Abstract
Resolution of inflammation has historically been viewed as a passive process, occurring as a result of the withdrawal of pro-inflammatory signals, including lipid mediators such as leukotrienes and prostaglandins. Thus, most anti-inflammatory drugs have traditionally targeted primarily mediator pathways that are engaged at the onset of inflammation. Only recently has it been established that inflammation resolution is an active process with a distinct set of chemical mediators. Several clinical and epidemiological studies have identified beneficial effects of polyunsaturated fatty acids (PUFAs) for a variety of inflammatory diseases, yet without mechanistic explanations for these beneficial effects. Resolvins and protectins are recently identified molecules that are generated from ω-3 PUFA precursors and can orchestrate the timely resolution of inflammation in model systems. Dysregulation of pro-resolving mediators is associated with diseases of prolonged inflammation, so designing pharmacological mimetics of naturally occurring pro-resolving mediators offers exciting new targets for drug design. This review describes the discovery and synthesis of these novel lipid mediators, their receptors and mechanisms of action, and summarizes the studies to date that have uncovered roles for resolvins and protectins in disease states.
This article is part of a themed issue on Mediators and Receptors in the Resolution of Inflammation. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Keywords: inflammation, resolvin, protectin, resolution, fish oils
Introduction
Inflammation is an essential biological process for maintenance of homeostasis and recovery from tissue injury or foreign pathogens. Prolonged inflammation, however, can be destructive and maladaptive, leading to disease and tissue destruction (Nathan, 2002). Despite many recent advances in the treatment of inflammatory disorders, mechanisms for the resolution of inflammation are still poorly understood and provide many new potential therapeutic targets in addressing diseases associated with unresolved inflammation (Gilroy et al., 2004; Serhan et al., 2008). Resolution has been well appreciated to be one of the four major outcomes for acute inflammation, along with progression to chronic inflammation, abscess development or scar formation (Cotran and Collins, 1999) (Figure 1). It was traditionally believed that resolution of inflammation was a passive process (Cotran and Collins, 1999), driven primarily by the declining levels of pro-inflammatory mediators over time and ‘fizzling out’ of the acute inflammatory response. Recent studies have demonstrated, however, that effective resolution of inflammation, including timely clearance of leukocytes and return of host stromal/parenchymal cells to a ‘non-inflammatory’ state, a process known as catabasis, is indeed an active process and is similar in complexity to the onset of inflammation (as reviewed in Serhan et al., 2004). Teleologically, the host can better control the onset and resolution of inflammatory responses by having both positive and negative regulatory inputs. It can then be reasoned that, in addition to excessive activation of pro-inflammatory cascades, perturbations of counter-regulatory circuits could also account for diseases characterized by runaway inflammation.
Figure 1.
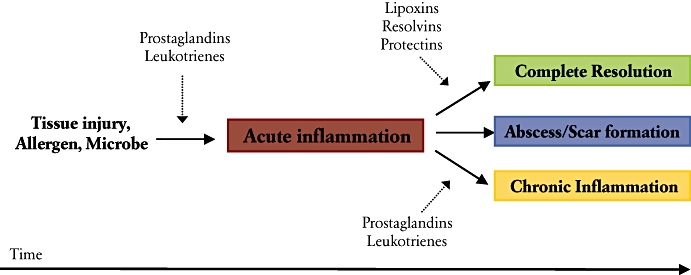
Illustration of the potential fates for acute inflammation. Tissue injury activates the release and formation of arachidonate-derived prostaglandins and leukotrienes, which regulate early events in the inflammatory response, such as changes in blood flow, oedema and leukocyte recruitment. Specialized counter-regulatory lipid mediators, such as lipoxins, resolvins and protectins, are generated at a later time and act in a tissue-specific manner to initiate the resolution of inflammation.
The resolution of acute inflammation is complex and involves several distinct cellular mechanisms. Cell clearance is critical to resolution and is driven both by apoptosis of leukocytes (Savill et al., 1989; Haslett, 1992; Rossi et al., 2006) and egress from tissues (Uller et al., 2006). Clearance of the inflammatory site is mediated in part via the non-phlogistic recruitment of monocytes that, as macrophages, participate in the phagocytosis of apoptotic cells and microbes (Godson et al., 2000; Schwab et al., 2007). In addition, mechanisms unique to mucosal surfaces exist to release neutrophils [polymorphonuclear leukocytes (PMNs)] from the apical surfaces of epithelial cells into the lumen for tissue clearance (Campbell et al., 2007).
A number of different indices have been developed to define and monitor resolution of acute inflammation, including the maximal number of PMNs, the time at which neutrophilic inflammation peaks, and the resolution interval (Ri) or time needed to decrease maximal PMN numbers by 50% (Bannenberg et al., 2005). As cell numbers decline, levels of pro-inflammatory cytokines decrease and eicosanoid class switching changes from generating pro-inflammatory lipid mediators [e.g., leukotrienes (LTs) and prostaglandins (PGs)] to anti-inflammatory mediators [e.g., lipoxins (LXs), resolvins (Rvs) and protectins (PDs)] (Serhan et al., 2000; 2002; Levy et al., 2001). In addition to anti-inflammatory actions on PMNs, such as inhibition of superoxide anion generation and endothelial transmigration, LXs, Rvs and PDs promote resolution by enhancing macrophage-mediated clearance of apoptotic PMNs (as reviewed in Serhan et al., 2008). In addition, apoptotic PMNs and T cells can sequester pro-inflammatory peptide mediators by CC chemokine receptor 5 (CCR5) expression (Ariel et al., 2006). Other pro-resolving cellular mechanisms include regulation of pro-inflammatory vascular adhesion molecule expression (Filep et al., 1999), angiogenesis (Cezar-de-Mello et al., 2008), fibrosis (Sodin-Semrl et al., 2000) and modulation of endogenous signal transduction pathways to terminate inflammatory responses (Levy et al., 1999; Lawrence et al., 2005). This range of actions can lead to potent regulation of Ri and other resolution indices in experimental models of acute inflammation (Bannenberg et al., 2005). In this review, we provide an update on recent progress in Rv and PD biosynthesis, sites of action and their anti-inflammatory and pro-resolving properties.
Rv and PD biosynthesis – lessons learned from the lipoxins and their 15-epimers
Distinct series of pro-resolving lipid mediators are generated, depending on the parent substrate and the presence or absence of aspirin. LXs and aspirin-triggered lipoxins (ATL), for example, are generated in a transcellular fashion from arachidonic acid (Fiore and Serhan, 1990; Claria and Serhan, 1995). While LX biosynthesis occurs via interactions between 5-lipoxygenase (5-LOX) and either 12-LOX or 15-LOX, the generation of closely related 15-epimer-LXs occurs via interactions between 5-LOX and aspirin-modified cyclooxygenase-2 (COX-2) (Claria and Serhan, 1995). Expression of COX-2 is increased during acute inflammation (Fukunaga et al., 2005) and the ingestion of aspirin leads to acetylation of COX-2, which blocks PG formation (Samuelsson, 1982). Acetylated COX-2 is not catalytically inactive, converting arachidonic acid to 15(R)-HETE rather than PGs (Claria and Serhan, 1995). 15(R)-HETE can serve as a substrate for 5-LOX for transformation to 15(R)-LXA4. Aspirin-triggered 15-epi-LXs are ∼twofold more potent than 15(S)-LXs (Takano et al., 1998; Serhan and Chiang, 2008). Of interest, 15-epi-LXs can also be generated in the absence of aspirin by cytochrome p450-mediated generation of 15(R)-HETE (Claria et al., 1996).
Similar to arachidonic acid-derived LXs and 15-epi-LXs, a series of novel compounds derived from 5Z,8Z,11Z,14Z,17Z- eicosapentaenoic acid (EPA) and 4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic acid (DHA), the most abundant omega-3 polyunsaturated fatty acids (PUFAs) in cold water marine fish oils, have recently been discovered in resolving murine inflammatory exudates (Serhan et al., 2000; 2002; and as reviewed in Serhan et al., 2008). These naturally occurring bioactive lipid mediators are termed Rvs (derived from ‘resolution phase interaction products’) (Figures 2 and 3) and PDs (Figure 4). Rvs are categorized as either E-series (from EPA) or D-series (from DHA) and aspirin-triggered epimers have been identified for each family (as reviewed in Serhan and Chiang, 2008). Similar to LXs, protectin D1 (PD1) is a product of 15-LOX-mediated conversion of a PUFA substrate, in this case DHA (Hong et al., 2003; Serhan et al., 2006). Rvs and PDs possess stereospecific and potent immunoregulatory actions that are protective in vitro and in vivo (Serhan et al., 2008). Further evidence has demonstrated roles for these compounds, in particular PD1, in protection of retinal epithelial cells (Mukherjee et al., 2004; Mukherjee et al., 2007b), experimental stroke-related ischaemia-reperfusion injury (Marcheselli et al., 2003) and animal models of Alzheimer's disease (Lukiw et al., 2005). Careful structure-function analyses for each Rv and PD1 have been determined using a murine experimental model of peritonitis (reviewed in Serhan and Chiang, 2008). Cellular and molecular actions for Rvs and PD1 are reviewed in Table 1.
Figure 2.
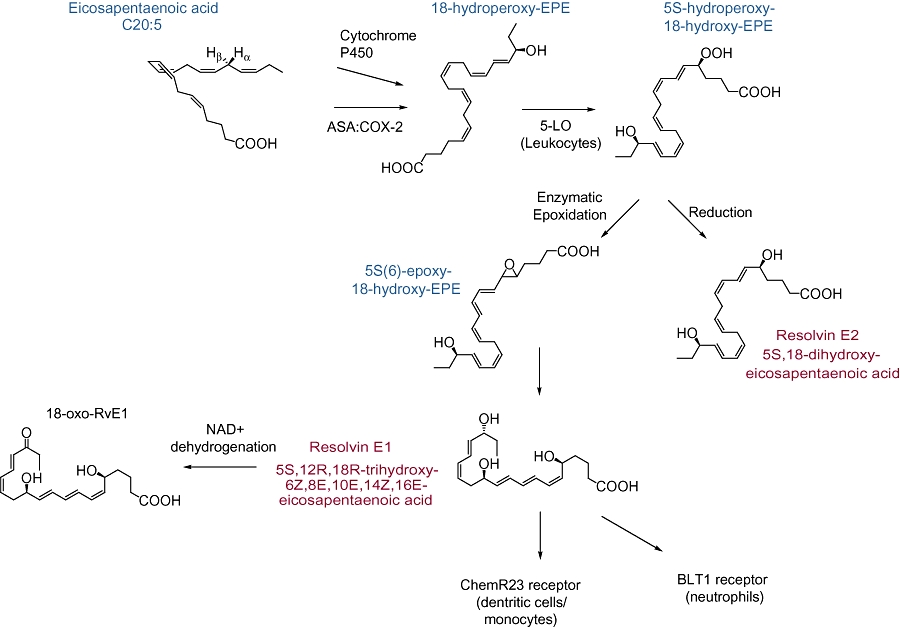
Biosynthesis of E-series resolvins (Rvs). Eicosapentaenoic acid is converted to 18R-hydroperoxy-EPE stereoselectively by cytochrome p450 in microbes or aspirin(ASA)-acetylated cyclooxygenase-2. This intermediate can then be further transformed by leukocyte 5-lipoxygenase to 5S-hydroperoxy-18-hydroxy-EPE for subsequent enzymatic epoxidation to RvE1 or reduction to RvE2. RvE1 can interact with either ChemR23 or BLT1 to mediate cell-type specific effects. Metabolism of RvE1 is initiated by conversion to the biologically inactive 18-oxo-RvE1. The receptor and inactivation of RvE2 are yet to be molecularly characterized.
Figure 3.
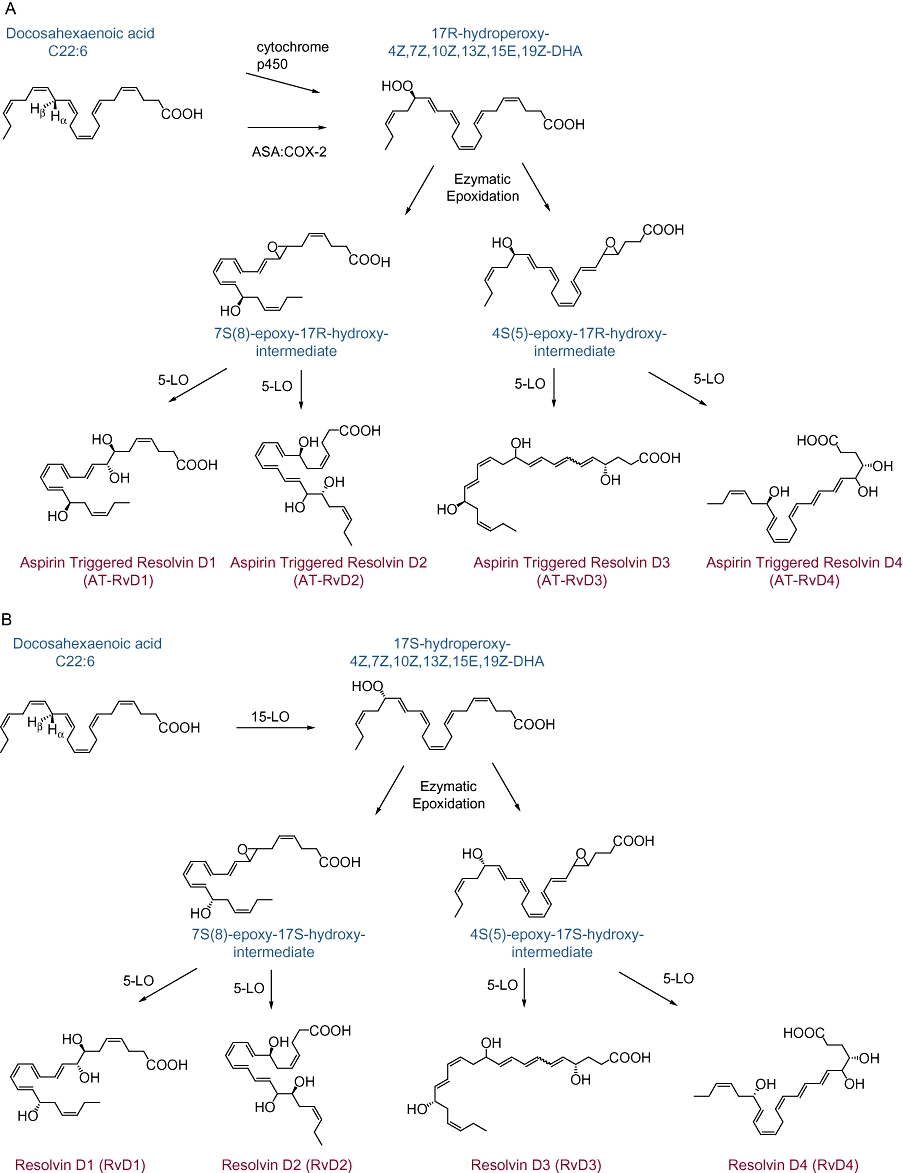
Biosynthesis of D-series resolvins (Rvs). DHA can be enzymatically converted to several distinct Rvs. (A) Similar to EPA, aspirin(ASA)-acetylated cyclooxygenase-2 or cytochrome p450 can convert docosahexaenoic acid (DHA) to a stereoselective intermediate, 17R-hydroperoxy-DHA, for subsequent enzymatic conversion to the 17R-RV D series (AT-RvD1-4). (B) Generation of RvD1-4 from DHA proceeds via a 15-lipoxygenase-mediated conversion to 17S-H(p)-DHA.
Figure 4.
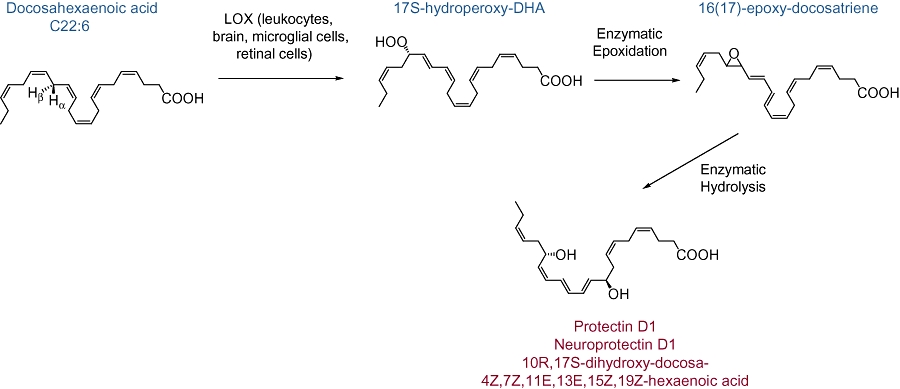
PD1/NPD1 biosynthesis. DHA conversion by 15-lipoxygenase (LOX) to 17S-H(p)-docosahexaenoic acid (DHA) can occur in several cell types and be further increased by cytokine induction of 15-LOX (e.g., IL-4). 17S-H(p)DHA can be further transformed to PD1/NPD1 via an epoxide intermediate.
Table 1.
Molecular and cellular effects of resolvins and protectins
| Parent PUFA | Mediator | Cell target | Action | Receptor | Reference |
|---|---|---|---|---|---|
| EPA | 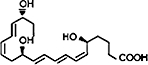 |
PMNs | Decreases transendothelial and transepithelial migration; attenuates BLT1 dependent pro- inflammatory signalling (NF-κB activation); reduces infiltration; protects from gastrointestinal inflammation in colitis | BLT1 | (Serhan et al., 2000; Arita et al., 2007; Campbell et al., 2007) (Bannenberg et al., 2005) (Arita et al., 2005b) |
| Platelets | Disrupts thromboxane-mediated platelet aggregation | (Dona et al., 2008) | |||
| T cells | Upregulates CCR5 expression | (Ariel et al., 2006) | |||
| Th17 cells | Decreases recruitment in response to allergen | (Haworth et al., 2008) | |||
| Dendritic cells | Inhibits migration and IL-12 production | ChemR23 | (Arita et al., 2005a) | ||
| Macrophages | Stimulates phagocytosis of apoptotic PMNs | ChemR23 | (Schwab et al., 2007) | ||
| Eosinophils | Decreases recruitment in response to allergen | (Aoki et al., 2008; Haworth et al., 2008) | |||
| EPA | 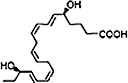 |
PMNs | Decreases infiltration | (Tjonahen et al., 2006) | |
| DHA | 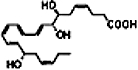 |
PMNs | Decreases infiltration in murine skin air pouch model; Limits infiltration in renal ischaemic injury | (Serhan et al., 2002; Duffield et al., 2006) | |
| Microglial cells | Inhibits IL-1β expression in vitro | (Serhan et al. (2002) | |||
| DHA | 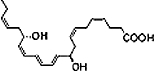 |
PMNs | Upregulates CCR5 expression; reduces tissue infiltration | (Marcheselli et al., 2003; Ariel et al., 2006) | |
| Macrophages | Stimulates phagocytosis of apoptotic PMNs | (Schwab et al., 2007) | |||
| T Cell | Promotes apoptosis in vitro | (Ariel et al., 2005) | |||
| Glial cells | Reduces cytokine production | (Hong et al., 2003) | |||
| Epithelial cells | Protects from oxidative-stress induced apoptosis (retinal pigment epithelium) | (Mukherjee et al., 2004) | |||
| Eosinophils | Decreases recruitment in response to allergen | (Levy et al., 2007a) | |||
| DHA + ASA | 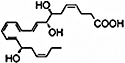 |
PMNs | Dose-dependent decrease in tissue infiltration | (Serhan et al., 2002; Sun et al., 2007) |
ASA, aspirin; CCR5, CC chemokine receptor 5; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PMNs, polymorphonuclear leukocytes; PUFA, polyunsaturated fatty acid.
Using the same COX and LOX enzymes operative during the initiation of acute inflammation in converting arachidonic acid (ω-6) to PGs and LTs, the host is able to shift the chemical mediator profile from pro-inflammatory to pro-resolving by modulating biosynthetic pathways to induce 15-LOX expression and activity to generate LXs, RVs and PD1. Disruption of this class switching process by COX-2 or LOX enzyme inhibitors or deficiency blocks the timely resolution of leukocyte clearance (Fukunaga et al., 2005; Schwab et al., 2007); demonstrating critical roles for pro-resolving COX-2 and LOX derived mediators in inflammation resolution. This experimentally derived resolution deficit can be rescued with exogenous administration of pro-resolving mediators at doses that are less than the inhibitor dose required to interrupt COX-2 and LOX enzymatic cascades (Schwab et al., 2007). In addition, animals can be protected from inflammatory diseases by overexpression of 15-LOX (Shen et al., 1996; Serhan et al., 2003) or expression of human anti-inflammatory receptors, such as the human lipoxin A4 receptor (ALX) (Levy et al., 2002; Devchand et al., 2003). These findings emphasize that the biochemical synthesis of these protective compounds is enzymatically mediated and leads to stereospecific molecules that interact with specific high affinity receptors. In this way, Rvs and PDs are distinct from auto-oxidation products of DHA and EPA, which are non-specific and can also be present during inflammation (Lee et al., 1984). The relationship between endogenous anti-inflammatory mediators and timely resolution is depicted in Figure 1.
Identification of novel compounds via lipidomic profiling
Rvs and PDs were initially identified by harvesting in vivo exudates during the resolution phase of acute inflammation, defined by the time period of rapidly declining PMN cell numbers (Cotran and Collins, 1999). The mouse dorsal air pouch model was selected for isolation of these mediators because it allows for the cellular and biochemical analysis of limited, self-resolving acute inflammatory responses, facilitating the isolation and discovery of molecules involved in this spontaneous resolution of inflammation (Winyard, 2003). Mice were injected with TNF-α and exudates were collected 4 h later, when PMN numbers were decreasing (Serhan et al., 2000; 2002;). These exudates were taken to liquid-chromatography-ultraviolet-mass spectrometry (LC-UV-MS-MS)-based lipidomic analyses (as reviewed in Serhan et al., 2007). In tandem, multiple lipid mediator libraries with physical properties, such as MS and MS/MS spectra, elution times and UV spectra were constructed to compare mediators isolated from exudates for matching purposes (Lu et al., 2005). As novel compounds were isolated, retrograde analysis was carried out using biogenic as well as total organic synthesis to identify structures and characterize stereochemistry (Serhan et al., 2000; 2002; 2006; Hong et al., 2003; Arita et al., 2005a; Sun et al., 2007). Using these methods, several classes of Rvs and PDs were identified.
EPA-derived E-series Rvs
Resolvin E1 (RvE1) and Resolvin E2 (RvE2), two major products in the family of EPA-derived resolvins, were originally isolated in vivo from murine dorsal air pouches treated with aspirin and EPA and were also generated in vitro from co-incubation of human endothelial cells with PMNs (Serhan et al., 2000). RvE1 is spontaneously produced in healthy subjects and levels are increased in individuals taking aspirin and/or EPA (Arita et al., 2005a). Transcellular formation of RvE1 can occur with the conversion of C20:5 to 18R-HEPE (18R-hydroxyeicosapentaenoic acid) by endothelial cells expressing COX-2 and treated with aspirin. Similar to 15(R)-HETE in 15-epi-LX formation, 18R-HEPE can be released from endothelial cells to neighboring leukocytes for subsequent conversion by 5-LOX to RvE1 via a 5(6) epoxide-containing intermediate (Serhan et al., 2000; Arita et al., 2005a) (Figure 2). This interaction is blocked by selective COX-2 inhibitors but not by indomethacin or acetaminophen (Serhan et al., 2000). Using gas chromatography-MS and liquid chromatography-tandem-MS-MS-based lipidomic analysis, the basic structure of this compound was elucidated as 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-EPA and complete stereochemistry was confirmed using total organic synthesis (Serhan et al., 2000; Arita et al., 2005a). This compound was found to be highly stereoselective both in vivo and in vitro. Synthesis of RvE1 is summarized in Figure 2.
RvE1 decreases PMN tissue accumulation by blocking human PMN transendothelial migration (Serhan et al., 2000) and facilitating apical PMN clearance from mucosal epithelial cells (Campbell et al., 2007). Other bioactions of RvE1 include attenuation of LTB4-BLT1 pro inflammatory signaling (NF-κB activation) (Arita et al., 2007), inhibition of PMN superoxide anion generation in response to TNFα or the bacterial surrogate peptide N-formyl-methionyl-leucyl-phenylalanine (Gronert et al., 2004), stimulation of macrophage phagocytosis of apoptotic PMNs (Schwab et al., 2007), inhibition of dendritic cell migration and cytokine release (Arita et al., 2005a; Haworth et al., 2008), and upregulation of CCR5 expression on leukocytes (Ariel et al., 2006). In addition to potent anti-inflammatory properties in acute inflammation, administration of RvE1 in a rabbit model of periodontitis also resulted in complete regeneration of damaged tissues, including bone, and normalization of systemic markers of inflammation, including C-reactive protein and IL-1β (Hasturk et al., 2007). Moreover, pre-treatment with RvE1, in contrast to molecules of the LX series, also conferred dramatic protection from inflammation-induced tissue and bone loss in periodontitis (Hasturk et al., 2006). In the murine dorsal air pouch model, RvE1 proved to be log-orders more potent than its dexamethasone or aspirin counterparts. Only nanogram amounts of RvE1 reduced leukocyte infiltration by 50–70% as compared with microgram amounts of dexamethasone or milligram amounts of aspirin (Serhan et al., 2000; Arita et al., 2005a). Recent studies have revealed that RvE1 is a potent modulator of pro-inflammatory leukocyte expression molecules, such as L-selectin, and selectively disrupts thromboxane-mediated platelet aggregation (Dona et al., 2008), adding additional mechanistic explanations to its anti-inflammatory and pro-resolving actions. LXA4 and RvE1 prevent pathological inflammation that can be detrimental, but also regulate physiological inflammation that is beneficial to the host, such as in wound healing. For example, when mouse cornea undergoes thermal injury, topically applied LXA4 or RvE1 increases the rate of re-epithelialization by 75% and decreases levels of the pro-inflammatory chemokine CXCL1 by 60% (Gronert et al., 2005). Overall, RvE1 initiates resolution of inflammation and causes decreased numbers of PMNs in exudates at earlier times than spontaneous resolution (as reviewed in Serhan et al., 2008).
Bioactive eicosanoids, such as PGs, LTs and LXs, transduce their signals via interactions with specific cognate receptors (Brink et al., 2003). Similarly, structure-activity assays suggested the presence of receptors for EPA-derived RvE1, and recently RvE1 cognate receptors were identified by screening G-protein-coupled receptors with assays of RvE1-mediated inhibition of TNF-α induced NF-κB activation as an endpoint (Arita et al., 2005a). [3H]-labeled RvE1 specifically binds to the receptor ChemR23, which shares a 36.4% homology of its amino acid sequence with ALX and is expressed on dendritic cells and monocytes (Arita et al., 2005a). In addition to blocking cytokine-induced NF-κB activation, RvE1 binding to leukocytes initiates specific arrays of intracellular signals, including activation of mitogen-activated protein (MAP) kinases (Arita et al., 2005a). ChemR23 was previously identified as a receptor for the peptide chemerin that also transduces anti-inflammatory signals (Cash et al., 2008). Thus, like ALX (Perretti et al., 2002), ChemR23 can interact with either lipid or peptide ligands. The specific binding of RvE1 to human PMN membrane occurs with high affinity with a Kd of 48.3 nM at 4oC. This [3H]-labeled RvE1 binding to PMN membranes can be displaced by unlabelled RvE1 (Ki = 34.3 nM), LTB4 (Ki = 0.08 nM), as well as LTB4 receptor 1 (BLT1) selective antagonist U-75302 (Ki = 1.5 nM). Interestingly, [3H]-RvE1 binding to human ChemR23 competed with homoligand RvE1 (Ki = 330 nM) or chemerin peptide (Ki = 429 nM), but not with LTB4. These findings suggest binding sites on human PMNs distinct from ChemR23 (Arita et al., 2007). RvE1 interactions at BLT1 lead to partial agonist/antagonist effects to locally dampen LTB4-BLT1 signals on PMNs (Arita et al., 2007). Therefore, it can be concluded that RvE1 is acting via at least two distinct receptors, exerting antagonist effects on LTB4-BLT1 signals on PMN activation and as a ChemR23 agonist to decrease cytokine production by dendritic cells and enhance clearance of apoptotic PMNs by macrophages.
Metabolic pathways for RvE1 inactivation have recently been uncovered and found to include enzymatic NAD+-dependent dehydrogenation that is regiospecific at carbon 18 to generate 18-oxo-RvE1 (Arita et al., 2006). These RvE1 inactivation pathways are species-, tissue- and cell-type specific with different metabolites generated in the lung compared with human PMNs in peripheral blood (Arita et al., 2006). Metabolomic profiles of RvE1 have identified additional products, including 10,11-dihydro-RvE1 and 20-carboxy-RvE1. Direct comparisons of 10,11-dihydro-RvE1, 18-oxo-RvE1 and 20-carboxy-RvE1 with RvE1 indicated decreased bioactivity in vivo for the metabolites relative to RvE1, which was active at concentrations as low as 1 nM, for increasing macrophage phagocytosis (Hong et al., 2008). Regulation of this pro-resolving activity by metabolic inactivation provides tissue and cell-localized control and mechanisms for restoration of homeostasis.
RvE2 is a second member of the EPA-derived family of E-series Rvs, and shares homology to RvE1 (Tjonahen et al., 2006). The full stereochemical structure for RvE2 is 5S,18(R/S)-dihydroxy-eicosapentaenoic acid (Figure 2). RvE2 is synthesized by human PMNs in amounts greater than RvE1, equipotent to RvE1 when given intravenously and additive to RvE1 at low doses when given intraperitoneally (Tjonahen et al., 2006). The RvE2 receptor is yet to be molecularly characterized. RvE2 stops zymosan-induced PMN infiltration and displays potent anti-inflammatory properties in murine peritonitis (Tjonahen et al., 2006).
D-series Rvs
D-series Rvs were also initially discovered in resolving exudates of mice given DHA plus aspirin. The aspirin-triggered Rv D1 (AT-RvD1) and RvD1 are derived from DHA, involving a pathway with sequential oxygenations, initiated by 15-LOX or aspirin-acetylated COX-2, respectively, followed by 5-LOX with an epoxide containing intermediate (Figure 3). For AT-RvDs, DHA is initially converted to 17R-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid (17R-HDHA). The stereochemistry at carbon 17 is conserved, so AT-RvDs each carry a 17R alcohol group configuration, resulting in a distinct series of AT-Rvs – AT-RvD1 to AT-RvD4 (Figure 3A). In the absence of aspirin, endogenous DHA substrate is enzymatically converted in vivo to 17S alcohol containing series of Rvs (RvD1-RvD4) (Figure 3B), and triene-containing structures via a LOX-mediated mechanism (Hong et al., 2003). The complete stereochemistry of RvD1 (7S,8R,17S,-trohydroxy-4Z,9E,11E,13Z,15E,9Z-docosahexaenoic acid) and AT-RvD1 (7S,8R,17SR-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) was recently confirmed (Serhan et al., 2002; Sun et al., 2007). RvD1 synthesis requires a two-step enzymatic process in vivo, yet it can be generated in vitro with a one-pot single enzyme incubation (Sun et al., 2007).
These products' counter-regulatory bioactions were determined in structure-function assays for control of peritoneal PMN infiltration (Serhan et al., 2002; Sun et al., 2007). Bioactive compounds were structurally characterized using retrograde analysis to determine the route of synthesis and origin of these compounds (Serhan et al., 2002). In murine peritonitis, RvD1 is equipotent to AT-RvD1, limiting PMN infiltration in a dose-dependent fashion (Sun et al., 2007). RvD1 is also more resistant to metabolic inactivation than LXA4 but it is eventually converted to 17-oxo-RvD1, which is biologically inactive (Sun et al., 2007).
PDs
DHA also serves as a precursor for the biosynthesis of additional bioactive counter-regulatory lipid mediators. For (neuro)protectin D1 (N)PD1 formation, DHA is rapidly released for conversion to 17S-hydroxy-DHA that serves as a biosynthetic precursor (Serhan et al., 2006) (Figure 4). The complete stereochemistry of PD1, the lead member of the PD family, was characterized by total organic synthesis of related isomers and matching studies with biologically derived compounds to show that PD1 (NPD1, when generated in neural tissues) is 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (Serhan et al., 2006). The geometry of the double bonds and intermediates confirmed that, in fact, PD1 is generated enzymatically by 15-LOX via an epoxide intermediate at the 16(17) position (Hong et al., 2003; Serhan et al., 2006).
PD1 is synthesized by human peripheral blood mononuclear cells and in Th2 CD4+ T-cells in a LOX-dependent manner (Hong et al., 2003; Serhan et al., 2006). This naturally occurring autacoid has been isolated from murine exudates, murine brain cells, human microglial cells (Serhan et al., 2002) and in peripheral human blood (Hong et al., 2003). PD1 is log orders of magnitude more potent than its parent compound DHA (Hong et al., 2003; Duffield et al., 2006; Serhan et al., 2006; Levy et al., 2007a) and demonstrates tissue-specific bioactivity. These effects are in contrast to the less potent and non-selective effects of positional isomers of PD1, including 4S,17S-diHDHA or 7S,17S-diHDHA (Hong et al., 2003).
In Alzheimer's disease, NPD1 biosynthesis is activated by soluble amyloid precursor protein-α (Lukiw et al., 2005). In this disorder, levels of DHA, NPD1 and 15-LOX are selectively decreased in the hippocampus, providing a plausible mechanism for decreased neuroprotection in Alzheimer's disease; that being less inhibition of apoptosis and subsequently, increased neuronal cell death (Marcheselli et al., 2003; Mukherjee et al., 2004; Lukiw et al., 2005). In a placebo-controlled randomized trial, Alzheimer's disease patients treated with DHA-rich dietary supplements had reduced release of IL-1β, IL-6 and granulocyte colony-stimulating factor from peripheral blood mononuclear cells (Vedin et al., 2008). Retinal pigment epithelium cells generate NPD1 when undergoing oxidative stress, allowing for up-regulation of anti-apoptotic proteins (BCL-2, BCL-XL), down-regulation of pro-apoptotic proteins (BAD and BAX) and toxic metabolite A2E-mediated apoptosis, protecting them from oxidative-stress-induced apoptosis and cell aging (Mukherjee et al., 2004; Mukherjee et al., 2007a,b;). Following an ischaemic stroke, 17-R D-series Rvs are generated (in the presence of aspirin) and leukocyte infiltration and pro-inflammatory gene expression are countered by the generation of NPD1 (Marcheselli et al., 2003).
Interestingly, airway mucosa is also enriched with DHA, and intriguing bioactivity for PD1 has recently been demonstrated in an experimental model of asthma (Levy et al., 2007a). Administration of PD1 (2–200 ng) intravenously to allergen-sensitized mice just prior to aerosol allergen challenge, protected the animals from the development of airway hyperresponsiveness and eosinophilic and T-cell-mediated inflammation. In this model, PD1 also dampened levels of Th2 inflammatory cytokines, pro-inflammatory lipid mediators and airway mucus (Levy et al., 2007a). These protective actions were also observed with PD1 when it was administered after airway inflammation was already established. In this setting, intravenous PD1 (20 ng) potently accelerated resolution of the allergic airway inflammation (Levy et al., 2007a). PD1's observed in vivo effects also include decreased T-cell migration, TNF and interferon-γ signalling and promotion of T-cell apoptosis (Ariel et al., 2005).
For purposes of comparison, LX-mediated resolution of lung injury and inflammation has been investigated in several animal models (Levy et al., 2002; Fukunaga et al., 2005; Levy et al., 2007b). With murine allergic airway responses, LXs and PD1 share similar protective actions, yet several differences also exist, supporting the notion that these counter-regulatory autacoids have distinct signalling pathways. While both LXs and PD1 block IL-13 and CysLT (cysteinyl leukotriene) generation without significant effects on IL-12 concentrations in bronchoalveolar lavage fluids (BALFs), the ∼IC50 dose for PD1 was approximately 1 log lower than for bioactive analogs of LXA4 (Levy et al., 2002; 2007a,b;). IL-5 production was reduced in BALFs by LX-stable analogs, but not by PD1 (Levy et al., 2002; 2007a,b;). Of note, administration of PD1 led to decreased levels of LXA4, indicative of a LX-independent signalling pathway. In addition, PD1 decreases levels of PGD2, a key prostanoid implicated in airway responses as well as the induction of 15-LOX, and consequently, LX biosynthesis (Levy et al., 2001; 2007a;). Together, these findings point to distinct pro-resolving signalling pathways for PDs and LXs. Relative to the prominent clinical asthma therapeutic montelukast, PD1, LXs, LX stable analogs and RvE1 are at least as potent and all display broader immunomodulatory actions (Levy et al., 2002; 2007a,b;Aoki et al., 2008; Haworth et al., 2008).
In addition to its role in protecting from tissue injury and inflammation in the lung (Levy et al., 2007a), similar actions for PD1 are observed in renal tissues. In response to ischaemia-reperfusion injury, murine kidneys spontaneously produce both D series Rvs and PD1 (Duffield et al., 2006), demonstrating that these compounds are endogenously generated in response to inflammation. Used as a rescue treatment when given 10 min following injury, RvD1 provides renoprotection, as evidenced by a lower peak serum creatinine value at 24 and 48 h following injury. At a cellular level, tissue leukocytes are reduced and there is inhibition of toll-like receptor-mediated macrophage activation by both RvD1 and PD1 in this model system (Duffield et al., 2006), indicating that RvD1 and PD1 can serve as ‘braking’ signals to prevent a runaway inflammatory response. Similarly to RvE1, PD1 also shifts the onset of resolution to an earlier time point than spontaneous resolution and decreases the Ri for PMNs. Both RvE1 and PD1 also up-regulate CCR5 on PMNs, which acts as a ‘stop signal’ to chemokine signalling by clearing pro-inflammatory CCR5 ligands from the inflammatory milieu (Ariel et al., 2006) (Table 1).
Molecular insights to clinical observations
Since 1929, it has been established that omega-3 fatty acids are essential to health, and dietary deficiency can lead to disease (Burr and Burr, 1929). Multiple epidemiological studies that followed this initial discovery have further demonstrated that omega-3 PUFAs are dysregulated in many disease states, including atherosclerosis, cystic fibrosis, asthma, cardiovascular disease, cancer and liver injury (Freedman et al., 1999; Nagakura et al., 2000; Marchioli et al., 2002) and ω-6 PUFAs can play pathological roles (Dwyer et al., 2004). Dietary supplementation of ω-3 fatty acids has proven to be directly beneficial in multiple scenarios (Das, 2008): feeding children EPA and DHA versus olive oil (control) resulted in markedly improved clinical outcomes of decreased asthma symptom scores and decreased airway responsiveness to inhaled methacholine challenge (Nagakura et al., 2000). Mice fed a DHA-enriched diet are protected from carbon-tetrachloride (CCl4)-induced induced necroinflammatory hepatic injury (Gonzalez-Periz et al., 2006). DHA and EPA supplementation to healthy subjects results in enhanced cardiac output, stroke volume and decreased systemic vascular resistance (Walser and Stebbins, 2008). EPA and DHA supplementation increases insulin sensitivity in rats (Andersen et al., 2008). Higher umbilical cord DHA concentration is associated with longer gestation, better visual acuity and mental and psychomotor performance at 11 months in children in arctic Quebec (Jacobson et al., 2008). Women with no history of vascular disease or risk factors were studied in a prospective cohort study, and those women who consumed more fish oils were found to have a lower risk of thrombotic stroke (Iso et al., 2001).
These studies of dietary supplementation with omega-3 PUFAs serve to highlight the regulation of pathological inflammation as a potential point for pharmacological intervention. Of particular note, a clinically significant difference between the rate of death, myocardial infarction and stroke among patients receiving both aspirin and ω-3 PUFAs versus controls was reported in a randomized controlled study conducted by the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI) group (GISSI investigators, 1999). There was no mechanistic explanation for the beneficial effect of aspirin in the presence of these PUFAs in this study. Given recent insights, the findings of the GISSI trial point to aspirin-triggered formation of Rvs from EPA and DHA in addition to its inhibition of PG and thromboxane pathways. If generated during myocardial infarction or stroke in higher amounts with aspirin and dietary ω-3 PUFA supplementation, Rvs would serve tissue-specific protective roles for catabasis.
In addition to cardiovascular, renal, hepatic and neural tissues, EPA and DHA may also serve important protective functions in injured and inflamed lung. A recent trial of enteric supplementation with a low-carbohydrate, high-fat diet enriched with EPA led to substantial improvement in clinical outcomes in the acute respiratory distress syndrome (ARDS) (Gadek et al., 1999). Patients receiving the intervention diet had significant decreases (approximately 2.5-fold) in total cells and PMNs per mL BALF, and significant improvements in oxygenation, mechanical ventilator support and length of stay in the intensive care unit (Gadek et al., 1999). In a separate analysis of these subjects' BALFs, decreased levels of IL-8 and LTB4 were identified and related to the decreased BALF PMNs and alveolar membrane protein permeability with the EPA-enriched enteral diet (Pacht et al., 2003). These exciting findings from a single centre are now being investigated in multiple sites as part of the EDEN-Omega ARDSnet trial. EPA-derived Rvs have not been studied in these patients, but RvE1 displays potent protective actions in airway inflammation (Aoki et al., 2008; Haworth et al., 2008) and RvE2 is a potent regulator of PMN functional responses (Tjonahen et al., 2006). Airway tissue concentrations of DHA are decreased in absolute mass and relative to arachidonic acid in other diseases of pathologic airway inflammation, including asthma and cystic fibrosis (Freedman et al., 2004). Recently, PD1 and its biosynthetic precursor 17S-hydroxy-DHA were identified in exhaled breath condensates from human subjects with decreased levels of PD1 produced during asthma exacerbations (Levy et al., 2007a). Together with results for RvE1 and PD1 in murine models of asthma, these findings support roles for EPA- and DHA-derived compounds in protection from excessive airway inflammation in several lung diseases.
If Rvs and PDs are the missing link to explain the salutary effects of dietary supplementation with EPA and DHA, then individual metabolism of these fatty acids could account for the variability observed in clinical studies that have had difficulty establishing benefit in oral supplementation with ω-3 fish oils (Van Biervliet et al., 2008). Gene by diet interactions has been established for ω-6 supplementation and risk for atherosclerosis (Dwyer et al., 2004). The important role that genetic variation can play in PUFA metabolism is emphasized in recent studies with FAT-1 transgenic mice. Compared with control animals, mice that are engineered to overexpress fatty acid desaturase produce higher levels of EPA and DHA plus increased amounts of Rvs and PDs when challenged with a pro-inflammatory stimulus (Hudert et al., 2006). These mice are relatively protected from gastrointestinal inflammation in colitis (Hudert et al., 2006) and demonstrate less tumour metastasis from melanoma (Xia et al., 2006).
Mimetics as emerging therapeutic targets for drug design in inflammatory diseases
Because they are endogenously generated compounds with protective actions, Rvs and PDs serve as rational templates for the design of molecular mimetics as potential therapeutics. Historically, the emphasis in development of anti-inflammatory drugs has been largely on interfering with the pro-inflammatory signalling cascade. Only recently has the focus shifted to augmenting naturally occurring pro-resolution responses in the host. As more counter-regulatory chemical mediators are isolated and characterized, newer pharmacological targets emerge for drug design of stable pro-resolving mimetics. Similar to the development of aspirin-triggered LXA4 stable analogs (ATLa) (Takano et al., 1997; Schottelius et al., 2002), an RvE1 stable analog has been designed and synthesized by chemical modification at the ω-end of RvE1 (Arita et al., 2006). The analog, 19-(p-fluorophenoxy)-RvE1 methyl ester, carries bioequivalence to native RvE1 in reducing pro-inflammatory cytokines and leukocyte numbers in murine periodontitis, and is highly resistant to ω-oxidation or rapid dehydrogenation (Arita et al., 2006).
These and other bioactive mimetics that resist metabolic inactivation are currently in development. The exciting possibility exists that this approach to drug development will lead to a new genus of pharmacological agents that serve as mimetics of natural resolution pathways (Serhan and Chiang, 2008). At this juncture, more research is needed on these new pro-resolving chemical mediators and their mechanisms of action to learn whether Rvs and PDs will ultimately provide long sought-after solutions to resolving pathological inflammation.
Acknowledgments
The work was supported in part by NIH grants AI068084 and P50-DE016191.
Glossary
Abbreviations:
- ATL
aspirin-triggered lipoxins
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- MS
mass spectrometry
- PD
protectin
- PG
prostaglandin
- PMN
polymorphonuclear leukocyte
- PUFA
polyunsaturated fatty acid
- Rv
resolvin
Statement of conflict of interest
BDL is an inventor on patents for the lipoxins, resolvins and protectins and their analogs that are assigned to Brigham and Women's Hospital and have been licensed for clinical development. PK has no conflict of interest to disclose.
References
- Andersen G, Harnack K, Erbersdobler HF, Somoza V. Dietary eicosapentaenoic acid and docosahexaenoic acid are more effective than alpha-linolenic acid in improving insulin sensitivity in rats. Ann Nutr Metab. 2008;52:250–256. doi: 10.1159/000140518. [DOI] [PubMed] [Google Scholar]
- Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005a;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005b;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, et al. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezar-de-Mello PF, Vieira AM, Nascimento-Silva V, Villela CG, Barja-Fidalgo C, Fierro IM. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br J Pharmacol. 2008;153:956–965. doi: 10.1038/sj.bjp.0707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- Cotran RVK, Collins T. Robbins Pathologic Basis of Disease. Philadelphia: Saunders; 1999. [Google Scholar]
- Das UN. Can essential fatty acids reduce the burden of disease(s)? Lipids Health Dis. 2008;7:9. doi: 10.1186/1476-511X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Arita M, Hong S, Bannenberg G, Moussignac RL, Gronert K, et al. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. Faseb J. 2003;17:652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN. Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94:4132–4142. [PubMed] [Google Scholar]
- Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J Exp Med. 1990;172:1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr(-/-) mice. Proc Natl Acad Sci USA. 1999;96:13995–14000. doi: 10.1073/pnas.96.24.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- GISSI investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, Titos E, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- Gronert K, Kantarci A, Levy BD, Clish CB, Odparlik S, Hasturk H, et al. A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. J Immunol. 2004;172:1856–1861. doi: 10.4049/jimmunol.172.3.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci (Lond) 1992;83:639–648. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A(4) to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the inuit of arctic Quebec. J Pediatr. 2008;152:356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- Lee TH, Mencia-Huerta JM, Shih C, Corey EJ, Lewis RA, Austen KF. Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophils. J Clin Invest. 1984;74:1922–1933. doi: 10.1172/JCI111612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Fokin VV, Clark JM, Wakelam MJ, Petasis NA, Serhan CN. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a ‘stop’ signaling switch for aspirin-triggered lipoxin A4. Faseb J. 1999;13:903–911. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007a;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, et al. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007b;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Hong S, Tjonahen E, Serhan CN. Mediator-lipidomics: databases and search algorithms for PUFA-derived mediators. J Lipid Res. 2005;46:790–802. doi: 10.1194/jlr.D400020-JLR200. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Chawla A, Loayza MS, Bazan NG. Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot Essent Fatty Acids. 2007a;77:233–238. doi: 10.1016/j.plefa.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007b;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J. 2000;16:861–865. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes. In: W Odelberg., editor. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Stockholm: Almqvist & Wiksell; 1982. pp. 153–174. [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J, et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Lu Y, Hong S, Yang R. Mediator lipidomics: search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol. 2007;432:275–317. doi: 10.1016/S0076-6879(07)32012-0. [DOI] [PubMed] [Google Scholar]
- Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim H-S, Kuhn H, et al. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Uller L, Persson CG, Erjefalt JS. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends Pharmacol Sci. 2006;27:461–466. doi: 10.1016/j.tips.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Van Biervliet S, Devos M, Delhaye T, Van Biervliet JP, Robberecht E, Christophe A. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukot Essent Fatty Acids. 2008;78:109–115. doi: 10.1016/j.plefa.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Vedin I, Cederholm T, Freund Levi Y, Basun H, Garlind A, Faxen Irving G, et al. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008;87:1616–1622. doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- Walser B, Stebbins CL. Omega-3 fatty acid supplementation enhances stroke volume and cardiac output during dynamic exercise. Eur J Appl Physiol. 2008;104:455–461. doi: 10.1007/s00421-008-0791-x. [DOI] [PubMed] [Google Scholar]
- Winyard PG, Willoughby, editors. Inflammation Protocols. Methods in molecular biology, Volume 225. Totowa, NJ: Humana Press; 2003. [Google Scholar]
- Xia S, Lu Y, Wang J, He C, Hong S, Serhan CN, et al. Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci USA. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]


