Abstract
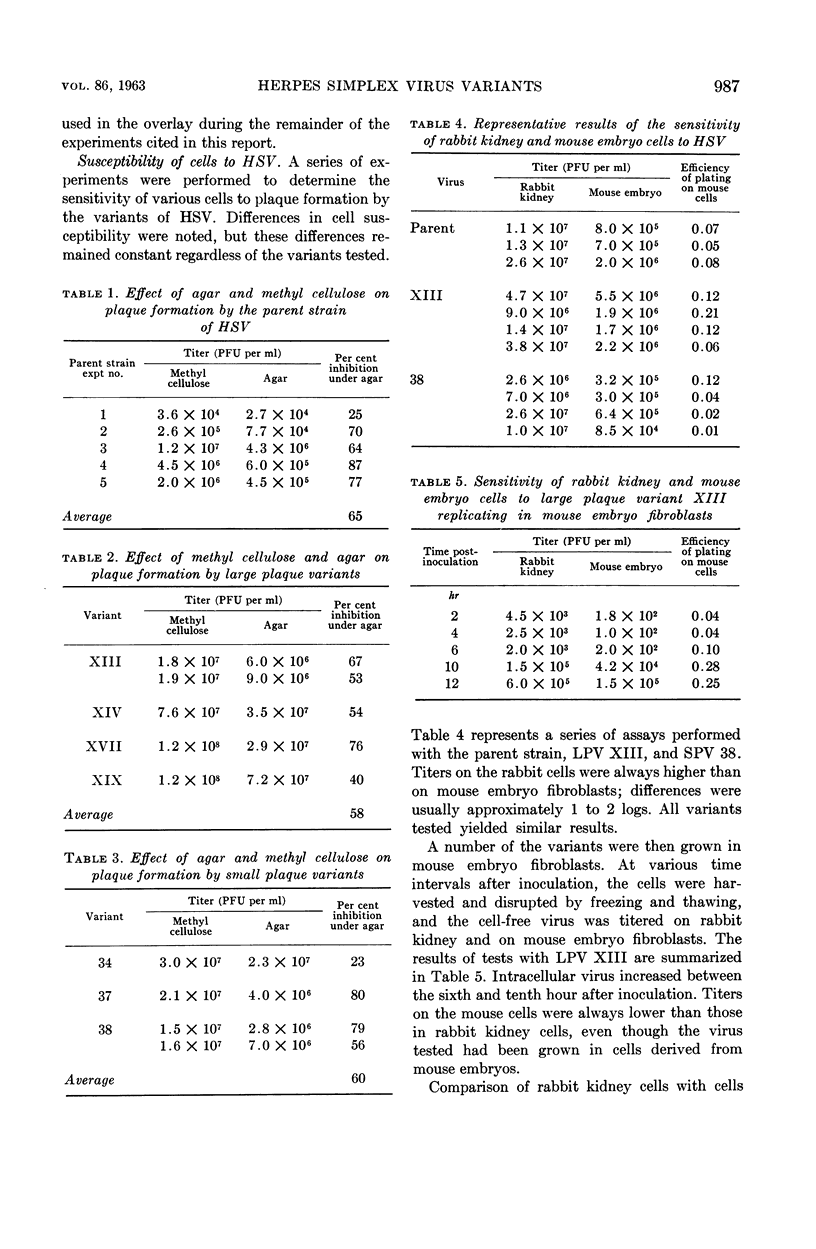
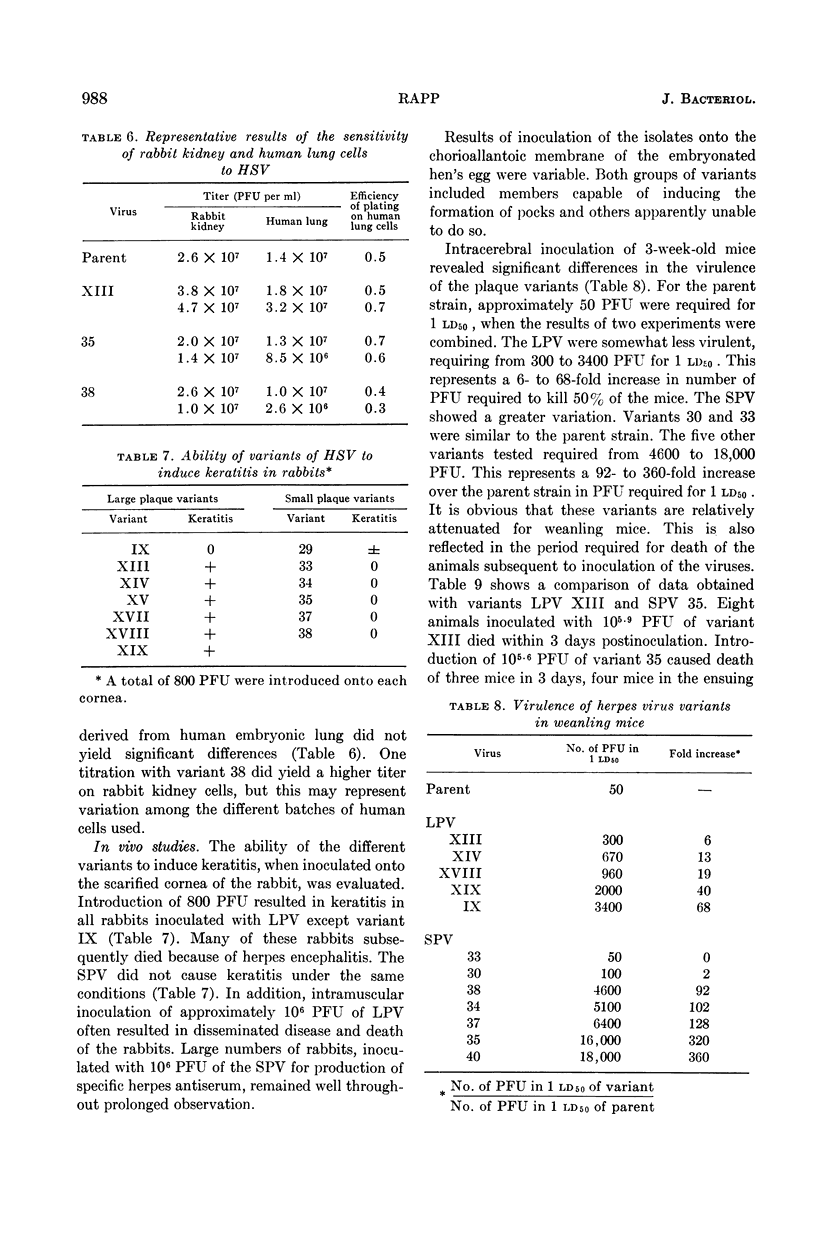
Rapp, Fred (Baylor University College of Medicine, Houston, Tex.). Variants of herpes simplex virus: isolation, characterization, and factors influencing plaque formation. J. Bacteriol. 86:985–991. 1963.—Variants of herpes simplex virus were isolated which differed in plaque size and in virulence for rabbits and mice. Keratitis on the rabbit cornea and generalized disease were associated with the large, but not the small, plaque variants. The large plaque variants were about as neurovirulent as was the parent strain for weanling mice, but the majority of the small plaque variants were only 1% as virulent for mice as was the parent virus. Agar inhibited the formation of plaques by the parent strain and all variants tested; development of plaques was retarded and the number of plaques was approximately 60% less than those developing under an overlay in which methyl cellulose had been substituted for the agar. Rabbit kidney cells and human embryonic lung fibroblasts were more sensitive than mouse embryo fibrolasts to infection with all variants tested.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHU L. W., WARREN G. H. Pathogenicity and immunogenicity of herpes simplex virus strains propagated in rabbit kidney tissue. Proc Soc Exp Biol Med. 1960 Nov;105:396–399. doi: 10.3181/00379727-105-26122. [DOI] [PubMed] [Google Scholar]
- HINZE H. C., WALKER D. L. Variation of herpes simplex virus in persistently infected tissue cultures. J Bacteriol. 1961 Oct;82:498–504. doi: 10.1128/jb.82.4.498-504.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B., ROANE P. R., Jr Further studies of variants of herpes simplex virus that produce syncytia or pocklike lesions in cell cultures. Am J Hyg. 1961 Jan;73:114–122. doi: 10.1093/oxfordjournals.aje.a120162. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. E. Use of methyl cellulose gel as a substitute for agar in tissue-culture overlays. Nature. 1955 Feb 19;175(4451):352–352. doi: 10.1038/175352a0. [DOI] [PubMed] [Google Scholar]
- JAWETZ E., COLEMAN V. R., MERRILL E. R. Studies on herpes simplex virus. VII. Immunological comparison of strains of herpes simplex. J Immunol. 1955 Jul;75(1):28–34. [PubMed] [Google Scholar]
- KAPLAN A. S. A study of the herpes simplex virus-rabbit kidney cell system by the plaque technique. Virology. 1957 Dec;4(3):435–457. doi: 10.1016/0042-6822(57)90078-8. [DOI] [PubMed] [Google Scholar]
- KOHLHAGE H., SIEGERT R. [2 genetically determined variants of a herpes simplex strain]. Arch Gesamte Virusforsch. 1962;12:273–286. [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. Alteration plaque morphology of EMC virus with polycations. Virology. 1961 Aug;14:502–504. doi: 10.1016/0042-6822(61)90349-x. [DOI] [PubMed] [Google Scholar]
- NII S. The difference in the cytopathic changes in FL cells infected with different strains of herpes simplex virus. Biken J. 1961 Sep;4:215–216. [PubMed] [Google Scholar]
- OSTERHOUT S., TAMM I. Measurement of herpes simplex virus by the plaque technique in human amnion cells. J Immunol. 1959 Oct;83:442–447. [PubMed] [Google Scholar]
- RAPP F., BENYESH-MELNICK M. Plaque assay for measurement of cells infected with zoster virus. Science. 1963 Aug 2;141(3579):433–434. doi: 10.1126/science.141.3579.433. [DOI] [PubMed] [Google Scholar]
- RAPP F., SELIGMAN S. J., JAROSS L. B., GORDON I. Quantitative determination of infectious units of measles virus by counts of immunofluorescent foci. Proc Soc Exp Biol Med. 1959 Jun;101(2):289–294. doi: 10.3181/00379727-101-24915. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr A physical difference between two strains of herpes simplex virus apparent on sedimentation in cesium chloride. Virology. 1961 Sep;15:75–79. doi: 10.1016/0042-6822(61)90079-4. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Inhibition of infectious and hemagglutinating properties of type 2 dengue virus by aqueous Agar extracts. Virology. 1963 Jan;19:49–57. doi: 10.1016/0042-6822(63)90023-0. [DOI] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology. 1963 Jan;19:40–48. doi: 10.1016/0042-6822(63)90022-9. [DOI] [PubMed] [Google Scholar]
- SCOTT T. F., McLEOD D. L. Cellular responses to infection with strains of herpes simplex virus. Ann N Y Acad Sci. 1959 Jul 21;81:118–128. doi: 10.1111/j.1749-6632.1959.tb49300.x. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. I. An agar polysaccharide determining plaque morphology of EMC virus. Virology. 1961 Aug;14:456–462. doi: 10.1016/0042-6822(61)90338-5. [DOI] [PubMed] [Google Scholar]
- TYTELL A. A., NEUMAN R. E. A medium free of agar, serum and peptone for plaque assay of herpes simplex virus. Proc Soc Exp Biol Med. 1963 Jun;113:343–346. doi: 10.3181/00379727-113-28362. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L., BIANCHI M. Factors influencing enterovirus and reovirus growth and plaque formation. Tex Rep Biol Med. 1962;20:693–702. [PubMed] [Google Scholar]


