Abstract
Glucose-6-phosphatase catalyzes the hydrolysis of glucose 6-phosphate (G6P) to glucose and inorganic phosphate. It is a multicomponent system located in the endoplasmic reticulum that comprises several integral membrane proteins, namely a catalytic subunit (G6PC) and transporters for G6P, inorganic phosphate, and glucose. The G6PC gene family contains three members, designated G6PC, G6PC2, and G6PC3. The tissue-specific expression patterns of these genes differ, and mutations in all three genes have been linked to distinct diseases in humans. This minireview discusses the disease association and transcriptional regulation of the G6PC genes as well as the biological functions of the encoded proteins.
General Features of the G6PC Family
In mammals, the highest levels of glucose-6-phosphatase activity are found in liver; however, progress in studying the enzyme responsible for this activity was impeded for many years because of its location in the ER2 membrane and its inherent instability (1, 2). Remarkably, the first cloning of a cDNA encoding an enzyme with glucose-6-phosphatase activity was achieved only in 1993 through the seminal work of Janice Chou and co-workers (3, 4), who took advantage of the observation that a unique mutant mouse strain had markedly reduced glucose-6-phosphatase activity. Differential screening of a hepatic cDNA library with probes representing wild-type and mutant mouse hepatic mRNAs led to the isolation of a glucose-6-phosphatase cDNA (3, 4).
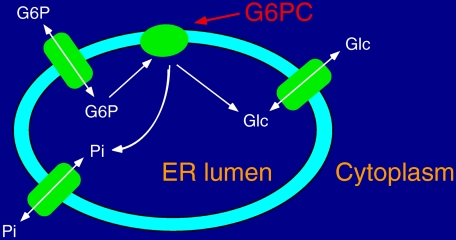
Various models have been proposed to explain the complex enzymology of the hepatic glucose-6-phosphatase system (1, 2). Fig. 1 shows the generally accepted model in which G6P hydrolysis occurs within the ER lumen and requires transporters to import the substrate, G6P, into the ER lumen and export the products, glucose and inorganic phosphate, back to the cytosol (1, 2). The analysis of hepatic microsomes isolated from mice in which the gene encoding the glucose-6-phosphatase cDNA had been mutated demonstrated that G6P hydrolysis and G6P transport into the ER were mediated by separate proteins (5). The cDNA originally isolated by Chou and co-workers represents the G6PC. A cDNA encoding a mammalian G6P transporter was subsequently isolated by van Schaftingen and Gerin (6) based on a data base analysis involving a search for mammalian expressed sequence tags homologous to a bacterial hexose phosphate transporter. Although the ER glucose transporter remains to be identified, recent data suggest that a single protein transports both G6P and inorganic phosphate (7).
FIGURE 1.
Model of the glucose-6-phosphatase multicomponent enzyme system. G6P enters the ER lumen through a G6P transporter. Once hydrolyzed to Glc and Pi by the G6PC, the products of the reaction return to the cytosol through specific transporters. Recent data suggest that the same protein transports both G6P and Pi.
The G6PC gene is expressed predominantly in liver and kidney but also at lower levels in intestine and pancreatic islets (1, 2, 6, 8). The latter also contain a second, distinct G6PC isoform that was initially called the islet-specific G6PC-related protein (IGRP) because of its selective expression in this tissue (9, 10). An IGRP cDNA was originally isolated through screening of a plasmid cDNA library prepared following subtraction of mouse insulinoma βTC-3 cDNA from mouse glucagonoma αTC-2 cDNA (9). The IGRP gene has now been renamed G6PC2. The third member of the G6PC gene family, termed G6PC3, was identified through data base analyses involving searches for expressed sequence tags with sequence homology to G6PC and G6PC2 (11). This isoform was initially called the ubiquitously expressed G6PC-related protein (UGRP) because, although predominantly expressed in brain, muscle, and kidney, it was found to be expressed in every tissue analyzed (11).
G6PC
Enzyme Activity and Function
G6PC catalyzes the terminal step in the gluconeogenic and glycogenolytic pathways. Although the liver is the primary site of gluconeogenesis in vivo (12), the actual contribution of the kidney and intestine, relative to the liver, to the overall rate of gluconeogenesis is the subject of continuing debate. However, because mutations in the G6PC gene lead to severe hypoglycemia (see below), G6PC clearly plays a critical role in maintaining euglycemia in the fasted state. Chou and co-workers (13) have performed extensive structure-function analyses to identify the catalytic site and other important residues within G6PC. Interestingly, Nordlie and co-workers (2) have shown that G6PC can also catalyze G6P synthesis, although the biological significance of this observation remains to be determined. The biology of G6PC has been discussed in detail in several earlier reviews (1, 2, 6, 8).
Disease Association
Mutations in the G6PC gene result in GSD type 1a, whereas mutations in the G6P transporter result in GSD type 1b. GSD type 1a is characterized primarily by severe hypoglycemia in the postabsorptive state but also by hyperlipidemia, hyperuricemia, and lactic acidemia (14). In addition, patients are prone to growth retardation, hepatic steatosis and cirrhosis, hepatic adenoma, and renal failure (14). Many features of GSD type 1a, although not all, are apparent in G6pc null mice (5). Chou and Mansfield (14) have extensively documented the mutations in the G6PC gene that give rise to this disease.
Whereas G6PC mutations cause GSD type 1a, overexpression of G6PC also affects glucose metabolism. Type 2 diabetes is characterized by defects in insulin secretion, peripheral glucose utilization, and HGP. The ability of insulin to stimulate peripheral glucose utilization and repress HGP in patients with type 2 diabetes is reduced as a consequence of insulin resistance. In addition, in some individuals with type 1 diabetes, HGP is increased either because of low circulating insulin levels or because poor glycemic control has led to the development of insulin resistance. Because G6PC catalyzes the final reaction in both the gluconeogenic and glycogenolytic pathways and because glucose leaves the liver through the facilitative GLUT2 glucose transporter, G6PC acts as the gatekeeper for glucose production by the liver.
In the postabsorptive state, HGP initially increases because of changes in substrate supply and liver metabolism. However, it is likely that during an overnight fast the rate of HGP will be affected by changes in G6PC expression (15). Because insulin normally inhibits G6PC expression (see below), in individuals with diabetes, G6PC expression is likely to be elevated as a consequence of insulin resistance or hypoinsulinemia. Indeed, in animal models of both type 1 and 2 diabetes, hepatic G6PC activity and G6PC mRNA levels are increased (6, 8). Similarly, the activity of the hepatic glucokinase/G6PC glucose cycle, the conversion of glucose to G6P and then back to glucose, is increased in patients with type 2 diabetes, and this is postulated to contribute to the elevated HGP (16).
Transcriptional Regulation
The transcriptional regulation of G6PC gene expression has been studied in all four tissues where it is expressed, namely liver, kidney, intestine, and pancreatic islets (1, 2, 6, 8), but because of space limitations, only regulation in liver will be discussed here. Not surprisingly, given the central role of G6PC in HGP, multiple hormones/metabolites regulate G6PC expression, including glucagon, which acts through cAMP, glucocorticoids, glucose, and fatty acids, which stimulate expression and insulin, and tumor necrosis factor-α and interleukin-6, which inhibit expression (15). Insulin is able to override the stimulatory effects of cAMP, glucocorticoids, glucose, and fatty acids (15). The induction of G6PC gene transcription by glucose seems counterintuitive because this would serve to further increase HGP. Rossetti and co-workers (17) have suggested that this action of glucose prevents excessive hepatic glucose storage and prepares for the transition to the postabsorptive period, when increased glucose output is needed.
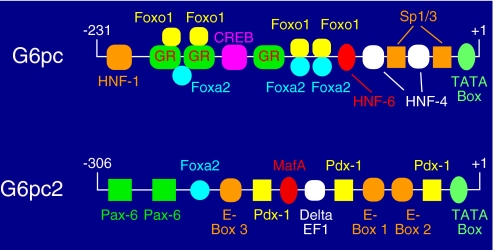
Promoter elements and associated transcription factors that are important for the action of glucagon (18), glucocorticoids (19), glucose (20), fatty acids (21), and insulin (22) have all been analyzed. Several recent studies have analyzed the effect of various co-activators, including PGC-1α (23, 24), CRTC2 (25), and SRC-2 (26), on G6PC expression. Fig. 2 shows a model of the G6PC promoter based on data from these studies.
FIGURE 2.
Comparison of transcription factors binding the G6pc and G6pc2 promoters. The proximal G6pc promoter binds multiple transcription factors, including HNF-1, Foxo1, the glucocorticoid receptor (GR), Foxa2, HNF-6, HNF-4, and Sp1/3. Some of the glucocorticoid receptor-binding sites overlap binding sites for Foxo1 and Foxa2. The proximal G6pc2 promoter also binds multiple transcription factors, including Pax-6, Pdx-1, Foxa2, MafA, and δEF1. At the G6pc2 promoter, E-Box 1 binds BETA-2, E-Box 2 binds USF-1 and -2, and the factor binding E-Box 3 is unknown. The diagrams are not drawn to scale.
G6PC2
Enzyme Activity and Function
G6PC2 is ∼50% identical at the amino acid level to G6PC (9, 11, 27); however, whether G6PC2 hydrolyzes G6P has been controversial (9, 11, 27–29). Our initial attempts to address this question, which involved expressing G6PC2 by transient transfection of COS cells, were unsuccessful (9, 11, 27), as were similar experiments by the Chou laboratory (28). However, Petrolonis et al. (29) were able to demonstrate a low rate of G6P hydrolysis by G6PC2 using somewhat different experimental conditions, although it is not clear where the discrepancy lies in methodological terms. Nevertheless, the observations of Petrolonis et al. (29) prompted us to re-examine this issue. We have recently devised an experimental protocol that involves permeabilization of COS cells to G6P with minimal perturbation of the intracellular membrane architecture, and we have achieved significant levels of G6P hydrolysis upon overexpression of G6PC2, although at a much lower rate than achieved with G6PC.3 This suggests that G6PC2 is easily denatured upon cell disruption or depends on critical components in the intracellular milieu or protein-protein interactions to maintain its activity.
To gain insight into the function of G6PC2 in vivo, we examined the phenotype of G6pc2 null mice. A small but significant decrease in blood glucose was observed in both male (−14%) and female (−11%) G6pc2−/− mice following a 6-h fast, whereas plasma insulin and glucagon concentrations were unchanged (30). We hypothesize that, consistent with a role for G6PC2 in G6P hydrolysis, deletion of the G6pc2 gene enhances glycolytic flux and hence increases the glucose sensitivity of GSIS. Glucokinase had been considered to be the glucose sensor in pancreatic beta cells (31), but these data suggest that G6PC2 should also be considered a component of this sensor. Such an arrangement would provide flexibility because the glucose sensitivity of GSIS could be fine-tuned through changes in either glucokinase or G6PC2 expression. The concept that G6PC2 functions primarily as a component of the beta cell glucose sensor is also consistent with the fact that pancreatic islets do not contribute significantly to whole body gluconeogenesis (12).
Various studies suggest that G6PC may have a similar function in rat islets, which contain a non-expressed G6pc2 pseudogene (27). For example, GSIS is impaired and G6PC expression is elevated, relative to controls, in islets isolated from partially pancreatectomized rats (32). Interestingly, the G6P hydrolytic activity in rat islet extracts displays distinct Km, pH dependence, and inhibitor profiles compared with that in rat liver extracts (9). The discovery of G6PC2 appeared to provide an explanation for these kinetic data. However, the subsequent demonstration that G6pc2 is a pseudogene in rats suggests that these kinetic data may instead be explained by the presence of an islet-specific factor that modulates G6PC activity.
Disease Association
The G6PC2 gene has been implicated in the pathophysiology of type 1 diabetes as well as cardiovascular-associated mortality. Thus, several reports have demonstrated that G6PC2 is an important target of cell-mediated autoimmunity in type 1 diabetes in both mice (33) and humans (34). In the nonobese diabetic mouse model of type 1 diabetes, G6PC2 is recognized by both CD8- and CD4-positive T cells infiltrating islets. Whether interventions directed at G6PC2 will be helpful in treating or preventing type 1 diabetes is unclear. In vivo administration of select G6PC2 epitope peptides to nonobese diabetic mice appears to abrogate or delay the disease process (35). However, other studies suggest that autoimmunity toward G6PC2 is a secondary event, with insulin being the primary autoantigen in nonobese diabetic mice (36).
A recent genome-wide association study has strongly linked SNPs in the G6PC2 gene to variations in fasting blood glucose levels in humans (37). This observation is clearly consistent with the observation that G6pc2 null mice have reduced fasting blood glucose levels (30), suggesting that G6PC2 plays a similar role in both mouse and human islets. Interestingly, fasting blood glucose levels are linearly correlated with cardiovascular-associated mortality rather than susceptibility to type 2 diabetes (38). Because fasting blood glucose levels are reduced only ∼15% in G6pc2 null mice, it is surprising that variations in G6PC2 expression or G6PC2 activity would impact cardiovascular-associated mortality, assuming a similar contribution of the G6PC2 gene to the control of fasting blood glucose in humans. However, various studies have shown that even mild variations in fasting glucose levels can have significant biological consequences in humans, specifically on the risk of cardiovascular-associated mortality. For example, in Europeans, an increase in fasting plasma glucose levels from <90 to between 99 and 108 mg/dl is associated with a 30% increased risk of cardiovascular-associated mortality (38). A key question that remains to be addressed by future studies is the identity of the causative SNP linking G6PC2 gene expression to fasting blood glucose levels.
Transcriptional Regulation
Immunohistochemical staining shows that G6PC2 is expressed almost exclusively in pancreatic islet beta cells, with possible expression in a subset of alpha cells (39). A short region of the proximal mouse G6pc2 promoter region extending from −306 to +3 is sufficient to confer islet-specific gene expression in transgenic mice initiating at embryonic day ∼12.5 (39), the same time as the endogenous G6pc2 gene (9). In newborn mouse islets, transgene expression is detected predominantly in beta cells, again like the endogenous G6pc2 gene (39). However, unlike the endogenous G6pc2 gene, transgene expression decreases after birth, indicating that this −306/+3 promoter region is unable to confer transgene expression in adult mice (39). The search for the transcriptional elements that mediate sustained G6pc2 expression in adult animals has led to the identification of multiple enhancers 5′, within, and 3′ of the G6pc2 gene; however, the transcriptional boundaries of the G6pc2 locus remain to be defined (40).
The identity of the transcription factors binding the proximal −306/+3 G6pc2 promoter region has been investigated in detail. Because the sequence of this promoter region is highly conserved in mice and humans (27), an in situ footprinting strategy was used initially to define key transcription factor-binding sites (41). Subsequent studies using chromatin immunoprecipitation assays demonstrated that Pdx-1, Pax-6, MafA, Foxa2, BETA-2, and USF all bind this promoter region in intact βTC-3 cells (42–44). Mutational analyses demonstrated that these factors all contribute to promoter activity (42–44), although the contribution of Pdx-1 varies between different islet-derived cell lines (44). This same group of factors has been shown to regulate, directly or indirectly, insulin gene transcription. Fig. 2 shows a model of the G6pc2 promoter based on the observations described above.
G6PC3
Enzyme Activity and Function
G6PC3 is ∼36% identical at the amino acid level to G6PC (11, 45). Our initial attempts to assess the ability of G6PC3 to hydrolyze G6P involved expressing G6PC3 by transient transfection of COS cells (11, 45). When expressed at levels equivalent to G6PC, G6PC3 did not confer enhanced hydrolysis of G6P in COS cell lysates (11, 45). In contrast, other groups were able to demonstrate G6P hydrolysis by G6PC3 when expressed at much higher levels using a stable transfection strategy (46) or adenoviral infection (47). The explanation as to why transient transfection fails to generate active G6PC3 is unclear. The Km values for G6P hydrolysis by G6PC and G6PC3 are similar (∼2 mm), whereas the Vmax is ∼6-fold higher for G6PC (47).
We have examined the phenotype of G6pc3 null mice to gain insight into the function of G6PC3 in vivo. G6P hydrolytic activity is decreased by ∼50% in homogenates of G6pc3−/− mouse brain and testis relative to wild-type tissue, consistent with the ability of G6PC3 to hydrolyze G6P (48). In addition, female, but not male, G6pc3−/− mice exhibit growth retardation, as do G6pc−/− mice and patients with GSD type 1a (48). However, in contrast to G6pc−/− mice and patients with GSD type 1a, G6pc3−/− mice exhibit no change in hepatic glycogen content or blood glucose or triglyceride levels. Although G6pc3−/− mice are not hypoglycemic, female G6pc3−/− mice have elevated (∼60%) plasma glucagon and reduced (∼20%) plasma cholesterol. We hypothesize that the hyperglucagonemia prevents hypoglycemia and that the hypocholesterolemia is secondary to the hyperglucagonemia (48). As such, the phenotype of G6pc3−/− mice is mild, indicating that G6PC is the major glucose-6-phosphatase of physiological importance for glucose homeostasis in vivo. Cheung et al. (49) reported a similar phenotype in their G6pc3−/− mice but also noted that the absence of G6PC3 led to neutropenia and defects in neutrophil function resulting in increased susceptibility to bacterial infection. Both of these studies failed to provide insight into the role of G6PC3 in the multiple other tissues in which it is expressed, although Chou and co-workers (50) have proposed the interesting hypothesis that the presence of G6PC3 in muscle may explain the improvement in endogenous glucose production and the decrease in susceptibility to hypoglycemia in patients with GSD type 1a after puberty.
Disease Association
A recent genetic analysis has identified mutations in G6PC3 as the cause of a severe congenital neutropenia syndrome associated with cardiac and urogenital malformations (51), consistent with the G6pc3−/− mouse data mentioned above (49).
Transcriptional Regulation
In contrast to the G6PC and G6PC2 promoters, the G6PC3 promoter does not contain a TATA box, and therefore, transcriptional initiation occurs at multiple locations (11, 45). Very little is known about the regulation of G6PC3 transcription. The human G6PC3 promoter region located between −474 and +1, relative to the translation start site, confers fusion gene expression in multiple cell lines (11), but no promoter elements have been functionally defined.
Future Directions
Several key questions remain to be addressed for each member of the G6PC gene family. With respect to G6PC, studies are ongoing to determine whether gene therapy will provide a cure for GSD type 1a (52). Another challenge will be understanding how the actions of the multiple factors that individually regulate G6PC transcription are integrated at the G6PC promoter. With respect to G6PC2, identification of the causative SNPs that explain the connection between variations in G6PC2 activity or G6PC2 expression and fasting blood glucose levels will be important, and ultimately, we would like to know whether inhibition of G6PC2 activity reduces the incidence of cardiovascular-associated mortality in humans. Finally, with respect to G6PC3, it will be of interest to gain further insight into the function of this protein in different tissues in vivo.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health (NIH) Grants DK61645, DK56374, and DK76027 (to R. M. O'B.); by NIH Grant P60 DK20593 (to the Vanderbilt Diabetes Center Core Laboratory); and by a Juvenile Diabetes Research Foundation Autoimmunity Prevention Center grant, NIH Grants DK076027 and DK052068, and the Barbara Davis Center Diabetes and Endocrinology Research Center (NIH Grant P30 DK57516) (to J. C. H.). This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
J. C. Hutton, unpublished data.
- ER
- endoplasmic reticulum
- G6P
- glucose 6-phosphate
- G6PC
- glucose-6-phosphatase catalytic subunit
- IGRP
- islet-specific G6PC-related protein
- GSD
- glycogen storage disease
- HGP
- hepatic glucose production
- GSIS
- glucose-stimulated insulin secretion
- SNP
- single nucleotide polymorphism.
REFERENCES
- 1.Mithieux G. (1997) Eur. J. Endocrinol. 136, 137–145 [DOI] [PubMed] [Google Scholar]
- 2.Foster J. D., Pederson B. A., Nordlie R. C. (1997) Proc. Soc. Exp. Biol. Med. 215, 314–332 [DOI] [PubMed] [Google Scholar]
- 3.Lei K. J., Shelly L. L., Pan C. J., Sidbury J. B., Chou J. Y. (1993) Science 262, 580–583 [DOI] [PubMed] [Google Scholar]
- 4.Shelly L. L., Lei K. J., Pan C. J., Sakata S. F., Ruppert S., Schutz G., Chou J. Y. (1993) J. Biol. Chem. 268, 21482–21485 [PubMed] [Google Scholar]
- 5.Lei K. J., Chen H., Pan C. J., Ward J. M., Mosinger B., Jr., Lee E. J., Westphal H., Mansfield B. C., Chou J. Y. (1996) Nat. Genet. 13, 203–209 [DOI] [PubMed] [Google Scholar]
- 6.van Schaftingen E., Gerin I. (2002) Biochem. J. 362, 513–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S. Y., Pan C. J., Nandigama K., Mansfield B. C., Ambudkar S. V., Chou J. Y. (2008) FASEB J. 22, 2206–2213 [DOI] [PubMed] [Google Scholar]
- 8.van de Werve G., Lange A., Newgard C., Méchin M. C., Li Y., Berteloot A. (2000) Eur. J. Biochem. 267, 1533–1549 [DOI] [PubMed] [Google Scholar]
- 9.Arden S. D., Zahn T., Steegers S., Webb S., Bergman B., O'Brien R. M., Hutton J. C. (1999) Diabetes 48, 531–542 [DOI] [PubMed] [Google Scholar]
- 10.Ebert D. H., Bischof L. J., Streeper R. S., Chapman S. C., Svitek C. A., Goldman J. K., Mathews C. E., Leiter E. H., Hutton J. C., O'Brien R. M. (1999) Diabetes 48, 543–551 [DOI] [PubMed] [Google Scholar]
- 11.Martin C. C., Oeser J. K., Svitek C. A., Hunter S. I., Hutton J. C., O'Brien R. M. (2002) J. Mol. Endocrinol. 29, 205–222 [DOI] [PubMed] [Google Scholar]
- 12.Cherrington A. D. (1999) Diabetes 48, 1198–1214 [DOI] [PubMed] [Google Scholar]
- 13.Pan C. J., Lei K. J., Annabi B., Hemrika W., Chou J. Y. (1998) J. Biol. Chem. 273, 6144–6148 [DOI] [PubMed] [Google Scholar]
- 14.Chou J. Y., Mansfield B. C. (2008) Hum. Mutat. 29, 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien R. M., Streeper R. S., Ayala J. E., Stadelmaier B. T., Hornbuckle L. A. (2001) Biochem. Soc. Trans. 29, 552–558 [DOI] [PubMed] [Google Scholar]
- 16.Efendic S., Karlander S., Vranic M. (1988) J. Clin. Invest. 81, 1953–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massillon D., Chen W., Barzilai N., Prus-Wertheimer D., Hawkins M., Liu R., Taub R., Rossetti L. (1998) J. Biol. Chem. 273, 228–234 [DOI] [PubMed] [Google Scholar]
- 18.Hornbuckle L. A., Everett C. A., Martin C. C., Gustavson S. S., Svitek C. A., Oeser J. K., Neal D. W., Cherrington A. D., O'Brien R. M. (2004) Am. J. Physiol. Endocrinol. Metab. 286, E795–E808 [DOI] [PubMed] [Google Scholar]
- 19.Vander Kooi B. T., Onuma H., Oeser J. K., Svitek C. A., Allen S. R., Vander Kooi C. W., Chazin W. J., O'Brien R. M. (2005) Mol. Endocrinol. 19, 3001–3022 [DOI] [PubMed] [Google Scholar]
- 20.Pedersen K. B., Zhang P., Doumen C., Charbonnet M., Lu D., Newgard C. B., Haycock J. W., Lange A. J., Scott D. K. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E788–E801 [DOI] [PubMed] [Google Scholar]
- 21.Xu C., Chakravarty K., Kong X., Tuy T. T., Arinze I. J., Bone F., Massillon D. (2007) J. Nutr. 137, 554–559 [DOI] [PubMed] [Google Scholar]
- 22.Onuma H., Vander Kooi B. T., Boustead J. N., Oeser J. K., O'Brien R. M. (2006) Mol. Endocrinol. 20, 2831–2847 [DOI] [PubMed] [Google Scholar]
- 23.Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. (2003) Nature 423, 550–555 [DOI] [PubMed] [Google Scholar]
- 24.Schilling M. M., Oeser J. K., Boustead J. N., Flemming B. P., O'Brien R. M. (2006) Nature 443, E10–E11 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J., 3rd, Olefsky J., Guarente L., Montminy M. (2008) Nature 456, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra A. R., Louet J. F., Saha P., An J., Demayo F., Xu J., York B., Karpen S., Finegold M., Moore D., Chan L., Newgard C. B., O'Malley B. W. (2008) Science 322, 1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin C. C., Bischof L. J., Bergman B., Hornbuckle L. A., Hilliker C., Frigeri C., Wahl D., Svitek C. A., Wong R., Goldman J. K., Oeser J. K., Leprêtre F., Froguel P., O'Brien R. M., Hutton J. C. (2001) J. Biol. Chem. 276, 25197–25207 [DOI] [PubMed] [Google Scholar]
- 28.Shieh J. J., Pan C. J., Mansfield B. C., Chou J. Y. (2004) FEBS Lett. 562, 160–164 [DOI] [PubMed] [Google Scholar]
- 29.Petrolonis A. J., Yang Q., Tummino P. J., Fish S. M., Prack A. E., Jain S., Parsons T. F., Li P., Dales N. A., Ge L., Langston S. P., Schuller A. G., An W. F., Tartaglia L. A., Chen H., Hong S. B. (2004) J. Biol. Chem. 279, 13976–13983 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Martin C. C., Oeser J. K., Sarkar S., McGuinness O. P., Hutton J. C., O'Brien R. M. (2007) Diabetologia 50, 774–778 [DOI] [PubMed] [Google Scholar]
- 31.Magnuson M. A., She P., Shiota M. (2003) J. Biol. Chem. 278, 32485–32488 [DOI] [PubMed] [Google Scholar]
- 32.Laybutt D. R., Sharma A., Sgroi D. C., Gaudet J., Bonner-Weir S., Weir G. C. (2002) J. Biol. Chem. 277, 10912–10921 [DOI] [PubMed] [Google Scholar]
- 33.Lieberman S. M., Evans A. M., Han B., Takaki T., Vinnitskaya Y., Caldwell J. A., Serreze D. V., Shabanowitz J., Hunt D. F., Nathenson S. G., Santamaria P., DiLorenzo T. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8384–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J., Danke N. A., Berger D., Reichstetter S., Reijonen H., Greenbaum C., Pihoker C., James E. A., Kwok W. W. (2006) J. Immunol. 176, 2781–2789 [DOI] [PubMed] [Google Scholar]
- 35.Han B., Serra P., Amrani A., Yamanouchi J., Marée A. F., Edelstein-Keshet L., Santamaria P. (2005) Nat. Med. 11, 645–652 [DOI] [PubMed] [Google Scholar]
- 36.Krishnamurthy B., Dudek N. L., McKenzie M. D., Purcell A. W., Brooks A. G., Gellert S., Colman P. G., Harrison L. C., Lew A. M., Thomas H. E., Kay T. W. (2006) J. Clin. Invest. 116, 3258–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouatia-Naji N., Rocheleau G., Van Lommel L., Lemaire K., Schuit F., Cavalcanti-Proença C., Marchand M., Hartikainen A. L., Sovio U., De Graeve F., Rung J., Vaxillaire M., Tichet J., Marre M., Balkau B., Weill J., Elliott P., Jarvelin M. R., Meyre D., Polychronakos C., Dina C., Sladek R., Froguel P. (2008) Science 320, 1085–1088 [DOI] [PubMed] [Google Scholar]
- 38.Khaw K. T., Wareham N., Luben R., Bingham S., Oakes S., Welch A., Day N. (2001) BMJ 322, 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigeri C., Martin C. C., Svitek C. A., Oeser J. K., Hutton J. C., Gannon M., O'Brien R. M. (2004) Diabetes 53, 1754–1764 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Flemming B. P., Martin C. C., Allen S. R., Walters J., Oeser J. K., Hutton J. C., O'Brien R. M. (2008) Diabetes 57, 133–141 [DOI] [PubMed] [Google Scholar]
- 41.Bischof L. J., Martin C. C., Svitek C. A., Stadelmaier B. T., Hornbuckle L. A., Goldman J. K., Oeser J. K., Hutton J. C., O'Brien R. M. (2001) Diabetes 50, 502–514 [DOI] [PubMed] [Google Scholar]
- 42.Martin C. C., Svitek C. A., Oeser J. K., Henderson E., Stein R., O'Brien R. M. (2003) Biochem. J. 371, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin C. C., Oeser J. K., O'Brien R. M. (2004) J. Biol. Chem. 279, 34277–34289 [DOI] [PubMed] [Google Scholar]
- 44.Martin C. C., Flemming B. P., Wang Y., Oeser J. K., O'Brien R. M. (2008) J. Mol. Endocrinol. 41, 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boustead J. N., Martin C. C., Oeser J. K., Svitek C. A., Hunter S. I., Hutton J. C., O'Brien R. M. (2004) J. Mol. Endocrinol. 32, 33–53 [DOI] [PubMed] [Google Scholar]
- 46.Guionie O., Clottes E., Stafford K., Burchell A. (2003) FEBS Lett. 551, 159–164 [DOI] [PubMed] [Google Scholar]
- 47.Shieh J. J., Pan C. J., Mansfield B. C., Chou J. Y. (2003) J. Biol. Chem. 278, 47098–47103 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Oeser J. K., Yang C., Sarkar S., Hackl S. I., Hasty A. H., McGuinness O. P., Paradee W., Hutton J. C., Powell D. R., O'Brien R. M. (2006) J. Biol. Chem. 281, 39982–39989 [DOI] [PubMed] [Google Scholar]
- 49.Cheung Y. Y., Kim S. Y., Yiu W. H., Pan C. J., Jun H. S., Ruef R. A., Lee E. J., Westphal H., Mansfield B. C., Chou J. Y. (2007) J. Clin. Invest. 117, 784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shieh J. J., Pan C. J., Mansfield B. C., Chou J. Y. (2004) J. Biol. Chem. 279, 26215–26219 [DOI] [PubMed] [Google Scholar]
- 51.Boztug K., Appaswamy G., Ashikov A., Schäffer A. A., Salzer U., Diestelhorst J., Germeshausen M., Brandes G., Lee-Gossler J., Noyan F., Gatzke A. K., Minkov M., Greil J., Kratz C., Petropoulou T., Pellier I., Bellanné-Chantelot C., Rezaei N., Mönkemöller K., Irani-Hakimeh N., Bakker H., Gerardy-Schahn R., Zeidler C., Grimbacher B., Welte K., Klein C. (2009) N. Engl. J. Med. 360, 32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou J. Y., Mansfield B. C. (2007) Curr. Gene Ther. 7, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.