Abstract
Aim
Nanosized particles (NPs) enriched in hydroxyapatite and protein isolated from calcified human tissue accelerate occlusion of endothelium-denuded arteries when injected intravenously into rabbits. Since platelet aggregation and secretory processes participate in normal hemostasis, thrombosis and vascular remodeling, experiments were designed to determine if these biologic NPs alter specific platelet functions in vitro.
Methods
Platelet-rich plasma was prepared from citrate anticoagulated human blood. Platelet aggregation and ATP secretion were monitored in response to thrombin receptor agonists peptide (10 μM) or convulxin (50 μg/ml) prior to and following 15 min incubation with either control solution, human-derived NPs, bovine-derived NPs or crystals of hydroxyapatite at concentrations of 50 and 150 nephelometric turbidity units.
Results
Incubation of platelets for 15 min with either human- or bovine-derived NPs reduced aggregation induced by thrombin receptor activator peptide and convulxin in a concentration-dependent manner. Hydroxyapatite caused a greater inhibition than either of the biologically derived NPs. Human-derived NPs increased ATP secretion by unstimulated platelets during the 15 min incubation period.
Conclusion
Effects of bovine-derived and hydroxyapatite NPs on basal release of ATP were both time and concentration dependent. These results suggest that biologic NPs modulate both platelet aggregation and secretion. Biologically derived NPs could modify platelet responses within the vasculature, thereby reducing blood coagulability and the vascular response to injury.
Keywords: ATP secretion, calcium phosphate, dense granules, fetuin, platelet aggregation, protein/mineral complexes, thrombin
Biologically derived nanoparticles (NPs), which are 20–500 nm vesicles are surrounded by a calcium phosphate shell, circulate in the blood. Their origin is uncertain, although they could be derived from activated or apoptotoic cells and/or chemical/mineral interactions [1–4]. Calcifying NPs are present in and have been isolated from diseased tissue of humans, including atherosclerotic plaque [5,6], kidney stones [7–10], psammoma bodies of ovarian cancer [11], saliva [12], mitral valve calciphylaxis [13] and prostate stones [14].
Three types of calcified NPs modify in vivo arterial remodeling [15]:
NPs derived from human calcified tissue – the composition of which may be complexes of proteins, lipids and mineral [16,17];
Calcified NPs derived from bovine blood – the composition of which is purported to be fetuin complexed to hydroxyapatite (HA) or calcium carbonate [18–20];
HA crystals [21].
When calcified NPs derived from human aneurysms and kidney stones were injected intravenously into rabbits, arterial occlusions and calcifications occurred within areas of endothelium-denuded arteries, whereas eccentric intimal hyperplasia occurred in injured arteries of animals injected with hydroxyapatite particles or NPs derived from bovine serum [15,22]. Therefore, the source and composition of NPs determine the pathophysiological consequences of their interactions with biological tissue, perhaps by initiating such actions as generation of oxygen-derived free radicals, inflammation, acting as nucleation sites for inorganic mineral deposition and altering protein structure [23–29].
Platelets aggregate at sites of vascular injury and secrete vasoactive and mitogenic substances that facilitate vascular remodeling [30]. Nano- and micro-sized carbon particles, positively charged NPs and latex NPs covered with collagen-related peptides activate platelets and enhance experimental thrombosis [28,31–33]. Alternatively, liposomes, PEGylated and nonPEGylated cetyl alcholol/polysorbate NPs inhibit agonist-induced platelet aggregation [34]. However, it is not known whether or not biologically derived NPs known to alter vascular remodeling in vivo affect platelet functions. Therefore, experiments were designed to investigate how these biologically derived NPs affect platelet reactivity in vitro. It was hypothesized that the ability of biologically derived NPs to modulate platelet aggregation and secretion would reflect the cellular origin and/or composition of the NPs.
Methods
Nanoparticle propagation & collection
Nanoparticles were prepared from human calcified aortic aneurysms and kidney stones, as previously described [5]. In brief, these NPs were seeded into standard tissue culture flasks containing Dulbecco’s modified Eagle medium (10–013; Mediatech, Inc., Manassas, VA, USA) supplemented with 10% γ-irradiated fetal calf serum (Atlanta Biologicals, Inc., Lawrenceville, GA, USA). In this medium, NPs exist in a floating or planktonic form prior to adhering to the surface of the culture flasks where they form visible domes encrusted with HA. Planktonic forms of human-derived NPs were collected from the culture media by centrifugation (20,000 g, 20 min, 4°C) 2 weeks after seeding the flasks. The supernatant was discarded and pellets were washed with phosphate-buffered saline (PBS); washed pellets from several flasks were combined. After two additional washes and centrifugations (20,000 g, 20 min, 4°C), the resulting pellet was suspended in PBS, with the volume adjusted to obtain a turbidity of 50 or 150 nephelometric turbidity units (NTU; Hach 2100N Turbidimeter, Loveland, CO, USA).
Stock solutions of bovine-derived NPs (Nanobac Oy, Finland) were diluted in sterile saline [20]. Inorganic HA crystals (1 μg; 3Ca3[PO4]2 ·Ca[OH]2; Sigma Chemical, MO, USA) were dissolved in PBS to 50 and 150 NTUs.
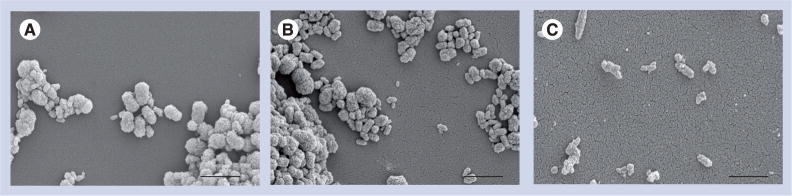
Turbidity reflects both the size and density of the NPs. Biologically derived NPs clump, making it impossible to determine actual number of particles/volume (Figure 1). Therefore, tubes containing stock solutions of NPs were vortexed, sonicated for 3 min and then vortexed again immediately prior to measurements of turbidity and pipetting aliquots needed for each experiment.
Figure 1. Scanning-electron micrographs of nanoparticles derived from (A) human aortic aneurysm, (B) fetal bovine serum and (C) hydroxyapatite.
Scale bars represent 1 μm.
Preparation of platelet-rich & -poor plasma
Venous blood from healthy male and female human volunteers (35–65 years of age) was collected into citrate anticoagulated tubes that were then centrifuged at 200 g for 15 min, 25°C to obtain platelet-rich plasma (PRP). Platelet-poor plasma (PPP) was prepared by centrifugation of PRP at 3000 g for 15 min. The platelet count of both PRP and PPP was monitored by Coulter counting. The PRP was adjusted with PPP to standardize the final platelet count at 250 × 103 platelets/μl.
Platelet aggregation
Platelet aggregation was monitored in PRP with and without NPs by the turbidimetric (light-transmission) method using a whole-blood aggregometer in optical mode (Chronolog Corp., Havertown, PA, USA). Human-derived, bovine-derived NPs or HA crystals (50 μl of stock dilutions) were added to PRP (400 μl; 250 × 103 platelets/μl) and the light transmission measured for 15 min to record changes in NP-induced basal aggregation. After 15 min, thrombin receptor activator peptide (TRAP; 10 μM) or convulxin (50 μg/ml) was added and light transmission was recorded for an additional 5 min. In some experiments, indomethacin (10−5 M) or NG-mono-methyl L-arginine (L-NMMA; 10−4 M; Sigma Chemical) was added 30 min prior to addition of the NPs.
Platelet-dense granule ATP secretion
Secretion of ATP from dense granules was determined by bioluminescence using 40 μl of diluted PRP (1:1000) in sterile Hanks’ balanced salt solution (GIBCO, CA, USA) buffered with 20 mM 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid (HEPES; pH 7.4) and containing 3 mg/ml bovine serum albumin, 0.5 g/l glucose and 1 μM tick anticoagulant peptide in the absence or presence of human-derived NPs, bovine NPs or HA crystals (50 or 150 NTU). ATP secretion was determined following 15 min incubation with NPs or HA alone.
Microscopy
Samples of NPs were examined by scanning-electron microscopy, as previously described [5,15]. Examination of human-derived NPs interacting with platelets by confocal laser-scanning microscopy was performed after the platelets were labeled with the thiol-reactive dye BODIPY®-FL maleimide by adding an aliquot of the dye stock, prepared in dimethly sulfoxide (DMSO), to an aliquot of platelets in suspension (1:10) and incubating them for 20 min at 25°C. A total of 10 μl of labeled platelets were then added to 80 μl Tyrode’s buffer together with 10 μl filtered (1.2 μm) NPs. After incubation for 20 min at 25°C, these platelets were fixed by adding 100 μl 4% paraformaldehyde, and maintaining the preparation at 4°C for 30 min. NPs not associated with platelets were then removed by centrifugation at 2500 g for 10 min. Resulting pellets were resuspended in 25 μl PBS and plated onto poly-L-lysine-coated slides for analysis by confocal laser-scanning microscopy. A set of unstained platelets were processed identically and used as a fluorescent signal blank.
To visualize platelet aggregation, platelet suspensions (100 μl) were prepared and maintained at 37°C for 35 min, either in the absence (control) or presence of 150 NTU of human-derived NPs. A second set of samples was treated identically, except that in these platelets aggregation was induced during the final 15 min of incubation by the addition of TRAP (10 μM final concentration). Platelet suspensions were then fixed and prepared for microscopy, as described earlier.
In experiments using BODIPY-FL maleimide-labeled platelets, each coverslip was observed using a Zeiss LSM 510 confocal laser-scanning system (Carl Zeiss, Inc., Oberkochen, Germany) equipped with a 488 nm argon laser and an Axiovert 200 M inverted microscope fitted with a 100×/1.4 Plan Apochromat oil-immersion objective. For fluorescence detection, the emission light was passed through a bandpass 505–550 filter. Differential interference contrast images were captured concurrent with fluorescence. In experiments using unlabeled platelets, only differential interference contrast images were taken. Images were captured and processed by using Zeiss LSM510 software.
Statistical analysis
All values are presented as mean ± standard error of the mean. PRP from a single individual was used in more than one type of experiment; n = the number of tests for each experiment. All experiments were carried out independently using blood from four to seven individuals. Different harvests of separate cultures of NPs derived from either human calcified aneurysms or kidney stones were used in these experiments. Statistical significance was evaluated by one-way analysis of variance or Student’s t-test unpaired observations; differences at a level of p < 0.05 were considered to be significant.
Results
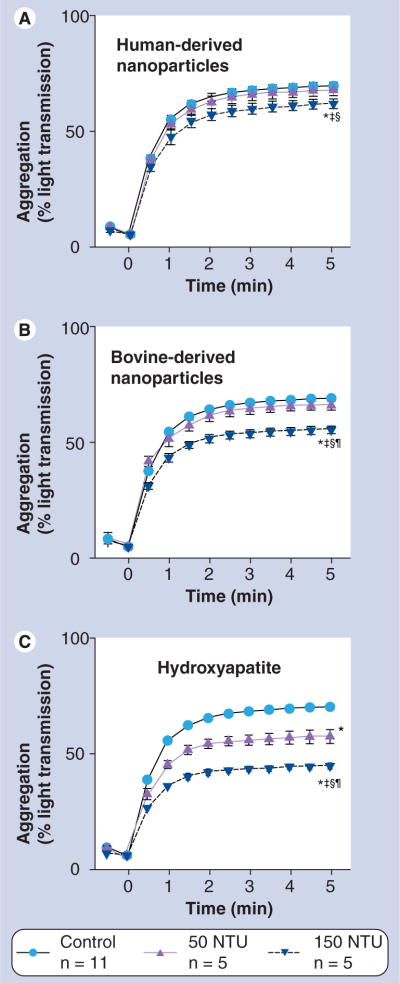
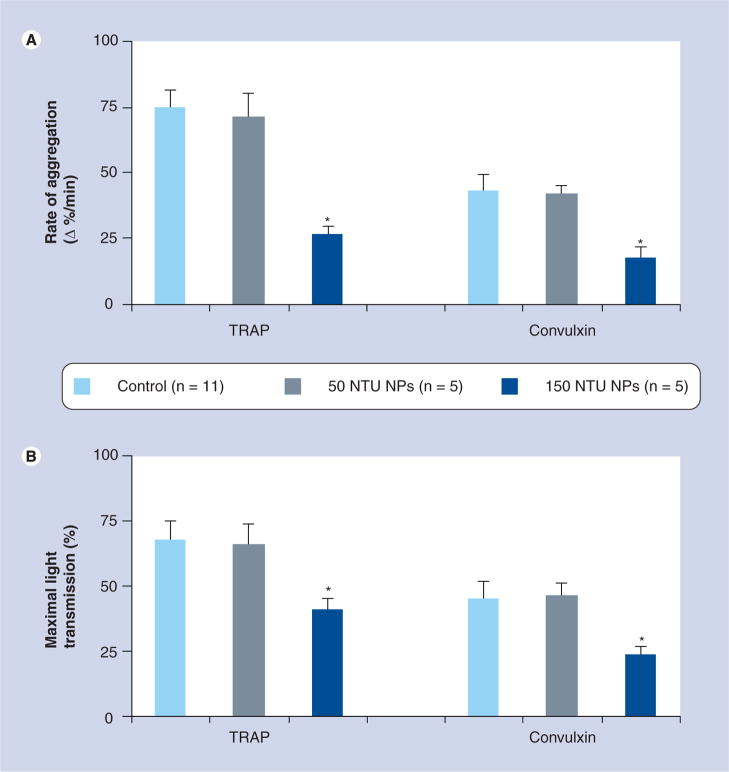
Nanoparticles associate with the platelet surface (Figure 2); however, addition of either NPs or HA crystals did not induce significant platelet aggregation without the concurrent agonist stimuation with TRAP (Figure 3). After 15 min of incubation with NPs and HA, both the rate and maximal aggregation to TRAP (Figures 3 & 4) were reduced in a concentration-dependent manner with HA having the highest potency and human NPs the lowest. Human-derived NPs also inhibited aggregation induced by convulxin, a less potent platelet aggregation agonist than TRAP (Figure 5). These inhibitory effects of human-derived NPs or HA (150 NTU) on platelet aggregation were not reversed by either L-NMMA or indomethacin (Table 1).
Figure 2. Representative images of human-derived nanoparticles interacting with platelets.
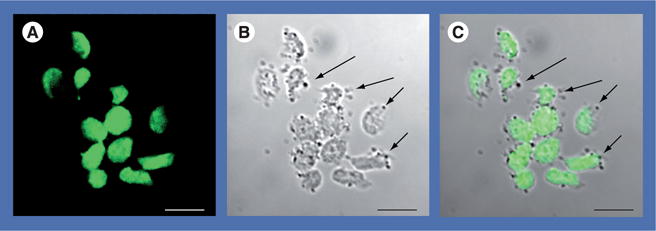
(A) The image taken using 488 nm laser excitation shows intense fluorescent label in platelets (green). (B) The differential interference contrast image shows that most platelets maintained discoid, inactivated shapes, whereas others were irregular and elongated when exposed to human-derived nanoparticles (nanoparticles image as dark circular forms, arrows). (C) The merged image shows human-derived nanoparticles (arrows) associated with the platelet exterior. Scale bars represent 5 μm.
Figure 3. Differential interference contrast analysis of effects of human-derived nanoparticles on activation and aggregation of human platelets.
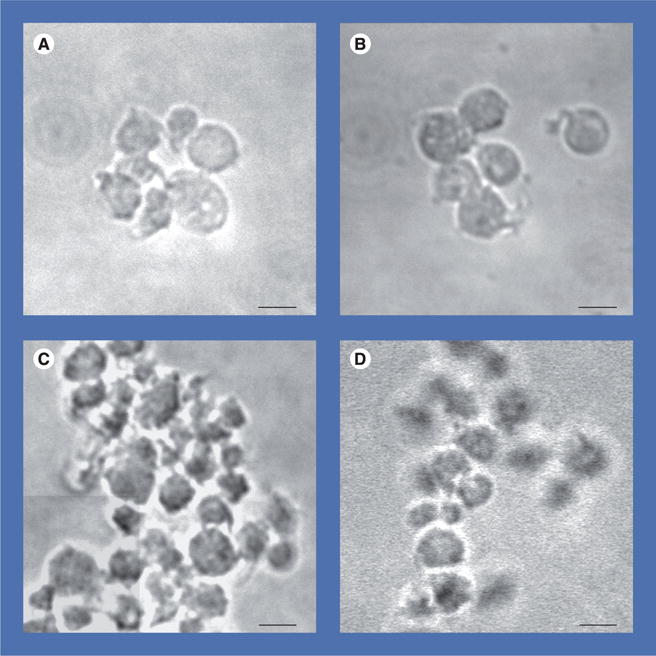
Representative images show unstimulated platelets (control) (A) maintained discoid morphology with moderate activation, whereas platelets coincubated with 150 nephelometric turbidity unit human-derived nanoparticles (B) exhibited a larger proportion of cells with spherical enlargement, together with shape changes and pseudopodia. Platelets exposed to thrombin receptor activator peptide (10 μM) (C) aggregated but platelets incubated with human-derived nanoparticles for 15 min prior to stimulation with thrombin receptor activator peptide (D) showed fewer and smaller aggregates. Cumulate data from these experiments are quantified in Figure 4. Scale bars represent 2 μm.
Figure 4. Averaged traces of thrombin receptor activator peptide-induced platelet aggregation (measured by light transmission using aggregometry) in the absence (control) or presence of (A) human-derived nanoparticles, (B) bovine-derived nanoparticles or (C) hydroxyapatite crystals.
Platelet suspensions were exposed to nanoparticles or hydroxyapatite for 15 min prior to aggregation induced by addition of thrombin receptor activator peptide (10 μM final concentration). Significant differences (paired t-test, p < 0.05) in amplitude of aggregation at 5 min: *Versus control; †Versus 50 NTU same treatment, and in slope of aggregation rate; ‡Versus untreated control; §Versus 50 NTU same treatment. Data are mean ± standard error of the mean, n = the number of tests conducted on aliquoted platelet-rich plasma derived from four or five individuals.
NTU: Nephelometric turbidity unit.
Figure 5. (A) Cumulative rate of and (B) maximal platelet aggregation in response to TRAP (10 μM) and convulxin (50 μg/ml) following 15 min incubation in the absence (control) or presence of human-derived nanoparticles.
Data are shown as mean ± standard error of the mean; n = the number of tests conducted on aliquoted platelet-rich plasma derived from five to seven individuals.
*Denote statistically significant decreases compared with control; p < 0.05.
NP: Nanoparticle; NTU: Nephelometric turbidity unit; TRAP: Thrombin receptor activator peptide.
Table 1.
Effects of L-NMMA and indomethacin on platelet aggregation in the presence of 150 nephelometric turbidity units of human-derived nanoparticles or hydroxyapatite*.
| Human-derived NPs | Hydroxyapatite | |
|---|---|---|
| Amplitude‡ | ||
| Control | 44.7 ± 0.7 | 37.3 ± 0.3 |
| L-NMMA (10−4 M) | 43.0 ± 0.6 | 36.0 ± 1.5 |
| Indomethacin (10−5 M) | 43.3 ± 1.7 | 33.3 ± 2.7 |
| Slope§ | ||
| Control | 48.7 ± 3.3 | 36.3 ± 2.0 |
| L-NMMA (10−4 M) | 47.0 ± 1.2 | 36.0 ± 2.0 |
| Indomethacin (10−5 M) | 46.0 ± 2.6 | 34.3 ± 2.0 |
Data are shown as mean ± standard error of the mean, n = 3/condition.
Percentage light transmission.
Change in percentage light transmission per min.
L-NNMA: NG-mono-methly L-arginine; NP: Nanoparticle.
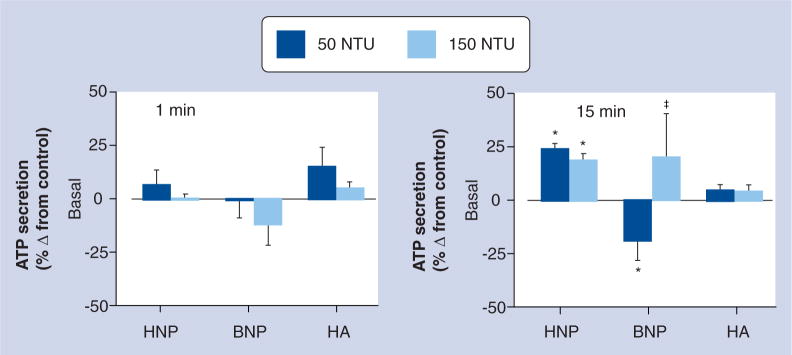
In contrast to the affects on aggregation, ATP secretion increased by 20% following 15 min incubation of platelets with human-derived NPs (p < 0.05), but was suppressed by 50 NTU of bovine-derived NPs. This effect was biphasic since ATP secretion returned to baseline after incubation of platelets with 150 NTU of bovine-derived NPs. HA did not have a statistically significant effect on basal ATP secretion either immediately, within 1 min or after 15 min incubation (Figure 6).
Figure 6. Changes in basal ATP secretion from human platelets immediately after (within 1 min) and following 15 min incubation of the platelets with either HNPs, BNPs or HA crystals at two different doses: 50 NTU and 150 NTU.
ATP secretion is presented as percentage change compared with vehicle-treated controls. Data are mean ± standard error of the mean of triplicate determinations on paired samples of blood (n = 5–7).
*Significantly different (p < 0.05) by paired t-test from corresponding value in untreated control.
‡50 NTU of the same treatment.
BNP: Bovine-derived nanoparticle; HA: Hydroxyapatite; HNP: Human-derived nanoparticle; NTU: Nephelometric turbidity unit.
Discussion
Results of this study indicate that NPs of biological origin differentially inhibit platelet aggregation in a concentration-dependent manner and alter dense granule ATP secretion. These results are consistent with the hypothesis that biological activity of NPs is dictated by their composition, size and charge [33,35]. This hypothesis is also supported by the differential degrees of platelet aggregation induced by multiwalled and single-walled nanotubes, C60 fullerenes, mixed carbon NPs or collagen-coated latex NPs [28,31] compared with inhibition of platelet aggregation by positively charged NPs or those formed from PEGylated and nonPEGylated cetyl alcohol/polysorbate NPs [32,34]. Results from this study suggest that some types of NPs with physiologically relevant compositions also inhibit rather than stimulate platelet functions. Bovine-derived NPs appear to be composed of fetuin–calcium complexes [20]. The exact chemical composition of biologically derived NPs cultured in bovine serum is complex and reflects, in part, the tissue of origin (serum compared with aneurysm or kidney stone) of the NPs and the conditions under which they are cultured [5,12,17,19]. The composition of human-derived NPs used in the present study consists of lipids, some nucleic acids and proteins of human and bacterial origin, including elongation factor tu [Lieske JC, Unpublished Data] [5,16].
These results have important biomedical and pathophysiological implications because NPs composed of bone matrix proteins and bovine serum albumin are being tested for delivery of agents for bone regeneration [36]. In addition, NPs of various compositions are being manufactured for use as carrier molecules for drugs in the treatment of cancers, bioimaging, modulation of angiogenesis and bone regeneration [36–39]. Based on the results of the present study, some NPs have the potential to inhibit platelet functions.
Platelet aggregation and secretion at sites of vascular endothelial injury contribute to vascular wound healing and the development of atherosclerosis [30]. The inhibitory effects of biologic NPs on platelet functions observed in the present study help to explain differences in vascular remodeling following injection of planktonic forms of these NPs into animals in which one carotid artery had been denuded of endothelium [15]. Intimal hyperplasia did not develop in the endothelium-denuded artery of animals inoculated with bovine-derived NPs and HA crystals [15]. These NPs had the greatest inhibitory effects on in vitro platelet aggregation in the present study. The disruption of the internal elastic lamina in arteries from these animals may reflect the fetuin–calcium composition of the bovine-derived NPs and HA crystals exposed to serum [20]. The heterogeneous nature of the human-derived NPs is reflected in the gradient in vascular healing from minimal intimal hyperplasia to luminal occlusion with calcification observed in animals injected with human-derived NPs [15]. These previous in vivo effects are consistent with the current in vitro observation of minimal inhibition of platelet aggregation but increased dense granule secretion when platelets were exposed to human-derived NPs.
The biologic NPs used in this study inhibited platelet aggregation induced by two different agonists, one that activates the thrombin receptor and the other that activates the collagen receptor, suggesting that the inhibitory effects may be nonspecific, perhaps by reducing platelet–platelet interaction as has been proposed by others [33,34] or by binding to these or other surface receptors (Figure 2). Platelet aggregation is inhibited by nitric oxide and prostacyclin. However, neither L-NMMA or indomethacin reversed the reduction in agonist-induced aggregation in the presence of NPs or HA, suggesting that neither activation of nitric oxide synthase or cyclooxygenase, respectively, contribute to inhibition. NPs interacting with the platelet surface membrane may activate other intracellular processes that affect ATP secretion from dense granules. Dense granule ATP could act as an autocrine factor to further modulate agonist-induced secretion or aggregation. Mechanisms of these processes remain to be explored.
Conclusion
Hydroxyapatite, as well as NPs from two different mammalian sources (bovine blood and human tissue homogenates) containing calcium phosphate complexed with protein, inhibit platelet aggregation. Greatest inhibition was observed with hydroxyapatite particles; the least inhibition was observed with human-derived NPs of heterogenous composition. Inhibition of aggregation may be achieved through decreased platelet–platelet interactions. Biologic NPs may also affect dense granule secretion. The combined effects of NPs on platelets will alter the ability of platelets to respond to vascular injuries.
Future perspective
Some authors have suggested that biologic NPs, a product of normal physiological processes, may not have pathophysiological potential [19]. However, the current studies suggest that biologic NPs have important physiologic effects on platelet activation. Microparticles/micro-vesicles found in human blood are similar in size to biologic NPs and their cellular origin and quantity in human blood varies with diseases such as atherosclerosis, thrombotic diseases and arterial calcification [2,4,36,40,41]. Therefore, as technologies to evaluate nanosized materials in the blood improve, evaluating the thrombogenic potential of various human-derived NPs or microvescicles/microparticles may increase understanding of the etiology of various diseases and facilitate development of diagnostic, preventive and curative NP modalities.
Conclusion
Experiments were designed to determine if NPs derived from calcified human tissue alter platelet aggregation and secretion in vitro. These human-derived NPs inhibited platelet aggregation but to a lesser degree than NPs of fetuin–calcium complexes or hydroxyapatite (calcium phosphate), most likely by reducing platelet–platelet interactions. Human-derived NPs also stimulated basal secretion of ATP from platelet-dense granules. These effects, if present in vivo, would modulate the ability of platelets to respond to intravascular injury.
Executive summary
Nanoparticles derived from calcified human tissue decreased aggregation of platelets stimulated with either thrombin receptor activator protein or convulxin, in part by reducing platelet–platelet interactions.
The inhibition of platelet aggregation by human-derived nanoparticles was less than that imparted by fetuin/mineral complexes or hydroxyapatite alone.
Human-derived nanoparticles increased basal secretion of ATP from platelet-dense granules.
Nanosized protein/mineral complexes and nano/microvesicles derived from activated and apoptotic cells or commensal bacteria, which circulate in the blood, could affect coagulability of the blood through interaction with platelets.
Utility and efficacy of nanoparticles currently under development for diagnostic and therapeutic purposes might be affected by how they interact with platelets and modulate platelet aggregation and secretory processes.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was funded by grants from the Fetzer Foundation, NIH HL88988 and the Mayo Foundation. Bovine-derived nanoparticles were provided by Nanobac OY, Finland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.Leroyer AS, Isobe H, Leseche G, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49(7):772–777. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 2▪.Jayachandran M, Litwiller RD, Owen WG, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in recently menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:931–938. doi: 10.1152/ajpheart.00193.2008. Identification of different populations of nano/microsized particles in persons with asymptomatic coronary calcification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toth B, Liebhardt S, Steinig K, et al. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb Haemost. 2008;100(4):663–669. [PubMed] [Google Scholar]
- 4▪▪.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21(3):157–171. doi: 10.1016/j.blre.2006.09.001. Excellent review of the issues related to the clinical implications of bloodborne nano/microsized particles as diagnostic and prognostic markers. [DOI] [PubMed] [Google Scholar]
- 5.Miller VM, Rodgers G, Charlesworth JA, et al. Evidence of nanobacterial-like structures in human calcified arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;287:H1115–H1124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 6▪.Amabile N, Guerin AP, Leroyer A, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16(11):3381–3388. doi: 10.1681/ASN.2005050535. One of the first papers to identify nanosized particles in blood. [DOI] [PubMed] [Google Scholar]
- 7.Akerman KK, Juronen I, Kajander EO. Scanning electron microscopy of nanobacteria – novel biofilm producing organisms in blood. Scan Electron Microsc. 1993;15(Suppl 3):90–91. [Google Scholar]
- 8.Ciftcioglu N, Bjorklund M, Kuorikoski K, Bergstrom K, Kajander EO. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 1999;56:1893–1898. doi: 10.1046/j.1523-1755.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 9.Drancourt M, Jacomo V, Lepidi H, et al. Attempted isolation of Nanobacterium sp. microorganisms from upper urinary tract stones. J Clin Microbiol. 2003;41:368–372. doi: 10.1128/JCM.41.1.368-372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjelle JT, Miller-Hjelle MA, Poxton IR, et al. Endotoxin and nanobacteria in polycystic kidney disease. Kidney Int. 2000;57:2360–2374. doi: 10.1046/j.1523-1755.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- 11.Sedivy R, Battistutti WB. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. APMIS. 2003;111:951–954. doi: 10.1034/j.1600-0463.2003.1111006.x. [DOI] [PubMed] [Google Scholar]
- 12▪.Cisar Jo, Xu D-Q, Thompson J, Swaim W, Hu L, Kopecko DJ. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci USA. 2000;97:11511–11515. doi: 10.1073/pnas.97.21.11511. Openly spurred the controversy regarding the biochemical composition of nanoparticles contributing to formation of calcific biofilm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jelic TM, Malas AM, Groves SS, et al. Nanobacteria-caused mitral valve calciphylaxis in a man with diabetic renal failure. South Med J. 2004;97:194–198. doi: 10.1097/01.SMJ.0000077067.44311.F0. [DOI] [PubMed] [Google Scholar]
- 14.Shoskes DA, Thomas KD, Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: preliminary experience. J Urol. 2005;173:474–477. doi: 10.1097/01.ju.0000150062.60633.b2. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Schwartz Mk, Lieske JC, Hunter LW, Miller VM. Systemic injection of planktonic forms of mammalian-derived nanoparticles alters arterial response to injury in rabbits. Am J Physiol Heart Circ Physiol. 2009;296:1434–1441. doi: 10.1152/ajpheart.00993.2008. Demonstrates differential in vivo pathogenic activity of nanoparticles of three different compositions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V, Farell G, Yu S, et al. Cell biology of pathologic renal calcification: Contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med. 2006;54(7):412–424. doi: 10.2310/6650.2006.06021. [DOI] [PubMed] [Google Scholar]
- 17.Benzerara K, Miller VM, Farell G, et al. Search for microbial signatures within human and microbial calcifications using soft-x-ray spectromicroscopy. J Invest Med. 2006;54:367–379. doi: 10.2310/6650.2006.06016. [DOI] [PubMed] [Google Scholar]
- 18.Martel J, Ding-E Young J. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc Natl Acad Sci USA. 2008;105(14):5549–5554. doi: 10.1073/pnas.0711744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪▪.Young JD, Martel J, Young L, Wu CY, Young A, Young D. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLoS ONE. 2009;4(2):e4417. doi: 10.1371/journal.pone.0004417. Description of biochemical manipulations of media that results in formation of calcific nanosized particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raoult D, Drancourt M, Azza S, et al. Nanobacteria are mineralo fetuin complexes. PLoS Pathog. 2008;4(2):e41. doi: 10.1371/journal.ppat.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieske JC, Norris R, Toback FG. Adhesion of hydroxyapatite crystals to anionic sites on the surface of renal epithelial cells. Am J Physiol. 1997;273:F224–F233. doi: 10.1152/ajprenal.1997.273.2.F224. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz Ma-K, Lieske JC, Kumar V, Farell-Baril G, Miller VM. Human-derived nanoparticles and vascular responses to injury in rabbit carotid arteries: proof of principle. Int J Nanomed. 2008;3:243–248. doi: 10.2147/ijn.s2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucki R, Pastore JJ. Bacterial endotoxin as inhibitor of the enzymatic activity of human thrombin. Eur J Haematol. 2006;76(6):510–515. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2448.x. [DOI] [PubMed] [Google Scholar]
- 24.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaldson K, Stone V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita. 2003;39:405–410. [PubMed] [Google Scholar]
- 26.Vertegel AA, Siegel RW, Dordick JS. Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir. 2004;20:6800–6807. doi: 10.1021/la0497200. [DOI] [PubMed] [Google Scholar]
- 27.Brown Dm, Stone V, Findlay P, Macnee W, Donaldson K. Increased inflammation and intracellular calcium caused by ultrafine carbon black is independent of transition metals or other soluble components. Occup Environ Med. 2000;57:684–691. doi: 10.1136/oem.57.10.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪▪.Radomski A, Jurasz P, Alonso-Escolano D, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146(6):882–893. doi: 10.1038/sj.bjp.0706386. Detailed description of effects of diverse nanoparticles on platelet activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraz N, Carlsson J, Hong J, Ott Mk. Influence of nanoporesize on platelet adhesion and activation. J Mater Sci Mater Med. 2008;19:3115–3121. doi: 10.1007/s10856-008-3449-7. [DOI] [PubMed] [Google Scholar]
- 30.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 31.Cejas MA, Chen C, Kinney WA, Maryanoff BE. Nanoparticles that display short collagen-related peptides. Potent stimulation of human platelet aggregation by triple helical motifs. Bioconjug Chem. 2007;18:1025–1027. doi: 10.1021/bc070105s. [DOI] [PubMed] [Google Scholar]
- 32.Mayer A, Vadon M, Rinner B, Novak A, Wintersteiger R, Frohlich E. The role of nanoparticle size in hemocompatibility. Toxicology. 2009;258:139–147. doi: 10.1016/j.tox.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto M, Sasakawa S, Ozawa T, Kawaguchi H, Ohtsuka Y. Platelet aggregation induced by latex particles. Effects of size, surface potential and hydrophobicity of particles. Biomaterials. 1989;10:251–257. doi: 10.1016/0142-9612(89)90101-4. [DOI] [PubMed] [Google Scholar]
- 34.Koziara J, Oh J, Akers W, Ferraris S, Mumper R. Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm Res. 2005;22:1821–1828. doi: 10.1007/s11095-005-7547-7. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Nel A. Air pollution – related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. Excellent summary of potential complex interactions of nanoparticles with biological tissue. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Siggers K, Zhang S, et al. Preparation of bmp-2 containing bovine serum albumin (BSA) nanoparticles stabilized by polymer coating. Pharm Res. 2008;25:2896–2909. doi: 10.1007/s11095-008-9692-2. [DOI] [PubMed] [Google Scholar]
- 37.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27:2798–2805. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Nahar M, Dutta T, Murugesan S, et al. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Crit Rev Ther Drug Carrier Syst. 2006;23(4):259–318. doi: 10.1615/critrevtherdrugcarriersyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 39.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hugel B, Socie G, Vu T, et al. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood. 1999;93(10):3451–3456. [PubMed] [Google Scholar]
- 41.Schoppet M, Shroff RC, Hofbauer LC, Shanahan CM. Exploring the biology of vascular calcification in chronic kidney disease: what’s circulating? Kidney Int. 2008;73(4):384–390. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]