Synopsis
Circulating levels of the BNP system can help in the diagnosis of cardiovascular disease and provide prognostic information not only in patients with HF but also the general population and other patient groups. Changes over time also carry prognostic information and studies are assessing BNP-guided treatment strategies. New insights regarding the biology of the BNP system are emerging with identification of circulating molecular forms of BNP, which may improve the diagnostic and prognostic value of BNP. Likewise, accounting for rs198389, a common single nucleotide polymorphism that increases BNP levels, may help to further refine our use of components of the BNP system as biomarkers.
Keywords: natriuretic peptides, biomarker, heart failure
The concept of the heart as an endocrine organ was advanced by DeBold and Matsuo three decades ago with the discovery that the heart synthesized and secreted atrial natriuretic peptide (ANP) (1,2). Others and we have demonstrated the production and release of ANP and also B-type natriuretic peptide (BNP) from models of experimental heart failure (HF) and from humans with HF (3,4). Thus, these hormones have emerged as cardiac biomarkers that can aid in the diagnosis, prognosis and management of HF. Here we will review the important clinical applications of these cardiac peptides with a special focus on BNP.
BNP: A Cardiac Hormone Activated in Heart Failure
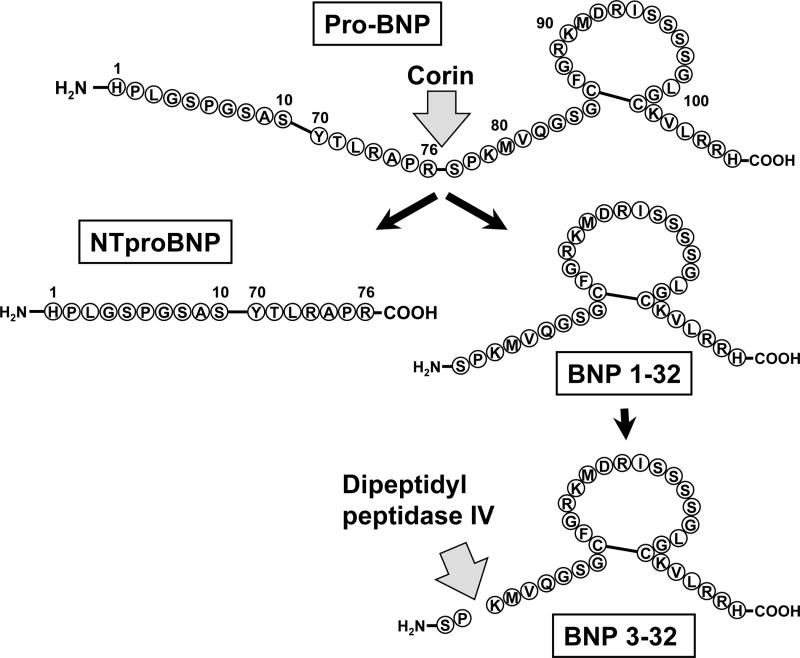
Within the heart, the BNP gene (NPPB) produces a 134 amino acid prepro-BNP precursor peptide, which after removal of a 26 amino acid signal peptide, results in the 108 amino acid prohormone – ProBNP (Figure 1). Subsequently, the enzyme corin cleaves ProBNP into the biologically active mature 32 amino acid BNP (BNP 1-32) containing the critical 17 amino acid disulfide ring. A second cleavage product is the linear 76 amino acid N-terminal peptide (NT)-proBNP 1-76. All studies suggest that mature BNP 1-32 binds to the natriuretic NRP-A receptor, activates the production of the second messenger cGMP and mediates the biological actions of BNP. These actions include natriuresis, vasodilatation, enhancement of ventricular relaxation, inhibition of fibroblast activation and suppression of the renin-angiotensin-aldosterone system. We and others have reported that NT-proBNP has no ability to activate NPR-A and generate cGMP and that the ability of proBNP to do so is markedly reduced (5,6). This reduced activation of NPR-A by proBNP and NT-proBNP is highly relevant to the pathophysiology of HF as increasing evidence demonstrates that proBNP and not BNP 1-32 is the predominant circulating form. Thus, chronic HF may be considered a relative BNP deficiency state.
Figure 1.
Schematic of proBNP and its processing to NTproBNP and BNP 1-32 by the protease corin, and processing of BNP 1-32 to BNP 3-32 by dipeptidyl peptidase IV.
Recent investigations have also documented that BNP 1-32 undergoes further processing by dipeptidyl peptidase IV, which removes the two N-terminal amino acids (Ser and Pro) producing BNP 3-32 (7). Importantly, BNP 3-32 circulates and its concentrations are increased in human HF (8). Its physiologic significance is high since BNP 3-32 as compared to BNP 1-32 has reduced natriuretic and diuretic properties and lacks renal vasodilatation (9).
Treatment of HF can be challenging, especially since common symptoms and signs have only limited specificity. For that reason, a sensitive, objective, and cost-effective measure of patient status is highly desirable. The cardiac-derived natriuretic peptide BNP and its related peptides may be such markers. Given that myocardial stretch stimulates BNP production and release, that the heart is the major source of BNP, and that BNP can easily be measured in plasma, there is a straightforward rationale for evaluating circulating BNP as a biomarker for cardiac overload (10,11). The following issues should be considered when interpreting data on BNP and its forms:
While BNP and NTproBNP have important biological differences, many studies yield qualitatively similar results for these two proBNP products (12,13).
While most BNP and NTproBNP assays were developed using antibodies directed against epitopes of these specific peptides, the forms ultimately detected in a sample can be expected to have considerable heterogeneity (5,8).
Many studies discussed in this review treat BNP as a stand-alone test while in practice clinical assessment and other test results would also be considered; e.g. accounting for conventional risk factors can reduce the additional prognostic information gained from novel biomarkers (14).
Rather than using BNP with a single cutpoint it may be better in many clinical applications to interpret BNP as a continuous variable; also, two cutpoints with an intermediate grey zone have been suggested (12,15).
BNP levels are not only affected by age, sex, cardiac load, and clearance, but also by genotype. For instance, a common single nucleotide polymorphism in the promoter region of the BNP gene (rs198389; also referred to as T-381C) was associated with 30% higher BNP levels per C-allele (16-21). Not only may this genetic BNP elevation confound our interpretation of assay results, it may, given BNP's unloading actions, even paradoxically be associated with improved outcomes.
Results observed in a specific population during a specific period of time cannot necessarily be extrapolated to other populations at other times with potentially different treatment standards.
The usefulness of BNP in the diagnosis of acute heart failure is described in a different chapter in this volume(Januzzi), so the present review will focus on the following potential applications of BNP assays in chronic HF: 1) diagnostic and prognostic significance of BNP; 2) prognostic value of changes over time; 3) prediction of therapy benefit; 4) BNP-guided therapy; and 5) what's on the horizon.
Diagnostic and Prognostic Significance of BNP
Given its association with cardiac load, it is not surprising that BNP has been found to convey diagnostic and prognostic information not only in acute HF but also in other settings. Of note, BNP levels are not only affected by cardiac overload but also by factors such as age, gender, renal function, obesity, and genetic factors (16-21). In the setting of acute HF the contribution of these factors is small compared to the impact of cardiac overload so that even single, unadjusted BNP values can provide very good diagnostic yield. In the setting of asymptomatic disease, however, the contribution of these other factors are more relevant and adjusting may significantly improve test characteristics (21,22).
The guidelines promulgated by the European Society of Cardiology (23) indicate that evidence supports the use of natriuretic peptides for the diagnosis, staging, making hospitalization/discharge decisions, and identifying patients at risk for clinical events. The evidence for their use in monitoring and adjusting drug therapy is less clearly established (23).
Wang et al showed in the Framingham Offspring Study that in asymptomatic individuals BNP elevations well below levels used in the diagnosis of acute HF were associated with increased risk of death, first cardiovascular event, HF, atrial fibrillation, and stroke or transient ischemic attack after adjustment for cardiovascular risk factors (24). McKie et al confirmed in a large random sample of the general population in Olmsted County, MN, that higher BNP and NT-proBNP levels are associated with increased mortality even after adjusting for risk factors and echocardiographic parameters including systolic and diastolic dysfunction, LV hypertrophy, and left atrial enlargement (25). The latter finding suggests that BNP can provide prognostic information beyond not only standard risk factors but also echocardiographic parameters. The authors also reported that individuals in the highest tertiles of BNP had a higher prevalence of cardiovascular drug use, hypertension, coronary artery disease, and history of myocardial infarction. Of note, there was a small number of subjects with BNP levels in the highest tertile without risk factors or echocardiographic abnormalities; these individuals did not show higher mortality (McKie, unpublished data). This could be explained by the impact of genetic contribution mentioned above (17-19); indeed, it would be worthwhile to formally evaluate the impact of genotype on outcomes.
In the PEACE trial, ACE inhibition with trandolapril compared to placebo in patients with established coronary artery disease and an LVEF>40% did not improve the primary end point, which was death from cardiovascular causes, myocardial infarction, or coronary revascularization, but it reduced the number of patients requiring hospitalization for or dying of HF (26). Omland et al evalutead in 3,761 of study participants whether baseline BNP and NTproBNP provided prognostic information in this patient population with coronary artery disease but preserved LV function. After adjusting for relevant parameters, the hazard ratio per standard deviation of log-transformed NTproBNP was significantly increased for CV mortality (1.69), fatal/non-fatal HF (2.35), fatal/non-fatal stroke (1.63), but not fatal/non-fatal myocardial infarction (1.02). The corresponding hazard ratios for BNP were 1.06, 1.62, 1.15, and 0.91, with only fatal/non-fatal HF being significant. The inability of BNPs to predict fatal/non-fatal myocardial infarction may reflect the complex pathophysiology of acute coronary syndromes, which include vascular and rheologic factors unlikely to be reflected by BNPs. Using the c-statistic as a measure of overall prognostic accuracy, NTproBNP in univariable analysis performed significantly better than BNP in the prediction of CV mortality, and fatal/non-fatal HF, and fatal/non-fatal stroke. Addition of NTproBNP to the best multivariable model significantly improved the c-statistic for prediction of CV mortality (from 0.74 to 0.77) and HF (from 0.82 to 0.85); for BNP this was only the case for prediction of HF (from 0.82 to 0.84). It would be interesting to know to what extent these findings would be affected if analyses were further adjusted for rs198389 genotype which, as will be discussed later, can influence circulating BNP. Also, it is unclear what therapeutic consequences, if any, elevated BNPs should have.
Epidemiologic studies have shown that about 50% of individuals with LV systolic dysfunction are asymptomatic (27,28). It has also been demonstrated that these patients with asymptomatic LV dysfunction are at increased risk of developing overt HF (29). These patients benefit from treatment, providing a powerful rationale to screen for asymptomatic LV dysfunction, and BNP has been evaluated for this purpose (21,22,30-32). Reviews and meta-analyses are also available (13,33,34).
As BNP can be elevated in a variety of cardiac conditions, a positive BNP test should be followed up by an imaging study to confirm a cardiac pathology. Thus, choosing a BNP cutoff value with good sensitivity to keep the false negative test results low may lead to a considerable number of false positive test results, i.e. a relatively large number of individuals will have normal imaging studies. While these individuals would be considered “false positive” from the perspective of screening for LV dysfunction, given the risk associated with elevated BNP levels in the general population discussed above, it should be evaluated whether these “false positives” actually have a worse prognosis than subjects with “true negatives” and may benefit from intervention.
The cost-effectiveness of BNP screening will be affected by the test characteristics of the BNP test, the cost of the assay and of the imaging study, and the disease prevalence in the population. Therefore, screening in specific populations at higher risk, e.g. patients with coronary artery disease, hypertension, diabetes, or the elderly, may be more cost-effective as screening the general population.
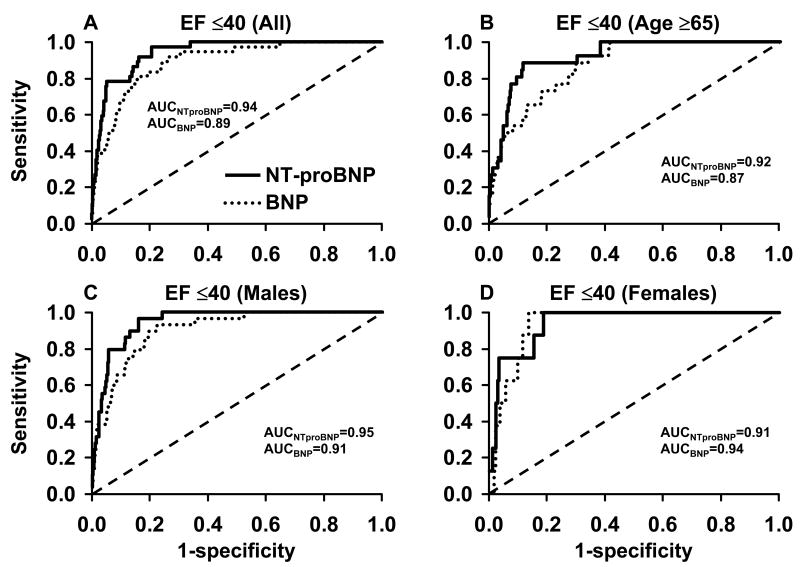
Figure 2 shows receiver operating characteristic analyses and area under the curves for BNP (Biosite Triage assay) and NTproBNP (Roche assay) in the total population and some subgroups of a random sample of the general population in Olmsted County, MN. As mentioned above, the common rs198389 SNP in the promoter region of the BNP gene has been shown to increase BNP values by about 30% per allele; this impact is comparable to that of gender, so that accounting for this SNP can be expected to improve test characteristics. However, genotyping would of course also affect the cost of the screening strategy.
Figure 2.
Receiver operating characteristic curves of NTproBNP and BNP for detecting an ejection fraction of ≤40% in a random sample of the general population ≥45 years of Olmsted County, MN, USA. (A) for the entire population, (B) for patients ≥65 years, (C) for male subjects, and (D) for female subjects. AUC, area under the curve, EF, ejection fraction. From Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al: Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 2006;47(2):349, with permission.
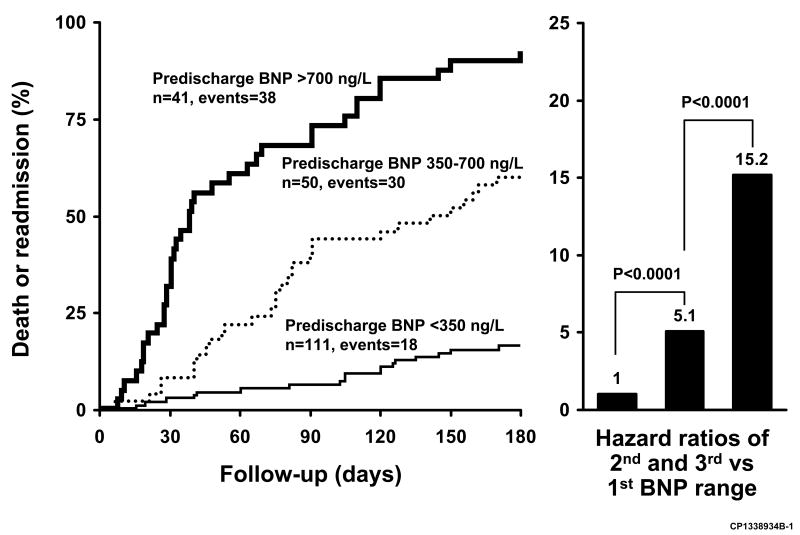
In patients hospitalized for acute HF, higher BNP values either at admission or at discharge, as well as percent change have been associated with worse outcomes. Logeart et al evaluated predictors of post-discharge outcomes of patients hospitalized for acute HF (35). The main outcome variable, which was death or rehospitalization for HF, was significantly predicted in univariate analysis by inotropic drug use, Doppler mitral inflow pattern, BNP values (Biosite triage assay) measured at admission, at discharge, and during the hospitalization, as well as percent decrease of BNP. In multivariate analysis, only pre-discharge BNP levels remained significant (hazard ratio=1.14 [1.02-1.28] per 100 ng/L increment). Further analysis showed that a BNP level of 350 ng/L best discriminated between patients with and without event, and that the range from 350 to 700 ng/L represented an intermediate risk (Fig. 3). Prospective studies are required to assess whether strategies that decrease pre-discharge BNP levels will lead to improved outcomes.
Figure 3.
Kaplan-Meier curves showing the cumulative incidence of death or hospital re-admission according to pre-discharge BNP ranges in patients hospitalized for decompensated heart failure; p<0.001 for trend among BNP ranges. Hazard ratios are shown on the right. From Logeart D, Thabut G, Jourdain P, et al: Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004;43(4):639, with permission.
Changes of BNP over Time
If cardiac overload negatively impacts prognosis and BNP reflects cardiac overload, then reductions in BNP would be expected to indicate an improved prognosis. If this were so, BNP could be used to help assess the efficacy of a therapeutic intervention in normal clinical practice as well as in clinical trials. Elevated levels could indicate impending clinical deterioration not yet apparent from symptoms and signs and trigger an earlier intervention, which could result in improved outcomes. With regard to BNP changes over time, there are important questions for clinical practice. How often should BNP values be determined? What constitutes a significant change that should trigger an intervention such as medication change or hospitalization?
Anand et al evaluated the prognostic information of BNP (Shionogi assay) and norepinephrine both in terms of baseline values as well as changes from baseline to 4 and 12 months in the Val-HeFT trial, which evaluated valsartan vs. placebo in patients with stable symptomatic HF with an LVEF <40% (mean age 63 years, mean LVEF 27%) (36). Higher quartiles of baseline BNP were associated with a higher likelihood of the composite of mortality and first morbid event, defined as death, sudden death with resuscitation, hospitalization for HF, or intravenous inotropic or vasodilator therapy for at least 4 hours. Mortality rates in the four BNP quartiles were 9.7, 14.3, 20.7, and 32.4% (BNP quartiles were <41, 41 to <97, 97 to <238 and ≥238 pg/mL); relative risks for all-cause mortality were 1.47, 2.27, and 4.0 for quartiles 2,3,4, respectively, vs. quartile 1; the corresponding values for relative risk of first morbid event were 1.50, 2.46, and 4.10. If change from baseline was expressed in absolute values, the highest mortality rates were found in the quartiles with the lowest and with the highest change.
As marked reductions of BNP can occur only in patients with high baseline values, which confer a higher risk, these results should not be surprising. Indeed, average baseline BNP was highest in the quartile with the largest reductions. If however changes from baseline were expressed as percent change, the baseline values in the quartiles of percent change were similar and mortality increased from the lowest to highest quartile, i.e. patients with larger percent decreases fared better. The relative risk of adverse outcome was also significantly increased when quartiles of percent change of BNP were adjusted for baseline BNP (1.30, 1.36, and 1.92 for quartiles 2, 3, 4, respectively, vs. quartile 1; the corresponding values for relative risk of first morbid event were 1.41, 1.67, and 2.2). The authors mention that the prognostic information of BNP was similar at different time points and in both randomization groups individually. To summarize, in this study both baseline BNP and percent change over time were able to reflect risk of adverse outcomes, with the baseline data providing more robust information.
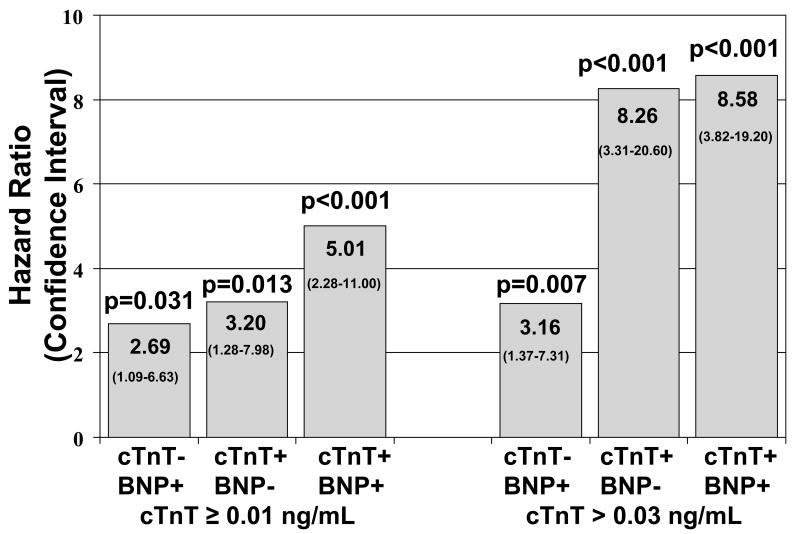
More recently Miller et al investigated whether baseline cardiac troponin T (cTnT) and BNP (Shionogi assay) as well as changes over time were predictive of outcome (death, cardiac transplantation, hospitalization) in stable HF patients in an outpatient setting (n=190, average age 71 years, median baseline BNP 305 pg/mL) (37). Blood samples were collected every three months and physicians were blinded to the results. For the analyses cTnT was divided into three categories (<0.01, ≥0.01 to ≤0.03, and >0.03 ng/mL). BNP values were dichotomized with an elevation being defined as >95th percentile of a normal, age- and gender matched population. Both elevated baseline cTnT and elevated BNP were associated with worse outcomes. An increase in cTnT over time increased risk while a decrease reduced risk. In contrast, a change from normal to elevated BNP indicated an increase in risk, but, once elevated, this increased risk persisted regardless of subsequent changes. Combination of the two markers further refined risk prediction (Fig. 4). Of note, this study did not assess whether monitoring the two biomarkers added information that was not already obvious from the clinical assessment.
Figure 4.
Hazard ratios for risk of death/cardiac transplantation in ambulatory patients with heart failure. Time dependent multivariate model with serial follow-up troponin T and BNP values (every 3 months). + indicates elevated BNP or troponin T ≥0.01 ng/mL or >0.03 ng/mL; - not elevated (HR, 1.0 for elevation of neither BNP or troponin T). From Miller WL, Hartman KA, Burritt MF, et al: Serial biomarker measurements in ambulatory patients with chronic heart failure: the importance of change over time. Circulation 2007;116(3):254, with permission.
In a second report Miller et al. used the same dataset to better define what degrees of change in BNP are associated with outcomes (38). For this analysis the investigators also measured NTproBNP. Changes were defined on a percent basis (>80% decrease, 20-80% decrease, no change, 20-80% increase, >80% increase) and on whether there was a movement from below to above a cutpoint or vice versa (500 pg/mL for BNP, 1000 pg/mL for NTproBNP). Impact of changes on outcomes are shown in Fig. 5. With regard to BNP, only a BNP reduction >80% was associated with a risk reduction, while an increase from below to above 500 pg/mL was associated with an increased risk. With regard to NTproBNP, only a reduction from above to below the 500 pg/mL cutpoint was associated with a reduced risk of death. The authors indicated that this analysis was meant to be hypothesis generating and did not correct for multiple comparisons. These findings are consistent with the high biological variability reported in other studies. They also noted that while there is considerable overlap in category changes of BNP and NTproBNP, there was also substantial variability. It remains to be established whether one assay is superior for monitoring HF patients or whether combining the information from both assays would be worthwhile.
Figure 5.
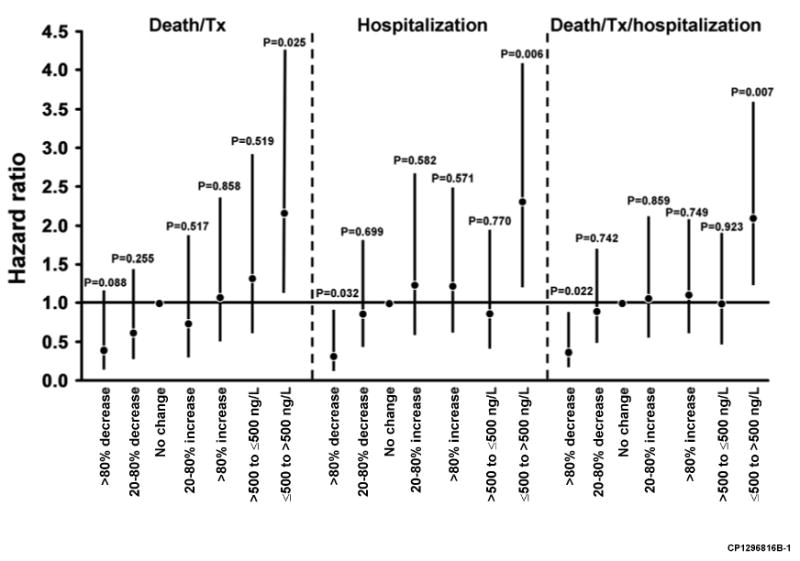
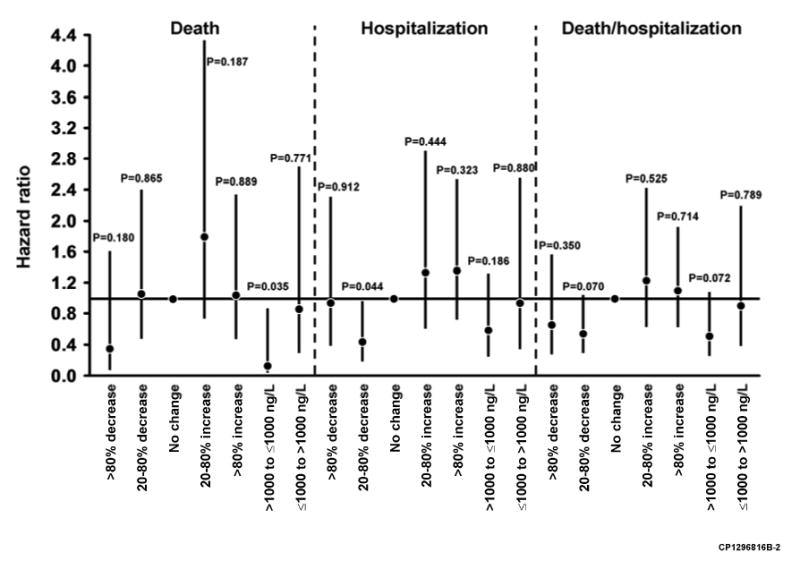
Cardiac-related event risks associated with changes in BNP concentration (A) and NTproBNP concentrations (B) in ambulatory patients with heart failure. Hazard ratios (95% confidence interval) from Cox models relative to no changes in BNP or NTproBNP or no crossing over from more than to less than the BNP (500 ng/L) or NTproBNP (1000 ng/L) cutpoint values. Tx, transplantation. From Miller WL, Hartman KA, Grill DE, et al: Only large reductions in concentrations of natriuretic peptides (BNP and NT-proBNP) are associated with improved outcome in ambulatory patients with chronic heart failure. Clin Chem 2008(1):81, with permission.
BNP-Guided Therapy
As discussed above, higher BNP levels are related to cardiac overload and have been associated with worse outcomes. This provides the rationale for the hypothesis that intensifying treatment so as to reduce BNP levels below a certain threshold could improve outcomes. Important study characteristics to consider are the treatment goal, i.e. target BNP value and the treatment algorithm to be followed. Some study characteristics discussed below are shown in Table 1.
Table 1.
Baseline parameters in studies evaluating NP guided therapy.
| Study | n | Age (years) |
NYHA class (mean) |
NYHA class≥III (%) |
LVEF (%) |
Baseline NP (pg/mL) |
Treatment goal (pg/mL) |
|---|---|---|---|---|---|---|---|
| BNP | |||||||
| Murdoch | 20 | 64/62 | 2.4/2.5 | NA | 25/25 | 139/111 pg/mL (mean) | <50 |
| STARS-BNP | 220 | 66/65 | 2.2/2.3 | 21/29 | 32/30 | NA/352 (mean) | <100 |
| NTproBNP | |||||||
| Troughton | 69 | 68/72 | 2.3/2.3 | 28/33 | 28/26 | 1835/2122 | <1700 |
| TIME-CHF | 499 | 77/76 | NA | 75/74 | 30/30 | 4657/3998 (median) | <400 (60-74 years) <800 (≥75 years) |
| BATTLESCARRED | 364 | 76-77 (median) | ∼2.2 | 24 | 37 | 1997-2021 (median) | <1300 |
Values are given for control group/hormone guided group or for groups combined. BATTLESCARRED consisted of three groups.
Murdoch et al in 1999 published a small pilot study (n=20) in which ACE inhibitor dose was increased in stable, well-compensated HF patients either according to target dose based on clinical trial data or to achieve a reduction of plasma BNP below 50 pg/mL (39). During the 8-week study drug dose was increased significantly more in the BNP-guided group. BNP levels were significantly lower between groups only at 4 weeks. Hemodynamic function including right atrial pressure, systemic and pulmonary vascular resistance assessed at baseline and at 8 weeks were not different with the exception of heart rate, which was lower in the BNP-guided group. The major limitation of this study was the small number of patients with the corresponding limited statistical power. Importantly, only 3 of 10 patients in the control group and 4 of 10 in the BNP-guided group achieved BNP levels below the goal of 50 pg/mL. Therefore, while one expectation prior to the study was that BNP-guidance may demonstrate that lower doses of ACE inhibitors may be sufficient to improve hemodynamic function and achieve “normal” BNP levels, the hormone guidance essentially forced maximization of therapy in most patients without necessarily achieving target BNP levels.
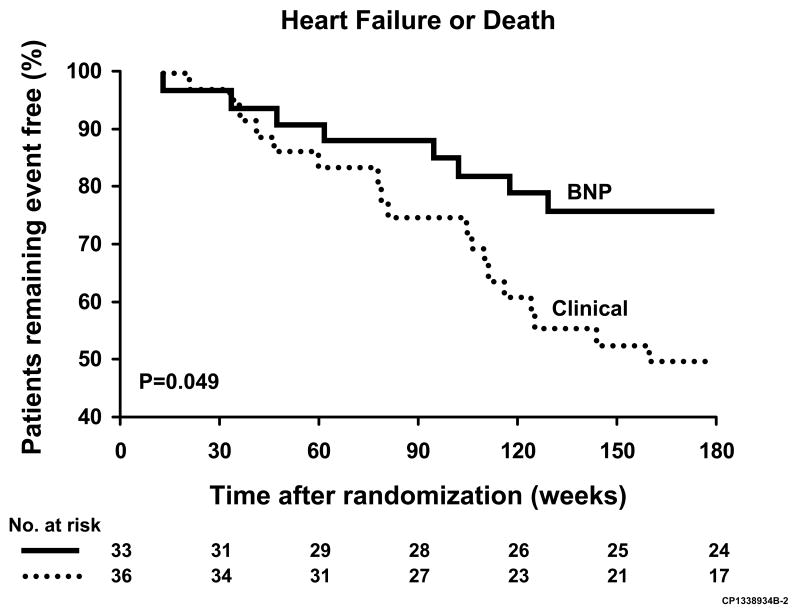
Troughton et al were the first to assess the impact of NTproBNP-guided therapy on long-term outcome in a study conducted from 1998 to 1999 (40). (Fig 6). Patients with an LVEF <40%, NYHA class II-IV HF, and plasma creatinine ≤200 μmol/L were randomized to a control group (n=36) or NTproBNP guided group (n=33). The control group received standard care which was uptitrated when clinical status as expressed by a HF score based on Framingham criteria indicated decompensation. In the NTproBNP guided group an additional aim was to reduce NTproBNP levels to less than 200 pmol/L (about 1700 pg/mL). Of note, less than 15% of patients received beta blockers during this study. During a median follow-up of about 9.5 months, the primary combined clinical endpoint (cardiovascular death, hospital admission, and outpatient heart failure) was significantly lower in the hormone-guided group as compared to the control group (19 vs 54 events), whereas changes in symptomatic and functional status did not differ between groups. NTproBNP levels decreased in the hormone guided group (-79 pmol/L) but remained unchanged in the control group (-3 pmol/L; p=0.16 between groups). ACE inhibitor doses were increased significantly more and more patients were started on spironolactone in the hormone-guided group. The average number of extra visits per patient was 1.7 in the BNP group and 0.8 in the clinical group (p=0.19). Of note, in this study a relatively high NTproBNP target was chosen compared to the other studies discussed in this section and the average baseline NTproBNP value was only slightly above these levels, so achieving the treatment goal in the hormone guided group was possible to a substantial degree.
Figure 6.
Kaplan-Meier event curves for time to heart failure event or death in heart failure patients randomized to a NTproBNP guided group or a clinical (control) group. From Troughton RW, Frampton CM, Yandle TG, et al: Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000;355(9210):1128, with permission.
The STARS-BNP trial enrolled 220 patients with stable HF (no hospital stay in the previous month), NYHA class II-III, LVEF<45%, stable (≥ 1month) HF medication, including diuretic and guideline-recommended doses of ACE inhibitors/angiotensin II receptor blockersB and beta blockers unless not tolerated (41). Patients with plasma creatinine >250 μmol/L were excluded. Patients were randomized to standard medical therapy or to a BNP-guided group, in which the goal was to lower BNP below 100 pg/mL. Adjustment of therapy in both groups was at the discretion of the investigator. In the first three months patients were seen every month (titration phase), thereafter every three months (follow-up phase). During the titration period, there were significantly more medication changes in the BNP-guided group as compared to the control group (134 vs. 66), and 79% of changes in the BNP guided group were triggered by elevated BNP values. At the end of the titration phase, dosages of ACE inhibitors and beta blocker were significantly higher in the BNP-guided group. While there were no differences in event rates between groups in the first three months, in the follow-up period the primary endpoint, which consisted of unplanned hospital stays for HF or death related to HF occurred significantly less often in the BNP group than in the control group (24% vs 52%). Event-free survival was also significantly better (84% vs. 73%). All-cause mortality (7 vs. 11) and all-cause hospital stays (52 vs 60 days) were not statistically different between BNP group and control. However, hospital stays for HF were lower in the BNP guided group (22 vs 48 patients) as were repeat HF hospitalizations (2 vs. 10 patients). Mean BNP levels decreased from 352 to 284 pg/mL with the proportion of patients with BNP<100 pg/mL increasing from 16% at baseline to 33% at 3 months.
The TIME-CHF (Trial of Intensified (BNP-guided) versus standard (symptom-guided) Medical therapy in Elderly patients with Congestive Heart Failure) trial was designed to assess BNP guided therapy both in younger (60-74 years, n=210) and older (≥75 years, n=289) HF patients, the latter a group that is frequently underrepresented in HF trials (42, 43). Patients were included if they had an LVEF<45%, had been hospitalized for HF in the previous year, were in NYHA class≥II, and had an NTproBNP value >400 or >800 pg/mL for the younger and older patients, respectively. Among the exclusion criteria were a body mass index >35 kg/m2 to reduce the impact of weight on symptoms, and plasma creatinine >220 μmol/L. The control group received standard medical therapy, and the aim was to reduce NYHA functional class to ≤II. In the intensified treatment group, an additional treatment aim was to reduce NTproBNP levels to <400 pg/mL and <800 pg/mL for younger and older patients, respectively. The older patient group was on average not only older but also more symptomatic, had worse renal function, more comorbidites, a higher proportion of females and patients with coronary artery disease, and higher NTproBNP values compared to the younger group (5063 vs 2998 pg/mL).
The primary endpoints were survival free of any hospitalizations and quality of life; overall they did not reach significance. The secondary endpoint was survival and survival without hospitalization for HF. Mortality was reduced with BNP guidance in the younger group, but not in the older group. Interestingly, while there was no difference in change in quality of life measures with intensified treatment in the younger group, patients in the older group with NTproBNP guidance improved significantly less than those randomized to standard therapy. NTproBNP-guided patients received significantly higher doses of ACE inhibitors (or ARBs) and beta blockers, while there was no difference with respect to diuretics, nitrates, and digoxin. NTproBNP levels decreased in all treatment groups, but the decrease tended to be greater with intensified treatment in the younger (p=0.056) but not the older patients (p=0.30); importantly, even in the hormone-guided group the majority of patients did not achieve levels below the target NTproBNP levels.
The findings in TIME-CHF show promising signals for NTproBNP guidance in younger patients and have provocative implications for the treatment of older patients. The major effect of BNP guidance was dose increase of ACE inhibitors and beta blockers and addition of mineralocorticoid receptor antagonists. All these interventions would generally have been considered to improve outcomes based on clinical trials, which however were frequently conducted in younger patients with fewer comorbidities. That this medication increase was not associated with a survival benefit but rather less improvement in quality of life poses the question whether targets in medication dose should be lower in at least some older patients. It should be noted that the lack of benefit in older patients may be due to their poorer health rather than their age.
As mentioned above, NTproBNP levels decreased in both treatment groups in both age categories. Importantly, however, NTproBNP tended to decrease more in the NTproBNP guided group than the control group in younger individuals (p=0.056), whereas this was not the case in the older patients (p=0.30). If one accepts that NTproBNP truly reflects cardiac load, then one could draw the conclusion that NTproBNP guided therapy did not improve outcomes because the therapeutic strategy used was not able to reduce cardiac load as compared to the control group. In other words, the lack of benefit seen in older patients does not disprove the concept of a hormone-guided treatment approach but rather questions the efficacy of the treatment.
The BATTLESCARRED trial was conducted at the Christchurch Hospital in New Zealand and enrolled patients within two weeks of a HF hospitalization (44). Patients with both reduced and preserved ejection fraction were eligible but they needed to have pre-randomization NTproBNP levels greater than 50 pmol/L (approximately 400 pg/mL) and a serum creatinine less than or equal to 250 μmol/L. The trial was designed to compare three treatment strategies: (a) usual care, (b) intensive standardized clinical assessment, and (c) intensive standardized clinical assessment plus NTproBNP guidance. In contrast to usual care, the two other treatments included a visit to the Christchurch Hospital research outpatient clinic at least once every three months with a thorough clinical assessment and, if appropriate, titration of medication according to a treatment algorithm. Intensification of drug therapy was triggered if a clinical HF score increased above a certain threshold indicating decompensation, and, in the hormone guided group, if the NTproBNP level was above 150 pmol/L (about 1300 pg/mL). Preliminary results results have been reported recently (45).
Compared to the TIME-CHF trial this study was conducted in a healthier patient population and had a less aggressive treatment goal. At 12 months after randomization, all-cause mortality was significantly lower with intensive follow-up compared to usual care, but without additional benefit from NTproBNP guidance (18.9% with usual care, 9.1% with intensive follow-up, and 9.1% with additional hormone guidance). At 2 and 3 years there were no significant differences between treatments overall. However, in patients ≤75 years, cumulative all-cause mortality was significantly reduced by NTproBNP guidance compared to usual care throughout follow-up (cumulative mortality at 1, 2, and 3 years were 1.7, 7.3, and 15.5% vs. 20.3, 23.4, and 31.3%, respectively) and also compared to intensive follow-up by 3 years (cumulative mortality at 1,2, and 3 years were 7.3, 20.0, and 30.9%; which was only significant compared to usual care at 1 year). The composite end-point of death or hospital admission with HF was reduced compared to usual care only in patients younger than 75 years with hormone guided therapy. No benefits were seen in patients older than 75 years.
Both TIME-CHF as well as BATTLESCARRED are very interesting studies. Important questions remain: Should there be individualized target NP values, e.g. considering age, sex, renal function, body mass index, and genotype? How many patients were able to reach the target range and what was their outcome? Is trying to lower the NP levels below the prespecified targets for most patients an exercise in futility that cannot be achieved and by treatment algorithm consequently leads to maximal tolerated therapy? What were the number of physician visits in the treatment groups? What were the changes in medication, e.g. diuretic dose? Is further uptitration not required if patients have values below the target level? To what degree are cardiac status and comorbidities rather than age responsible for the differential results in younger vs. older patients? One of the major conclusions may be that maximization of therapy beneficial in younger patients may be of less or no benefit in older individuals with more comorbidities.
HF therapies not only come with a financial cost but also with a risk of complications and side effects. For that reason it would be desirable to have a biomarker that could indicate in what patients the treatment is not required because of low risk or what patients are unlikely to benefit from a risky intervention. For instance, cardiac resynchronization therapy (CRT) significantly improves symptomatic status and survival, but a substantial percentage of patients appear to be “non-responders”. In the CARE-HF trial, elevated NTproBNP was a significant predictor of adverse outcome, but patients both below and above the median derived benefit from CRT relative to the control group without CRT (46). Thus, NTproBNP levels do not seem to help in deciding for or against CRT.
In the PEACE trial, ACE inhibition with trandolapril compared to placebo in patients with established coronary artery disease and an LVEF>40% did not improve the primary end point, which was death from cardiovascular causes, myocardial infarction, or coronary revascularization, but it reduced the number of patients requiring hospitalization for or dying of HF. Given the association of BNPs with outcomes it was hypothesized that patients with higher baseline NPs would have been more likely to benefit from ACE inhibition. However, no such interaction was found (26).
On the Horizon
What exactly are we measuring?
As mentioned above, most current assay systems use antibodies directed against epitopes of BNP 1-32 or NTproBNP. This approach can only have limited specificity, and indeed, several conventional assays also detect circulating proBNP (5,6). As proBNP has reduced biological activity compared to BNP 1-32, non-specific assays may not accurately reflect biological activity (5,6). Recently, a proBNP assay has been developed which uses an antibody directed against the hinge region of proBNP so that better differentiation between prohormone and cleavage products is possible (47). This may be important because there may be impaired processing of proBNP to BNP because of peptide glycosylation or decreased enzymatic activity (48,49). Also, if a BNP assay uses antibodies directed against the ring structure and the carboxy terminus, this assay will not be able to differentiate between BNP forms with a full as compared to a cleaved amino terminus. BNP 3-32, which at least in vivo is produced when BNP 1-32 is cleaved by the ubiquitous aminopeptidase dipeptidyl peptidase IV, can be detected in plasma either by specific assays, biochemically, or by mass spectrometry (7,8,50,51). While BNP 3-32 and BNP 1-32 had similar cGMP-activating properties in vitro in canine cardiac fibroblasts, bioactivity of BNP 3-32 in vivo in healthy canines was reduced, presumably due to a shorter half-life (9).
Other cleavage products of BNP 1-32 have been described by mass spectrometry, but their biological activity and significance remains undefined (8). It should be noted that in general peptide cleavage may not only mean a quantitative reduction in bioactivity, but it may also qualitatively change the activity profile of a hormone (52). At this time it is unclear whether more specific characterization of BNP immunoreactivity will yield higher diagnostic and prognostic information for the clinician than currently available assays which seem to provide a composite of different molecular forms. However, tools like mass spectrometry will most certainly improve our understanding of the biology of the natriuretic peptide system and may provide the rationale for more specific assay developments and innovative therapies (8,53).
Personalized medicine with BNP
As mentioned above, BNP is a good example of how diagnostic tests can be affected by genetic variation. Accounting for genotype may improve test characteristics and thus patient management / prevention strategies. It can be expected that similar test modifying genetic variants will be found in the future as has been for prostate-specific antigen (54), so that it may become worthwhile to develop a chip with a multitude of genetic markers that will help to individualize and refine the interpretation of diagnostic tests and the approach to prevention and treatment. In addition, it would be worthwhile to investigate whether genetically elevated BNP values have a protective effect regarding cardiovascular disease and especially HF. Such findings could provide a rationale for supplementing BNP or similar NPR-A agonists in subjects with relatively reduced endogenous levels.
Acknowledgments
This research was supported by PO1 HL76611, RO1 HL36634 (JCB), and T32 HL07111 (GB)
Footnotes
Financial Disclosures: JCB has research support from BioRad. No disclosures (GB, LCC-B).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.de Bold AJ, Borenstein HB, Veress AT, et al. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Kangawa K, Matsuo H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP) Biochem Biophys Res Commun. 1984;118(1):131–9. doi: 10.1016/0006-291x(84)91077-5. [DOI] [PubMed] [Google Scholar]
- 3.Burnett JC, Jr, Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231(4742):1145–7. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 4.Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87(4):1402–12. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heublein DM, Huntley BK, Boerrigter G, et al. Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49(5):1114–9. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 6.Liang F, O'Rear J, Schellenberger U, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49(10):1071–8. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Brandt I, Lambeir AM, Ketelslegers JM, et al. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52(1):82–7. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 8.Niederkofler E, Kiernan U, O'Rear J, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail. 2008;1:258–64. doi: 10.1161/CIRCHEARTFAILURE.108.790774. [DOI] [PubMed] [Google Scholar]
- 9.Boerrigter G, Costello-Boerrigter LC, Harty GJ, et al. Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R897–901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload J Clin Invest. 1995;96(3):1280–7. doi: 10.1172/JCI118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825–32. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 12.Maisel A, Mueller C, Adams K, Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–39. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Clerico A, Fontana M, Zyw L, et al. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: a systematic review. Clin Chem. 2007;53(5):813–22. doi: 10.1373/clinchem.2006.075713. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 15.Coste J, Jourdain P, Pouchot J. A gray zone assigned to inconclusive results of quantitative diagnostic tests: Application to the use of brain natriuretic peptide for diagnosis of heart failure in acute dyspneic patients. Clin Chem. 2006;52(12):2229–35. doi: 10.1373/clinchem.2006.072280. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJ, Larson MG, Levy D, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108(1):13–6. doi: 10.1161/01.CIR.0000081657.83724.A7. [DOI] [PubMed] [Google Scholar]
- 17.Meirhaeghe A, Sandhu MS, McCarthy MI, et al. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16(11):1343–50. doi: 10.1093/hmg/ddm084. [DOI] [PubMed] [Google Scholar]
- 18.Lanfear DE, Stolker JM, Marsh S, et al. Genetic variation in the B-type natiuretic peptide pathway affects BNP levels. Cardiovasc Drugs Ther. 2007;21(1):55–62. doi: 10.1007/s10557-007-6007-5. [DOI] [PubMed] [Google Scholar]
- 19.Costello-Boerrigter LC, Boerrigter G, Ameenuddin S, et al. The B-type natriuretic peptide T-381C polymorphism is associated with increased BNP plasma immunoreactivity and higher prevalence of type 2 diabetes mellitus and atrial fibrillation. Eur Heart J. 2008;29(Abstract Supplement):766. [Google Scholar]
- 20.Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5):976–82. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 21.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47(2):345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109(25):3176–81. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 23.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 25.McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47(5):874–80. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omland T, Sabatine MS, Jablonski KA, et al. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol. 2007;50(3):205–14. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 28.McDonagh TA, Morrison CE, Lawrence A, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997;350(9081):829–33. doi: 10.1016/S0140-6736(97)03033-X. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8):977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 30.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351(9095):9–13. doi: 10.1016/s0140-6736(97)03034-1. [DOI] [PubMed] [Google Scholar]
- 31.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288(10):1252–9. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 32.Hedberg P, Lonnberg I, Jonason T, et al. Electrocardiogram and B-type natriuretic peptide as screening tools for left ventricular systolic dysfunction in a population-based sample of 75-year-old men and women. Am Heart J. 2004;148(3):524–9. doi: 10.1016/j.ahj.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 33.Ewald B, Ewald D, Thakkinstian A, et al. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38(2):101–13. doi: 10.1111/j.1445-5994.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia M, Pewsner D, Juni P, et al. Accuracy of B-type natriuretic peptide tests to exclude congestive heart failure: systematic review of test accuracy studies. Arch Intern Med. 2006;166(10):1073–80. doi: 10.1001/archinte.166.10.1073. [DOI] [PubMed] [Google Scholar]
- 35.Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–41. doi: 10.1016/j.jacc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 36.Anand IS, Fisher LD, Chiang YT, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107(9):1278–83. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 37.Miller WL, Hartman KA, Burritt MF, et al. Serial biomarker measurements in ambulatory patients with chronic heart failure: the importance of change over time. Circulation. 2007;116(3):249–57. doi: 10.1161/CIRCULATIONAHA.107.694562. [DOI] [PubMed] [Google Scholar]
- 38.Miller WL, Hartman KA, Grill DE, et al. Only large reductions in concentrations of natriuretic peptides (BNP and NT-proBNP) are associated with improved outcome in ambulatory patients with chronic heart failure. Clin Chem. 2009;55(1):78–84. doi: 10.1373/clinchem.2008.108928. [DOI] [PubMed] [Google Scholar]
- 39.Murdoch DR, McDonagh TA, Byrne J, et al. Titration of vasodilator therapy in chronic heart failure according to plasma brain natriuretic peptide concentration: randomized comparison of the hemodynamic and neuroendocrine effects of tailored versus empirical therapy. Am Heart J. 1999;138(6 Pt 1):1126–32. doi: 10.1016/s0002-8703(99)70079-7. [DOI] [PubMed] [Google Scholar]
- 40.Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355(9210):1126–30. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 41.Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49(16):1733–9. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 42.Brunner-La Rocca HP, Buser PT, Schindler R, et al. Management of elderly patients with congestive heart failure--design of the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) Am Heart J. 2006;151(5):949–55. doi: 10.1016/j.ahj.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Pfisterer M, Buser P, Rickli H, et al. TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301(4):383–92. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 44.Lainchbury JG, Troughton RW, Frampton CM, et al. NTproBNP-guided drug treatment for chronic heart failure: design and methods in the “BATTLESCARRED” trial. Eur J Heart Fail. 2006;8(5):532–8. doi: 10.1016/j.ejheart.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Richards AM, Lainchbury JG, Troughton RW, et al. NTproBNP Guided Treatment for Chronic Heart Failure: Results from the BATTLESCARRED Trial. Circulation. 2008;118(18):S1035–5946. [Google Scholar]
- 46.Cleland J, Freemantle N, Ghio S, et al. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. J Am Coll Cardiol. 2008;52(6):438–45. doi: 10.1016/j.jacc.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 47.Giuliani I, Rieunier F, Larue C, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52(6):1054–61. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 48.Schellenberger U, O'Rear J, Guzzetta A, et al. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006;451(2):160–6. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Liao X, Fukuda K, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103(5):502–8. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam CS, Burnett JC, Jr, Costello-Boerrigter L, et al. Alternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general population. J Am Coll Cardiol. 2007;49(11):1193–202. doi: 10.1016/j.jacc.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu H, Masuta K, Aono K, et al. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316(12):129–35. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 52.Ban K, Noyan-Ashraf MH, Hoefer J, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 53.Hawkridge AM, Heublein DM, Bergen HR, 3rd, et al. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102(48):17442–7. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cramer SD, Chang BL, Rao A, et al. Association between genetic polymorphisms in the prostate-specific antigen gene promoter and serum prostate-specific antigen levels. J Natl Cancer Inst. 2003;95(14):1044–53. doi: 10.1093/jnci/95.14.1044. [DOI] [PubMed] [Google Scholar]