Abstract
Intracellular Ca2+ mobilization plays an important role in a wide variety of cellular processes, and multiple second messengers are responsible for mediating intracellular Ca2+ changes. Here we explored the role of one endogenous Ca2+-mobilizing nucleotide, cyclic adenosine diphosphoribose (cADPR), in the proliferation and differentiation of neurosecretory PC12 cells. We found that cADPR induced Ca2+ release in PC12 cells and that CD38 is the main ADP-ribosyl cyclase responsible for the acetylcholine (ACh)-induced cADPR production in PC12 cells. In addition, the CD38/cADPR signaling pathway is shown to be required for the ACh-induced Ca2+ increase and cell proliferation. Inhibition of the pathway, on the other hand, accelerated nerve growth factor (NGF)-induced neuronal differentiation in PC12 cells. Conversely, overexpression of CD38 increased cell proliferation but delayed NGF-induced differentiation. Our data indicate that cADPR plays a dichotomic role in regulating proliferation and neuronal differentiation of PC12 cells.
Mobilization of intracellular Ca2+ stores is involved in diverse cell functions, including fertilization, cell proliferation, and differentiation (1–4). At least three endogenous Ca2+-mobilizing messengers have been identified, including inositol trisphosphate (IP3),3 nicotinic adenine acid dinucleotide phosphate (NAADP), and cyclic adenosine diphosphoribose (cADPR). Similar to IP3, cADPR can mobilize calcium release in a wide variety of cell types and species, from protozoa to animals. The cADPR-mediated Ca2+ signaling has been indicated in a variety of cellular processes (5–7), from abscisic acid signaling and regulation of the circadian clock in plants, to mediating long-term synaptic depression in hippocampus.
Ample evidence shows that the ryanodine receptors are the main intracellular targets for cADPR (1, 2, 8). Ryanodine receptors (RyRs) are intracellular Ca2+ channels widely expressed in various cells and tissues, including muscles and neurons. It is the major cellular mediator of Ca2+-induced Ca2+ release (CICR) in cells. There are three isoforms of ryanodine receptors: RyR1, RyR2, and RyR3, all of which have been implicated in the cADPR signaling (1, 2, 8). However, evidence regarding cADPR acting directly on the receptors is lacking (9). It has been suggested that accessory proteins, such as calmodulin and FK506-binding protein (FKBP), may be involved instead (10–15).
cADPR is formed from nicotinamide adenine dinucleotide (NAD) by ADP-ribosyl cyclases. Six ADP-ribosyl cyclases have been identified so far: Aplysia ADP-ribosyl cyclase, three sea urchin homologues (16, 17), and two mammalian homologues, CD38 and CD157 (18). CD38 is a membrane-bound protein and the main mammalian ADP-ribosyl cyclase. As a novel multifunctional enzyme, CD38 catalyzes the synthesis and hydrolysis of both cADPR and NAADP, two structurally and functionally distinct Ca2+ messengers. Virtually all mammalian tissues ever examined have been shown to express CD38. CD38 knock-out mice exhibit multiple physiological defects, ranging from impaired immune responses, metabolic disturbances, to behavioral modifications (1, 6, 18).
CD38 was originally identified as a lymphocyte differentiation antigen (18). Indeed, CD38/cADPR has been linked to cell differentiation (5). For example, in human HL-60 cells, CD38 expression and the consequential accumulation of cADPR play a causal role in mediating granulocytic differentiation (19). In addition, expression of CD38 in HeLa and 3T3 cells not only increased intracellular Ca2+ concentration but also induced cell proliferation by significantly reducing the S phase duration, leading to shortened cell doubling time (20). The ability of cADPR to increase cell proliferation has also been observed in human T cells (21), human hemopoietic progenitors (22), human peripheral blood mononuclear cells (23), human mesenchymal stem cells (24), and murine mesangial cells (25).
The PC12 cell line was derived from rat adrenal medulla and has been used extensively as a neuronal model, since it exhibits many of the functions observed in primary neuronal cultures (26). Most importantly, PC12 cells can be induced by nerve growth factor (NGF) to differentiate into cells with extensive neurite outgrowths, resembling neuronal dendritic trees (26, 27). In contrast to NGF, numerous growth factors and neurotransmitters can induce the proliferation of PC12 cells instead (26). Both IP3 receptor- and ryanodine receptor-mediated Ca2+ stores have been shown to be present in PC12 cells (28–31). The type 2 ryanodine receptor is expressed in PC12 cells and activation of the NO/cGMP pathway in PC12 cells results in calcium mobilization, which is mediated by cADPR and similar to that seen in sea urchin eggs (32). It has been demonstrated that NAADP, another Ca2+-mobilizing messenger, is also a potent neuronal differentiation inducer in PC12 cells, while IP3 exhibits no such role (33, 34). Whether cADPR is involved in the proliferation and differentiation of PC12 cells is unknown.
Here we show that activation of the CD38/cADPR/Ca2+ signaling is required for the ACh-induced proliferation in PC12 cells, while inhibition of the pathway accelerates NGF-induced neuronal differentiation. Our data indicate that cADPR is important in regulating cell proliferation and neuronal differentiation in PC12 cells.
EXPERIMENTAL PROCEDURES
Cell Culture
PC12 cells were maintained in DMEM plus 7.5% horse serum, 7.5% fetal bovine serum, and 100 units/ml of penicillin/streptomycin at 7.5% CO2 and 37 °C. The medium was changed every 48 h.
Cell Proliferation Assay
PC12 cells were plated at a density of 6000 cells/cm2 in 48-well plates in DMEM medium. Cells were rinsed twice with phosphate-buffered saline the next day, and incubated in a low serum (0.5% fetal bovine serum) medium for 12 h before addition of ACh (50 μm) or NGF (50 ng/ml) for various time periods, followed by treatments with [H3]thymidine (25 μCi) or BrdUrd (10 μm) for last 1 or 4 h of indicated treatment period, respectively. Incorporation of [H3]thymidine into DNA was determined as described previously (68). BrdUrd incorporation into PC12 cells was determined by an immunolabeling assay and quantified using a fluorometer according to the manufacturer's instructions (HTS01, EMD Chemicals, Inc.).
Intracellular Ca2+ Measurement
Cells were cultured in 24-well plates in DMEM and were labeled with 4 μm Fura-2 AM in Hanks' balanced salt solution (HBSS) at room temperature for 60 min. The cells were then washed with HBSS three times and incubated at room temperature for another 10 min. Cells were put on the stage of an Olympus inverted epifluorescence microscope and visualized using a 20× objective. Fluorescence images were obtained by alternate excitation at 340 and 380 nm with emission set at 510 nm. Images were collected by a CCD camera every 3 s and analyzed by a Cell R imaging software.
Western Blot Analysis
Cells were plated and treated as described for the proliferation assay above. Cells were lysed in an ice-cold EBC lysis buffer (50 mm Tris-HCl pH 8.0, 120 mm NaCl, 0.5% Nonidet P-40, 100 μm NaF, 200 μm Na3VO4, 100 μg/ml aprotinin, 20 μg/ml leupeptin, 150 μm phenylmethylsulfonyl fluoride, 0.5% sodium deoxycholate, and 0.5% SDS), and the lysates were passed through a 21-gauge needle several times to disperse any large aggregates. Protein concentrations of the cell lysates were determined by the Bradford assay. Proteins (30 or 40 μg per lane) were diluted in the standard SDS-sample buffer and subjected to electrophoresis on 10 or 15% SDS-polyacrylamide gels. Proteins were transferred to an Immobilon-P blotting membrane (Millipore, Billerica, MA), blocked with 5% milk in Tris-buffered saline (20 mm Tris, 150 mm NaCl, pH 7.6), and incubated with the primary antibody (CD38, SC-7048, Santa Cruz Biotechnology, 1:500 dilution; phospho-MAPK, 9106, Cell Signaling Technology, Beverly MA, 1:1000 dilution; cyclin D1, 05-815, Upstate Biotechnology, 1:1000 dilution; RyRs, SC-13942, Santa Cruz Biotechnology, 1:1000 dilution; cyclin E, SC-481, Santa Cruz Biotechnology, 1:1000 dilution; p21Cip1, SC-756, Santa Cruz Biotechnology, 1:1000 dilution; and 662 MEK1 antibody, 1:1000 dilution) for 1 h. After washing, the blots were probed with a secondary antibody for detection by chemiluminescence.
CD38-shRNA Lentivirus Production and Infection
Two optimal 21-mer targets: gttaaattggttactcaaacc and agcaacactgtcgggaaagca, in the rat CD38 gene were selected by following the published criteria (35, 36). The 21-mer target in the GFP gene: tacaacagccacaacgtctat, was also selected as a control. These three 21-mers were then cloned into pLKO.1, a replication-incompetent lentiviral vector for expressing shRNA. The shRNA lentivirus production was performed in 293T cells as described (37). For infection, PC12 cells were plated at a density of 6000 cells/cm2 in 6-cm dishes. On the next day, 100-μl pools of two CD38 shRNAs lentiviruses or control GFP-shRNAs lentivirus were added to the cells in fresh medium containing 8 μg/ml polybrene. Two days later, cells were selected in fresh medium containing puromycin (1 μg/ml) for 3–5 days. The puromycin-resistant cells were pooled and the knockdown efficiency was verified by both quantitative real-time RT-PCR and Western blot analysis.
Quantitative Real-time RT-PCR
The quantitative real-time RT-PCR using the iScriptTM One-Step Kit with SYBR® Green (Invitrogen) was performed normally in Bio-Rad MiniOpticonTM Real-time PCR Detection System according to the manufacturer's instructions.
cADPR Measurement
PC12 cells were seeded in 10-cm dishes at a density of 1 × 105 cells/cm2. After 24 h, the cells were washed with HBSS once and incubated with HBSS for 5 min. Cells were then treated with ACh (50 μm) for the indicated times at room temperature. Cells were lysed with ice-cold 0.6 m PCA, and the lysates were centrifuged at 4 °C to remove proteins. The lysates were then extracted with chloroform:Tri-n-octylamine (3:1) and the aqueous phase containing cADPR was obtained. The cADPR concentration in the extracts was finally measured by a sensitive enzymatic cycling assay as described previously (38).
Selection of PC12 Cells Expressing Human CD38-GFP
The pEGFP-CD38 or empty vector was transfected into PC12 cells by LipofectamineTM 2000 (Invitrogen). Two days after transfection, cells were selected with G418 (200 μg/ml) for 3–4 days. Antibiotic-resistant clones were pooled by trypsinization and maintained in medium containing G418 (100 μg/ml).
Quantification of Neurite Outgrowth
Cells were seeded in a 96-well plate at a density of 2000 cells/well. Next day, the medium was changed into a low serum medium (0.5% fetal bovine serum) for 12 h before treating with NGF (50 ng/ml). Cells were scored as neurite-bearing cells if their neurite lengths were at least two times longer than the length of the cell body. Percentages of cells with neurites were calculated. 80–100 cells were randomly sampled from each experiment. At least three independent experiments were performed for each treatment.
RESULTS
cADPR Is Required for Acetylcholine-induced Calcium Increase in PC12 Cells
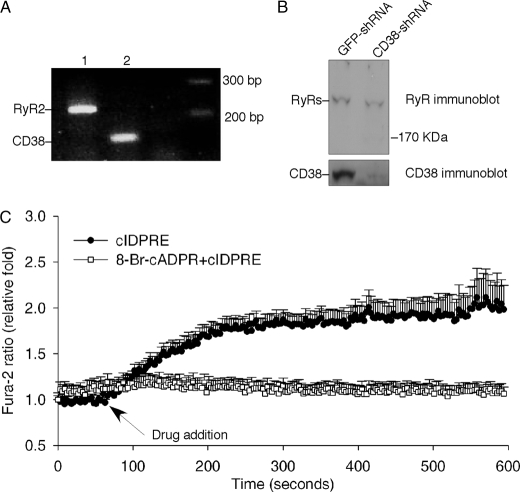
It has been shown previously that NO donors release Ca2+ in PC12 cells via the cADPR pathway (32). To test the possible role of cADPR in proliferation and differentiation in PC12 cells, we first examined the expression of CD38 and type 2 ryanodine receptors (RyR2) in PC12 cells by RT-PCR and immunoblot analysis. As shown in Fig. 1, A and B, both CD38 and RyR2 are expressed. Moreover, a cell-permeant cADPR agonist, cIDPRE (39), induced Ca2+ release in intact PC12 cells, which was blocked by pretreatment of the cells with a cADPR antagonist, 8-Br-cADPR (40) (Fig. 1C). In addition, cADPR itself induced Ca2+ release in permeabilized PC12 cells (data not shown). These data indicate that the PC12 cells are responsive to cADPR and possess the key components of the cADPR signaling pathway.
FIGURE 1.
Components of the cADPR/CD38 signaling pathway in PC12 cells. A, expression of CD38 and RyR2 mRNA in PC12 cells were determined by RT-PCR. B, protein expression of CD38 and RyR2 in GFP-shRNA or CD38-shRNA-infected PC12 cells were determined by immunoblot analysis using either anti-CD38 or anti-Ryr antibody. C, Ca2+ increases in intact PC12 cells induced by a cell-permeant cADPR agonist, cIDPRE (200 μm), in the absence or presence of an antagonist of cADPR, 8-Br-cADPR (200 μm).
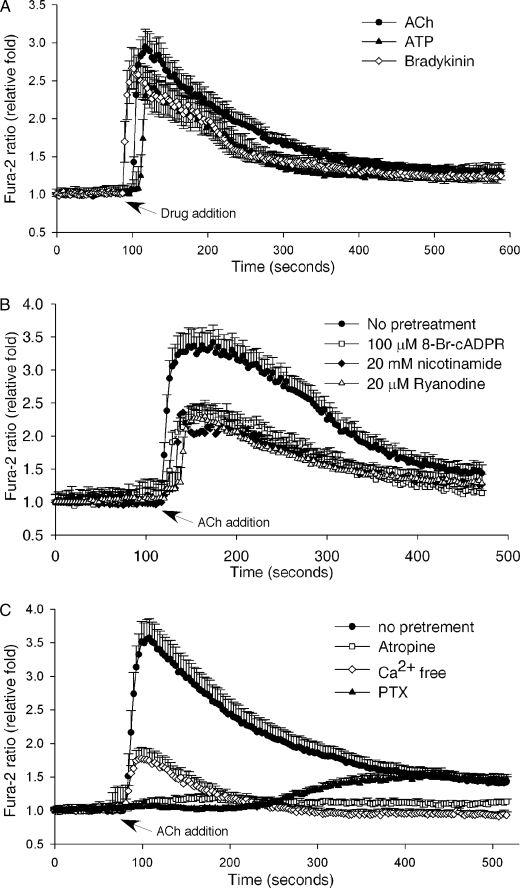
It is known that neurotransmitters and growth factors can induce cell proliferation in PC12 cells while NGF promotes cell differentiation (26). We tested whether any of theses factors can induce intracellular Ca2+ changes in PC12 cells via the cADPR pathway. We screened a series of neurotransmitters, including ACh, oxytocin, histamine, and glutamate, as well as other extracellular stimuli, such as bradykinin, ATP, and NGF, for their ability to induce Ca2+ release in the cells. Although ACh, bradykinin, and ATP all induced Ca2+ spikes, only ACh-induced Ca2+ changes were inhibited significantly by 8-Br-cADPR (Fig. 2, A and B). Moreover, pretreatment of the cells with high concentrations of nicotinamide, which is commonly used to inhibit the ADP-ribosyl cyclase activity thereby preventing cADPR production, or ryanodine, a RyR antagonist, inhibited ACh-induced Ca2+ release (Fig. 2B). These data indicate that cADPR is responsible for mediating about 50% of the ACh-induced Ca2+ increase in PC12 cells. That cADPR is the messenger for the ACh-induced Ca2+ release has also been reported in several other cell types (41–46).
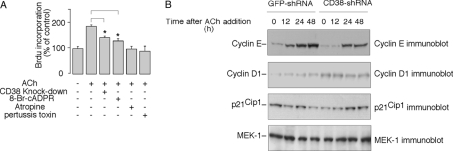
FIGURE 2.
Acetylcholine-induced Ca2+ increase in PC12 cells is mediated by cADPR. A, ACh (50 μm), ATP (100 μm), or bradykinin (50 μm) induced a Ca2+ increase in PC12 cells as measured by the Ca2+ indicator, Fura-2 AM. B, pretreatment of PC12 cells with either 8-Br-cADPR (100 μm), nicotinamide (20 mm), or ryanodine (20 μm) inhibited the ACh-induced Ca2+ increase compared with untreated cells. C, inhibition of the ACh-induced Ca2+ increase in Fura-2-loaded PC12 cells by either removal of external Ca2+ (Ca2+-free HBSS with 0.2 mm EGTA), pretreatment with PTX (1 μg/ml) or atropine (10 μm). The graphs represent data from five independent experiments expressed as means ± S.E., n = 20–50 cells.
In addition, the ACh-induced Ca2+ increases were blocked by atropine, an antagonist of the muscarinic receptor. Preincubation of PC12 cells with pertussis toxin (PTX), which uncouples G-proteins, significantly inhibited ACh-induced Ca2+ release, indicating the requirement of the activation of the muscarinic G protein-coupled receptor (GPCR). When the extracellular Ca2+ was removed with the inclusion of EGTA, the ACh-induced Ca2+ increases were also significantly inhibited, indicating Ca2+ influx is required as well (Fig. 2C).
CD38 Is Required for Acetylcholine-induced Ca2+ Increase in PC12 Cells
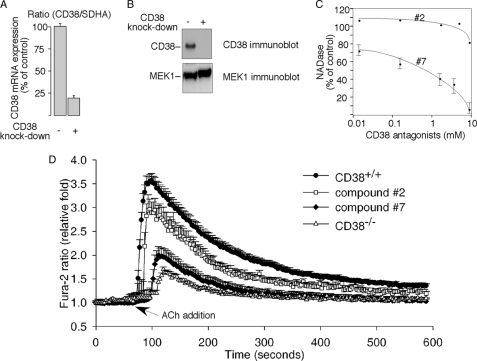
Because CD38 is likely responsible for the synthesis of cADPR and is expressed in PC12 cells (Fig. 1, A and B), we next determined whether it is needed for the ACh-induced Ca2+ increase. Two approaches were used to inhibit the CD38 activity in the cells. First, CD38 expression was efficiently knocked-down by using lentivirus-mediated short hairpin RNA (shRNA), targeting the CD38 gene (Fig. 3, A and B). The ACh-induced Ca2+ increase was significantly inhibited in the CD38 knockdown cells (Fig. 3D). Second, a CD38 inhibitor newly developed by us, compound 7 (N-[5′-(8′-quinolinoxy)ethoxy-methyl]-nicotinamide chloride), was used to inhibit CD38 activity. In vitro, compound 7, but not its close structural analog, compound 2 (N-(5′-acetoxyethoxy-methyl)-nicotinamide chloride), significantly inhibited the NADase activity of CD38 (Fig. 3C). Consistently, when applied in vivo, only compound 7 significantly inhibited the ACh-induced Ca2+ increase in PC12 cells, while compound 2, its less potent analog, had no significant effect on the ACh-induced Ca2+ changes (Fig. 3D). The structures and synthesis of these compounds will be reported elsewhere.
FIGURE 3.
Requirement of CD38 for the acetylcholine-induced Ca2+ increase in PC12 cells. A, quantitative real-time RT-PCR data of CD38-knockdown in PC12 cells. SDHA was used as the internal control. Data are expressed as means ± S.D., n = 3. B, immunoblot analysis of CD38-knockdown in PC12 cells. MEK-1 immunoblot was used as the internal control. C, CD38 antagonist, compound 7, inhibited the NADase activity of the recombinant CD38 in vitro, while its structurally related inactive analog, compound 2, had much less effect. D, CD38-knockdown with CD38-shRNA or pretreatment with compound 7 (50 μm) inhibited the ACh-induced Ca2+ increase in Fura-2-loaded PC12 cells compared with the responses of respective control cells either infected with GFP-shRNA or pretreated with the inactive analog compound 2 (50 μm). The graphs represent data from three independent experiments expressed as means ± S.E., n = 20–50 cells.
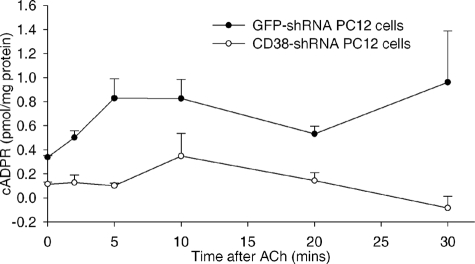
It has been shown previously that ACh increases cADPR synthesis in several other cell types (41–46). As shown in Fig. 4, ACh treatments also induced elevation of cADPR levels in PC12 cells in a time-dependent manner, and CD38 knockdown completely abrogated the ACh-induced production of cADPR, supporting that CD38 is the dominant ADP-ribosyl cyclase in the cells. Taken together, these data indicate that the CD38/cADPR signaling pathway is involved in the ACh-induced Ca2+ increase in PC12 cells.
FIGURE 4.
Elevation of endogenous cADPR in PC12 cells by ACh. Intracellular cADPR concentrations were measured in either CD38-shRNA or GFP-shRNA (control) expressed PC12 cells that were subsequently treated with 50 μm ACh for the indicated times. The graphs represent data from six independent experiments expressed as means ± S.D.
CD38/cADPR Signaling Is Required for Acetylcholine-stimulated PC12 Cell Proliferation
Recent reports on the ability of ACh to stimulate the proliferation of neuronal stem cells (47–49) prompted us to examine whether ACh can also stimulate growth of PC12 cells and, if so, whether the CD38/cADPR signaling is involved. As shown in Fig. 5A, ACh treatment for 24 h significantly induced PC12 cell proliferation as demonstrated by the increase of BrdUrd incorporation into the cells. As expected, pretreatment PC12 cells with either atropine or pertussis toxin abolished the increase. Both CD38-knockdown and pretreatment with 8-Br-cADPR partly but significantly inhibited ACh-induced BrdUrd incorporation into PC12 cells as well. We then examined the effects of CD38-knockdown on some regulators of the G1/S cell cycle. As shown in Fig. 5B, CD38 knockdown significantly inhibited the ability of ACh to induce cyclin E expression. The effect was most prominent at the 12-h time point. Although ACh did not significantly change the cyclin D1 expression levels in PC12 cells, the levels were slightly elevated in the CD38-knockdown cells. Neither the expression levels of p21Cip1 (Fig. 5B) nor p27Kip1 (data not shown) were affected by ACh. Taken together, these data demonstrate that the CD38/cADPR signaling is required for ACh-induced proliferation in PC12 cells.
FIGURE 5.
The ACh-stimulated cell proliferation is mediated by the CD38/cADPR signaling pathway. A, inhibition of the ACh (50 μm)-induced BrdUrd incorporation into PC12 cells by 8-Br-cADPR (100 μm), CD38-knockdown, atropine (10 μm), or PTX (1 μg/ml). Data are expressed as means ± S.D., n = 3. The * symbols indicate the results of Student's t test analysis, p < 0.05, compared with PC12 treated with ACh alone. B, expression of cyclin E, cyclin D1, and p21Cip1 in CD38-shRNA-expressed PC12 cells, or control cells expressed GFP-shRNA, in response to ACh (50 μm) treatment for the indicated times. MEK-1 immunoblot was used as the internal control.
The CD38/cADPR Signaling Is Required for Acetylcholine-induced Ca2+ Increase in Differentiated PC12 Cells
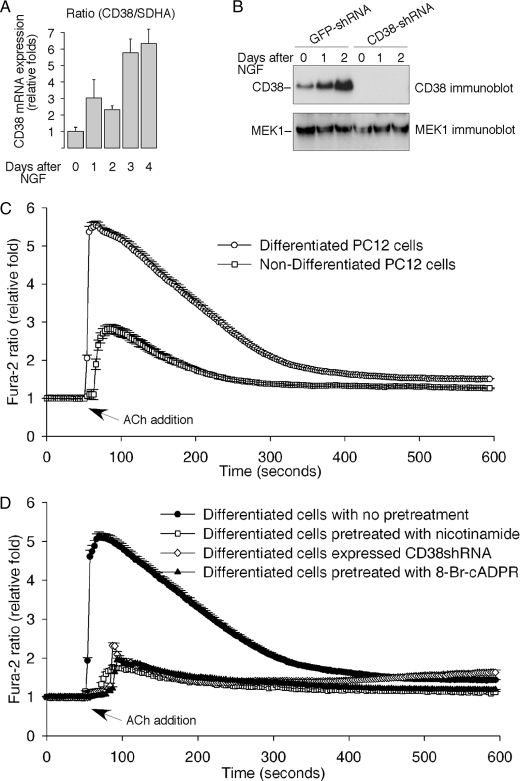
PC12 cells can be induced to differentiate and to adopt a neuronal morphology by NGF (26). We found that NGF dramatically increased CD38 expression in PC12 cells (Fig. 6, A and B). Moreover, the ability of ACh to induce a Ca2+ increase was significantly greater in the NGF-differentiated cells as compared with the undifferentiated cells (Fig. 6C). 8-Br-cADPR, nicotinamide, and CD38-knockdown all significantly inhibited the ACh-induced Ca2+ increase in the differentiated PC12 cells (Fig. 6D). These data not only support a role of cADPR/CD38 in mediating the Ca2+ signaling, but also imply a role of the pathway in regulating neuronal plasticity.
FIGURE 6.
The ACh-induced Ca2+ release in differentiated PC12 cells is mediated by the CD38/cADPR signaling pathway. A, real-time RT-PCR data of NGF-induced CD38 expression in PC12 cells expressed as means ± S.D., n = 3. SDHA was used as an internal control. B, immunoblot analysis of CD38 expression in CD38-shRNA-expressed PC12 cells, or control cells expressed GFP-shRNA, in response to stimulation by NGF (50 ng/ml) for the indicated times. MEK1 expression was used as internal controls. C, NGF-differentiated wild-type PC12 cells showed enhanced Ca2+ response to ACh (50 μm) as compared with the non-differentiated cells. D, CD38-knockdown (CD38 shRNA expression) or pretreatments with either a CD38 inhibitor, nicotinamide (20 mm) or a cADPR antagonist, 8-Br-cADPR (100 μm), all inhibited the ACh-induced Ca2+ increase in differentiated PC12 cells. Neuronal differentiation was induced by NGF (50 ng/ml) for 7 days. The graphs represent data from three independent experiments expressed as means ± S.E., n = 30–70 cells.
The Role of CD38/cADPR Signaling in NGF-induced Neuronal Differentiation
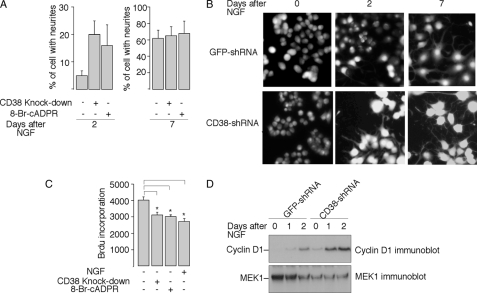
As NGF dramatically induced CD38 expression in PC12 cells, and NAADP, another potent Ca2+ messenger and product of CD38, has been shown to induce PC12 differentiation (33, 34), we next examined the effect of the CD38/cADPR signaling on NGF-induced neuronal differentiation. Control GFP shRNA- or CD38 shRNA-expressed cells were treated with NGF in the presence or absence of 8-Br-cADPR for 7 days. The neurite length was monitored daily. As shown in Fig. 7, A and B, both CD38-knockdown and the 8-Br-cADPR treatment surprisingly accelerated NGF-induced neuronal differentiation, as evidenced by faster neurite outgrowth in PC12 cells on day 2. As shown in Fig. 7C, concomitant to acceleration of neurite outgrowth, the treatments also inhibited PC12 cell proliferation.
FIGURE 7.
The role of the CD38/cADPR signaling in NGF-induced neuronal differentiation. A, CD38-knockdown or treatment with 8-Br-cADPR (100 μm) accelerated NGF-induced neurite outgrowth in PC12 cells. Data are expressed as means ± S.D., n = 3. B, representative fluorescence images of Fura-2 labeled wild-type or CD38-knockdown PC12 cells, both treated with NGF (50 ng/ml) for the indicated number of days. C, inhibition of the BrdUrd incorporation into PC12 cells by 8-Br-cADPR, CD38-knockdown, or NGF. Cells were plated as described under “Experimental Procedures,” and treated with or without NGF (50 ng/ml) or 8-Br-cADPR (100 μm) for 24 h before assayed for BrdUrd incorporation. The data are expressed as means ± S.D., n = 3. The * symbols indicate the results of the Student's t test analysis, p < 0.005, compared with PC12 wild-type cells without treatment. D, expression of cyclin D1 in CD38-shRNA expressed PC12 cells, or control cells expressed GFP-shRNA, in response to NGF (50 ng/ml) treatment for the indicated times. MEK-1 immunoblot was used as the internal control.
It is known that NGF induces cyclin D1 expression, which may contribute to the cell cycle arrest and differentiation in PC12 cells. Overexpression of cyclin D1 has also been shown to induce neurite growth in PC12 cells (50–56). Because cyclin D1 expression levels in the CD38-knockdown cells were elevated (Fig. 5B), NGF-induced cyclin D1 expression was examined in these cells. As shown in Fig. 7D, the expression of cyclin D1 was significantly higher in the NGF-treated CD38-knockdown cells compared with the wild-type cells, and is in line with the faster neurite growth in CD38-knockdown cells.
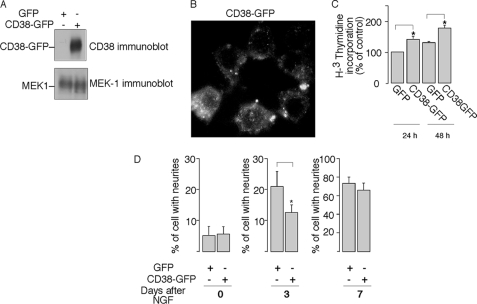
To further validate the role of CD38 in cell proliferation and differentiation, we examined the effects of overexpression of CD38 in the PC12 cells. Vectors encoding human CD38 fused with GFP or GFP alone were transfected into PC12 cells, and pools of stably transfected cells were selected (Fig. 8, A and B). [H3]Thymidine incorporation assay was then performed on the asynchronized cells at 24 h or 48 h after plating. As shown in Fig. 8C, the proliferation of PC12 cells expressing CD38-GFP was significantly increased. Finally, we examined the role of overexpression of CD38 on NGF-induced PC12 differentiation. As shown in Fig. 8D, after 3 days of treatment with NGF, neurite outgrowth was significantly delayed in cells expressing CD38-GFP as compared with the control cells expressing GFP alone. However, by day 7, the majority of the CD38-GFP expressed cells, like the control GFP cells, exhibited long neurites, indicating that NGF can overpower the negative effects of CD38 expression to induce neuronal differentiation eventually. Nevertheless, these data indicate the ability of cADPR to promote cell proliferation antagonizes the NGF-induced differentiation in PC12 cells.
FIGURE 8.
The effects of CD38 overexpression on proliferation and NGF-induced differentiation in PC12 cells. A, overexpression of human CD38 in PC12 cells. PC12 cells were transfected CD38-GFP, or GFP as control. The expression of CD38 was determined by immunoblot using anti-CD38 antibody. MEK1 expression was used as a loading control. B, membrane localization of CD38 in CD38-GFP-transfected PC12 cells. C, expression CD38-GFP correlated with cell proliferation. [H3]Thymidine incorporation was assayed in CD38-GFP expressed PC12 cells, or control cells expressed GFP, 1 or 2 days after cell plating. The * symbols indicate the results of Student's t test analysis, p < 0.05, compared with GFP-expressed PC12 at 24 h or 48 h, respectively. D, CD38-GFP expression delayed NGF (50 ng/ml)-induced neuronal differentiation in PC12 cells. Data are expressed as means ± S.D., n = 3. The * symbols indicate the results of Student's t test analysis, p < 0.005, compared with GFP-expressed PC12 cells after 3 days of NGF treatment.
DISCUSSION
ACh muscarinic receptors are a family of five G-protein-coupled receptors widely distributed in the central nervous system and in peripheral organs. In several cell types, ACh has been shown to induce cell proliferation (47, 57, 58). More recently, a study by Diamandis et al. (48) reported that ACh can potently promote proliferation in neuronal stem cells. The ability of ACh to modulate DNA synthesis through muscarinic receptors may be relevant in the context of brain development and neoplastic growth (59). Here our results clearly demonstrate that ACh activated the CD38/cADPR signaling in PC12 cells, resulting in intracellular Ca2+ changes. Moreover, disruption of the cADPR signaling pathway by either a cADPR antagonist or by CD38-knockdown inhibited ACh-induced proliferation in the cells. These data indicate that the CD38/cADPR/Ca2+ signaling is required for ACh-induced proliferation in PC12 cells.
The activation of MAPKs by ACh has been shown to be important for cell proliferation (47, 60). However, in PC12 cells, CD38 knockdown did not have any significant effects on ACh-induced MAPKs activation (supplemental Fig. S1A), suggesting cADPR may affect ACh-induced cell growth not related to or downstream of MAPKs. It is possible that cADPR signaling activated by ACh utilizes the calmodulin (CaM) and CaM-dependent protein kinases (CaMKs) to regulate gene expression or protein stability of cyclins or Cdk inhibitors (CKIs), thereby promoting cell proliferation. Along this line, our data show that the ability of ACh to induce cyclin E expression is impaired by disrupting the cADPR signaling (Fig. 5B). Whether ACh could activate CaMKs and, if so, whether the cADPR signaling is involved in ACh-activated CaMKs remains to be determined.
Interestingly, the positive effect of cADPR/CD38 on cell proliferation is shown to antagonize differentiation. Thus, overexpression of CD38 delayed NGF-induced differentiation (Fig. 8E), while a cADPR-antagonist or CD38-knockdown actually accelerated the ability of NGF to induce neurite growth in PC12 cells (Fig. 7, A and B). These data demonstrate that the cADPR signaling is negatively involved in NGF-induced differentiation. It is well established that NGF activates the Ras/Raf/MEK1/MAPKs cascade, and NGF-induced sustained MAPK activation is essential for neuronal differentiation in PC12 cells (61, 62). However, CD38-knockdown or 8-Br-cADPR treatment had no significant effects on the NGF-induced MAPK activity (supplemental Fig. S1B), indicating CD38/cADPR/Ca2+ signaling is targeting a downstream event other than the Ras/MAPK pathway to influence the role of NGF in PC12 cells.
In general, NGF dramatically inhibits Cdks kinase activity by induction of Cdk inhibitor (CKI) p21Cip1 and cyclin D1, arresting cells at the Go/G1 phase, and stopping division. Overexpression of p21Cip1 or cyclin D1 in PC12 cells induces differentiation similar to NGF (50–56, 63–66). Here we found that CD38-knockdown or the 8-Br-cADPR treatment not only enhanced NGF-induced differentiation (Fig. 7, A and B) but also inhibited cell proliferation (Fig. 7C). Moreover, in the CD38-shRNA-expressed PC12 cells the cyclin D1 expression levels were elevated (Fig. 5B), and the NGF-induced cyclin D1 expressions were significantly accelerated as well (Fig. 7D). Therefore, it is possible that in the absence of NGF, the CD38/cADPR-mediated Ca2+ signaling is positively involved in PC12 cell proliferation by suppressing the expression levels of some differentiation factors, such as cyclin D1. Inhibition of the CD38/cADPR-mediated Ca2+ signaling can then lead to up-regulation of these factors, resulting in growth inhibition, which in turn potentiates the ability of NGF to induce cell differentiation.
Although NGF-induced differentiation has been under active study for many years, the mechanisms by which cells exit from the cell cycle and enter differentiation remain elusive. Treatment of PC12 cells with NGF results in sequential induction of immediate early genes, such as c-fos (67), delayed early genes, such as the activation of tyrosine hydroxylase (68), and late structure genes, such as neuron-specific intermediate filament protein and tau-1 (69, 70). It is possible that the cADPR pro-proliferation activity is not related to its anti-differentiation activity. Instead, cADPR may affect NGF-induced differentiation by negatively controlling the expression or activities of some latter genes. It is known that these proteins, such as Tau, are required for neurite outgrowth. Along this line, it has been shown that phosphorylation of Tau by CaMKs leads to the loss of cytoskeletal integrity and neurite instability (71). Perhaps, it is more likely that both cADPR pro-proliferation activity and its effects on the expression of these structure proteins contribute to its anti-differentiation effects.
Last, we also found that NGF induces CD38 expression in PC12 cells and that the ability of ACh to induce a Ca2+ increase was significantly increased in the NGF-differentiated cells as compared with the undifferentiated cells (Fig. 6). Moreover, more apoptotic cells were observed in CD38 shRNA-infected cells than that in control cells (data not shown). These data suggest that the induction of CD38 by NGF plays a role in neuronal survival and protection as well. In summary, our results show that the cADPR/CD38 signaling pathway is important in regulating diverse cellular functions in PC12 cells.
Supplementary Material
Acknowledgments
We thank Christina Leung, Rich Graeff, and other members of the laboratory for advice on the manuscript.
This work was supported by Research Grant Council (RGC) grants from Hong Kong (to H. C. L. and J. Y.), National Natural Science Foundation of China (NSFC)/RGC Grant N_HKU 722/08 (to H. C. L.), NSFC grants from China (20831160506, to L. H. Z. and 90713005, to L. R. Z.), and a Special Fellow Award from the Leukemia and Lymphoma Society of America (to J. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- IP3
- inositol trisphosphate
- cADPR
- cyclic adenosine diphosphoribose
- ACh
- acetylcholine
- NGF
- nerve growth factor
- NAADP
- nicotinic adenine acid dinucleotide phosphate
- DMEM
- Dulbecco's modified Eagle's medium
- BrdUrd
- bromodeoxyuridine
- MAPK
- mitogen-activated protein kinase
- CaMK
- calmodulin kinase
- shRNA
- short hairpin RNA
- GFP
- green fluorescent protein.
REFERENCES
- 1.Lee H. C. (2004) Curr Mol. Med. 4, 227–237 [DOI] [PubMed] [Google Scholar]
- 2.Guse A. H. (2005) Febs J. 272, 4590–4597 [DOI] [PubMed] [Google Scholar]
- 3.Galione A., Churchill G. C. (2002) Cell Calcium 32, 343–354 [DOI] [PubMed] [Google Scholar]
- 4.Berridge M. J., Galione A. (1988) Faseb. J. 2, 3074–3082 [DOI] [PubMed] [Google Scholar]
- 5.Lee H. C. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 317–345 [DOI] [PubMed] [Google Scholar]
- 6.Guse A. H. (2004) Curr. Med. Chem. 11, 847–855 [DOI] [PubMed] [Google Scholar]
- 7.Galione A., Churchill G. C. (2000) Sci. STKE 2000, PE1. [DOI] [PubMed] [Google Scholar]
- 8.Morgan A. J., Galione A. (2008) Methods 46, 194–203 [DOI] [PubMed] [Google Scholar]
- 9.Thomas J. M., Masgrau R., Churchill G. C., Galione A. (2001) Biochem. J. 359, 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H. C., Aarhus R., Graeff R., Gurnack M. E., Walseth T. F. (1994) Nature 370, 307–309 [DOI] [PubMed] [Google Scholar]
- 11.Noguchi N., Takasawa S., Nata K., Tohgo A., Kato I., Ikehata F., Yonekura H., Okamoto H. (1997) J. Biol. Chem. 272, 3133–3136 [DOI] [PubMed] [Google Scholar]
- 12.Tang W. X., Chen Y. F., Zou A. P., Campbell W. B., Li P. L. (2002) Am. J. Physiol. Heart Circ Physiol. 282, H1304–H1310 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y. X., Zheng Y. M., Mei Q. B., Wang Q. S., Collier M. L., Fleischer S., Xin H. B., Kotlikoff M. I. (2004) Am. J. Physiol. Cell Physiol. 286, C538–C546 [DOI] [PubMed] [Google Scholar]
- 14.Morita K., Kitayama T., Kitayama S., Dohi T. (2006) J. Pharmacol. Sci. 101, 40–51 [DOI] [PubMed] [Google Scholar]
- 15.Thomas J. M., Summerhill R. J., Fruen B. R., Churchill G. C., Galione A. (2002) Curr. Biol. 12, 2018–2022 [DOI] [PubMed] [Google Scholar]
- 16.Churamani D., Boulware M. J., Ramakrishnan L., Geach T. J., Martin A. C., Vacquier V. D., Marchant J. S., Dale L., Patel S. (2008) Cell. Signal. 20, 2347–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churamani D., Boulware M. J., Geach T. J., Martin A. C., Moy G. W., Su Y. H., Vacquier V. D., Marchant J. S., Dale L., Patel S. (2007) PLoS ONE 2, e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malavasi F., Deaglio S., Funaro A., Ferrero E., Horenstein A. L., Ortolan E., Vaisitti T., Aydin S. (2008) Physiol. Rev. 88, 841–886 [DOI] [PubMed] [Google Scholar]
- 19.Munshi C. B., Graeff R., Lee H. C. (2002) J. Biol. Chem. 277, 49453–49458 [DOI] [PubMed] [Google Scholar]
- 20.Zocchi E., Daga A., Usai C., Franco L., Guida L., Bruzzone S., Costa A., Marchetti C., De Flora A. (1998) J. Biol. Chem. 273, 8017–8024 [DOI] [PubMed] [Google Scholar]
- 21.Guse A. H., da Silva C. P., Berg I., Skapenko A. L., Weber K., Heyer P., Hohenegger M., Ashamu G. A., Schulze-Koops H., Potter B. V., Mayr G. W. (1999) Nature 398, 70–73 [DOI] [PubMed] [Google Scholar]
- 22.Podestà M., Zocchi E., Pitto A., Usai C., Franco L., Bruzzone S., Guida L., Bacigalupo A., Scadden D. T., Walseth T. F., De Flora A., Daga A. (2000) Faseb. J. 14, 680–690 [DOI] [PubMed] [Google Scholar]
- 23.Bruzzone S., De Flora A., Usai C., Graeff R., Lee H. C. (2003) Biochem. J. 375, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarfì S., Ferraris C., Fruscione F., Fresia C., Guida L., Bruzzone S., Usai C., Parodi A., Millo E., Salis A., Burastero G., De Flora A., Zocchi E. (2008) Stem Cells 26, 2855–2864 [DOI] [PubMed] [Google Scholar]
- 25.Kim S. Y., Gul R., Rah S. Y., Kim S. H., Park S. K., Im M. J., Kwon H. J., Kim U. H. (2008) Am. J. Physiol. Renal Physiol. 294, F982–F989 [DOI] [PubMed] [Google Scholar]
- 26.Greene L. A., Tischler A. S. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi A., Biocca S., Cattaneo A., Calissano P. (1988) Mol. Neurobiol. 2, 201–226 [DOI] [PubMed] [Google Scholar]
- 28.Koizumi S., Bootman M. D., Bobanovic L. K., Schell M. J., Berridge M. J., Lipp P. (1999) Neuron 22, 125–137 [DOI] [PubMed] [Google Scholar]
- 29.Barry V. A., Cheek T. R. (1994) Biochem. J. 300, 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry V. A., Cheek T. R. (1994) J. Cell Sci. 107, 451–462 [DOI] [PubMed] [Google Scholar]
- 31.Bennett D. L., Bootman M. D., Berridge M. J., Cheek T. R. (1998) Biochem. J. 329, 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clementi E., Riccio M., Sciorati C., Nisticò G., Meldolesi J. (1996) J. Biol. Chem. 271, 17739–17745 [DOI] [PubMed] [Google Scholar]
- 33.Brailoiu E., Hoard J. L., Filipeanu C. M., Brailoiu G. C., Dun S. L., Patel S., Dun N. J. (2005) J. Biol. Chem. 280, 5646–5650 [DOI] [PubMed] [Google Scholar]
- 34.Brailoiu E., Churamani D., Pandey V., Brailoiu G. C., Tuluc F., Patel S., Dun N. J. (2006) J. Biol. Chem. 281, 15923–15928 [DOI] [PubMed] [Google Scholar]
- 35.Schwarz D. S., Hutvágner G., Du T., Xu Z., Aronin N., Zamore P. D. (2003) Cell 115, 199–208 [DOI] [PubMed] [Google Scholar]
- 36.Khvorova A., Reynolds A., Jayasena S. D. (2003) Cell 115, 209–216 [DOI] [PubMed] [Google Scholar]
- 37.Moffat J., Grueneberg D. A., Yang X., Kim S. Y., Kloepfer A. M., Hinkle G., Piqani B., Eisenhaure T. M., Luo B., Grenier J. K., Carpenter A. E., Foo S. Y., Stewart S. A., Stockwell B. R., Hacohen N., Hahn W. C., Lander E. S., Sabatini D. M., Root D. E. (2006) Cell 124, 1283–1298 [DOI] [PubMed] [Google Scholar]
- 38.Graeff R. M., Walseth T. F., Lee H. C. (1997) Methods Enzymol. 280, 230–241 [DOI] [PubMed] [Google Scholar]
- 39.Gu X., Yang Z., Zhang L., Kunerth S., Fliegert R., Weber K., Guse A. H., Zhang L. (2004) J. Med. Chem. 47, 5674–5682 [DOI] [PubMed] [Google Scholar]
- 40.Walseth T. F., Lee H. C. (1993) Biochim. Biophys. Acta 1178, 235–242 [DOI] [PubMed] [Google Scholar]
- 41.Higashida H., Yokoyama S., Hashii M., Taketo M., Higashida M., Takayasu T., Ohshima T., Takasawa S., Okamoto H., Noda M. (1997) J. Biol. Chem. 272, 31272–31277 [DOI] [PubMed] [Google Scholar]
- 42.Higashida H., Yokoyama S., Hoshi N., Hashii M., Egorova A., Zhong Z. G., Noda M., Shahidullah M., Taketo M., Knijnik R., Kimura Y., Takahashi H., Chen X. L., Shin Y., Zhang J. S. (2001) Biol. Chem. 382, 23–30 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J. S., Jin D., Higashida H. (2005) Biochem. Biophys. Res. Commun. 335, 920–924 [DOI] [PubMed] [Google Scholar]
- 44.Higashida H., Bowden S. E., Yokoyama S., Salmina A., Hashii M., Hoshi N., Zhang J. S., Knijnik R., Noda M., Zhong Z. G., Jin D., Higashida K., Takeda H., Akita T., Kuba K., Yamagishi S., Shimizu N., Takasawa S., Okamoto H., Robbins J. (2007) Neurosci Res. 57, 339–346 [DOI] [PubMed] [Google Scholar]
- 45.Yamasaki M., Thomas J. M., Churchill G. C., Garnham C., Lewis A. M., Cancela J. M., Patel S., Galione A. (2005) Curr. Biol. 15, 874–878 [DOI] [PubMed] [Google Scholar]
- 46.Ge Z. D., Zhang D. X., Chen Y. F., Yi F. X., Zou A. P., Campbell W. B., Li P. L. (2003) J. Vasc Res. 40, 28–36 [DOI] [PubMed] [Google Scholar]
- 47.Ma W., Maric D., Li B. S., Hu Q., Andreadis J. D., Grant G. M., Liu Q. Y., Shaffer K. M., Chang Y. H., Zhang L., Pancrazio J. J., Pant H. C., Stenger D. A., Barker J. L. (2000) Eur J. Neurosci 12, 1227–1240 [DOI] [PubMed] [Google Scholar]
- 48.Diamandis P., Wildenhain J., Clarke I. D., Sacher A. G., Graham J., Bellows D. S., Ling E. K., Ward R. J., Jamieson L. G., Tyers M., Dirks P. B. (2007) Nat. Chem. Biol. 3, 268–273 [DOI] [PubMed] [Google Scholar]
- 49.Resende R. R., Alves A. S., Britto L. R., Ulrich H. (2008) Exp. Cell Res. 314, 1429–1443 [DOI] [PubMed] [Google Scholar]
- 50.Yan G. Z., Ziff E. B. (1995) J. Neurosci. 15, 6200–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Billon N., Carlisi D., Datto M. B., van Grunsven L. A., Watt A., Wang X. F., Rudkin B. B. (1999) Oncogene 18, 2872–2882 [DOI] [PubMed] [Google Scholar]
- 52.Galderisi U., Jori F. P., Giordano A. (2003) Oncogene 22, 5208–5219 [DOI] [PubMed] [Google Scholar]
- 53.Marampon F., Casimiro M. C., Fu M., Powell M. J., Popov V. M., Lindsay J., Zani B. M., Ciccarelli C., Watanabe G., Lee R. J., Pestell R. G. (2008) Mol. Biol. Cell 19, 2566–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erhardt J. A., Pittman R. N. (1998) Oncogene 16, 443–451 [DOI] [PubMed] [Google Scholar]
- 55.Poluha W., Schonhoff C. M., Harrington K. S., Lachyankar M. B., Crosbie N. E., Bulseco D. A., Ross A. H. (1997) J. Biol. Chem. 272, 24002–24007 [DOI] [PubMed] [Google Scholar]
- 56.van Grunsven L. A., Thomas A., Urdiales J. L., Machenaud S., Choler P., Durand I., Rudkin B. B. (1996) Oncogene 12, 855–862 [PubMed] [Google Scholar]
- 57.Resende R. R., Gomes K. N., Adhikari A., Britto L. R., Ulrich H. (2008) Cell Calcium 43, 107–121 [DOI] [PubMed] [Google Scholar]
- 58.Martins A. H., Resende R. R., Majumder P., Faria M., Casarini D. E., Tárnok A., Colli W., Pesquero J. B., Ulrich H. (2005) J. Biol. Chem. 280, 19576–19586 [DOI] [PubMed] [Google Scholar]
- 59.Costa L. G., Guizzetti M., Oberdoerster J., Yagle K., Costa-Mallen P., Tita B., Bordi F., Vitalone A., Palmery M., Valeri P. (2001) Growth Factors 18, 227–236 [DOI] [PubMed] [Google Scholar]
- 60.Berkeley J. L., Levey A. I. (2000) J. Neurochem. 75, 487–493 [DOI] [PubMed] [Google Scholar]
- 61.Burry R. W. (2001) J. Neurosci. Res. 63, 45–53 [DOI] [PubMed] [Google Scholar]
- 62.Santos S. D., Verveer P. J., Bastiaens P. I. (2007) Nat. Cell Biol. 9, 324–330 [DOI] [PubMed] [Google Scholar]
- 63.Billon N., van Grunsven L. A., Rudkin B. B. (1996) Oncogene 13, 2047–2054 [PubMed] [Google Scholar]
- 64.Park D. S., Levine B., Ferrari G., Greene L. A. (1997) J. Neurosci. 17, 8975–8983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erhardt J. A., Pittman R. N. (1998) J. Biol. Chem. 273, 23517–23523 [DOI] [PubMed] [Google Scholar]
- 66.Yan G. Z., Ziff E. B. (1997) J. Neurosci. 17, 6122–6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg M. E., Greene L. A., Ziff E. B. (1985) J. Biol. Chem. 260, 14101–14110 [PubMed] [Google Scholar]
- 68.Gizang-Ginsberg E., Ziff E. B. (1994) Mol. Endocrinol. 8, 249–262 [DOI] [PubMed] [Google Scholar]
- 69.Drubin D. G., Feinstein S. C., Shooter E. M., Kirschner M. W. (1985) J. Cell Biol. 101, 1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troy C. M., Greene L. A., Shelanski M. L. (1992) J. Cell Biol. 117, 1085–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. (1990) EMBO J. 9, 3539–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.