Abstract
Protease-activated receptors (PARs) are a novel family of G protein-coupled receptors. Signalling through PARs typically involves the cleavage of an extracellular region of the receptor by endogenous or exogenous proteases, which reveals a tethered ligand sequence capable of auto-activating the receptor. A considerable body of evidence has emerged over the past 20 years supporting a prominent role for PARs in a variety of human physiological and pathophysiological processes, and thus substantial attention has been directed towards developing drug-like molecules that activate or block PARs via non-proteolytic pathways. PARs are widely expressed within the respiratory tract, and their activation appears to exert significant modulatory influences on the level of bronchomotor tone, as well as on the inflammatory processes associated with a range of respiratory tract disorders. Nevertheless, there is debate as to whether the principal response to PAR activation is an augmentation or attenuation of airways inflammation. In this context, an important action of PAR activators may be to promote the generation and release of prostanoids, such as prostglandin E2, which have well-established anti-inflammatory effects in the lung. In this review, we primarily focus on the relationship between PARs, prostaglandins and inflammatory processes in the lung, and highlight their potential role in selected respiratory tract disorders, including pulmonary fibrosis, asthma and chronic obstructive pulmonary disease.
This article is part of a themed issue on Mediators and Receptors in the Resolution of Inflammation. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Keywords: protease-activated receptors, prostaglandins, airway inflammation, asthma, allergy, fibrosis
Introduction
Protease-activated receptors (PARs) are a family of G protein-coupled receptors that possess a unique mechanism of activation (Hollenberg and Compton, 2002; Steinhoff et al., 2005). The emergence of PARs as a novel receptor family was stimulated by the discovery that thrombin specifically cleaves the extracellular N-terminal region of its receptor to create a new receptor amino terminus that functions as an activating tethered ligand (Vu et al., 1991). PAR activation can also occur in the absence of proteolytic activity, by synthetic peptides called PAR-activating peptides (PAR-APs) that mimic the final five to seven amino acids of the tethered ligand sequence. At present, four distinct subtypes of PAR have been characterized and designated PAR1, PAR2, PAR3 and PAR4, in chronological order of their discovery (Rasmussen et al., 1991; Vu et al., 1991; Nystedt et al., 1995a; Ishihara et al., 1997; Kahn et al., 1998a; Xu et al., 1998). Of particular interest, numerous studies have recently demonstrated the involvement of PARs in a wide variety of physiological and pathophysiological processes (see recent review by Ramachandran and Hollenberg, 2008). The actions of PARs and their activating proteinases in the airways have also been studied extensively to determine their role in various lung diseases (Sokolova and Reiser, 2007). Among their actions, PARs induce cyclooxygenase (COX) activation and expression in a variety of cell types, resulting in the synthesis and release of various prostanoids (Cocks et al., 1999). PAR-mediated prostaglandin production represents an attractive target for the development of treatments for inflammatory lung diseases, and is the principal focus of this review.
Expression and signalling of PARs in the lung
The human respiratory tract appears to express all four PAR subtypes. Within the lung, PARs are expressed on many cell types including alveoli, fibroblasts, airway smooth muscle, nerves, epithelial cells, endothelial cells, mesothelial cells, goblet cells, as well as on various leukocytes (Sokolova and Reiser, 2007; Ramachandran and Hollenberg, 2008). Of particular interest, exposure of the lung to inflammatory stimuli may enhance the expression of PARs, as well as of PAR-activating proteases. Proteases that may be present in the respiratory tract and activate PARs include the endogenous enzymes mast cell tryptase (activates PAR2), trypsin (PAR1, PAR2 and PAR4), chymase (PAR1) and cathepsin G (PAR4), as well as exogenous enzymes such as Der p1 (PAR2) that are inhaled. However, these and other enzymes within the respiratory tract may also inactivate or disarm various PARs by cleaving them at other sites that remove the tethered ligand sequence (Loew et al., 2000).
PAR1 is the primary cell-surface receptor responsible for thrombin-mediated platelet aggregation in humans (Rasmussen et al., 1991; Vu et al., 1991; Ahn et al., 2000). PAR1 is also activated by many other proteases, including activated protein C (APC), and by selective PAR1-APs such as TFLLR-NH2 (Vu et al., 1991; Ramachandran and Hollenberg, 2008). Non-peptide agonists for PAR1 are currently unavailable. Nevertheless, several potent and selective non-peptidic PAR1 antagonists have been developed (Ramachandran and Hollenberg, 2008). One such antagonist, SCH530348, is currently undergoing phase III clinical trials as a preventative medication for atherothrombosis (Clasby et al., 2007; Butler, 2008; Severino et al., 2008).
PAR1 is expressed on many cell types within the lungs, including airway smooth muscle, epithelial cells, platelets, macrophages, mast cells, CD3+ T lymphocytes and fibroblasts (Knight et al., 2001; Hollenberg and Compton, 2002; Lan et al., 2002; Steinhoff et al., 2005; Li and He, 2006; Sokolova and Reiser, 2007; Ramachandran and Hollenberg, 2008). Although PAR1 expression does not appear to be altered in asthmatic airways, increased expression is seen following exposure of cells to influenza A, cockroach allergen and various PAR-APs (Knight et al., 2001; Lan et al., 2004; Ostrowska et al., 2007; Zhang et al., 2008). PAR1 expression is decreased in fibroblasts following PGI2 or prostglandin E2 (PGE2) exposure (Sokolova et al., 2005), possibly involving a cAMP-dependent mechanism as demonstrated in vascular smooth muscle cells (Sokolova et al., 2005; Pape et al., 2008). Gq/11 appears to be the primary signalling G protein for PAR1, although it has also been reported to signal via Gi and G12/13 pathways depending on cell type (see review by Ramachandran and Hollenberg, 2008).
The cloning and characterization of PAR1 was followed by the discovery of PAR2, which was shown to be activated by trypsin but resistant to thrombin (Nystedt et al., 1995a,b;). In addition to endogenous proteases such as trypsin and tryptase, PAR2 can be activated by exogenous proteases, such as Der p1 from the house dust mite Dermatophagoides Pteronyssinus and by PAR2-APs such as SLIGKV-NH2 (Molino et al., 1997; Asokananthan et al., 2002; Page et al., 2003). Selective small-molecule agonists, as well as an antagonist, have recently been developed; however, they have low potency and are not yet widely available (Kelso et al., 2006; Gardell et al., 2008; Seitzberg et al., 2008).
PAR2 is expressed on a range of cell types including mesothelial cells of the pleura, bronchial glands, epithelial cells, endothelial cells, smooth muscle cells, nerves and immune cells such as CD3+ T lymphocytes, eosinophils, mast cells and neutrophils (Knight et al., 2001; Miotto et al., 2002; Henry, 2006; Li and He, 2006). PAR2 expression is elevated following exposure to a variety of inflammatory stimuli, including respiratory tract viruses, smoke, bacterial products and allergens (Knight et al., 2001; Ostrowska et al., 2007). PAR2 signals primarily through activation of Gq/11, but has recently been shown to signal through G protein-independent mechanisms via β-arrestins (Zoudilova et al., 2007).
The discovery of a second platelet thrombin receptor (Connolly et al., 1996) was soon followed by the cloning and characterization of PAR3 (Ishihara et al., 1997). PAR3 mRNA is expressed in airway smooth muscle cells, epithelial cells, CD3+ T lymphocytes and fibroblasts (Hauck et al., 1999; Shimizu et al., 2000; Li and He, 2006; Ramachandran et al., 2006), and its expression on epithelial cells is increased following exposure to influenza A. Somewhat surprisingly, the corresponding tethered ligand sequence does not activate PAR3. At first it appeared that proteolytic cleavage of PAR3 and exposure of its tethered ligand did not induce signalling per se; rather PAR3 appeared to act as a co-factor for the activation of other PARs (Kahn et al., 1998b). For example, PAR3 dimerized with PAR1 and PAR4 to amplify their signalling (Nakanishi-Matsui et al., 2000; Weiss et al., 2002; McLaughlin et al., 2007). A recent study, however, has shown that thrombin can signal through PAR3 independently of other PARs, to induce interleukin (IL)-8 release from HEK-293 cells, via ERK1/2 phosphorylation (Ostrowska and Reiser, 2008).
The observation that thrombin could stimulate the aggregation of mouse platelets in the absence of PAR1 and PAR3 provided evidence for the existence of a fourth PAR subtype (Kahn et al., 1998b; Xu et al., 1998). Although thrombin activates PAR4, it is much less potent at PAR4 than at PAR1 (Kahn et al., 1998b; Xu et al., 1998). A PAR-AP, HYPGKF-NH2 also activates PAR4. No non-peptidic agonists for PAR4 are available; however, a non-peptidic antagonist has been reported (Wu et al., 2002). A low potency peptide antagonist has been developed, although it exhibits low selectivity and is known to produce non-PAR effects (Hollenberg and Saifeddine, 2001; Hollenberg et al., 2004). P4pal-10 is a high-potency pepducin antagonist for PAR4, although it also partially inhibits activation of PAR1 by SFLLRN-NH2 (Covic et al., 2002a,b; Kuliopulos and Covic, 2003; Hansen et al., 2008).
PAR4 is widely expressed in the lung, present on endothelial and epithelial cells, airway smooth muscle cells as well as alveolar macrophages (Lan et al., 2000; Shimizu et al., 2000; Asokananthan et al., 2002; Kataoka et al., 2003). PAR4 expression on bronchial fibroblasts was recently reported to be elevated following exposure to inflammatory stimuli, such as tumour necrosis factor-α (TNF-α) (Ramachandran et al., 2007). PAR4 signals through a Gq/11-mediated pathway (Xu et al., 1998).
PAR-mediated effects in the lung
PAR-mediated inhibition of airway smooth muscle tone
PAR2 activators can modulate bronchomotor tone, with the predominant effect being bronchodilatation. For example, intravenous administration of PAR2-APs attenuated methacholine-, serotonin- and histamine-induced increases in airway resistance in mice, rats and guinea pigs respectively (Cicala et al., 1999; Cocks et al., 1999; Lan et al., 2004). Consistent with this, PAR2-APs induce concentration-dependent relaxation response in isolated airway preparations from these animals (Cocks et al., 1999; Chow et al., 2000; Lan et al., 2000; Ricciardolo et al., 2000; Kawabata et al., 2004b; Franchi-Micheli et al., 2005). In general, inhibitors of COX such as indomethacin block PAR2-induced bronchodilatory effects, indicating a prominent mediator role for relaxant prostanoids in this response (Cicala et al., 1999; Cocks et al., 1999; Lan et al., 2004). Direct evidence that PGE2 was an important mediator in PAR-induced relaxation responses came from studies showing that exposure of murine airways to PAR2-APs caused concentration-dependent increases in PGE2 release, which correlated strongly and positively with the magnitude of the relaxation response (Lan et al., 2001). PAR2-mediated release of PGE2 is likely to cause bronchodilator responses via activation of airway smooth muscle EP2 receptors, which signal through Gαs, adenylate cyclase and cAMP (Fortner et al., 2001; Lan et al., 2001).
PAR-mediated production of the anti-inflammatory prostanoid PGE2
In most organ systems, PGE2 promotes inflammatory processes, whereas it produces predominantly anti-inflammatory effects in the lung (Vancheri et al., 2004). This section will cover the specific prostaglandins released by PAR subtypes, the mechanisms involved in PAR-mediated generation of prostaglandins and the modulatory effects of these prostaglandins on inflammatory processes in the airways. As indicated above, PAR activators induce the rapid and sustained formation and release of prostanoids from a wide variety of cell and tissue types. For example, PAR1-APs cause PGE2 release from human bronchial epithelial cells (Asokananthan et al., 2002) and human lung fibroblasts (Sokolova et al., 2005; Sokolova et al., 2008). Human bronchial airway epithelial cell cultures release PGE2 following exposure to PAR2-APs; however, cultured airway smooth muscle cells, while capable of PGE2 production, do not do so in response to PAR2 activation (Pang and Knox, 1997; Asokananthan et al., 2002; Chambers et al., 2003; Dulon et al., 2005; Sharma et al., 2006). Tryspin induces PGE2 release from airway smooth muscle cells independently of PAR2, suggesting that PAR4 may induce PGE2 release from airway smooth muscle (Chambers et al., 2003). PAR4-APs can also induce PGE2 release from bronchial epithelial cell cultures (Asokananthan et al., 2002). PAR1, PAR2 and PAR4-APs increased PGE2 levels in murine isolated tracheal preparations, with PAR2-APs inducing the largest response (Lan et al., 2001). As PAR2 expression on airway epithelial cells increases in response to a variety of inflammatory stimuli, PAR2-mediated generation of PGE2 may be amplified in inflammatory lung disorders (Knight et al., 2001).
PAR-mediated PGE2 production occurs via a complex signalling pathway, which has been studied in greatest detail in cultures of A549 cells (a transformed human airway epithelial cell line) (Kawao et al., 2005), and in mouse isolated tracheal preparations (Kawabata et al., 2004b). In these systems, PAR2-APs induce a rapid, transient increase in PGE2 levels, which is thought to sequentially involve the activation of PAR2, an increase in [Ca2+]i, activation of cytosolic phospholipase A2, release of arachidonic acid and downstream processing of arachidonic acid by COX-1 and prostaglandin E synthase (PGES). This is followed by a second, sustained phase of PGE2 generation involving COX-1-dependent up-regulation of COX-2 and microsomal prostaglandin E synthase-1 (mPGES-1) (Kawabata et al., 2004b; Kawabata and Kawao, 2005; Kawao et al., 2005; Nagataki et al., 2007; Sekiguchi et al., 2007; Hsieh et al., 2008; Rastogi et al., 2008; Wang et al., 2008). PAR2-mediated production of PGE2 appears to be dependent on mPGES-1 but not mPGES-2 or cPGES (Nagataki et al., 2007). Figure 1 shows a simplified representation of PAR2-mediated generation of PGE2.
Figure 1.
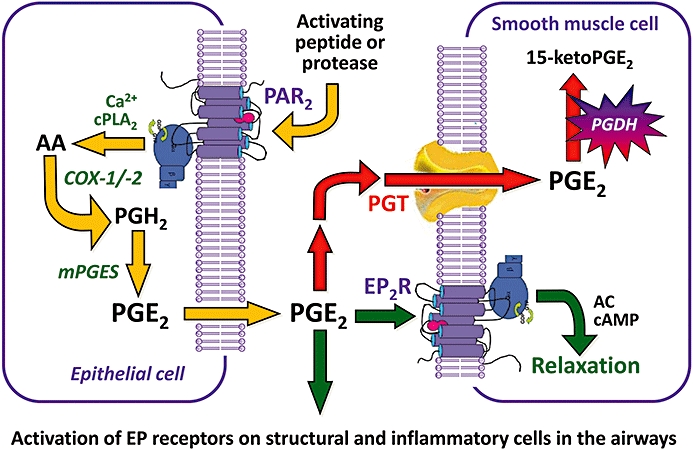
PAR2-mediated generation of PGE2 in the airways.
PGE2 induces an extensive array of effects within the respiratory tract ( Table 1), affecting the activity of most structural and inflammatory cells. These effects are mediated by E prostanoid receptors, a family of four G protein-coupled receptors (EP1–EP4) linked to Gq/11 (EP1 receptors), Gs (EP2 and EP4 receptors) and Gi/o (EP3 receptors) (Alexander et al., 2008). As highlighted by Vancheri and coworkers (Vancheri et al., 2004) and the data presented in Table 1, PGE2 appears to have a role in limiting the immune-inflammatory response and tissue repair processes. These findings indicate that PAR-mediated production of PGE2 may be beneficial in instances when dysregulated inflammatory and tissue repair processes contribute to disease.
Table 1.
Summary of effects produced by prostglandin E2 (PGE2) in isolated cells of the respiratory tract, in animal models of airway disease, and in humans with airway disease
| Response to PGE2 | EP receptor (species if not human) | Reference | |
|---|---|---|---|
| In vitro effects of PGE2 on | |||
| Airway smooth muscle | Relaxation | EP2 | Norel et al. (1999) |
| ↓Proliferation | EP2 | Burgess et al. (2004); Kassel et al. (2008) | |
| ↓Migration | ? | Goncharova et al. (2003) | |
| ↓RANTES, ICAM, GM-CSF, Il-8, eotaxin, MCP-1 | ? | Ammit et al. (2000); Lazzeri et al. (2001); Wuyts et al. (2003); Kaur et al. (2008) | |
| ↑VEGF, G-CSF, Il-6 | EP2/EP4 | Ammit et al. (2000); Bradbury et al. (2005); Clarke et al. (2005) | |
| Epithelial | ↑Cilia beat frequency | ? | Bonin et al. (1992); Schuil et al. (1995); Haxel et al. (2001) |
| ↑Cl-channel conductance | EP4 (frog, cow) | Clayton et al. (2005); Palmer et al. (2006); Joy and Cowley (2008); Seto et al. (2008) | |
| ↑Na+ transport | EP1/EP2 (frog) | Berk et al. (2004) | |
| ↑Mucin secretion | EP4 | Kook Kim et al. (2006) | |
| ↑MUC5A/8 expression | EP4 | Cho et al. (2005); Kook Kim et al. (2006); Song et al. (2009) | |
| ↑Rate of wound closure | EP1/EP4/EP2? | Savla et al. (2001) | |
| ↓Il-8 & ET-1 secretion | EP3/EP4 | Pelletier et al. (2001); Hattori et al. (2008) | |
| ↑Il-6 release | EP2/EP4 | Tavakoli et al. (2001) | |
| Submucosal gland | ↑Ionic currents & secretory function | ? (pig) | Liu et al. (2005) |
| ↑Sensitivity to acetylcholine | EP2 (pig) | Liu and Farley (2007) | |
| Cholinergic nerve | ↓Acetylcholine release | EP3 (guinea pig, dog) | Deckers et al. (1989); Zhao et al. (1994); Spicuzza et al. (1998); Clarke et al. (2004) |
| Sensory nerve (pulmonary C-fibre afferents) | ↑Sensitivity to chemical stimulants | EP2 (rat) | Ho et al. (2000); Kwong and Lee (2002; 2005;) |
| Alveolar type II | ↑Surfactant secretion | EP1 (rat) | Marino and Rooney (1980); Morsy et al. (2001) |
| ↑Na+ uptake | EP3 | Mukhopadhyay et al. (1998) | |
| Pulmonary endothelial | ↑Barrier function | Birukova et al. (2007) | |
| Alveolar macrophage | ↓Phagocytosis | EP2 (mouse/rat) | Canning et al. (1991); Aronoff et al. (2004); Canetti et al. (2007); Brock et al. (2008); Lee et al. (2009); Medeiros et al. (2009) |
| ↓Bacterial killing | EP2/EP4 | Serezani et al. (2007) | |
| ↓Mitochondrial inner membrane perturbation and necrosis | EP2 | Chen et al. (2008) | |
| ↓TNF-α | EP2/EP4 | Ratcliffe et al. (2007) | |
| ↑Il-10 & NO release | ? (rat) | Menard et al. (2007) | |
| Fibroblast | ↓proliferation | EP2 | Bitterman et al. (1986); Liu et al. (2004); Huang et al. (2007); Huang et al. (2008) |
| ↓Collagen production | EP2 | Saltzman et al. (1982); Fine et al. (1989); Liu et al. (2004); Huang et al. (2007); Huang et al. (2008) | |
| ↓Fibroblast to myofibroblast transition | ? | Kolodsick et al. (2003) | |
| ↓Myofibroblast differentiation | Dunkern et al. (2007) | ||
| ↓Migration | EP2 | Kohyama et al. (2001); White et al. (2005) | |
| ↓Smoke-induced apoptosis | EP2 | Sugiura et al. (2007) | |
| Mast cell | ↓Migration | EP3? | Duffy et al. (2008) |
| ↓Histamine release | EP2 | Drury et al. (1998); Kay et al. (2006); Duffy et al. (2008) | |
| Dendritic cell | ↑Podosome dissolution | van Helden et al. (2008) | |
| ↑Migration | EP2/EP4 | Legler et al. (2006) | |
| ↑Maturation | EP2/EP4 | Kubo et al. (2004) | |
| ↓CCL3 and CCL4 | EP2/EP4 (mouse) | Jing et al. (2003) | |
| ↑Resistance to apoptosis | EP2/EP4 | Baratelli et al. (2005a) | |
| ↓TNF-α release & antigen presentation | ? (mouse) | Kambayashi et al. (2001) | |
| T cells | ↓Proliferation | ? | Jarvinen et al. (2008) |
| ↑Il-10 expression | ? | Benbernou et al. (1997) | |
| Treg | ↑Inhibitory function & differentiation | ? | Baratelli et al. (2005b) |
| ↑Expansion | ? | Garg et al. (2008) | |
| Eosinophils | ↓Migration | EP2/EP4 | Sturm et al. (2008) |
| ↓Release from bone marrow | ? (guinea pig) | Sturm et al. (2008) | |
| ↓Degranulation | EP2/EP4 | Sturm et al. (2008) | |
| ↓PAF-induced aggregation | EP2 (guinea pig) | Teixeira et al. (1997) | |
| Neutrophils | ↓Chemotaxis | EP2? | Armstrong (1995) |
| B cells | ↓Proliferation | EP4 (mouse) | Murn et al. (2008) |
| Promotes differentiation and Il-4 and LPS-driven class switching to IgE | EP2/EP4 (mouse) | Fedyk and Phipps (1996) | |
| In vivo effects of PGE2 in | |||
| Normal animals or subjects | Bronchodilatation | EP2 (mouse) | Mathe and Hedqvist (1975); Smith et al. (1975); Sheller et al. (2000); Tilley et al. (2003) |
| Asthmatics (allergic) | Bronchodilatation | Smith et al. (1975) | |
| ↓Early and late response to allergen | Pavord et al. (1993); Gauvreau et al. (1999) | ||
| ↓Airway hyperresponsiveness and sputum eosinophils | Gauvreau et al. (1999) | ||
| ↓BAL PGD2, ↓BAL eosinophils | Hartert et al. (2000) | ||
| Asthmatic (exercise) | ↓Exercise-induced bronchoconstriction | Melillo et al. (1994) | |
| Allergic inflammation | ↓Allergen-induced bronchoconstriction | EP2/EP4 (guinea pig) | Martin et al. (2002); Tanaka et al. (2005) |
| ↓BAL eosinophils | ? (mouse, rat) | Martin et al. (2002); De Campo and Henry (2005); Sturm et al. (2008) | |
| ↓BAL LTs, ↓T cell cytokine expression | ? (rat) | Martin et al. (2002) | |
| Bleomycin-induced fibrosis | PGE2 synthetic analogue 16,16-dimethyl-PGE2↓infiltration by leukocytes, ↓myeloperoxidase activity, ↓Il-1, TNF-α and nitrotyrosine, ↓lung edema & injury, ↓collagen deposition, ↓weight loss and mortality | ? (mouse) | Failla et al. (2009) |
Not all references to PGE2-induced responses are cited, with preference given to studies that have used human cells, tissues or subjects. Animal studies are cited where human studies have not been reported, or to indicate which E-prostanoid (EP) receptor subtype mediated the response.
PAR-mediated production of pro-inflammatory cytokines
PAR activation may also promote inflammatory processes in the respiratory tract, as indicated by reports that PAR-APs induce the release of pro-inflammatory cytokines and mediators in cell cultures relevant to airways disease. For example, PAR1, PAR2 and PAR4-APs induced IL-6 and IL-8 release from airway epithelial cells (Asokananthan et al., 2002). Additionally, PAR1- and PAR2-APs, but not PAR3- or PAR4-APs, induced IL-6, but not IL-13, release from cultured human T cells (Li and He, 2006). PAR1 activators have also been reported to induce CCL2, IL-1β and TNF-α release from macrophages (Naldini et al., 1998; 2002; Riewald et al., 2002; Colognato et al., 2003), and PAR2-APs induced MMP-9 release from primary cultures of human airway epithelial cells (Vliagoftis et al., 2000). The PAR2-AP, SLIGKV-NH2, induced GM-CSF mRNA expression but did not increase protein expression in human fibroblasts. SLIGKV-NH2 also increased cell surface expression of VCAM-1 on primary human bronchial fibroblasts (Ramachandran et al., 2006). Furthermore, PAR2 activation promoted eosinophil degranulation and superoxide production (Miike et al., 2001), and may promote IL-8 release (Temkin et al., 2002).
PAR-mediated modulation of allergic, eosinophilic inflammation
Consistent with PAR-mediated production of pro-inflammatory cytokines in vitro, several in vivo studies have demonstrated a pro-inflammatory role for PARs in the airways. For example, mice lacking PAR2 exhibit decreased inflammation in an allergen challenge model, whereas overexpression of PAR2 was associated with increased inflammation (Schmidlin et al., 2002; Takizawa et al., 2005). In subsequent studies, co-administration of PAR2-APs caused increased cell influx, IL-5, IL-13 and TNF-α in bronchoalveolar lavage (BAL) fluid, while lowering IL-10, in allergen-sensitized and challenged mice (Ebeling et al., 2005; 2007;). In these latter studies, a single dose of PAR2-AP did not induce an inflammatory response; however, multiple doses did. Other studies have suggested that PAR2 may induce airway inflammation through a neuropeptide-dependent mechanism; however, this requires further investigation given that PAR2-APs are known to activate NK1 receptors (Su et al., 2005; Abey et al., 2006).
On the contrary, there is also a significant body of evidence indicating that PAR activators suppress inflammatory processes within the lung. Of particular relevance to the current review, the anti-inflammatory effects produced by PAR activators appear to be mediated by prostaglandins such as PGE2 (Asokananthan et al., 2002; Henry, 2006). For example, intratracheally administered PAR2-APs reduced bronchoconstriction, airway hyperresponsiveness and eosinophil influx in a murine model of allergic inflammation (De Campo and Henry, 2005). This PAR-mediated effect was suppressed by inhibitors of COX and mimicked by PGE2 (De Campo and Henry, 2005). Administration of PAR2-AP to antigen-sensitized and challenged rabbits was also associated with reduced bronchoconstriction, airway hyperresponsiveness and eosinophilia (D'Agostino et al., 2007), although the role of COX and PGE2 is uncertain. Nevertheless, this latter study showed a marked increase in IL-10 mRNA from CD4+ T cells recovered from PAR2-AP-treated animals, as well as decreased interferon (IFN)-γ and IL-2 production to control levels (D'Agostino et al., 2007), consistent with an anti-inflammatory response.
Thus, a puzzling, yet not uncommon trait of research investigating the role of PARs in inflammatory processes in the respiratory system is the existence of seemingly contradictory observations, with some reports demonstrating that PAR-APs induce inflammatory responses, and others demonstrating overt anti-inflammatory effects. Although there is unlikely to be single reason that adequately explains these inconsistencies, a contributing factor is likely to be the lack of subtype-selective small-molecule agonists and antagonists for PARs, often necessitating the use of indirect methods to determine the role of PARs in these processes.
As PAR-APs are typically between five and seven amino acids long, the development of small-molecule lead compounds that selectively bind to PARs is problematic. Thus, there is a current paucity of agents capable of selectively activating and inhibiting these receptors. Until recently, this has necessitated the use of one or more of the following approaches to explore PARs: (i) use of PAR-activating proteases; (ii) use of relatively selective but low-potency PAR-APs; and (iii) manipulation of PAR expression.
Although numerous proteases are well-established activators of PARs, their use in characterizing subtypes of PARs is often limited. One particular limitation is that proteases frequently possess a multitude of effects – being capable of activating more than one PAR subtype as well as inducing PAR-independent effects. For example, thrombin can activate PAR1, PAR3 and PAR4, as well as induce smooth muscle proliferation in a PAR-independent fashion (Tran and Stewart, 2002). The currently used PAR-APs are typically more selective than proteases, but most lack absolute subtype specificity and may activate non-PAR receptors (Abey et al., 2006). Furthermore, aminopeptidases can degrade most PAR-APs, which limits their effectiveness. An exception is the aminopeptidase-resistant PAR2-AP 2-furoyl-LIGRLO-NH2 (Kawabata et al., 2004a). Gene knockout and overexpression approaches have been used to investigate the role of PARs, although the ubiquitous expression of PARs, and their central role in platelet aggregation, coagulation and inflammation makes clear interpretation of findings difficult. The development of potent, subtype-selective, small-molecule ligands for PARs will provide valuable information on the roles of these receptors, and may help clarify current inconsistencies in the airway PAR literature.
Potential role of PARs in asthma
Asthma is a chronic inflammatory airway disease, characterized by shortness of breath and repeated wheezing episodes (Masoli et al., 2004; Hamid and Tulic, 2009). Allergic asthma involves an inappropriately large immune response to one or more inhaled allergens (Busse and Lemanske, 2001). In asthmatic individuals, otherwise innocuous stimuli trigger a Th2 cell-driven immune response frequently involving antigen-specific IgE production, release of mast cell-derived mediators and recruitment of eosinophils to the airways. Allergen-induced inflammation is typically associated with acute bronchoconstriction, airway hyperresponsiveness and eventually, airway remodelling. The current mainstay of asthma treatment remains glucocorticoids as a preventative medication, with short- or long-acting β2-adrenoceptor agonists as a reliever from acute attacks (Adcock et al., 2008). Recently, newer medications such as anti-leukotrienes and IgE inhibitors have been used in the clinical management of asthma (Holgate and Polosa, 2008). While many of the current medications are useful in managing the clinical symptoms of asthma, they are not curative and the search for better asthma treatments remains an important focus.
Increased immunization, antibiotic use, altered diet and decreased pathogen exposure contribute to an immune system that is more susceptible to developing allergies (Strachan, 1989; Anderson et al., 2001; Kaiser, 2004; Devereux, 2006). Of the immune cells involved in allergic asthma, the CD4+ T cells appear to play a central role in the development and maintenance of allergic sensitization (Fischer et al., 2007; Anderson, 2008; Burchell et al., 2008; Pucci and Incorvaia, 2008). CD4+ T cells can be broadly categorized into four subsets, namely, T helper type 1 (Th1), Th2, Th17 and regulatory T cells (Treg). Subtype selection of undifferentiated T cells (Th0) is controlled by the cytokine signals present upon stimulation of the Th0 cell (Murphy and Reiner, 2002; Curiel, 2007; McGeachy and Cua, 2008; Korn et al., 2009). These cells serve varied and distinct functions in immune responses (Broide, 2008). Th1 cells are primarily responsible for coordinating the immune response to intracellular infections such as viruses (Murphy and Reiner, 2002). Th2 cells are primarily involved in the destruction of extracellular pathogens such as helminth parasites (Fallon and Mangan, 2007). Th17 cells have not been studied as extensively due to their recent discovery; however, an increasing body of evidence suggests their involvement in asthma and allergy (Infante-Duarte et al., 2000; Korn et al., 2008; Lochner et al., 2008; McKinley et al., 2008; Oboki et al., 2008). Treg cells suppress inflammation and are involved in maintaining peripheral tolerance (Cohn, 2008; Vignali et al., 2008). A polarization towards Th2-type responses appears central to the pathogenesis of asthma (Salvi et al., 2001; O'Byrne et al., 2004; Strickland et al., 2006; Galli et al., 2008; Oboki et al., 2008; Pucci and Incorvaia, 2008; Broide, 2009; Hamid and Tulic, 2009). While recent evidence suggests that Th2 cells are not the only T helper lineage involved in allergic asthma, they play a significant role and therefore, inhibiting their actions may prove useful in asthma (Holt et al., 2005; Fischer et al., 2007; Holt and Sly, 2007; Caramori et al., 2008; Schmidt-Weber, 2008). Very few controlled clinical trials have determined the effect of currently used medications on Th2 cell responses (Caramori et al., 2008).
PAR activation may alter CD4+ cell polarization in the airways and inhibit allergic inflammation through a variety of mechanisms, including via production of PGE2. While PGE2 favours Th1 differentiation in vitro, the converse appears to be true in vivo (Betz and Fox, 1991; Snijdewint et al., 1993; Martin et al., 2002; Nagamachi et al., 2007). Administering PGE2in vivo appears to inhibit Th2 activation and cytokine expression (Martin et al., 2002), and inhibits T cell proliferation through EP2 receptors (Nataraj et al., 2001). PGE2 also inhibits transendothelial migration of lymphocytes into the airways, likely via increased intracellular cAMP (Pober et al., 1993; Oppenheimer-Marks et al., 1994; Panettieri et al., 1995). Consistent with this, mice deficient in COX-1 or COX-2 show increased inflammation upon allergen challenge, and inhibitors of COX-1 or COX-2 produce similar effects (Gavett et al., 1999; Stokes Peebles et al., 2002; Carey et al., 2003). Epidemiological studies have also shown that frequent use of COX inhibitors may increase the risk of developing asthma and allergy (Allmers, 2005). Furthermore, a recent study has shown that exposing mice to indomethacin during allergic sensitization increases primary and memory Th2 responses in vivo (Zhou et al., 2008). Thus, the activation and up-regulation of COX may well increase prostaglandin levels in the lung, thereby reducing Th2 polarization and inflammation.
Elevating PGI2 levels represents another distinct pathway by which PARs could alter T helper cell polarization in the airways. Activation of endothelial PAR1- and PAR2-APs promotes PGI2 release (Syeda et al., 2006), which may alter Th2 immune function by stimulating Th2 cells to release IL-10, an anti-inflammatory cytokine that serves to suppress immune responses (Jaffar et al., 2002). Additionally, PGI2 markedly inhibits CCL17-induced chemotaxis of Th2 cells, perhaps by inhibiting CCR4 and/or CCR8 signalling, resulting in fewer Th2 cells being recruited to the airways following allergen challenge (Jaffar et al., 2007). Furthermore, pre-treating Th2 cells with PGI2 before adoptive transfer markedly decreased inflammation in mice following allergen challenge (Jaffar et al., 2007). Interestingly, Th2 cells exhibit increased expression of prostanoid IP receptors compared with Th1 cells. This allows for selective inhibition of Th2 cells by PGI2, which may reduce the ratio of Th2 cells present in the airways (Jaffar et al., 2002). Thus, PAR-mediated production of PGI2 may selectively inhibit Th2 immune responses.
IP receptor-deficient mice exhibit increased eosinophil, lymphocyte and neutrophil influx in an allergen challenge model. These IP-null mice also had increased total and antigen-specific serum levels of IgE as well as total IgG. Allergen challenge of isolated spleenocytes from these IP-null mice resulted in increased IL-4 compared with wild-type mice (Takahashi et al., 2002). These findings are supported by studies using an IP receptor agonist, iloprost, in an allergen challenge model. Iloprost decreased allergen-induced BAL fluid levels of eosinophils, lymphocytes, IL-4, IL-5 and IL-13; and these effects were abolished by an IP receptor antagonist. Additionally, iloprost inhibited allergen-induced increases in airway resistance, as well as decreases in compliance (Idzko et al., 2007). Iloprost also altered dendritic cell (DC) function. DCs incubated with iloprost and then adoptively transferred to mice showed markedly decreased Th2-like responses upon subsequent allergen challenge compared with vehicle-treated DCs. Iloprost treatment of DCs prior to adoptive transfer inhibited allergen-induced BAL numbers of macrophages, lymphocytes and eosinophils; as well as inhibiting IL-4, IL-5 and IL-13; and increasing IL-10 and IFN-γ production (Idzko et al., 2007). Thus, PGI2 decreases Th2 cell mediator release, DC induction of Th2 cell differentiation and allergen-induced cell influx. Additionally, PGI2 increases levels of the anti-inflammatory cytokine IL-10. Thus, agents capable of increasing airway PGI2 levels, such as PARs, may prove to be useful in the treatment of asthma.
In addition to inhibition of Th2 cells, it is likely that induction of Treg would reduce airway inflammation in allergic asthma (McGee and Agrawal, 2006; Bohle et al., 2007; Adcock et al., 2008; Burchell et al., 2008; Jin et al., 2008). In this context, PAR2 activation induced the maturation of immature murine DCs (iDCs) into mature DCs (mDCs) (Fields et al., 2003). In addition, recent evidence suggests that PGE2 may induce immune tolerance via induction of Treg (Li et al., 2008; Muthuswamy et al., 2008). Pulmonary iDCs exposed to PGE2 during maturation resulted in mDCs that attracted Treg with increased affinity (Muthuswamy et al., 2008). This effect was mediated by PGE2-induced hypersecretion of CCL22, a proposed selective Treg chemokine (Curiel et al., 2004; Muthuswamy et al., 2008). IFN-α ablated this effect, suggesting that if viral infection were concomitant, an effective immune response would still develop (Muthuswamy et al., 2008). Neither PGE2 nor lipopolysaccharide (LPS) alone induced nearly as strong an effect; co-stimulation was necessary for hypersecretion of CCL22. This suggests a potential mechanism to explain the allergy resistance conferred by early life exposure to LPS (Cochran et al., 2002).
Potential role of PARs in neutrophilic inflammation
Elevated numbers of neutrophils are a characteristic feature of numerous inflammatory lung diseases, including chronic obstructive pulmonary disease (COPD) and acute respiratory distress syndrome (ARDS), as well as certain forms of asthma (Pettersen and Adler, 2002; Jeffery, 2004; Kamath et al., 2005; Cepkova and Matthay, 2006; Noma et al., 2008). Signals for the influx of neutrophils into the lung are likely to include LTB4 and IL-8, whose levels become elevated in response to inflammatory stimuli such as bacterial LPS. While the exact effects of LPS exposure in asthma remain to be elucidated, it appears to be involved in the development and severity of asthma (Michel, 2003). LPS exposure early in life may provide some protection against developing asthma; however, in established asthma, LPS exposure levels appear to be correlated with disease severity (Lapa e Silva et al., 2000; Cochran et al., 2002; Jung et al., 2006; Kim et al., 2007). Inhibiting neutrophil influx in asthmatics may be useful as neutrophils cause significant damage to the airways and are associated with severe and treatment-resistant forms of the disease (Delclaux et al., 1997; Pettersen and Adler, 2002). Additionally, ARDS and COPD are also characterized by intense neutrophilia and may benefit from agents that reduce neutrophil influx into the lung. In this context, activators of PAR2 reduced the airway neutrophilia associated with LPS exposure in mice (Moffatt et al., 2002; Saleh et al., 2008). PGE2 also inhibited LPS-induced neutrophilia, indicating this product as a likely mediator of PAR2-AP-induced inhibition of LPS-induced neutrophilia (Goncalves de Moraes et al., 1996; Saleh et al., 2008). Isolated bronchi from LPS-treated rats showed increased relaxation in response to PAR2-APs, as well as increased PGE2 release (Morello et al., 2005). Thus PAR2-mediated reductions in LPS-induced neutrophilia may be mediated by PGE2; however, this remains to be shown experimentally. As effective medications for both ARDS and COPD are lacking, exploration of the possible benefits of novel therapeutic strategies such as activators of PARs is opportune.
Respiratory tract virus-induced exacerbations of asthma are typically associated with airway neutrophilia (Dougherty and Fahy, 2009). PAR activators can modulate host responses to respiratory tract viral infection, although the role of prostaglandins in these responses is unknown. For example, a recent study using cultured human monocytes revealed that PAR2-APs were able to increase IFN-γ-induced effects, resulting in lower titres of influenza A virus, indicating a potentially protective role of PAR2 activation during the progression of influenza A virus infection (Feld et al., 2008). There are no published reports of the in vivo effects of PAR activation on the time-course of a respiratory tract viral infection. Nevertheless, influenza A virus infection in mice has been associated with elevated epithelial PAR expression, and augmented PAR2-mediated inhibition of methacholine-induced bronchoconstriction (Lan et al., 2004). In non-respiratory systems, PAR1-APs have been shown to increase the viral infectivity of herpes simplex virus in human foreskin fibroblasts and human umbilical vein endothelial cells; however, neither PAR2 nor PAR4-APs increased viral infectivity (Sutherland et al., 2007).
Changes in the levels or activity of the enzymes involved in the synthesis of prostaglandins can alter the host response to respiratory tract viral infection. Carey and coworkers (2005) revealed that deficiency of COX-1 is associated with an enhanced inflammatory response and earlier increases in the levels of the pro-inflammatory cytokines TNF-α and IL-1β (Carey et al., 2005). In contrast, deficiency of COX-2 resulted in reduced inflammation and pro-inflammatory cytokine release, reduced morbidity and enhanced survival (Carey et al., 2005). Thus, COX-1 and COX-2 appear to exert important but contrasting effects on the host immune response to influenza viral infection, which may be due to altered production of prostaglandins and leukotrienes. In a related study, mice that overexpress PGI2 synthase selectively in bronchial epithelium had less respiratory syncytial virus-induced illness (Hashimoto et al., 2004). In contrast, IP receptor-null mice showed increased mortality, weight loss and viral titers (Hashimoto et al., 2004). This is consistent with earlier reports that exogenous PGI2 administration greatly enhanced survival of mice exposed to viral infection and interestingly, this effect was reduced by COX inhibition (Zavagno et al., 1987). As PARs increase prostaglandin production, more research is justified to examine the in vivo effects of PAR activators during viral infection of the lungs.
Potential role of PARs in pulmonary fibrosis
Pulmonary fibrosis is a characteristic pathologic feature of many lung diseases, including asthma, ARDS, COPD and idiopathic pulmonary fibrosis (Wynn, 2007). Fibrosis is difficult to reverse pharmacologically and is a major factor in the morbidity and mortality associated with these lung diseases (Rogliani et al., 2008). Pulmonary fibrosis is a complex process involving varying extents of epithelial and endothelial injury, a state of hypercoagulation, fibroblast activation and differentiation, epithelial-mesenchymal transition, fibrocyte recruitment, extracellular matrix deposition, angiogenesis and aberrant repair mechanisms (Scotton and Chambers, 2007). A key cell-type involved in extracellular matrix deposition is the myofibroblast, whose numbers are elevated in fibrotic disease due to increased proliferation and decreased apoptosis. IL-4, IL-5 and IL-13 are important cytokines in pulmonary fibrosis as they induce release of active transforming growth factor-β (TGF-β), a potent fibrotic agent, capable of causing fibroblast proliferation and inflammatory cell recruitment through MCP-1 activation of CCR2 (Strutz, 2001; Szardening-Kirchner et al., 2008). Along with these potent fibrotic effects, however, TGF-β is also involved in Treg cell differentiation (Huber and Schramm, 2006; Wahl, 2007; Chen et al., 2008). Treg cells release IL-10, which suppresses inflammation and inhibits fibrosis, making their induction an attractive target for the treatment of fibrosis (Holsti et al., 2004; Nakagome et al., 2006; Couper et al., 2008). The pathogenesis, aetiology and regulation of pulmonary fibrosis have recently been expertly reviewed (Wilson and Wynn, 2009). Here we will focus on the role of PARs and prostaglandins in pulmonary fibrosis.
Activation of the coagulation cascade, with the resultant generation of coagulation proteases such as thrombin, plays a central role in acute and chronic phases of fibrotic lung disease. For example, continuous infusion of a direct thrombin inhibitor significantly reduced lung collagen accumulation in a bleomycin model of pulmonary fibrosis (Howell et al., 2002). A prominent role for PAR1 in this model was subsequently established in studies showing that gene knockout of PAR1 was protective against bleomycin-induced fibrosis (Howell et al., 2005). It is not entirely clear how thrombin promotes fibrosis, although it induces PAR1-dependent fibroblast to myofibroblast proliferation dependent upon PKC-α (Bogatkevich et al., 2001), and inhibits apoptosis of fibroblasts through a PKC-ε-dependent mechanism (Bogatkevich et al., 2005). Recent studies indicate that PAR4 may also play a profibotic role. Stimulation of epithelial PAR4 with thrombin or a PAR4 AP-induced epithelial-mesenchymal transition, as evidenced by changes in cell morphology and changes in the expression of epithelial (e-cadherin) and myofibroblast (α-smooth muscle actin) markers (Ando et al., 2007). In contrast, there is evidence that PAR4 may suppress pulmonary fibrosis by countering PAR1-stimulated proliferation of fibroblasts. In these studies, exposure of human primary bronchial fibroblasts to pro-inflammatory stimuli induced expression of functional PAR4 on fibroblasts (Ramachandran et al., 2007). In these TNF-α-stimulated fibroblasts, thrombin no longer induced proliferation, and a PAR4-AP caused a reduction in fibroblast cell number (Ramachandran et al., 2007). In this setting, a specific PAR1-AP retained its mitogenic effects, indicating that thrombin activation of PAR4 appears to suppress thrombin-mediated PAR1 signalling (Ramachandran et al., 2007). Furthermore, induction of PAR4 expression enables cathespin G signalling, a proteinase that silences PAR1 and PAR2 but activates PAR4. Interestingly, in TNF-α-treated fibroblasts, tryptase silenced PAR2, rather than activating it (Ramachandran et al., 2007).
Intratracheal administration of APC, a coagulation cascade inhibitory protein, is protective in bleomycin-induced pulmonary fibrosis (Yasui et al., 2001). This is of particular interest because APC can activate PAR1 via its coreceptor, the endothelial cell protein C receptor (Riewald et al., 2002). Furthermore, this group has introduced the concept that when activating PAR1, APC can stimulate signalling pathways distinct from those activated by thrombin, that is, the paradoxical condition that these two key coagulation proteases can mediate opposite effects on endothelial biology through the same receptor, PAR1 (Riewald and Ruf 2005; Schuepbach et al., 2008). Thus, stimulation of PAR1 could inhibit or enhance fibrotic effects, depending on the method of activation.
While PAR1 activation is typically associated with the development of fibrosis, the prostanoids PGE2 and PGI2 are potent anti-fibrotic agents. Suppression of COX activity increases bleomycin-induced fibrosis in mice (Keerthisingam et al., 2001; Bonner et al., 2002), and CCR2-null mice are protected from bleomycin-induced fibrosis, due to increased PGE2 production from airway epithelial cells (Moore et al., 2001; 2003; Lama et al., 2002). EP2 receptor activation by PGE2 inhibits fibroblast proliferation and migration, transition to myofibroblast and collagen synthesis (Kolodsick et al., 2003; Moore et al., 2005; White et al., 2005). PGI2 also appears to be anti-fibrotic, with IP receptor-null mice being more susceptible to bleomycin-induced lung fibrosis (Lovgren et al., 2006). Interestingly, in these studies, E-prostanoid receptor-null and mPGES-1-null mice did not exhibit any increase in bleomycin-induced fibrosis (Lovgren et al., 2006). Consistent with this, no increase in bleomycin-induced fibrosis was observed in COX-2-null mice, despite lung dysfunction (Card et al., 2007).
PAR activation induces PGE2 release from fibroblasts, which down-regulate PAR1 expression on these cells via a negative feedback loop (Sokolova et al., 2005; 2008;). PAR2-mediated generation of PGE2 by airway epithelial cells (Lan et al., 2001) may also suppress fibroblast PAR1 expression. Together, these findings indicate that PAR-mediated PGE2 production within the airways may inhibit fibrosis through a number of mechanisms – indirectly via PGE2-mediated suppression of fibroblast PAR1 expression and directly by inhibiting fibroblast function as described above.
In summary, it appears that antagonists of PAR1 may yield effective therapies in fibrosis. The role of other PAR subtypes is less clear with preliminary data suggesting that PAR2-mediated generation of PGE2 and PGI2 inhibits fibrosis. Thus, a combination of PAR1 antagonists, PAR2-APs and protease inhibitors may be beneficial in suppressing fibrosis, although the specific pharmacological agents required to fully test this hypothesis are still in the development stage.
PAR interactions with respiratory system pharmacotherapies
Glucocorticoids are the mainstay therapy for inflammatory airway diseases and are likely to remain so for the foreseeable future. Thus, it is necessary for any new medications to maintain therapeutic activity when co-administered with glucocorticoids. As glucocorticoids suppress many of the pathways of PGE2 production, the interaction between dexamethasone and PAR2-APs has been investigated. While dexamethasone suppressed SLIGRL-induced increases in PGE2 in cell culture, long- or short-term dexamethasone did not affect the PGE2-dependent, SLIGRL-induced relaxation of isolated tracheal preparations (Saleh et al., 2008). Furthermore, dexamethasone pre-treatment did not ablate PAR2-AP-induced reductions in LPS-induced neutrophilia in mice (Saleh et al., 2008). Interestingly, glucocorticoids were able to suppress PAR2-mediated MMP-9 release from epithelial cells in vitro; however, this effect has not been examined in vivo (Vliagoftis et al., 2000). This raises the possibility that glucocorticoids may be able to suppress PAR-mediated inflammatory pathways, but not anti-inflammatory pathways, although additional in vivo studies are required to confirm this. Although the interaction between PAR2-APs and glucocorticoids has not been evaluated in other models of airway inflammation, these findings indicate that agents capable of increasing endogenous PGE2 production are likely to retain their effectiveness in the presence of glucocorticoids.
Other therapeutic agents also modulate PAR-mediated PGE2 production in the airways. Inhibition of prostaglandin metabolism by the thiazolidinedione compound rosiglitazone (Cho and Tai, 2002) augmented PAR2-mediated increases in PGE2 levels and relaxation of isolated tracheal preparations (Henry et al., 2005). Whether these effects persist in vivo is unknown, but warrants further investigation. Rosiglitazone is more widely recognized as an activator of PPAR-γ, and this class of drug alters a variety of inflammatory processes within the airways (Belvisi et al., 2006; Ward and Tan, 2007). Further studies are required to investigate the potentially useful anti-inflammatory effects produced by combinations of glucocorticoids, PARs and PPAR-γ agonists (Belvisi et al., 2006; Usami et al., 2006).
Conclusions
While PARs are capable of inducing a wide range of inflammatory processes and cytokines within the lung, PAR-mediated prostaglandin production represents a distinct anti-inflammatory pathway. In the case of allergic inflammation, activation of PARs on epithelial cells causes PGE2 production, resulting in lowered inflammatory cell recruitment and airway hyperresponsiveness, with PAR2 being the most prevalent inducer of PGE2 release (De Campo and Henry, 2005). Thus, inhaled agonists of PAR2 may prove to be useful agents in the treatment of allergic airway inflammation. Additionally, the inhibition of neutrophil recruitment by PAR2 is also likely to be prostaglandin-dependent. Coupled to their other anti-inflammatory properties, this effect would likely prove useful in the treatment of inflammatory airway diseases characterized by intense neutrophilia, such as ARDS, COPD and some forms of asthma (Moffatt et al., 2002). Additionally, in vivo retention of anti-inflammatory effects upon concomitant glucocorticoid treatment makes PAR-mediated prostaglandin production an even more attractive target for the development of inflammatory airway disease treatments (Saleh et al., 2008). Further investigation is therefore justified to determine whether PAR-mediated anti-inflammatory effects are retained in other models of airway inflammation in vivo. Additionally, the emerging role of PGI2 in lung inflammation and its production by PARs warrant further investigation.
Acknowledgments
The authors wish to acknowledge the financial support of the National Health and Medical Research Council (Australia).
Glossary
Abbreviations:
- AC
adenylate cyclase
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- COX
cyclooxygenase
- DC
dendritic cell
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- mPGES
microsomal prostaglandin E synthase
- NK
neurokinin
- PAR
protease-activated receptor
- PAR-AP
PAR-activating peptide
- PGE2
prostglandin E2
- PGI2
prostglandin I2
- PGT
prostaglandin transporter
- TGF-β
transforming growth factor-β
- TNF-α
tumour necrosis factor-α
- VCAM
vascular cell adhesion molecule
References
- Abey HT, Fairlie DP, Moffatt JD, Balzary RW, Cocks TM. Protease-activated receptor-2 peptides activate neurokinin-1 receptors in the mouse isolated trachea. J Pharmacol Exp Ther. 2006;317:598–605. doi: 10.1124/jpet.105.097121. [DOI] [PubMed] [Google Scholar]
- Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. The Lancet. 2008;372:1073–1087. doi: 10.1016/S0140-6736(08)61449-X. [DOI] [PubMed] [Google Scholar]
- Ahn H, Foster C, Boykow G, Stamford A, Manna M, Graziano M. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-{[4-(1-methylethyl)phenyl]methyl}-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem Pharmacol. 2000;60:1425–1434. doi: 10.1016/s0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmers H. Frequent acetaminophen use and allergic diseases: is the association clear? J Allergy Clin Immunol. 2005;116:859–862. doi: 10.1016/j.jaci.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DW, Torphy TJ, et al. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells. Modulation by cyclic adenosine monophosphate. Am J Respir Cell Mol Biol. 2000;23:794–802. doi: 10.1165/ajrcmb.23.6.4184. [DOI] [PubMed] [Google Scholar]
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- Anderson WJA, Watson L, Lemanske RF, Jr, Busse WW. Asthma and the Hygiene Hypothesis. N Engl J Med. 2001;344:1643–1644. doi: 10.1056/NEJM200105243442116. [DOI] [PubMed] [Google Scholar]
- Ando S, Otani H, Yagi Y, Kawai K, Araki H, Fukuhara S, et al. Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir Res. 2007;8:31. doi: 10.1186/1465-9921-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA. Investigation of the inhibitory effects of PGE2 and selective EP agonists on chemotaxis of human neutrophils. Br J Pharmacol. 1995;116:2903–2908. doi: 10.1111/j.1476-5381.1995.tb15943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, et al. Activation of Protease-Activated Receptor (PAR)-1, PAR-2, and PAR-4 Stimulates IL-6, IL-8, and Prostaglandin E2 Release from Human Respiratory Epithelial Cells. J Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- Baratelli F, Krysan K, Heuze-Vourc'h N, Zhu L, Escuadro B, Sharma S, et al. PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. J Leukoc Biol. 2005a;78:555–564. doi: 10.1189/jlb.1004569. [DOI] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005b;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006;533:101–109. doi: 10.1016/j.ejphar.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Benbernou N, Esnault S, Shin HC, Fekkar H, Guenounou M. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997;91:361–368. doi: 10.1046/j.1365-2567.1997.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A, Fronius M, Clauss W, Schnizler M. Prostaglandin E2 induces upregulation of Na+ transport across Xenopus lung epithelium. J Comp Physiol [B] 2004;174:83–89. doi: 10.1007/s00360-003-0391-3. [DOI] [PubMed] [Google Scholar]
- Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, et al. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986;77:700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- Bogatkevich GS, Gustilo E, Oates JC, Feghali-Bostwick C, Harley RA, Silver RM, et al. Distinct PKC isoforms mediate cell survival and DNA synthesis in thrombin-induced myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;288:L190–L201. doi: 10.1152/ajplung.00448.2003. [DOI] [PubMed] [Google Scholar]
- Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Bonin SR, Phillips PP, McCaffrey TV. The effect of arachidonic acid metabolites on the ciliary beat frequency of human nasal mucosa in vitro. Acta Otolaryngol. 1992;112:697–702. doi: 10.3109/00016489209137462. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, et al. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury D, Clarke D, Seedhouse C, Corbett L, Stocks J, Knox A. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J Biol Chem. 2005;280:29993–30000. doi: 10.1074/jbc.M414530200. [DOI] [PubMed] [Google Scholar]
- Brock TG, Serezani CH, Carstens JK, Peters-Golden M, Aronoff DM. Effects of prostaglandin E2 on the subcellular localization of Epac-1 and Rap1 proteins during Fcgamma-receptor-mediated phagocytosis in alveolar macrophages. Exp Cell Res. 2008;314:255–263. doi: 10.1016/j.yexcr.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide D. New perspectives on mechanisms underlying chronic allergic inflammation and asthma in 2007. J Allergy Clin Immunol. 2008;122:475–480. doi: 10.1016/j.jaci.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced Airway Hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol. 2008;296:L307–L319. doi: 10.1152/ajplung.00521.2007. [DOI] [PubMed] [Google Scholar]
- Burgess JK, Ge Q, Boustany S, Black JL, Johnson PR. Increased sensitivity of asthmatic airway smooth muscle cells to prostaglandin E2 might be mediated by increased numbers of E-prostanoid receptors. J Allergy Clin Immunol. 2004;113:876–881. doi: 10.1016/j.jaci.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Busse WW, Lemanske RF. Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- Canetti C, Serezani CH, Atrasz RG, White ES, Aronoff DM, Peters-Golden M. Activation of phosphatase and tensin homolog on chromosome 10 mediates the inhibition of FcgammaR phagocytosis by prostaglandin E2 in alveolar macrophages. J Immunol. 2007;179:8350–8356. doi: 10.4049/jimmunol.179.12.8350. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Hmieleski RR, Spannhake EW, Jakab GJ. Ozone reduces murine alveolar and peritoneal macrophage phagocytosis: the role of prostanoids. Am J Physiol. 1991;261:L277–L282. doi: 10.1152/ajplung.1991.261.4.L277. [DOI] [PubMed] [Google Scholar]
- Caramori G, Groneberg D, Ito K, Casolari P, Adcock I, Papi A. New drugs targeting Th2 lymphocytes in asthma. J Occup Med Toxicol. 2008;3:S6. doi: 10.1186/1745-6673-3-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JW, Voltz JW, Carey MA, Bradbury JA, DeGraff LM, Lih FB, et al. Cyclooxygenase-2 deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am J Respir Cell Mol Biol. 2007;37:300–308. doi: 10.1165/rcmb.2007-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, et al. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1-/- but not cyclooxygenase-2-/- mice. Am J Respir Crit Care Med. 2003;167:1509–1515. doi: 10.1164/rccm.200211-1383OC. [DOI] [PubMed] [Google Scholar]
- Carey MA, Bradbury JA, Seubert JM, Langenbach R, Zeldin DC, Germolec DR. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006;21:119–143. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers LS, Black JL, Ge Q, Carlin SM, Au WW, Poniris M, et al. PAR-2 activation, PGE2, and COX-2 in human asthmatic and nonasthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L619–L627. doi: 10.1152/ajplung.00416.2002. [DOI] [PubMed] [Google Scholar]
- Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Tai H-H. Thiazolidinediones as a novel class of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase inhibitors. Arch Biochem Biophys. 2002;405:247–251. doi: 10.1016/s0003-9861(02)00352-1. [DOI] [PubMed] [Google Scholar]
- Cho KN, Choi JY, Kim CH, Baek SJ, Chung KC, Moon UY, et al. Prostaglandin E2 induces MUC8 gene expression via a mechanism involving ERK MAPK/RSK1/cAMP response element binding protein activation in human airway epithelial cells. J Biol Chem. 2005;280:6676–6681. doi: 10.1074/jbc.M412722200. [DOI] [PubMed] [Google Scholar]
- Chow JM, Moffatt JD, Cocks TM. Effect of protease-activated receptor (PAR)-1, -2 and -4-activating peptides, thrombin and trypsin in rat isolated airways. Br J Pharmacol. 2000;131:1584–1591. doi: 10.1038/sj.bjp.0703738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, et al. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Giembycz MA, Patel HJ, Belvisi MG. E-ring 8-isoprostanes inhibit ACh release from parasympathetic nerves innervating guinea-pig trachea through agonism of prostanoid receptors of the EP3-subtype. Br J Pharmacol. 2004;141:600–609. doi: 10.1038/sj.bjp.0705648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DL, Belvisi MG, Smith SJ, Hardaker E, Yacoub MH, Meja KK, et al. Prostanoid receptor expression by human airway smooth muscle cells and regulation of the secretion of granulocyte colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2005;288:L238–L250. doi: 10.1152/ajplung.00313.2004. [DOI] [PubMed] [Google Scholar]
- Clasby MC, Chackalamannil S, Czarniecki M, Doller D, Eagen K, Greenlee WJ, et al. Himbacine derived thrombin receptor antagonists: discovery of a new tricyclic core. Bioorg Med Chem Lett. 2007;17:3647–3651. doi: 10.1016/j.bmcl.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Clayton A, Holland E, Pang L, Knox A. Interleukin-1beta differentially regulates beta2 adrenoreceptor and prostaglandin E2-mediated cAMP accumulation and chloride efflux from Calu-3 bronchial epithelial cells. Role of receptor changes, adenylyl cyclase, cyclo-oxygenase 2, and protein kinase A. J Biol Chem. 2005;280:23451–23463. doi: 10.1074/jbc.M502242200. [DOI] [PubMed] [Google Scholar]
- Cochran JR, Khan AM, Elidemir O, Xue H, Cua B, Fullmer J, et al. Influence of lipopolysaccharide exposure on airway function and allergic responses in developing mice. Pediatr Pulmonol. 2002;34:267–277. doi: 10.1002/ppul.10161. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- Cohn M. What roles do regulatory T cells play in the control of the adaptive immune response? Int Immunol. 2008;20:1107–1118. doi: 10.1093/intimm/dxn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–2652. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- Connolly AJ, Ishihara H, Kahn ML, Farese RV, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA. 2002a;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002b;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Regulatory T-cell development: is Foxp3 the decider? Nat Med. 2007;13:250–253. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- D'Agostino B, Roviezzo F, De Palma R, Terracciano S, De Nardo M, Gallelli L, et al. Activation of protease-activated receptor-2 reduces airways inflammation in experimental allergic asthma. Clin Exp Allergy. 2007;37:1436–1443. doi: 10.1111/j.1365-2222.2007.02793.x. [DOI] [PubMed] [Google Scholar]
- De Campo BA, Henry PJ. Stimulation of protease-activated receptor-2 inhibits airway eosinophilia, hyperresponsiveness and bronchoconstriction in a murine model of allergic inflammation. Br J Pharmacol. 2005;144:1100–1108. doi: 10.1038/sj.bjp.0706150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers IA, Rampart M, Bult H, Herman AG. Evidence for the involvement of prostaglandins in modulation of acetylcholine release from canine bronchial tissue. Eur J Pharmacol. 1989;167:415–418. doi: 10.1016/0014-2999(89)90451-2. [DOI] [PubMed] [Google Scholar]
- Delclaux C, Rezaiguia-Delclaux S, Delacourt C, Brun-Buisson C, Lafuma C, Harf A. Alveolar neutrophils in endotoxin-induced and bacteria-induced acute lung injury in rats. Am J Physiol. 1997;273:L104–L112. doi: 10.1152/ajplung.1997.273.1.L104. [DOI] [PubMed] [Google Scholar]
- Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury DE, Chong LK, Ghahramani P, Peachell PT. Influence of receptor reserve on beta-adrenoceptor-mediated responses in human lung mast cells. Br J Pharmacol. 1998;124:711–718. doi: 10.1038/sj.bjp.0701897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SM, Cruse G, Cockerill SL, Brightling CE, Bradding P. Engagement of the EP2 prostanoid receptor closes the K+ channel KCa3.1 in human lung mast cells and attenuates their migration. Eur J Immunol. 2008;38:2548–2556. doi: 10.1002/eji.200738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon S, Leduc D, Cottrell GS, D'Alayer J, Hansen KK, Bunnett NW, et al. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2005;32:411–419. doi: 10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- Dunkern TR, Feurstein D, Rossi GA, Sabatini F, Hatzelmann A. Inhibition of TGF-beta induced lung fibroblast to myofibroblast conversion by phosphodiesterase inhibiting drugs and activators of soluble guanylyl cyclase. Eur J Pharmacol. 2007;572:12–22. doi: 10.1016/j.ejphar.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H, Ng J. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–630. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. 2007;179:2910–2917. doi: 10.4049/jimmunol.179.5.2910. [DOI] [PubMed] [Google Scholar]
- Failla M, Genovese T, Mazzon E, Fruciano M, Fagone E, Gili E, et al. 16,16-dimethyl prostaglandin E2 efficacy on prevention and protection from bleomycin-induced lung injury and fibrosis. Am J Respir Cell Mol Biol. 2009;41:50–58. doi: 10.1165/rcmb.2007-0438OC. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- Fedyk ER, Phipps RP. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc Natl Acad Sci USA. 1996;93:10978–10983. doi: 10.1073/pnas.93.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld M, Shpacovitch VM, Ehrhardt C, Kerkhoff C, Hollenberg MD, Vergnolle N, et al. Agonists of proteinase-activated receptor-2 enhance IFN-{gamma}-inducible effects on human monocytes: role in influenza a infection. J Immunol. 2008;180:6903–6910. doi: 10.4049/jimmunol.180.10.6903. [DOI] [PubMed] [Google Scholar]
- Fields RC, Schoenecker JG, Hart JP, Hoffman MR, Pizzo SV, Lawson JH. Protease-activated receptor-2 signaling triggers dendritic cell development. Am J Pathol. 2003;162:1817–1822. doi: 10.1016/S0002-9440(10)64316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine A, Poliks CF, Donahue LP, Smith BD, Goldstein RH. The differential effect of prostaglandin E2 on transforming growth factor-beta and insulin-induced collagen formation in lung fibroblasts. J Biol Chem. 1989;264:16988–16991. [PubMed] [Google Scholar]
- Fischer R, Tome D, McGhee JR, Boyaka PN. Th1 and Th2 cells are required for both eosinophil- and neutrophil-associated airway inflammatory responses in mice. Biochem Biophys Res Commun. 2007;357:44–49. doi: 10.1016/j.bbrc.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortner CN, Breyer RM, Paul RJ. EP2 receptors mediate airway relaxation to substance P, ATP, and PGE2. Am J Physiol Lung Cell Mol Physiol. 2001;281:L469–L474. doi: 10.1152/ajplung.2001.281.2.L469. [DOI] [PubMed] [Google Scholar]
- Franchi-Micheli S, Mazzetti L, Cantore M, Ciuffi M, Zilletti L, Failli P. Influence of resting tension on protease-activated receptor-mediated relaxation in guinea-pig tracheas. Pulm Pharmacol Ther. 2005;18:141–150. doi: 10.1016/j.pupt.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Ma J-N, Seitzberg JG, Knapp AE, Schiffer HH, Tabatabaei A, et al. Identification and characterization of novel small molecule Protease Activated Receptor 2 (PAR2) agonists. J Pharmacol Exp Ther. 2008;327:799–808. doi: 10.1124/jpet.108.142570. [DOI] [PubMed] [Google Scholar]
- Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, Garcia VE, et al. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–469. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau GM, Watson RM, O'Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, et al. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves de Moraes VL, Boris Vargaftig B, Lefort J, Meager A, Chignard M. Effect of cyclo-oxygenase inhibitors and modulators of cyclic AMP formation on lipopolysaccharide-induced neutrophil infiltration in mouse lung. Br J Pharmacol. 1996;117:1792–1796. doi: 10.1111/j.1476-5381.1996.tb15356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, et al. Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol. 2003;29:19–27. doi: 10.1165/rcmb.2002-0254OC. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Tulic M. Immunobiology of Asthma. Annual Review of Physiology. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- Hansen KK, Oikonomopoulou K, Baruch A, Ramachandran R, Beck P, Diamandis EP, et al. Proteinases as hormones: targets and mechanisms for proteolytic signaling. Biol Chem. 2008;389:971–982. doi: 10.1515/BC.2008.120. [DOI] [PubMed] [Google Scholar]
- Hartert TV, Dworski RT, Mellen BG, Oates JA, Murray JJ, Sheller JR. Prostaglandin E(2) decreases allergen-stimulated release of prostaglandin D(2) in airways of subjects with asthma. Am J Respir Crit Care Med. 2000;162:637–640. doi: 10.1164/ajrccm.162.2.9904038. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Graham BS, Geraci MW, FitzGerald GA, Egan K, Zhou W, et al. Signaling through the prostaglandin I2 receptor IP protects against respiratory syncytial virus-induced illness. J Virol. 2004;78:10303–10309. doi: 10.1128/JVI.78.19.10303-10309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori R, Shimizu S, Majima Y, Shimizu T. EP4 agonist inhibits lipopolysaccharide-induced mucus secretion in airway epithelial cells. Ann Otol Rhinol Laryngol. 2008;117:51–58. doi: 10.1177/000348940811700111. [DOI] [PubMed] [Google Scholar]
- Hauck RW, Schulz C, Schomig A, Hoffman RK, Panettieri RA., Jr alpha -Thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am J Physiol Lung Cell Mol Physiol. 1999;277:L22–L29. doi: 10.1152/ajplung.1999.277.1.L22. [DOI] [PubMed] [Google Scholar]
- Haxel BR, Schafer D, Klimek L, Mann WJ. Prostaglandin E2 activates the ciliary beat frequency of cultured human nasal mucosa via the second messenger cyclic adenosine monophosphate. Eur Arch Otorhinolaryngol. 2001;258:230–235. doi: 10.1007/s004050100339. [DOI] [PubMed] [Google Scholar]
- van Helden SF, Oud MM, Joosten B, Peterse N, Figdor CG, van Leeuwen FN. PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J Cell Sci. 2008;121:1096–1106. doi: 10.1242/jcs.020289. [DOI] [PubMed] [Google Scholar]
- Henry PJ. The protease-activated receptor2 (PAR2)-prostaglandin E2-prostanoid EP receptor axis: a potential bronchoprotective unit in the respiratory tract? Eur J Pharmacol. 2006;533:156–170. doi: 10.1016/j.ejphar.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Henry PJ, D'Aprile A, Self G, Hong T, Mann TS. Inhibitors of prostaglandin transport and metabolism augment protease-activated receptor-2-mediated increases in prostaglandin E2 levels and smooth muscle relaxation in mouse isolated trachea. J Pharmacol Exp Ther. 2005;314:995–1001. doi: 10.1124/jpet.105.086124. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E(2) enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med. 2000;162:528–533. doi: 10.1164/ajrccm.162.2.9910059. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M. Proteinase-activated receptor 4 (PAR4): activation and inhibition of rat platelet aggregation by PAR4-derived peptides. Can J Physiol Pharmacol. 2001;79:439–442. [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Sandhu S, Houle S, Vergnolle N. Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br J Pharmacol. 2004;143:443–454. doi: 10.1038/sj.bjp.0705946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsti MA, Chitnis T, Panzo RJ, Bronson RT, Yagita H, Sayegh MH, et al. Regulation of postsurgical fibrosis by the programmed death-1 inhibitory pathway. J Immunol. 2004;172:5774–5781. doi: 10.4049/jimmunol.172.9.5774. [DOI] [PubMed] [Google Scholar]
- Holt PG, Sly PD. Prevention of allergic respiratory disease in infants: current aspects and future perspectives. Curr Opin Allergy Clin Immunol. 2007;7:547–555. doi: 10.1097/ACI.0b013e3282f14a17. [DOI] [PubMed] [Google Scholar]
- Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Howell DC, Laurent GJ, Chambers RC. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem Soc Trans. 2002;30:211–216. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Howell DCJ, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, et al. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H-L, Sun C-C, Wang T-S, Yang C-M. PKC-[delta]/c-Src-mediated EGF receptor transactivation regulates thrombin-induced COX-2 expression and PGE2 production in rat vascular smooth muscle cells. Biochim Biophys Acta. 2008;1783:1563–1575. doi: 10.1016/j.bbamcr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L405–L413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39:482–489. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Schramm C. TGF-beta and CD4+CD25+ regulatory T cells. Front Biosci. 2006;11:1014–1023. doi: 10.2741/1859. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hodgsteden HC, et al. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest. 2007;117:464. doi: 10.1172/JCI28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th Cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Connolly AJ, Zeng D, Kahn ML, Wu Zheng Y, Timmons C, et al. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- Jaffar Z, Wan KS, Roberts K. A key role for prostaglandin I2 in limiting lung mucosal Th2, but not Th1, responses to inhaled allergen. J Immunol. 2002;169:5997–6004. doi: 10.4049/jimmunol.169.10.5997. [DOI] [PubMed] [Google Scholar]
- Jaffar Z, Ferrini ME, Buford MC, FitzGerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol. 2007;179:6193–6203. doi: 10.4049/jimmunol.179.9.6193. [DOI] [PubMed] [Google Scholar]
- Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–183. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- Jin H, Kang Y, Zhao L, Xiao C, Hu Y, She R, et al. Induction of adaptive T regulatory cells that suppress the allergic response by coimmunization of DNA and protein vaccines. J Immunol. 2008;180:5360–5372. doi: 10.4049/jimmunol.180.8.5360. [DOI] [PubMed] [Google Scholar]
- Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol. 2003;74:868–879. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- Joy AP, Cowley EA. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am J Respir Cell Mol Biol. 2008;38:143–152. doi: 10.1165/rcmb.2006-0295OC. [DOI] [PubMed] [Google Scholar]
- Jung YW, Schoeb TR, Weaver CT, Chaplin DD. Antigen and lipopolysaccharide play synergistic roles in the effector phase of airway inflammation in mice. Am J Pathol. 2006;168:1425–1434. doi: 10.2353/ajpath.2006.050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn ML, Hammes SR, Botka C, Coughlin SR. Gene and locus structure and chromosomal localization of the protease-activated receptor gene family. J Biol Chem. 1998a;273:23290–23296. doi: 10.1074/jbc.273.36.23290. [DOI] [PubMed] [Google Scholar]
- Kahn ML, Zheng Y-W, Huang W, Bigornia V, Zeng D, Moff S, et al. A dual thrombin receptor system for platelet activation. Nature. 1998b;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kaiser HB. Risk factors in allergy/asthma. Allergy Asthma Proc. 2004;25:7. [PubMed] [Google Scholar]
- Kamath AV, Pavord ID, Ruparelia PR, Chilvers ER. Is the neutrophil the key effector cell in severe asthma? Thorax. 2005;60:529–530. doi: 10.1136/thx.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Wallin RP, Ljunggren HG. cAMP-elevating agents suppress dendritic cell function. J Leukoc Biol. 2001;70:903–910. [PubMed] [Google Scholar]
- Kassel KM, Wyatt TA, Panettieri RA, Jr, Toews ML. Inhibition of human airway smooth muscle cell proliferation by beta 2-adrenergic receptors and cAMP is PKA independent: evidence for EPAC involvement. Am J Physiol Lung Cell Mol Physiol. 2008;294:L131–L138. doi: 10.1152/ajplung.00381.2007. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng Y-W, Cheng A, et al. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224–3231. doi: 10.1182/blood-2003-04-1130. [DOI] [PubMed] [Google Scholar]
- Kaur M, Holden NS, Wilson SM, Sukkar MB, Chung KF, Barnes PJ, et al. Effect of beta2-adrenoceptor agonists and other cAMP-elevating agents on inflammatory gene expression in human ASM cells: a role for protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2008;295:L505–L514. doi: 10.1152/ajplung.00046.2008. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kawao N. Physiology and pathophysiology of proteinase-activated receptors (PARs): PARs in the respiratory system: cellular signaling and physiological/pathological roles.[see comment] J Pharmacol Sci. 2005;97:20–24. doi: 10.1254/jphs.fmj04005x4. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kanke T, Yonezawa D, Ishiki T, Saka M, Kabeya M, et al. Potent and metabolically stable agonists for protease-activated receptor-2: evaluation of activity in multiple assay systems in vitro and in vivo. J Pharmacol Exp Ther. 2004a;309:1098–1107. doi: 10.1124/jpet.103.061010. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kubo S, Ishiki T, Kawao N, Sekiguchi F, Kuroda R, et al. Proteinase-activated receptor-2-mediated relaxation in mouse tracheal and bronchial smooth muscle: signal transduction mechanisms and distinct agonist sensitivity. J Pharmacol Exp Ther. 2004b;311:402–410. doi: 10.1124/jpet.104.068387. [DOI] [PubMed] [Google Scholar]
- Kawao N, Nagataki M, Nagasawa K, Kubo S, Cushing K, Wada T, et al. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin E2 formation in human lung epithelial cells. J Pharmacol Exp Ther. 2005;315:576–589. doi: 10.1124/jpet.105.089490. [DOI] [PubMed] [Google Scholar]
- Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, et al. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-{beta} in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–1024. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- Kim Y-K, Oh S-Y, Jeon SG, Park H-W, Lee S-Y, Chun E-Y, et al. Airway exposure levels of lipopolysaccharide determine type 1 versus Type 2 experimental asthma. J Immunol. 2007;178:5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, et al. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- Kook Kim J, Hoon Kim C, Kim K, Jong Jang H, Jik Kim H, Yoon JH. Effects of prostagladin E(2) on gel-forming mucin secretion in normal human nasal epithelial cells. Acta Otolaryngol. 2006;126:174–179. doi: 10.1080/00016480500280033. [DOI] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kubo S, Takahashi HK, Takei M, Iwagaki H, Yoshino T, Tanaka N, et al. E-prostanoid (EP)2/EP4 receptor-dependent maturation of human monocyte-derived dendritic cells and induction of helper T2 polarization. J Pharmacol Exp Ther. 2004;309:1213–1220. doi: 10.1124/jpet.103.062646. [DOI] [PubMed] [Google Scholar]
- Kuliopulos A, Covic L. Blocking receptors on the inside: pepducin-based intervention of PAR signaling and thrombosis. Life Sciences. 2003;74:255–262. doi: 10.1016/j.lfs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Kwong K, Lee L-Y. PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol. 2002;93:1419–1428. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- Kwong K, Lee LY. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol. 2005;564:437–450. doi: 10.1113/jphysiol.2004.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27:752–758. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- Lan RS, Stewart GA, Henry PJ. Modulation of airway smooth muscle tone by protease activated receptor-1,-2,-3 and -4 in trachea isolated from influenza A virus-infected mice. Br J Pharmacol. 2000;129:63–70. doi: 10.1038/sj.bjp.0703007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan RS, Knight DA, Stewart GA, Henry PJ. Role of PGE(2) in protease-activated receptor-1, -2 and -4 mediated relaxation in the mouse isolated trachea. Br J Pharmacol. 2001;132:93–100. doi: 10.1038/sj.bjp.0703776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan RS, Stewart GA, Henry PJ. Role of protease-activated receptors in airway function: a target for therapeutic intervention? Pharmacol Ther. 2002;95:239–257. doi: 10.1016/s0163-7258(02)00237-1. [DOI] [PubMed] [Google Scholar]
- Lan RS, Stewart GA, Goldie RG, Henry PJ. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L388–L398. doi: 10.1152/ajplung.00286.2003. [DOI] [PubMed] [Google Scholar]
- Lapa e Silva JR, Possebon da Silva MD, Lefort J, Vargaftig BB. Endotoxins, asthma, and allergic immune responses. Toxicology. 2000;152:31–35. doi: 10.1016/s0300-483x(00)00289-4. [DOI] [PubMed] [Google Scholar]
- Lazzeri N, Belvisi MG, Patel HJ, Chung KF, Yacoub MH, Mitchell JA. RANTES release by human airway smooth muscle: effects of prostaglandin E(2) and fenoterol. Eur J Pharmacol. 2001;433:231–235. doi: 10.1016/s0014-2999(01)01520-5. [DOI] [PubMed] [Google Scholar]
- Lee SP, Serezani CH, Medeiros AI, Ballinger MN, Peters-Golden M. Crosstalk between prostaglandin E2 and leukotriene B4 regulates phagocytosis in alveolar macrophages via combinatorial effects on cyclic AMP. J Immunol. 2009;182:530–537. doi: 10.4049/jimmunol.182.1.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol. 2008;38:2751–2761. doi: 10.1002/eji.200838542. [DOI] [PubMed] [Google Scholar]
- Li T, He S. Induction of IL-6 release from human T cells by PAR-1 and PAR-2 agonists. Immunol Cell Biol. 2006;84:461–466. doi: 10.1111/j.1440-1711.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Farley JM., Sr Prostaglandin E2 enhances acetylcholine-induced, Ca2+-dependent ionic currents in swine tracheal mucous gland cells. J Pharmacol Exp Ther. 2007;322:501–513. doi: 10.1124/jpet.107.120154. [DOI] [PubMed] [Google Scholar]
- Liu H, Mamoon AM, Farley JM., Sr Prostanoids secreted by alveolar macrophages enhance ionic currents in swine tracheal submucosal gland cells. J Pharmacol Exp Ther. 2005;315:729–739. doi: 10.1124/jpet.105.088542. [DOI] [PubMed] [Google Scholar]
- Liu X, Ostrom RS, Insel PA. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol. 2004;286:C1089–C1099. doi: 10.1152/ajpcell.00461.2003. [DOI] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ ROR{gamma}t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew D, Perrault C, Morales M, Moog S, Ravanat C, Schuhler S, et al. Proteolysis of the exodomain of recombinant protease-activated receptors: prediction of receptor activation or inactivation by MALDI mass spectrometry. Biochemistry. 2000;39:10812–10822. doi: 10.1021/bi0003341. [DOI] [PubMed] [Google Scholar]
- Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, FitzGerald GA, et al. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L144–L156. doi: 10.1152/ajplung.00492.2005. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- McGee H, Agrawal D. TH2 cells in the pathogenesis of airway remodeling: regulatory T cells a plausible panacea for asthma. Immunol Res. 2006;35:219–231. doi: 10.1385/IR:35:3:219. [DOI] [PubMed] [Google Scholar]
- McKinley L, Alcorn JF, Peterson A, DuPont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci USA. 2007;104:5662. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino PA, Rooney SA. Surfactant secretion in a newborn rabbit lung slice model. Biochimica et Biophysica Acta. 1980;620:509–519. doi: 10.1016/0005-2760(80)90143-5. [DOI] [PubMed] [Google Scholar]
- Martin JG, Suzuki M, Maghni K, Pantano R, Ramos-Barbon D, Ihaku D, et al. The immunomodulatory actions of prostaglandin E2 on allergic airway responses in the rat. J Immunol. 2002;169:3963–3969. doi: 10.4049/jimmunol.169.7.3963. [DOI] [PubMed] [Google Scholar]
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- Mathe AA, Hedqvist P. Effect of prostaglandins F2 alpha and E2 on airway conductance in healthy subjects and asthmatic patients. Am Rev Respir Dis. 1975;111:313–320. doi: 10.1164/arrd.1975.111.3.313. [DOI] [PubMed] [Google Scholar]
- Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo E, Woolley KL, Manning PJ, Watson RM, O'Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med. 1994;149:1138–1141. doi: 10.1164/ajrccm.149.5.8173753. [DOI] [PubMed] [Google Scholar]
- Menard G, Turmel V, Bissonnette EY. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol. 2007;150:340–348. doi: 10.1111/j.1365-2249.2007.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel O. Role of lipopolysaccharide (LPS) in asthma and other pulmonary conditions. J Endotoxin Res. 2003;9:293–300. doi: 10.1179/096805103225002539. [DOI] [PubMed] [Google Scholar]
- Miike S, McWilliam AS, Kita H. Trypsin Induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- Miotto D, Hollenberg MD, Bunnett NW, Papi A, Braccioni F, Boschetto P, et al. Expression of protease activated receptor-2 (PAR-2) in central airways of smokers and non-smokers. Thorax. 2002;57:146–151. doi: 10.1136/thorax.57.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JD, Jeffrey KL, Cocks TM. Protease-activated receptor-2 activating peptide SLIGRL inhibits bacterial lipopolysaccharide-induced recruitment of polymorphonuclear leukocytes into the airways of mice. Am J Respir Cell Mol Biol. 2002;26:680–684. doi: 10.1165/ajrcmb.26.6.4693. [DOI] [PubMed] [Google Scholar]
- Molino M, Woolkalis MJ, Reavey-Cantwell J, Pratico D, Andrade-Gordon P, Barnathan ES, et al. Endothelial cell thrombin receptors and PAR-2. Two protease-activated receptors located in a single cellular environment. J Biol Chem. 1997;272:11133–11141. doi: 10.1074/jbc.272.17.11133. [DOI] [PubMed] [Google Scholar]
- Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, et al. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284:342–349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, et al. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174:5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- Morello S, Vellecco V, Roviezzo F, Maffia P, Cuzzocrea S, Cirino G, et al. A protective role for proteinase activated receptor 2 in airways of lipopolysaccharide-treated rats. Biochem Pharmacol. 2005;71:223–230. doi: 10.1016/j.bcp.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Morsy MAM, Isohama Y, Miyata T. Prostaglandin E2 increases surfactant secretion via the EP1 receptor in rat alveolar type II cells. Eur J Pharmacol. 2001;426:21–24. doi: 10.1016/s0014-2999(01)01211-0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Dutta-Roy AK, Fyfe GK, Olver RE, Kemp PJ. G protein-coupled prostaglandin receptor modulates conductive Na+ uptake in lung apical membrane vesicles. Am J Physiol. 1998;274:L567–L572. doi: 10.1152/ajplung.1998.274.4.L567. [DOI] [PubMed] [Google Scholar]
- Murn J, Alibert O, Wu N, Tendil S, Gidrol X. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205:3091–3103. doi: 10.1084/jem.20081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Muthuswamy R, Urban J, Lee J-J, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamachi M, Sakata D, Kabashima K, Furuyashiki T, Murata T, Segi-Nishida E, et al. Facilitation of Th1-mediated immune response by prostaglandin E receptor EP1. J Exp Med. 2007;204:2865–2874. doi: 10.1084/jem.20070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagataki M, Moriyuki K, Sekiguchi F, Kawabata A. Evidence that PAR2-triggered prostaglandin E2 formation involves the ERK-cytosolic phospholipase A2-COX-1-microsomal PGE synthase-1 cascade in human lung epithelial cells. Cell Biochem Funct. 2007;26:279–282. doi: 10.1002/cbf.1434. [DOI] [PubMed] [Google Scholar]
- Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki J, Yamamoto K. In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-{beta} in the lung. Thorax. 2006;61:886–894. doi: 10.1136/thx.2005.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Zheng Y-W, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Naldini A, Sower L, Bocci V, Meyers B, Carney DH. Thrombin receptor expression and responsiveness of human monocytic cells to thrombin is linked to interferon-induced cellular differentiation. J Cell Physiol. 1998;177:76–84. doi: 10.1002/(SICI)1097-4652(199810)177:1<76::AID-JCP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Naldini A, Pucci A, Carney DH, Fanetti G, Carraro F. Thrombin enhancement of interleukin-1 expression in mononuclear cells: involvement of proteinase-activated receptor-1. Cytokine. 2002;20:191–199. doi: 10.1006/cyto.2002.2001. [DOI] [PubMed] [Google Scholar]
- Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, et al. Receptors for prostaglandin E(2) that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y, Hosoki K, Nagao M, Tokuda R, Fujisawa T. Neutrophil proteases activate eosinophil function in vitro. J Allergy Clin Immunol. 2008;121:S45–S45. doi: 10.1159/000126055. [DOI] [PubMed] [Google Scholar]
- Norel X, Walch L, Labat C, Gascard J-P, Dulmet E, Brink C. Prostanoid receptors involved in the relaxation of human bronchial preparations. Br J Pharmacol. 1999;126:867–872. doi: 10.1038/sj.bjp.0702392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995a;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Larsson AK, Aberg H, Sundelin J. The mouse proteinase-activated receptor-2 cDNA and gene. Molecular cloning and functional expression. J Biol Chem. 1995b;270:5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Inman MD, Adelroth E. Reassessing the Th2 cytokine basis of asthma. Trends Pharmacol Sci. 2004;25:244–248. doi: 10.1016/j.tips.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008;57:121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N, Kavanaugh AF, Lipsky PE. Inhibition of the transendothelial migration of human T lymphocytes by prostaglandin E2. J Immunol. 1994;152:5703–5713. [PubMed] [Google Scholar]
- Ostrowska E, Reiser G. The protease-activated receptor-3 (PAR-3) can signal autonomously to induce interleukin-8 release. Cell Mol Life Sci. 2008;65:970–981. doi: 10.1007/s00018-008-7555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowska E, Sokolova E, Reiser G. PAR-2 activation and LPS synergistically enhance inflammatory signaling in airway epithelial cells by raising PAR expression level and interleukin-8 release. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1208–L1218. doi: 10.1152/ajplung.00137.2007. [DOI] [PubMed] [Google Scholar]
- Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J Allergy Clin Immunol. 2003;112:1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Palmer ML, Lee SY, Maniak PJ, Carlson D, Fahrenkrug SC, O'Grady SM. Protease-activated receptor regulation of Cl- secretion in Calu-3 cells requires prostaglandin release and CFTR activation. Am J Physiol Cell Physiol. 2006;290:C1189–C1198. doi: 10.1152/ajpcell.00464.2005. [DOI] [PubMed] [Google Scholar]
- Panettieri RA, Jr, Lazaar AL, Pure E, Albelda SM. Activation of cAMP-dependent pathways in human airway smooth muscle cells inhibits TNF-alpha-induced ICAM-1 and VCAM-1 expression and T lymphocyte adhesion. J Immunol. 1995;154:2358–2365. [PubMed] [Google Scholar]
- Pang L, Knox AJ. Effect of interleukin-1[beta], tumour necrosis factor-[alpha] and interferon-[gamma] on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br J Pharmacol. 1997;121:579–587. doi: 10.1038/sj.bjp.0701152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape R, Rauch BH, Rosenkranz AC, Kaber G, Schrör K. Transcriptional inhibition of protease-activated receptor-1 expression by prostacyclin in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:534–540. doi: 10.1161/ATVBAHA.107.159483. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am Rev Respir Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- Pelletier S, Dube J, Villeneuve A, Gobeil F, Jr, Yang Q, Battistini B, et al. Prostaglandin E(2) increases cyclic AMP and inhibits endothelin-1 production/secretion by guinea-pig tracheal epithelial cells through EP(4) receptors. Br J Pharmacol. 2001;132:999–1008. doi: 10.1038/sj.bjp.0703886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen CA, Adler KB. Airways inflammation and COPD*: epithelial-neutrophil interactions. Chest. 2002;121:142S–150. doi: 10.1378/chest.121.5_suppl.142s. [DOI] [PubMed] [Google Scholar]
- Pober JS, Slowik MR, De Luca LG, Ritchie AJ. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993;150:5114–5123. [PubMed] [Google Scholar]
- Pucci S, Incorvaia C. Allergy as an organ and a systemic disease. Clin Exp Immunol. 2008;153:1–2. doi: 10.1111/j.1365-2249.2008.03712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153:S263–S282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Morice AH, Compton SJ. Proteinase-activated receptor2 agonists upregulate granulocyte colony-stimulating factor, IL-8, and VCAM-1 expression in human bronchial fibroblasts. Am J Respir Cell Mol Biol. 2006;35:133–141. doi: 10.1165/rcmb.2005-0362OC. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Sadofsky LR, Xiao Y, Botham A, Cowen M, Morice AH, et al. Inflammatory mediators modulate thrombin and cathepsin-G signaling in human bronchial fibroblasts by inducing expression of proteinase-activated receptor-4. Am J Physiol Lung Cell Mol Physiol. 2007;292:L788–L798. doi: 10.1152/ajplung.00226.2006. [DOI] [PubMed] [Google Scholar]
- Rasmussen UB, Vouret-Craviari V, Jallat S, Schlesinger Y, Pagès G, Pavirani A, et al. cDNA cloning and expression of a hamster [alpha]-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Rastogi P, Young DM, McHowat J. Tryptase activates calcium-independent phospholipase A2 and releases PGE2 in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L925–L932. doi: 10.1152/ajplung.90230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe MJ, Walding A, Shelton PA, Flaherty A, Dougall IG. Activation of E-prostanoid4 and E-prostanoid2 receptors inhibits TNF-{alpha} release from human alveolar macrophages. Eur Respir J. 2007;29:986–994. doi: 10.1183/09031936.00131606. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FLM, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- Riewald M, Ruf W. Protease-activated Receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- Rogliani P, Mura M, Assunta Porretta M, Saltini C. Review: new perspectives in the treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2008;2:75–93. doi: 10.1177/1753465808089363. [DOI] [PubMed] [Google Scholar]
- Saleh SM, Mann TS, Peters T, Betts RJ, Henry PJ. Influence of dexamethasone on protease-activated receptor 2-mediated responses in the airways. J Pharmacol Exp Ther. 2008;324:622–630. doi: 10.1124/jpet.107.132753. [DOI] [PubMed] [Google Scholar]
- Saltzman LE, Moss J, Berg RA, Hom B, Crystal RG. Modulation of collagen production by fibroblasts. Effects of chronic exposure to agonists that increase intracellular cyclic AMP. Biochem J. 1982;204:25–30. doi: 10.1042/bj2040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi SS, Suresh Babu K, Holgate ST. Is asthma really due to a polarized T cell response toward a helper T cell type 2 Phenotype? Am J Respir Crit Care Med. 2001;164:1343–1346. doi: 10.1164/ajrccm.164.8.2103080. [DOI] [PubMed] [Google Scholar]
- Savla U, Appel HJ, Sporn PH, Waters CM. Prostaglandin E(2) regulates wound closure in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;280:L421–L431. doi: 10.1152/ajplung.2001.280.3.L421. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- Schmidt-Weber CB. Th17 and treg cells innovate the TH1/TH2 concept and allergy research. Chem Immunol Allergy. 2008;94:1–7. doi: 10.1159/000154844. [DOI] [PubMed] [Google Scholar]
- Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111:2667. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuil PJ, Ten Berge M, Van Gelder JM, Graamans K, Huizing EH. Effects of prostaglandins D2 and E2 on ciliary beat frequency of human upper respiratory cilia in vitro. Acta Otolaryngol. 1995;115:438–442. doi: 10.3109/00016489509139344. [DOI] [PubMed] [Google Scholar]
- Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- Seitzberg JG, Knapp AE, Lund BW, Mandrup Bertozzi S, Currier EA, Ma J-N, et al. Discovery of potent and selective small-molecule PAR-2 Agonists. J Med Chem. 2008;51:5490–5493. doi: 10.1021/jm800754r. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F, Saito S, Takaoka K, Hayashi H, Nagataki M, Nagasawa K, et al. Mechanisms for prostaglandin E2 formation caused by proteinase-activated receptor-1 activation in rat gastric mucosal epithelial cells. Biochem Pharmacol. 2007;73:103–114. doi: 10.1016/j.bcp.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto V, Hirota C, Hirota S, Janssen LJ. E-ring isoprostanes stimulate a Cl conductance in airway epithelium via prostaglandin E2-selective prostanoid receptors. Am J Respir Cell Mol Biol. 2008;38:88–94. doi: 10.1165/rcmb.2007-0117OC. [DOI] [PubMed] [Google Scholar]
- Severino B, Fiorino F, Perissutti E, Frecentese F, Cirino G, Roviezzo F, et al. Synthesis and pharmacological evaluation of peptide-mimetic protease-activated receptor-1 antagonists containing novel heterocyclic scaffolds. Bioorg Med Chem. 2008;16:6009–6020. doi: 10.1016/j.bmc.2008.04.059. [DOI] [PubMed] [Google Scholar]
- Sharma A, Goh HL, Asokananthan N, Bakker A, Stewart GA, Mitchell HW. Delayed and persistent suppression of bronchoconstriction by trypsin in the airway lumen. Eur Respir J. 2006;27:20–28. doi: 10.1183/09031936.06.00041605. [DOI] [PubMed] [Google Scholar]
- Sheller JR, Mitchell D, Meyrick B, Oates J, Breyer R. EP2 receptor mediates bronchodilation by PGE2 in mice. J Appl Physiol. 2000;88:2214–2218. doi: 10.1152/jappl.2000.88.6.2214. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L503–L510. doi: 10.1152/ajplung.2000.279.3.L503. [DOI] [PubMed] [Google Scholar]
- Smith AP, Cuthbert MF, Dunlop LS. Effects of inhaled prostaglandins E1, E2, and F2alpha on the airway resistance of healthy and asthmatic man. Clin Sci Mol Med. 1975;48:421–430. doi: 10.1042/cs0480421. [DOI] [PubMed] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- Sokolova E, Reiser G. A novel therapeutic target in various lung diseases: airway proteases and protease-activated receptors. Pharmacol Ther. 2007;115:70–83. doi: 10.1016/j.pharmthera.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Grishina Z, Buhling F, Welte T, Reiser G. Protease-activated receptor-1 in human lung fibroblasts mediates a negative feedback downregulation via prostaglandin E2.[see comment] Am J Physiol Lung Cell Mol Physiol. 2005;288:L793–L802. doi: 10.1152/ajplung.00343.2004. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Hartig R, Reiser G. Downregulation of protease-activated receptor-1 in human lung fibroblasts is specifically mediated by the prostaglandin E receptor EP2 through cAMP elevation and protein kinase A. FEBS J. 2008;275:3669–3679. doi: 10.1111/j.1742-4658.2008.06511.x. [DOI] [PubMed] [Google Scholar]
- Song KS, Choi YH, Kim JM, Lee H, Lee TJ, Yoon JH. Suppression of prostaglandin E2-induced MUC5AC overproduction by RGS4 in the airway. Am J Physiol Lung Cell Mol Physiol. 2009;296:L684–L692. doi: 10.1152/ajplung.90396.2008. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Giembycz MA, Barnes PJ, Belvisi MG. Prostaglandin E2 suppression of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by interacting with prostanoid receptors of the EP3-subtype. Br J Pharmacol. 1998;123:1246–1252. doi: 10.1038/sj.bjp.0701720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Stokes Peebles R, Jr, Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, et al. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2002;165:1154–1160. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2) Kidney Int. 2001;59:579–592. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- Sturm EM, Schratl P, Schuligoi R, Konya V, Sturm GJ, Lippe IT, et al. Prostaglandin E2 inhibits eosinophil trafficking through E-Prostanoid 2 receptors. J Immunol. 2008;181:7273–7283. doi: 10.4049/jimmunol.181.10.7273. [DOI] [PubMed] [Google Scholar]
- Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Liu X, Togo S, Kobayashi T, Shen L, Kawasaki S, et al. Prostaglandin E(2) protects human lung fibroblasts from cigarette smoke extract-induced apoptosis via EP(2) receptor activation. J Cell Physiol. 2007;210:99–110. doi: 10.1002/jcp.20825. [DOI] [PubMed] [Google Scholar]
- Sutherland MR, Friedman HM, Pryzdial ELG. Thrombin enhances herpes simplex virus infection of cells involving protease-activated receptor 1. J Thromb Haemost. 2007;5:1055–1061. doi: 10.1111/j.1538-7836.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, et al. Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-{kappa}B pathways. J Biol Chem. 2006;281:11792–11804. doi: 10.1074/jbc.M509292200. [DOI] [PubMed] [Google Scholar]
- Szardening-Kirchner C, Konrad L, Hauck EW, Haag SM, Eickelberg O, Weidner W. Upregulation of mRNA expression of MCP-1 by TGF-beta1 in fibroblast cells from Peyronie's disease. World J Urol. 2008;27:123–130. doi: 10.1007/s00345-008-0320-x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tokuoka S, Masuda T, Hirano Y, Nagao M, Tanaka H, et al. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br J Pharmacol. 2002;137:315–322. doi: 10.1038/sj.bjp.0704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa T, Tamiya M, Hara T, Matsumoto J, Saito N, Kanke T, et al. Abrogation of bronchial eosinophilic inflammation and attenuated eotaxin content in protease-activated receptor 2-deficient mice. J Pharmacol Sci. 2005;98:99–102. doi: 10.1254/jphs.scz050138. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kanako S, Abe S. Prostaglandin E2 receptor selective agonists E-prostanoid 2 and E-prostanoid 4 may have therapeutic effects on ovalbumin-induced bronchoconstriction. Chest. 2005;128:3717–3723. doi: 10.1378/chest.128.5.3717. [DOI] [PubMed] [Google Scholar]
- Tavakoli S, Cowan MJ, Benfield T, Logun C, Shelhamer JH. Prostaglandin E(2)-induced interleukin-6 release by a human airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2001;280:L127–L133. doi: 10.1152/ajplung.2001.280.1.L127. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, al-Rashed S, Rossi AG, Hellewell PG. Characterization of the prostanoid receptors mediating inhibition of PAF-induced aggregation of guinea-pig eosinophils. Br J Pharmacol. 1997;121:77–82. doi: 10.1038/sj.bjp.0701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin V, Kantor B, Weg V, Hartman M-L, Levi-Schaffer F. Tryptase activates the mitogen-activated protein kinase/activator protein-1 pathway in human peripheral blood eosinophils, causing cytokine production and release. J Immunol. 2002;169:2662–2669. doi: 10.4049/jimmunol.169.5.2662. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, et al. Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am J Physiol Lung Cell Mol Physiol. 2003;284:L599–L606. doi: 10.1152/ajplung.00324.2002. [DOI] [PubMed] [Google Scholar]
- Tran T, Stewart AG. Protease-activated receptor (PAR)-independent growth and pro-inflammatory actions of thrombin on human cultured airway smooth muscle. Br J Pharmacol. 2002;138:865–875. doi: 10.1038/sj.bjp.0705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami A, Ueki S, Ito W, Kobayashi Y, Chiba T, Mahemuti G, et al. Theophylline and dexamethasone induce peroxisome proliferator-activated receptor-Î3 expression in human eosinophils. Pharmacology. 2006;77:33–37. doi: 10.1159/000092376. [DOI] [PubMed] [Google Scholar]
- Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends in Immunol. 2004;25:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, et al. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol. 2000;106:537–545. doi: 10.1067/mai.2000.109058. [DOI] [PubMed] [Google Scholar]
- Vu T-KH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Wang H, Wen S, Bunnett NW, Leduc R, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor-2 induces cyclooxygenase-2 expression through beta-catenin and cyclic AMP-response element-binding protein. J Biol Chem. 2008;283:809–815. doi: 10.1074/jbc.M703021200. [DOI] [PubMed] [Google Scholar]
- Ward JE, Tan X. Peroxisome proliferator activated receptor ligands as regulators of airway inflammation and remodelling in chronic lung disease. PPAR Res. 2007;2007:14983. doi: 10.1155/2007/14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–3244. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, et al. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Hwang TL, Liao CH, Kuo SC, Lee FY, Lee CY, et al. Selective inhibition of protease-activated receptor 4-dependent platelet activation by YD-3. Thromb Haemost. 2002;87:1026–1033. [PubMed] [Google Scholar]
- Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. Modulation by cAMP of IL-1beta-induced eotaxin and MCP-1 expression and release in human airway smooth muscle cells. Eur Respir J. 2003;22:220–226. doi: 10.1183/09031936.03.00112002. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, et al. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H, Gabazza EC, Tamaki S, Kobayashi T, Hataji O, Yuda H, et al. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am J Respir Crit Care Med. 2001;163:1660–1668. doi: 10.1164/ajrccm.163.7.9911068. [DOI] [PubMed] [Google Scholar]
- Zavagno G, Jaffe B, Esteban M. Role of prostaglandins and non-steroid anti-inflammatory drugs in the pathogenicity of vaccinia virus. J Gen Virol. 1987;68:593–600. doi: 10.1099/0022-1317-68-2-593. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Yang H, Zhang L, Chen X, Zheng X, et al. Induction of T-helper type 2 cytokine release and up-regulated expression of protease-activated receptors on mast cells by recombinant American cockroach allergen Per a 7. Clin Exp Allergy. 2008;38:1160–1167. doi: 10.1111/j.1365-2222.2008.02991.x. [DOI] [PubMed] [Google Scholar]
- Zhao WW, Robinson NE, Yu MF. PGE2 inhibits acetylcholine release from cholinergic nerves in canine but not equine airways. Prostaglandins Leukot Essent Fatty Acids. 1994;51:347–355. doi: 10.1016/0952-3278(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Zhou W, Newcomb DC, Moore ML, Goleniewska K, O'Neal JF, Peebles RS., Jr Cyclooxygenase inhibition during allergic sensitization increases STAT6-independent primary and memory Th2 responses. J Immunol. 2008;181:5360–5367. doi: 10.4049/jimmunol.181.8.5360. [DOI] [PubMed] [Google Scholar]
- Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. beta-Arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]


