Abstract
The transnitrosylating nitric oxide (NO) donor nitrocysteine (CysNO) induced a disulfide bond between the two regulatory RI subunits of protein kinase A (PKA). The conventional NO donor S-nitroso-N-acetylpenicillamine failed to do this, consistent with our observation that it also did not promote protein S-nitrosylation. This disulfide oxidation event activated PKA and induced vasorelaxation independently of the classical β-adrenergic or NO signaling pathway. Activation of PKA had also been anticipated to exert a positive inotropic effect on the myocardium but did not. The lack of positive inotropy was explained by CysNO concomitantly activating protein kinase G (PKG) Iα. PKG was found to exert a partial negative inotropic influence regardless of whether PKA was activated by classical β-receptor stimulation or by disulfide bond formation. This work demonstrates that NO molecules that can induce S-nitrosylation directly activate type I PKA, providing a novel cross-talk to β-adrenergic-like signaling without receptor or adenylate cyclase stimulation. However, the expected positive inotropic consequences of PKA activation by this novel mechanism are countermanded by the simultaneous dual activation of PKGIα, which is also activated by CysNO.
Nitric oxide (NO) initiates cell signaling by binding and activating soluble guanylate cyclase (sGC)2 to produce the second messenger cGMP. cGMP primarily allosterically activates protein kinase G (PKG) but can also regulate other proteins. Although this NO-sGC-cGMP-PKG pathway is well defined (1), a second major mechanism of NO-dependent regulation has subsequently emerged. This involves NO covalently adducting to protein thiols, a process known as S-nitrosylation or S-nitrosation (2).
Significant evidence continues to accumulate supporting protein S-nitrosylation as a fundamental regulator of protein and thus cell function (3). NO is produced in a regulated way (4), with a defined structural basis for selectivity in the proteins it covalently modifies (5, 6). Additional regulatory control can be achieved by the localization of NO synthase enzymes proximal to target proteins (6) and by reverse denitrosylation being enzymatically controlled (7). Indeed, many proteins appear to be basally S-nitrosylated, offering the potential for attenuation (8) as well as potentiation of signaling.
Although stable regulatory S-nitrosylation occurs in some proteins, in others, it serves as an intermediate prior to transition to other redox states, especially disulfides (9). Previously, we searched for proteins that form interprotein disulfides in response to hydrogen peroxide (H2O2), identifying the regulatory RI subunit of protein kinase A (PKA) as such a protein (10, 11). This appears to activate the kinase (11), although the mechanism is not yet precisely defined. There is a rational structural basis for interprotein disulfide formation in PKA RI in response to H2O2. The RI dimer is held together by an N-terminal amphipathic leucine zipper in which the monomers are aligned antiparallel to each other with both Cys17 residues directly facing the corresponding Cys38 residues on the opposite chains (12). H2O2-mediated RI disulfide formation is likely via protein sulfenic acid formation by one thiol in the Cys17 and Cys38 disulfide-forming pair, prior to reduction by the other cysteine to yield the covalently conjugated dimer. Intriguingly, this pair of thiol-disulfide switches in RI is located directly on either side of the protein kinase A anchor protein-binding domain (13). This provides a rational structural basis for the PKA RI-protein kinase A anchor protein interaction being redox-modulated, as the interaction is strongly anticipated to change depending on the oxidation state of the cysteine switches, which flank the interaction locus (11).
We hypothesized that NO may also be able to drive RI disulfide formation via an S-nitrosylated catalytic redox intermediate in a mechanism analogous to transient sulfenation formation during H2O2-induced covalent conjugation. This conceptual link between NO and PKA was investigated by comparing the biochemical and functional responses of cardiovascular tissue to the NO donors S-nitroso-N-acetylpenicillamine (SNAP) and nitrocysteine (CysNO). The authentic NO donor SNAP did not promote RI disulfide formation, whereas CysNO did so efficiently, consistent with its established thiol-oxidizing transnitrosylating ability. We show that disulfide-mediated activation of PKA significantly contributes to vasorelaxation induced by CysNO. However, disulfide activation of PKA failed to exert a positive inotropic influence in isolated hearts exposed to CysNO, which was difficult to reconcile with the kinase being truly activated by oxidation. Further investigations showed that this lack of positive inotropy following CysNO-induced oxidation is explained by the co-activation of PKGIα, which we demonstrated previously can be disulfide-activated (15). PKGIα serves as a master regulator of cardiac inotropy, dominating the system to prevent increases in cardiac contractility. Thus, thiol-oxidizing derivatives of NO can activate PKA and so exert β-adrenergic-like signaling, although dual activation of PKG prevents the anticipated positive inotropy.
EXPERIMENTAL PROCEDURES
Isolated Heart Perfusion Protocols
Isolated rat hearts were stabilized in Langendorff mode for 30 min before being perfused at a constant flow with a selected drug. To maintain the time matching in some perfusion protocols, hearts were stabilized for 20 min and then perfused at a constant flow with the PKG inhibitor KT5823 (10 μm) for 10 min before further treatment. In one series of experiments, after stabilization, hearts were perfused at a constant flow for 5 min with isoprenaline before 5 min of continued treatment either with isoprenaline alone or with both isoprenaline and SNAP. In some experiments, isolated hearts were frozen in liquid nitrogen at the end of the perfusion protocol for protein analysis.
Detection of S-Nitrosylated Proteins
Each heart was prepared by weighing and then homogenizing in 10% (w/v) buffer containing 100 mm Tris (pH 7.4), 0.2 mm neocuproine, 1 mm diethylenetriaminepentaacetic acid, 100 mm maleimide, and protease inhibitors (Roche Applied Science). A variation on the original biotin-switch method was used to determine the extent of protein S-nitrosylation in each heart homogenate. The chelators neocuproine (0.2 mm) and diethylenetriaminepentaacetic acid (1 mm) were used throughout the protocol. Maleimide was removed after 25 min of incubation at 50 °C using protein spin desalting columns (Pierce). S-Nitrosylated proteins were reduced and then labeled using 30 mm ascorbate and 0.1 mm biotin-maleimide. Labeled proteins were then detected by probing samples with horseradish peroxidase-conjugated streptavidin.
N-terminal PKGIα Expression
Primers were designed to subclone the first 100 amino acids of N-terminal wild-type PKGIα into the pMSV vector (kind gift from Dr. Martin Singleton, Cancer Research UK) using a construct for full-length PKGIα (provided by Dr. Darren Browning, Medical College of Georgia, Augusta, GA). The N-terminal 100 amino acids of PKGIα was expressed as a His-tagged fusion protein in Rosetta 2(DE3)pLysS cells and purified using Ni2+-Sepharose following a standard protocol. In experiments to assess disulfide dimerization in response to CysNO, purified N-terminal PKG was reduced by preincubation with 2 mm dithiothreitol for 10 min. This was followed by the addition of either buffer or 1 mm CysNO for 5 min at room temperature before quenching the reaction with SDS sample buffer containing 100 mm maleimide.
Adult Rat Ventricular Myocyte Isolation
Adult rat ventricular myocytes were isolated from male Wistar rat hearts as described previously (14). The isolated adult rat ventricular myocytes were kept in modified Tyrode's buffer for 3 h at room temperature before treatment. To assess kinase disulfide dimerization, cells were treated either with 100 μm SNAP or 100 μm cysteine or with both 100 μm SNAP and 100 μm cysteine for 20 min at room temperature. Reactions were quenched by the addition of sample buffer containing 100 mm maleimide.
Immunoblotting
Phospholamban phosphorylation at Ser16 was detected in hearts perfused with SNAP from reducing SDS-polyacrylamide gels transferred to polyvinylidene difluoride membranes using a standard immunoblotting protocol with an antibody supplied by Dr. William Fuller (University of Dundee). Kinase disulfide dimerization in hearts perfused with CysNO was determined by transferring proteins from nonreducing SDS-polyacrylamide gels to polyvinylidene difluoride membranes and then immunoblotting for PKGIα (E-17, Santa Cruz Biotechnology) or PKA RI (BD Biosciences) using specific antibodies.
Measurement of Tension in Isolated Aorta
Tension in isolated rat aortas was determined using organ chambers and a method that has been described previously (15). In short, vessels were set at a resting tension of 1 g, which was maintained over 1 h before priming twice with 48 mm KCl. The phenylephrine concentration required to obtain 80% constriction was determined by carrying out a dose-response curve on each vessel. The integrity of the endothelium for each vessel was assessed using 10 μm acetylcholine. If the acetylcholine did not generate 50% relaxation after phenylephrine treatment, vessels were discarded. All vessels underwent a relaxation dose-response curve to either SNAP or CysNO before being treated with inhibitors. Vessels were incubated for 30 min in the absence or presence of the sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 5 μm) or the adenylate cyclase inhibitor 2′,5′-dideoxyadenosine (10 μm). In addition, some vessels were incubated with ODQ in combination with either the PKG inhibitor (Rp)-8-bromo-cGMP (100 μm) or the PKA inhibitor (Rp)-8-bromo-cAMP (100 μm) for 30 min. After incubation, vessels were constricted to 80% using phenylephrine and then treated with an increasing dose of either SNAP or CysNO.
RESULTS
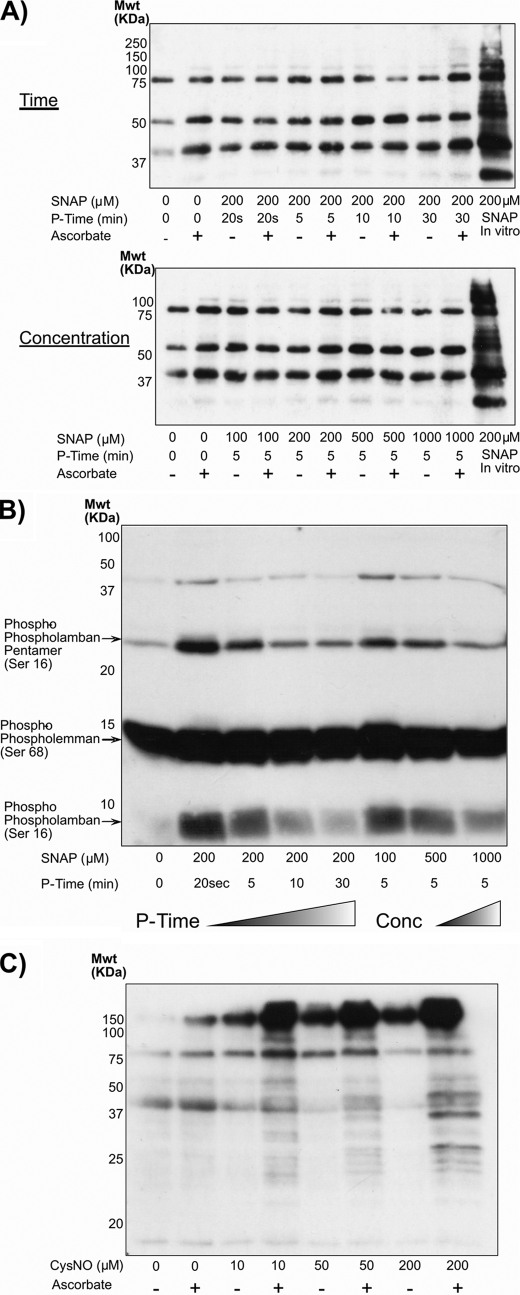
Isolated perfused rat hearts were exposed to the NO donor SNAP and assessed using the biotin-switch method for protein S-nitrosylation. A range of SNAP treatment times (0.5–30 min) were assessed, as was a dose response (Fig. 1A), but no alteration in protein S-nitrosylation was observed. If a cardiac homogenate was directly exposed to SNAP in vitro, marked protein S-nitrosylation resulted, confirming that the biotin-switch method was indeed operational. The lack of SNAP-induced S-nitrosylation may have been explained by its rapid degradation when prepared in the aqueous perfusion buffer. Consequently, the cardiac tissue samples analyzed using the biotin-switch method were also assessed by Western immunoblotting for PKG substrate phosphorylation (Fig. 1B). It was evident that phospholamban became rapidly phosphorylated in hearts exposed to SNAP, showing the NO donor is bioavailable to the tissue and induces phosphosignaling but does not promote S-nitrosylation.
FIGURE 1.
A, isolated perfused rat hearts were exposed to the NO donor SNAP for different durations or concentrations before being assayed for protein S-nitrosylation using the biotin-switch method. All attempts to induce protein S-nitrosylation by administering SNAP to intact hearts failed. However, if a cardiac homogenate was exposed to SNAP in vitro, S-nitrosylation was observed. B, although SNAP failed to induce protein S-nitrosylation in the perfused heart, it did modulate the phosphorylation status of phospholamban in a time- and dose-dependent manner, consistent with activation of the NO-cGMP-PKG pathway and demonstrating that the NO donor was indeed bioavailable to the tissue. C, in marked contrast to SNAP, when the NO donor CysNO was perfused into the heart, it induced a dose-dependent increase in protein S-nitrosylation. Mwt, molecular weight; P-Time, perfusion time.
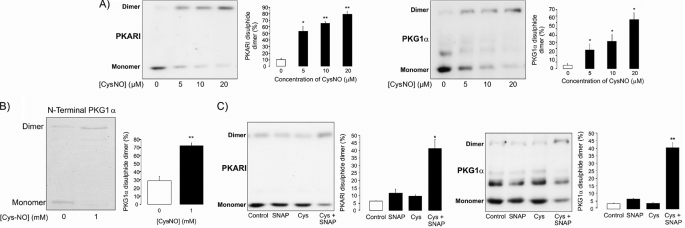
In notable contrast to SNAP, the NO donor CysNO promoted robust protein S-nitrosylation in a dose-responsive manner (Fig. 1C). Consistent with this CysNO-induced protein oxidation by S-nitrosylation, we also observed disulfide oxidation of the redox-modulated kinases PKGIα and PKA RI (Fig. 2A). In contrast, SNAP did not induce the disulfide in either of these kinases. The N terminus of PKGIα was also exposed to CysNO in vitro, causing disulfide formation as shown in the Coomassie-stained gel (Fig. 2B). This illustrates that CysNO itself has the potential to drive directly disulfide formation (via transient S-nitrosylation) without a requirement for other components and so could also be the mechanism that operates in cells. Consistent with the lack of SNAP-induced protein S-nitrosylation or kinase disulfide bond formation in isolated perfused hearts, this NO donor also failed to induce these oxidation events in a primary culture of isolated ventricular myocytes (Fig. 2C). However, when the myocytes were exposed to SNAP in combination with cysteine, this efficiently induced disulfides in both PKA and PKG. In control experiments in which myocytes were exposed to cysteine alone, there was no disulfide formation (Fig. 2C).
FIGURE 2.
A, consistent with CysNO inducing widespread S-nitrosylation, this treatment also caused disulfide oxidation of the redox-modulated kinases PKGIα and PKA RI. B, CysNO also efficiently induced disulfide formation in recombinant N-terminal PKGIα, demonstrating that this oxidation state can result as a direct reaction of the oxidizing NO donor and the kinase. This supports the possibility that CysNO may directly S-nitrosylate the kinases in vivo prior to self-reduction and consequential disulfide bond formation. C, by themselves, SNAP and cysteine failed to induce PKGIα or PKA RI interprotein disulfide bond formation. However, together, they efficiently induced disulfides into both kinases, consistent with SNAP S-nitrosylating cysteine extracellularly before transport into the cell, where it modifies proteins.
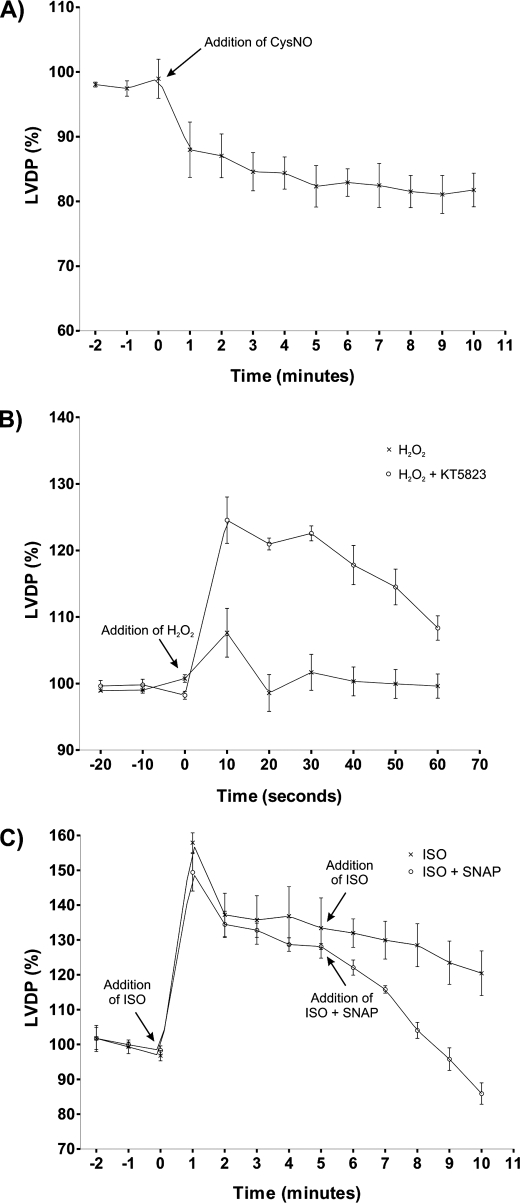
We monitored the contractile performance of isolated hearts exposed to CysNO, a treatment that generates the disulfide oxidation state in PKA and PKG (Fig. 3A). The left ventricular developed pressure (LVDP) was rapidly depressed by 16.6 ± 2.4% upon exposure to the oxidizing NO donor. This was surprising because we had anticipated a positive inotropic response, as we equate disulfide PKA RI with activation of this kinase, which couples to increased cardiac output. As PKG is capable of exerting partial negative inotropic effects (16–18), we hypothesized that its concurrent activation might override the influence of PKA activation and explain why LVDP was not increased. To examine this, we promoted disulfides in PKA and PKG by H2O2 treatment, choosing this as a “cleaner” intervention than CysNO, which is more complex, as it simultaneously triggers the classical NO-sGC-cGMP-PKG pathway. Again, H2O2 treatment did not elevate LVDP, but this again was hypothesized to be a result of simultaneous PKG activation, possibly countermanding the PKA activation. To test this idea, we compared the LVDP response to H2O2 in the presence and absence of the PKG inhibitor KT5823. Indeed, inhibition of PKG activity during H2O2 treatment led to a positive inotropic LVDP increase (Fig. 3B). To further assess the ability of PKG to dominate PKA and prevent positive inotropy, we exposed hearts to isoprenaline with or without co-treatment with SNAP. Isoprenaline induced a marked increase in LVDP as expected, but the co-administration of SNAP rapidly exerted a negative inotropic effect, lowering contractile performance below basal levels (Fig. 3C). This depression of LVDP below basal levels is consistent with the NO donor assessed in this model, namely CysNO (Fig. 3A).
FIGURE 3.
A, the LVDP of isolated rat hearts was rapidly depressed by 16. 6 ± 2.4% when exposed to CysNO. A positive inotropic response had been anticipated, as we equate disulfide PKA RI with activation of this kinase, which should increase cardiac work. We hypothesized that the negative inotropy was due to the simultaneous activation of PKG by CysNO, which activates this kinase classically by its NO donor capability and also by disulfide oxidation. B, to avoid the additional complexity of CysNO being an NO donor as well as a disulfide inducer, LVDP was monitored in hearts exposed to H2O2 with or without co-treatment with the PKG inhibitor KT5823. H2O2 induced disulfide bond activation of PKGIα and PKA RI without stimulating the NO-sGC pathway. By inhibiting PKG during the H2O2 treatment, an underlying activation of PKA by disulfide bond formation was unveiled, resulting in the observed positive inotropy. C, to examine the possibility that PKG dominates in the face of PKA activation and prevents positive inotropy, we exposed hearts to isoprenaline (ISO) with or without co-treatment with SNAP. Isoprenaline increased LVDP as expected, whereas the co-administration of SNAP rapidly exerted a negative inotropic effect.
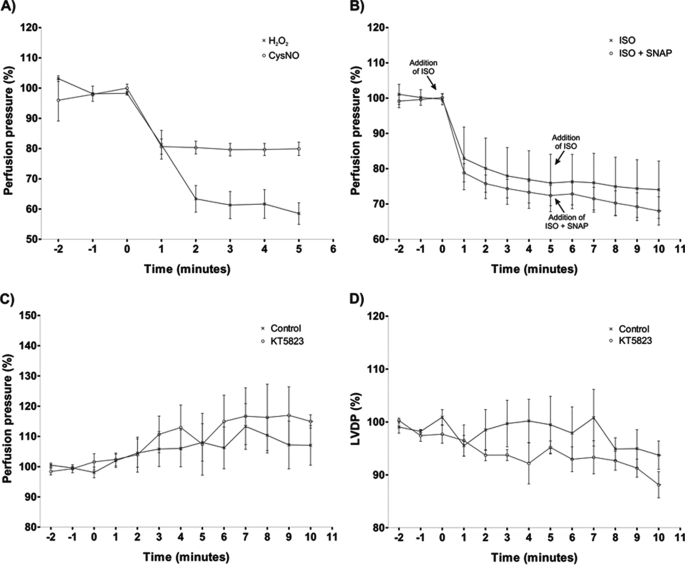
In isolated hearts, the LVDP contractile response to CysNO and H2O2 is complicated by their also promoting coronary vasorelaxation. A reduction in coronary flow may depress contractility via a hydraulic mechanism that is independent of kinase activation (19). To assess this, we examined the influence of CysNO and H2O2 on coronary perfusion pressure (Fig. 4A). Despite both agents lowering perfusion pressure as anticipated, it is evident from Fig. 3 (A and B) that only CysNO reduced LVDP, whereas H2O2 did not affect it. This is despite H2O2 inducing essentially double the vasodilation of CysNO. To further address this issue, we monitored perfusion pressure in hearts treated with isoprenaline alone or with isoprenaline and then both isoprenaline and SNAP. It is clear that isoprenaline had a positive inotropic action (Fig. 3A), and this actually occurred at a time when perfusion pressure was reduced (Fig. 4B). When SNAP was co-administered in the presence of isoprenaline, it depressed LVDP compared with the isoprenaline-alone control. This depression in LVDP again was not explained by the lowering of perfusion pressure, as this was already markedly reduced and was unaffected by co-treatment with SNAP. This is consistent with activation of the PKG pathway exerting a negative inotropic effect that can override the otherwise positive influence of the PKA pathway.
FIGURE 4.
A, both CysNO and H2O2 rapidly lowered the perfusion pressure in rat coronary vessels, with the former exerting a more marked vasodilatory response. B, isoprenaline rapidly reduced coronary perfusion pressure. When SNAP was then administered simultaneously with isoprenaline (ISO), perfusion pressure remained depressed and similar to that observed with isoprenaline alone. This lack of effect of SNAP on perfusion pressure was in marked contrast to its negative influence on LVDP (Fig. 3C). C, aerobically perfused isolated hearts exposed to the PKG inhibitor KT5823 responded with a marginal time-dependent increase in perfusion pressure, which may be consistent with a limited contribution of this kinase to coronary tone under basal conditions. D, during the treatment of the aerobic heart with the KT5823 inhibitor, there was a small time-dependent depression in LVDP contractility.
The intricate interrelationship of perfusion pressure and contractility in the isolated hearts also complicates our observations with the PKG inhibitor KT5823. The ability of KT5823 to induce a positive inotropic response when co-administered in the presence of H2O2 (Fig. 3B) could be due to inhibition of a basal unstimulated PKG activity that couples to a partial negative inotropy. When we aerobically perfused isolated hearts with KT5823, there was indeed a marginal increase in perfusion pressure (Fig. 4C). However, at that time, there was a corresponding minor decrease in LVDP (Fig. 4D) and certainly not an increase, which may be anticipated with an elevation in perfusion pressure.
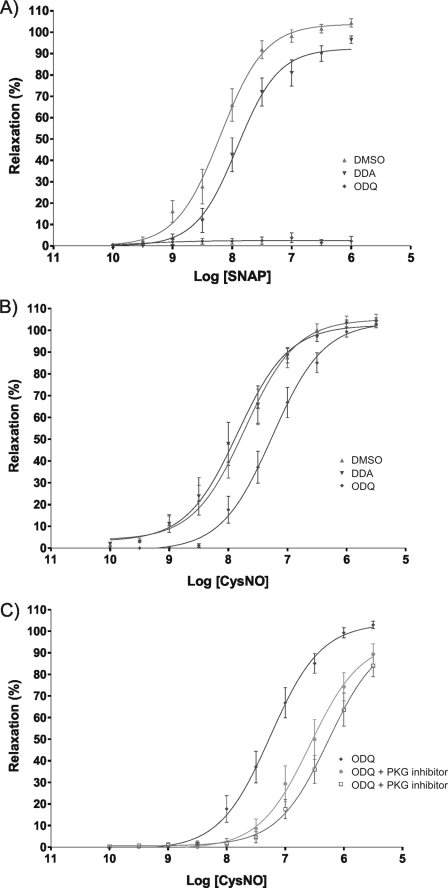
Regulation of vasotone is predominantly associated by many with activation of PKG, primarily through the NO-sGC-cGMP pathway. Previously, we have shown that disulfide activation of PKG can also promote vasorelaxation and that this is independent of elevations in cGMP (15). In addition, although perhaps less well appreciated, classical cAMP-dependent PKA activation also couples to vasorelaxation. Thus, PKA activation involving RI disulfide oxidation may also contribute to the vasorelaxive responses to oxidants. To examine this, we compared the vasorelaxive properties of SNAP and the thiol-oxidizing CysNO donor in isolated vascular rings from rat thoracic aorta. SNAP elicited a classical dose-response vasorelaxation curve, which was significantly inhibited (rightward shift) by the adenylate cyclase inhibitor 2′,5′-dideoxyadenosine (Fig. 5A). These SNAP-induced relaxations were fully prevented by the sGC inhibitor ODQ. In contrast, CysNO relaxations were not modulated by 2′,5′-dideoxyadenosine at all, and only ODQ had a partial inhibitory effect (Fig. 5B). In essence, CysNO induced vasorelaxation independently of the classical NO-sGC signaling paradigm. As CysNO induces disulfide activations of PKA RI and also PKG and as both kinases signal to vasorelaxation, we sought to determine the relative contribution of each to this phenomenon. Thus, CysNO-induced relaxations of rat aorta were compared in the presence and absence of either a PKA or PKG inhibitor. The dose response to CysNO was significantly rightward-shifted by inhibitors of both kinases, but more so by the PKA antagonist (Rp)-8-bromo-cAMP.
FIGURE 5.
A, the vasorelaxive properties of SNAP and the thiol-oxidizing CysNO donor were compared in isolated vascular rings from rat thoracic aorta. SNAP elicited a classical dose-response vasorelaxation curve, which was significantly inhibited (rightward shift) by the adenylate cyclase inhibitor 2′,5′-dideoxyadenosine (DDA). The SNAP-induced relaxations were entirely prevented by the sGC inhibitor ODQ. DMSO, dimethyl sulfoxide. B, CysNO also induced vasorelaxation, albeit this was only partially sensitive to ODQ. Thus, CysNO can induce vasorelaxation independently of the classical NO-sGC signaling paradigm, consistent with it directly activating PKA and PKG by disulfide oxidation. C, CysNO-induced relaxations of rat aorta were examined in the presence and absence of either a PKA or PKG inhibitor. The dose response to CysNO was significantly rightward-shifted by inhibitors of either kinase, but more so by the PKA antagonist (Rp)-8-bromo-cAMP. Overall, CysNO induced vasorelaxation independently of sGC, involving the activation of both PKA and PKG by interprotein disulfide bond formation.
DISCUSSION
Evidence supporting protein S-nitrosylation as a fundamental regulator of proteins and cell function continues to emerge (3). Many proteins are controlled by stable S-nitrosylation, which can be induced and reversed in a regulated way by NO synthase and thioredoxin enzyme systems, respectively (7). The regulatory RI subunit of PKA exists as a homodimer, with two pairs of precisely aligned cysteine thiols that allow two interchain disulfides to form under pro-oxidizing conditions (11). PKGIα is also a homodimer, with the Cys42 residues from each of the polypeptides correctly aligned with each other to form a disulfide (20), which they do when exposed to oxidants such as H2O2 (15).
We reasoned that NO may promote interchain disulfides in each of these kinases in a scenario whereby S-nitrosylation occurs at one of the redox-active thiols before reduction by the adjacent thiol on the other polypeptide. SNAP completely failed to induce disulfide formation in PKA or PKG, whereas CysNO did so very efficiently in a dose-dependent manner. This is consistent with the observations of others, who found that donors that release authentic NO did not promote protein S-nitrosylation (21, 22). In contrast, the transnitrosylating molecule CysNO promoted the modification effectively. SNAP delivers NO into the cell after breaking down extracellularly and then diffusing inward, after which it is rapidly sequestered by protein heme groups, preventing widespread adduction to thiols. Heme is crucial for NO binding to and activating cGMP production by sGC, an event that clearly happens in SNAP-perfused hearts, as evidenced by phosphorylation of downstream targets of PKG. Unlike SNAP, CysNO can be transported into the cell by the amino acid transporters (22), subsequently directly transferring its NO moiety to induce efficient S-nitrosylation (21). The transport process and the transnitrosylating chemistry are dual requisites for S-nitrosylation, as nitrosoglutathione (which is not transported) poorly nitrosylates intracellular proteins (22). The crucial role of transport into the cell is supported by the observation that SNAP treatment can induce a disulfide in PKA and PKG when carried out in the presence of cysteine, consistent with the extracellular formation of the nitrosothiol before subsequent inward transport. Of course, when CysNO enters the cell, it is exposed to a complex pro-reducing redox environment, which provides potential for undefined secondary reactions involving undefined chemistry. However, we found that CysNO is capable of directly inducing a disulfide in recombinant PKGIα in vitro, supporting the prospect of these direct reaction events in cells.
Previous work from our laboratory demonstrated that when PKA or PKG forms an interprotein disulfide in response to oxidant stress, this activates the kinases (11, 15). These disulfide oxidations enable activation of kinases independently of cellular elevations in their respective cyclic nucleotides, leading to phosphorylation of their substrates and replication at least in part of some of the hallmarks of the classical activation of these kinases. Thus, activation of PKG couples vasorelaxation either by the conventional NO-sGC-cGMP pathway or by direct disulfide activation of the kinase. Although we found that PKG can be directly activated by oxidation in a cGMP-independent manner, we did not definitively show the same for PKA. Type I PKA is sensitized to a fixed cAMP concentration by an increased concentration of the substrates it phosphorylates (23), a phenomenon not featured by type II PKA (24). As disulfide oxidation of RI translocates from the cytosol to locations enriched with PKA substrates (membranes and myofilaments) (11), it is possible that this relocation process engages this kinase sensitization process. This may mean that disulfide activation of PKA is still dependent on its cyclic nucleotide second messenger, which is not the case for PKG.
The direct disulfide-mediated activation of PKA without elevations in cAMP was anticipated to also contribute to oxidant-induced vasorelaxation. This was reasoned on the basis that classical activation of PKA by cAMP contributes to vasorelaxation in response to prostanoids or β-receptor stimulation (25). Consistent with these ideas, we found that CysNO treatment induced vasorelaxation of rat thoracic aorta that was sensitive to pharmacological inhibition of either PKA or PKG. Thus, disulfide activation of both kinases contributes to the resulting vasorelaxation following treatment with thiol-oxidizing forms of NO. These observations are consistent with the CysNO vasorelaxation being only partially inhibited by the sGC inhibitor ODQ and not by adenylate cyclase inhibition by 2′,5′-dideoxyadenosine. This CysNO-induced vasorelaxation is independent of elevations in cyclic nucleotides, as also observed with H2O2-induced activation of these kinases (11, 15). CysNO vasorelaxation is expected to be only partially ODQ-sensitive, as this NO donor also oxidatively activates PKA and PKG as well as releases NO to initiate the classical sGC-cGMP pathway. The relative insensitivity of CysNO vasorelaxation to ODQ is in striking contrast to that induced by SNAP, which is fully blocked by sGC inhibition. This conspicuous difference between the mechanisms of action of these two NO donors reflects their differing chemical properties outlined above. Thus, SNAP and CysNO both activate sGC, but only the latter directly oxidizes the kinases to yield disulfide bond formation and activation. Thus, NO (in a form that induces thiol oxidation) can activate PKA to yield vasorelaxation, events normally triggered by β-adrenergic signaling.
If thiol-oxidizing NO donors truly mimic the events of β-adrenergic signaling by direct activation of type I PKA, a rational expectation is that they should exert a positive inotropic effect on the heart. This was not the case, as CysNO was in fact partially negatively inotropic, attenuating the LVDP contractile response. Similarly, H2O2 also failed to enhance LVDP, despite both interventions inducing RI disulfide formation, an oxidative modification that we advocate activates PKA. This observation confounds our hypothesis regarding PKA activation via RI disulfide formation, as enhanced contractility is essentially a gold standard marker for activation of this kinase. However, a review of the literature highlighted the potential for PKG activation to exert a negative inotropic influence (17, 18), capable of overriding the otherwise positive effects of PKA (16). Consequently, we hypothesized that the failure of RI-oxidizing compounds (CysNO and H2O2) to be positively inotropic may be because they also simultaneously co-activate PKGIα. To assess this, we monitored hearts before and after H2O2 treatment and found that LVDP did not change. However, when H2O2 treatment was repeated in the presence of a PKG inhibitor, we found that it was positively inotropic. Furthermore, the positive inotropy induced by the β-agonist isoprenaline was markedly attenuated when an NO donor was co-administered to an isolated heart. Even in the presence of robust PKA stimulation by isoprenaline-induced cAMP production, NO reduced the contractility below basal levels. The presence of NO donors (either CysNO or SNAP) reduced contractility by ∼17% of basal levels regardless of the activation state of PKA (i.e. with or without the presence of isoprenaline). This suggests that PKG controls a “master switch” governing excitation-contraction coupling, overriding the phosphorylation events induced by PKA. It is interesting to speculate on the molecular identity of this switch, which could be the L-type Ca2+ channel. Recently, PKGIα, the redox-active isoform investigated here, was shown to phosphorylate Cav1.2α1c and β2 subunits of the L-type Ca2+ channel to negatively regulate it (26). This would reduce trigger calcium and likely explain the negative inotropic influence of activating PKG via the NO-sGC-cGMP pathway or by disulfide oxidation.
There is a complex relationship between contractile function and coronary perfusion pressure in the myocardium. This complicates the mechanistic interpretation of drugs such as H2O2 and CysNO, which modulate both contractility and vasotone. Increased coronary perfusion pressure may increase contractility as a result of the Gregg phenomenon (19). When the coronary pressure in isolated hearts becomes elevated, there can be a resulting increase in contractility caused by a hydraulic mechanism, often referred to as the garden hose effect (19). Thus, the reduction in contractility upon CysNO treatment may be simply due to these phenomena, meaning that the reduced contractility with CysNO may be simply a physical hydraulic consequence of lowering the tone of the coronary vessels and independent of kinase activation in the ventricular myocytes. Indeed, when we perfused hearts with CysNO, we observed vasorelaxation, and this correlated with a depression in LVDP. However, when we perfused hearts with H2O2, we also observed relaxation, but this was notably without any alteration in LVDP. Thus, the depression of LVDP by CysNO is consistent with PKG activation as suggested and not simply a physical consequence of lowering coronary tone.
Further insight into this issue was provided by hearts treated with isoprenaline alone or with isoprenaline followed by isoprenaline and SNAP. Isoprenaline promoted a strong positive inotropic action and a marked vasodilation. These PKA-mediated events clearly override the Gregg phenomenon/garden hose effect (19). Furthermore, when SNAP and isoprenaline were given together, LVDP was depressed compared with the isoprenaline-alone control. Again, this cannot be explained simply by physical consequences of reducing perfusion pressure, as this was already markedly reduced by the preceding isoprenaline treatment and was unaffected when SNAP was administered. Overall, it is clear that PKA activation (with isoprenaline) exerts positive inotropic effects. However, when the NO-sGC-PKG pathway was stimulated with SNAP, there was an overriding negative inotropy. It is evident that these contractile responses cannot be explained by the Gregg phenomenon/garden hose effect (19) but are consistent with our contention that classical or oxidative disulfide activation of PKG overrides the otherwise positive inotropy induced by PKA.
Previous studies have investigated the influence of NO and related molecules on sGC-independent modulation of contractility. For example, Paolocci et al. (27) found that SIN-1 and peroxynitrite (OONO−) promoted positive inotropy independently of cGMP production. Although these agents may have exerted their actions via S-nitrosylation reactions, this oxidative modification was not measured. SIN-1 is a dual NO and superoxide donor that efficiently yields OONO−, which nitrates proteins at tyrosine residues and promotes alternate thiol oxidation states such as S-glutathiolation (28). Such modifications may be more likely to occur than S-nitrosylation in tissue exposed to OONO− and so may underlie the functional responses.
This complexity of mixed redox state, along with the concomitant classical NO-sGC-cGMP signaling depending on the precise NO donor/oxidant utilized, may help explain differences between studies. For example, Chesnais et al. also observed a positive inotropy following OONO− treatment (29), whereas in other studies, they found that SIN-1 (which can also yield OONO−) along with the NO donors sodium nitroprusside and SNAP exerted a negative inotropy effect (30). An additional complexity of the effects of OONO− on contractility results from its interfering with β-adrenergic signaling, attenuating isoprenaline-induced phosphorylation of phospholamban (31). Precisely how OONO− interferes with PKA-mediated phosphorylations at a molecular level is unclear, but a variety of thiol- and tyrosine-targeted modifications may be responsible. With regard to protein S-nitrosylation, several muscle proteins that are integral to regulated contractile function are modulated in this way, including the ryanodine receptor (32), potassium channels (33), the L-type calcium channel, and SERCA (34).
Oxidative and nitrosative stress is associated with multiple cardiovascular diseases, and it is interesting to speculate that disulfide modifications of PKA and PKG may participate in the etiology of these. Thus, these events may contribute to injury in which loss of redox homeostasis occurs, such as ischemia and reperfusion injury, sepsis, and heart failure. In addition, it remains possible that these disulfide-mediated events that are driven by thiol-oxidizing forms of NO contribute to homeostatic regulation of function during health. Finally, it is possible that drugs that regulate cardiovascular function, such as glycerol trinitrate and nitroxyl, exert some of their effects via kinase disulfide formation, consistent with evidence suggesting that they can oxidize thiols (35, 36), although the enhanced cardiac contractility resulting from nitroxyl treatment is independent of PKA and PKG activity (37). Further work is required to assess the role of kinase disulfide activation in cardiovascular health and disease.
Overall, we conclude that thiol-oxidizing NO molecules promote β-adrenergic-like signaling by promoting intermolecular disulfide bonds in type I PKA. Although this new NO-PKA pathway couples to vasorelaxation, the influence on cardiac contractility is complex, as PKGIα is concurrently activated, and this overrides the anticipated positive inotropy.
This work was supported by a Sir Henry Wellcome postdoctoral fellowship from The Wellcome Trust (Sponsor Reference 085483/Z/08/Z; to J. R. B.) and the Medical Research Council (Sponsor Reference G0700320).
- sGC
- soluble guanylate cyclase
- PKG
- protein kinase G
- PKA
- protein kinase A
- SNAP
- S-nitroso-N-acetylpenicillamine
- CysNO
- nitrocysteine
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- LVDP
- left ventricular developed pressure.
REFERENCES
- 1.Moncada S., Higgs E. A. (2006) Br. J. Pharmacol. 147, Suppl. 1, S193–S201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamler J. S., Lamas S., Fang F. C. (2001) Cell 106, 675–683 [DOI] [PubMed] [Google Scholar]
- 3.Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S. (2005) Nat. Rev. Mol. Cell Biol. 6, 150–166 [DOI] [PubMed] [Google Scholar]
- 4.Griffith O. W., Stuehr D. J. (1995) Annu. Rev. Physiol. 57, 707–736 [DOI] [PubMed] [Google Scholar]
- 5.Hess D. T., Matsumoto A., Nudelman R., Stamler J. S. (2001) Nat. Cell Biol. 3, E46–E49 [DOI] [PubMed] [Google Scholar]
- 6.Greco T. M., Hodara R., Parastatidis I., Heijnen H. F., Dennehy M. K., Liebler D. C., Ischiropoulos H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7420–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benhar M., Forrester M. T., Hess D. T., Stamler J. S. (2008) Science 320, 1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gow A. J., Chen Q., Hess D. T., Day B. J., Ischiropoulos H., Stamler J. S. (2002) J. Biol. Chem. 277, 9637–9640 [DOI] [PubMed] [Google Scholar]
- 9.Eaton P. (2006) Free Radic. Biol. Med. 40, 1889–1899 [DOI] [PubMed] [Google Scholar]
- 10.Brennan J. P., Wait R., Begum S., Bell J. R., Dunn M. J., Eaton P. (2004) J. Biol. Chem. 279, 41352–41360 [DOI] [PubMed] [Google Scholar]
- 11.Brennan J. P., Bardswell S. C., Burgoyne J. R., Fuller W., Schröder E., Wait R., Begum S., Kentish J. C., Eaton P. (2006) J. Biol. Chem. 281, 21827–21836 [DOI] [PubMed] [Google Scholar]
- 12.Kinderman F. S., Kim C., von Daake S., Ma Y., Pham B. Q., Spraggon G., Xuong N. H., Jennings P. A., Taylor S. S. (2006) Mol. Cell 24, 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banky P., Huang L. J., Taylor S. S. (1998) J. Biol. Chem. 273, 35048–35055 [DOI] [PubMed] [Google Scholar]
- 14.Brennan J. P., Miller J. I., Fuller W., Wait R., Begum S., Dunn M. J., Eaton P. (2006) Mol. Cell. Proteomics 5, 215–225 [DOI] [PubMed] [Google Scholar]
- 15.Burgoyne J. R., Madhani M., Cuello F., Charles R. L., Brennan J. P., Schröder E., Browning D. D., Eaton P. (2007) Science 317, 1393–1397 [DOI] [PubMed] [Google Scholar]
- 16.Ebihara Y., Karmazyn M. (1996) Cardiovasc. Res. 32, 622–629 [PubMed] [Google Scholar]
- 17.Angelone T., Filice E., Quintieri A. M., Imbrogno S., Recchia A., Pulera E., Mannarino C., Pellegrino D., Cerra M. C. (2008) Acta Physiol. 193, 229–239 [DOI] [PubMed] [Google Scholar]
- 18.Sandirasegarane L., Diamond J. (1999) J. Mol. Cell. Cardiol. 31, 799–808 [DOI] [PubMed] [Google Scholar]
- 19.Schipke J. D., Frehen D. (2001) Z. Kardiol. 90, 319–326 [DOI] [PubMed] [Google Scholar]
- 20.Schnell J. R., Zhou G. P., Zweckstetter M., Rigby A. C., Chou J. J. (2005) Protein Sci. 14, 2421–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Hogg N. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7891–7896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Hogg N. (2005) Free Radic. Biol. Med. 38, 831–838 [DOI] [PubMed] [Google Scholar]
- 23.Vigil D., Blumenthal D. K., Brown S., Taylor S. S., Trewhella J. (2004) Biochemistry 43, 5629–5636 [DOI] [PubMed] [Google Scholar]
- 24.Viste K., Kopperud R. K., Christensen A. E., Døskeland S. O. (2005) J. Biol. Chem. 280, 13279–13284 [DOI] [PubMed] [Google Scholar]
- 25.Wright D. H., Abran D., Bhattacharya M., Hou X., Bernier S. G., Bouayad A., Fouron J. C., Vazquez-Tello A., Beauchamp M. H., Clyman R. I., Peri K., Varma D. R., Chemtob S. (2001) Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1343–R1360 [DOI] [PubMed] [Google Scholar]
- 26.Yang L., Liu G., Zakharov S. I., Bellinger A. M., Mongillo M., Marx S. O. (2007) Circ. Res. 101, 465–474 [DOI] [PubMed] [Google Scholar]
- 27.Paolocci N., Ekelund U. E., Isoda T., Ozaki M., Vandegaer K., Georgakopoulos D., Harrison R. W., Kass D. A., Hare J. M. (2000) Am. J. Physiol. Heart Circ. Physiol. 279, H1982–H1988 [DOI] [PubMed] [Google Scholar]
- 28.Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schöneich C., Cohen R. A. (2004) Nat. Med. 10, 1200–1207 [DOI] [PubMed] [Google Scholar]
- 29.Chesnais J. M., Fischmeister R., Mery P. F. (1999) J. Physiol. 521, 375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesnais J. M., Fischmeister R., Méry P. F. (1999) J. Physiol. 518, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohr M. J., Wang H., Wheeler D. G., Velayutham M., Zweier J. L., Ziolo M. T. (2008) Cardiovasc. Res. 77, 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L., Eu J. P., Meissner G., Stamler J. S. (1998) Science 279, 234–237 [DOI] [PubMed] [Google Scholar]
- 33.Asada K., Kurokawa J., Furukawa T. (2009) J. Biol. Chem. 284, 6014–6020 [DOI] [PubMed] [Google Scholar]
- 34.Sun J., Morgan M., Shen R. F., Steenbergen C., Murphy E. (2007) Circ. Res. 101, 1155–1163 [DOI] [PubMed] [Google Scholar]
- 35.Wheatley R. M., Dockery S. P., Kurz M. A., Sayegh H. S., Harrison D. G. (1994) Am. J. Physiol. Heart Circ. Physiol. 266, H291–H297 [DOI] [PubMed] [Google Scholar]
- 36.Paolocci N., Jackson M. I., Lopez B. E., Miranda K., Tocchetti C. G., Wink D. A., Hobbs A. J., Fukuto J. M. (2007) Pharmacol. Ther. 113, 442–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tocchetti C. G., Wang W., Froehlich J. P., Huke S., Aon M. A., Wilson G. M., Di Benedetto G., O'Rourke B., Gao W. D., Wink D. A., Toscano J. P., Zaccolo M., Bers D. M., Valdivia H. H., Cheng H., Kass D. A., Paolocci N. (2007) Circ. Res. 100, 96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]