Abstract
Background and purpose:
The effects of systemic treatment with indomethacin-loaded nanocapsules (IndOH-NC) were compared with those of free indomethacin (IndOH) in rat models of acute and chronic oedema.
Experimental approach:
The following models of inflammation were employed: carrageenan-induced acute oedema (measured between 30 min and 4 h), sub-chronic oedema induced by complete Freund's adjuvant (CFA) (determined between 2 h and 72 h), and CFA-induced arthritis (oedema measured between 14 and 21 days).
Key results:
IndOH or IndOH-NC produced equal inhibition of carrageenan-elicited oedema. However, IndOH-NC was more effective in both the sub-chronic (33 ± 4% inhibition) and the arthritis (35 ± 2% inhibition) model of oedema evoked by CFA, when compared with IndOH (21 ± 2% and 14 ± 3% inhibition respectively) (P < 0.01). In the CFA arthritis model, treatment with IndOH-NC markedly inhibited the serum levels of the pro-inflammatory cytokines tumour necrosis factor α and IL-6 (by 83 ± 8% and 84 ± 11% respectively), while the levels of the anti-inflammatory cytokine IL-10 were significantly increased (196 ± 55%). The indices of gastrointestinal damage in IndOH-NC-treated animals were significantly less that those after IndOH treatment (58 ± 16%, 72 ± 6% and 69 ± 2%, for duodenum, jejunum and ileum respectively).
Conclusions and implications:
IndOH-NC produced an increased anti-inflammatory efficacy in long-term models of inflammation, allied to an improved gastrointestinal safety. This formulation might represent a promising alternative for treating chronic inflammatory diseases, with reduced undesirable effects.
This article is part of a themed issue on Mediators and Receptors in the Resolution of Inflammation. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Keywords: indomethacin, polymeric nanocapsules, drug delivery, inflammation, gastrointestinal damage
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) represent a group of approximately 50 different medicines widely prescribed for the management of pain, which display variable anti-inflammatory, anti-pyretic and analgesic activities. Their effects are mainly mediated by the inhibition of cyclooxygenases 1 and 2 (COX-1 and COX-2), with a consequent decrease in the formation of central and peripheral prostanoids (Burian and Geisslinger, 2005). Furthermore, most NSAIDs share a number of adverse effects, especially those related to gastrointestinal complications (Kean and Buchanan, 2005). Patients taking systemic NSAIDs for the treatment of chronic inflammatory diseases (such as rheumatoid arthritis and osteoarthritis) show variable relief of painful symptoms and they have increased risk of developing gastric or duodenal ulcers and bleeding, which might preclude their long-term use (Langford et al., 2006; Fiorucci et al., 2007).
The development of new drugs and/or new formulations for treating chronic inflammatory and painful diseases continues to be an issue of high interest. The pharmacological response to a drug is directly related to its concentration at the required site of action. A non-specific distribution leads to high drug concentration in healthy organs, tissues and cells, leading to toxicity (Soppimath et al., 2001; Couvreur et al., 2002). One method of restricting the drug to the required site is to associate it with a carrier system (Couvreur et al., 2002; Vauthier and Couvreur, 2007). Among the different nanocarrier systems, biodegradable nanoparticles have received considerable attention as potential drug delivery vehicles over the last few years. Polymeric nanoparticles are colloidal structures below 1 µm, which have been designed to encapsulate lipophilic drugs in order to target organs or tissues, to avoid drug degradation, to improve its efficacy or to circumvent the toxicity (Allémann et al., 1998; Pinto-Alphandary et al., 2000; Guterres, 2001; Couvreur et al., 2002; Vila et al., 2002). In this regard, it has been suggested that NSAIDs-loaded nanocapsules might display an increased efficacy, associated with a marked reduction of adverse effects (Guterres et al., 2001; Bansal JoshiBansal et al., 2007). A recent publication from our group reinforced this idea, by showing that indomethacin-loaded nanocapsules (IndOH-NC) were more potent than free indomethacin in decreasing the viability and proliferation of glioma cell lines, without exerting significant cytotoxic effects on normal cells (Bernardi et al., 2008).
In order to provide additional evidence on the effects of alternative delivery systems for NSAIDs, the present study was designed to characterize the effects of systemic treatment with IndOH-NC in rat models of acute or chronic inflammation. Attempts have also been made to determine the gastrointestinal effects of IndOH-NC. Additionally, we have aimed to compare both the anti-inflammatory and the adverse effects of IndOH-NC, with those displayed by free indomethacin in solution.
Methods
Preparation of nanocapsules
Nanocapsules were prepared by interfacial deposition of polymer as previously described (Fessi et al., 1989). At 40°C, indomethacin (0.010 g), poly(ε-caprolactone) (0.100 g), capric/caprylic triglyceride (0.33 mL) and sorbitan monostearate (0.077 g) were dissolved in acetone (27 mL). In a separate flask, polysorbate 80 (0.077 g) was added to 53 mL of water. The organic solution was injected into the aqueous phase under magnetic stirring at room temperature. After 10 min, the acetone was evaporated and the suspensions concentrated under reduced pressure. The final volume was adjusted to 10 mL. Control formulation (unloaded nanocapsules) was prepared by omitting the drug (indomethacin).
Characterization of nanocapsules
After preparation, the pH of the suspensions was determined using a potentiometer (Micronal B-474). Particle size, polydispersity and zeta potential of the suspensions were determined using a Zetasizer®nano-ZS ZEN 3600 model (Malvern, UK). The samples were diluted in water (MilliQ®) (particle size) or in 10 mmol·L−1 NaCl aqueous solution (zeta potential). The measurements were made in triplicate. The total concentrations of indomethacin in the formulations were measured by reverse phase high-performance liquid chromatography (HPLC) (Perkin-Elmer S-200, with injector S-200, detector UV-Vis, a guard-column and a Lichrospher 100 RP-18 column of 250 mm, 4 mm and 5 µm; Merck). The mobile phase consisted of acetonitrile/water (70:30, v/v) adjusted to apparent pH 5.0 ± 0.5 with 10% (v/v) acetic acid. Each suspension (100 µL) was treated with acetonitrile (10 mL); the solution was filtered (Millipore 0.45 µm) and injected (20 µL). The HPLC method was previously validated (Pohlmann et al., 2004). Linear calibration curves for indomethacin were obtained in the range of 1–25 µg·mL−1 presenting correlation coefficients higher than 0.9992.
Animals
All animal care and experimental procedures used in the present study followed the ‘Principles of Laboratory Animal Care’ from NIH publication No. 85–23 and were approved by the Ethics Committee of Pontifícia Universidade Católica do Rio Grande do Sul. The number of animals and intensity of noxious stimuli used were the minimum necessary to demonstrate consistent effects of the drug treatment. Male Wistar rats (180–200 g) were obtained from the Central Biotery of the Federal University of Pelotas (Brazil). Animals were housed under conditions of optimum light (12:12 h light–dark cycle), temperature (22 ± 1°C) and humidity (50 to 60%), with food and water provided ad libitum. For the experiments, the animals were acclimatized to the laboratory for at least 1 h, and they were used only once in each test.
Carrageenan-induced rat paw oedema-acute protocol
The experiments were conducted according to the method described by Tratsk et al. (1997). Under light anaesthesia with oxygen (3%) and isoflurane (2%), the animals received an intradermal (i.d.) injection in the right hindpaw of saline (0.9%) containing carrageenan (300 µg per paw; 100 µL). As a control, the contralateral paw (left paw) received 100 µL of saline. Oedema was measured by means of a plethysmometer (Ugo Basile) at several time points after carrageenan injection (30, 60, 120 and 240 min). Oedema is expressed in mL as the difference between the right and left paws.
In this model, two distinct schedules of treatment have been adopted. In the prophylactic scheme, the animals were pretreated with IndOH-NC group or indomethacin in solution (solubilized in calcium carbonate 3%) (IndOH group) (both at 1 mg·kg−1, i.p.), 30 min before carrageenan injection. In the therapeutic scheme, the animals received IndOH or IndOH-NC (1 mg·kg−1, i.p.), 60 min after the injection of carrageenan. The control groups received the vehicle solutions: calcium carbonate 3% (control group) or unloaded nanocapsules (NC group) (1 mL·kg−1, i.p.), at the same schedules of administration.
Complete Freund's adjuvant (CFA)-induced rat paw oedema – sub-chronic protocol
The protocol used was similar to that described by Stein et al. (1988), with minor modifications. Briefly, isoflurane-anaesthetized animals received an i.d. injection in one hindpaw (right paw) of CFA (1 mg·mL−1; 100 µL; heat-killed and dried Mycobacterium tuberculosis, each millilitre of vehicle containing 0.85 mL paraffin oil plus 0.15 mL mannide monooleate), which was suspended in a 1:1 oil/saline emulsion (in a total volume of 200 µL per paw). As a control, the contralateral paw (left paw) received 200 µL of saline. In this model, the animals were treated with IndOH or IndOH-NC (1 mg·kg−1, i.p.), 2 h post-CFA injection, and once a day for 3 days. The control groups received the corresponding vehicle solutions at the same intervals of time. The oedema was measured by using a plethysmometer (Ugo Basile) at several time points following CFA injection (2, 4, 6, 8, 24, 48 and 72 h), and it is expressed in mL as the difference between the right and left paws.
CFA-elicited oedema – arthritis model
The adjuvant-induced arthritis model employed in the present study was similar to that described by Lorton et al. (2000), with some modifications. For this purpose, the oedema was induced by CFA injection, as indicated above, and it was assessed daily in a plethysmometer, between days 14 and 21 post-CFA administration. Animals were treated with IndOH or IndOH-NC (1 mg·kg−1, i.p.), twice a day, for 8 days, starting at the 14th day of CFA injection, until the 21st day. Control groups received the respective vehicle solutions.
Determination of cytokine levels in serum
In the arthritis group, the animals were killed on the 21st day by isoflurane inhalation, and blood samples were collected by cardiac puncture. The blood samples were centrifuged at 1300 g at 4°C for 10 min. The supernatant was rapidly frozen and stored at −70°C for later measurement of tumour necrosis factor α (TNF-α), interleukin (IL)-6 and IL-10 levels using specific enzyme-linked immunosorbent assay (ELISA) kits, according to the recommendations of the supplier (R&D Systems).
Evaluation of gastrointestinal damage
The occurrence of gastrointestinal lesions was evaluated in the arthritis group, at the end of experiments (21 days after arthritis induction by CFA). For this purpose, rats were killed, and the intestine (duodenum, jejunum and ileum) was slit open opposite the attached mesenteric tissue. The organs were washed with saline and the mucosal surfaces were macroscopically examined according to an arbitrary scale previously reported (Guterres et al., 2001). Accordingly, the number and the gravity of erosions were scored on a scale of five grades: grade 0, no lesion; grade 0.5, hemorrhagic point; grade 1, ulcer length <2 mm; grade 2, ulcer length >2 mm; grade 3, lesion with perforation and haemorrhage. Experimental data were obtained by multiplying the score by the number of lesions. The mean scores for each group were calculated and expressed as lesion indexes.
Statistical analysis
The results are presented as the mean ± SEM of 5–8 animals. The statistical significance between groups was assessed by means of one-way analysis of variance (anova) followed by Tukey's test. P-values less than 0.05 (P < 0.05) were considered significant. The percentages of inhibition between groups IndOH and IndOH-NC were determined in percentage, on the basis of the area under the curve. The statistical significance between groups was assessed by means of unpaired Student's t-test. P-values less than 0.05 were considered significant.
Results
Physico-chemical characterization of nanocapsule formulations
The nanocapsule formulations were prepared by interfacial deposition of polymer without the need of any subsequent step of purification. IndOH-NC and unloaded nanocapsules presented a macroscopic homogeneous aspect, such as white bluish opalescent liquids. After preparation, the average particle sizes were 240 nm (IndOH-NC) and 226 nm (unloaded nanocapsules). The suspensions showed monomodal size distributions and polydispersity indexes lower than 0.19, indicating narrow size distributions. The pH values were 5.95 (IndOH-NC) and 6.05 (unloaded nanocapsules). The zeta potential values were −6.9 and −7.3 mV respectively. The indomethacin content was 0.991 ± 0.012 mg·mL−1 and the encapsulation efficiency was close to 100%.
Carrageenan-induced paw oedema – acute protocol
We firstly examined the effects of IndOH or IndOH-NC treatment, on the oedema induced by carrageenan. The results demonstrated that prophylactic administration of IndOH or IndOH-NC (1 mg·kg−1, i.p., 30 min before carrageenan) markedly inhibited the oedema induced by carrageenan, when compared with the respective control groups, with inhibition of 61 ± 4% and 63 ± 3% respectively (Figure 1). In addition, the oedema elicited by i.d. injection of carrageenan into the rat paw was significantly reduced by the therapeutic administration of IndOH or IndOH-NC (1 mg·kg−1, i.p.) administered 60 min after carrageenan, with inhibition of 31 ± 11% and 44 ± 7% respectively (Figure 2). Comparison of the inhibition observed for IndOH and IndOH-NC did not reveal any significant difference in the effect of the tested formulations of indomethacin, in either the prophylactic or therapeutic schedules of treatment (P > 0.05) (Figures 1 and 2).
Figure 1.
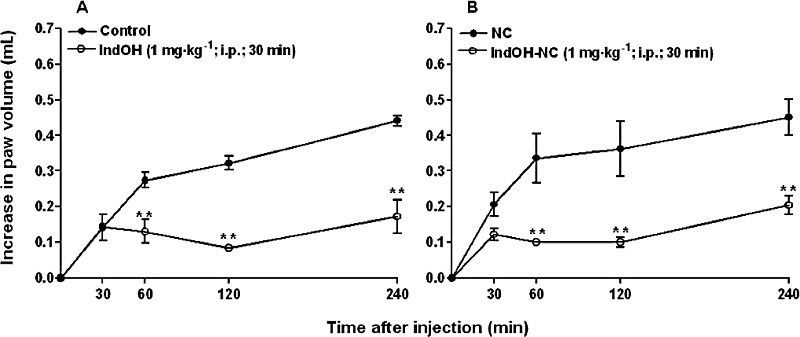
Effect of indomethacin-loaded nanocapsules (IndOH-NC, 1 mg·kg−1, i.p., 30 min before), on rat paw oedema induced by carrageenan (300 µg·paw−1, acute model – prophylactic treatment). (A) Indomethacin in solution (IndOH) and calcium carbonate 3% (Control); (B) Indomethacin-loaded nanocapsules (IndOH-NC) and unloaded nanocapsules (NC). Each point represents the mean of 6–8 animals and vertical lines show the SEM. Asterisks denote the significance levels in comparison to respective control values: **P < 0.01.
Figure 2.
Effect of indomethacin-loaded nanocapsules (IndOH-NC, 1 mg·kg−1, i.p., after 60 min), on rat paw oedema induced by carrageenan (300 mg paw−1, acute model – therapeutic treatment). (A) Indomethacin in solution (IndOH) and calcium carbonate 3% (Control); (B) Indomethacin-loaded nanocapsules (IndOH-NC) and unloaded nanocapsules (NC). Each point represents the mean of 6–8 animals and vertical lines show the SEM. Asterisks denote the significance levels in comparison to respective control values: *P < 0.05, **P < 0.01.
CFA-induced rat paw oedema – sub-chronic protocol
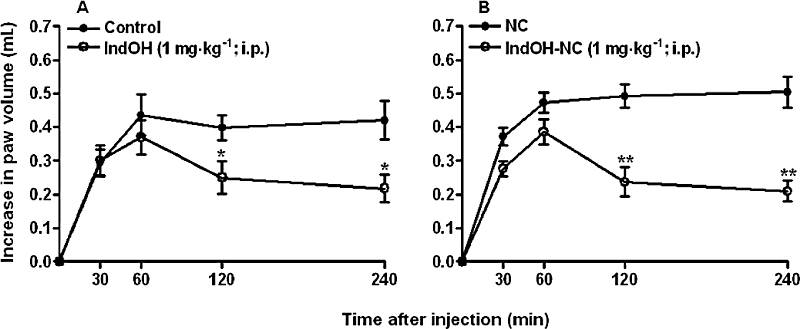
In this experimental set, we compared the effects of IndOH and IndOH-NC in a short period of evaluation following CFA application (until 3 days). The results depicted in Figure 3 show that administration of IndOH or IndOH-NC (1 mg·kg−1, i.p., 2 h after induction of paw oedema, and once a day, for 3 days) significantly reduced the oedema induced by CFA injection, according to assessment in the sub-chronic protocol. The calculated inhibition was 21 ± 2% (IndOH) and 33 ± 4% (IndOH-NC) and these values were significantly different (P < 0.05; Figure 3).
Figure 3.
Effect of indomethacin-loaded nanocapsules (IndOH-NC, 1 mg·kg−1, i.p. daily, 2 h after induction of paw oedema by CFA, for 3 days), on rat paw oedema induced by CFA (sub-chronic model). (A) Indomethacin in solution (IndOH) and calcium carbonate 3% (control); (B) Indomethacin-loaded nanocapsules (IndOH-NC) and unloaded nanocapsules (NC). Each point represents the mean of 6–8 animals and vertical lines show the SEM. Asterisks denote the significance levels in comparison to respective control values. *P < 0.05, **P < 0.01.
CFA-elicited oedema – arthritis model
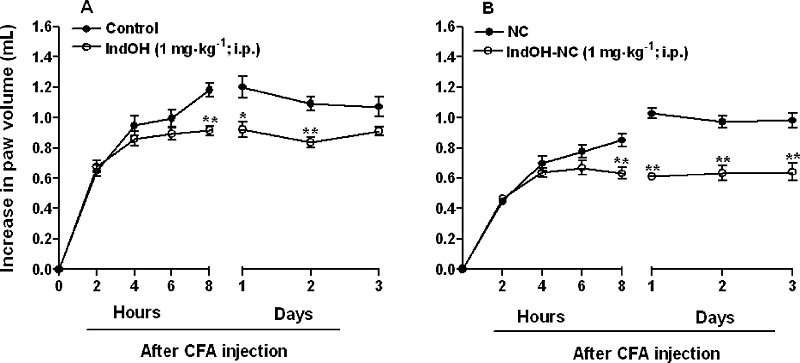
It is well known that CFA injection into the rat paw evokes a marked and time-related local oedema, which is observed as early as 2 h after and persists for up to 28 days, and presenting signs of systemic alterations. Hence, assessment of CFA-induced oedema in the later phases (after 14 days) is widely adopted as an arthritis model (Lorton et al., 2000). In this study, animals received either IndOH or IndOH-NC (1 mg·kg−1, i.p., twice a day, for 8 days), between the 14th and the 21st day after CFA injection. Both indomethacin formulations were able to significantly reduce the long-term oedema caused by CFA. In these experiments, IndOH-NC exhibited a greater inhibition (35 ± 2%), than IndOH (14 ± 2%) (P < 0.01) (Figure 4).
Figure 4.
Effect of indomethacin-loaded nanocapsules (IndOH-NC, 1 mg·kg−1, i.p., 14 days after induction of paw oedema, twice a day, for 8 days), on rat paw oedema induced by CFA (arthritis model). (A) Indomethacin in solution (IndOH) and calcium carbonate 3% (control); (B) Indomethacin-loaded nanocapsules (IndOH-NC) and unloaded nanocapsules (NC). Each point represents the mean of 6–8 animals and vertical lines show the SEM. Asterisks denote the significance levels in comparison to respective control values. *P < 0.05, **P < 0.01.
Determination of cytokine levels in serum
The serum of animals in the arthritis group was collected at 21 days after CFA injection, and it was used for determining effects of the different indomethacin formulations on the systemic alterations of cytokine levels. The results demonstrate that the treatment with IndOH-NC (1 mg·kg−1, i.p., twice a day, for 8 days), between the 14th and the 21st day after CFA injection, produced a striking inhibition in the production of the pro-inflammatory cytokines TNF-α and IL-6 in serum of CFA-injected rats, by 83 ± 8% and 84 ± 11% respectively (Figure 5A and B). Furthermore, the same treatment with IndOH-NC induced a marked increase of the anti-inflammatory cytokine IL-10 by 196 ± 55% (Figure 5C). Conversely, the administration of IndOH (at the same schedule of administration) failed to significantly alter the systemic production of all analysed cytokines (Figure 5).
Figure 5.
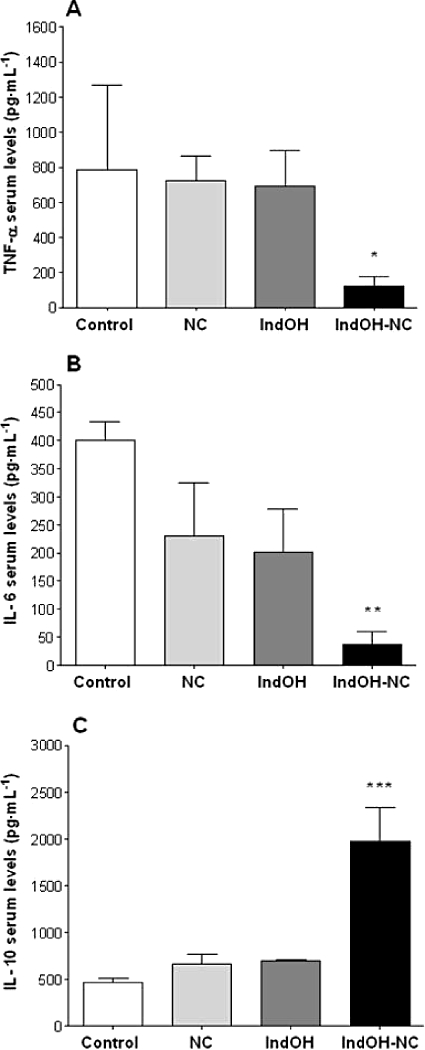
Effect of indomethacin-loaded nanocapsules (IndOH-NC) (1 mg·kg−1 IndOH-NC i.p., 14 days after induction of paw oedema, twice a day, for 8 days) on (A) TNF-α and (B) IL-6 and IL-10 levels in serum of animals in the arthritis model. Each point represents the mean of 5–8 animals and vertical lines show the SEM. Significantly different from the control, unloaded nanocapsules (NC) and indomethacin in solution (IndOH) groups for *P < 0.05, **P < 0.01 and ***P < 0.001.
Evaluation of gastrointestinal damage
This series of experiments was designed to evaluate the gastrointestinal toxicity of IndOH-NC in relation to IndOH, after the long-term administration of both formulations. For the first time, the efficacy and the toxicity were determined using the same groups treated with nanoencapsulated NSAIDs. For this purpose, the intestines in the arthritis group of rats (killed at 21 days) were analysed and the indices of damage were determined separately for duodenum, jejunum and ileum. As shown in the Figure 6, the lesion indices in the animals treated with IndOH-NC were significantly reduced when compared with the IndOH group, by 58 ± 16%, 72 ± 6% and 69 ± 2%, for duodenum, jejunum and ileum respectively. When the total lesion index was calculated (i.e. the total score for the three intestinal regions) the reduction was 68 ± 5% in the IndOH-NC group, compared with the IndOH-treated rats (Figure 6). The animals treated with NC presented a low but significant increase (10 ± 2%) in the total lesion indexes when compared with the control group (Figure 6).
Figure 6.
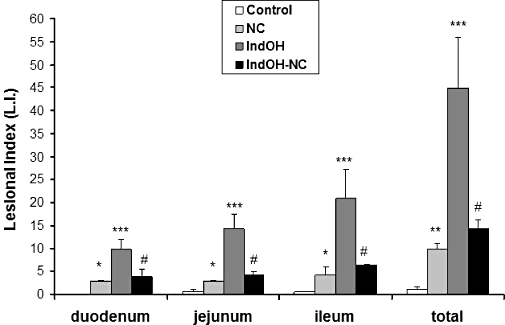
Effect of indomethacin-loaded nanocapsules (IndOH-NC) (1 mg·kg−1 IndOH-NC i.p. every 12 h, 14 days after induction of paw oedema, for 8 days) on intestine lesional index (LI) of animals in the arthritis group. Each point represents the mean of 6–8 animals and vertical lines show the SEM. *Significantly different from the control group (P < 0.05). **Significantly different from the control group (P < 0.01). ***Significantly different from the control and unloaded nanocapsules (NC) groups (P < 0.001). #Significantly different from the indomethacin in solution group (IndOH) (P < 0.001).
Discussion
The present study was conducted to investigate the potential actions of IndOH-NC in experimental models of inflammation in rats. To this end, three classical models of infmallation in vivo were employed to evaluate the short and long-term effects of IndOH-NC, in comparison with IndOH: carrageenan-induced acute oedema, CFA-induced sub-chronic inflammation and CFA-induced arthritis. We have also attempted to compare the gastrointestinal toxicity found in rats chronically treated with either IndOH-NC or IndOH.
The injection of carrageenan into the rat hindpaw represents a model commonly employed to study acute inflammation and pain. The application of carrageenan causes a rapid formation of oedema, allied to an exacerbated sensitivity to thermal and mechanical stimuli (Rocha et al., 2006). In this regard, carrageenan-induced rat paw oedema is widely used to characterize the mechanisms of action of new anti-inflammatory drugs or formulations, including NSAIDs (Velo et al., 1973; Kawamura et al., 2000; Quintão et al., 2005). We assessed the effects of IndOH-NC in comparison to IndOH, when both formulations were dosed by two distinct schedules of administration, before (prophylactic) or after (therapeutic), the i.d. injection of carrageenan. Our results indicate that IndOH-NC displays an anti-inflammatory efficacy, which is similar to that observed for IndOH, according to assessment in both regimens of treatment. However, no significant difference was observed between the anti-inflammatory efficacy for IndOH-NC and IndOH in the carrageenan acute model of inflammation.
Considering the kinetic properties of polymeric nanocapsules, we decided to investigate whether IndOH-NC might exhibit increased efficacy in long-term models of inflammation. First, we have assessed its effects in the sub-chronic model of inflammation induced by CFA, in which the oedema was measured until 3 days after the application of the inflammatory agent. This experimental set revealed that IndOH-NC presented a significantly higher efficacy in comparison to IndOH. This encouraging result prompted us to test the anti-inflammatory efficacy of IndOH-NC in an experimental model of clinical relevance: CFA-induced arthritis. Repeated treatment with IndOH-NC produced a marked inhibition of CFA-induced long-term oedema formation (between 14 and 21 days), which was significantly greater than that obtained with IndOH. One plausible explanation for these effects is that, the nanoencapsulation improves drug efficacy and drug bioavailability (Couvreur et al., 2002; Schaffazick et al., 2003) by providing a more sustained drug release to the inflamed site, according to evaluation in the CFA-arthritis model. It is important to note that the dose of indomethacin used in the present study (1 mg·kg−1) is sub-therapeutic, showing that anti-inflammatory actions of IndOH-NC were noticeably enhanced when compared with the same dose of indomethacin in solution.
It is well known that lowered pH is one of the hallmarks of rheumatoid arthritis (Andersson et al., 1999; Levick, 1990). This pH decline may lead a delay in the indomethacin release from the nanocapsules, enhancing its anti-inflammatory effect. Furthermore, plasma protein binding is known to limit indomethacin cellular uptake, by reducing the free fraction of the drug in the circulation (Parepally et al., 2006). In this regard, some characteristics of nanoparticulated systems such as the carrier size, the polymer type, as well as their surface features, might induce steric stabilization of nanoparticles, thus inhibiting protein binding and increasing blood circulation time (Brigger et al., 2002; Brioschi et al., 2007). In this context, a recent publication (Zhang et al., 2007) revealed that indomethacin concentrations in plasma were prolonged in the group treated subcutaneously with indomethacin-loaded micelles, compared with indomethacin in aqueous solution. Furthermore, the nanoparticles can accumulate in inflamed tissues due to the greater microvascular permeability in those sites. Additionally in our study, the polymeric nanocapsules were prepared with polysorbate 80, a hydrophilic coating able to delay the protein plasma binding, increasing the particle blood circulation time. Accordingly, all of these factors might well have contributed to the increased efficacy of IndOH-NC observed in the present study.
As reported in the literature, CFA injection can elicit the release of a series of inflammatory mediators, including cytokines. Cytokine production is an important event related to the onset and/or maintenance of inflammatory diseases, such as asthma, arthritis, sepsis and inflammatory bowel disease, among others (Laufer, 2003; Meyer, 2003; Stokkers and Hommes, 2004; Ulloa and Tracey et al., 2005; Woodfolk, 2006). Herein, we sought to determine whether the anti-inflammatory effects of IndOH-NC in the CFA-arthritis model, were associated with changes in cytokine generation. Interestingly, our data demonstrate that treatment with IndOH-NC was able to produce a significant decrease of the pro-inflammatory cytokines TNF-α and IL-6, in the serum of arthritic rats. More relevantly, the administration of IndOH-NC also induced a marked increase in the serum levels of the anti-inflammatory cytokine IL-10. Conversely, no significant effect on cytokine production was observed when rats were treated with indomethacin in solution. Thus, on the basis of this series of results, it is possible to infer that increased efficacy of IndOH-NC in comparison to free IndOH, is likely to be related to its ability to alter cytokine production in the inflammatory scenario.
Treatment with NSAIDs has been associated with development of adverse and severe gastrointestinal effects (Asako et al., 1992; Tries et al., 2002). Accordingly, another important aspect assessed in our study was the gastrointestinal toxicity of IndOH-NC, when dosed in a chronic schedule of administration. Present data clearly demonstrated that animals treated with IndOH-NC showed a significant reduction of intestinal lesion indices, when compared with the animals that received indomethacin in solution. This allows us to suggest that IndOH-NC formulation displayed a lower level of adverse effects than that after IndOH, presenting a desirable, increased gastrointestinal tolerance. Surprisingly, the animals treated with NC also exhibited a significant increase in the intestinal lesion indices when compared with the control group. As previously reported by our group, acute treatment with the NC formulation did not present a significant gastrointestinal toxicity (Guterres et al., 2001; Schaffazick et al., 2003). It is important to note that in the present study the animals were treated chronically (1 mg·kg−1 i.p. every 12 h for 8 days). Therefore, the low gastrointestinal toxicity caused by NC formulations was probably due to this prolonged period of treatment. A relevant point to be discussed is that cytokines might exhibit an important role in mucosal defence (Robinson et al., 2008). Therefore, the reduction of TNFα and IL-6 production, associated with the elevation of IL-10 levels might well contribute to the reduced gastrointestinal toxicity observed in the IndOH-NC-treated group.
In summary, the data reported herein clearly demonstrate that polymeric nanocapsules are able to successfully carry indomethacin into the inflammatory sites. Of note, in long-term models of inflammation following CFA injection, IndOH-NC presented an increased anti-inflammatory efficacy, allied to an improved gastrointestinal safety. Thus, the present findings allow us to suggest that IndOH-NC might constitute a relevant and apparently gastrointestinal safe therapeutic alternative for the treatment of chronic inflammatory diseases, such as rheumatoid arthritis with NSAIDs.
Acknowledgments
This study was supported by the Brazilian agencies: CNPq/Brazil (Edital Universal), FINEP, Rede Nanocosméticos/CNPq-MCT. A.B. and E.J. were recipients of Brazilian Capes and CNPq fellowships. A.C.C.V.Z. was recipient of a Brazilian BPA-PUCRS fellowship. The authors thank Juliano Soares for his excellent technical assistance.
Glossary
Abbreviations:
- CFA
complete Freund's adjuvant
- COX-1
cyclooxygenase 1
- COX-2
cyclooxygenase 2
- IL-6
interleukin 6
- IL-10
interleukin 10
- IndOH-NC
indomethacin-loaded nanocapsules
- NC
unloaded nanocapsules
- NSAIDs
non-steroidal anti-inflammatory drugs
- TNF-α
tumour necrosis factor α
Conflict of interest
None.
References
- Allémann E, Leroux JC, Gurny R. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Del Rev. 1998;34:171–189. doi: 10.1016/s0169-409x(98)00039-8. [DOI] [PubMed] [Google Scholar]
- Andersson SE, Lexmuller K, Johansson A, Ekstrom GM. Tissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the rat. J Rheumatol. 1999;26:2018–2024. [PubMed] [Google Scholar]
- Asako H, Kubes P, Wallace J, Gaginella T, Wolf RE, Granger DN. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol. 1992;262:903–908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- Bansal SS, Joshi A, Bansal AK. New dosage formulations for targeted delivery of cyclo-oxygenase-2 inhibitors: focus on use in the elderly. Drugs Aging. 2007;6:441–451. doi: 10.2165/00002512-200724060-00001. [DOI] [PubMed] [Google Scholar]
- Bernardi A, Frozza RL, Jäger E, Figueiro F, Bavaresco L, Salbego C, et al. Selective cytotoxicity of indomethacin and indomethacin ethyl ester-loaded nanocapsules against glioma cell lines: an in vitro study. Eur J Pharmacol. 2008;586:24–34. doi: 10.1016/j.ejphar.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Brioschi A, Zenga F, Zara CP, Gasco MR, Ducati A, Mauro A. Solid lipid nanoparticles: could they help to improve the efficacy of pharmacologic treatments for brain tumors? Neurolog Res. 2007;29:324–330. doi: 10.1179/016164107X187017. [DOI] [PubMed] [Google Scholar]
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Del Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther. 2005;2:139–154. doi: 10.1016/j.pharmthera.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carrier Syst. 2002;19:99–134. doi: 10.1615/critrevtherdrugcarriersyst.v19.i2.10. [DOI] [PubMed] [Google Scholar]
- Fessi H, Puisieux F, Devissaguet JP, Amoury N, Benita S. Nanocapsules formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;113:r1–r4. [Google Scholar]
- Fiorucci S, Santucci L, Distrutti E. NSAIDs, coxibs, CINOD and H2S-releasing NSAIDs: what lies beyond the horizon. Dig Liver Dis. 2007;12:1043–1051. doi: 10.1016/j.dld.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Guterres SS. Spray-dried diclofenac-loaded poly(epsilon-caprolactone) nanocapsules and nanospheres: preparation and physicochemical characterization. Pharmazie. 2001;56:864–867. [PubMed] [Google Scholar]
- Guterres SS, Muller CB, Michalowski CB, Pohlmann AR, Dalla Costa T. Gastro-intestinal tolerance after oral administration of spray-dried diclofenac-loaded nanocapsules and nanospheres. S.T.P. Pharma Sci. 2001;11:229–233. [Google Scholar]
- Kawamura M, Hatanaka K, Saito M, Ogino M, Ono T, Ogino K, et al. Are the anti-inflammatory effects of dexamethasone responsible for inhibition of the induction of enzymes involved in prostanoid formation in rat carrageenan-induced pleurisy? Eur J Pharmacol. 2000;400:127–135. doi: 10.1016/s0014-2999(00)00377-0. [DOI] [PubMed] [Google Scholar]
- Kean WF, Buchanan WW. The use of NSAIDs in rheumatic disorders 2005: a global perspective. Inflammopharmacology. 2005;4:343–370. doi: 10.1163/156856005774415565. [DOI] [PubMed] [Google Scholar]
- Langford R, McKenna F, Ratcliffe S, Vojtassák J, Richarz U. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis: a randomized, placebo-controlled trial. Arthritis Rheum. 2006;6:1829–1837. doi: 10.1002/art.21884. [DOI] [PubMed] [Google Scholar]
- Laufer S. Role of eicosanoids in structural degradation in osteoarthritis. Curr. Opin. Rheumatol. 2003;15:623–627. doi: 10.1097/00002281-200309000-00017. [DOI] [PubMed] [Google Scholar]
- Levick JR. Hypoxia and acidosis in chronic inflammatory arthritis: relation to vascular supply and dynamic effusion pressure. J Rheumatol. 1990;17:579–582. [PubMed] [Google Scholar]
- Lorton D, Lubahn C, Engan C, Schaller J, Felten DL, Bellinger DL. Local application of capsaicin into the draining lymph nodes attenuates expression of adjuvant-induced arthritis. Neuroimmunomodulation. 2000;3:115–125. doi: 10.1159/000026429. [DOI] [PubMed] [Google Scholar]
- Meyer O. Role of TNFα and cytokines in the physiopathology of rheumatoid arthritis. Therapeutic perspectives. Bull Acad Natl Med. 2003;187:935–954. [PubMed] [Google Scholar]
- Parepally JM, Mandula H, Smith QR. Brain uptake of nonsteroidal anti-inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharm Res. 2006;23:873–881. doi: 10.1007/s11095-006-9905-5. [DOI] [PubMed] [Google Scholar]
- Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents. 2000;13:155–168. doi: 10.1016/s0924-8579(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Pohlmann AR, Soares LU, Cruz L, Da Silveira NP, Guterres SS. Diffusion and mathematical modeling of release profiles from nanocarriers. Curr Drug Deliv. 2004;1:103–110. [Google Scholar]
- Quintão NL, Medeiros R, Santos AR, Campos MM, Calixto JB. The effects of diacerhein on mechanical allodynia in inflammatory and neuropathic models of nociception in mice. Anesth Analg. 2005;6:1763–1769. doi: 10.1213/01.ane.0000184182.03203.61. [DOI] [PubMed] [Google Scholar]
- Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;10:1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- Rocha AC, Fernandes ES, Quintão NL, Campos MM, Calixto JB. Relevance of tumour necrosis factor-alpha for the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw. Br J Pharmacol. 2006;5:688–695. doi: 10.1038/sj.bjp.0706775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffazick SR, Pohlmann AR, Dalla-Costa T, Guterres SS. Freeze-drying colloidal suspensions: nanocapsules, nanospheres and nanodispersion. A comparative study. Eur J Pharm and Biopharm. 2003;56:501–505. doi: 10.1016/s0939-6411(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988;2:451–455. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- Stokkers PC, Hommes DW. New cytokine therapeutics for inflammatory bowel disease. Cytokine. 2004;28:167–173. doi: 10.1016/j.cyto.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Tratsk KS, Campos MM, Vaz ZR, Filho VC, Schlemper V, Yunes RA, et al. Anti-allergic effects and oedema inhibition caused by the extract of Drymis winteri. Inflamm Res. 1997;46:509–514. doi: 10.1007/s000110050234. [DOI] [PubMed] [Google Scholar]
- Tries S, Neupert W, Laufer S. The mechanism of action of the new compound ML3000: inhibition of 5-LOX and COX-1/2. Inflamm Res. 2002;51:135–143. doi: 10.1007/pl00000285. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Tracey KJ. The ‘cytokine profile’: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Vauthier C, Couvreur P. Nanomedicines: a new approach for the treatment of serious diseases. J Biomed Nanotechnol. 2007;3:223–234. [Google Scholar]
- Velo GP, Dunn CJ, Giroud JP, Timsit J, Willoughby DA. Distribuition of prostaglandins in inflammatory exudates. J Pathol. 1973;111:149–158. doi: 10.1002/path.1711110302. [DOI] [PubMed] [Google Scholar]
- Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002;78:15–24. doi: 10.1016/s0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- Woodfolk JA. Cytokines as a therapeutic target for allergic diseases: a complex picture. Curr Pharm Des. 2006;12:2349–2363. doi: 10.2174/138161206777698936. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Yan MQ, Li XH, Qi LY, Li XD, Li XJ, et al. Local delivery of indomethacin to arthritis-bearing rats through polymeric micelles based on amphiphilic polyphosphazenes. Pharm Res. 2007;24:1944–1953. doi: 10.1007/s11095-007-9322-4. [DOI] [PubMed] [Google Scholar]


