Abstract
A comprehensive and chronological account of dendrimers based on [1,3,5]-triazines is provided. Synthetic strategies to install the triazine through cycloaddition, cyclotrimerization, and nucleophilic aromatic substitution of cyanuric chloride are discussed. Motivations and applications of these architectures are surveyed, including the preparation of supra-molecular assemblies in the solution and solid states and their use in medicines, advanced materials, and separations when anchored to solid supports.
Keywords: dendrimers, functionalization of polymers, polytriozines, synthesis
INTRODUCTION
Uniquely new ideas are rare. Co-invention occurs with slightly greater frequency. Rediscovery is becoming more common with the widespread availability of the scientific and patent literature and ease with which it can be efficiently sieved. The history of triazine dendrimers reflects the rare, significant convergence at co-invention and rediscovery. The versatility of triazine chemistry suggests that these reports represent the beginning of a multilaboratory investigation of these systems. The timeline is consistent with the increasing interest that this system is attracting. We, the Texas A&M group, have been working in what we perceived to be a new area for 5 years, a period of time that has allowed us to gain deeper appreciation for its roots. More general accounts of dendrimers, their nomenclature, and synthetic routes useful for their preparation can be found elsewhere.1–5
WHY TRIAZINES?
Specifically, we ask, “Why 1,3,5-triazines?” as we will use the term triazine solely to refer to this subclass of the triazine family. The attraction of the triazine building block derives from opportunities for molecular recognition and synthetic versatility.
Molecular Recognition
Triazines—especially melamines—can recognize other molecules by the donation and acceptance of hydrogen bonds, metal chelation, and π–π interactions (Chart 1). This opportunity has enabled various supramolecular structures to be prepared on the basis of hydrogen-bonding interactions to form ribbons and other types of interesting oligomers and polymers. The synthesis and characterization of these types of triazine-based supramolecular structures have been reviewed elsewhere.6–9
Chart 1.
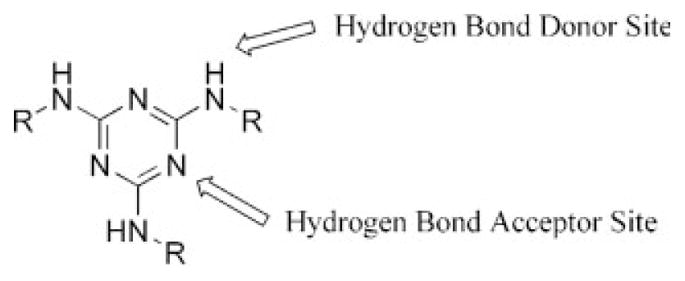
Sites of triazine/melamine derivatives that can participate in hydrogen bonding.
Synthesis
The synthesis of triazine derivatives is well established and has been reviewed elsewhere in detail.10,11 Triazines can be synthesized by a variety of routes; however, for the sake of brevity, we will focus on the two most common methods that have been employed for the preparation of triazine-based dendrimers. Initial efforts in this field focused on cycloaddition reactions to form the triazine ring (Scheme 1). This reaction is carried out at higher temperatures by the treatment of the nitrile of interest with a nitrile-substituted guanidine derivative in the presence of a base, typically sodium or potassium hydroxide.
Scheme 1.
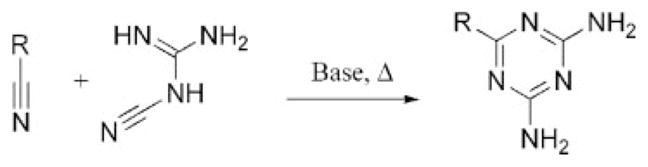
Cycloaddition reaction to form a diaminotriazine derivative.
Later efforts describe the nucleophilic aromatic substitution of cyanuric chloride (C3N3Cl3) in a chemoselective fashion using temperature and the judicious choice of the nucleophile to produce a single product with high chemical complexity (Scheme 2). Thus, when architectures that feature different types of peripheral groups are desired, triazine-based dendrimers offer a powerful and versatile synthetic strategy to well-defined products. The nucleophilic aromatic substitution of alkoxy-substituted triazine derivatives by amine nucleophiles is also a route that has been exploited; however, higher temperatures in excess of 100 °C are required to achieve the conversion to the desired product.
Scheme 2.
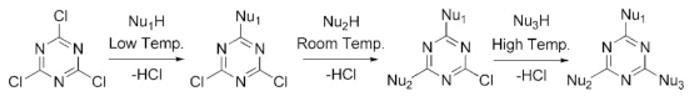
Chemoselective reactivity of cyanuric chloride.
Triazines can also be prepared by cyclotrimerization of organic cyanates, but to date, the use of this route for the synthesis of dendrimers has not been pursued. Rather, this chemistry is employed to prepare hyper-branched polymers.12
The goal of this review is to trace the origins of triazine dendrimers, a field that we perceive is expanding (Fig. 1). The advances in the syntheses of these materials receive the majority of the attention. Numerous examples of multiple triazine units tethered to a single core are included because these can be construed as first-generation dendrimers and can be readily elaborated to larger structures because of the functionality at the periphery. A number of applications have evolved in which this class of polymers has demonstrated enormous potential. Overall, this review is intended to provide a nearly comprehensive account of the evolution of the synthesis and applications of triazine-based dendrimers.
Figure 1.
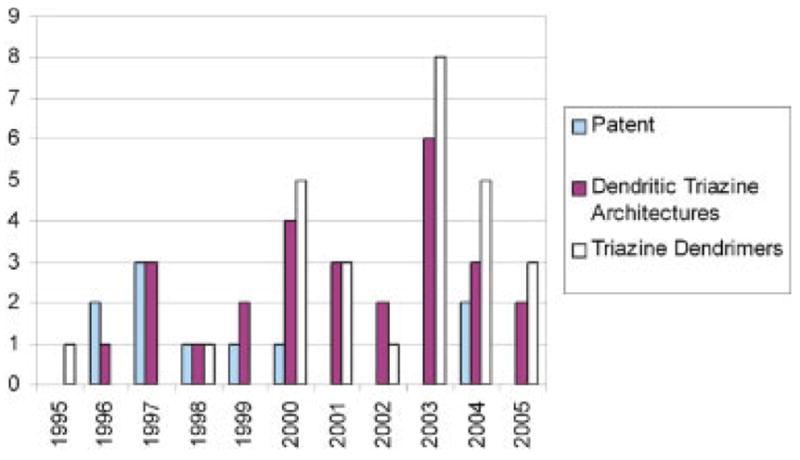
Graph showing that reports of triazine-based dendritic structures have increased during the previous decade (2005 data are as of April 29, 2005).
SYNTHETIC ROUTES TO TRIAZINE DENDRIMERS
Much of the early work in the area focused on the optimization of routes for the synthesis of these species using either cycloaddition reactions or triazine substitution reactions.
Cycloaddition and Cyclotrimerization Methods
To the best of our knowledge, the first example of the synthesis of a triazine-based dendrimer using a divergent approach was detailed in two patents that were submitted in 1994,13 one of which was reviewed in a separate account in 1995 (Scheme 3).14 In both cases, an iterative synthesis was developed in which the triazine units were prepared by the cycloaddition of terminal nitrile-functional groups, 1, with nitrile-substituted guanidine, 2, to afford an amino-terminated dendrimer, 3. Iteration produces higher generations such as 4. The synthesis outlined in the patent by Meijer et al.13 is of particular interest because it details a procedure that can be used to prepare commercially relevant quantities of dendrimer product. More recent reports have demonstrated the utility of the cycloaddition method to afford triazine-based dendritic and hyperbranched materials,15 some of which have found application in the construction of porous, hydrogen-bonded networks (see the Molecular Recognition and Supramolecular Self-Assembly section). In addition, cyclotrimerization routes have been used to produce materials relevant for integrated optics (see the Energy-Harvesting and -Emitting Applications section).16
Scheme 3.
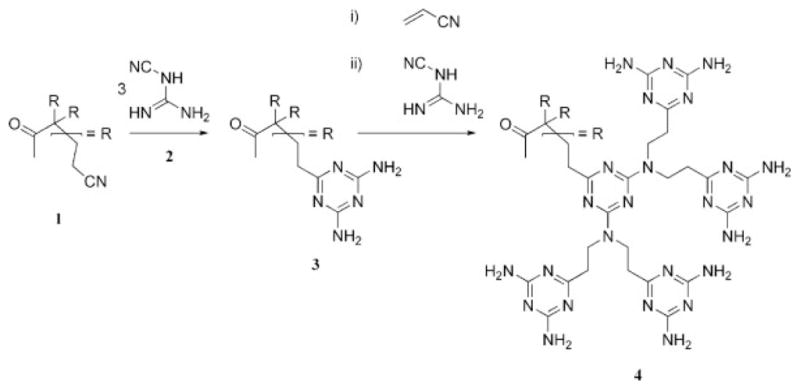
Initial efforts to prepare triazine-based dendrimers employed cycloaddition methods.
In all the cycloaddition procedures, the chemistry of the iterative process is the same: cyanoethylation of a pendant amine forms a dendron with twice the number of nitrile groups. Subsequent elaboration of the dendron by cycloaddition between the peripheral nitriles and nitrile-substituted guanidine derivatives increases the dendrimer generation. Maciejewski17 wrote one of the earliest theoretical articles on the subject of dendrimers that could be prepared with this method in 1982. Moreover, cyanoethylation was the method used by Vögtle18 in what is commonly described as the first dendrimer synthesis, although his dendrimer did not contain triazine derivatives.
A method resulting in compound 6, with features strikingly similar to that of Vögtle’s, was described in a patent by Niederhauser19 in 1951, 27 years before Vögtle’s work (Scheme 4). Niederhauser knew of the ability to reduce nitrile groups to amines: he described such a method in a patent filed in 1945.20 Either through the reduction of the nitriles to amines or further cycloaddition reactions with the nitriles, Niederhauser might have laid claim to the first dendrimer. Here was a near miss to the beginning of dendrimer chemistry. Although a more exhaustive (and wholly impractical) search of the literature may provide more near misses, our interest in triazines necessitates its inclusion. The elaboration of Niederhauser’s tetracyanoethyl benzoguanamine to afford a structurally imperfect generation-four dendrimer, however, took over 40 years to accomplish.
Scheme 4.
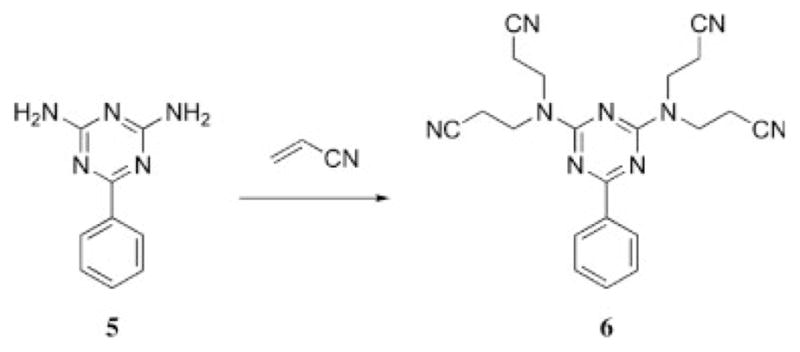
Niederhauser’s route to tetracyanoethyl benzo guanamine.
In 1993, two groups described the successful synthesis of Vögtle’s route to give dendrimers through iterative reactions.21,22 These methods eventually led to the commercial production of this class of dendrimers known as poly(propylene imine) dendrimers. In general, the cycloaddition/cyanoethylation method is attractive because it requires no functional group interconversions or the use of protecting groups as long as there are no other functional groups present that can interfere with this iterative process.
Nucleophilic Aromatic Substitution
The vast majority of triazine-based dendrimers are synthesized by nucleophilic aromatic substitution on cyanuric chloride. One of the earliest examples of the use of nucleophilic aromatic substitution to afford a dendritic product that incorporated triazine groups was reported in 1996 and described the treatment of m-bis(methylami-no)benzene with 2 equiv of cyanuric chloride. A subsequent treatment with excess amine afforded the desired product, 7. These dendritic structures were tested as chelating ligands for Gd in magnetic resonance imaging (MRI) applications (Chart 2).23
Chart 2.
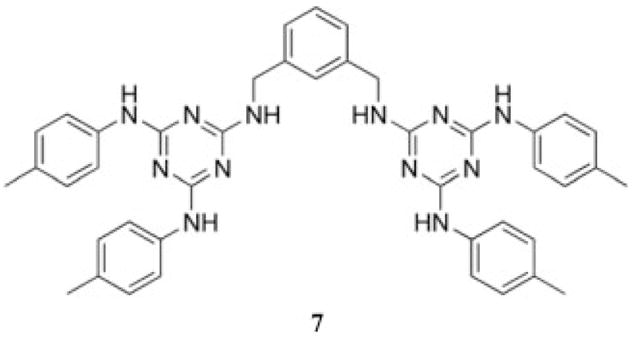
Dendritic molecule designed for use as a chelating agent for Gd in MRI applications.
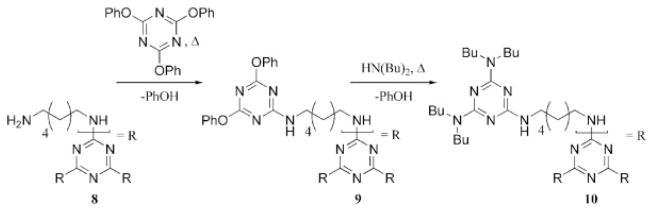
The first report concerned specifically with the construction of dendrimers by the substitution of cyanuric chloride with diamine linkers is a German patent filed in 1995 that describes a series of linear and dendritic polymer architectures synthesized with divergent and convergent methods.24 Trisphenoxytriazine was used instead of trichlorotriazine to reduce hydrolysis. These derivatives still display a gradient of reactivity that has been investigated with activation energies measured in N-methylpyrrolidine with octylamine of 25, 53, and 82 kJ/mol. A separate account released in 2000 described the synthesis of a first-generation dendrimer by the treatment of a symmetrical melamine derivative, 8, with tris(phenoxy)triazine (Scheme 5).25 Once complete substitution had been achieved to give 9, the phenoxy groups were exchanged by a treatment with excess secondary amine to afford the product, 10. The products of this synthesis, derived from dibutylamine (10), dihexylamine and dioctylamine, were viscous oils. Additional complexity was incorporated with a similar divergent approach that commenced with an excess of HN(CH2CH2NH2)2 and trisphenoxytriazine.
Scheme 5.
Convergent synthesis of a triazine-based dendrimer with nucleophilic aromatic substitution, with dibutylamine as an example of a precursor amine.
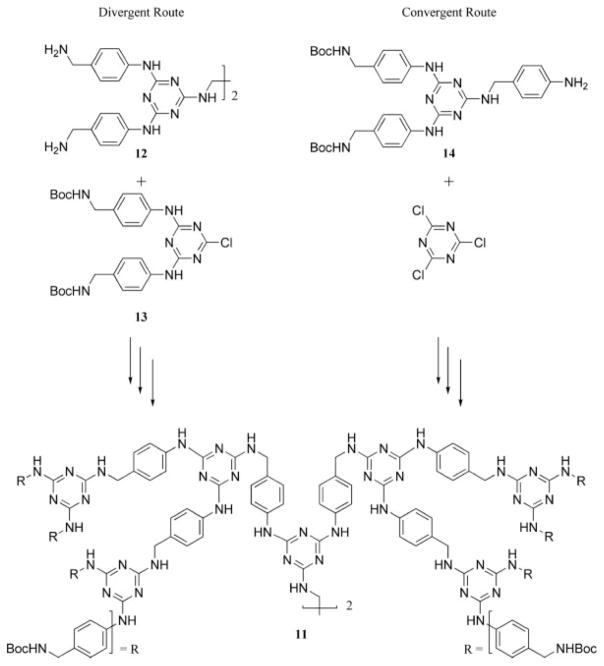
In 2000, we described methods for the construction of melamine-based dendrimers with diamine linkers and cyanuric chloride.26,27 A third-generation dendrimer, 11, was constructed with divergent (Scheme 6) and convergent routes. The divergent route connected a tetraamine core, 12, with a BOC-protected monochlorotriazine synthon, 13 (BOC = t-butoxycarbonyl). The convergent route used a BOC-protected peripheral group, 14, and an iterative reaction with cyanuric chloride and p-amino-benzylamine. Impurities that were implied by a tailing in the size exclusion chromatography (SEC) data were removed with chromatography purification techniques to obtain materials that had a single ion peak in the matrix-assisted laser desorption/ionization mass spectrometry spectrum. To us, this report represents a significant advance in triazine-based dendrimer synthesis because it was the first example reported in the mainstream literature of nucleophilic aromatic substitution under moderate synthetic conditions to yield a triazine dendrimer. These efforts complement the original work described in the dissertation work of Jens Neumann-Rodekirch of the University of Bremen.28
Scheme 6.
Convergent and divergent syntheses of a melamine-based dendrimer.
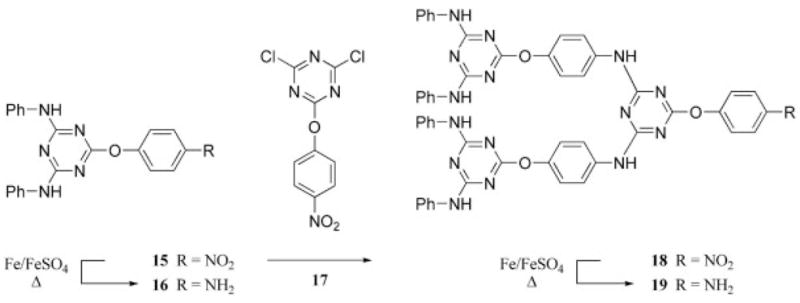
Takagi and coworkers29,30 published two articles on the syntheses of structurally related triazine dendrimers. Their first report described the convergent synthesis of a second-generation dendrimer and several third-generation dendrons. The synthesis involved the treatment of a disubstituted triazine derivative with p-nitroaniline (15), followed by the reduction of the nitro group to unmask an amine group (16). The product of this reaction was then treated with a dichlorotriazine derivative, 17, to increase the generation of the dendron (18 and 19, Scheme 7).29 The second article described convergent and divergent methods for the preparation of second-generation triazine-based dendrimers with a different linkage group to accomplish each approach. The divergent route described in the second article employed the same iterative process as the convergent route outlined in their first article. However, the second-generation dendron prepared by the convergent route was prepared with aryloxy substitution of cyanuric chloride. Subsequent generations of dendrons were achieved through the double substitution of the dichlorotriazine building block by an aryl amine.30
Scheme 7.
Iterative synthesis of a dendron with functional group interconversions.
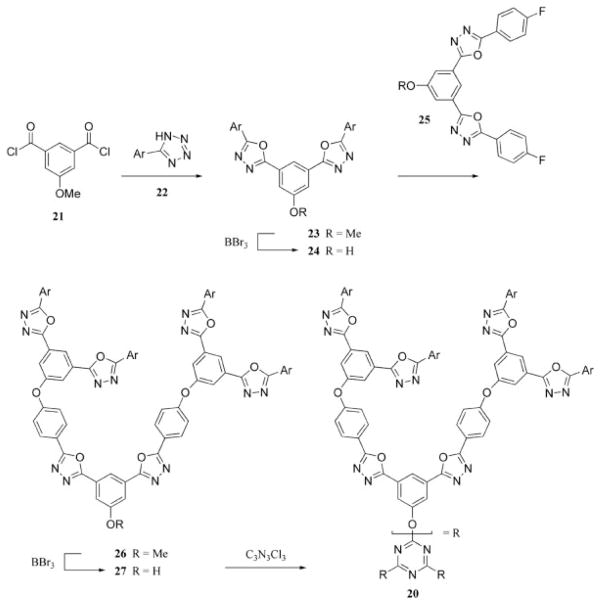
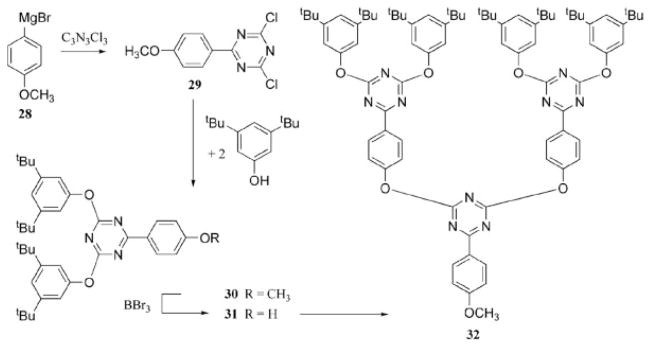
Verheyde and Dehaen31 synthesized dendrimers made up of 1,3,4-oxadiazole repeating units with a single triazine at the core (20, through 21–27, Scheme 8). In a separate account, Dehaen et al.32 produced dendrimers with triazines as the branching units. The first anisole was added to the triazine ring with a Grignard reagent, 28, to give the dichlorotriazine product, 29. Surface groups were attached to the triazine ring through aryloxy nucleophilic displacement of the chlorides to prepare 30. The methoxy functionality of anisole was unmasked to reveal a phenol (31) that was subsequently treated with a half-equivalent of cyanuric chloride to afford the next generation of dendron (32, Scheme 9). A more recent report describes the use of click chemistry to prepare a similar dendritic structure, also from a triazine core.33
Scheme 8.
Triazine as the core of a dendrimer.
Scheme 9.
Different nucleophiles are used to synthesize a dendron.
In 2001, the synthesis of the first tailored triazine-based dendrimer, 33, was described. A convergent approach permitted access to pure melamine dendrons (Chart 3) or dendrimers in which one or two of the peripheral sites (out of a possible 16) were different from the remaining peripheral groups.34 Interestingly, the addition of only one oligo(ethyleneoxy)ethylamine group to the exterior dramatically influenced the ability of the dendrimers to be characterized by SEC. This clearly illustrates that even subtle changes imparted by tailored dendrimers can result in properties dramatically different from those of their monofunctionalized counterparts.
Chart 3.
Tailored dendron.
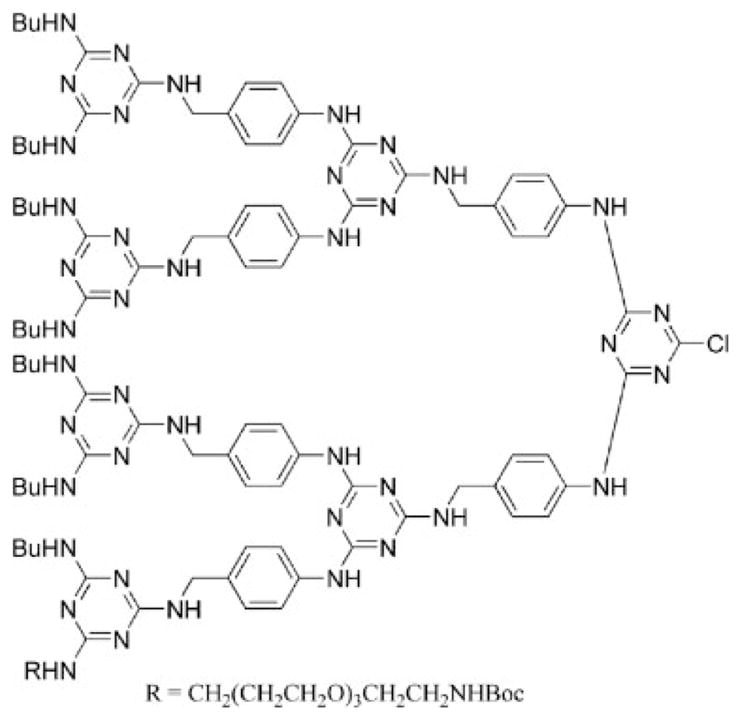
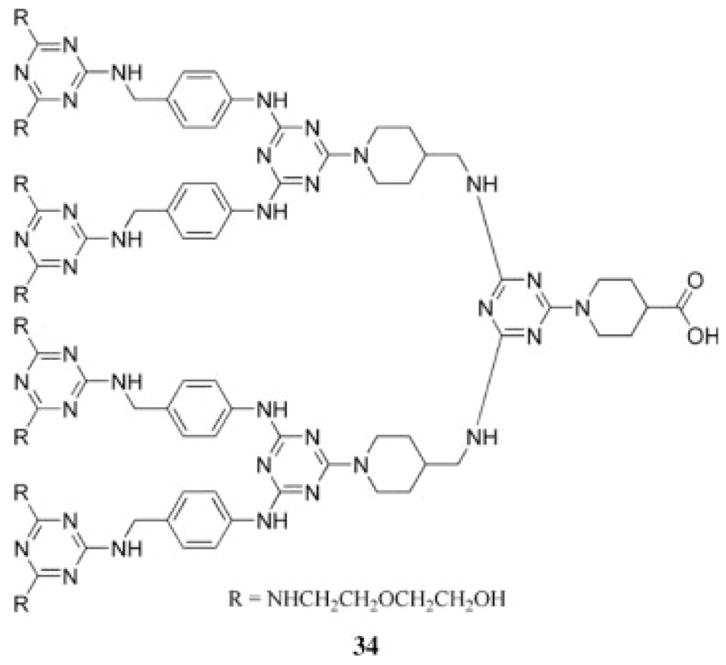
Another improvement in dendrimer synthesis was achieved when a third-generation dendron, 34, was prepared in the absence of protecting groups or functional group manipulations (Chart 4).35,36 The strategy expanded the use of chemoselective diamines from p-amino-benzyl amine to additional diamines. Diamines comprising amines with reactivity differences greater than 20 were found to be useful for chemoselective reactions.
Chart 4.
Dendron that was synthesized without protecting group manipulations or functional group interconversions.
The same strategy that was applied for the synthesis of a tailored dendrimer was also successfully employed to prepare a dendrimer or dendron with five (35) or six (36) orthogonal reactive sites, respectively (Chart 5).37 The inference to the potential utility of this dendrimer for drug delivery and related biological applications was based on the demonstration that the functional groups could be manipulated after the synthesis of the dendrimer with little formation of byproducts. The power of triazine chemistry is best exemplified in these targets.
Chart 5.
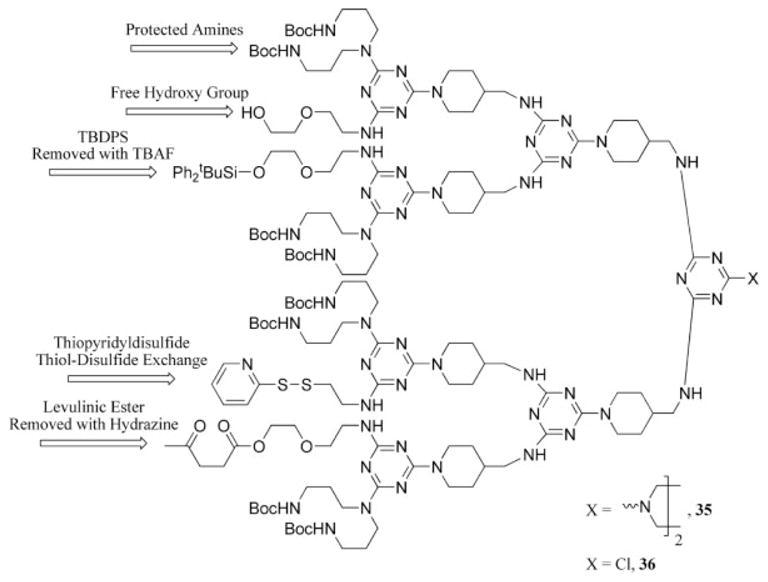
Pure dendron and dendrimer with a high degree of functional group diversity for postsynthetic manipulation (TBAF = tetrabutylammonium fluoride; TBDPS = tert-butyl diphenylsilane).
The most recent example of the use of nucleophilic aromatic substitution to prepare a triazine-based dendrimer was demonstrated by the treatment of a poly(ethylene glycol) (PEG) diamine derivative with cyanuric chloride.38 An iterative treatment with ethanol amine and cyanuric chloride resulted in the formation of a third-generation dendrimer, 37, tethered by a PEG oligomer (Chart 6). The result was an amphiphilic material, and the critical micelle concentrations of the first-and second-generation versions of the block copolymer were 5.5 × 10−5 M and 7.6 × 10−4 M, respectively.
Chart 6.
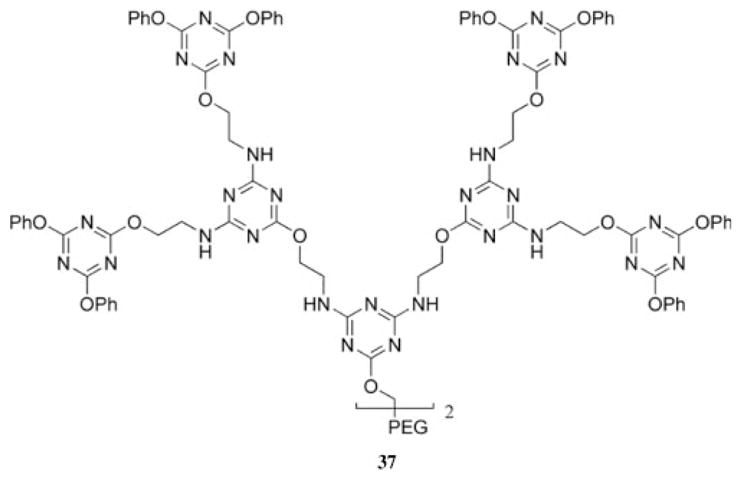
Triazine-based dendritic block copolymer.
Hyperbranched Molecules
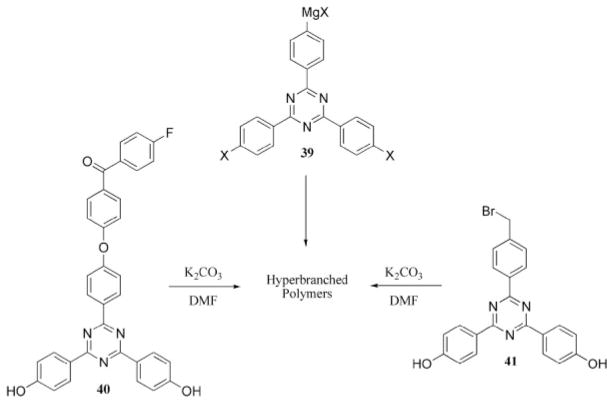
A third synthetic route to triazine-containing dendritic materials involves the preparation of hyperbranched molecules. Polycyanurate ester resins dating to the 1960s could be regarded as progenitors of these materials.12 Early efforts by Neumann-Rodekirch28 using the homopolycondensation of diphenyoxytriazines substituted with amines, including p-aminobenzylamine, N-methyl-p-aminobenzylamine, and masked amines such as acetylaniline, mono-BOC protected hydrazine, and hexane diamine, were conducted in N-methylpyrrolidine at 200–240 °C with the intent of producing stationary phases for chromatography. More recent examples of hyperbranched triazines include work by Kim et al.,39 who prepared hyperbranched triazine polymers by performing a Heck coupling of an AB2 triazine monomer, 38, to produce materials with a weight-average molecular weight (Mw) of 6000–10,000 g mol−1, as estimated by SEC (Scheme 10). This novel route to hyperbranched triazine products is of interest because of the ability to prepare relatively high-molecular-weight material with a single-pot procedure. A related protocol has been reported in which hyperbranched triazine polymers are prepared from an AB2 triazine monomer (39, Scheme 11) that incorporates a Grignard reagent.40,41
Scheme 10.
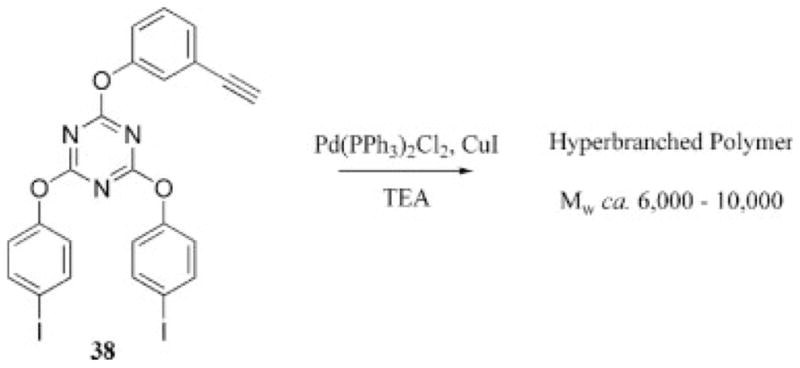
Heck coupling of a triazine AB2 monomer results in hyperbranched materials (TEA = triethylamine).
Scheme 11.
Additional routes to hyperbranched materials (DMF = dimethylformamide).
This separate series of articles were published by Kim and coworkers,42–45 who described a similar strategy to obtain hyperbranched polymers from AB2 triazine monomers 40 and 41. In these cases, the polymer was obtained by heating the monomer in the presence of potassium carbonate to 140 °C (Scheme 11). The polymers had Mw values of 11,000–15,000 g mol−1, as estimated by gel permeation chromatography, and displayed excellent thermal stability, as evidenced by thermogravimetric analysis (TGA). More recently, the free hydroxyl end groups of one of the hyperbranched polymers were functionalized with Mitsunobu or dicyclohexylcarbodiimide reactions to install oligoethyleneoxy or stearyl groups, respectively.44 The incorporation of these groups significantly lowered the glass-transition temperature of these materials with respect to the precursor polymer.
APPLICATIONS
Our discussion of applications is divided into five categories: molecular recognition and supramolecular self-assembly, decoration of inorganic supports, energy-harvesting and -emitting applications, surfactants, and medicinal applications.
Molecular Recognition and Supramolecular Self-Assembly
Dendritic structures based on melamine usually present periphery groups that are able to participate in hydrogen bonding. In an elegant example, Fréchet and coworkers46,47 demonstrated the self-assembly of higher ordered dendrimer structures by attaching two complementary hydrogen-bonding moieties to the focal point of different dendrons, 42. In this case, melamine and cyanuric acid derivatives were separately appended to dendrons of generations 2–4, and the supramolecular assembly of these dendrons with each other was investigated. Second-generation dendrons formed the expected hexameric structure as a result of the complementary hydrogen bonding of the melamine and cyanuric acid derivatives (Chart 7), but solutions of the larger dendrons appeared to be an equilibrium mixture of different aggregates, presumably to accommodate steric constraints. A related dendritic assembly of this type was reported by van Veggel et al.48
Chart 7.
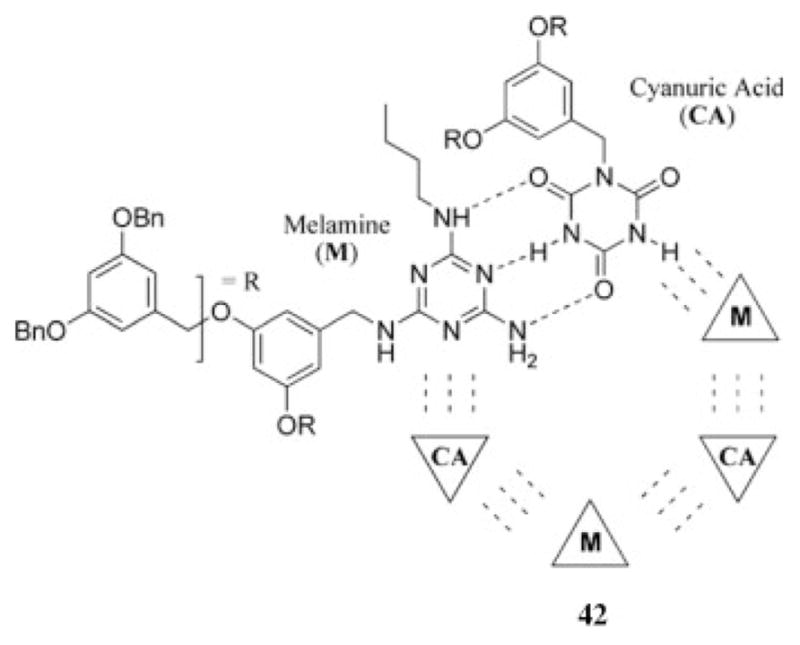
Self-assembly affords higher order dendrimer structures.
One of the applications of the smaller generation triazine-decorated dendrimers pioneered by Wuest and coworkers has been their use as tectons in solid-state networks of controlled porosity (43–47). The initial report detailed the use of a tecton (43, Chart 8) that was prepared by the cycloaddition of the precursor nitrile with dicyandiamide in a 90% yield.49 Interestingly, when this tecton was crystallized from solutions of formic acid and dioxane, inclusion solids resulted that appeared to be relatively insensitive to the guest because of an unusually robust hydrogen-bond network. The cycloaddition method was also used to produce boron-containing tectons50 and larger dendritic tectons (44–46, Chart 8)51–54 that in turn produced solid-state networks of high porosity. The authors observed that when the dendritic nature of the tecton increased, an increase in the number of hydrogen bonds did not necessarily correlate with increased stability or a higher porosity network following attempted guest-exchange experiments. With a different synthetic approach, nucleophilic aromatic substitution was also exploited to prepare tectons.55–58 In one case (47, Chart 8),55 the triazine ring was substituted by three different functional groups, which gave rise to a solid-state network comprising interconnected helical channels when crystallized from a solution of acetone, dimethyl sulfoxide, and water. The development of this technology has already demonstrated potential in practical applications, particularly for use in inkjet printing as phase-changing inks.59,60
Chart 8.
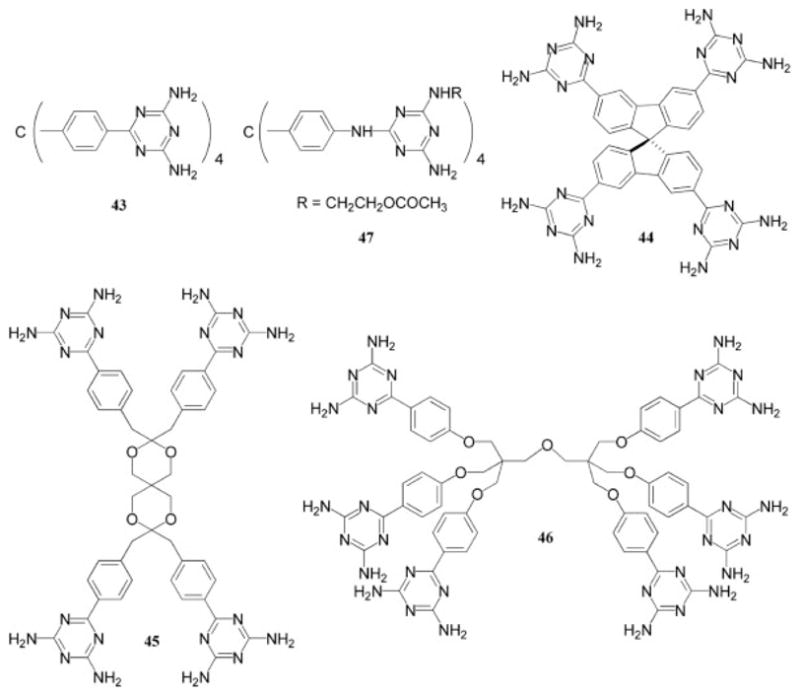
Tectons used for the construction of highly porous, solid-state networks.
Dendrimers based on melamine also aggregate in solution because of the extensive hydrogen-bonding sites that are available. Remarkably, the addition of copper(II) to a solution of a dendrimer comprising triazines linked by p-aminobenzyl groups induces a line-sharpening effect in SEC traces.61 In related work, the SEC trace obtained for a dendrimer in which the interior and peripheral groups had N—H sites available to participate in hydrogen bonding (48–54, Chart 9) had characteristically broad peaks when the analysis was performed in acidified solvents.62 However, when neutral solvents were used, a similar line-sharpening effect was observed, and the sample eluted with longer retention times than in acidified solvents. These results are consistent with the formation of higher molecular weight aggregates in acidified solvents, and gels of 50, 51, and 52 were observed under these conditions. No gelation of smaller dendrimers (48 and 49) or dendrimers deficient in hydrogen-bonding sites (53 or 54) was observed.
Chart 9.
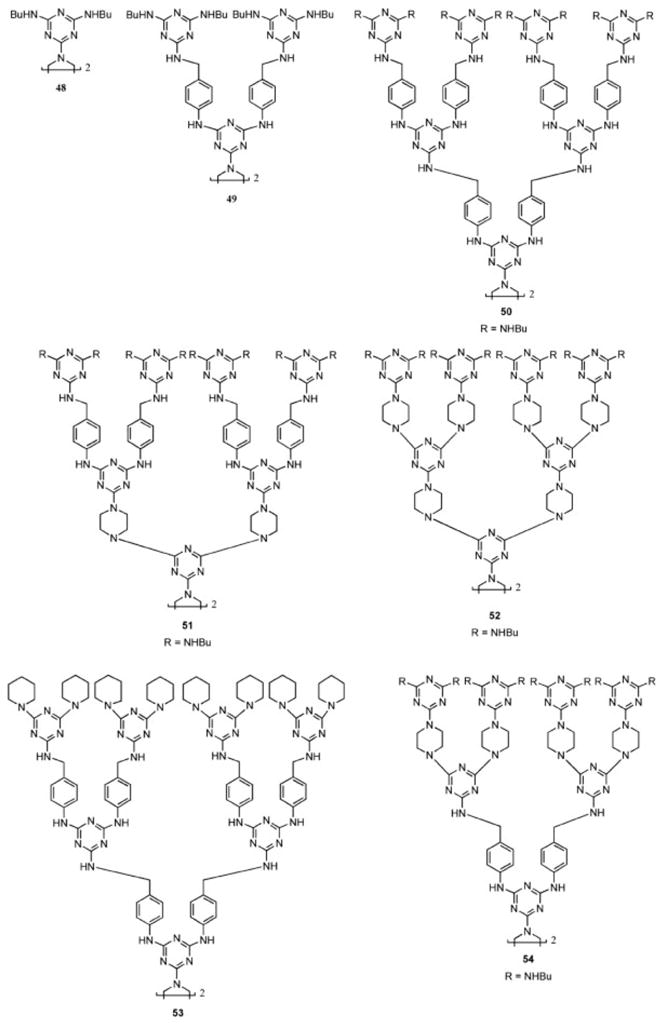
Dendrimers that show a range of hydrogen bonding in acidified solvents.
Dendritic resins with the triazine unit tethered to an alcohol-functionalized Wang resin have been prepared for the explicit purpose of capitalizing on the ability of melamine dendrimers to scavenge protons (55 and 56, Chart 10).63 The dendritic dichlorotriazine precursors were also examined to determine their ability to scavenge nucleophiles from solution. Both materials performed with similar efficiency in comparison with commercially available scavenging resins. The relative increase in reactive functional groups per gram of resin allowed for less resin to be used.
Chart 10.
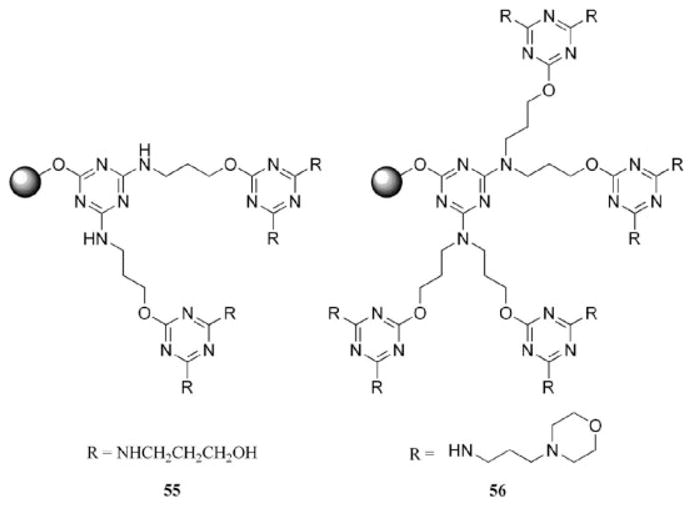
Modified resins successful for the scavenging of protons.
Inorganic Supports and Molecules
The patent literature has many accounts that detail the use of dendritic materials to modify silica surfaces for various purposes. One of the earliest accounts of the incorporation of triazines into a dendrimer was published in 1997 when triazine-based dendrimers were used to modify a silica surface for use in chromatographic applications.64 More recently, Su et al.65 reported a fourth-generation melamine dendrimer that was synthesized on a treated silica surface by an iterative process in which 1,6-diaminohexane and cyanuric chloride were alternated to generate a dendrimer with amine groups at the periphery. The modified silica gel was used in a microcolumn to effect the preconcentration and separation of platinum from heterogeneous samples, demonstrating potential utility in analytical applications. We have also prepared third- and fourth-generation dendrimers on a treated silica surface using piperazine (57)66 and 4-aminomethylpiperidine,67 respectively, as diamine links to the triazine units. A comparison was made in which the dendrimers with piperazine as the diamine were synthesized and characterized by a convergent method in solution and then tethered to the silica support, as opposed to being prepared in a divergent fashion directly on the silica surface. TGA evidence demonstrated that the materials derived from the convergent approach had less organic content, whereas those derived from the divergent method had structural defects (Scheme 12). Both of the materials, however, sequestered atrazine, a monochlorotriazine, from aqueous solutions, demonstrating their utility as solid-phase scavenging reagents.
Scheme 12.
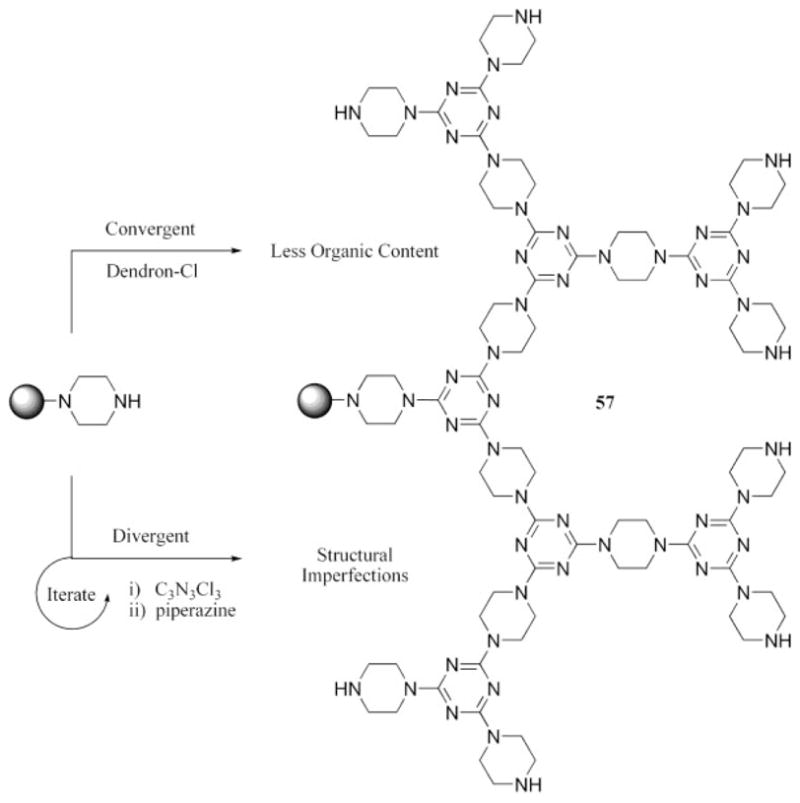
Convergent and divergent methods of tethering a third-generation dendrimer to the surface afford very different materials.
There are several accounts of discrete inorganic complexes that contain dendritic structures that are composed of triazine functional groups. A dendritic ruthenium complex was prepared upon the treatment of tris(-pyridyl)triazine with 3 equiv of the metal precursor (58, Chart 11).68 The product was characterized with synchrotron radiation, and efforts to investigate the photo-physical properties have not yet been disclosed. More recently, the research groups of Gamez and Reedikj69,70 designed and prepared a series of ligands based on the triazine scaffold by nucleophilic aromatic substitution, several of which are dendritic in nature. When these ligands were treated with copper(II) in water, the catalyst systems that were generated in situ successfully performed the oxidation of 3,5-di-t-butylcatechol.70 The copper complex generated from ligand 59 (Chart 11) resulted in the most active and most stable catalyst system, and the authors attributed this observation to additional stabilization of the active catalyst through the use of a dendritic ligand.
Chart 11.
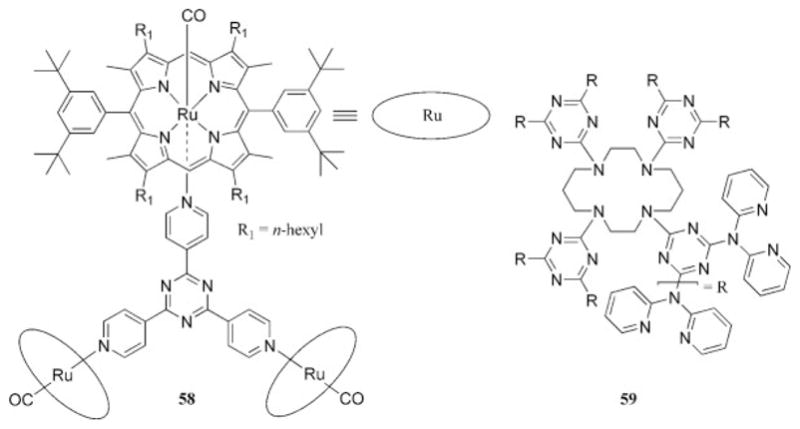
Dendritic ligands and metal complexes.
Energy-Harvesting and -Emitting Applications
In 1998, an early report described the use of triazine-based dendrimers as potential light-harvesting antennae.71 The first-generation dendrimer had six surface groups that were tethered to triazine branching units through a silyloxy phthalocyanine bond (60, Chart 12). In a related report, Burn and coworkers72,73 used the alkoxy and aryloxy substitution of cyanuric chloride to form the surface groups of a dendrimer linked by a distyrylbenzene core (61, Chart 12). The electroluminescence was investigated to determine its potential as a light-emitting diode by the preparation of luminescent films from the dendritic material. The devices had external quantum efficiencies of 0.003% but only limited stability; therefore, the authors suggested that a subsequent device might perform better if they incorporated a dendritic linkage without the phenoxy group present. More recently, a series of dendritic polymers based on the triazine unit were investigated for integrated optics applications.16 A preliminary examination of the materials indicates that they have good thermal stability and optical losses at 1550 nm of 0.28–0.44 dB/cm. The patent literature likely harbors many triazines for use in integrated optics, but this topic is outside the focus of this review as most of these molecules appear not to be dendrimers but polymeric products derived from the community interested in cyanate esters.
Chart 12.
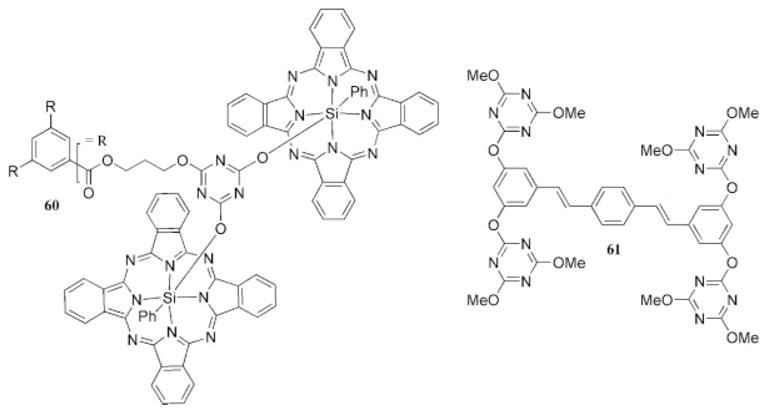
Dendritic structures prepared to investigate their optical properties.
Surfactants
Limited data exist detailing the use of dendritic materials with incorporated triazine units as surfactants. A preliminary account details the syntheses of zero- and first-generation-type dendrimers based on a triazine core that display a high number of alcohol functional groups for use as a nonionic surfactant (62, Chart 13).74,75 We reported evidence for a new organoclay morphology when smectite clay was treated with a modified version of a dendritic surfactant (63, Chart 13).76,77 This evidence originates in part from X-ray powder diffraction data, which indicate a small increase in the interlayer distance of the clay. In addition, IR spectroscopy of the clay with a surfactant larger than 63 showed that a significant amount of water remained in the interlayer space. TGA corroborated this observation: a minimal amount of organic content was present. For this reason, the phrase frustrated intercalation was used to explain the nontraditional behavior of the clay–surfactant composite, in which only a portion of the surfactant could penetrate the interlayer space.
Chart 13.
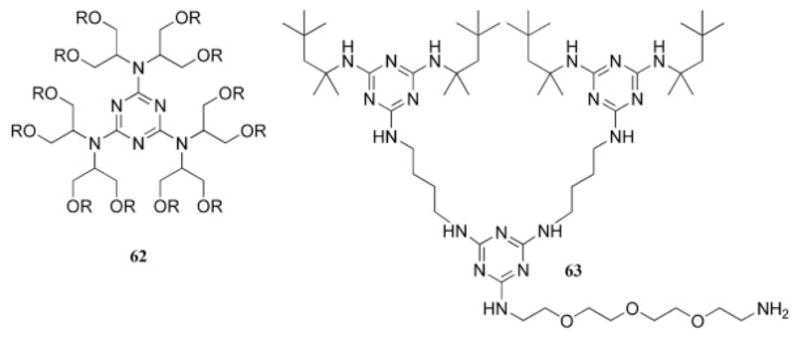
Representative examples of triazine-based dendritic materials examined for use as surfactants.
Biologically Relevant Molecules
Specific cases have been reported in which a dendritic structure containing multiple triazine groups has displayed efficacy as antiviral agents.78 In 1992, Wyeth-Ayerst initiated a program to identify novel inhibitors of the human respiratory syncytial virus (RSV).79 The only active compound identified from the screening of a library of 20,000 compounds using a whole virus and cell-based assay was a dendritic structure. The identified compound, termed CL 309623 (64, Chart 14), contains a disulfonated stilbene core with a triazine tethered at each end installed with standard nucleophilic aromatic substitution protocols. This class of compounds was originally reported in 1962 as potential optical brighteners.80 A patent was filed in 1997 to use CL 309623 and related derivatives to treat viral infections.78 In 1998, a comprehensive structure–activity relationship study was undertaken, which resulted in the identification of even more potent molecules, all of which retained two triazine units.81 An extension of this study conducted in 2001 identified RFI-641 (65, Chart 14) as a potent and selective inhibitor of RSV.82 The mode of action of these dendrimer-like compounds is the interruption of F-protein-mediated cell fusion of RSV with the target cell through a specific interaction of the antiviral agent with the fusion protein of the RSV virus.
Chart 14.
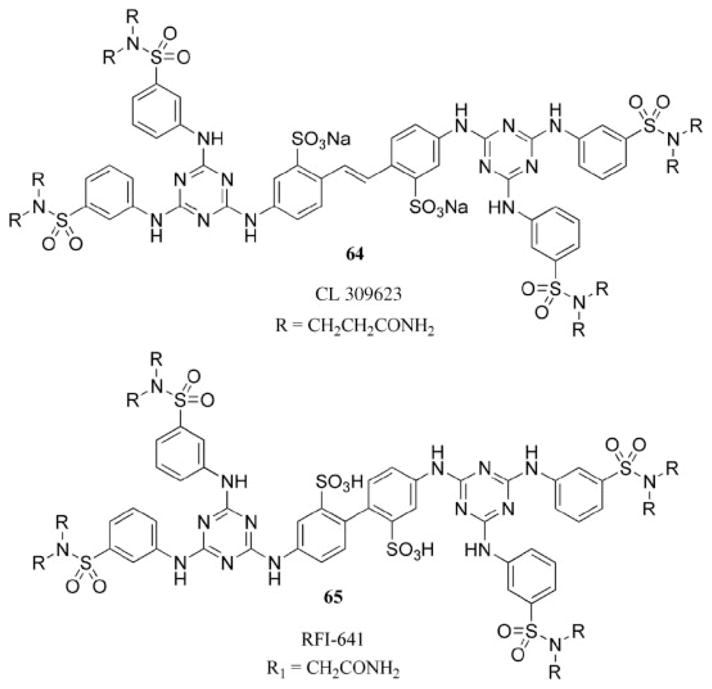
Potent dendritic antiviral agents.
Promising triazine-based antibiotics have been developed by their examination as part of a dendrimer bound to a polystyrene resin.83 The dendrimer was not constructed from repeating triazine units but instead had a variety of triazine derivatives tethered to the periphery (66, Chart 15). A modified second-generation Newkome-type dendrimer with nine surface amines was constructed on a solid polystyrene support, effectively increasing the loading capacity of the resin. The surface amines were modified to present a phenol and then treated with cyanuric chloride. This resulted in the incorporation of nine dichlorotriazine units at the surface of each dendron. These dendrons were subsequently treated with various amine nucleophiles to produce a library of compounds. The triazines were liberated from the solid support by the heating of the resin in the presence of morpholine or piperidine because the aryloxy–triazine bond is susceptible to attack by these secondary amines. This design permitted single-bead screening of a library of potential antibiotics and thus represents a significant advance in this type of assay because a higher concentration of the agent of interest could be produced due to the dendritic nature of the resin.
Chart 15.
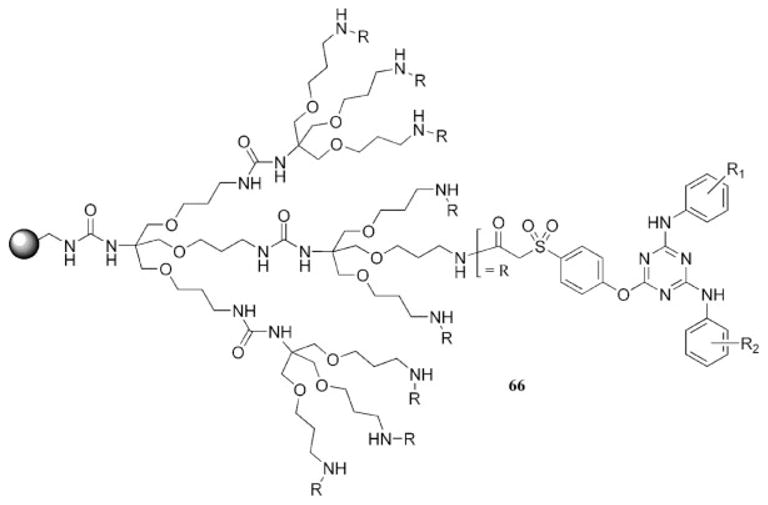
Dendritic design that facilitates single-bead screening of potential antibacterial agents.
A significant advance in the application of triazine-based dendrimer chemistry has been the preparation of dendrimers that present disulfide linkages at the periphery. Several precursor dendrimers and dendrons, which were prepared with the nucleophilic aromatic substitution strategy, were decorated with pyridyl disulfide groups at the periphery or at the terminal of a dendron. These disulfide groups readily underwent exchange with biotin, captopril, a small peptide sequence, or even a DNA oligonucleotide (67 and 68, Chart 16), although the characterization and purification of the latter were challenging, and no general protocols emerged.37,84,85
Chart 16.
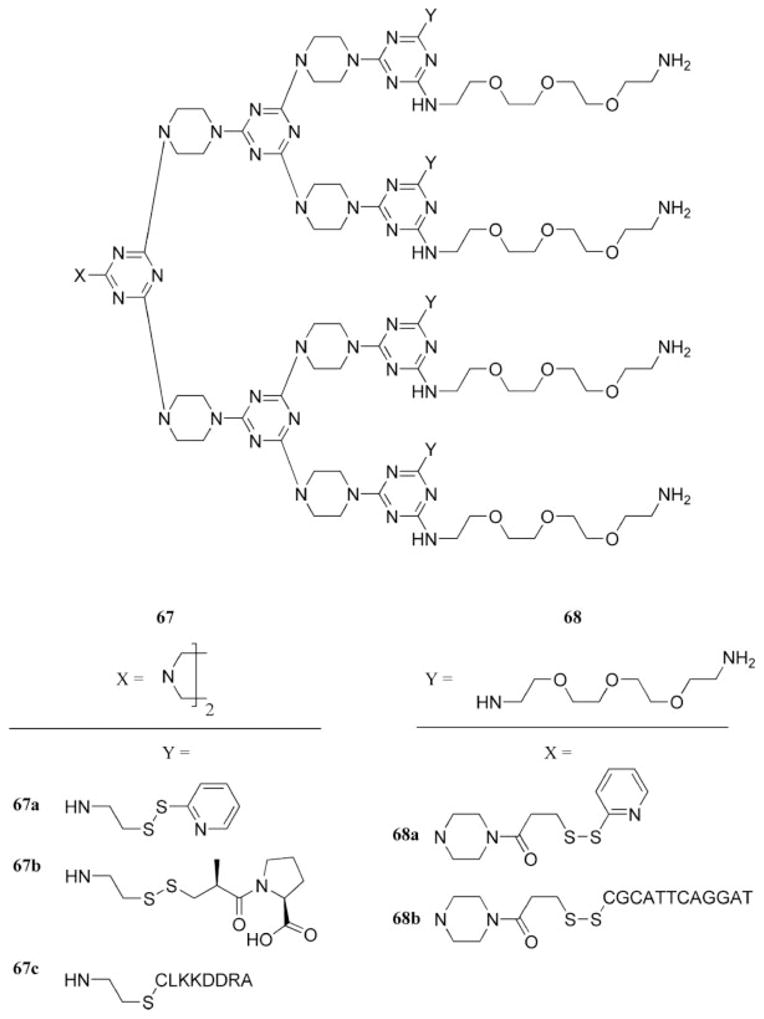
Precursors and products of disulfide exchange experiments.
Qualitative observations resulting from these experiments indicated that steric factors arising from the size of the substrate or the dendrimer influenced the degree of exchange that occurred. A related kinetic study of disulfide exchange on the periphery of other melamine-derived dendrimers confirmed that the rate of exchange increases as the size of the dendrimer decreases (69–73, Chart 17).86 Interestingly, in the two cases (71 and 73) that were followed by mass spectrometry, rate constants were identical within a factor of 100× for each of the disulfides of a dendrimer but increased as dansyl groups were shed from the architecture. These findings are intriguing: disulfide linkages can be used to tether pharmacophores, targeting agents, or imaging agents to a dendrimer for drug delivery and in vivo monitoring applications.
Chart 17.
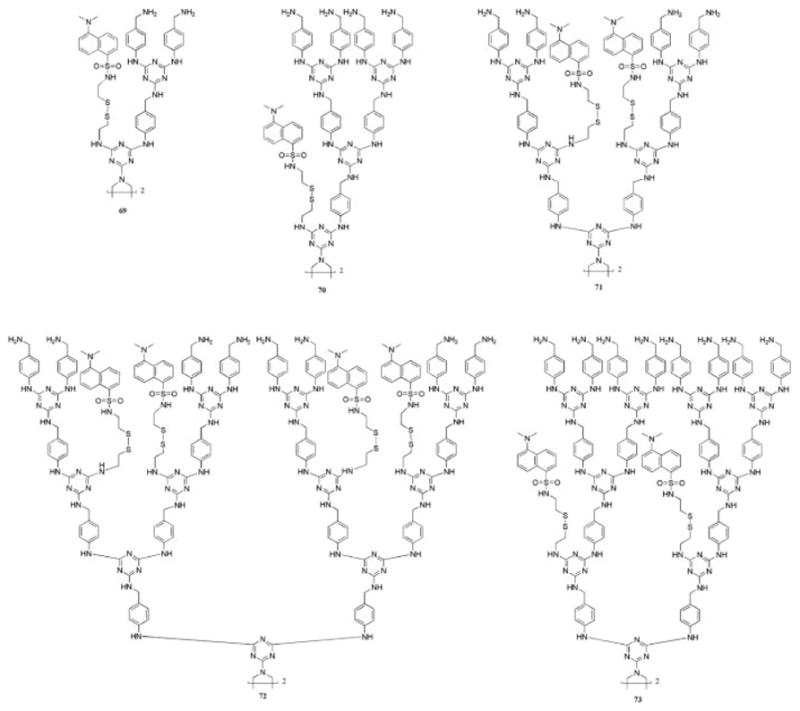
The rate of disulfide exchange with dithiothreitol depends on the size of the dendrimer: 69 ~ 70 ~ 71 > 72 > 73.
Another method of drug delivery that could be facilitated by dendrimers is noncovalent transport and delivery. To this end, a preliminary study showed that the treatment of a cationic melamine-based dendrimer sequestered drugs as a function of drug composition when mixed with indomethacin, methotrexate, or 10-hydroxycamptothecin.87 The degree of solubilization was dependent on the properties of the drug; only in the case of 10-hydroxycamptothecin and a bisindole methane was any significant solubility enhancement observed (3.7 molecules of drug solubilized/dendrimer). However, studies with pyrene showed that for dendrimers of related molecular weights, triazine dendrimers performed similarly to Fréchet’s original aryl ethers by solubilizing 0.1 and 0.2 molecules of pyrene/dendrimer. This represents a 10–20-fold increase over the more hydrophilic poly(amidoamine) and poly(propylene imine) dendrimers.
To more thoroughly examine the toxicity of melamine-derived dendrimers in vitro and in vivo, seven different dendrimers were prepared that displayed groups at the periphery that were cationic, anionic, or neutral and could still convey sufficient solubility in water (74–80, Chart 18).88 The results of the cell viability study indicated that the neutral dendrimer decorated with PEG, 80, was the least hemolytic over a concentration range of 0.001–10 mg/mL. Subsequent acute dosing experiments with the PEG-functionalized dendrimer indicated no toxicity in mice at concentrations up to 2.56 g/kg (intraperitoneal administration) or 1.28 g/kg (intravenous administration). Related studies with a cationic dendrimer demonstrated no detectable toxicity in vivo until mice were dosed with 40 mg/kg.89 Additional recent results are promising because the data suggest that the hepatoxicity of the anticancer drugs methotrexate and 6-mercaptopurine were reduced upon noncovalent encapsulation of these pharmacophores by a melamine-based dendrimer.90 The accumulation of these data and other animal studies91 suggests that dendrimers based on melamine have potential for a variety of biomedical applications.
Chart 18.
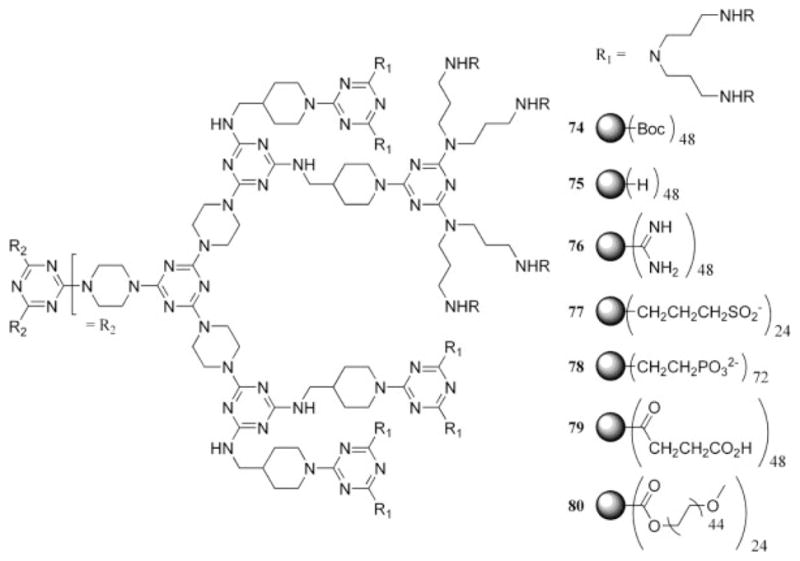
Variety of dendrimers that were subjected to cell viability studies.
CONCLUSIONS
The use of triazine derivatives in an enormous number of applications is well documented. However, with the advent of dendrimer chemistry, the role of triazines as potential constructs for the synthesis of dendritic materials had initially been limited. Recently, the benefits that arise from both the structural complexity and ease of synthetic manipulation that can be achieved with triazine units as building blocks have caught the interest of an increasing number of research groups in industrial and academic settings. In a relatively short period of time, synthetic strategies have been developed, and a number of applications are currently being investigated in which triazine-based dendritic materials play an essential role. It is likely that the convergence of co-invention and rediscovery that has occurred with triazine-based dendritic materials will only continue to generate interesting materials for current applications and result in the development of new applications.
Challenges remain for the application of triazine-based dendrimers. Scalable syntheses that are industrially compatible are generally lacking. These materials are not commercially available, and this limits the widespread evaluation by scientists and engineers that is generally required to facilitate advances and realize applications. Biomedical applications are held in check for lack of metabolic data and toxicological profiling. Despite these challenges, the interest in this class of materials appears to be increasing, and in time, these challenges will be met.
Biographies

Mackay B. Steffensen (Ph.D. 2004) joined Simanek’s laboratory after completing undergraduate work at Southern Utah University. Steffensen is currently completing postdoctoral studies with Hagan Bayley at the University of Oxford.

Dr. Eric E. Simanek is an Associate Professor of Chemistry at Texas A&M University. He joined the faculty in 1998 after completing postdoctoral studies with Chi-Huey Wong at the Scripps Research Institute; graduate work under George Whitesides at Harvard University; and undergraduate studies under Kenneth Rinehart, Jr., at the University of Illinois in Urbana–Champaign. The research focus of Simanek’s group has been dendrimers based on triazines and their applications to separation science and drug delivery.

Frank Kuschel received his Ph.D. in vapor-liquid equilibria of nonelectrolyte mixtures and in 1978 his Habilitation in physical chemistry. Since that time he has been involved in research on liquid crystals and their electro-optical behavior. In 1982 he was appointed full professor of physical chemistry at Martin-Luther-University Halle-Wittenberg, Germany. He focused his research on synthesis and phase structures of thermotropic polymers and amphiphilic lyotropics. From 1992 to 2000, he was one of the founders of MLS GmbH (Materials for Light Controlling Systems) at Leuna, Germany. He joined Fraunhofer Institute IZM, Branch Lab Polymeric Materials and Composites, Teltow, Germany, in 2001. Now he is working as senior advisor at the Fraunhofer Institute IZM.

Monika Bauer received her Ph.D. (Dr. rer. nat.) at the Academy of Science, Central Institute for Organic Chemistry, Berlin/Germany, in 1979 and Dr. sc. nat. 1987 at the same place. Since 1998, Dr. Bauer is the Head of the Branch Lab Polymeric Materials and Composites of the Fraunhofer Institute for Reliability and Microintegration, Teltow, Germany and Professor of the chair of Polymeric Materials at the Brandenburg University of Technology, Cottbus, Germany. Her research activity is focused on synthesis and characterization of network and linear polymers (e.g. polycyanurates, epoxies, bis maleimides, polyacrylates, polyethers, polyimides) for lightweight structures and opto-microelectronics; flame resistance toughening; polymer formation mechanism; structure-property relationships; development of adhesives, casting resins, bistable reflective displays, integrated optics.

Emily Hollink was trained as an inorganic chemist, performing research under the direction of Dr. Deryn E. Fogg at the University of Ottawa (B.Sc.) and Dr. Douglas W. Stephan at the University of Windsor (Ph.D.). She has been working as an NSERC postdoctoral fellow under the direction of Dr. Eric E. Simanek for two years while exploring the beautiful Texas wilderness.
REFERENCES AND NOTES
- 1.Dendrimers, et al. In: Dendritic Polymers. Fréchet JMJ, Tomalia DA, editors. Wiley; New York: 2001. [Google Scholar]
- 2.Grayson SM, Fréchet JMJ. Chem Rev. 2001;101:3819–3868. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]
- 3.Tomalia DA, Fréchet JMJ. J Polym Sci Part A: Polym Chem. 2002;40:2719–2728. [Google Scholar]
- 4.Fréchet JMJ. J Polym Sci Part A: Polym Chem. 2003;41:3713–3725. [Google Scholar]
- 5.Hecht S. J Polym Sci Part A: Polym Chem. 2003;41:1047–1058. [Google Scholar]
- 6.Timmerman P, Prins LJ. Eur J Org Chem. 2001;17:3191–3205. [Google Scholar]
- 7.Pons M, Millet O. Prog Nucl Magn Reson Spectrosc. 2001;38:267–324. [Google Scholar]
- 8.Simanek EE, Mammen M, Gordon DM, Chin D, Mathias JP, Seto CT, Whitesides GM. Tetrahedron. 1995;51:607–619. [Google Scholar]
- 9.Whitesides GM, Simanek EE, Mathias JP, Seto CT, Chin D, Mammen M, Gordon DM. Acc Chem Res. 1995;28:37–44. [Google Scholar]
- 10.Quirke JME. In: Comprehensive Heterocyclic Chemistry. part 2B. Katritzky AR, Rees CW, editors. Vol. 3. Pergamon; Elmsford, NY: 1984. pp. 457–530. Chapter 2.20. [Google Scholar]
- 11.Bartholomew D. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 6. Pergamon; Elmsford, NY: 1996. pp. 575–636. Chapter 6.12. [Google Scholar]
- 12.Hamerton I. Chemistry and Technology of Cyanate Ester Resins. Blackie; London: 1994. [Google Scholar]
- 13.(a) Maciejewski M, Janiszewski J Instytut Chemii Przemyslowej Ignacego Moscickiego. 176865. Polish Patent PL. 1999:8.; (b) Meijer EW, Bosman HJM, Vandenbooren FHAM, De Brabander-van Den Berg E, Castelijns AMCF, De Man HCJ, Reintjens RWEG, Stoelwinder CJC, Nijenhuis AJ. U.S. Patent. 1996:19. (DSM N.V.) (contained in part of U.S. series 117,004 and abandoned)
- 14.Maciejewski M. Polimery (Warsaw) 1995;40:404–409. [Google Scholar]
- 15.Maciejewski M, Bednarek E, Janiszewska J, Janiszewski J, Szczygiel G, Zapora M. J Macromol Sci Pure Appl Chem. 2000;37:753–783. [Google Scholar]
- 16.Dreyer C, Schneider J, Göcks K, Beuster B, Bauer M, Keil N, Yao H, Zawadzki C. Macromol Symp. 2003;199:307–319. [Google Scholar]
- 17.Maciejewski M. J Macromol Sci Chem. 1982;17:689–703. [Google Scholar]
- 18.Buhleier E, Wehner W, Voegtle F. Synthesis. 1978:155–158. [Google Scholar]
- 19.Niederhauser WD. 2,577,477. U.S. Patent. 1951
- 20.Bruson HA, Niederhauser WD Rohm Haas Co. 2,460,733. U.S. Patent. 1949
- 21.Woerner C, Muelhaupt R. Angew Chem Int Ed Engl. 1993;32:1306–1308. [Google Scholar]
- 22.de Brabander-van den Berg EMM, Meijer EW. Angew Chem Int Ed. 1993;32:1308–1311. [Google Scholar]
- 23.Keana JFW, Martin V, Ralston WH University of Oregon. 5,567,411. U.S. Patent. 1996
- 24.Bauer J, Bauer M, Neumann J Fraunhofer-Gesellschaft zur Foerderung der Angewandten Forschung e.V. 95-19528882. German Patent DE. 1995:19.
- 25.Dreyer C, Blume A, Bauer M, Bauer J, Neumann-Rodekirch J. Proc ECSO. 2000;4:1196–1213. [Google Scholar]
- 26.Zhang W, Simanek EE. Org Lett. 2000;2:843–845. doi: 10.1021/ol005585g. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Simanek EE. Polym Prepr (Am Chem Soc Div Polym Chem) 2000;41:1579. [Google Scholar]
- 28.Neumann-Rodekirch J. Ph.D. Thesis. University of Bremen; Bremen, Germany: 1997. Darstellung und Charakterisierung von hochverweigten, molekularuneinjeitlichen Polymelaminstrukturen. [Google Scholar]
- 29.Takagi K, Uchikura K, Hattori T, Kunisada H, Yuki Y. Kobunshi Ronbunshu. 2000;57:646–651. [Google Scholar]
- 30.Takagi K, Hattori T, Kunisada H, Yuki Y. J Polym Sci Part A: Polym Chem. 2000;38:4385–4395. [Google Scholar]
- 31.Verheyde B, Dehaen W. J Org Chem. 2001;66:4062–4064. doi: 10.1021/jo005772s. [DOI] [PubMed] [Google Scholar]
- 32.Verheyde B, Maes W, Dehaen W. Mater Sci Eng C. 2001;18:243–245. [Google Scholar]
- 33.Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Fréchet JMJ, Sharpless KB, Fokin VV. Angew Chem Int Ed. 2004;43:3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Nowlan DT, III, Thomson LM, Lackowski WM, Simanek EE. J Am Chem Soc. 2001;123:8914–8922. doi: 10.1021/ja0041369. [DOI] [PubMed] [Google Scholar]
- 35.Steffensen M, Simanek EE. Abstracts of Papers, 225th ACS National Meeting; New Orleans, LA. March 23–27, 2003; Washington, DC: American Chemical Society; 2003. [Google Scholar]
- 36.Steffensen MB, Simanek EE. Org Lett. 2003;5:2359–2361. doi: 10.1021/ol0347491. [DOI] [PubMed] [Google Scholar]
- 37.Steffensen MB, Simanek EE. Angew Chem Int Ed. 2004;43:5178–5180. doi: 10.1002/anie.200460031. [DOI] [PubMed] [Google Scholar]
- 38.Namazi H, Adeli M. J Polym Sci Part A: Polym Chem. 2005;43:28–41. [Google Scholar]
- 39.Kim C, Chang Y, Kim JS. Macromolecules. 1996;29:6353–6355. [Google Scholar]
- 40.Tanaka S, Kumei M Agency of Industrial Sciences and Technology of Japan; Stanley Electric Co., Ltd. 96-114891, 09302073. Japanese Patent JP. 1997:8.
- 41.Tanaka S, Kumei M Agency of Industrial Sciences and Technology of Japan; Stanley Electric Co., Ltd. 9302073. Japanese Patent JP. 1997
- 42.Kim C, Cho SY, Kim JS, Chang Y. Polym Mater Sci Eng. 1997;77:191–192. [Google Scholar]
- 43.Chang Y, Kwon YC, Park K, Kim C. Korea Polym J. 2000;8:142–146. [Google Scholar]
- 44.Cho SY, Chang Y, Kim JS, Lee SC, Kim C. Macromol Chem Phys. 2001;202:263–269. [Google Scholar]
- 45.Chang Y, Kim YN, Noh I, Kim C. Macromol Chem Phys. 2000;201:1808–1812. [Google Scholar]
- 46.Freeman AW, Vreekamp R, Fréchet JMJ. Book of Abstracts, 214th ACS National Meeting; Las Vegas, NV. Sept 7–11, 1997; Washington, DC: American Chemical Society; 1997. PMSE-128. [Google Scholar]
- 47.Freeman AW, Vreekamp RH, Fréchet JMJ. Polym Mater Sci Eng. 1997;77:138–139. [Google Scholar]
- 48.Huck WTS, Hulst R, Timmerman P, van Veggel FCJM, Reinhoudt DN. Angew Chem Int Ed Engl. 1997;36:1006–1008. [Google Scholar]
- 49.Brunet P, Simard M, Wuest JD. J Am Chem Soc. 1997;119:2737–2738. [Google Scholar]
- 50.Malek N, Maris T, Simard M, Wuest JD. J Am Chem Soc. 2005;127:5910–5916. doi: 10.1021/ja042233m. [DOI] [PubMed] [Google Scholar]
- 51.Sauriat-Dorizon H, Maris T, Wuest JD, Enright GD. J Org Chem. 2003;68:240–246. doi: 10.1021/jo026267t. [DOI] [PubMed] [Google Scholar]
- 52.Laliberte D, Maris T, Sirois A, Wuest JD. Org Lett. 2003;5:4787–4790. doi: 10.1021/ol035712j. [DOI] [PubMed] [Google Scholar]
- 53.Laliberte D, Maris T, Wuest JD. J Org Chem. 2004;69:1776–1787. doi: 10.1021/jo035311h. [DOI] [PubMed] [Google Scholar]
- 54.Demers E, Maris T, Wuest JD. Cryst Growth Des. 2005;5:1227–1235. [Google Scholar]
- 55.Le Fur E, Demers E, Maris T, Wuest JD. Chem Commun. 2003:2966–2967. doi: 10.1039/b308355a. [DOI] [PubMed] [Google Scholar]
- 56.Brunet P, Demers E, Maris T, Enright GD, Wuest JD. Angew Chem Int Ed. 2003;42:5303–5306. doi: 10.1002/anie.200352252. [DOI] [PubMed] [Google Scholar]
- 57.Boils D, Perron ME, Monchamp F, Duval H, Maris T, Wuest JD. Macromolecules. 2004;37:7351–7357. [Google Scholar]
- 58.Fournier JH, Maris T, Wuest JD. J Org Chem. 2004;69:1762–1775. doi: 10.1021/jo0348118. [DOI] [PubMed] [Google Scholar]
- 59.Boils-Boissier DC, Breton MP, Thomas JW, Titterington DR, Banning JH, Goodbrand HB, Wuest JD, Perron M-E, Monchamp F, Duval H Xerox Corp. 2,004,050,295. U.S. Patent. 2004:34.
- 60.Breton MP, Boils-Boissier DC, Thomas JW, Titterington DR, Goodbrand HB, Banning JH, Wuest JD, Laliberte D, Perron M-E Xerox Corp. 2,004,075,723. U.S. Patent. 2004:68.
- 61.Zhang W, Simanek EE. Tetrahedron Lett. 2001;42:5355–5357. [Google Scholar]
- 62.Zhang W, Gonzalez SO, Simanek EE. Macromolecules. 2002;35:9015–9021. [Google Scholar]
- 63.Marsh A, Carlisle SJ, Smith SC. Tetrahedron Lett. 2001;42:493–496. [Google Scholar]
- 64.Neumann-Rodekirch J, Bauer J, Bauer M Fraunhofer-Gesellschaft zuer Foerderung der Angewandten Forschung e.V. 19621741. German Patent DE. 1996:8.
- 65.Wu X, Liu P, Pu Q, Su Z. Anal Lett. 2003;36:2229–2241. [Google Scholar]
- 66.Acosta EJ, Gonzalez SO, Simanek EE. J Polym Sci Part A: Polym Chem. 2005;43:168–177. [Google Scholar]
- 67.Acosta EJ, Carr CS, Simanek EE, Shantz DF. Adv Mater (Weinheim, Ger) 2004;16:985–989. [Google Scholar]
- 68.Darling SL, Ching Mak C, Bampos N, Feeder N, Teat SJ, Sanders JKM. New J Chem. 1999;23:359–364. [Google Scholar]
- 69.de Hoog P, Gamez P, Driessen WL, Reedijk J. Tetrahedron Lett. 2002;43:6783–6786. [Google Scholar]
- 70.Gamez P, de Hoog P, Lutz M, Spek AL, Reedijk J. Inorg Chim Acta. 2003;351:319–325. [Google Scholar]
- 71.Kraus GA, Louw SV. J Org Chem. 1998;63:7520–7521. doi: 10.1021/jo980244q. [DOI] [PubMed] [Google Scholar]
- 72.Lupton JM, Hemingway LR, Samuel IDW, Burn PL. J Mater Chem. 2000;10:867–871. [Google Scholar]
- 73.Samuel IDW, Halim M, Burn PL, Pillow JNG ISIS Innovation, Ltd. 9921935. International Patent WO. 1999:71.
- 74.Finn M, Krulle TM, Scott DA, Fleet GWJ. Book of Abstracts, 217th ACS National Meeting; Anaheim, CA. March 21–25, 1999; Washington, DC: American Chemical Society; 1999. ORGN-088. [Google Scholar]
- 75.Fleet G, Scott DA, Finn M, Krulle TM. 00/55111. International Patent WO. 2000
- 76.Acosta EJ, Deng Y, White GN, Dixon JB, McInnes KJ, Senseman SA, Frantzen AS, Simanek EE. Chem Mater. 2003;15:2903–2909. [Google Scholar]
- 77.Acosta EJ, Simanek EE. Abstracts of Papers, 225th ACS National Meeting; New Orleans, LA. March 23–27, 2003; Washington, DC: American Chemical Society; 2003. MTLS-038. [Google Scholar]
- 78.Gluzman Y, LaRocque JP, O’Hara BM, Morin JE, Ellestad GA, Mitsner B, Ding W-D, Raifeld YE, Nikitenko AA. 5,852,015. U.S. Patent. 1998
- 79.Gazumyan A, Mitsner B, Ellestad GA. Curr Pharm Des. 2000;6:525–546. doi: 10.2174/1381612003400704. [DOI] [PubMed] [Google Scholar]
- 80.Gehn R, Schmidt O, Mertens H, Grunwald W, Hehl M Badische Anilin-. Soda-Fabrik AG. 908229. Patent GB. 1962:8.
- 81.Ding WD, Mitsner B, Krishnamurthy G, Aulabaugh A, Hess CD, Zaccardi J, Cutler M, Feld B, Gazumyan A, Raifeld Y, Nikitenko A, Lang SA, Gluzman Y, O’Hara B, Ellestad GA. J Med Chem. 1998;41:2671–2675. doi: 10.1021/jm980239e. [DOI] [PubMed] [Google Scholar]
- 82.Huntley CC, Weiss WJ, Gazumyan A, Buklan A, Feld B, Hu W, Jones TR, Murphy T, Nikitenko AA, O’Hara B, Prince G, Quartuccio S, Raifeld YE, Wyde P, O’Connell JF. Antimicrob Agents Chemother. 2002;46:841–847. doi: 10.1128/AAC.46.3.841-847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lebreton S, Newcombe N, Bradley M. Tetrahedron. 2003;59:10213–10222. [Google Scholar]
- 84.Umali AP, Simanek EE. Org Lett. 2003;5:1245–1247. doi: 10.1021/ol034161u. [DOI] [PubMed] [Google Scholar]
- 85.Bell SA, McLean ME, Oh SK, Tichy SE, Zhang W, Corn RM, Crooks RM, Simanek EE. Bioconjugate Chem. 2003;14:488–493. doi: 10.1021/bc020075n. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W, Tichy SE, Perez LM, Maria GC, Lindahl PA, Simanek EE. J Am Chem Soc. 2003;125:5086–5094. doi: 10.1021/ja0210906. [DOI] [PubMed] [Google Scholar]
- 87.Zhang W, Jiang J, Qin C, Perez LM, Parrish AR, Safe SH, Simanek EE. Supramol Chem. 2003;15:607–616. [Google Scholar]
- 88.Chen HT, Neerman MF, Parrish AR, Simanek EE. J Am Chem Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 89.Neerman MF, Zhang W, Parrish AR, Simanek EE. Int J Pharm. 2004;281:129–132. doi: 10.1016/j.ijpharm.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 90.Neerman MF, Chen HT, Parrish AR, Simanek EE. Mol Pharm. 2004;1:390–393. doi: 10.1021/mp049957p. [DOI] [PubMed] [Google Scholar]
- 91.Neerman MF, Umali AP, Chen HT, Waghela SD, Parrish AR, Simanek EE. J Drug Delivery Sci Technol. 2005;15:31–40. [Google Scholar]