Abstract
Although the unsaturated fatty acid (UFA) synthetic pathway of Escherichia coli is the prototype of such pathways, several unresolved issues have accumulated over the years. The key players are the fabA and fabB genes. Earlier studies of fabA transcription showed that the gene was transcribed from two promoters, with one being positively regulated by the FadR protein. The other weaker promoter (which could not be mapped with the technology then available) was considered constitutive because its function was independent of FadR. However, the FabR negative regulator was recently shown to represses fabA transcription. We report that the weak promoter overlaps the FadR-dependent promoter and is regulated by FabR. This promoter is strictly conserved in all E. coli and Salmonella enterica genomes sequenced to date and is thought to provide insurance against inappropriate regulation of fabA transcription by exogenous saturated fatty acids. Also, the fabAup promoter, a mutant promoter previously isolated by selection for increased FabA activity, was shown to be a promoter created de novo by a four-base deletion within the gene located immediately upstream of fabA. Demonstration of the key UFA synthetic reaction catalyzed by FabB has been elusive, although it was known to catalyze an elongation reaction. Strains lacking FabB are UFA auxotrophs indicating that the enzyme catalyzes an essential step in UFA synthesis. Using thioesterases specific for hydrolysis of short chain acyl-ACPs, the intermediates of the UFA synthetic pathway have been followed in vivo for the first time. These experiments showed that a fabB mutant strain accumulated less cis-5-dodecenoic acid than the parental wild-type strain. These data indicate that the key reaction in UFA synthesis catalyzed by FabB is elongation of the cis-3-decenoyl-ACP produced by FabA.
The fabA gene of Escherichia coli encodes 3-hydroxydecanoyl-acyl carrier protein (ACP)2 dehydratase/isomerase, a bifunctional enzyme that introduces the unsaturated fatty acid (UFA) double bond at the C10 level (1–3) (see Fig. 1). As expected fabA mutants require supplementation with UFA for growth (4). Although UFA synthesis can be considered a housekeeping function, fabA transcription is subject to an unexpectedly complex regulation. Work from this laboratory and others showed that fabA transcription is positively regulated by FadR, a protein that also functions as the repressor of the β-oxidation regulon (5–7). In that work the FadR-dependent promoter was identified as well as a second weaker promoter that was unaffected by FadR and thus was considered constitutively active (5, 6). The FadR-independent promoter is thought to ensure continued expression of fabA under conditions where FadR activation is compromised. E. coli fabA mutants lyse in the absence of UFA (3). Because FadR responds to CoA thioesters of both unsaturated and saturated fatty acids, E. coli could shut down UFA synthesis in response to saturated fatty acid (SFA) availability, a potential disaster. We have proposed that E. coli avoids this calamity by the presence of the weaker promoter (8). This promoter has an activity equivalent to that of the main promoter in the absence of FadR activation (6). The basal transcription initiated at the FadR-regulated promoter plus the weak constitutive promoter accounts for the UFA synthesis remaining in FadR strains. FadR strains have about 30% less unsaturated fatty acid than wild-type cells (9) and because a 10-fold decrease in FabA levels produces only a modest decrease in UFA synthesis, wild-type cells must produce FabA in excess. Hence, we believe that this promoter arrangement provides insurance against repression of UFA synthesis by saturated fatty acids.
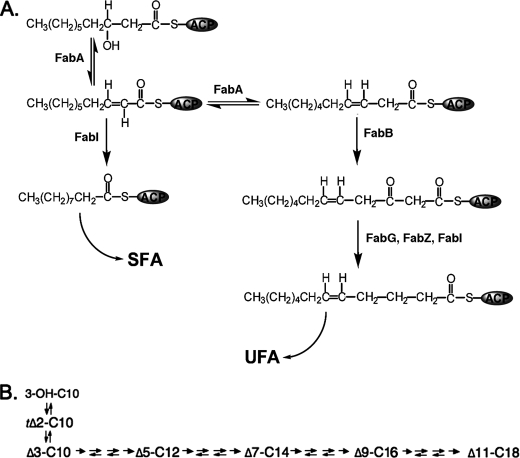
FIGURE 1.
UFA synthesis in E. coli. Panel A shows the reactions catalyzed by FabA and the proposed step catalyzed by FabB. Panel B shows the carbon flow in the UFA pathway. The arrows between each intermediate denote in order the 3-ketoacyl-ACP synthase (fabB or FabF), 3-ketoacyl-ACP reductase (FabG), the 3-hydroxyacyl-ACP dehydratase (FabZ), and the enoyl-ACP reductase (FabI). The first and last arrows denote irreversible reactions. The 3-ketoacyl-ACP synthase is truly irreversible due to decarboxylation, whereas FabI is reversible but the equilibrium lies very strongly in favor of reduction of the trans-2 double bond (21). The FabG and FabZ reactions are freely reversible (16).
However, our picture of fabA transcription has recently become more complex. A second protein called FabR was found to negatively regulate fabA and fabB (10, 11). In the prior work the weak fabA promoter could not be mapped due to insensitivity of the methods then available, this raised the possibility that FabR might regulate function of this promoter. We report that this is the case.
A related question was the nature of the lesion in a cis-acting mutation (called fabAup) isolated by selection for greatly increased FabA activity (12). The increased fabA transcription was shown due to a promoter located well upstream of the fabA coding sequence hypothesized to represent a mutation of the constitutive promoter that increased its efficiency. We report that this is incorrect. The fabAup mutation is a deletion within the nonessential gene located immediately upstream of fabA that results in creation of a new promoter.
Another major question in E. coli unsaturated fatty acid synthesis is the reaction catalyzed by the FabB protein, inactivation of which results in UFA auxotrophy (13). FabB is a well studied 3-ketoacyl-ACP synthase, a class of enzymes that catalyze the elongation (condensation) reaction of fatty acid synthesis (14, 15). E. coli has three such enzymes, FabB, FabF, and FabH (16). The UFA auxotrophy of strains lacking FabB indicates that this enzyme catalyzes a step in UFA synthesis that cannot be performed by FabF (FabH is not a candidate because it synthesizes only short chain acyl-ACPs). It has long been supposed that the elongation step uniquely catalyzed by FabB is extension of cis-3-decenoyl-ACP produced by the FabA dehydratase/isomerase to 3-keto-cis-5-dodecenoyl-ACP (17) (Fig. 1). However, this supposition was based on negative evidence because in vitro substrate specificity studies and high-resolution crystal structures of FabB and FabF have failed to fully explain the phenotypes of mutants lacking these enzyme activities (14, 18, 19). We approached this question in vivo by intercepting the intermediates of UFA synthesis using plant thioesterases active on short chain acyl-ACPs (20). Based on the equilibria of the fatty acid synthetic reactions (21) we predicted that the intermediate acyl chains released by thioesterase action would be cis-5-dodecenoic acid and cis-7-tetradecenoic acid. It has not previously been possible to detect cis-5-dodecenoic acid in E. coli because this acid is not incorporated into the complex lipids of the bacterium, whereas only traces of cis-7-tetradecenoic acid are found in the E. coli phospholipids unless elongation is inhibited by precursor limitation (22). We report that expression of a modified form of a plant thioesterase results in release of both of these short chain UFAs during de novo fatty acid synthesis thereby allowing assignment of the step catalyzed by FabB. These data indicate that the key reaction in UFA synthesis catalyzed by FabB is elongation of the cis-3-decenoyl-ACP produced by FabA.
EXPERIMENTAL PROCEDURES
Materials
Standard chemical reagents were obtained from Sigma. The iQ SYBR Green Supermix was from Bio-Rad. The 5′-RACE (version 2.0) and PCR kits, vector pCR2.1-TOPO, and restriction enzymes were purchased from Invitrogen. The Ambion FirstChoice RLM-RACE kit was supplied by Applied Biosystems. Qiagen provided plasmid isolation and PCR product purification kits plus Fast T4 DNA ligase and RNase-free DNase. Promega supplied the ImProm-II reverse transcription system, the pGEM-T Easy vector, and DNA markers. Oligonucleotide primers were synthesized by Integrated DNA Technologies and DNA sequencing was done by the Keck Genomics Center core sequencing facility of this university using a 3730XL DNA analyzer. The synthesis of cis-3-decenoic acid began with the acetate ester of cis-3-decenol (Z-3-decen-1-yl acetate from Bedoukian Research), which was hydrolyzed with 1 m KOH in 50% aqueous ethanol at room temperature overnight to give cis-3-decenol. The ethanol was then evaporated under a stream of nitrogen, the aqueous phase was saturated with KCl and the cis-3-decenol was extracted into ether/pentane (1/1). The extracts were pooled and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the cis-3-decenol was converted to cis-3-decenoic acid by chromic trioxide-catalyzed periodate oxidation (23). After quenching the reaction by addition of ethanol the acid was extracted first into ether/pentane (1/1) and then from that solution into 1 m potassium bicarbonate to remove any remaining cis-3-decenol. After extraction with pentane the bicarbonate solution was acidified with HCl to decompose the fatty acid salts and the acidified solution was saturated with KCl. The fatty acid was then extracted into ether/pentane (1/1). Thin layer chromatography showed that each step of the synthesis proceeded quantitatively. The other fatty acid standards were either synthesized as described previously (14) or were obtained from Aldrich.
Strains and Media
All strains are derivatives of E. coli K12 and are given under supplemental Table S1. Strains Ymel and K27 were from the Coli Genetic Stock Center of Yale University, whereas P. Overath provided strain K113. Strains DC170, DC308, and CY272 were from our laboratory collection. Strain CY272 was constructed by transduction of the Tn10 element from DC334 (24) into strain UFAts (25) with selection for tetracycline resistance at 30 °C followed by screening for temperature-sensitive growth. Strains CY1924 and CY1925 were constructed by phage P1vir transduction of strain K27 with phage stocks grown on strains DC308 and CY272, respectively, followed by selection for a Tn10 element closely linked to the fab mutation of the donor strain. The transductants were then screened for temperature sensitivity and rescue of growth at high temperature with oleic acid. The complex media used were LB medium (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter) or RB (LB with one-fifth of the yeast extract). The defined medium was medium E supplemented with 0.001% thiamine, 0.1% vitamin-free casamino acids, and 0.4% glucose as carbon source. Antibiotics were added to (in μg/ml) sodium ampicillin, 100; kanamycin, 50 μg/ml; and ticarillin/clavulanate, 25 (15/1, from Research Products International). Cultures were grown with vigorous shaking at 37 °C unless otherwise stated. For fatty acid analyses of phospholipids and β-galactosidase assays bacterial cultures were grown in minimal medium E (26) supplemented with 0.001% thiamine, 0.1% vitamin-free casamino acids, and 0.4% dextrose as carbon source. Bacterial growth was determined by measuring optical density at 600 nm.
DNA Manipulations
The thioesterase expression plasmids were constructed as follows. Plasmid pXL49 (27), which encodes a version of the Cinnamomum camphorum thioesterase (GenBankTM U31813) optimized for E. coli expression was the kind gift of H. Vora and C. Khosla. Plasmid pXL49 was cut with NdeI and HindIII and ligated to vector pQE-2 (Qiagen) cut with the same enzymes to give plasmid pCY792. The C. camphorum thioesterase coding sequence was inserted into several other vectors to facilitate alterations done by cassette mutagenesis in which synthetic double-stranded mutant sequences were exchanged for the wild-type sequences using restriction sites within the coding sequence. After confirmation by sequencing, the modified genes were then inserted into vector pQE-2 as described above to give plasmids pCY793 and pCY798 that encode the R197M/R199H and R197M/R199H/K231T thioesterases, respectively.
RNA Isolation and RT-PCR
Exponentially growing cultures of three strains (Ymel, K113, and DC170) were used for RNA isolation performed as recommended by the manufacturer with minor modifications. The RNA samples were separated by electrophoresis on 0.8% agarose gels to assess RNA quality from the rRNA profiles. To rule out the possibility of residual DNA contamination, general PCR-based detection was carried out using the total RNA as template and two pairs of specific primers (16S-F/16S-R, fabA-F/fabA-R). RNA samples that satisfied the quality criteria were subjected to RT-PCR analyses by the following protocol. A mixture of RNA (1 μg) and random primers (0.5 μg) in a volume of 22 μl was heated at 70 °C for 5 min and then chilled on ice for 5 min. To this mixture was added 8 μl of ImProm-IITM 5× reaction buffer, 5 μl of the supplied MgCl2 solution, 2 μl of the supplied dNTP mix, 1μl of the supplied recombinant RNasin ribonuclease inhibitor solution, and 1 and 2 μl of ImProm-II reverse transcriptase solution to give a total volume of 40 μl. This mixture was equilibrated at 25 °C for 5 min, extended at 42 °C for 60 min, and the enzyme was inactivated at 70 °C for 15 min. The cDNA (1 μl) generated was directly used as template for a 50-μl general PCR amplification containing the corresponding primers (supplemental Table S2) using an Eppendorf thermal cycler.
Real Time Quantitative PCR
To quantify fabA transcription levels, real time quantitative PCR was adopted using the SYBR Green dye method (28, 29). The reaction mixtures (20 μl) were composed of iQ SYBR Green Supermix, 12.5 μl; primers of 1 μl each; and diluted cDNA sample, 1 μl. All reactions were performed in triplicate on a Mastercycler ep realplex (Eppendorf), in which the program was a denaturing cycle at 95 °C for 15 min, followed by 45 cycles comprising 94 °C for 20 s, 60 °C for 20 s, and 72 °C for 20 s. The 16 S rRNA here served as an internal standard (supplemental Table S2) and a blank sample containing only water as template monitored cross-contamination of the cDNA samples. The relative levels of fabA transcription were calculated as described by Livak and Schmittgen (30).
β-Galactosidase Assays
Overnight E. coli cultures (e.g. MFH66) were subcultured in 10 ml of glucose minimal medium E and incubated with shaking at 37 °C. When the culture reached mid-log phase the cells were pelleted and twice washed with Z buffer (31). The optical density at 600 nm of the resuspended cell suspension was determined and the cells were permeabilized by chloroform/SDS treatment and β-galactosidase was assayed according to Miller (31).
5′-RACE Analyses
To fine map the various fabA transcription start sites two RACE kits from different manufacturers (the Invitrogen 5′-RACE kit and the Ambion RLM-RACE kit) were employed. Although the Ambion procedure is designed to detect only those RNA molecules having a 5′-triphosphate indicating they are primary transcription products we obtained the same 5′ ends with both procedures indicating the absence of RNA processing events. For the Invitrogen kit the total RNAs extracted from cultures of strains Ymel, K113, and DC170 were prepared for first strand cDNA synthesis with the reverse primer fabA-GSP (supplemental Table S2). After purification of the cDNA via the supplied column, an oligo(dC) tail was attached to the 5′-end of the first strand of cDNA via a terminal deoxynucleotidyl transferase-mediated reaction at 65 °C. The resulting dC-tailed cDNA functioned as the template for PCR amplification using an abridged anchor primer together with the fabA-GSP primer. Using the nested primer, fabA-R and AUAP (supplemental Table S2), nested PCR was carried out with the template of the above PCR products diluted 20-fold. The same cycling protocol adopted in the above two rounds of PCR amplifications was characterized with a pre-denaturing step at 94 °C for 5 min, and 35 cycles consisting of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, followed by a final extension step at 72 °C for 8 min. The resulting PCR products were purified by agarose gel electrophoresis and directly cloned into pGEM-T-Easy or pCR2.1 vectors for DNA sequencing. The first nucleotide that followed the oligo(dG) sequence was regarded as the putative transcriptional initiation site. For the Ambion 5′-RLM-RACE kit the RNA samples were treated with phosphatase to remove 5′-phosphates from degraded mRNA, rRNA, and tRNA species. Following inactivation of the phosphatase the RNA samples were treated with tobacco acid pyrophosphatase to convert mRNA triphosphates to the monophosphates needed for RNA ligase addition of the 5′-RACE adaptor. Reverse transcription was then performed using random primers as recommended by the manufacturer followed by nested PCR using outer 5′-RLM-RACE PCR (with the 5′-RACE Outer and fabA-GSP) primers and inner 5′-RLM RACE PCR (with the 5′-RACE Inner and fabA-R primers) (supplemental Table S2). The PCR products were then analyzed by gel electrophoresis followed by and insertion into vector pCR2.1 TOPO and DNA sequencing. The first nucleotide following the 5′-RACE adaptor was taken as the transcription start site.
Labeling and Analysis of Fatty Acids during Thioesterase Expression
Log phase cultures of the thioesterase encoding strains (about 3 × 108 cells/ml) were grown at 30 °C in RB medium containing ticarillin/clavulanate and then shifted to the non-permissive temperature of 43 °C for 0.5–1 h to inactivate the thermolabile mutant enzymes. The cultures were then diluted 3-fold into fresh 43 °C medium containing 1 mm isopropyl 1-thio-β-d-galactopyranoside to induce thioesterase expression together with [1-14C]acetate to label the newly synthesized fatty acids. The cultures were incubated at 43 °C with shaking for 5–6 h. After labeling the cells were pelleted at 2500 × g for 10 min and each 3-ml of supernatant was decanted into a second tube. Glacial acetic acid (0.15 ml) was added to break down any fatty acid salts followed by 9 ml of methanol/chloroform (2/1). The resulting single-phase mixture was then supplemented with 10 μg each of cis-3-decenoic acid, cis-5-dodecenoic acid, and cis-7-tetradecenoic acid. Three ml each of chloroform and water were added to each tube and after mixing was allowed to settle to give a two-phase mixture. The bottom (chloroform) phase was then removed to a new tube and carefully taken to dryness under nitrogen at 40 °C. To each tube 0.2 ml of ethyl ether/methanol (9/1) was added sufficient trimethylsilyldiazomethane (2 m in hexanes from Aldrich) to give a stable yellow solution. The tubes were capped and allowed to stand for at least 1 h. The excess trimethylsilyldiazomethane was then consumed by addition of 5-μl aliquots of glacial acetic acid until the solution was colorless and the resulting mixture was then either directly spotted on a silver nitrate TLC plate or carefully taken to dryness under nitrogen at 20 °C, taken up in hexane, and applied to a silver ion chromatographic column. Argentation (silver ion) TLC was done on 250-μm thick Silica Gel G plates impregnated with 20% silver nitrate (Analtech) activated at 110 °C for 1 h before use. The plates were developed three times in toluene at −15 °C in an explosion-proof freezer and dried between developments with a hair dryer (32). Argentation column chromatography was done on 750-mg Discovery Ag-ION columns (Sigma) as described by the manufacturer except that elution of the saturated methyl esters was done with hexane/acetone (98/2) rather than the recommended hexane/acetone (96/4) because the latter solvent eluted methyl cis-11-octadecenoate and methyl cis-3-decenoate, the most weakly binding of the cis-unsaturated species synthesized. Subsequent elution of test columns with hexane/acetone (96/4) showed that the hexane/acetone (98/2) solvent quantitatively eluted the saturated species and that no detectable cis- or trans-unsaturated esters were present in the hexane/acetone (96/4) fraction. Reverse phase chromatography was done on Partisil KC18 plates of octadecyl-modified Silica Gel 60 (Whatman) using a solvent system of acetonitrile/methanol/water (65/35/0.5). All thin layer plates were divided into lanes using a layer scriber to prevent cross-contamination.
For mass spectral analyses unsaturated methyl ester samples in hexane were converted to their dimethyldisulfide adducts by treatment with 200 μl of dimethyldisulfide and one drop of 6% iodine solution in diethyl ether for 14 h at 45 °C. The samples were cooled, diluted with 200 μl of hexane, and extracted with 50 μl of 10% aqueous Na2S2O3 to remove iodine. The aqueous extract was then extracted with hexane. The hexane extracts were pooled and concentrated to 50 μl under N2. Gas chromatography-mass spectroscopy analyses were done on an Agilent system consisting of a 5975C Mass Selective Detector, a 7683B autosampler, and a 7890A gas chromatograph equipped with an Agilent 60-m HP-5MS column with 0.25-mm inner diameter and 0.25-mm film thickness. Injection temperature and the Mass Selective Detector transfer line were set to 280 °C, the ion source and MS quadrupole were adjusted to 230 and 150 °C, respectively. The helium carrier gas was set at a constant flow rate of 1.6 ml/min. The temperature program was: 2 min of isothermal heating at 140 °C, followed by an oven temperature increase of 20 °C/min until 325 °C was reached followed by 10 min at that temperature. A 1-μl sample was injected with a split ratio of 10/1. The spectra acquired were recorded in the m/z 25–800 scanning range and processed using AMDIS (National Institute of Standards and Technology) and MSD ChemStation D.02.00.275 (Agilent) software and compared with standard mass spectrum libraries NIST08 (National Institute of Standards and Technology), WILEY8n (Palisade Corporation), and a custom library.
Fatty acid methyl esters were analyzed on the same instrument except that a ZB-WAX (Phenomenex Inc) capillary column (30 m × 0.25 mm inner diameter and 250-μm film thickness) was used. A 1-μl sample was injected with the split ratio of 5/1. Inlet temperature was 230 °C and the interface temperature was 250 °C. Helium as the carrier gas was set at a constant flow rate of 1/ml. The temperature program was: 140 °C for 5 min, 5 °C/min to 265 °C, and then held at 265 °C for 10 min. Mass spectra were obtained as described above. Fatty acid compositions of membrane phospholipids were determined as previously described (33).
RESULTS
The fabA Promoters
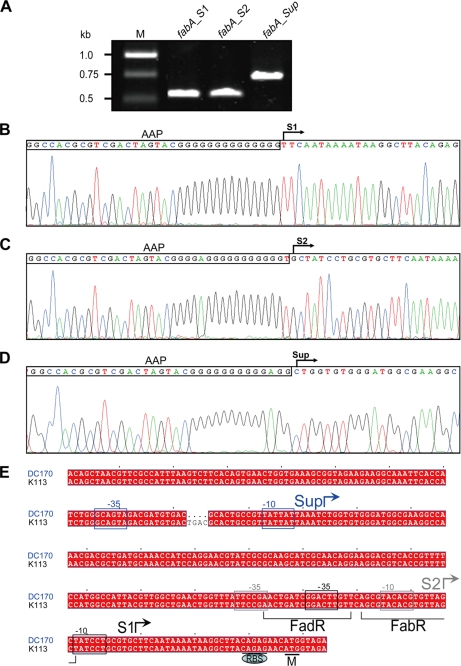
Prior work from this laboratory identified the FadR-dependent fabA promoter and the 5′-end of the fabAup transcript (5, 6). We also had evidence for a weaker promoter that did not depend on FadR activation and would function when activation by FadR was not available (see Introduction). We were unable to map the weaker promoter due to low sensitivity of the mapping methods then available (6). At that time we proposed that the fabAup promoter was a version of the weak promoter that had been improved by mutation. We now report this is not the case. The 5′-end of the fabAup transcript previously mapped by primer extension (6) was confirmed as the initiation site by 5′-RACE analysis using two different methods. Although one procedure was designed to detect only those RNA molecules that have a 5′-triphosphate (thereby indicating they are primary transcription products) we obtained the same 5′-ends with both procedures indicating the absence of RNA processing events. This approach was also used to determine the initiation site of the weaker promoter (Fig. 2). Because the transcripts of the two promoters began at sequences separated by 162 bp, our prior hypothesis was clearly incorrect. This raised the question of the origin of the fabAup promoter (PfabAup). We determined the DNA sequences upstream of the initiation site of PfabAup in both the fabAup mutant strain and its parent and found that the mutant strain carried a four-base deletion within the coding sequence of the upstream gene, ycbZ, which encodes a nonessential (34) Lon protease homologue (Fig. 2). The data characterizing this mutant promoter are given under supplemental Results and Figs. S1 and S2).
FIGURE 2.
Analyses of the three promoters from which the fabA gene is transcribed. Panel A, RT-PCR analysis of fabA transcription versions in different strains. M, DNA marker. fabA_S1, transcript of the wild-type strain, Ymel; fabA_S2, transcript of the fadR strain K113; fabA_Sup, transcript of the fabAup fadR strain DC170. Panel B, mapping of transcription start site, S1 for the FadR-dependent promoter, P1 from the wild-type strain. Panel C, mapping of transcription start site, S2 for the promoter, P2 of the fadR strain K113. Panel D, mapping of transcription start site Sup for promoter, PfabAup, of the fabAup fadR strain DC170. The sequencing traces of plasmids carrying the 5′-RACE products are shown. Abridged anchor primer (AAP) is denoted by the rectangle. Panel E, sequence alignments of fabA promoter regions of strains K113 and DC170. −10 and −35 (in rectangles) are two conserved hexamers of E. coli σ70 promoters. Initiation site Sup (in blue) is specific to the fabAup fadR strain DC170 (blue). Initiation site S2 (in gray) is found in fadR strain K113, whereas S1 (black) is functional in the wild-type strain Ymel. The binding sites of FadR and FabR are indicated with square brackets. “CAGAG” is a ribosome binding site (RBS). The fabA initiation codon is designated M.
P2, the FadR-independent fabA Promoter
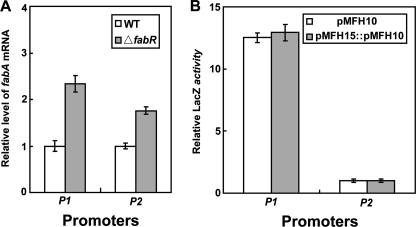
This promoter was largely defined by residual transcriptional activity observed in the absence of FadR that seemed to originate upstream of the P1 promoter (hence its association with the fabAup mutation), but we were unable to map the 5′-end of the transcript at that time (5, 6). Both RT-PCR experiments clearly showed a fabA transcript in cells lacking FadR that was shorter than the fabAup transcript of strain DC170, but slightly longer than that made from P1 (Fig. 2A). Hence, even by use of these very sensitive methods we were unable to confirm the faint Northern blot bands seen in the absence of FadR that seemed to denote a longer transcript similar in size to the fabAup transcript (5). The initiation site of the weak promoter (now called P2) was mapped by 5′-RACE and found to begin 13 bp upstream of the P1 initiation site (Fig. 2). Therefore, fabA was transcribed from two overlapping promoters. Moreover, the −10 region of the P2 promoter was located within the FabR binding site suggesting that P2 promoter function was repressed by FabR. This was confirmed by quantitative RT-PCR, which showed that the degree of regulation was modest; deletion of FabR resulted in about an 80% increase in P2 transcripts, whereas P1 transcripts showed about a 2-fold increase (Fig. 3). FabR also blocked transcription originating well upstream because the level of fabAup transcripts were increased over 3-fold in the strain lacking FabR (data not shown). In contrast, the FadR protein had no obvious effect on expression from P2 (Fig. 3) and failed to block expression from the fabAup promoter (data not shown).
FIGURE 3.
Relative activities of the fabA promoters in vivo. Panel A, real time quantitative RT-PCR assays of fabA expression normalized to the mRNA level expressed from the fabA gene of fadR strain K113 in the presence or absence of FabR. To monitor cross-contamination of different cDNA samples, a blank sample was utilized in which no cDNA was added. Each sample was assayed four times, and the data are expressed in mean ± S.D. Panel B, β-galactosidase activities of fusion constructs in which the fabA gene was expressed from either P1 (in the presence of FadR, strain Ymel) or P2 (in the absence of FadR, strain K113). Two different plasmids carrying the same fusion construct at different copy numbers are shown.
The Block in fabB Mutants
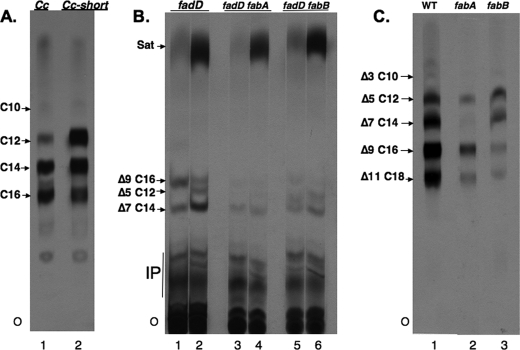
The early intermediates in E. coli UFA synthesis, cis-5-dodecenoic acid and cis-7-tetradecenoic acid, have not been demonstrated in vivo because these acids are not incorporated into the complex lipids of this bacterium (although in the case of cis-7-tetradecenoic acid it has been shown to accumulate in the phospholipids of cells grown under conditions of acetyl-CoA limitation (22)). Therefore, we used plant thioesterases that cleave short chain acyl-ACPs to intercept the growing acyl chains before they were elongated to the acyl moieties that are substrates for incorporation into complex lipids. The cleavage products of acyl-ACP intermediates are FFA and ACP (35). The parent thioesterase was encoded by a codon-optimized version of the C. camphorum (camphor tree) thioesterase (27). This enzyme preferentially releases C14 fatty acids together with lesser amounts of C12 and C16 fatty acids (27, 36). Based on prior work with the closely related (92% sequence identity) seed oil thioesterase from Umbellularia californica (the California bay tree) (36), which preferentially releases C12 fatty acids with lesser amounts of C14 fatty acids, we mutated specific codons of the C. camphorum thioesterase gene with the aim of increasing the levels of short chain fatty acids released. This mutant enzyme was used because expression of the U. californica thioesterase in E. coli was reported to be toxic at 37 °C and lead to plasmid instability (37). In contrast, the C. camphorum thioesterase had been reported to function well at 37 °C (36) and thus seemed better suited to the high temperatures required in our experiments (see below). In prior work with U. californica thioesterase, three residues were converted to those found in the C. camphorum enzyme resulting in a specificity switch to longer chain lengths (36). We reversed this process and converted C. camphorum thioesterase residues Arg-197 and Arg-199 to methionine and histidine, respectively, resulting in the double mutant (R197M/R199H) enzyme we call the Cc-short thioesterase. These mutations resulted in increased release of short chain fatty acids in both the saturated (Fig. 4A) and unsaturated (Fig. 4B) branches of the fatty acid synthetic pathway. We also introduced a third mutation (Lys-231 to threonine) into the Cc-short thioesterase gene. Although in the U. californica enzyme the reverse mutation gave a further shift toward longer chain specificity (but only in the presence of the first two mutations) (36), in the present case the additional mutation gave no further increase in cleavage of short chain substrates and resulted in an enzyme that was considerably less active in fatty acid release in E. coli (data not shown). Efficient recovery of the released fatty acids requires inactivation of the cellular β-oxidation system. If β-oxidation is active, fatty acids are converted to their CoA esters and degraded (38, 39). The β-oxidation system has generally been blocked by introduction of a mutation in fadD, the enzyme encoding the aerobic acyl-CoA synthetase, the first step of the β-oxidation pathway (38). This mutation not only blocks β-oxidation but also prevents conversion of the released fatty acids to their acyl-CoA thioesters thereby allowing recovery of acids that would otherwise be sequestered as intracellular acyl-CoA esters. Based on prior work (37, 40) we used the fadD strain K27 because this strain is known to release the liberated fatty acids to the medium (another E. coli strain is reported to retain the FFA within the cells (27)). It should be noted that plant thioesterases have long been characterized by their expression in β-oxidation-deficient E. coli strains (20, 35, 37, 39, 41). The acids released to the culture medium (both unsaturated and saturated acids of chain lengths 8–18 carbon atoms) accurately reflect the substrate specificities observed for the purified enzymes in vitro (20, 35, 37, 39, 41–44). When examined export was essentially quantitative, although no discrete export system has been identified. We introduced temperature-sensitive (ts) mutations in either fabB or fabA into strain K27 by phage P1-mediated transduction using closely linked Tn10 insertions as selectable markers. Temperature-sensitive mutations were used rather than null mutations because a strain having fabB or a fabA null mutation has an obligate requirement for an unsaturated fatty acid and FabD is required for uptake of the supplement. That is, the combination of null mutations in fadD and fabA or fabB was expected to be synthetically lethal even in the presence of oleic acid supplementation. Indeed, the fabB(ts) fadD and fabA(ts) fadD strains constructed (supplemental Table S1) were unable to grow at the nonpermissive temperature either in the presence or absence of oleic acid, whereas both strains grew well at the permissive temperature of 30 °C. It should be noted that although the inviability of mutant strains lacking FadD and either FabA or FabB has long been assumed, this appears to be the first report of the expected synthetic lethal phenotype. The experimental design was to introduce a plasmid encoding a thioesterase under a controllable promoter into fadD, fadD, fabB(ts), and fadD fabA(ts) strains. It should be noted that prior work on the expression of plant thioesterases in E. coli had noted the accumulation of monounsaturated C12 and C14 acids in the FFA fraction that were interpreted as UFA synthetic intermediates (27, 37). However, because neither the positions nor the configurations of the double bonds of these fatty acids were determined, these acids could have been species other than UFA synthetic intermediates such as the trans-2-enoic acids formed as intermediates in the fatty acid elongation cycle.
FIGURE 4.
Analysis of the [1-14C]acetate-labeled fatty acids excreted into the medium during thioesterase expression. The extracted fatty acids were converted to their methyl esters prior to chromatography. Panel A, effects of thioesterase mutations on chain length specificity. The methyl esters of the saturated fatty acids excreted by fadD strain K27 expressing either the wild-type C. camphorum or mutant Cc-short thioesterases were recovered by argentation column chromatography and separated by reverse phase TLC. The number following the C denotes the acyl chain length. Panel B, argentation TLC analysis. The plate was developed three times in toluene at −15 °C and then exposed to x-ray film. The spots were identified by co-chromatography with authentic standards detected under ultraviolet light after spraying the plate with 2,7-dichlorofluorescein (0.1% in 95% ethanol). Each pair of lanes is from a different E. coli strain as denoted at the top of the figure. In the odd numbered lanes the thioesterase expressed was the wild-type C. camphorum enzyme, whereas in the even numbered lanes the Cc-short thioesterase was expressed. The nomenclature indicates the position of the double bond by the number following the Δ symbol, whereas the chain length is as above. Hence, Δ5 C12 is cis-5-dodecenoic acid. The material marked IP are probably isoprenoid compounds. O denotes the origin. Note that the trans isomer of each of these unsaturates migrates well ahead of the cis isomer (32, 46). Panel C, the UFA methyl ester fraction resulting from argentation column chromatography was analyzed by reverse phase TLC. All three strains expressed the Cc-short thioesterase and were fadD. The fab phenotype of each strain is shown at the top of the figure. The spots were identified as in panel A except that the plates were sprayed with 0.03% 1,6-diphenyl-2,3,5-hexatriene in chloroform. Note that due to the increased polarity imparted by the double bond an unsaturated fatty acid ester migrates slightly more rapidly than the saturated fatty acid that is two carbons shorter. There was no isoprenoid contamination because these compounds fail to elute from argentation chromatographic matrix. All of the lanes of a panel are from the same plate, but the lanes were sometimes rearranged to facilitate presentation. The data of panels B and C are characteristic of those obtained in seven independent experiments.
Our experiments used vector pQE-2 to give high-level thioesterase expression intended to increase interception of the shorter (e.g. C10) acyl-ACP species. Transcription was from a phage T5 promoter controlled by two lacZYA operators and the vector-encoded LacI repressor. Following thioesterase induction at the non-permissive temperature of the mutant strains, we used incorporation of [1-14C]acetate to label the newly made fatty acids. The primary reason for radioactive labeling was to allow addition of nonradioactive short chain UFAs as carriers to counter the volatility of these acids and (especially) their methyl esters. However, another advantage was that analysis of radioactive FFA restricted the products observed to those FFA formed at the non-permissive temperature of the mutant strains (basal thioesterase expression gave low levels of FFA accumulation at the permissive temperature). Log phase cultures of the thioesterase-encoding strains were grown at 30 °C and briefly shifted to the non-permissive temperature of 43 °C to inactivate the thermolabile mutant enzymes. The cultures were then diluted into fresh 43 °C medium containing isopropyl β-d-1-thiogalactopyranoside to induce thioesterase expression and [1-14C]acetate to label the newly synthesized fatty acids followed by incubation at 43 °C to allow fatty acid accumulation. This protocol was chosen because most of the fatty acid accumulation occurs during the entry into stationary phase (27, 37) and the cessation of growth in stationary phase prevents the growth-dependent cell lysis characteristic of UFA-starved cultures (3). Following incubation the cultures were centrifuged and the supernatants were supplemented with a mixture of nonradioactive short chain UFAs as carrier, acidified and subjected to lipid extraction. The extracted lipids were then treated with trimethylsilyldiazomethane. This reagent converts FFA to their methyl esters, but leaves the acyl chains of any complex lipids unchanged. The resulting methyl ester preparations were then resolved by argentation (silver ion) and/or reversed phase chromatography. Argentation thin layer chromatography (TLC) resolves monoenoic UFA methyl esters based on double bond configuration and position and also resolves cis-unsaturated esters from trans-unsaturated and saturated esters (45). Based on the prediction of Morris and co-workers (32) we expected methyl cis-5-dodecenoate to migrate behind methyl cis-7-tetradecenoate, but instead found that it migrated between the cis-7 and cis-9 monounsaturated methyl esters (see Fig. 1) as was reported by other workers using a different solvent system (46). Because the methyl cis-5-dodecenoate spot often overlapped either the cis-9 or cis-11 monounsaturated methyl ester spots, we adopted a scheme in which the UFA methyl esters were first separated from the saturated species as a group using argentation column chromatography followed by reverse phase TLC resolution of the component acids based on their differing chain lengths (see Fig. 4C). These analyses showed that the culture medium of wild-type strain K27 contained the following 14C-labeled unsaturated species: cis-5-dodecenoate, cis-7-tetradecenoate, cis-9-hexadecenoate, and cis-11-octadecenoate. Small amounts of cis-3-decenoate were also seen. These same fatty acids were found in the culture medium of fabA(ts) fadD and fabB(ts) fadD strains, the important result being the lack of a marked accumulation of cis-5-dodecenoate in the FabB-deficient strain (see Fig. 4). Phosphorimaging analysis of the lanes of Fig. 4C showed that relative to the wild-type strain the fabB(ts) and fabA(ts) strains accumulated only 66 and 34% as much cis-5-dodecenoate, respectively, as the wild-type strain. The wild-type strain also accumulated 2.7-fold more cis-7-tetradecenoate than the fabB(Ts) strain, whereas the value for the fabA(ts) strain was 14.1-fold. As will be discussed below, the lack of accumulation of cis-5-dodecenoate was evidence as the key FabB reaction is elongation of the cis-3-decenoyl-ACP produced by FabA. Note that both the fabA(ts) fadD and fabB(ts) fadD strains produced low levels of UFA at the non-permissive temperature consistent with prior analyses of their phospholipid acyl chains (3, 15).
The identities of the unsaturated acids were confirmed by mass spectral analysis of the fatty acids excreted into the medium of the fadD strain K27 expressing either the wild-type or Cc-short thioesterases. The fatty acids were extracted, converted to methyl esters, and the cis-unsaturated esters resolved from the saturated (and any trans unsaturated) species by argentation column chromatography. The unsaturated esters were then converted to their dimethyldisulfide adducts that were analyzed by gas chromatography-mass spectroscopy. Fragmentation of the adducts in the mass spectrometer cleaved each chain between the carbons that originally constituted the double bond to yield two diagnostic fragments that unequivocally demonstrated the position of each double bond. All four unsaturated species gave a fragment of m/z = 145 derived from the methyl ends of the molecules. The second fragment indicated the distance from the double bond to the carboxyl end of the chain with each two-carbon chain extension adding the expected 28 atomic mass units (Fig. 5). These analyses showed that the four species were the methyl esters of cis-5-dodecenoic acid, cis-7-tetradecenoic acid, cis-9-hexadecenoic (palmitoleic) acid, and cis-11-octadecenoic (cis-vaccenic) acid in agreement with the TLC analyses (the assignments of the double bond conformations as cis were based on argentation chromatography behavior). We did not detect methyl cis-3-dodecenoic acid probably due to the volatility of the acid and its derivatives. Gas chromatography of the saturated fatty acid methyl ester fraction (data not shown) detected the same species seen by reverse phase TLC.
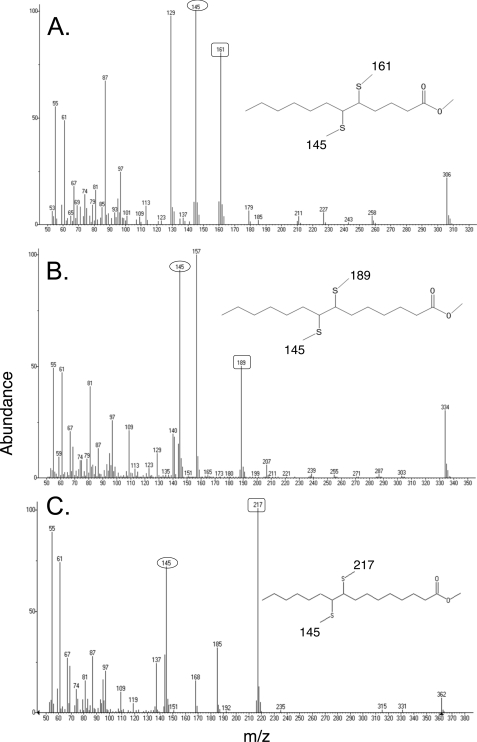
FIGURE 5.
Mass spectroscopy of the unsaturated fatty acids extracted from the medium of wild-type strain K27 expressing the C. camphorum thioesterase. The unsaturated species obtained as described under “Experimental Procedures” were then derivatized first to their methyl esters and then to their dimethyl disulfide adducts that were analyzed by gas chromatography-mass spectroscopy. The mass spectra are of: panel A, methyl cis-5-dodecenoic acid; panel B, methyl cis-7-tetradecenoic acid; and panel C, methyl cis-9-hexadecenoic acid. Each of the unsaturated esters gave a cleavage fragment of m/z 145 (numbers in ovals) corresponding to the methyl end of the molecule and a second fragment corresponding to the ester end of the molecule (numbers in squares). Not shown is the spectrum of methyl cis-11-octadecenoic acid, which gave the m/z 145 fragment plus the expected m/z 245 ester end fragment. Essentially identical fragmentation patterns were obtained for the fatty acids released by strain K27 expressing the Cc-short thioesterase (data not shown).
DISCUSSION
Our analyses show that fabA is transcribed from two overlapping promoters, both of which show modest regulation by the FabR repressor protein. Only the upstream promoter is regulated by FadR consistent with the role of the protein as a positive activator that binds at the −40 region of the promoter (6). We found the prior hypothesis (6) that the fabAup mutant promoter was an improved version of a natural promoter was incorrect. The PfabAup promoter arose from a deletion event that created a new promoter from an otherwise inactive DNA sequence (supplemental Figs. S1 and S2). It is not clear why transcription of fabA, a housekeeping gene, is so complex. A small stretch of DNA binds two regulatory proteins and is able to bind RNA polymerase at two overlapping sites. FadR regulation provides a means to decrease UFA synthesis when UFAs are present in the medium. Exogenous UFAs are converted to CoA esters that bind FadR and release it from the P1 promoter resulting in decreased fabA transcription (6). However, the same regulation occurs when a long chain SFA is added to the medium. How then can E. coli retain sufficient UFA synthesis in the presence of exogenous SFAs? Promoter P2 may provide the needed fail-safe mechanism by ensuring some fabA expression in the absence of FadR activation (8). However, this scenario is somewhat complicated by the weak FabR-mediated repression of P2. The FabR regulatory ligand is unknown and thus the physiological role of the protein is unclear and probably will remain so until the extreme tendency of FabR to form insoluble aggregates (data not shown) can be overcome. However, the importance of the P2 promoter and the overlapping FabR binding site is supported by its absolute conservation in all 58 E. coli and Shigella genomes in GenBank as well as in all 44 Salmonella enterica genome sequences plus those of strains of Citrobacter, Yersinia, and Enterobacter. The fact that the exact sequence has been maintained during the 30 million years since the divergence of E. coli and S. enterica argues that promoter P2 plays an important role in fabA expression.
The use of plant thioesterases specific for short chain acyl-ACPs has allowed the intermediates of UFA synthesis to be followed in vivo for the first time. Our finding that strains deficient in FabB activity release a spectrum of UFA intermediates similar to that released by a strain deficient in FabA activity indicates that the two enzymes catalyze steps at the same level of the pathway. If the synthetic block in FabB strains was exerted after formation of cis-5-dodecenoyl-ACP, then cis-5-dodecenoic acid (and perhaps cis-7-tetradecenoic acid) would have accumulated at the expense of the longer UFA species (Fig. 1). A parallel situation arises in mutants lacking the other E. coli long chain 3-ketoacyl-ACP synthase, FabF, which are defective in elongation of cis-9-hexadecenoate to cis-11-octadecenoate (14, 15, 47). Extracts of the fabF mutant strain synthesize 7-fold more cis-9-hexadecenoate than wild-type extracts (47). However, no comparable accumulation of cis-5-dodecenoic acid in the medium of the fabB(ts) strain was seen. Indeed, the levels were lower than those of the wild-type strain (Fig. 4). Therefore, the block in fabB strains must be prior to the synthesis of cis-5-dodecenoyl-ACP (see Fig. 1). From the reactions catalyzed by FabA and the knowledge that FabB is a 3-ketoacyl-ACP synthase, it follows that FabB catalyzes the elongation of cis-3-decenoyl-ACP produced by FabA to generate the first 12-carbon cis-unsaturated fatty acid intermediate, 3-keto-cis-5-decenoyl-ACP, which is converted to cis-5-dodecenoyl-ACP (Fig. 1). The fabB(ts) strain released cis-5-dodecenoic acid rather than another possible 12 carbon-unsaturated intermediate such as 3-hydroxy-cis-5-dodecenoic acid. This can be attributed to the equilibrium of the FabI reaction, which dictates that the hydroxy and keto intermediates do not accumulate (21). Another possibility is that plant thioesterases are thought to have evolved for cleavage of saturated acyl-ACPs (20) and thus their active sites may be unable to accommodate bulky hydroxyl and ketone groups.
It might be expected that because FabB-deficient cultures elongate cis-3-decenoyl-ACP poorly, they should release high levels of cis-3-decenoic acid. However, only low levels of cis-3-decenoic acid were detected by radioactive labeling (Fig. 4). The lack of accumulation of this acid is readily explained by the fact that all of the reactions catalyzed by FabA are freely reversible (1, 48) (Fig. 1). Therefore, the bulk of the cis-3-decenoyl-ACP formed would be isomerized back to trans-2-decenoyl-ACP. The trans-2-decenoyl-ACP would be reduced by the FabI enoyl-ACP reductase and the acyl chain would enter the SFA synthetic branch. Indeed, it has been shown that overproduction of FabA increases the levels of SFA moieties rather than the levels of phospholipid UFA moieties in vivo (12). This effect was nullified when both FabA and FabB were overproduced (12) indicating that FabB is the limiting step in UFA synthesis and any excess cis-3-decenoyl-ACP produced by FabA would be diverted to the saturated fatty acid synthetic pathway (12). It should also be noted that the chain lengths of the FFA released from the fabB(ts) strain are somewhat shorter than those released by the wild-type and fabA(ts) strains (see Fig. 4). This is to be expected because the FabB-deficient strain would have less than one-third of the normal level of long chain 3-ketoacyl-ACP synthase activity (14, 15, 49). (The fabB strain has only the FabF elongation activity, whereas the other strains contain both FabF and FabB.) Slowing elongation would increase the probability that the thioesterase would cleave an acyl-ACP before its elongation to chain lengths where it would become noncleavable (due either to thioesterase specificity or incorporation of the acyl chain into complex lipids). Therefore, limitation of FabB activity should result in shortening of the chains released. Indeed, the fabA(Ts) strain accumulated the longer chain UFAs at the expense of cis-7-tetradecenoic acid, whereas the fabB(Ts) strain accumulated predominately cis-7-tetradecenoic acid.
Supplementary Material
Acknowledgment
We thank Dr. Alex Ulanov of the Carver Metabolomics Center for help with the mass spectral analyses.
This work was supported, in whole or in part, by National Institutes of Health Grant AI15650 from the NIAID.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Results,” Figs. S1 and S2, and Tables S1 and S2.
- ACP
- acyl carrier protein
- UFA
- unsaturated fatty acid
- SFA
- saturated fatty acid
- RT
- reverse transcriptase
- FFA
- free fatty acid
- 5′-RACE
- 5′-rapid amplification of cDNA ends.
REFERENCES
- 1.Bloch K. (1971) in The Enzymes (Boyer P. D. ed) 3rd Ed., pp. 441–464, Academic Press, New York [Google Scholar]
- 2.Cronan J. E., Jr., Li W. B., Coleman R., Narasimhan M., de Mendoza D., Schwab J. M. (1988) J. Biol. Chem. 263, 4641–4646 [PubMed] [Google Scholar]
- 3.Cronan J. E., Jr., Gelmann E. P. (1973) J. Biol. Chem. 248, 1188–1195 [PubMed] [Google Scholar]
- 4.Silbert D. F., Vagelos P. R. (1967) Proc. Natl. Acad. Sci. U.S.A. 58, 1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry M. F., Cronan J. E., Jr. (1991) J. Mol. Biol. 222, 843–849 [DOI] [PubMed] [Google Scholar]
- 6.Henry M. F., Cronan J. E., Jr. (1992) Cell 70, 671–679 [DOI] [PubMed] [Google Scholar]
- 7.DiRusso C. C., Metzger A. K., Heimert T. L. (1993) Mol. Microbiol. 7, 311–322 [DOI] [PubMed] [Google Scholar]
- 8.Cronan J. E., Jr., Subrahmanyam S. (1998) Mol. Microbiol. 29, 937–943 [DOI] [PubMed] [Google Scholar]
- 9.Nunn W. D., Giffin K., Clark D., Cronan J. E., Jr. (1983) J. Bacteriol. 154, 554–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y. M., Marrakchi H., Rock C. O. (2002) J. Biol. Chem. 277, 15558–15565 [DOI] [PubMed] [Google Scholar]
- 11.McCue L., Thompson W., Carmack C., Ryan M. P., Liu J. S., Derbyshire V., Lawrence C. E. (2001) Nucleic Acids Res. 29, 774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark D. P., DeMendoza D., Polacco M. L., Cronan J. E., Jr. (1983) Biochemistry 22, 5897–5902 [DOI] [PubMed] [Google Scholar]
- 13.Cronan J. E., Jr., Birge C. H., Vagelos P. R. (1969) J. Bacteriol. 100, 601–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garwin J. L., Klages A. L., Cronan J. E., Jr. (1980) J. Biol. Chem. 255, 11949–11956 [PubMed] [Google Scholar]
- 15.Garwin J. L., Klages A. L., Cronan J. E., Jr. (1980) J. Biol. Chem. 255, 3263–3265 [PubMed] [Google Scholar]
- 16.Cronan J., Rock C. (2008) in EcoSal, Escherichia coli and Salmonella: Cellular and Molecular Biology (Böck A., Curtiss R., 3rd, Kaper J., Karp P., Neidhardt F., Nyström T., Slauch J., Squires C., Ussery D. eds) Chapter 3.6.4, ASM Press, Washington, DC [Google Scholar]
- 17.Rock C. O., Cronan J. E. (1996) Biochim. Biophys. Acta 1302, 1–16 [DOI] [PubMed] [Google Scholar]
- 18.Edwards P., Nelsen J. S., Metz J. G., Dehesh K. (1997) FEBS Lett. 402, 62–66 [DOI] [PubMed] [Google Scholar]
- 19.White S. W., Zheng J., Zhang Y. M., Rock C. O. (2005) Annu. Rev. Biochem. 74, 791–831 [DOI] [PubMed] [Google Scholar]
- 20.Jones A., Davies H. M., Voelker T. A. (1995) Plant Cell 7, 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heath R. J., Rock C. O. (1995) J. Biol. Chem. 270, 26538–26542 [DOI] [PubMed] [Google Scholar]
- 22.Batchelor J. G., Cronan J. E., Jr. (1973) Biochem. Biophys. Res. Commun. 52, 1374–1380 [DOI] [PubMed] [Google Scholar]
- 23.Zhao M., Li J., Song Z., Desmond R., Tschaen D. M., Grabowski E. J. J., Reider P. J. (1998) Tetrahedron Lett. 39, 5323–5326 [Google Scholar]
- 24.Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. (1989) Microbiol. Rev. 53, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshikawa W., Akutsu H., Kyogoku Y., Akamatsu Y. (1985) Biochim. Biophys. Acta 821, 277–285 [DOI] [PubMed] [Google Scholar]
- 26.Vogel H. J., Bonner D. M. (1956) J. Biol. Chem. 218, 97–106 [PubMed] [Google Scholar]
- 27.Lu X., Vora H., Khosla C. (2008) Metab. Eng. 10, 333–339 [DOI] [PubMed] [Google Scholar]
- 28.Cho B. K., Knight E. M., Palsson B. Ø. (2006) Microbiology 152, 2207–2219 [DOI] [PubMed] [Google Scholar]
- 29.Feng Y., Li M., Zhang H., Zheng B., Han H., Wang C., Yan J., Tang J., Gao G. F. (2008) J. Bacteriol. 190, 7567–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 31.Miller J. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 32.Morris L. J., Wharry D. M. (1965) J. Chromatogr. 20, 27–37 [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Cronan J. E. (2003) J. Bacteriol. 185, 4930–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voelker T. (1996) Genet. Eng. 18, 111–133 [DOI] [PubMed] [Google Scholar]
- 36.Yuan L., Voelker T. A., Hawkins D. J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10639–10643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voelker T. A., Davies H. M. (1994) J. Bacteriol. 176, 7320–7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark D., Cronan J. (2005) in EcoSal, Escherichia coli and Salmonella: Cellular and Molecular Biology (Böck A., Curtiss R., 3rd, Kaper J., Karp P., Neidhardt F., Nyström T., Slauch J., Squires C., Ussery D. eds) Chapter 3.4.4, ASM Press, Washington, DC [Google Scholar]
- 39.Voelker T. A., Worrell A. C., Anderson L., Bleibaum J., Fan C., Hawkins D. J., Radke S. E., Davies H. M. (1992) Science 257, 72–74 [DOI] [PubMed] [Google Scholar]
- 40.Mayer K. M., Shanklin J. (2007) BMC Plant Biol. 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salas J. J., Ohlrogge J. B. (2002) Arch. Biochem. Biophys. 403, 25–34 [DOI] [PubMed] [Google Scholar]
- 42.Dörmann P., Voelker T. A., Ohlrogge J. B. (1995) Arch. Biochem. Biophys. 316, 612–618 [DOI] [PubMed] [Google Scholar]
- 43.Ghosh S. K., Bhattacharjee A., Jha J. K., Mondal A. K., Maiti M. K., Basu A., Ghosh D., Ghosh S., Sen S. K. (2007) Plant Physiol. Biochem. 45, 887–897 [DOI] [PubMed] [Google Scholar]
- 44.Jha J. K., Maiti M. K., Bhattacharjee A., Basu A., Sen P. C., Sen S. K. (2006) Plant Physiol. Biochem. 44, 645–655 [DOI] [PubMed] [Google Scholar]
- 45.Christie W. W. (2003) Lipid Analysis, The Oily Press, PJ Barnes & Associates, Bridgwater, UK [Google Scholar]
- 46.Gunstone F. D., Ismail I. A. (1967) Chem. Phys. Lipids 1, 376–385 [DOI] [PubMed] [Google Scholar]
- 47.Gelmann E. P., Cronan J. E., Jr. (1972) J. Bacteriol. 112, 381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerra D. J., Browse J. A. (1990) Arch. Biochem. Biophys. 280, 336–345 [DOI] [PubMed] [Google Scholar]
- 49.D'Agnolo G., Rosenfeld I. S., Vagelos P. R. (1975) J. Biol. Chem. 250, 5289–5294 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.