Abstract
Background and purpose:
The intracellular signalling kinase, extracellular signal-regulated kinase 1/2 (ERK1/2) is required for new memory formation, suggesting that control of ERK signalling might be a target for the treatment of cognitive dysfunction. Previously, we reported that tanshinone congeners have ameliorating effects on drug-induced memory impairment in mice. Here, we have investigated possible modes of action of tanshinone I on learning and memory, associated with ERK phosphorylation.
Experimental approach:
Using immunohistochemical, Western blot techniques, and behavioural testing, we studied the effect of tanshinone I on memory impairment induced by diazepam or dizocilpine (MK-801) in mice.
Key results:
Tanshinone I (2 or 4 mg·kg−1, p.o.) increased latency times versus vehicle-treated control group in the passive avoidance task. Western blot analysis and immunohistochemical data showed that tanshinone I (4 mg·kg−1) increased levels of phosphorylated cAMP response element binding protein (pCREB) and phosphorylated ERK (pERK) in the hippocampus. These increases in pCREB and pERK were blocked by U0126 (inhibitor of ERK1/2), which also prevented the increase in passive avoidance task latency time after tanshinone I. In models of learning and memory impairment induced by diazepam and MK-801, tanshinone I (4 mg·kg−1) reversed learning and memory impairments detected by the passive avoidance test. Western blot analysis showed that tanshinone I reversed the diazepam- and MK-801-induced inhibitions of ERK and CREB activation in hippocampal tissues. These effects were also blocked by U0126.
Conclusions and implications:
Tanshinone I ameliorates the learning and memory impairments induced by diazepam and MK-801 through activation of ERK signalling.
Keywords: tanshinone I, diazepam, MK-801, ERK pathway
Introduction
Extracellular signal-regulated kinase 1/2 (ERK1/2) is a member of the family of MAP kinases, and the cascade has been reported to activate cAMP response element binding protein (CREB) and other transcription factors involved in the regulation of novel protein synthesis that is required for the stabilization of new memories (Bernabeu et al., 1997; Taubenfeld et al., 1999; Kida et al., 2002; Pittenger et al., 2002) and in the regulation of long-term synaptic plasticity (Xia et al., 1996; Impey et al., 1999; Roberson et al., 1999; Vanhoutte et al., 1999). Furthermore, ERK1/2 signalling appears to facilitate transcriptional events, and thus, to regulate the distributions and functions of synaptic proteins that control many forms of synaptic plasticity, including long-term potentiation (LTP), a cellular model of learning and memory (Lisman et al., 2002; Malenka, 2003; Lynch, 2004). The ERK pathway participates in the transcription of genes essential for the induction and/or maintenance of LTP (English and Sweatt, 1997; Poser and Storm, 2001; Komiyama et al., 2002; Schmitt et al., 2005). Furthermore, the CREB-mediated transcriptional pathway is strongly implicated in memory consolidation (Silva and Giese, 1994; Impey et al., 1999; Tully et al., 2003), and CREB activity is essential for long-term facilitation in Aplysia (Dash et al., 1990; Martin et al., 1997), and for long-term memory (LTM) in Drosophila (Yin et al., 1994; 1995;) and in mice (Bourtchuladze et al., 1994; Pittenger et al., 2002).
Previously, we reported that tanshinone I and its congeners isolated from the roots of Salvia miltiorrhiza Bunge (Labiatae) have memory-enhancing and ameliorating effects on scopolamine-induced memory impairment in mice (Kim et al., 2007). In addition, tanshinone I has also been reported to inhibit [3H]flunitrazepam binding (IC50= 36.2 mM) (Lee et al., 1991) and to prevent diazepam-induced memory deficits (Bourtchuladze et al., 1994). These previous reports suggest that memory enhancement by tanshinone I, like that of bicuculline, is mediated by its antagonist activity at GABAA receptors (receptor nomenclature follows Alexander et al., 2008). However, although we looked for evidence of GABAA receptor blockade by tanshinone I using an electrophysiological technique, the inward chloride current induced by GABA was not affected by tanshinone I, except at concentrations above 500 µM (unpublished data). These findings suggest that the antagonism shown by tanshinone I against diazepam-induced memory deficits might not be directly derived from GABAA receptor blockade. We hypothesized that the memory-ameliorating effect of tanshinone I against diazepam is not due to antagonism at GABAA receptors, but rather to the sharing or convergence of an intracellular signalling pathway, such as the ERK–CREB signalling pathway. In a pilot study, we found that tanshinone I and other tanshinone congeners, namely, tanshinone I, tanshinone IIA, cryptotanshinone and 15,16-dihydrotanshinone I (4 mg·kg−1, respectively), increased ERK phosphorylation within 1 h in normal mice. Here, we investigated the mode of action of tanshinone I with respect to ERK–CREB phosphorylation, and sought to determine whether tanshinone I treatment affects memory. In the present study, we also used models of learning and memory impairment in mice induced by a GABAA receptor agonist (diazepam) or an NMDA receptor antagonist (MK-801).
Methods
Animals
All animal procedures and maintenance were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985) and with the Animal Care and Use Guidelines issued by Kyung Hee University, Korea. Male ICR mice, weighing 25–30 g, were purchased from the Orient Co., Ltd, a branch of Charles River Laboratories (Seoul, Korea). The animals were housed four or five per cage, allowed access to water and food ad libitum and maintained at constant temperature (23 ± 1°C) and humidity (60 ± 10%) under a 12 h light/dark cycle (light on 0730–1930 h). We used a total of 320 mice in these experiments; different mice were used in each experiment. All efforts were made to minimize the number of animals as well as their suffering.
Step-through passive avoidance task
Passive avoidance performance was carried out in two identical light and dark square boxes (20 × 20 × 20 cm, respectively) separated by a guillotine door (5 × 5 cm), as described in our previous report (Kim et al., 2006). The illuminated compartment contained a 50 W bulb, and its floor was composed of 2 mm stainless steel rods spaced with centres 1 cm apart. A mouse was initially placed in the illuminated compartment for the acquisition trial, and the door between the two compartments was opened 10 s later. When the mouse entered the dark compartment, the guillotine door was automatically closed and an electrical foot shock (0.5 mA) of 3 s duration was delivered through the stainless steel rods. The mice were given tanshinone I (1, 2 or 4 mg·kg−1, p.o.) 40 min before the acquisition trial. Memory impairment was induced by diazepam (1 mg·kg−1, i.p.), a selective antagonist of the benzodiazepine site of the GABAA receptor or MK-801 (0.1 mg·kg−1, i.p.), an NMDA receptor channel blocker, which was administered 10 min after tanshinone I or vehicle (10% Tween 80). Control animals were administered vehicle solution only. Twenty-four hours after a single acquisition trial, the mice were subjected to retention trial and placed again in the illuminated compartment. The times taken for a mouse to enter the dark compartment after door opening was defined as latency time for both acquisition and retention trials. Latency to enter the dark compartment was recorded for up to 300 s. To investigate the effect of tanshinone I alone on memory, tanshinone I was given to mice 40 min before the acquisition trial. To avoid a ceiling effect in unimpaired animals, foot shock intensity was set at 0.25 mA. This lower intensity shock allowed a behavioural window to determine whether tanshinone I enhances learning and memory. The effect of U0126 on memory impairment in the passive avoidance task was also investigated. Our pilot studies confirmed that the effective dose that could induce memory impairment was over 1 nmol (i.c.v.). Thereafter, we adopted 1 nmol for further study. U0126 was manually injected into lateral ventricle (1 nmol, i.c.v.) under anaesthesia [using a mixture of N2O and O2 (70:30) containing 2.5% isoflurane], as previously described (Laursen and Belknap, 1986), 30 min before the acquisition trial, and animals were then returned to their home cages. The control animals were injected in the same manner with 5 µL of 0.2% DMSO.
Spontaneous locomotor behaviour test
It is acknowledged that a general increase in locomotor activities induces a skewing of latency times measured in the passive avoidance task (Konat et al., 2008), and that stress caused by i.c.v. injection and anaesthetic agents also affects those parameters (Irifune et al., 1997; Jones and Hess, 2003). In the present study, we measured the spontaneous locomotor behaviour, as described previously (Jung et al., 2006), to assess whether the anaesthetic agent or stress by i.c.v. injection with or without U0126 changed the general locomotor behaviour, and whether tanshinone I alone or combined with diazepam or MK-801 changed general locomotor behaviour. Briefly, the mice were placed in the centre of a horizontal locomotor activity box (40 × 40 × 40 cm), and their locomotor activity was measured for 10 min using the video-based Ethovision System (Noldus, Wageningen, The Netherlands). All tests were conducted 30 min after the last treatment. Horizontal locomotor activity was converted to total ambulatory distance.
Western blot analysis
A pilot study was conducted to examine the effect of tanshinone congeners on ERK phosphorylation. In the pilot study, tanshinone IIA, cryptanshinone, tanshinone I or 15,16-dihydrotanshinone I (2 mg·kg−1, p.o.) were given 40 min before death. To determine the effects of tanshinone I on the expressions of brain-derived neurotrophic factor (BDNF), phospho-CREB (pCREB) and phospho-ERK (pERK), tanshinone I (1, 2 or 4 mg·kg−1, p.o.) was also administered 40 min before death. To determine the temporal effects of tanshinone I on pCREB and pERK protein levels, tanshinone I (4 mg·kg−1, p.o.) was also given 0, 10, 30, 60, 120, 180 and 240 min before killing the mice.
During the main study programme, some mice were killed immediately after the acquisition trial in the passive avoidance task. Hippocampal tissues were homogenized in buffer [10 mM Tris–HCl (pH 7.4) containing 0.5 mM EDTA (pH 8), 0.25 M sucrose, 1 mM phenylmethylsulphonyl fluoride, 1 mM Na4VO3] containing a protease inhibitor cocktail (Complete Roche-Boehringer-Mannheim, Roche, IN, USA). After centrifugation at 18 000×g for 15 min at 4°C, supernatants were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Proteins were loaded (25 µg) and size separated by 8–10% SDS–PAGE, and gels were processed for antigens and blotted onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA) for 1 h. Blots were blocked with Tris-buffered saline containing 5% non-fat dry milk and 0.01% Tween 20, incubated with anti-pERK, anti-ERK, anti-pCREB, anti-CREB or anti-BDNF antibodies (1:1000 dilution), and then with secondary antibody conjugated to horseradish peroxidase (1:2000 dilution). Blots were detected using an ECL-detection system (Amersham Pharmacia Biotech, Sunnyvale, CA, USA).
Immunohistochemistry
The mice (n= 4 per group) were anaesthetized with pentobarbital sodium (60 mg·kg−1, i.p.) 1 h after tanshinone I administration (4 mg·kg−1, p.o.), and then perfused transcardially with 0.1 M phosphate buffer (pH 7.4) followed by ice-cold 4% paraformaldehyde. Brains were removed and post-fixed in phosphate buffer (50 mM, pH 7.4) containing 4% paraformaldehyde overnight, immersed in 30% sucrose solution [in 50 mM, phosphate-buffered saline (PBS)], and stored at 4°C until required for sectioning. Frozen brains were coronally sectioned on a cryostat at 30 µm, and stored in storage solution at 4°C until required.
Free-floating sections were incubated for 24 h in PBS (4°C) containing polyclonal anti-BDNF antibody (1:1000 dilution), anti-pCREB antibody (1:1000 dilution) or anti-pERK (1:1000 dilution), and 3% Triton X-100, 0.5 mg·mL−1 of bovine serum albumin and 1.5% normal horse serum, as previously described (Kim et al., 2006). The sections were then incubated with biotinylated secondary antibody (1:200 dilution) for 90 min, avidin–biotin–peroxidase complex (1:100 dilution) at room temperature for 1 h. The sections were then reacted with 0.02% 3,3′-diaminobenzidine and 0.01% H2O2 for about 3 min. Finally, they were mounted on gelatin-coated slides, dehydrated in an ascending alcohol series and cleared in xylene. After each incubation step mentioned earlier, the sections were washed three times with PBS.
Quantitative immunostaining and Western blotting
Cell counts in the hippocampal CA1 layer were determined using a computerized image analysis system (Leica Microsystems AG, Wetzlar, Germany) in six sections per mouse by one person unaware of the treatments given. Film densitometry analysis of Western blots was performed using a Quantity One Image Analysis System (version 4.6.3, Bio-Rad Laboratories, Hercules, CA, USA). Levels of phosphorylated ERK and CREB expression were determined by calculating the ratio of phosphor-protein density to total protein density in same membranes. BDNF expression levels were normalized to the actin levels in same membranes.
Statistics
Values are expressed as means ± SEM. The Kruskal–Wallis non-parametric test was used to analyse passive avoidance task data. When results were significant, treatment groups were compared using Tukey's post hoc test. One-way analysis of variance (anova) was used to analyse Western blot, immunohistochemical and spontaneous locomotor behavioural data, and when results were found to be significant, Tukey's post hoc test was used to compare treatment groups. Two-way anova was used to analyse group interaction, and when results were significant, Tukey's post hoc test was used to compare treatment groups. Statistical significance was accepted for P values of <0.05.
Materials
Tanshinone I (Figure 1) and its congeners (tanshinone IIA, cryptotanshinone and 15,16-dihydrotanshinone I) were isolated by the authors (Kim et al., 2007), and the chemical purity of tanshinone I was 96.1%. MK-801 (an NMDA receptor channel antagonist) and U0126 (an ERK1 and ERK2 inhibitor) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Diazepam and pentobarbital sodium were obtained from DaeWon Pharmaceutical Co. (Seoul, Korea) and ChoongWae Pharma Co. (Seoul, Korea) respectively. Anti-BDNF, anti-ERK, anti-pERK, anti-CREB and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and anti-pCREB was purchased from Upstate Lake Placid (Upstate, Lake Placid, NY, USA). Biotinylated secondary antibody and avidin–biotin–peroxidase complex were obtained from Vector (Burlingame, CA, USA). All other materials were of the highest grade commercially available. Tanshinone I and its congeners were suspended in a 10% aqueous Tween 80 solution.
Figure 1.
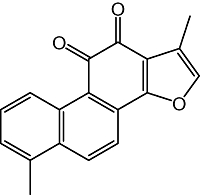
Structure of tanshinone I.
Results
Effect of tanshinone I on ERK–CREB signalling
Of the tanshinone congeners, namely, tanshinone I (2 mg·kg−1), tanshinone IIA (2 mg·kg−1), cryptotanshinone (2 mg·kg−1) and 15,16-dihydrotanshinone I (2 mg·kg−1), only tanshinone I (2 mg·kg−1, p.o.) was found to markedly increase ERK phosphorylation in the hippocampus within 40 min (Figure 2A). To determine the effective doses of tanshinone I on ERK–CREB signalling, it was administered at 1, 2 or 4 mg·kg−1, and 40 min later the mice were killed for Western blot and immunohistochemical analyses. Tanshinone I at 2 or 4 mg·kg−1 was found to significantly increase pERK protein levels in the hippocampus over those in vehicle-treated control mice [F (3, 12) = 14.497, P < 0.001, Figure 2B,C]. Furthermore, these results were supported by immunohistochemical findings [F (1, 6) = 15.015, P= 0.008, Figure 3D]. The transcription factor CREB is a key signalling molecule activated by pERK and is involved in learning and memory (Tully et al., 2003; Carlezon et al., 2005). Tanshinone I (2 or 4 mg·kg−1) was found to increase pCREB protein levels in the hippocampus versus vehicle-treated controls [F (3, 12) = 15.221, P < 0.001, Figure 2B,C], and our immunohistochemical analysis results supported this finding [F (1, 6) = 41.336, P < 0.001, Figure 3C]. On the other hand, levels of BDNF, a target protein of pCREB, appeared to increase, but this did not reach statistical significance by Western blotting [F (3, 12) = 1.347, P= 0.306, Figure 2B,C] or by immunostaining [F (1, 6) = 10.791, P= 0.654, Figure 3B]. In addition, tanshinone I (4 mg·kg−1) increased ERK–CREB signalling within 30 min in the hippocampus (Figure 2D). Thus, in subsequent experiments undertaken to investigate its memory-related activity, tanshinone I was given 40 min before testing.
Figure 2.
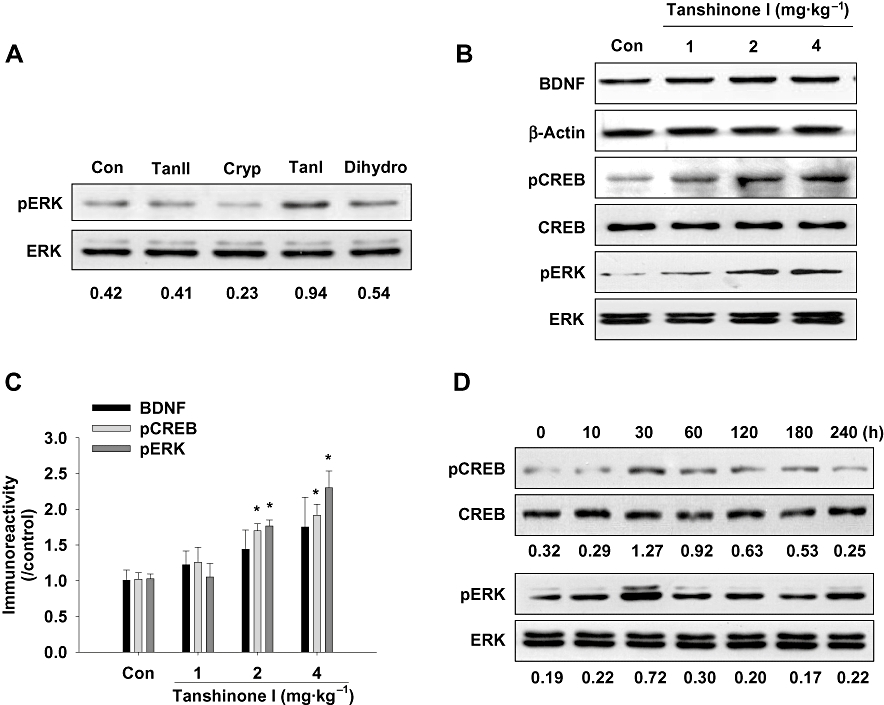
Tanshinone I increases brain-derived neurotrophic factor (BDNF), phosphorylated cAMP response element binding protein (pCREB) and phosphorylated extracellular signal-regulated kinase (pERK) protein levels in the hippocampus. (A) pERK immunoreactivities in hippocampal tissues 40 min after treatment with tanshinone IIA (TanII, 4 mg·kg−1, p.o.), cryptotanshinone (Cryp, 4 mg·kg−1, p.o.), tanshinone I (TanI, 4 mg·kg−1, p.o.) or 15,16-dihydrotanshinone I (Dihydro, 4 mg·kg−1, p.o.). (B and C) BDNF, pCREB and pERK immunoreactivities as determined by Western blotting and densitometry. In graph C, the BDNF scale shows normalized ratios versus β-actin, whereas the other normalized ratios were calculated versus their respective total levels. Tanshinone I (1, 2 or 4 mg·kg−1, p.o.) or vehicle solution (the same volume of 10% Tween 80) was administered to the mice 40 min before death. Data represent means ± SEM (n= 4 per group). *P < 0.05 versus respective vehicle controls. (D) Representative immunoreactivities of pCREB and pERK in hippocampal tissues at various times after tanshinone I treatment (4 mg·kg−1, p.o.). Values under the immunoblots represent immunoreactivity ratios versus corresponding individual total protein levels.
Figure 3.
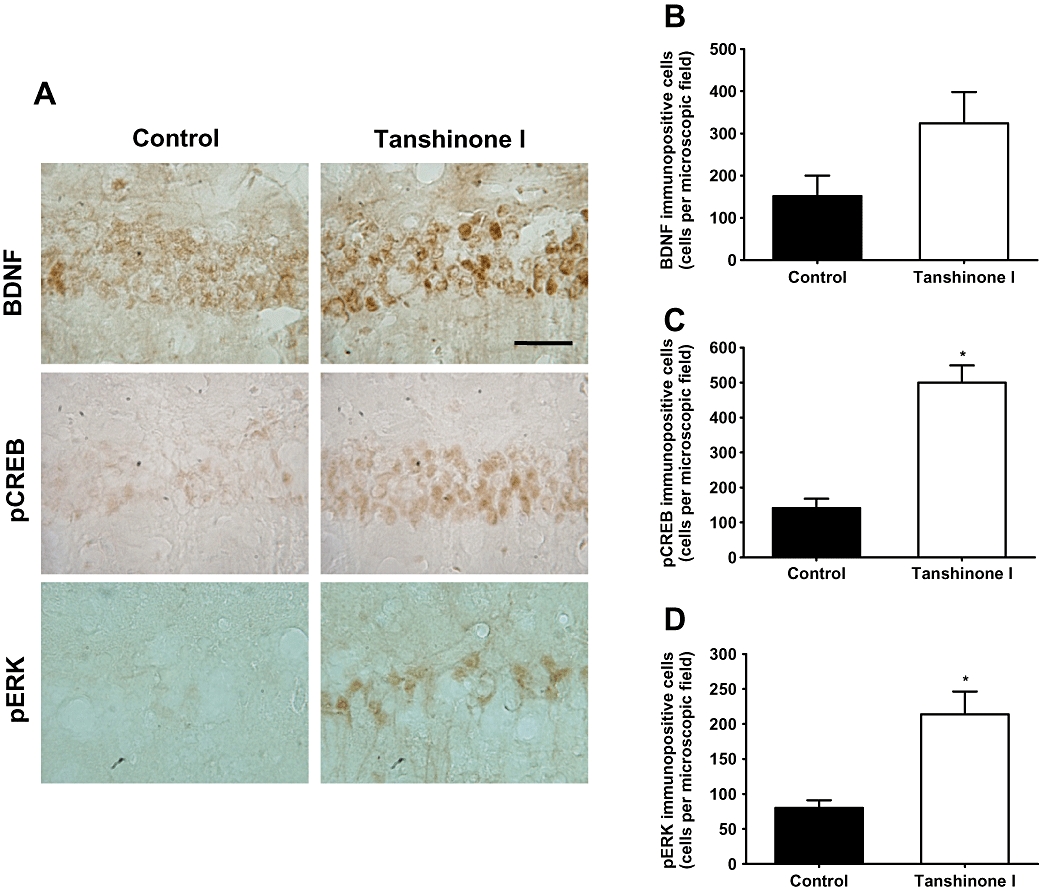
Photomicrographs of brain-derived neurotrophic factor (BDNF)-, phosphorylated cAMP response element binding protein (pCREB)- and phosphorylated extracellular signal-regulated kinase (pERK)-positive cells in the hippocampal CA1 region (A). Tanshinone I (4 mg·kg−1, p.o.) was administered 40 min before transcardial perfusion. Summary data of the numbers of BDNF- (B), pCREB- (C) and pERK- (D) positive cells in CA1. Data represent means ± SEM for six determinations in each region for four animals. *P < 0.05, compared with untreated controls. Magnification: 1000×. Bar = 50 µm.
Effect of tanshinone I on learning and memory in the passive avoidance task
We measured the effects of stress caused by i.c.v. injection with or without U0126 or anaesthetic agent [a mixture of N2O and O2 (70:30) containing 2.5% isoflurane] on the general locomotor behaviour. As shown in Figure 4A, anaesthetic agent and i.c.v. injection did not affect general locomotor activities. For this lack of effect, U0126 was delivered into the system as outlined earlier. U0126 induced memory impairment at over 1 nmol (in 5 µL) as measured in the passive avoidance task [H (4) = 22.278, P < 0.001, Figure 4B].
Figure 4.
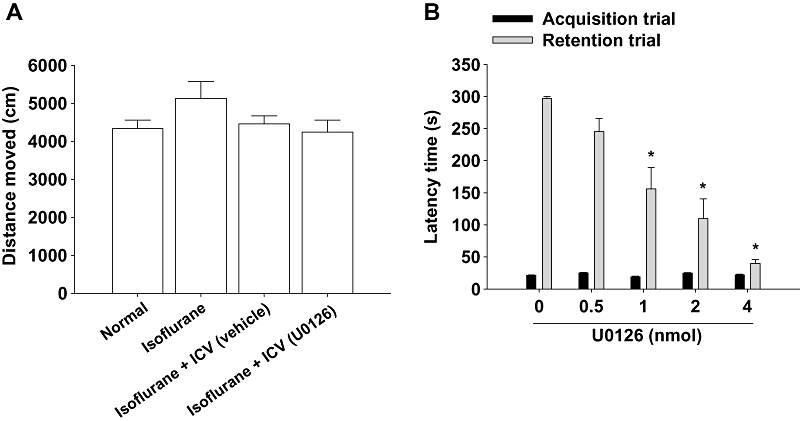
(A) Effect of anaesthesia, i.c.v. injection of vehicle or U0126 on spontaneous locomotor behaviour in open field. All treatments were conducted 30 min before the test. Total distance moved was measured for 10 min using a video-based Ethovision System (Nodulus, Wageningen, The Netherlands) as described in Methods. Data represent means ± SEM (n= 7 per group). Normal, group without any treatment; Isoflurane, group treated with a mixture of N2O and O2 (70:30) containing 2% isoflurane for 5 min; Isoflurane + vehicle (i.c.v.), group treated with 5 µL of 0.2% DMSO solution by i.c.v. injection under a mixture of N2O and O2 (70:30) containing 2% isoflurane within 5 min; Isoflurane + U0126 (i.c.v.), group treated with 1 nmol of U0126 in 5 µL of 0.2% DMSO solution by i.c.v. injection under the same anaesthetic conditions. (B) Effect of U0126 on the memory performances in the passive avoidance task. U0126 (0.5, 1, 2 or 4 nmol in 5 µL, i.c.v.) was injected 30 min before acquisition trial of the passive avoidance task. The control animals were treated with vehicle solution (0.2% DMSO solution) in the same manner. Data represent means ± SEM (n= 7 per group). *P < 0.05 versus vehicle-treated controls.
To investigate whether the effect of tanshinone I on ERK–CREB signalling affects learning and memory, tanshinone I (1, 2 or 4 mg·kg−1, p.o.) was given 40 min before the acquisition trial. Tanshinone I (2 or 4 mg·kg−1) was found to significantly increase latency time in the passive avoidance task versus vehicle-treated controls [H (4) = 11.671, P= 0.020, Figure 5A]. However, this effect of tanshinone I at 4 mg·kg−1 was blocked by U0126 (1 nmol) [H (3) = 43.070, P < 0.001, Figure 5B]. Furthermore, this tanshinone I × U0126 interaction showed a significant group effect [F (1, 56) = 6.532, P= 0.013]. To investigate ERK–CREB signal changes in the hippocampus, the mice were killed immediately after the acquisition trial and Western blot analysis was conducted. It was found that tanshinone I significantly increased pERK protein levels [F (3, 20) = 38.479, P < 0.001], and that this increase was blocked by U0126 (Figure 5C,D). In addition, similar results were observed for pCREB protein levels in the hippocampus [F (3, 20) = 46.861, P < 0.001, Figure 5C,D]. Furthermore, the interaction between tanshinone I and U0126 showed a significant group effect on pERK [F (1, 20) = 6.142, P= 0.022] and pCREB levels [F (1, 20) = 11.319, P= 0.003]. Low levels of pERK and pCREB were shown in normal mice that had not undergone the acquisition trial in the passive avoidance box (Figure 5E).
Figure 5.
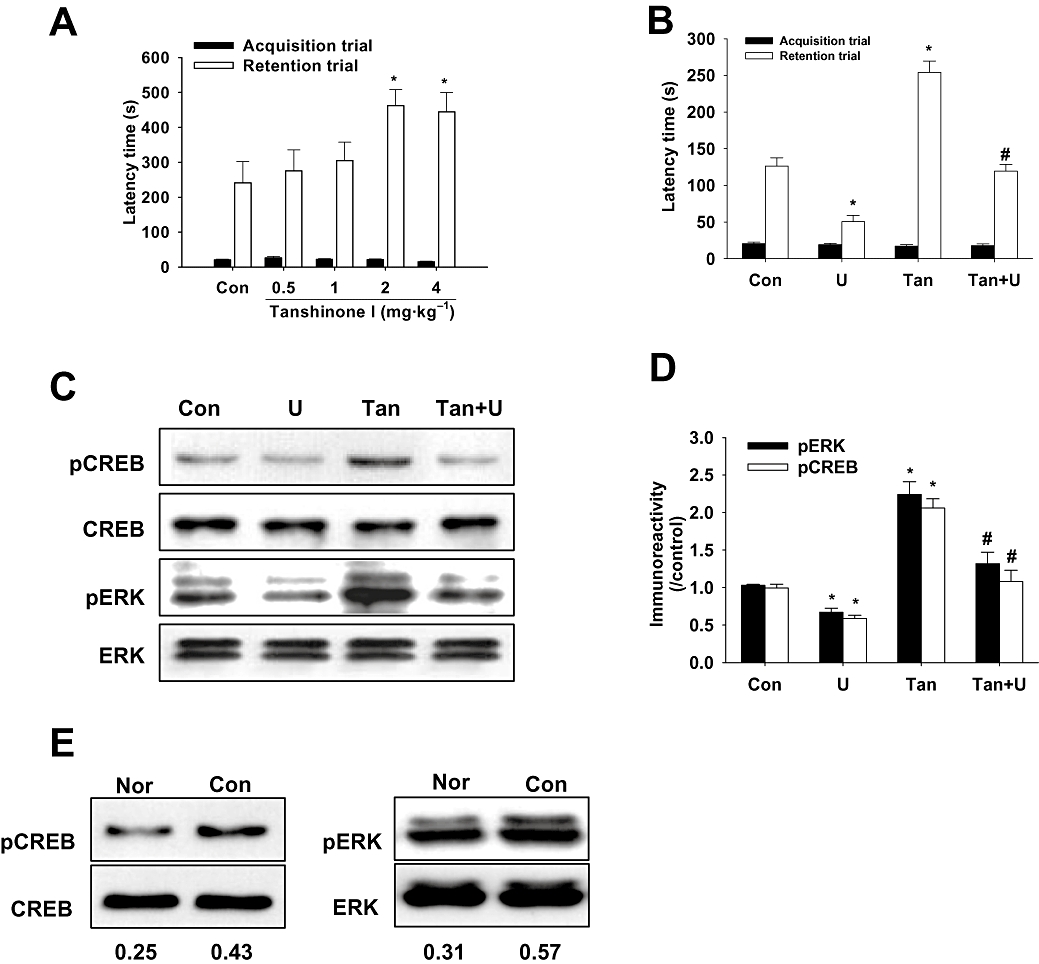
The effects of tanshinone I on memory and extracellular signal-regulated kinase (ERK)–cAMP response element binding protein (CREB) signalling pathway involvement. Tanshinone I (0.5, 1, 2 or 4 mg·kg−1, p.o.) increased passive avoidance task latency (A). Data represent means ± SEM (n= 10 per group). *P < 0.05 versus vehicle controls. Increased latency by tanshinone I (4 mg·kg−1, p.o.) was reversed by U0126 (1 nmol, i.c.v.), an ERK1 and ERK2 inhibitor (B). Data represent means ± SEM (n= 15 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus tanshinone I only-treated animals. Immunoreactivities of phospho-CREB (pCREB) and phosphorylated ERK (pERK) by Western blotting after treatment with tanshinone I and/or U0126 (C) and densitometry results (D). Data represent means ± SEM (n= 6 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus tanshinone I-treated mice. Con, control; U, U0126; Tan, tanshinone I. (E) Representative immunoreactivities of pCREB and pERK in the hippocampal tissues of non-trained normal mice (Nor) and of passive avoidance test-trained control mice (Con). Values under the immunoblots represent immunoreactivity ratios versus corresponding individual total protein levels.
Effect of tanshinone I on the memory impairment induced by diazepam in the passive avoidance task
We examined whether tanshinone I affects the memory impairments induced by diazepam (a GABAA receptor site agonist), and whether diazepam inhibits the activations of ERK and CREB in the hippocampus. Tanshinone I (2 or 4 mg·kg−1, p.o.) significantly prevented the reduction in latency times caused by diazepam administration [H (4) = 29.019, P < 0.001, Figure 6A] without any changes in locomotor activity (Figure 6F). Moreover, these effects of tanshinone I on memory impairment induced by diazepam were blocked by U0126 (1 nmol) [H (3) = 18.176, P < 0.001, Figure 6B], and tanshinone I × U0126 interaction showed a significant group effect [F (1, 25) = 5.505, P= 0.027]. Moreover, in the ERK–CREB signalling study, diazepam reversed the pERK and pCREB protein up-regulation induced by the acquisition trial, and tanshinone I significantly enhanced diazepam-induced pERK [F (3, 8) = 19.186, P < 0.001] and pCREB down-regulation [F (3, 8) = 27.843, P < 0.001] (Figure 6C,D). Moreover, these effects of tanshinone I on pERK and pCREB protein levels during diazepam-induced signal impairment were blocked by U0126 (P < 0.05). In addition, the interaction between tanshinone I and U0126 showed a significant group effect on pERK [F (1, 16) = 11.561, P= 0.004] and on pCREB levels [F (1, 16) = 5.123, P= 0.038]. Low levels of pERK and pCREB were shown in the normal mice that did not undergo the acquisition trial in the passive avoidance box (Figure 6E).
Figure 6.
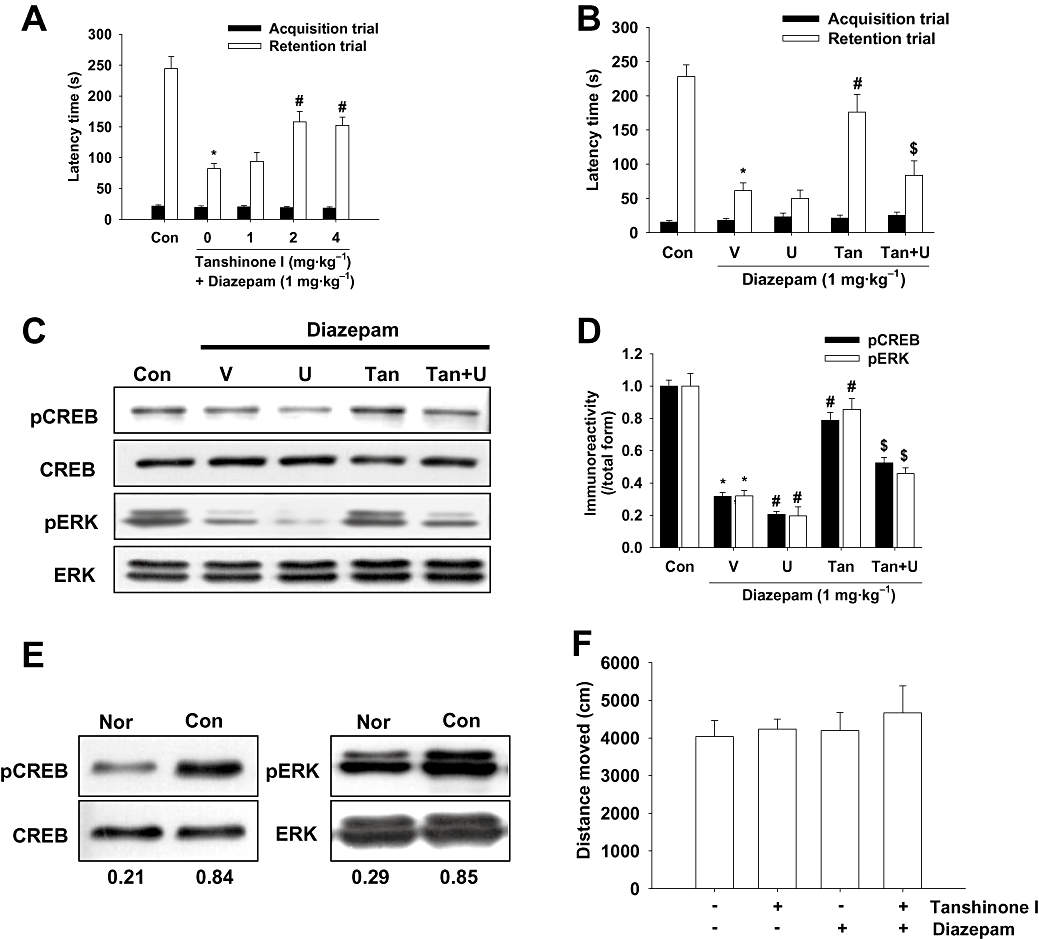
The effect of tanshinone I on memory impairment induced by diazepam via extracellular signal-regulated kinase (ERK)–cAMP response element binding protein (CREB) signalling. (A) Effect of tanshinone I (1, 2 and 4 mg·kg−1, i.p.) on passive avoidance task latency. Tanshinone I was administered 40 min before, and diazepam 30 min before the acquisition trial. Data represent means ± SEM (n= 10 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus diazepam-treated mice. (B) Increased passive avoidance task latency induced by tanshinone I (4 mg·kg−1, p.o.) was reversed by U0126 (U, 1 nmol, i.c.v.). Tanshinone I was administered 40 min before, and diazepam and/or U0126 30 min before the acquisition trial. Latency times were determined as described in Methods. Data represent means ± SEM (n= 7 or 8 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus diazepam-treated mice, $P < 0.05 versus diazepam and tanshinone I-co-treated mice. Immunoreactivities of phospho-CREB (pCREB) and phosphorylated ERK (pERK) by Western blot analysis in tanshinone I-, diazepam- and/or U0126-treated mice (C), and corresponding densitometric analysis results (D). Tanshinone I was administered 40 min before, and diazepam (1 mg·kg−1, i.p.) and/or U0126 (1 nmol, i.c.v.) 30 min before the acquisition trial for the passive avoidance trial. The mice were killed immediately after the acquisition trial. Data represent means ± SEM (n= 3 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus diazepam-treated mice, $P < 0.05 versus diazepam and tanshinone I-co-treated mice. Con, control; V, vehicle; U, U0126; Tan, tanshinone I. (E) Representative immunoreactivities of pCREB and pERK in the hippocampal tissues of non-trained normal mice (Nor) and passive avoidance test-trained control mice (Con). Values under the immunoblots represent immunoreactivity ratios versus corresponding individual total protein levels. (F) Effect of tanshinone I alone or combined with diazepam on spontaneous locomotor behaviour in the open field. The test was conducted as described in Methods. Data represent means ± SEM (n= 10 per group).
Effect of tanshinone I on memory impairment induced by MK-801 in the passive avoidance task
Several studies have reported that MK-801, an NMDA receptor antagonist, blocks both associative learning and ERK activation in the hippocampus (Atkins et al., 1998; Cammarota et al., 2000). We tested whether tanshinone I affects memory impairments induced by MK-801 and whether MK-801 inhibits ERK or CREB activation in the hippocampus. In the pilot study, we observed that MK-801 significantly decreased latency time when administered at over 0.1 mg·kg−1 in the passive avoidance task (data not shown). Based on these findings, we used a dose of 0.1 mg·kg−1 of MK-801 for MK-801-induced memory impairment testing. Tanshinone I (2 or 4 mg·kg−1, p.o.) significantly reversed the latency time reduction induced by MK-801 [H (4) = 35.140, P < 0.001, Figure 7A]. As shown in Figure 7F, tanshinone I did not affect MK-801-induced hyperactivity, suggesting that the ameliorating effects of tanshinone I on the MK-801-induced memory impairments are not derived from the changes of locomotor behaviour. Moreover, the effect of tanshinone I on memory impairment induced by MK-801 was blocked by U0126 (1 nmol) [H (3) = 17.569, P < 0.001, Figure 7B], and the tanshinone I × U0126 interaction showed a significant group effect [F (1, 20) = 4.375, P= 0.049]. In the ERK–CREB signalling study, MK-801 was found to block the pERK and pCREB protein up-regulation induced by the acquisition trial, and tanshinone I significantly reversed MK-801-induced pERK [F (3, 8) = 10.499, P= 0.001] and pCREB [F (3, 8) = 24.640, P < 0.001] down-regulation at the protein level (Figure 7C,D). In addition, this effect of tanshinone I on pERK and pCREB protein levels during MK-801-induced signal impairment was blocked by U0126 (P < 0.05). Moreover, the interaction between tanshinone I and U0126 showed a significant group effect on pERK [F (1, 16) = 7.359, P= 0.015] and on pCREB levels [F (1, 16) = 16.260, P < 0.001]. Low levels of pERK and pCREB were shown in the normal mice that did not undergo the acquisition trial in the passive avoidance box (Figure 7E).
Figure 7.
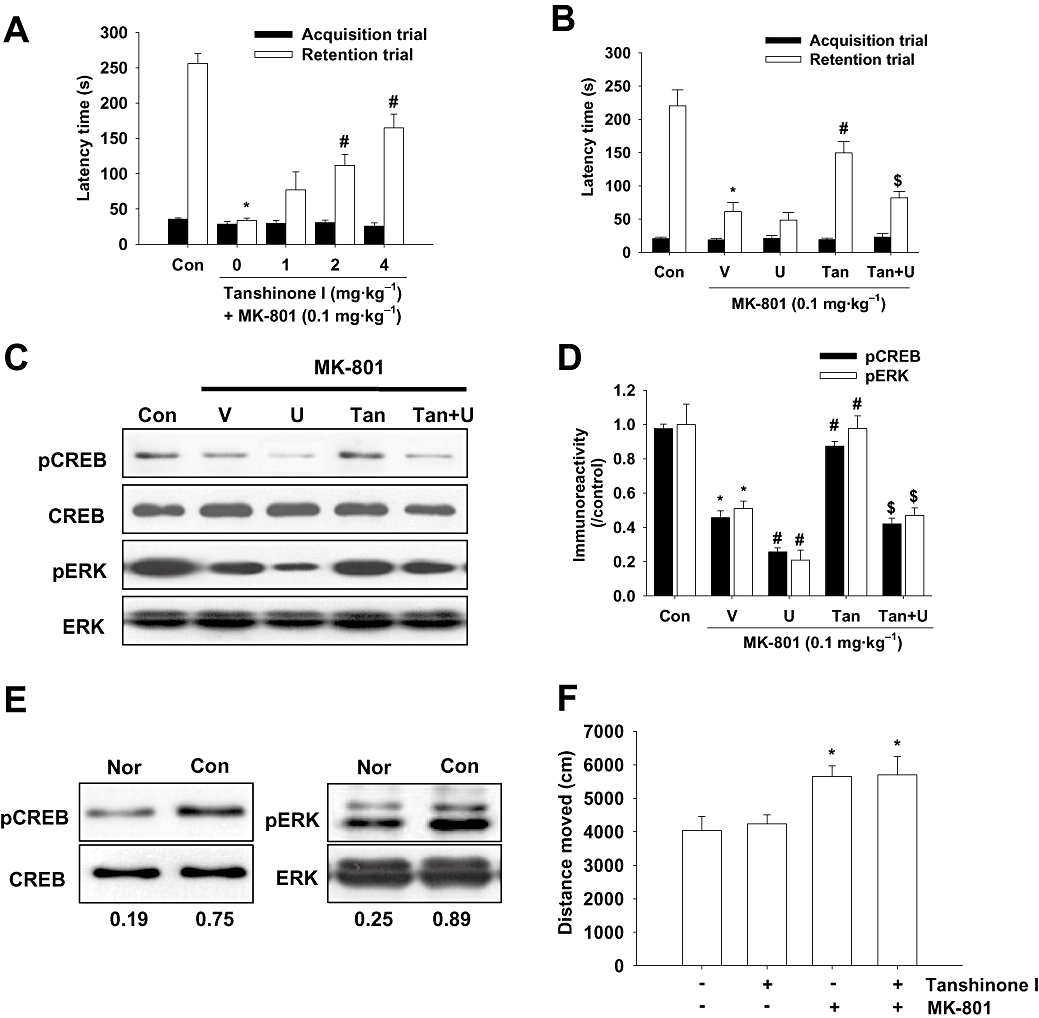
The effects of tanshinone I on memory impairment induced by MK-801 via extracellular signal-regulated kinase (ERK)–cAMP response element binding protein (CREB) signalling. (A) Effect of tanshinone I (1, 2 or 4 mg·kg−1, i.p.) on passive avoidance task latency. Tanshinone I was administered 40 min before, and MK-801 30 min before the acquisition trial. Data represent means ± SEM (n= 10 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus diazepam-treated mice. Latency increases induced by tanshinone I (4 mg·kg−1, p.o.) were reversed by U0126 (U, 1 nmol, i.c.v.) (an ERK1 and ERK2 inhibitor) (B). Tanshinone I was administered 40 min before, and MK-801 and/or U0126 30 min before the acquisition trial. Latencies were measured as described in Methods. Data represent means ± SEM (n= 6 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus diazepam-treated mice, $P < 0.05 versus diazepam and tanshinone I-co-treated mice. Immunoreactivities of phospho-CREB (pCREB) and phosphorylated ERK (pERK) by Western blotting analysis after treatment with tanshinone I and/or MK-801 and/or U0126 (C), and by densitometric analysis (D). Tanshinone I was administered 40 min before, and MK-801 (1 mg·kg−1, i.p.) and/or U0126 (1 nmol, i.c.v.) were administered 30 min before the acquisition trial. The mice were killed immediately after the acquisition trial for the passive avoidance task. Data represent means ± SEM (n= 3 per group). *P < 0.05 versus vehicle controls, #P < 0.05 versus diazepam-treated mice, $P < 0.05 versus MK-801 and tanshinone I-co-treated mice. Con, control; V, vehicle; U, U0126; Tan, tanshinone I. (E) Representative immunoreactivities of pCREB and pERK in the hippocampal tissues of non-trained normal mice (Nor) and trained control mice (Con). Values under the immunoblots represent immunoreactivity ratios versus corresponding individual total protein levels. (F) Effect of tanshinone I alone or combined with MK-801 on spontaneous locomotor behaviour in the open field. The test was conducted as described in Methods. Data represent means ± SEM (n= 10 per group). *P < 0.05 versus vehicle controls.
Discussion
The present study demonstrated that tanshinone I activated ERK–CREB signalling pathways in normal mice and ameliorated memory impairments induced by a GABAA receptor agonist or an NMDA receptor antagonist, accompanied by the inhibition of learning-associated ERK and CREB activation in the mouse hippocampus.
Recently, ERK1 and 2, which are important downstream signalling mediators of several receptors, have been implicated in learning and memory (Thiels and Klann, 2001). Furthermore, rats subjected to avoidance learning showed significant and specific increases in the activated forms of ERK1 and 2 in the hippocampus (Cammarota et al., 2005), which concur with the results of the present study (Figures 5E, 6E and 7E). CREB, a transcription factor, is also required for hippocampus-dependent LTM formation (Silva et al., 1998), and the activation of CREB by phosphorylation requires the activation of ERKs, PKA or CaMKII. Furthermore, this phosphorylation of CREB results in BDNF or c-fos expression, and these genes are targets of CREB (Tao et al., 1998). Previously, we found that a group of tanshinone congeners isolated from Salvia miltiorrhiza enhanced learning and memory in the passive avoidance task. If these effects were mediated by ERK signalling, these tanshinone congeners would be expected to activate ERK or its downstream pathway including CREB. In the present study, only tanshinone I was found to increase ERK phosphorylation in the hippocampus over vehicle-treated controls, which suggests that the learning and memory-enhancing effects of tanshinone I were associated with the ERK pathway. Therefore, we used tanshinone I to study the mechanism of learning and memory associated with ERK–CREB signalling, and found that tanshinone I significantly enhanced learning and memory in the passive avoidance task, and ameliorated spatial learning and memory impairment induced by scopolamine in the Morris water maze task (data not shown), which concurs with our previous findings (Kim et al., 2007). Furthermore, tanshinone I significantly increased CREB phosphorylation (a memory formation marker) in the hippocampus, which suggests that CREB activation by tanshinone I was mediated via ERK phosphorylation. Moreover, similar results were also observed in the amygdala region (data not shown), which suggests that tanshinone I is also associated with emotion-related passive avoidance memory, because the amygdala region is believed to play a role in emotional responses (Ambrogi Lorenzini et al., 1997; Cimadevilla et al., 2007).
The inhibition of ERK phosphorylation causes cognitive impairments, and previous observations suggest that MEK inhibition perturbs working memory in the rat and that hippocampal ERK phosphorylation plays a crucial role in spatial working memory (Nagai et al., 2006). These findings suggest that the inhibition of ERK activation might reverse tanshinone I-induced ERK and CREB phosphorylations, and attenuate learning and memory. As was expected, in the present study, U0126 (an ERK1 and ERK2 inhibitor) reduced the phosphorylation of ERK and CREB in the hippocampal tissues of foot-shocked mice and in those of tanshinone I-treated mice. Furthermore, U0126 antagonized the learning and memory-enhancing effects of tanshinone I. Taken together, these findings suggest that the learning and memory-enhancing effects of tanshinone I are associated with the phosphorylation of ERK and CREB (Maher et al., 2006).
Extensive evidence now indicates that GABAA receptor agonists or antagonists affect learning and memory (Zarrindast et al., 2002; 2004; Möhler et al., 2004). Recently, Kalluri and Ticku (2002) demonstrated a decrease in phosphorylated MAP kinase staining by flurazepam (a GABAA receptor agonist). These findings suggest the possibility that GABAA receptor agonists, like diazepam, decrease ERK phosphorylation, and that this results in decreased learning and memory associated with CREB phosphorylation, as has been reported for flurazepam. In the present study, diazepam reduced ERK phosphorylation by 73%, and CREB phosphorylation by 79% in the hippocampal region compared with the control mice. Furthermore, tanshinone I significantly prevented the reductions in the phosphorylation of ERK and CREB induced by diazepam. In addition, tanshinone I ameliorated diazepam-induced memory impairment, which concurs with a previous report (Kim et al., 2007). However, as yet, we have been unable to identify any corresponding Cl- current changes in hippocampal slices (data not shown). Furthermore, the binding affinity of tanshinone I to GABAA receptors is only moderate (Lee et al., 1991), and thus, it is unlikely that the ameliorating effect of tanshinone I on diazepam-induced learning and memory impairment is directly derived from its binding to GABAA receptors. In addition, it is unclear whether tanshinone I or its active metabolite(s) are responsible for these results. Further research is needed to clarify these issues.
The ERK signalling pathway is also linked to NMDA receptor activation via a cAMP-dependant mechanism (Okuyama et al., 2004; Seo et al., 2007). Moreover, activation of NMDA receptors and the resulting Ca2+ influx activate CaMKII, which in turn activates Ras-GTP, which initiates a series of kinase cascades, including the Raf-1, MAP kinase/ERK kinase and ERK cascades (Thomas and Huganir, 2004). Accordingly, blockade of the NMDA receptor can reduce ERK activation (Chandler et al., 2001). Conversely, increased ERK activation can attenuate NMDA receptor blockade-induced physical and behavioural changes. Furthermore, in the present study, we found that ERK and CREB were hyperphosphorylated in the hippocampal tissues of mice that had completed the acquisition trial in the passive avoidance task, but that this phosphorylation was lower in MK-801-treated mice (Nakajima et al., 2007). In addition, tanshinone I reversed the MK-801-induced inhibition of ERK and CREB phosphorylation in the hippocampal tissues of mice that performed the acquisition trial. Furthermore, the ameliorating effect of tanshinone I on MK-801-induced memory impairment was blocked by U0126. Accordingly, these results suggest that the ameliorating effect of tanshinone I on MK-801-induced cognitive impairment was related to ERK activation in the hippocampus.
Given previous findings on this topic (Atkins et al., 1998; Cammarota et al., 2000; Athos et al., 2002; Levenson et al., 2004; Chwang et al., 2006), our data indicate that inhibition of the ERK cascade hinders learning and memory augmentation by tanshinone I. As we previously described, tanshinone I reverses the cognitive impairments induced by scopolamine (a muscarinic receptor antagonist) and diazepam (a GABAA receptor agonist) (Kim et al., 2007). In the present study, we also found that tanshinone I ameliorated the learning and memory deficits induced by MK-801 (an NMDA receptor antagonist). In particular, the reversal by tanshinone I of the effects of diazepam or MK-801 was blocked by U0126, which inhibits ERK phosphorylation. These results suggest that ERK phosphorylation and downstream CREB phosphorylation play crucial roles in tanshinone I-induced learning and memory enhancement. Moreover, ERK phosphorylation should be a common pathway for the learning and memory-related behavioural changes observed after GABAA receptor agonist or NMDA receptor antagonist treatment, which suggests that the ERK cascades in the hippocampus are a potential target for the development of a cognitive improvement agent.
In conclusion, the present study demonstrates that tanshinone I can increase signalling by ERK/CREB in the hippocampus, and enhance learning and memory. Moreover, tanshinone I was found to reverse the learning and memory impairments associated with NMDA or GABAA receptors by activating ERK signalling in the hippocampus. We conclude that tanshinone I is a potential candidate for pre-clinical studies aimed at treating cognitive deficits associated with the ERK and CREB pathways.
Acknowledgments
This research was supported by the Korean Food and Drug Administration (#S-06-02-2-CHM-230-0-B, 2005).
Glossary
Abbreviations:
- BDNF
brain-derived neurotrophic factor
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CREB
cAMP response element binding protein
- ERK1/2
extracellular signal-regulated kinase 1/2
- LTM
long-term memory
- LTP
long-term potentiation
- MEK
mitogen-activated protein kinase kinase
- PMSF
phenylmethylsulphonyl fluoride
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. 2008 revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response memory trace. Brain Res. 1997;768:242–248. doi: 10.1016/s0006-8993(97)00651-3. [DOI] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signalling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041–7016. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, et al. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Mol Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Barros DM, Vianna MR, Izquierdo LA, Medina JH, et al. Retrieval and the extinction of memory. Cell Mol Neurobiol. 2005;25:465–474. doi: 10.1007/s10571-005-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl d-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Mendez M, Mendez-Lopez M, Arias JL. Unilateral hippocampal blockade reveals that one hippocampus is sufficient for learning a passive avoidance task. J Neurosci Res. 2007;85:1138–1142. doi: 10.1002/jnr.21222. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signalling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Irifune M, Sato T, Nishikawa T, Masuyama T, Nomoto M, Fukuda T, et al. Hyperlocomotion during recovery from isoflurane anesthesia is associated with increased dopamine turnover in the nucleus accumbens and striatum in mice. Anesthesiology. 1997;86:464–475. doi: 10.1097/00000542-199702000-00022. [DOI] [PubMed] [Google Scholar]
- Jones MD, Hess EJ. Norepinephrine regulates locomotor hyperactivity in the mouse mutant coloboma. Pharmacol Biochem Behav. 2003;75:209–216. doi: 10.1016/s0091-3057(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Jung JW, Ahn NY, Oh HR, Lee BK, Lee KJ, Kim SY, et al. Anxiolytic effects of the aqueous extract of Uncaria rhynchophylla. J Ethnopharmacol. 2006;108:193–197. doi: 10.1016/j.jep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Ticku MK. Role of GABA(A) receptors in the ethanol-mediated inhibition of extracellular signal-regulated kinase. Eur J Pharmacol. 2002;451:51–54. doi: 10.1016/s0014-2999(02)02100-3. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kim DH, Hung TM, Bae KH, Jung JW, Lee S, Yoon BH, et al. Gomisin A improves scopolamine-induced memory impairment in mice. Eur J Pharmacol. 2006;542:129–135. doi: 10.1016/j.ejphar.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jeon SJ, Jung JW, Lee S, Yoon BH, Shin BY, et al. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur J Pharmacol. 2007;574:140–147. doi: 10.1016/j.ejphar.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, et al. SynGAP regulates ERK/MAPK signalling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konat GW, Kraszpulski M, James I, Zhang HT, Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008;23:325–333. doi: 10.1007/s11011-008-9100-y. [DOI] [PubMed] [Google Scholar]
- Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Lee CM, Wong HN, Chui KY, Choang TF, Hon PM, Chang HM. Miltirone, a central benzodiazepine receptor partial agonist from a Chinese medicinal herb Salvia miltiorrhiza. Neurosci Lett. 1991;127:237–241. doi: 10.1016/0304-3940(91)90802-z. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, et al. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Crestani F, Hensch T, Rudolph U. Specific GABA(A) circuits in brain development and therapy. Biochem Pharmacol. 2004;68:1685–1690. doi: 10.1016/j.bcp.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Nagai T, Kamei H, Dohniwa M, Takayanagi M, Suzuki M, Matsuya T, et al. Involvement of hippocampal extracellular signal-regulated kinase 1/2 in spatial working memory in rats. Neuroreport. 2006;17:1453–1457. doi: 10.1097/01.wnr.0000233095.74913.88. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamakuni T, Matsuzaki K, Nakata N, Onozuka H, Yokosuka A, et al. Nobiletin, a citrus flavonoid, reverses learning impairment associated with N-methyl-d-aspartate receptor antagonism by activation of extracellular signal-regulated kinase signalling. J Pharmacol Exp Ther. 2007;321:784–790. doi: 10.1124/jpet.106.117010. [DOI] [PubMed] [Google Scholar]
- Okuyama N, Takagi N, Kawai T, Miyake-Takagi K, Takeo S. Phosphorylation of extracellular-regulating kinase in NMDA receptor antagonist-induced newly generated neurons in the adult rat dentate gyrus. J Neurochem. 2004;88:717–725. doi: 10.1046/j.1471-4159.2003.02215.x. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, et al. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Poser S, Storm DR. Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. Int J Dev Neurosci. 2001;19:387–394. doi: 10.1016/s0736-5748(00)00094-0. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JM, Guire ES, Saneyoshi T, Soderling TR. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J Neurosci. 2005;25:1281–1290. doi: 10.1523/JNEUROSCI.4086-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MS, Kim SH, Ahn YM, Kim Y, Jeon WJ, Yoon SC, et al. The effects of repeated administrations of MK-801 on ERK and GSK-3β signalling pathways in the rat frontal cortex. Int J Neuropsychopharmacol. 2007;10:359–368. doi: 10.1017/S1461145706006869. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Giese KP. Plastic genes are in! Curr Opin Neurobiol. 1994;4:413–420. doi: 10.1016/0959-4388(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Bear MF, Alberini CM. A molecular correlate of memory and amnesia in the hippocampus. Nat Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Thiels E, Klann E. Extracellular signal-regulated kinase, synaptic plasticity, and memory. Rev Neurosci. 2001;12:327–345. doi: 10.1515/revneuro.2001.12.4.327. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pagès C, Besson MJ, Hipskind RA, et al. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. J Psychopharmacol. 2002;16:313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Shamsi T, Azarmina P, Rostami P, Shafaghi B. GABAergic system and imipramine-induced impairment of memory retention in rats. Eur Neuropsychopharmacol. 2004;14:59–64. doi: 10.1016/s0924-977x(03)00068-3. [DOI] [PubMed] [Google Scholar]


