular space, because immunization of tau transgenic mice with the PHF1 phospho-tau-peptide (which contains the phospho-sites Ser396 and Ser404) causes a reduction of aggregated tau levels, a shift from the insoluble to the soluble pool of tau and a slowing of the progression of an NFT-related motor phenotype (Asuni et al., 2007).
The fact that our way of life affects our health in old age comes as no surprise. Several recent studies in mice address stress, diet, exercise and environmental enrichment. One of the earliest studies addressing the impact of an enriched environment used the APPsw/PS1ΔE9 mice with a plaque pathology. Housing of the mice was in large cages and they were given running wheels, coloured tunnels, toys and chewable material to play with. This caused reductions in Aβ levels and plaque load; furthermore, levels of the Aβ-degrading enzyme neprilysin and of learning-related genes were up-regulated (Lazarov et al., 2005). When plaque-forming Tg2576 mice were fed with the green tea active compound EGCG (epigallocatechin gallate), this had similar effects: again, Aβ levels and plaque load were reduced, and this was dependent on the up-regulation of the α-secretase ADAM (a disintegrin and metalloproteinase) 10 (Rezai-Zadeh et al., 2005; Obregon et al., 2006).
The deacetylase Sir2 is a critical regulator of the ageing process. SIR2 is a longevity gene and SIRT1 is its human homologue. Resveratrol, a well-known active compound in red wine, activates SIRT1. When p25 transgenic mice [p25 is an activator of the kinase cdk5 (cyclin-dependent kinase 5)] were intra-hippocampally injected with SIRT1 lentiviruses, this protected the p25 mice from neurodegeneration (Kim et al., 2007). Finally, the typical Western diet is not well balanced with a ratio of unsaturated fatty acids of n−3 (omega-3)/n−6 (omega-6) = 1:24, whereas the ideal diet would be 1:5. When Tg2576 mice were fed with n−3, this reduced Aβ levels by 70% and the plaque burden by 40% (Lim et al., 2005). Taken together this indicates a role for environmental factors in the progression of AD.
Both tau and APP are phospho-proteins, and phosphorylation is regulated by a balanced interplay of kinases and phosphatases; hence, numerous molecules which interfere with their function have been tested in vitro and in animals (Pei et al., 2008). Very recently, a zebrafish model has been established which reproduced tau hyperphosphorylation, NFT formation, and neuronal and behavioural disturbances, as well as cell death. Of the many inhibitors of the kinase GSK3β (glycogen synthase kinase 3β) tested in the fish model, AR-534 turned out to reduce tau hyperphosphorylation in vivo (Paquet et al., 2009).
Another strategy is the targeting of phosphatases as has been suggested recently (Iqbal and Grundke-Iqbal, 2008). Here, PP2A (protein phosphatase 2A) is particularly attractive, as instead of modulating general catalytic activity it may be possible to activate specifically the one of its many regulatory subunits that confers specificity for tau as a substrate (Kins et al., 2001; Schild et al., 2006). Together this presents a strategy that modulates either kinases or phosphatases as an attractive therapeutic approach (Iqbal and Grundke-Iqbal, 2008).
WHAT HAVE WE ACHIEVED SO FAR AND WHAT DOES THE FUTURE HOLD?
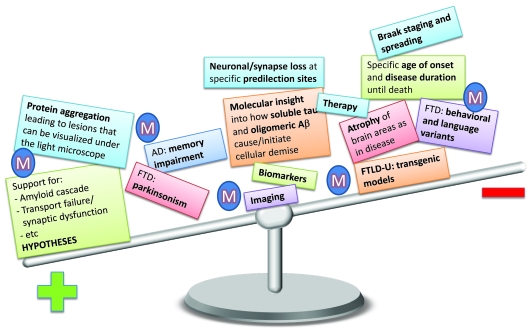
What has been achieved so far? Depending on how much emphasis is placed on the different aspects of AD modelling in animals (symbolized by the ‘M’ in Figure 4), the balance of ‘achievement’ will vary. The difference in emphasis is not meant to be taken at face value, but should rather provide an idea for further opportunities of advancement.
Figure 4. Modelling in animals and what has been achieved so far.
Depending on how much weight we put on the different aspects of AD modelling (symbolized by the ‘M’) in animals the balance of ‘achievement’ will vary. The distribution is not meant to be taken at face value, but thought to provide an idea of which aspects of the human disease need further development. Protein aggregation has been very faithfully modelled in animals, as have aspects of behavioural impairment. Support has been provided for all hypotheses proposed for AD and FTD. Some insight has been achieved into the molecular mechanisms of how soluble tau and the different assembly states of Aβ cause and initiate cellular demise, but a real understanding is still lacking. The more we move to the right, the less has been authentically modelled. There are indeed aspects which, for obvious reasons, cannot be modelled in animals at all, such as the language variants of FTD.
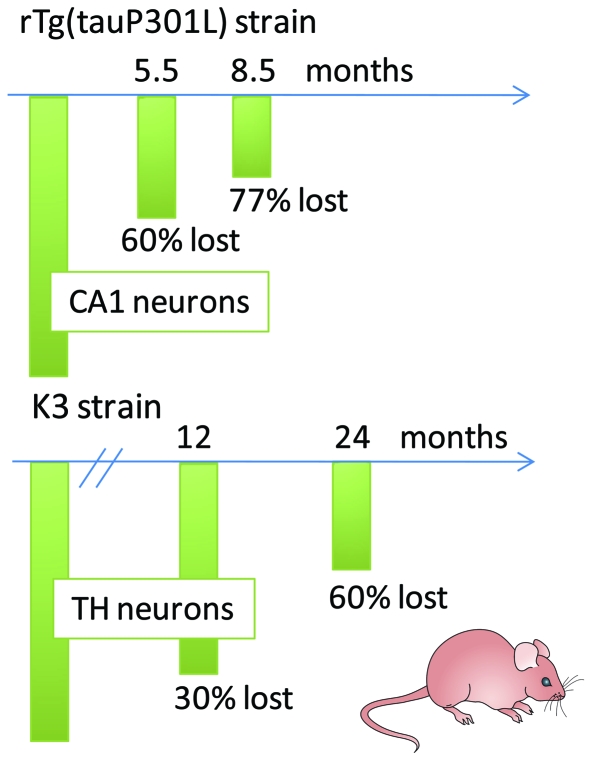
In discussing what the future holds, we would like to highlight two selected aspects, selective vulnerability and the functional domains of APP. Selective vulnerability characterizes AD and FTD but the cellular and molecular basis of it is not at all understood (Götz et al., 2009). In AD, both the Braak staging outlined above (Braak and Braak, 1991) and the distribution of NFTs reflect selective vulnerability. Interestingly, in the AD brain some areas are spared from degeneration until very late in disease. Also, in the basal forebrain all neurons that die appear to contain NFTs (Cullen and Halliday, 1998), whereas in the CA1 region, up to 20% of the neuronal loss cannot be explained by NFT formation, suggesting that modes of death differ (Gomez-Isla et al., 1997; Kril et al., 2002; Giannakopoulos et al., 2003). How can transgenic mouse models be exploited to dissect selective vulnerability and identify genes and proteins that either protect from degeneration or confer an increased risk? This is exemplified for two selected mouse models, one characterized by memory impairment and the other in addition by parkinsonism (Figure 5): rTg(tauP301L)4510 mice with an inducible and hence very strong P301L mutant tau expression are characterized by massive brain weight loss and gross atrophy of the forebrain (Santacruz et al., 2005). By 5.5 months, 60% of CA1 hippocampal pyramidal neurons are lost, and by 8.5 months only 23% remain (Santacruz et al., 2005). K3 mice are characterized by loss of TH-positive, K369I tau-expressing neurons in the substantia nigra, long after the first clinical symptoms become apparent (Ittner et al., 2008, 2009). Interestingly, this loss is only partial, with 60% of the TH neurons lost by 24 months of age. What protects a subset of morphologically indistinguishable neurons within this brain area from cell death while others degenerate?
Figure 5. Selective vulnerability examined in two mouse models with neuronal loss.
Selective vulnerability characterizes two selected mouse strains, one characterized by memory impairment [rTg(tauP301L)4510 line] and the other, in addition, by parkinsonism (K369I tau mutant K3 strain). The rTg(tauP301L)4510 mice lose 60% of CA1 hippocampal pyramidal neurons by 5.5 months, and only 23% remain by 8.5 months. In the K3 mice, loss of TH neurons is also only partial, with 60% lost by 24 months of age. What protects a subset of morphologically indistinguishable neurons within the respective brain area from cell death while others degenerate?
We previously used the tools of functional genomics to dissect pathogenic mechanisms, including mitochondrial dysfunction and AGE formation that not only operate in the transgenic mouse but also in human diseased brain (Chen et al., 2004b; Hoerndli et al., 2004; David et al., 2005a, 2005b; Hoerndli et al., 2005; David et al., 2006; Hoerndli et al., 2007). Functional genomics not only assists in dissecting disease mechanisms, but also emerges as a powerful tool in identifying cell-type-specific gene expression, which ultimately may be useful to understand selective vulnerability. In an impressive study, Sugino and colleagues used four GFP (green fluorescent protein)-expressing transgenic mouse lines to obtained eleven fluorescently labelled neuronal populations from different brain areas (Sugino et al., 2006). They triturated the neurons and by an elaborate panning process, manually isolated between 30 and 120 neurons from each brain area that they had characterized previously using current-clamp recordings. The subsequent analysis of the transcriptomic profile allowed them to construct a taxonomic tree that showed clear distinctions between neuronal cell types such as cortical interneurons and projection neurons (Sugino et al., 2006). We believe that crossing GFP-marked mice with AD or FTD mouse models such as those described above should allow identification of genes that confer susceptibility to or protection from neuronal degeneration. Ultimately, these genes can be reintroduced into the germline of mice for a functional validation (Figure 5).
Another aspect which is likely to receive more attention in the future is the realization that in pathogenesis functional domains of APP, other than Aβ, may have a role. APP overexpression causes an axonal transport defect (Stokin et al., 2005, 2008). However, by fusing the Aβ domain with the BRI protein, it could be shown that the APP-induced axonal defects are not caused by Aβ as one might have expected (Stokin et al., 2008). Similarly, in more recent work, APP was found to be a ligand of the DR (death receptor). Binding of an N-terminal APP fragment was further shown to trigger neuronal degeneration, leading to the notion that an extracellular APP fragment, in addition to Aβ, contributes to AD (Nikolaev et al., 2009).
Therefore it is obvious that more transgenic models are needed to dissect the role of the different functional domains of APP. Certainly, for any gene implicated in AD and FTD, more sophisticated cell-type-specific and/or inducible transgenic systems need to be developed. Animals need to be humanized by using BAC-transgenic approaches as has been done previously (Andorfer et al., 2003). More tools need to be developed, such as additional conformation- and epitope-specific antibodies, to better characterize and isolate toxic species (Habicht et al., 2007; Glabe, 2008). Finally, insight gained from the various animal species needs to be fully integrated, taking into consideration the species-inherent limitations, but also the complementary potential. In conclusion, animal models will continue to be an important tool in AD and FTD research. While the mouse is likely to remain the major species, a larger contribution of invertebrate models can be foreseen.
ACKNOWLEDGEMENTS
J.G. is a Medical Foundation Fellow.
Footnotes
The work in the authors' laboratory is supported by the University of Sydney; the National Health and Medical Research Council (NHMRC); the Australian Research Council (ARC); the New South Wales Government through the Ministry for Science and Medical Research (BioFirst Program); the Medical Foundation (University of Sydney); and the Judith Jane Mason and Harold Stannett Williams Memorial Foundation.
REFERENCES
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Tschape JA, Kopietz F, Braun G, Baade JK, Wiederhold KH, Staufenbiel M, Prinz M, Deller T, Kalinke U, Buchholz CJ, Muller UC. Vaccination with Aβ-displaying virus-like particles reduces soluble and insoluble cerebral Aβ and lowers plaque burden in APP transgenic mice. J Immunol. 2009;182:7613–7624. doi: 10.4049/jimmunol.0803366. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Hickey GA, Skoch J, Kajdasz ST, Wang Y, Huang GF, Mathis CA, Klunk WE, Hyman BT. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-β ligand in transgenic mice. Proc Natl Acad Sci USA. 2003;100:12462–12467. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP×Tau transgenic mice. Am J Pathol. 2007;171:2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brandt R, Gergou A, Wacker I, Fath T, Hutter H. A Caenorhabditis elegans model of tau hyperphosphorylation: induction of developmental defects by transgenic overexpression of Alzheimer's disease-like modified tau. Neurobiol Aging. 2009;30:22–33. doi: 10.1016/j.neurobiolaging.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Chen F, David D, Ferrari A, Götz J. Posttranslational modifications of tau: role in human tauopathies and modeling in transgenic animals. Curr Drug Targets. 2004a;5:503–515. doi: 10.2174/1389450043345236. [DOI] [PubMed] [Google Scholar]
- Chen F, Wollmer MA, Hoerndli F, Münch G, Kuhla B, Rogaev EI, Tsolaki M, Papassotiropoulos A, Götz J. Role for glyoxalase I in Alzheimer's disease. Proc Natl Acad Sci USA. 2004b;101:7687–7692. doi: 10.1073/pnas.0402338101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet. 2008;24:186–194. doi: 10.1016/j.tig.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM. Neurofibrillary degeneration and cell loss in the nucleus basalis in comparison to cortical Alzheimer pathology. Neurobiol Aging. 1998;19:297–306. doi: 10.1016/s0197-4580(98)00066-9. [DOI] [PubMed] [Google Scholar]
- David D, Hoerndli F, Götz J. Functional genomics meets neurodegenerative disorders Part I: transcriptomic and proteomic technology. Prog Neurobiol. 2005a;76:153–168. doi: 10.1016/j.pneurobio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Dröse S, Brandt U, Müller WE, Eckert E, Götz J. Proteomic and functional analysis reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem. 2005b;280:23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- David DC, Ittner LM, Gehrig P, Nergenau D, Shepherd C, Halliday G, Götz J. β-Amyloid treatment of two complementary P301L tau-expressing Alzheimer's disease models reveals similar deregulated cellular processes. Proteomics. 2006;6:6566–6577. doi: 10.1002/pmic.200600634. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Chen L, Vitek MP. The tau N279K exon 10 splicing mutation recapitulates frontotemporal dementia and parkinsonism linked to chromosome 17 tauopathy in a mouse model. J Neurosci. 2007;27:9155–9168. doi: 10.1523/JNEUROSCI.5492-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deters N, Ittner LM, Götz J. Divergent phosphorylation pattern of tau in P301L tau transgenic mice. Eur J Neurosci. 2008;28:137–147. doi: 10.1111/j.1460-9568.2008.06318.x. [DOI] [PubMed] [Google Scholar]
- Deters N, Ittner LM, Götz J. Substrate-specific reduction of PP2A activity exaggerates tau pathology. Biochem Biophys Res Commun. 2009;379:400–405. doi: 10.1016/j.bbrc.2008.12.140. [DOI] [PubMed] [Google Scholar]
- Dickey C, Kraft C, Jinwal U, Koren J, Johnson A, Anderson L, Lebson L, Lee D, Dickson D, de Silva R, Binder LI, Morgan D, Lewis J. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. Am J Pathol. 2009;174:228–238. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Eckert A, Hauptmann S, Scherping I, Meinhardt J, Rhein V, Drose S, Brandt U, Fandrich M, Muller WE, Götz J. Oligomeric and fibrillar species of β-amyloid (Aβ42) both impair mitochondrial function in P301L tau transgenic mice. J Mol Med. 2008;86:1255–1267. doi: 10.1007/s00109-008-0391-6. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Götz J. β-Amyloid induces PHF-like tau filaments in tissue culture. J Biol Chem. 2003;278:40162–40168. doi: 10.1074/jbc.M308243200. [DOI] [PubMed] [Google Scholar]
- Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci. 2005;25:3539–3550. doi: 10.1523/JNEUROSCI.0081-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Baur CP, Ahringer J, Jakes R, Hasegawa M, Spillantini MG, Smith MJ, Hill F. PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. J Cell Sci. 1996;109:2661–2672. doi: 10.1242/jcs.109.11.2661. [DOI] [PubMed] [Google Scholar]
- Golde TE, Petrucelli L, Lewis J. Targeting Aβ and tau in Alzheimer's disease, an early interim report. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Götz J. Tau and transgenic animal models. Brain Res Brain Res Rev. 2001;35:266–286. doi: 10.1016/s0165-0173(01)00055-8. [DOI] [PubMed] [Google Scholar]
- Götz J, Nitsch RM. Compartmentalized tau hyperphosphorylation and increased levels of kinases in transgenic mice. Neuroreport. 2001;12:2007–2016. doi: 10.1097/00001756-200107030-00045. [DOI] [PubMed] [Google Scholar]
- Götz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L Tau. J Biol Chem. 2001a;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science. 2001b;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Götz J, Ittner LM, Kins S. Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer's disease? J Neurochem. 2006;98:993–1006. doi: 10.1111/j.1471-4159.2006.03955.x. [DOI] [PubMed] [Google Scholar]
- Götz J, Deters N, Doldissen A, Bokhari L, Ke Y, Wiesner A, Schonrock N, Ittner LM. A decade of tau transgenic animal models and beyond. Brain Pathol. 2007;17:91–103. doi: 10.1111/j.1750-3639.2007.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Götz J, Ittner LM, Fandrich M, Schonrock N. Is tau aggregation toxic or protective: a sensible question in the absence of sensitive methods? J Alzheimers Dis. 2008a;14:423–429. doi: 10.3233/jad-2008-14410. [DOI] [PubMed] [Google Scholar]
- Götz J, Ittner LM, Schonrock N, Cappai R. An update on the toxicity of Aβ in Alzheimer's disease. Neuropsychiatr Dis Treat. 2008b;4:1033–1042. doi: 10.2147/ndt.s3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Schonrock N, Vissel B, Ittner LM. Alzheimer's disease selective vulnerability and modelling in transgenic mice. J Alzheimers Dis. 2009;18:243–251. doi: 10.3233/JAD-2009-1143. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem. 2006;97:1005–1014. doi: 10.1111/j.1471-4159.2006.03784.x. [DOI] [PubMed] [Google Scholar]
- Habicht G, Haupt C, Friedrich RP, Hortschansky P, Sachse C, Meinhardt J, Wieligmann K, Gellermann GP, Brodhun M, Götz J, Halbhuber KJ, Rocken C, Horn U, Fandrich M. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Aβ protofibrils. Proc Natl Acad Sci USA. 2007;104:19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GF, Cohen MJ. Extensive dendritic sprouting induced by close axotomy of central neurons in the lamprey. Science. 1983;222:518–521. doi: 10.1126/science.6623092. [DOI] [PubMed] [Google Scholar]
- Hall GF, Lee VM, Lee G, Yao J. Staging of neurofibrillary degeneration caused by human tau overexpression in a unique cellular model of human tauopathy. Am J Pathol. 2001;158:235–246. doi: 10.1016/S0002-9440(10)63962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9:151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Zhang B, Forman MS, Yoshiyama Y, Trojanowski JQ, Lee VM. Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J Neurosci. 2005;25:9434–9443. doi: 10.1523/JNEUROSCI.2691-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerndli FJ, Toigo M, Schild A, Götz J, Day PJ. Reference genes identified in SH-SY5Y cells using custom-made gene arrays with validation by quantitative polymerase chain reaction. Anal Biochem. 2004;335:30–41. doi: 10.1016/j.ab.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Hoerndli F, David D, Götz J. Functional genomics meets neurodegenerative disorders. Part II: application and data integration. Prog Neurobiol. 2005;76:169–188. doi: 10.1016/j.pneurobio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hoerndli FJ, Pelech S, Papassotiropoulos A, Götz J. Aβ treatment and P301L tau expression in an Alzheimer's disease tissue culture model act synergistically to promote aberrant cell cycle re-entry. Eur J Neurosci. 2007;26:60–72. doi: 10.1111/j.1460-9568.2007.05618.x. [DOI] [PubMed] [Google Scholar]
- Hornsten A, Lieberthal J, Fadia S, Malins R, Ha L, Xu X, Daigle I, Markowitz M, O'Connor G, Plasterk R, Li C. APL-1, a Caenorhabditis elegans protein related to the human β-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci USA. 2007;104:1971–1976. doi: 10.1073/pnas.0603997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer's disease. J Neurosci. 2004;24:7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12:38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Götz J. Pronuclear injection for the generation of transgenic mice. Nat Protoc. 2007;2:1206–1215. doi: 10.1038/nprot.2007.145. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Fath T, Ke YD, Bi M, van Eersel J, Li KM, Gunning P, Götz J. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc Natl Acad Sci USA. 2008;105:15997–16002. doi: 10.1073/pnas.0808084105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Götz J. Phosphorylated tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer's disease. J Biol Chem. 2009;284:20909–20916. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WH, Lee S, Jung C, Ahmed A, Lee G, Hall GF. Interneuronal transfer of human tau between lamprey central neurons in situ. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1273. [DOI] [PubMed] [Google Scholar]
- Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Götz J. Reduced PP2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Koson P, Zilka N, Kovac A, Kovacech B, Korenova M, Filipcik P, Novak M. Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. Eur J Neurosci. 2008;28:239–246. doi: 10.1111/j.1460-9568.2008.06329.x. [DOI] [PubMed] [Google Scholar]
- Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD. From the Cover: neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci USA. 2003;100:9980–9985. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bogh IB, Holm IE, Jakobsen JE, Johansen MG, Purup S, Bolund L, Vajta G, Jorgensen AL. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer's disease-causing dominant mutation APPsw. Transgenic Res. 2009;18:545–558. doi: 10.1007/s11248-009-9245-4. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Patel S, Harding AJ, Halliday GM. Neuron loss from the hippocampus of Alzheimer's disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol (Berl) 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- Kurosinski P, Götz J. Glial cells under physiologic and pathological conditions. Arch Neurol. 2002;59:1524–1528. doi: 10.1001/archneur.59.10.1524. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci USA. 2006;103:9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Murphy PM, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin W-L, Chisholm L, Corral A, Jones G, Yen S-H, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant Tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Li X, Greenwald I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc Natl Acad Sci USA. 1997;94:12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani E, Fan J, Gasparini L, Golding M, Williams M, Schiavo G, Goedert M, Amos LA, Spillantini MG. Interaction of tau protein with the dynactin complex. EMBO J. 2007;26:4546–4554. doi: 10.1038/sj.emboj.7601878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JB, Aamodt S, Aamodt E. ptl-1, a Caenorhabditis elegans gene whose products are homologous to the tau microtubule-associated proteins. Biochemistry. 1996;35:9415–9423. doi: 10.1021/bi952646n. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka T, Ding Z, Gengyo-Ando K, Oue M, Yamaguchi H, Mitani S, Ihara Y. Progressive neurodegeneration in C. elegans model of tauopathy. Neurobiol Dis. 2005;20:372–383. doi: 10.1016/j.nbd.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Mocanu MM, Nissen A, Eckermann K, Khlistunova I, Biernat J, Drexler D, Petrova O, Schonig K, Bujard H, Mandelkow E, Zhou L, Rune G, Mandelkow EM. The potential for β-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J Neurosci. 2008;28:737–748. doi: 10.1523/JNEUROSCI.2824-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos M, Hutter H. The model Caenorhabditis elegans in diabetes mellitus and Alzheimer's disease. J Alzheimers Dis. 2009;16:897–908. doi: 10.3233/JAD-2009-0977. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Schulz-Schaeffer W, Crowther RA, Smith MJ, Spillantini MG, Goedert M, Kretzschmar HA. Pick's disease associated with the novel Tau gene mutation K369I. Ann Neurol. 2001;50:503–513. doi: 10.1002/ana.1223. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Noorbakhsh F, Overall CM, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. doi: 10.1016/j.tins.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Obregon DF, Rezai-Zadeh K, Bai Y, Sun N, Hou H, Ehrhart J, Zeng J, Mori T, Arendash GW, Shytle D, Town T, Tan J. ADAM10 activation is required for green tea (−)-epigallocatechin-3-gallate-induced α-secretase cleavage of amyloid precursor protein. J Biol Chem. 2006;281:16419–16427. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles. Intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Aβ and tau, but not soluble Aβ alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, LaFerla FM. Blocking Aβ42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein 70-interacting protein: a mechanistic link between Aβ and tau pathology. J Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet D, Bhat R, Sydow A, Mandelkow EM, Berg S, Hellberg S, Falting J, Distel M, Koster RW, Schmid B, Haass C. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest. 2009;119:1382–1395. doi: 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Sjogren M, Winblad B. Neurofibrillary degeneration in Alzheimer's disease: from molecular mechanisms to identification of drug targets. Curr Opin Psychiatry. 2008;21:555–561. doi: 10.1097/YCO.0b013e328314b78b. [DOI] [PubMed] [Google Scholar]
- Pennanen L, Welzl H, D'Adamo P, Nitsch RM, Götz J. Accelerated extinction of conditioned taste aversion in P301L tau transgenic mice. Neurobiol Dis. 2004;15:500–509. doi: 10.1016/j.nbd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Pennanen L, Wolfer DP, Nitsch RM, Götz J. Impaired spatial reference memory and increased exploratory behavior in P301L tau transgenic mice. Genes Brain Behav. 2006;5:369–379. doi: 10.1111/j.1601-183X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid β. Proc Natl Acad Sci USA. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A, Götz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol (Berl) 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schild A, Ittner LM, Götz J. Altered phosphorylation of cytoskeletal proteins in mutant protein phosphatase 2A transgenic mice. Biochem Biophys Res Commun. 2006;343:1171–1178. doi: 10.1016/j.bbrc.2006.03.066. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shulman JM, Feany MB. Genetic modifiers of tauopathy in Drosophila. Genetics. 2003;165:1233–1242. doi: 10.1093/genetics/165.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL, Feany MB. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol Biol Cell. 2007;18:5060–5068. doi: 10.1091/mbc.E07-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Stokin GB, Almenar-Queralt A, Gunawardena S, Rodrigues EM, Falzone T, Kim J, Lillo C, Mount SL, Roberts EA, McGowan E, Williams DS, Goldstein LS. Amyloid precursor protein-induced axonopathies are independent of amyloid-β peptides. Hum Mol Genet. 2008;17:3474–3486. doi: 10.1093/hmg/ddn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5:525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 2007;27:2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam D, De Deyn PP. Drug discovery in dementia: the role of rodent models. Nat Rev Drug Discov. 2006;5:956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]
- van de Hoef DL, Hughes J, Livne-Bar I, Garza D, Konsolaki M, Boulianne GL. Identifying genes that interact with Drosophila presenilin and amyloid precursor protein. Genesis. 2009;47:246–260. doi: 10.1002/dvg.20485. [DOI] [PubMed] [Google Scholar]
- Wang J, Farr GW, Hall DH, Li F, Furtak K, Dreier L, Horwich AL. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000350. doi: 10.1371/journal.pgen.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T. Amyloid β-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:4755–4759. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilka N, Filipcik P, Koson P, Fialova L, Skrabana R, Zilkova M, Rolkova G, Kontsekova E, Novak M. Truncated tau from sporadic Alzheimer's disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006;580:3582–3588. doi: 10.1016/j.febslet.2006.05.029. [DOI] [PubMed] [Google Scholar]