Abstract
Dental pulp tissue is vulnerable to infection. Entire pulp amputation followed by pulp-space disinfection and filling with an artificial rubber-like material is employed to treat the infection – commonly known as root-canal therapy. Regeneration of pulp tissue has been difficult as the tissue is encased in dentin without collateral blood supply except from the root apical end. However, with the advent of the concept of modern tissue engineering and the discovery of dental stem cells, regeneration of pulp and dentin has been tested. This article will review the early attempts to regenerate pulp tissue and the current endeavor of pulp and dentin tissue engineering, and regeneration. The prospective outcome of the current advancement in this line of research will be discussed.
Keywords: dental pulp stem cells, mesenchymal stem cells, stem cells from apical papilla, stem cells from human exfoliated deciduous teeth, tissue engineering, tissue regeneration
A tooth is a complex structure built by cells originated from both ectoderm and mesoderm. Enamel is made by ameloblasts, which are derived from ectoderm, and these cells disappear after hard-tissue formation is complete. Dentin is produced by odontoblasts that are derived from ectomesenchyme. These cells continue to exist in the pulp throughout a person’s life. Recent advances in tissue engineering have drawn scientists to test the possibility of tooth engineering and regeneration. Tooth regeneration is normally referred to as the regeneration of the entire tooth or root that can be integrated into the jaw bone. This technology is still at its infancy and when it matures, it may be used to restore missing teeth and replace artificial dental implants [1–5]. When the tooth is damaged but still in a reparable condition, regeneration of parts of the tooth structure can prevent or delay the loss of the whole tooth.
Dental pulp is a soft connective tissue and its main functions are to produce dentin, and maintain the biological and physiological vitality of the dentin. In addition, it possesses a highly responsive sensory nervous system that generates unbearable pain when the tooth is inflicted by mechanical trauma, chemical irritation or microbial invasion. Owing to the small volume of pulp tissue encased in dentin – an unyielding space, with blood supply only from one end at the root apex – pulp tissue is vulnerable to external insults. When infected, it is difficult for the immune system to eradicate the infection owing to the lack of a collateral blood supply. Partially removing the infected pulp, a procedure termed partial pulpectomy, has been proven to be ineffective as infection may still be left behind. Therefore, this approach is no longer commonly practised by clinicians. Clinically, when pulp is diagnosed with irreversible pulpitis, in other words, no treatment can reverse the situation regardless of the amount of remaining normal pulp tissue, the entire pulp is amputated by pulpectomy. The pulp space is then disinfected and replaced with a rubber-like material, gutta percha. This treatment protocol has been a common clinical practice for decades. Many breakthroughs have been made in clinical endodontics since its recognition by the American Dental Association as a dental specialty in 1963, however, the innovations have been heavily focused on the technical aspects. For example, the constant improvement of instruments that are used to clean and enlarge the root canal space, and modification of the filling materials that are used to fill the canal space.
As mentioned, the volume of the mature pulp tissue is very small (~10–100 μl). The regeneration of such a small volume of soft tissue should be relatively simple compared with that for larger organs or tissues. However, it is considered a difficult task to engineer and regenerate the entire pulp and its main product, dentin, owing to: the unique anatomical location of the pulp tissue, which is encased within dentin with mainly one apical foramen to allow angiogenesis for the engineered tissue; the unique microstructure of pulp tissue, in other words, the different types of cells (e.g., odontoblasts) in different layers or zones and complex innervation; the specific location of dentin, which is located only peripherally of the pulp tissue; and the highly organized structure of dentin with well-aligned dentinal tubules.
Attempts to regenerate pulp tissue were tested in the 1960s and 70s but without success. It was not until the late 1990s when modern tissue-engineering technology began to emerge that pulp-tissue regeneration was re-investigated [6–8]. This endeavor halted owing to the lack of isolation and characterization of pulp stem cells that potentially may differentiate into odontoblasts. Regenerated pulp tissue should be functionally competent, for example, capable of forming dentin to repair lost structure. Reports have shown that isolated pulp cells can be induced to differentiate into odontoblast-like cells and generate dentin-like mineral structure in vitro [9,10]. The in vivo evidence of pulp cells capable of generating dentin was demonstrated by Gronthos et al., who showed that a human pulp/dentin complex can be formed ectopically in immunocompromised mice [11]. This discovery shed new light on the possibility of regenerating pulp/dentin for clinical applications.
Clinical advantage with pulp/dentin regeneration
Although the success rate of endodontic treatment is relatively high (78–98%) [12–15], there are many problems associated with this aggressive treatment, including the following:
Endodontic procedures are technically sensitive; mishaps occur including blockage of the root-canal space, and breakage of instruments in the canals and perforations [16–21], which will leave infected tissue behind or even cause loss of the tooth;
If dealing with an immature tooth that has little dentin structure after loss of pulp tissue, endodontic treatment cannot prevent its susceptibility to fracture from traumatic injuries [22–26];
Teeth after endodontic treatment lose a significant amount of tooth structure. Post-space plus crown preparation sacrifices more tooth structure, which weakens the tooth [27–29];
Pulpless teeth have no sensation to irritations, rendering caries progression unnoticed by patients. Long-term studies have demonstrated that tooth loss is higher for endodontically treated teeth than nontreated teeth owing to secondary caries and complex restoration-associated problems [30–33].
The potential of pulp tissue to regenerate lost dentin is well known. When pulp tissue is exposed owing to the loss of the overlaying dentin, direct pulp-capping therapy can allow the pulp to form new dentin, which is termed a dentin bridge. Using various cement-based materials for pulp capping, such as calcium hydroxide and more recently mineral trioxide aggregate (MTA), has been well documented and studied [34,35]. When the tooth is further damaged, regeneration of dentin becomes difficult as it requires a healthy pulp, which may be compromised by the disease. Ideally, the regenerated dentin should not replace the pulp space.
Two types of pulp engineering/regeneration can be considered based on the clinical situations: one is partial pulp regeneration and the other is de novo synthesis of pulp. It has been observed that pulpal infection and inflammation is compartmentalized until the entire pulp tissue undergoes necrosis [36,37]. Before complete pulp necrosis, the remaining pulp tissue may be recoverable after disinfection and help to regenerate the lost portion. To enhance the regeneration, engineered pulp tissues may be inserted into the pulp space to facilitate the entire recovery of pulp tissue and the generation of new dentin. When the entire pulp tissue is lost, de novo synthesis of pulp must take place in order to regenerate the tissue.
Early attempts at pulp regeneration
Regeneration of any tissue back to its original condition has been a long quest in all medical disciplines. To enhance regeneration, blood clots have been used as a rich source of growth factors to help tissue repair. Creating hemorrhage to fill into the surgical site has been a routine practice for certain conditions in surgery. In endodontics, this idea was tested by Ostby in the 1960s to determine if filling the canal space with a blood clot could lead to regeneration of pulp tissue [38]. In the 1970s, similar experiments were conducted by another group and generation of soft connective tissue was observed. The average ingrowth of the tissue into the canal was only 0.1–1.0 mm [39]. The pitfall of this approach is that pulp tissue is different from the periapical tissue, therefore pulp regeneration made by cells from the adjacent tissues is unlikely. Evidence has shown that when there is a total loss of pulp tissue due to trauma, the canal space is filled in by periapical tissues including bone, periodontal ligament and cementum, but not pulp [40,41].
Modern concept of pulp tissue engineering
The modern concept of tissue engineering emerged in the late 1980s and one of the key components of this modern concept is the utilization of synthetic biodegradable materials as a scaffold to hold ex vivo-expanded tissue cells. The scaffold provides a 3D environment for cells to attach and grow, therefore mimicking the in vivo condition. In addition, these synthetic matrices can be fabricated such that it may form any desired shape and carry required growth factors to guide the process of cell differentiation and tissue formation. Generally, tissue-engineering technology involves generating tissue or organ constructs in vitro for subsequent implantation. The biodegradable material can be synthetic polymers (e.g., poly(D-L-lactide-co-glycolide) [PLG]) or processed biological products (e.g., collagen matrix or gel) [42–44].
Research teams led by Mooney and Rutherford tested pulp regeneration using modern tissue-engineering concepts by growing pulp cells onto a synthetic polymer scaffold of poly(glycolic acid) (PGA) and performed in vitro and in vivo analyses [6–8]. Their approaches are basically a proof-of-principle to test whether cultured pulp cells can grow well and produce matrix on PGA, and whether the engineered pulp can be vascularized using in vivo study models. However, owing to the lack of the technology to isolate stem cells in pulp tissues that can give rise to odontoblasts and make dentin in vivo, this line of research was discontinued.
Several papers that signified the possibility of regeneration of pulp and dentin clinically were reported by Shi and colleagues. A type of pulp cells termed postnatal dental pulp stem cells (DPSCs) were found to exhibit the characteristics of mesenchymal stem cells (MSCs) and, most importantly, the ability to form an ectopic human pulp-/dentin-like complex, as well as to form dentin-like structures on an existing human dentin surface in immuno-compromised mice [11,45,46]. Besides DPSCs, several other types of stem cells or progenitor cells from dental tissues have been isolated and characterized, these are stem cells from exfoliated deciduous teeth (SHED) [47], periodontal ligament stem cells [48], stem cells from apical papilla (SCAP) [5,49] and dental follicle progenitor cells [50]. These postnatal populations have MSC-like qualities, including the capacity for self-renewal and multilineage differentiation potential [51].
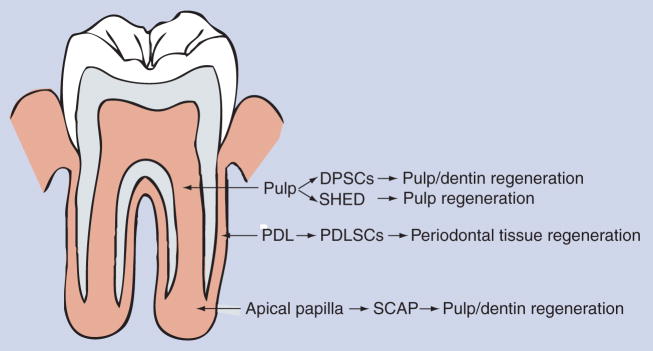
Dentistry has taken note on the potential of these cells for clinical applications. In the field of endodontics, the term regenerative endodontics has been coined and it advocates the requirement for research on the understanding of these stem cells, and their use for the regeneration of endodontic tissues including pulp and dentin [52–54] (see Figure 1, the potential applications of dental stem cells for dental tissue regeneration).
Figure 1. Source of dental stem cells and use for dental tissue regeneration.
Note DPSCs are from pulp of permanent teeth, SHED from exfoliated primary teeth. SHED have been shown to regenerate pulp, but not dentin as yet [75].
DPSC: Dental pulp stem cell; PDL: Periodontal ligament; PDLSC: Periodontal ligament stem cell; SCAP: Stem cells from apical papilla; SHED: Stem cells from exfoliated deciduous teeth.
The biology of dental stem cells
Stem cell biology has emerged as one of the fundamental underpinnings for regenerative medicine. In order to effectively regenerate tissues via tissue-engineering technologies, understanding the biology of stem cells has become an essential basis for the planning and execution of the engineering and regeneration process.
Stem cell niche
The stem cell niche, in general, is still an elusive term as the exact location of niches of specific stem cells is still largely unknown. The stem cell niche for hematopoietic stem cells is better understood [55], whereas the niches for MSCs are still unclear. Limited information has suggested that the location of the MSC niche is the perivascular areas [56,57]. The existence of stem/progenitor cells in pulp tissue that can give rise to newly differentiated pulp cells, especially the highly specialized cells odontoblasts that produce dentin, has been long known. Using STRO-1, CD146 and pericyte-associated antigen (3G5) as markers, the DPSC niche in human dental pulp was found to be localized in the perivascular and perineural sheath regions [57]. These STRO-1+/CD146+ DPSCs form a dentin–pulp-like complex in vivo, similar to the multiple colony-derived DPSCs. The STRO-1-positive region in the pulp of deciduous teeth is similar to that of permanent teeth, also in the perivascular regions. STRO-1 staining of apical papilla has shown that the positive stain is located in the perivascular region as well as other regions scattered in the tissue [5]. STRO-1/CD146/CD44 staining of the periodontal ligament has shown that it is mainly located in the paravascular region and small clusters of cells are in the extravascular region [58], suggesting that these are the niches of periodontal ligament stem cells.
Stem cell markers
Dental stem cells are considered a population of MSC-like cells, therefore markers that have been used for identifying MSCs are also used for dental stem cells, such as the positive markers: STRO-1, CD13, CD44, CD24, CD29, CD73, CD90, CD105, CD106, CD146, Oct4, Nanog and β2 integrin, and the negative markers: CD14, CD34, CD45 and HLA-DR [57,59–64]. Like all MSCs, dental stem cells are also heterogeneous and the various markers listed previously may be expressed by subpopulations of these stem cells [45]. Subpopulations expressing c-kit+/CD34+/STRO-1+ DPSCs or SHED were reported to be multipotent stem cells, although c-kit and CD34 are known to be markers for hematopoietic lineages of cells [65,66]. Side population cells exist in porcine dental pulp exhibiting stem cell properties with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis and neurogenesis [67]. In a dog animal model, a subfraction of the side population of pulp cells, CD31−/CD146− cells, were found to regenerate partially removed pulp tissue in the pulp chamber [68]. The human counterparts of these subpopulations of DPSCs are yet to be reported.
Multiple differentiation potential
Dental stem cells display multidifferentiation potential with the capacity to give rise to at least three distinct cell lineages: osteo/odontogenic, adipogenic and neurogenic. Differences have been noted between the dental stem cell populations and bone marrow-derived MSCs, where dental stem cells appear to be more committed to odontogenic rather than osteogenic development [11]. Subpopulations of DPSCs and SHED appear to have osteognic potential as well as chodrogenic, adipogenic and neurogenic potentials [47,65,69,70]. Table 1 summarizes the known differentiation potentials for dental stem cells. Further characterization of subpopulations of DPSCs has been reported demonstrating that distinct stem/progenitor cells expressing embryonic neural-crest cell makers appear to exist and possess multiple differentiation potential [71].
Table 1.
Multiple differentiation potential of human dental stem cells (in vitro analysis).
| Cell type | Multipotentiality | Ref. | |
|---|---|---|---|
| DPSC | Osteo/dentinogenic | + | [11,45] |
| Adipogenic | + | ||
| Chondrogenic | + | ||
| Myogenic | + | ||
| Neurogenic | + | ||
| SHED | Dentinogenic | + | [47,69,87] |
| Adipogenic | + | ||
| Chondrogenic | + | ||
| Myogenic | + | ||
| Neurogenic | + | ||
| Osteo-inductive | + | ||
| SCAP | Dentinogenic | + | [5,49] |
| Adipogenic | + | ||
| Chondrogenic | ND | ||
| Myogenic | ND | ||
| Neurogenic | + | ||
| PDLSC | Osteo/cementogenic | + | [48] |
| Adipogenic | + | ||
| Chondrogenic | + | ||
| Myogenic | ND | ||
| Neurogenic | + | ||
| DFPC | Cementogenic | + | [50] |
| Odontogenic | + | ||
| Adipogenic | + | ||
| Chondrogenic | + | ||
| Myogenic | ND | ||
| Neurogenic | ND | ||
| BMMSC | Odontogenic | − | [88] |
| Osteogenic | + | ||
| Adipogenic | + | ||
| Chondrogenic | + | ||
| Myogenic | + | ||
| Neurogenic | + | ||
BMMSC: Bone marrow-derived mesenchymal stem cell; DFPC: Dental follicle progenitor cell; DPSC: Dental pulp stem cell; ND: Not determined; PDLSC: Periodontal ligament stem cell; SCAP: Stem cells from apical papilla; SHED: Stem cells from exfoliated deciduous teeth.
Suitable cell types for pulp/dentin engineering & regeneration
Among the identified dental stem cells mentioned previously, DPSCs, SHED and SCAP are potentially suitable cell sources for pulp/dentin regeneration because they are derived from pulp tissue or the precursor of pulp. DPSCs and SCAP are known to form a pulp–dentin complex when transplanted into immunocompromised mice [5,11,45], whereas SHED form mineralized tissue without the distinct pulp–dentin complex [47]. Whether other types of stem cells, such as bone marrow-derived MSCs, can differentiate into odonto-blasts and make dentin is questionable. Hu et al. demonstrated that mouse crude bone marrow cells rarely give rise to dental cells and only c-kit+-enriched bone marrow cells can acquire the characteristics of odontoblasts. Nonetheless, this phenomenon requires the interactions between oral epithelial cells and the enriched bone marrow cells [72]. Using a rat model, Yu et al. compared the odontogenic capability between bone marrow mesenchymal stromal cells and DPSCs by co-culturing these cells with apical bud cells (ABCs). They discovered that recombined DPSCs/ABCs formed typical tooth-shaped tissues with balanced amelogenesis and dentinogenesis, whereas bone marrow mesenchymal stromal cells/ABC recombinants developed into atypical dentin–pulp complexes without enamel formation [73]. These findings indicate that utilizing bone marrow cells to regenerate pulp/dentin is a less straightforward approach than using DPSCs, SCAP and SHED. Multipotent dermal cells incubated with a conditioned medium of embryonic tooth germ cells can behave similarly to DPSCs by undergoing odontogenic differentiation, demonstrated in a rat model [74]. Therefore, other sources of MSCs may be guided to become a source of dentinogenic cells by embryonic tooth germ cells.
De novo regeneration of pulp
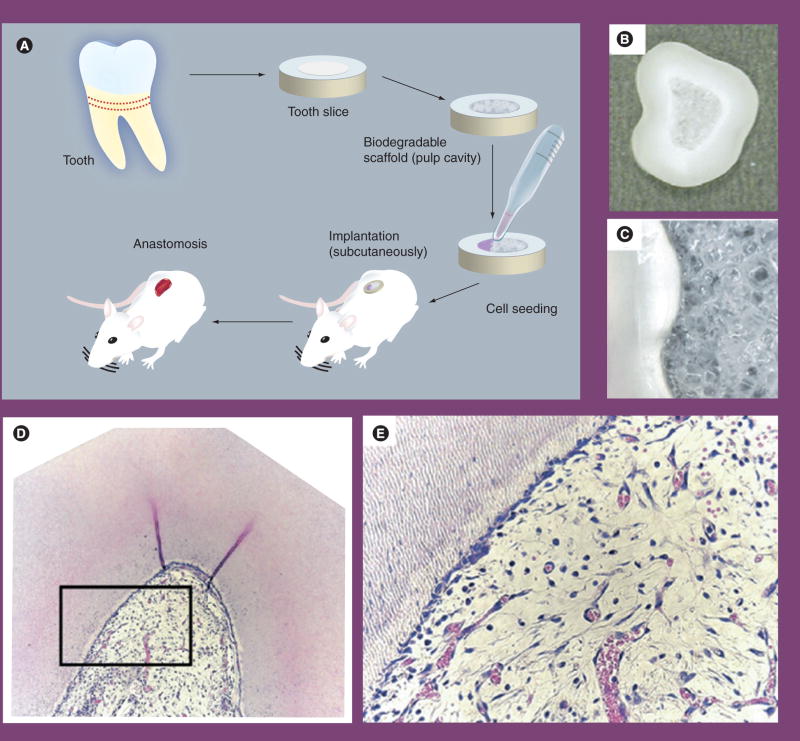
Total loss of pulp tissue can result from infection or trauma. To regenerate pulp tissue from scratch, pulp stem cells seeded into a scaffold and implanted into the canal space is a practical approach. Since the blood supply is an issue for pulp regeneration in the dead-end root-canal space, a proof-of-principle approach to test pulp regeneration disregarding this blood supply issue was undertaken using a tooth-slice model [75]. In this approach, a horizontal section of a tooth slice, 1 mm thick, was generated from human teeth. The pulp-chamber space of the slice was then cast and filled with a biosynthetic scaffold, poly-L-lactic acid (PLLA). SHED were then seeded onto the scaffolds and the tooth slices transplanted into the subcutaneous space of immunocompromised mice. Well-vascularized pulp-like tissue was formed in the pulp chamber space after 2–4 weeks of implantation (Figure 2). In addition, odontoblast-like cells expressing DSP, an odontoblast-specific gene in pulp tissue, were found to localize against the existing dentin surface. No new dentin, however, was observed to form on the existing dentin surface.
Figure 2. Engineering of dental pulp tissue with dental pulp stem cells.
(A) Strategy for dental pulp tissue engineering. (B) Biodegradable scaffold is prepared within the root canal and then seeded with stem cells from exfoliated deciduous teeth (SHED) only or SHED mixed with endothelial cells. A tooth slice containing cells is then implanted into the subcutaneous tissue of immunodeficient mice. (C) High magnification of the tooth slice/scaffold showing the interface between scaffold and predentin. (D) Low magnification (×100) of a dental pulp engineered with SHED and primary human dermal microvascular endothelial cells 14 days after implantation in an immunodeficient mouse. (E) High magnification (×400) of the boxed area of the engineered dental pulp presented in (D). Adapted with permission from [75].
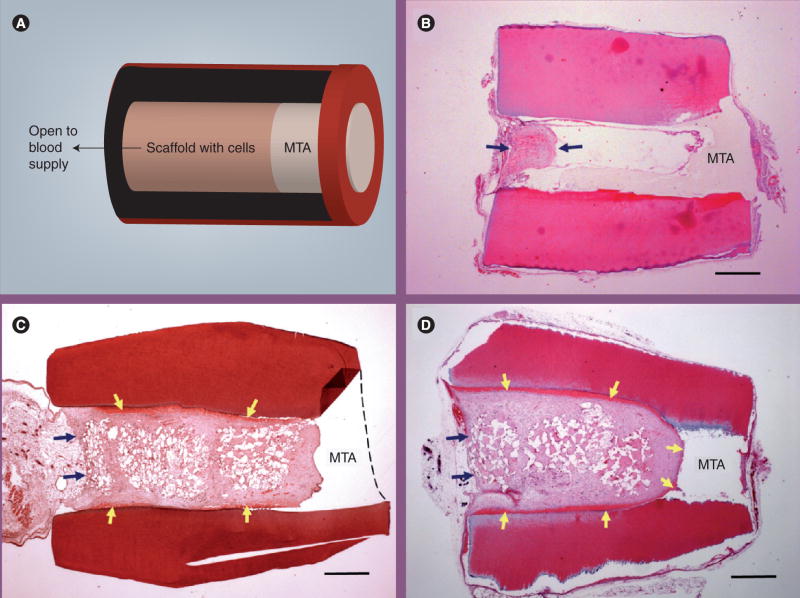
Dental pulp stem cells were also used to regenerate pulp-like tissue using a similar approach as the above with a tooth-slice model. Collagen was the scaffold to carry the DPSCs along with a growth-factor dentin matrix protein-1. Pulp-like tissue was formed 6 weeks after the transplantation of the construct in the subcutaneous space of immunocompromised mice [76]. The quality of the pulp-like tissue with collagen used as the scaffold appears to be less optimal compared with using PLLA. Moreover, no odontoblast-like cells were found located against the existing dentin surface. The problem with using collagen gel as a scaffold for carrying DPSCs to regenerate pulp is that severe contraction occurs. It was discussed in our previous study that dental pulp cells seeded into collagen gel caused dramatic contraction with a size reduction down to approximately 30% and the level of contraction is proportional to the density of cells seeded [52]. We performed a similar in vivo study except a tooth-fragment model was used in which a section of human tooth roots, approximately 6–8 mm in length, were obtained. The canal content was totally removed and the space enlarged to 1–3 mm in diameter with one end of the canal opening sealed with a cement, MTA (Figure 3A). Collagen gels containing DPSCs were filled into the canal space and the tooth fragment constructs implanted into the subcutaneous space of severe combined immunodeficiency mice. It was revealed that using collagen as a scaffold for pulp regeneration in the pulp-canal space, the collagen/cell constructs appeared to have contracted and failed to fill into the deeper part of canal space. No odontoblast-like cells were observed on the dentinal wall either (Figure 3B). We later utilized PLG as a scaffold to carry DPSCs, packed into the canal space and the constructs transplanted into the subcutaneous space of severe combined immunodeficiency mice. The emptied canal space was filled with regenerated well-vascularized pulp-like tissue 4 months later (Figure 3C). In addition, a layer of dentin-like mineral tissue deposited onto the canal’s dentin wall was observed.
Figure 3. Root fragment In vivo model for pulp/dentin regeneration.
(A) A human tooth root fragment with enlarged canal space was generated. One end of the canal opening was sealed with MTA cement and the canal filled with poly(D-L-lactide-co-glycolide) (PLG) scaffold seeded with stem cells. The construct was transplanted into the subcutaneous space of severe combined immunodeficiency (SCID) mice. The implanted tooth fragment containing cells/scaffold was removed after several months in the subcutaneous space of SCID mice and processed for analysis. A longitudinal section of the decalcified sample was stained with hematoxylin and eosin. (B) Pulp regeneration using human dental pulp stem cells cast in collagen hydrogel. The implanted tooth fragment containing cells/collagen gel was removed after 3 months in the subcutaneous space of SCID mice and processed for analysis. Arrows indicate the regenerated pulp-like tissue only located near the canal opening. (C) Pulp/dentin regeneration using human dental pulp stem cells seeded in PLG. The implanted tooth fragment containing cells/PLG was removed after 4 months in the subcutaneous space of SCID mice and processed for analysis. Black arrows indicate the demarcation between the subcutaneous tissue from the mouse and the regenerated pulp-like tissue in the canal. Yellow arrows indicate the newly deposited dentin-like mineral tissue onto the canal dentinal walls. (D) Pulp/dentin regeneration using human stem cells from apical papilla seeded in PLG. The implanted tooth fragment containing cells/PLG was removed after 3 months in the subcutaneous space of SCID mice and processed for analysis. Black arrows indicate the demarcation between the subcutaneous tissue from the mouse and the regenerated pulp-like tissue in the canal. Yellow arrows indicate the newly deposited dentin-like mineral tissue onto the canal dentinal walls and MTA surface. Scale bar: 1 mm.
MTA: Mineral trioxide aggregate.
Another type of cell that could potentially be a better source for regeneration of pulp/dentin are SCAP. SCAP are cells from the apical papilla, which is a developing tissue, and these cells are relatively more robust than DPSCs in terms of population doubling capacity, proliferation rate, telomerase activity and cell migration ability [5]. Using the same study model as we used for pulp regeneration with DPSCs, we tested pulp regeneration with SCAP. A high-quality vascularized pulp-like tissue was formed and, more importantly, a uniformed thickness of a newly generated dentin-like layer was deposited onto the canal dentin wall, as well as onto the MTA cement (Figure 3D).
Partial regeneration of pulp
Stem cells may be used to regenerate partially lost pulp and dentin. Nakashima illustrated the possibility of generating a piece of pulp–dentin complex in vitro as a filling material [77]. This approach appears difficult as engineering and generating a 3D structure of pulp–dentin complex in vitro is very technically challenging. No report so far has demonstrated a success with this design. However, Nakashima’s group was able to demonstrate partial regeneration of pulp using a dog model by using two approaches:
Autologous DPSCs were grown as a 3D pellet treated with the growth factor bone morphogenetic protein-2 and implanted into the space of a partially amputated pulp chamber. This approach was able to stimulate reparative dentin formation by the newly differentiated odontoblasts [78];
A subfraction of a side population of pulp cells (CD31−/CD146−) were mixed with a collagen scaffold and inserted into the pulp-chamber space where tissues were removed by pulpotomy. Formation of regenerated pulp tissue with good vacularity and new dentin deposition was observed in the pulp chamber [68].
Noncell-based pulp/dentin tissue regeneration
The major hurdle of cell-based therapy is its complexity, including the availability of cells and the procedures involved in processing the cells. For an extensive tissue defect, cell-based therapy is inevitable if a complete regeneration of the lost tissue is desired. When the defect is less extensive, utilization of materials that can induce the mobilization of stem cells from the adjacent tissue to migrate into the defected site and initiate tissue regeneration is a more favorable approach with fewer ramifications. Application of recombinant growth factors to the injured site to enhance the regeneration of dentin has been investigated for repair of the small amount of dentin lost [79]. Evidence on more extensive regeneration using a noncell-based approach is still lacking.
Issues on regeneration of functional pulp/dentin
Functional tissue engineering and regeneration is the ultimate goal for regenerative medicine. Regenerated pulp tissue in the pulp space of a tooth should be vascularized, contain similar cell density and architecture of extracellular matrix to those of natural pulp, be capable of giving rise to new odontoblasts lining against the existing dentin surface, produce new dentin and be innervated. Vascularization may be difficult for teeth in which the apical canal opening for blood vessel entrance is small (<1 mm). The size of the apical opening would affect the ingrowth of blood vessels into the engineered pulp tissue. Obviously, the larger the opening, the more likely that the angiogenesis can occur. Immature teeth with open apices are therefore the best candidates for pulp tissue regeneration. It was considered that the use of angiogenic-inducing factors, such as VEGF and/or PDGF, should enhance and accelerate the pulp angiogenesis. Synthetic scaffolds, such as PLG, can be fabricated with impregnated growth factors [42,80–83]. Alternatively, the insertion of engineered pulp tissue may have to be separated into multiple steps [54].
If a good blood supply can be achieved, optimal cell density and the laying down of high-quality extracellular matrix should occur. New odontoblasts will form against the existing dentinal wall that has been chemically disinfected as evidenced by the findings from Nör’s group [75] and ours [52]. Furthermore, we have demonstrated, as presented herein, new dentin-like tissue can be deposited onto the canal’s dentinal wall, although the nature of the new mineral tissue remains to be determined in terms of its mechanical and chemical properties. The newly formed dentin is known to be weaker than primary dentin during natural pulp healing. Therefore, it is possible that newly formed dentin from engineering approaches is also weaker. With respect to innervation, it is likely that generated/regenerated pulp contains ingrown nerve fibers from and adjacent to natural tissues. DPSCs have been shown to either produce neurotrophic factors or possess neural differentiation potential [45,84]. However, the specific innervation at the pulp–dentin complex makes the issue more complicated. The reason why dentin is so sensitive to various irritations is because of the hydrodynamic activities of the dentinal tubules in association with the sensory A-δ fibers extending into the dentinal tubules in the predentin layer. Since the newly generated dentin does not appear to have well-organized dentinal tubules, even if the regenerated A-δ fibers reach the pulp–dentin junction, it may not cause the normal dentin sensitivity that natural teeth do.
Future perspective
For cell-based tissue regeneration, the infrastructure required to support stem cell-based therapy for pulp and dentin regeneration in the clinical practice has to be established. Tooth and dental stem cell banking are essential to make cell-based therapy practical either for autologous or allogenic applications. Ultimately, the paucity of stem cells may be resolved by the generation of induced pluripotent stem cells from somatic cells [85,86] such as dental stem cells [Huang GTJ, Unpublished Data]. These cells are potentially immortal and may serve as an unlimited cell source for regenerative medicine as well as for pulp/dentin regeneration.
Executive summary
Clinical advantage of pulp/dentin regeneration
The current clinical protocol of endodontic treatment sacrifices tissues in order to disinfect. After which, no regenerative process can take place as the lost tooth structure is replaced by artificial materials, which do not strengthen the tooth.
Regeneration of lost pulp and dentin tissues can reverse the deteriorated tooth and avoid following more aggressive procedures that cause more tooth structure loss.
Modern concept of pulp/dentin tissue regeneration
3D scaffold systems that carry growth factors or cells for regeneration have been tested in the engineering process of pulp/dentin tissues.
Discovering dental pulp stem cells catapulted the progress of pulp/dentin regeneration. Several different stem cells from pulp tissues have been discovered: dental pulp stem cells from the pulp of permanent teeth, pulp stem cells from human exfoliated deciduous teeth and stem cells from apical papilla. These cells have demonstrated characteristics of mesenchymal stem cells with multiple differentiation potentials.
Dental pulp stem cells, stem cells from apical papilla and pulp stem cells from human exfoliated deciduous teeth demonstrate the capability to de novo regenerate pulp and the former two are also capable of generating new dentin.
Issues on regeneration of functional pulp/dentin
Owing to the anatomical location of the pulp, which is encased within the dentin tissue and receives its blood supply only from the apical end, establishing vascularity may be difficult in cases where the apical opening is less than 1 mm.
The newly formed dentin is known to be weaker than primary dentin during natural pulp healing and lacks the ability of stimuli transfer. Regenerated dentin via tissue-engineering approaches lacks highly organized dentinal tubules. Its mechanical strength and sensory innervation into the pulp/dentin complex remain to be investigated.
Future perspective
For cell-based therapy, the source of cells is an issue. Dental stem cell supply is limited especially from autologous sources. Not every individual who needs the regeneration treatment has the cells readily available.
Establishing dental stem cell banking may be a necessary step and further progress on establishing individualized induced pluripotent stem cells for dental tissue regeneration is imminent.
Acknowledgments
The author wishes to thank LD Shea (Northwestern University, Evanston, IL, USA) for providing the PLG scaffolds and RS Tuan (National Institutes of Health, Bethesda, MD) for providing imaging facilities.
Financial & competing interests disclosure
This study was supported in part by an Endodontic Research Grant from American Association of Endodontists Foundation and National Institutes of Health R01 DE019156–01. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Bibliography
- 1.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 2.Young CS, Abukawa H, Asrican R, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 3.Yen A, Sharpe P. Stem cells and tooth tissue engineering. Cell Tissue Res. 2008;331:359–372. doi: 10.1007/s00441-007-0467-6. [DOI] [PubMed] [Google Scholar]
- 4.Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 5.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mooney DJ, Powell C, Piana J, Rutherford B. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12:865–868. doi: 10.1021/bp960073f. [DOI] [PubMed] [Google Scholar]
- 7.Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. J Biomater Sci Polym Ed. 1998;9:749–764. doi: 10.1163/156856298x00127. [DOI] [PubMed] [Google Scholar]
- 8.Buurma B, Gu K, Rutherford RB. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999;107:282–289. doi: 10.1046/j.0909-8836.1999.eos107408.x. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto Y, Fukutani S, Shin-Ike T, et al. Mineralized nodule formation by cultures of human dental pulp-derived fibroblasts. Arch Oral Biol. 1992;37:1045–1055. doi: 10.1016/0003-9969(92)90037-9. [DOI] [PubMed] [Google Scholar]
- 10.About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. Human dentin production in vitro. Exp Cell Res. 2000;258:33–41. doi: 10.1006/excr.2000.4909. [DOI] [PubMed] [Google Scholar]
- 11.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alley BS, Kitchens GG, Alley LW, Eleazer PD. A comparison of survival of teeth following endodontic treatment performed by general dentists or by specialists. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:115–118. doi: 10.1016/j.tripleo.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Marending M, Peters OA, Zehnder M. Factors affecting the outcome of orthograde root canal therapy in a general dentistry hospital practice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:119–124. doi: 10.1016/j.tripleo.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 14.Kojima K, Inamoto K, Nagamatsu K, et al. Success rate of endodontic treatment of teeth with vital and nonvital pulps. A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:95–99. doi: 10.1016/j.tripleo.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Dammaschke T, Steven D, Kaup M, Ott KH. Long-term survival of root-canal-treated teeth: a retrospective study over 10 years. J Endod. 2003;29:638–643. doi: 10.1097/00004770-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ruddle CJ. Broken instrument removal. The endodontic challenge. Dent Today. 2002;21:70–72. 74. 76 passim. [PubMed] [Google Scholar]
- 17.Ward JR. The use of an ultrasonic technique to remove a fractured rotary nickel-titanium instrument from the apical third of a curved root canal. Aust Endod J. 2003;29:25–30. doi: 10.1111/j.1747-4477.2003.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 18.Suter B, Lussi A, Sequeira P. Probability of removing fractured instruments from root canals. Int Endod J. 2005;38:112–123. doi: 10.1111/j.1365-2591.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 19.Bargholz C. Perforation repair with mineral trioxide aggregate: a modified matrix concept. Int Endod J. 2005;38:59–69. doi: 10.1111/j.1365-2591.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 20.Zenobio EG, Shibli JA. Treatment of endodontic perforations using guided tissue regeneration and demineralized freeze-dried bone allograft: two case reports with 2–4 year post-surgical evaluations. J Contemp Dent Pract. 2004;5:131–141. [PubMed] [Google Scholar]
- 21.Yoldas O, Oztunc H, Tinaz C, Alparslan N. Perforation risks associated with the use of Masserann endodontic kit drills in mandibular molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:513–517. doi: 10.1016/j.tripleo.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 23.Feely L, Mackie IC, Macfarlane T. An investigation of root-fractured permanent incisor teeth in children. Dent Traumatol. 2003;19:52–54. doi: 10.1034/j.1600-9657.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 24.Andreasen JO, Andreasen FM, Mejare I, Cvek M. Healing of 400 intra-alveolar root fractures. 1 Effect of pre-injury and injury factors such as sex, age, stage of root development, fracture type, location of fracture and severity of dislocation. Dent Traumatol. 2004;20:192–202. doi: 10.1111/j.1600-9657.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 25.Bastone EB, Freer TJ, McNamara JR. Epidemiology of dental trauma: a review of the literature. Aust Dent J. 2000;45:2–9. doi: 10.1111/j.1834-7819.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva AC, Passeri LA, Mazzonetto R, De Moraes M, Moreira RW. Incidence of dental trauma associated with facial trauma in Brazil: a 1-year evaluation. Dent Traumatol. 2004;20:6–11. doi: 10.1111/j.1600-4469.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 27.Sedgley CM, Messer HH. Are endodontically treated teeth more brittle? J Endod. 1992;18:332–335. doi: 10.1016/S0099-2399(06)80483-8. [DOI] [PubMed] [Google Scholar]
- 28.Huang GT, Schilder H, Nathanson D. Effects of moisture content and endodontic treatment on some mechanical properties of human dentin. J Endodont. 1992;18:209–215. doi: 10.1016/S0099-2399(06)81262-8. [DOI] [PubMed] [Google Scholar]
- 29.Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15:512–516. doi: 10.1016/S0099-2399(89)80191-8. [DOI] [PubMed] [Google Scholar]
- 30.Piwowarczyk A, Lauer HC, Sorensen JA. Microleakage of various cementing agents for full cast crowns. Dent Mater. 2005;21:445–453. doi: 10.1016/j.dental.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Caplan DJ, Cai J, Yin G, White BA. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent. 2005;65:90–96. doi: 10.1111/j.1752-7325.2005.tb02792.x. [DOI] [PubMed] [Google Scholar]
- 32.Pappen AF, Bravo M, Gonzalez-Lopez S, Gonzalez-Rodriguez MP. An in vitro study of coronal leakage after intraradicular preparation of cast-dowel space. J Prosthet Dent. 2005;94:214–218. doi: 10.1016/j.prosdent.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Demirel F, Saygili G, Sahmali S. Microleakage of endodontically treated teeth restored with prefabricated posts and tooth-colored restorative materials. Int J Periodontics Restorative Dent. 2005;25:73–79. [PubMed] [Google Scholar]
- 34.Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008;139:305–315. doi: 10.14219/jada.archive.2008.0160. [DOI] [PubMed] [Google Scholar]
- 35.Olsson H, Petersson K, Rohlin M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int Endod J. 2006;39:429–442. doi: 10.1111/j.1365-2591.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- 36.Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. 1963;16:969–977. doi: 10.1016/0030-4220(63)90201-9. [DOI] [PubMed] [Google Scholar]
- 37.Trowbridge H. Histology of pulpal Inflammation. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender’s Dental Pulp. Quintessence Publishing Co., Inc; Carol Stream, IL, USA: 2002. pp. 227–245. [Google Scholar]
- 38.Ostby BN. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol Scand. 1961;19:324–353. [PubMed] [Google Scholar]
- 39.Myers WC, Fountain SB. Dental pulp regeneration aided by blood and blood substitutes after experimentally induced periapical infection. Oral Surg Oral Med Oral Pathol. 1974;37:441–450. doi: 10.1016/0030-4220(74)90119-4. [DOI] [PubMed] [Google Scholar]
- 40.Ellis E, 3rd, Cox CF, Hitchcock R, Baker J. Vital apicoectomy of the teeth: a 1–4 week histopathological study in Macaca mulatta. J Oral Pathol. 1985;14:718–732. doi: 10.1111/j.1600-0714.1985.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 41.Hitchcock R, Ellis E, 3rd, Cox CF. Intentional vital root transection: a 52-week histopathologic study in Macaca mulatta. Oral Surg Oral Med Oral Pathol. 1985;60:2–14. doi: 10.1016/0030-4220(85)90205-1. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan MH, Shea LD, Peters MC, Mooney DJ. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J Control Release. 2000;64:91–102. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 43.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 44.Nerem RM. Tissue engineering in the USA. Med Biol Eng Comput. 1992;30:CE8–CE12. doi: 10.1007/BF02446171. [DOI] [PubMed] [Google Scholar]
- 45.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 46.Batouli S, Miura M, Brahim J, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–981. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- 47.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 49.Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morsczeck C, Gotz W, Schierholz J, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs from other sources: the biology and role in regenerative medicine. J Dent Res. 2009 doi: 10.1177/0022034509340867. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang GTJ, Sonoyama W, Chen J, Park S. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225–236. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 53.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Zannettino ACW, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 57.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 58.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodont Res. 2006;41:547–553. doi: 10.1111/j.1600-0765.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 59.Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by oct4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 60.Gronthos S, Zannettino ACW. A method to isolate and purify human bone marrow stromal stem cells. In: Prockop DJ, Bunnell BA, Phinney DG, editors. Mesenchymal Stem Cells Methods and Protocols. Humana Press; USA: 2008. pp. 45–57. [DOI] [PubMed] [Google Scholar]
- 61.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 62.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 63.Battula VL, Bareiss PM, Treml S, et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007;75:279–291. doi: 10.1111/j.1432-0436.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 64.Miura Y, Miura M, Gronthos S, et al. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci USA. 2005;102:14022–14027. doi: 10.1073/pnas.0409397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laino G, d’Aquino R, Graziano A, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 66.Laino G, Graziano A, d’Aquino R, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 67.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 68.Iohara K, Zheng L, Ito M, et al. Regeneration of dental pulp after pulpotomy by transplantation of CD31−/CD146− side population cells from a canine tooth. Regen Med. 2009;4:377–385. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 69.Kerkis I, Kerkis A, Dozortsev D, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 70.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 71.Waddington RJ, Youde SJ, Lee CP, Sloan AJ. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs. 2009;189:268–274. doi: 10.1159/000151447. [DOI] [PubMed] [Google Scholar]
- 72.Hu B, Unda F, Bopp-Kuchler S, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–421. doi: 10.1177/154405910608500504. [DOI] [PubMed] [Google Scholar]
- 73.Yu J, Wang Y, Deng Z, et al. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99:465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- 74.Huo N, Tang L, Yang Z, et al. Differentiation of dermal multipotent cells into odontogenic lineage induced by embryonic and neonatal tooth germ cell conditioned medium. Stem Cells Dev. 2009 doi: 10.1089/scd.2009.0048. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 75.Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Prescott RS, Alsanea R, Fayad MI, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421–426. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–718. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 78.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 79.Rutherford RB, Gu K. Treatment of inflamed ferret dental pulps with recombinant bone morphogenetic protein-7. Eur J Oral Sci. 2000;108:202–206. doi: 10.1034/j.1600-0722.2000.108003202.x. [DOI] [PubMed] [Google Scholar]
- 80.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 81.Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22:1110–1116. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 82.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 83.Stiver SI, Tan X, Brown LF, Hedley-Whyte ET, Dvorak HF. VEGF-A angiogenesis induces a stable neovasculature in adult murine brain. J Neuropathol Exp Neurol. 2004;63:841–855. doi: 10.1093/jnen/63.8.841. [DOI] [PubMed] [Google Scholar]
- 84.Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388–2398. doi: 10.1111/j.0953-816X.2004.03314.x. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 86.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 87.Seo BM, Sonoyama W, Yamaza T, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14:428–434. doi: 10.1111/j.1601-0825.2007.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]