Abstract
Tristetraprolin (TTP) is a prototypic family member of CCCH-type tandem zinc-finger domain proteins that regulate mRNA destabilization in eukaryotic cells. TTP binds to AU-rich elements (AREs) in the 3′-untranslated region of certain mRNAs, including tumor necrosis factor α, granulocyte macrophage colony-stimulating factor, and immediate early response 3, thereby facilitating their ARE-mediated decay. Expression of TTP is up-regulated by a variety of agents, including inflammatory mediators such as tumor necrosis factor α, a prominent activator of the nuclear factor κB (NF-κB) family of transcription factors. Accordingly, TTP is involved in the negative feedback regulation of NF-κB through promoting mRNA degradation. We describe here a novel, ARE-mediated decay-independent function of TTP on the termination of NF-κB response: TTP suppresses the transcriptional activity of NF-κB-dependent promoters independent of its mRNA-destabilizing property. In TTP knock-out mouse embryonic fibroblasts, lack of TTP leads to enhanced nuclear p65 levels, which is associated with the up-regulation of specific, ARE-less NF-κB target genes. We find that attenuation of NF-κB activity is at least in part due to an interference of TTP with the nuclear import of the p65 subunit of the transcription factor. This novel role of TTP may synergize with its mRNA-degrading function to contribute to the efficient regulation of proinflammatory gene expression.
Tristetraprolin (TTP)4 was first identified in the late 1980s as an immediate early gene rapidly induced by phorbol 12-myristate 13-acetate (1–3) and a variety of other stimuli, including serum, insulin, and growth factors (4–7). The finding that this phosphoprotein is composed of a tandem zinc-finger structure, as well as its reported nuclear localization, initially led to the assumption that it might function as a transcriptional regulator (8–10). However, investigations over the last decade have demonstrated that TTP binds to and accelerates the degradation of mRNAs exhibiting high turnover rates (e.g. cytokines, growth factors, or proto-oncogenes) (11, 12). These mRNAs are characterized by AU-rich element (ARE) motifs in their 3′- untranslated regions that serve as docking sites for RNA-binding proteins. TTP was shown to bind a subset of these AREs, and the nonamer UUAUUUAUU was identified as the minimal complete binding site (13). The motif is recognized by the tandem CCCH-type zinc-finger domain of TTP, and the integrity of this domain has been shown to be indispensable for mRNA degradation because a single mutation within either zinc-finger abolishes the ARE binding activity (14). TTP exhibits no enzymatic activity itself but recruits various components of the basic RNA decay machinery via its N- and C-terminal domains (15, 16). The degradation of ARE-containing mRNAs is regulated mainly by extracellular cues. Typically, TTP target mRNAs are degraded rapidly in unstimulated cells but become transiently stabilized in response to activation. Several signaling pathways were shown to be involved in the control of ARE-mediated decay, including the p38 mitogen-activated protein kinase-MK2 pathway which accounts for direct phosphorylation of mouse TTP in response to lipopolysaccharide. Importantly, this phosphorylation stabilizes the TTP protein but abrogates its ability to degrade mRNAs, resulting in the accumulation of the known target tumor necrosis factor α (TNFα) (17–19). TNFα, a principal mediator of inflammation in acute and chronic inflammatory diseases (20), is up-regulated in TTP knock-out (ko) mice, leading to a complex syndrome of inflammatory arthritis (21). Likewise, mutations in TTP have been associated with autoimmune diseases in humans (22). In addition to TNFα, the turnover of several other mRNAs is controlled by TTP, including granulocyte macrophage colony-stimulating factor, cyclooxygenase-2, immediate early response 3, as well as the interleukins IL-2, -3, -6, and -10 (11, 12, 23, 24).
One of the main signaling pathways triggered by TNFα is the NF-κB pathway. NF-κB comprises a family of transcription factors (p65 (RelA), c-Rel, RelB, p50 (NF-κB1), p52 (NF-κB2)) that form homo- and heterodimers, as well as the inhibitory subunits IκBα, -β, and -ϵ. Engagement of TNF receptor superfamily members leads to the activation of the classical NF-κB signaling cascade via recruitment of adaptor proteins (FADD (Fas-associated death domain), TRADD (TNF receptor-associated death domain), and TRAFs (TNF receptor-associated factors)) to the cytoplasmic part of the receptor complex (25). The TRAF·receptor-interacting protein complex subsequently recruits and activates TAK1, leading to further activation of the IκB kinase (IKK) complex (26). IKKs in turn phosphorylate IκBs, thus promoting their ubiquitination and subsequent degradation by the 26 S proteasome. This results in unmasking of the NF-κB/p65 nuclear localization signal (NLS), enabling its nuclear translocation. p65 contains a classical monopartite NLS, which is characterized by 4–6 basic amino acid residues, and it has been shown that the karyopherin importin α3 specifically binds the p65-NLS (27), whereas importin β is responsible for docking to the nuclear pore complex. Nevertheless, it is not well established so far how nuclear shuttling of the known diversity of transcription factors is selectively timed and regulated, but the variety of differently structured nuclear localization signals is known to contribute to the specific recognition by various karyopherins (28, 29).
Besides the tight regulation of p65 nuclear import, further activation of NF-κB is achieved through posttranslational modifications. For example, phosphorylation of serine-threonine residues in either the N-terminal Rel homology domain of p65, which accounts for dimerization and DNA binding, or the C-terminal transcriptional activation domains (TA1 and TA2), is linked to enhanced binding of coactivator proteins, increased nuclear localization and stability, as well as to supporting the connection with the basal transcriptional apparatus (reviewed in reference 30). In addition, other signaling pathways and transcription factors, such as activator protein-1 (AP-1), cAMP-responsive element-binding protein (CREB) or CCAAT enhancer-binding protein (C/EBP), often act in cooperation with NF-κB to contribute to the expression of inflammatory genes. Furthermore, the recruitment of nuclear coactivators, acting mainly on chromatin relaxation (e.g. the histone acetyltransferases CREB-binding protein (CBP) and p300 and the nuclear kinase MSK1), is a critical step in building an “enhanceosome” to modulate general levels of gene expression (31).
We show here that TTP attenuates NF-κB activity independently of its ability to destabilize mRNAs in human and murine cells. Further, we demonstrate that TTP interferes with NF-κB activation at least in part by impairing nuclear translocation of p65 leading to the selective regulation of NF-κB-dependent gene expression. These findings reveal a previously unknown function of TTP and suggest that this protein might play a broader role in the regulation of diverse cellular processes.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
Reporter plasmids 5×NF-κB-luc, p53-luc, NFAT-luc, and pFR-luc were obtained from Stratagene (PathDetect). Il-8-luc, the truncated version of the IL-8 promoter, TF-luc as well as expression vectors for p65, IKK2, and TAK1 have been described elsewhere (32–34). TAB1 was purchased from Invitrogen. The Gal4-p65 mutant constructs (p65*, p65 amino acids 286–551; M1, p65 amino acids 286–520; M2, p65 amino acids 521–551; and M3, p65 amino acids 450–520) were kind gifts from L. Schmitz (35) as well as the pRC/CMVp65 plasmid used for generation of p53NLSΔNLSp65. Briefly, a synthetic p53NLS linker (5′HindIII-3′NcoI) was fused to the N terminus of p65. The p65 NLS sequence was excised by BstEII-AleI restriction and exchanged with the corresponding fragment from the pCDNA3.1/myc-Hisp65NLSmut vector kindly provided by R. Fagerlund (27). The expression plasmids for SV40NLSp65 and SV40NLSΔNESp65 were gifts from Shao-Cong Sun (36). Human mycTTP was generated by PCR from the RZPD clone IRAK p961M0614Q2 and ligated into the EcoRI/SalI sites of pCMV-myc (Clontech). MycTZF (amino acids 97–173) and mycC124R were generated by subcloning and in vitro mutagenesis, respectively. Oligonucleotide sequences are shown in supplemental Table 1. The integrity of all constructs generated by PCR was confirmed by sequencing. Expression plasmids for TRAF2, CBP/p300, and pRSGFP-C1-NFATc were kindly provided by H. Wajant, C. Brostjan, and L. Gerace, respectively.
Cell Culture and Transfections
HEK 293 cells and HeLa cells were obtained from ATCC. Wild-type (WT) and TTP ko mouse embryonic fibroblasts (MEF) were kindly provided by P. J. Blackshear. Cells were cultured in Dulbecco's modified Eagle's medium (Bio-Whittaker) supplemented with 10% fetal calf serum (Sigma), 2 mm l-glutamine (Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin. Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords as described (37) and maintained in medium 199 (Lonza) supplemented with 20% fetal calf serum, 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml Fungizone, 5 units/ml heparin, and 25 μg/ml endothelial cell growth supplement (Promocell). HEK 293 cells were transfected by the calcium phosphate method as described (38). MEF as well as HUVEC were transfected using the polyethyleneimine method (39). Small interfering RNA (siRNA)-mediated knockdown of TTP was performed using the Stealth® custom siRNA pool for TTP (Invitrogen). The sequences are given in supplemental Table 1.
Reporter Gene Assays
HEK 293 cells were grown in 24-well plates, MEF and HUVEC in 12-well plates. Cells were transfected as described with the indicated reporter and/or expression plasmids using 4 μg of total DNA. Cells were stimulated with 10 ng/ml TNFα for 16 h prior to harvesting. All experiments were performed in triplicate and are representative of at least three independent experiments. Luciferase values were normalized for cotransfected β-galactosidase or GFP and are illustrated as mean fold induction. Error bars represent standard deviation of the mean.
Statistical Significance Calculations
Differences between samples were analyzed using a paired Student's t test. Two-tailed probability values of <0.05 and <0.01 were considered significant and highly significant, respectively. p values are given within the figure legends.
Cell Extracts, Western Blotting, and Densitometry
For p65 nuclear translocation and IκBα degradation, cells were grown and transfected in 6-cm (HEK 293 cells) or 10-cm dishes (MEF). Forty-eight hours after transfection, cells were stimulated with 10 ng/ml TNFα (mouse or human rTNFα; R&D Systems) for the indicated time points. Cells were harvested, and nuclear extracts were prepared as described elsewhere (40). For determination of total p65, cells were lysed in 1× passive lysis buffer (Promega). Proteins were separated by 10% SDS-PAGE, transferred electrophoretically onto a nitrocellulose membrane (Hybond-C; Amersham Biosciences), and blocked in 5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20 for 1 h at room temperature before incubation with the first antibody overnight (4 °C). The following antibodies were used: α-myc (sc-40), α-β-actin (sc-1616), α-p65 (sc-109), α-IκBα (sc-203), and α-SP1 (sc-59), all obtained from Santa Cruz Biotechnology. α-Glyceraldehyde-3-phosphate dehydrogenase was from Chemicon (monoclonal antibody 374). The α-TNFα blocking antibody (AF-410-NA; R&D Systems) was used at a concentration of 5 μg/ml. The α-TTP antibody was kindly provided by P. Kovarik. Horseradish peroxidase-conjugated antibodies α-rabbit IgG, α-mouse IgG (Amersham Biosciences), and α-goat IgG (Santa Cruz Biotechnology) were used as secondary reagents. Blots were developed using chemiluminescence (West Pico, Pierce & ECL Detection Reagents; Amersham Biosciences). Densitometric analysis of nuclear p65 levels was done using the background-corrected integrated densities of the p65 bands normalized to the SP-1 bands assessed by ImageJ software (rsb.info.nih.gov/ij/).
Immunocytochemistry
Cells were seeded onto fibronectin-coated Lab Tek II chamber slides (Nunc), transfected with either empty vector or mycTTP, and stimulated 24 h later with 20 ng/ml TNFα for the indicated times. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Immunostaining of p65 was done using the rabbit α-p65 antibody (sc-109, 1:500; Santa Cruz Biotechnology), mycTTP was detected with mouse α-myc antibody (sc-40; Santa Cruz Biotechnology) followed by goat α-rabbit Alexa Fluor 488 (Molecular Probes) and APC goat α-mouse (BD Biosciences) secondary reagents at dilutions of 1:5000 and 1:500, respectively. For NFAT translocation, cells were transfected with GFP-NFATc and mycTTP expression vectors as described above and stimulated with ionomycin (500 mm final concentration; Sigma).
Real Time PCR
Total RNA was isolated from either HEK 293 cells or MEF grown in 6-well plates using the High Pure RNA isolation kit (Roche Applied Science), according to the manufacturer's protocol. One microgram of RNA was reverse transcribed using the TaqMan reverse transcription kit (Applied Biosystems) with random hexamers and murine leukemia virus reverse transcriptase following the manufacturer's recommendations. Real time PCR was performed in a LightCycler (Roche Applied Science) using SYBR Green for detection. Experiments were done in triplicate, and the relative amount of mRNA was calculated using the Pfaffl method and normalization to β2- micoglobulin. Error bars represent the standard deviation of the mean. Primer sequences are given in supplemental Table 1.
RESULTS
TTP Specifically Attenuates NF- κB-dependent Transcription
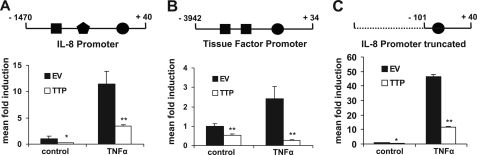
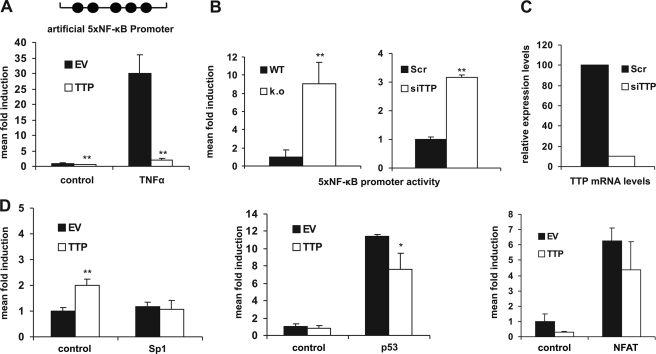
Our investigations of negative feedback regulators and potential contributors to the resolution phase of the inflammatory process resulted in the observation that TTP has the capacity to suppress the transcriptional activity of different promoters of inflammatory genes. TTP reduced basal as well as TNFα-stimulated luciferase expression driven by the human IL-8 (Fig. 1A) or tissue factor promoter (Fig. 1B) in HEK 293 cells. These promoters contain binding sites for various transcription factors, including C/EBP, activator protein-1, SP-1, and NF-κB. To gain mechanistic insight into which transcription factor or signaling pathway was affected by TTP, we used a truncated version of the IL-8 promoter containing only one NF-κB site ∼100 bp upstream of the transcriptional start. This modified promoter responded similarly to TTP as did the full-length promoters (Fig. 1C), suggesting that the NF-κB site accounted for TTP sensitivity. Consequently, we analyzed a minimal artificial promoter containing a multimerized NF-κB binding site and observed that it was also potently inhibited by TTP (Fig. 2A). These findings could be reproduced in other cell types such as HeLa and HUVEC (data not shown). Conversely, the lack of TTP generates conditions of a “preactivated” state in ko fibroblasts, reflected by a ∼10-fold higher basal NF-κB level in TTP-deficient cells (Fig. 2B, left). Furthermore, knock-down of endogenous TTP in HUVEC by RNA interference enhanced NF-κB activity (Fig. 2B, right), recapitulating the situation observed in TTP ko MEF. Similar results were obtained in HEK 293 cells (supplemental Fig. 1A). The functionality of the siRNA was verified on the mRNA (Fig. 2C) and protein level (supplemental Fig. 1A, bottom panels). In addition, reconstitution of TTP expression by transient transfection into TTP ko cells resulted in a 5×NF-κB activity that was comparable with that observed in WT cells (supplemental Fig. 1B). Importantly, TTP did not decrease the basal and induced transcriptional activity of either minimal SP-1 or p53 or NFAT-dependent promoters, as shown in Fig. 2D, demonstrating specificity and ruling out possible effects on the reporter plasmids. Additionally, we did not observe any influence of TTP on the degradation of luciferase mRNA (supplemental Fig. 1C). Together, this demonstrates that TTP specifically interferes with transcriptional activation triggered by the transcription factor NF-κB.
FIGURE 1.
TTP attenuates NF-κB activity. HEK 293 cells were transfected with luciferase reporter genes driven by the IL-8 promoter (A), the tissue factor promoter (B), or a truncated version of the IL-8 promoter (C) as shown schematically on top (●, NF-κB; ■, activator protein-1; and, C/EBP transcription factor binding sites). Luciferase activity was analyzed before (control) and after TNFα stimulation (10 ng/ml, 16 h) in the absence (EV, empty vector) or presence of coexpressed TTP and normalized to the activity of cotransfected β-galactosidase. Mean fold induction was calculated in relation to unstimulated conditions (control). *, p < 0.05; **, p < 0.01, compared with the respective empty vector samples. Error bars represent standard deviation of the mean.
FIGURE 2.
Specificity toward NF-κB activity. A, HEK 293 cells were transfected with a luciferase reporter gene driven by a minimal NF-κB promoter containing five NF-κB binding sites (5×NF-κB-luc; top), and luciferase activity was analyzed as described in Fig. 1. **, p < 0.01, compared with the respective empty vector (EV) samples. B, lack of TTP triggers NF-κB activity. Left, 5×NF-κB promoter activity was analyzed in WT versus TTP ko MEF. Luciferase levels were normalized to cotransfected β-galactosidase. Right, HUVEC were transfected with the 5×NF-κB-luc reporter along with a siRNA directed against TTP (siTTP) or a scrambled control (Scr), and luciferase activity normalized to coexpressed GFP was assessed 48 h after transfection. **, p < 0.01, compared with WT (left) or the scrambled control (right). C, real time PCR analysis of TTP mRNA levels normalized to β2-microglobulin in HUVEC was done 48 h after transfection with either siTTP or the scrambled control used in B. A representative experiment is shown. D, TTP does not bias NF-κB-independent promoters. HEK 293 cells were transfected with a SP-1 (left), a p53 (center), or a NFAT promoter-dependent (right) luciferase reporter gene in the presence (TTP) or absence of TTP (EV). Luciferase activities were analyzed in the absence (control) or presence of cotransfected SP-1, p53, or NFAT expression plasmids, respectively. *, p < 0.05; **, p < 0.01, compared with the respective empty vector samples. Error bars represent standard deviation of the mean.
TTP Interferes with NF-κB Signaling Downstream of the IKK Signalosome, at the Level of p65
Based on the results above, we sought to examine whether TTP interferes with NF-κB signaling upstream or downstream of the IKK signalosome. Activation of NF-κB by TRAF2 or TAK1/TAB1, acting upstream of the IKK complex, did not result in the abrogation of TTP-mediated NF-κB inhibition (Fig. 3A). Moreover, TTP also potently inhibited IKK2- and even NF-κB/p65-mediated activation of the 5×NF-κB reporter in HEK 293 cells (Fig. 3B), suggesting that it acts downstream or at the level of p65. In addition, overexpression of p65 did not impede TTP action in WT or in reconstituted ko MEF (supplemental Fig. 2A). Further examination of TTP effects on p65-induced IL-8 promoter activity (supplemental Fig. 2B) confirmed the results obtained for the artificial NF-κB promoter. Increasing amounts of p65 expression levels could not overcome the TTP-mediated inhibition (Fig. 3C), and in addition, attenuation of NF-κB activity occurred in a dose-dependent manner (Fig. 3D). We observed 50% inhibition of p65-mediated luciferase activity at ∼7 ng of cotransfected TTP. Because overexpression of p65 might bypass its natural regulation through the IκBs, we examined a possible influence of TTP on IκBα degradation. We found that TTP did not prevent TNFα-induced IκBα degradation, neither in HEK 293 cells after overexpression (Fig. 3E, top), nor was it altered in TTP ko compared with WT MEF (Fig. 3E, bottom).
FIGURE 3.
TTP interferes with NF-κB signaling downstream of p65 transactivation. HEK 293 cells were transfected with 5×NF-κB-luc alone (control) or in combination with TAB1/TAK1, TRAF2 (A), p65 or IKK2 expression plasmids (B), and luciferase activities were analyzed in the absence (empty vector; EV) or presence of coexpressed TTP. Mean fold induction was calculated in relation to basal 5×NF-κB activity (control). **, p < 0.01, compared with the respective empty vector samples. C, increased p65 levels do not compensate for the inhibition of NF-κB by TTP. HEK 293 cells were transfected with 5×NF-κB-luc either in the absence (EV) and presence of TTP (TTP) (**, p < 0.01, compared with the EV sample) or together with increasing amounts of p65 (0.1–0.4 μg) and a constant amount of TTP (100 ng) expression plasmids, as indicated. (**, p < 0.01, compared with the p65-induced sample). 5×NF-κB promoter activity was analyzed as above. Protein levels of p65 as well as Myc-tagged TTP were assessed by Western blotting. β-Actin represents the loading control (middle panels). D, TTP reduces NF-κB activity in a dose-dependent manner. HEK 293 cells were transfected with increasing amounts of TTP as indicated (1–100 ng) in combination with a constant amount (0.1 μg) of p65, and 5×NF-κB activity was determined as described above. **, p < 0.01, compared with the sample without TTP coexpression. E, IκBα degradation is not affected by TTP. Upper, HEK 293 cells were transfected with either empty vector (EV) or myc-tagged TTP and stimulated with 10 ng/ml TNFα for the indicated time points followed by preparation of cytoplasmic extracts. IκBα degradation (top panel) as well as ectopic TTP expression (middle panel) was assessed by Western blotting. β-Actin was used as loading control (bottom panel). Lower, WT and ko MEF were stimulated with 10 ng/ml TNFα for 15 min, and cytoplasmic extracts were subjected to Western blot analysis for IκBα degradation. β-Actin served as loading control (bottom panel). F, nuclear p65-coactivators cannot counteract TTP. HEK 293 cells were transfected with a 5×NF-κB reporter in combination with p65 (EV+p65) along with CBP, p300, or MSK1 expression vectors. Luciferase activities were analyzed in the presence (TTP) and absence of coexpressed TTP. Fold induction was calculated in relation to the basal reporter activity (data not shown). **, p < 0.01, compared with the respective p65-induced EV samples. Error bars represent standard deviation of the mean.
Another important step prior to transcriptional activation by NF-κB is the recruitment of nuclear coactivators, such as CBP/p300 or activating kinases like MSK1, into the transcriptional activation complex. Analysis of these nuclear signaling components in transient transfection experiments, using the respective expression vectors, revealed that neither of them could dampen the inhibitory TTP effect (Fig. 3F). To exclude that TTP affects p65 mRNA or protein, we analyzed these levels in HEK 293 cells after TTP overexpression (supplemental Fig. 2C, left) and in TTP ko MEF (supplemental Fig. 2C, right) and could not detect significant differences.
mRNA Binding Ability of TTP Is Dispensable for Its Inhibitory Effect on NF-κB Promoter Activity
One simple explanation of our results could have been that TTP affects the stability of an ARE-containing mRNA that encodes a protein necessary for full NF-κB activation. Therefore, we took advantage of the TTP mutant C124R (Fig. 4A), which is deficient in ARE binding and therefore cannot destabilize e.g. TNFα mRNA (41–43) (supplemental Fig. 3A). TTP-C124R retained the capacity to inhibit 5×NF-κB activity, both after stimulation with cotransfected p65 (Fig. 4B) as well as under basal conditions (Fig. 4C). In contrast, the TTP construct comprising the zinc-finger domain only (TZF; Fig. 4A), which binds mRNAs efficiently (44) but fails to induce degradation (supplemental Fig. 3A), displayed no influence on luciferase activity (Fig. 4C). This suggests that the ability of TTP to inhibit NF-κB is independent of its mRNA binding capacity but requires the presence of the N- and/or C-terminal domains. As already observed for TTP (Fig. 2D), the C124R mutant did not affect SP-1-dependent or p53-dependent promoter activities (supplemental Fig. 3B).
FIGURE 4.
Attenuation of NF-κB activity is independent of the mRNA binding ability of TTP. A, shown is a schematic depiction of TTP constructs used in reporter gene assays. Numbers represent amino acid residues of human TTP. A single amino acid mutation within the first zinc-finger (C124R) abolishes RNA binding. CCCH, cysteine-cysteine-cysteine-histidine zinc-finger structure. All proteins are N-terminally linked to a myc tag. B, HEK 293 cells were transfected with 5×NF-κB-luc alone (EV) or in combination with a p65 expression vector as indicated. The effect of coexpression of either TTP or the RNA-binding mutant C124R on p65-stimulated 5×NF-κB promoter activity was monitored by analysis of luciferase expression levels as described above. Mean fold induction was calculated in relation to the empty vector (EV) sample. The bottom panel represents Western blot analysis of the same cell lysates and shows equal protein expression levels of both TTP and C124R by detection with a α-myc antibody. **, p < 0.01, compared with the p65-induced sample. C, HEK 293 cells were transfected with 5×NF-κB-luc alone (EV) or in combination with increasing amounts of TTP-C124R or TTP-TZF expression plasmids. Luciferase activities were analyzed, and cell lysates were subjected to Western blotting (bottom panel) to verify expression of TTP constructs as described in B. Numbers at the left side represent molecular mass in kDa. *, p < 0.05, compared with the EV sample. Error bars represent standard deviation of the mean.
TTP Impairs Cytoplasmic-Nuclear Transition of p65
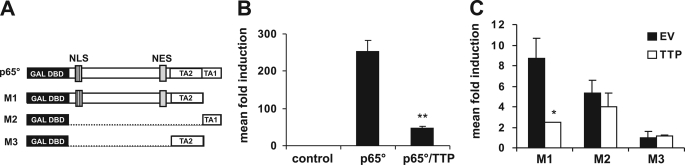
Having established that TTP inhibits NF-κB downstream or at the level of p65, we sought to gain further insight into its mode of action. Because one of the additional regulatory steps of NF-κB activation occurs at the level of transactivation, we utilized different hybrid constructs where parts of p65 are fused to the yeast Gal4 DNA binding domain (DBD) (Fig. 5A). When we tested the ability of these constructs to influence Gal4-dependent luciferase expression we found that the exchange of the N-terminal Rel homology domain with the yeast Gal4 DBD retained the inhibitory effect of TTP (Fig. 5B), indicating that neither p65 dimerization nor DNA binding was impaired. Further truncations of the p65 protein revealed that TTP could not suppress the activity of each of the two transactivation domains when they were fused directly to the Gal4 DBD. The only region of p65 that was sensitive to TTP was the central part, which contains the NLS as well as the nuclear export sequence (NES), suggesting that TTP may prevent import or expedite export of the transcription factor (Fig. 5C). Comparable results were obtained in HeLa cells (data not shown). To substantiate these data, we investigated the possible influence of TTP on p65 subcellular localization. Therefore, we analyzed p65 protein levels in cytoplasmic and nuclear fractions of TNFα-induced HEK 293 cells and found that the overexpression of TTP impaired nuclear accumulation of p65 (Fig. 6A, top). Additionally, and in line with previous reporter results (Fig. 2B, left, and supplemental Fig. 1B), the nuclear localization of p65 was enhanced in TTP ko MEF (Fig. 6B, top) supporting the notion of a “prestimulated” scenario. Although these cells do not express detectable amounts of TNFα (43) (real time PCR; data not shown), we wanted to rule out that potential minute amounts of TNFα are the cause for enhanced NF-κB activity in ko cells. Therefore, we performed this experiment in the presence of a neutralizing α-TNFα antibody. Furthermore, immunocytochemical analysis of HUVEC, transiently transfected with TTP, showed reduced translocation of endogenous p65 after TNFα stimulation (Fig. 6C and supplemental Fig. 4A); in contrast, shuttling of NFAT was not affected (supplemental Fig. 4B).
FIGURE 5.
The p65 region containing the NLS/NES motifs accounts for TTP sensitivity. A, shown is a schematic representation of expression constructs containing different p65 domains fused to the yeast Gal4 DBD. TA1 and TA2, transactivation domains 1 and 2. For a detailed description, see Experimental Procedures and reference 35. B, HEK 293 cells were transfected with the yeast-based Gal4 reporter plasmid pFR-luc (control) together with p65° (see A) to enable promoter activity, and the effect of TTP coexpression on p65°-induced luciferase expression was analyzed (p65°/TTP). The impact of TTP coexpression on promoter activity induced by the p65 mutants M1-M3 (see A) is depicted in C. *, p < 0.05 compared with the respective EV sample; **, p < 0.01, compared with the p65°-induced sample. Error bars represent standard deviation of the mean.
FIGURE 6.
TTP impairs p65 cytoplasmic-nuclear shuttling. A: Upper, Western blot analysis of endogenous p65 and myc-tagged TTP in cytoplasmic and nuclear extracts prepared from HEK 293 cells stimulated with TNFα for the indicated time points, 48 h after transfection with empty vector (EV) or TTP (mycTTP expression plasmid). LC*, loading control. β-Actin was used for the cytoplasmic and SP-1 for the nuclear fraction. Lower, densitometric analysis of nuclear p65 levels based on the Western blot shown in the upper panel using the ImageJ software (for detailed information, see Experimental Procedures). p65 levels were normalized to SP1 levels in the same sample. Relative p65 levels are shown in relation to time point 0. Numbers on the top of the bars represent the percentage reduction of nuclear p65 levels in the presence of TTP. B: Upper, TTP ko MEF exhibit increased nuclear p65 levels. Cytoplasmic (Cyt) and nuclear (Nuc) extracts prepared from WT and TTP ko MEF were analyzed by Western blotting (top panels) for p65 levels in the presence of an α-TNFα antibody. SP-1 is shown as loading control and for the purity of the nuclear fraction. Lower, densitometric analysis of nuclear p65 levels based on the Western blot on top. Analysis was done as described in A. C, immunocytochemical analysis showing TTP influence on p65 nuclear translocation in HUVEC. Cells were transfected with a TTP expression plasmid (mycTTP), stimulated with 20 ng/ml TNFα for 15 min, and endogenous p65 (green, left panels) as well as ectopically expressed TTP (red, right panels) were detected by immunocytochemistry. TTP-transfected cells are indicated by an arrow.
TTP Impairs p65 Nuclear Import and Affects Transcriptional Onset of Specific NF-κB Target Genes in an ARE-independent Manner
NF-κB/p65 was shown to shuttle constitutively between cytoplasm and nucleus under basal conditions (45). The abundance of regulatory proteins like IκBα, in addition to the appropriate composition of the nuclear pore complex, as well as the accessibility of NLS and NES, is indispensable for the proper timing of import and export. The NLS of p65 belongs to the monopartite class of leader motifs (46, 47) that further includes those of the SV40 T antigen and the human c-Myc. Recently it was described by Li et al. (48) that the transcription factor p53 shuttles through a specific nuclear import pathway that seems to be selective for the bipartite p53-NLS and different from the pathway used by the monopartite SV40 T antigen. Accordingly, we tested different p65-NLS/NES mutants, as summarized schematically in Fig. 7A, in reporter gene assays. The N-terminal fusion of the SV40NLS (PKKKRKV) to WT p65 resulted in activation of 5×NF-κB activity similar in strength to the induction achieved with the WT protein, and in both cases TTP had a comparable inhibitory effect (Fig. 7B). In contrast, the fusion of the bipartite nuclear leader of p53 (see Fig. 7A), which is distinguished from the monopartite class of p65 and SV40 nuclear localization signals, significantly abrogated the inhibitory effect of TTP (Fig. 7C). Mutation of the nuclear export signal of p65 (ΔNES) resulted in strongly enhanced promoter activity but could nevertheless be suppressed by TTP (Fig. 7B). This suggests that TTP does not act via enhancing p65 export, but through regulation of nuclear import that is dependent on the nature of the p65 NLS.
FIGURE 7.
TTP affects p65 nuclear import. A, schematic representation of p65 expression constructs used for reporter gene assays in B and C. SV40NLS p65, the monopartite SV40 NLS (PKKKRKV) fused to the N terminus of WT p65; SV40NLSΔNES p65, mutation of the NES within the SV40NLSp65 construct; p53NLSΔNLS p65, the p53 bipartite NLS (KRALPNNTSSSPQPKKKP) fused to the N terminus of WT p65, the internal WT p65 NLS KRKR mutated to AAAR (for detailed cloning information, see Experimental Procedures). B and C, HEK 293 cells transfected with 5×NF-κB-luc alone (control) or in combination with either WT p65 (WT), SV40NLSp65 (SV40NLS) and SV40NLSΔNESp65 (ΔNES) (B) or WT p65 (WT) and p53NLSΔNLSp65 (p53NLS) (C). Luciferase activities were analyzed in the absence (EV) or presence of coexpressed TTP. Fold induction was calculated in relation to the basal reporter activity (control). *, p < 0.05; **, p < 0.01, compared with the respective EV samples. Error bars represent standard deviation of the mean.
To study the biological consequences of altered NF-κB activity, we analyzed the levels of NF-κB-dependent target genes in WT and TTP ko MEF. To exclude any contribution of ARE-mediated decay, NF-κB target genes were selected from the Boston University People Web site with regard to the absence of any ARE in their respective mRNAs. Six NF-κB-dependent genes, belonging to diverse groups of NF-κB targets, were chosen (Table 1), and the absence of any AU cluster was confirmed bioinformatically. They were analyzed by real time PCR for their relative expression levels in WT compared with TTP-deficient MEF (Fig. 8). Although MCP-1, also known as the macrophage chemotactic protein, as well as the antioxidant genes FTH1 and TXN2 were unaffected (Fig. 8, left), the chemokine RANTES as well as Gadd45β and TRAF1 were substantially up-regulated in TTP ko cells (Fig. 8, right).
TABLE 1.
Bioinformatic analysis of selected NF-κB target genes
NF-κB dependent target genes, involved in a variety of different cellular functions, were preselected and analyzed for the absence of an ARE in the 3′-untranslated region of the respective mRNAs using the ARED database. Targets lacking an ARE motif were analyzed further for the presence/absence of the minimal AU pentamer AUUUA.
| Gene | Human gene name | Function | RefSeqa | AREDb | AUUUAc |
|---|---|---|---|---|---|
| Cytokines and modulators | |||||
| MCP-1 | CCL2 | Macrophage chemotactic protein | NM_011333 | No | 1 Pentamer |
| RANTES | CCL5 | Regulated upon activation normal T lymphocyte | NM_013653 | No | No |
| Stress response genes | |||||
| Ferritin heavy chain | FTH1 | Antioxidant | NM_010239 | No | No |
| Thioredoxin | TXN2 | Redox-active protein/antioxidant | NM_019913 | No | No |
| Regulators of apoptosis | |||||
| TRAF1 | TRAF1 | TNF receptor-associated factor 1 | NM_009421 | No | 1 Pentamer |
| Miscellaneous | |||||
| Gadd45β | GADD45β | DNA repair/cell cycle control | NM_008655 | No | No |
Mus musculus RefSeq.
ARED, ARE database; the ARED organism search engine was used for preanalysis.
AUUUA, minimal pentamer, core sequence of classI/II/III ARE clusters.
FIGURE 8.
TTP controls the expression of ARE-less NF-κB target genes. Real time PCR analysis of selected, ARE-less NF-κB-dependent genes (Table 1) in WT and TTP ko MEF is shown. Relative expression levels were normalized to β2-microglobulin, and the results shown are representatives of two independent experiments done in triplicate. **, p < 0.01, compared with the respective WT samples. Error bars represent standard deviation of the mean.
DISCUSSION
The notion that the zinc-finger protein TTP might act as a negative regulator of transcription initially emerged more than 10 years ago, when the first suppressive effects on promoters were described (11). Nevertheless, this view has taken a back seat because of the concomitant finding that TTP acts as a mRNA-destabilizing protein. However, evidence has accumulated that TTP might have a broader function. Examples include the influence on other RNA-binding proteins, e.g. the KH-type splicing regulatory protein (KSRP) (49) as well as the proposed multifunctional activity in the control of glucocorticoid-mediated gene expression (50).
We describe here that TTP is involved in the negative transcriptional regulation of promoters containing NF-κB binding sites in different cell types. TTP inhibits the induced activity of these promoters about 70–95% whereas the influence on the basal levels fluctuates below 50%. This can be explained by the fact that NF-κB constitutively shuttles between cytoplasm and nucleus (45), leading to a basal promoter activity with its magnitude being determined by the actual situation and condition of the cell. The negative regulatory role of TTP was further supported by the analysis of TTP-deficient MEF as well as by siRNA-mediated knockdown of endogenous TTP in HUVEC.
TTP exhibited a dose-dependent effect on NF-κB with only ∼7 ng of transfected TTP expression plasmid resulting in half-maximal reduction of promoter activity, an amount that was below our detection limit on Western blots (whereas endogenous TTP could be detected at least after stimulation in other cell types; data not shown). This indicates that the transfected amounts are well below the induced, endogenous TTP levels of other cell types. Lai et al. (14) have shown previously that the overexpression of human TTP exhibits different effects on the degradation of TNFα mRNA depending on the expression levels: although low levels of TTP efficiently destabilize the TNFα message, they observed that higher levels lead to the stabilization of the mRNA, which is explained by the possibility that TTP might form inactive dimers at higher concentrations or sequester components of the RNA degradation machinery.
We initially hypothesized that TTP might trigger the down-regulation of the expression of one or more components of the NF-κB signaling pathway through its known mRNA- destabilizing activity. However, the use of TTP constructs, which either carry a mutation in the zinc-finger domain (C124R) that impairs ARE binding and mRNA degradation or lack the N-and C-terminal domains (TZF), provided evidence that TTP inhibits NF-κB independent of its well described mRNA-destabilizing function. The fact that the RNA-binding mutant C124R could not diminish, but rather augments TNFα mRNA levels has already been observed by Lai et al. and was confirmed in our experiments (supplemental Fig. 3A). It can be explained by the inhibition of mRNA turnover caused by the dominant negative function of this mutant (41). Further examination of the mechanism of TTP action unveiled that TTP influences the activation of NF-κB at the level of p65: no upstream mediator of NF-κB signaling (TRAF2, TAK1/TAB1, or IKK2) could overcome the strong, inhibitory TTP effect; IκBα degradation was not affected; and overexpression of the known p65 nuclear coactivators CBP, p300, and MSK1 did not prohibit TTP action. Besides, neither total p65 protein amounts nor mRNA levels were influenced, and no physical interaction of TTP with p65 could be demonstrated by coimmunoprecipitation (data not shown). However, the results of follow-up experiments revealed that the nuclear import of NF-κB/p65 was affected by TTP: biochemical analysis demonstrated that p65 appeared in the nucleus 15 min after TNFα stimulation in control cells, whereas TTP-transfected cells showed a severely impaired nuclear translocation. Densitometric analysis of nuclear p65 levels revealed that already under unstimulated conditions less p65 was present in the nucleus, and the difference became even more evident after stimulation with TNFα, in agreement with the results obtained by the reporter gene assays. These findings were further confirmed at the single-cell level by immunofluorescent analysis of endogenous p65 in primary HUVEC.
In line with the promoter studies performed in TTP ko MEF, we found that p65 exhibited increased nuclear localization under unstimulated conditions in these cells. Here, the lack of TTP seems to allow enhanced nuclear shuttling of NF-κB, leading to a “prestimulated” situation that is reflected by the up-regulation of certain NF-κB target genes. It has to be pointed out that the target genes analyzed in untreated TTP ko cells provide an insight into the prestimulated situation generated by TTP deficiency. It is in all probability that under e.g. TNFα-stimulated conditions other and/or additional target genes might be differentially regulated. The fact that only a subset of NF-κB-regulated genes is affected in our experiments can be explained by the complexity of NF-κB activation: the selective transcriptional onset of specifically required target genes is dependent on the formation of distinct NF-κB dimer/heterodimer combinations, the abundance and distribution of essential cofactors, as well as the epigenetic regulation of the accessibility of the promoters. The proper timing and interplay of these components may facilitate the differential expression of a plethora of NF-κB-dependent genes.
Our analysis revealed the ARE-less NF-κB targets RANTES, Gadd45β, and TRAF1 being up-regulated in TTP ko MEF. RANTES is a chemokine that appears chemotactic for T cells, eosinophils, and basophils and plays an active role in recruiting leukocytes into inflammatory sites. TRAF1 is a member of the TNFR2 signaling complex, which exerts multiple biological effects on cells such as proliferation, cytokine production, and cell death. Gadd45β in turn is known to be involved in the regulation of DNA repair and cell cycle control. In light of the fact that TTP ko cells grow somewhat faster than WT MEF, it appears interesting that all of these genes have been connected to the regulation of cell proliferation or apoptosis, a decision that is predominantly controlled by the cross-talk of NF-κB with c-Jun N-terminal kinase signaling. The elucidation of a possible influence of TTP in this cross-talk will be subject of further studies.
Concerning the mechanism of TTP-mediated inhibition of the nuclear import of NF-κB, it is interesting to note that in Drosophila, nup214, a component of the nuclear pore complex, has been implicated in the modulation of NF-κB activation. Nup214 is a member of the so-called FG-Nups that provide specificity and abundance of transport factor binding sites at the nuclear pore complex and have been shown to facilitate the karyopherin-cargo movement across the nuclear pore. Nup214 mutants impaired the nuclear accumulation of the p65/p50 homologs Dorsal and Dif after bacterial challenge (51). A separate study showed that TTP was able to interact with nup214 in THP-1 cells (52). It is therefore tempting to speculate that the mechanism of TTP action might involve the selective prevention of NF-κB import via regulation of a Nup214-containing nuclear pore. Selectivity could thereby be achieved through the nature of the monopartite p65 NLS as opposed to e.g. the bipartite leader of p53 which, when exchanged, abrogated the inhibitory function of TTP (Fig. 7).
Taken together, these observations reveal an additional, and, to our knowledge, novel activity for TTP. To date we cannot completely exclude that TTP triggers the activation or repression of other ARE-binding proteins through protein-protein interactions or that the loss of function mutation in the zinc-finger concerns only a subset of ARE-containing targets. In this regard, a recent finding revealed that TTP does not exclusively bind to ARE sequences, but additionally recognizes a non-ARE cis element in the major histocompatibility complex class I mRNA (53). However, 3′-untranslated region analysis of this mRNA demonstrated a binding deficiency of the zinc-finger mutant as well, suggesting that the loss of function is not restricted to a certain ARE motif.
In conclusion, TTP contributes to the regulation of NF-κB-mediated inflammatory responses at least on two levels: through the destabilization of cytokine mRNAs (e.g. TNFα) and through the impairment of NF-κB/p65 nuclear translocation. It thus controls the selective, transcriptional onset of a subset of NF-κB-dependent genes and acts as a negative feedback regulator during a variety of NF-κB-dependent pathophysiological situations.
Supplementary Material
Acknowledgments
We thank Pavel Kovarik for sharing the α-TTP antibody, M. Lienhard Schmitz for providing the p65-TAD mutant constructs, R. Fagerlund for the pcDNA3.1/Myc-Hisp65NLSmut vector, Shao-Cong Sun for the expression plasmids SV40NLSp65 and SV40NLSΔNESp65 and Perry J. Blackshear for the TTP ko MEF. We thank Christof Lemberger for helpful comments on the manuscript.
This work was supported by a grant from the Austrian Science Foundation, Project P19217-B13 (to R. d. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4 and Table 1.
- TTP
- tristetraprolin
- ARE
- AU-rich element
- CBP
- CREB-binding protein
- C/EBP
- CCAAT enhancer-binding protein
- CREB
- cAMP-responsive element-binding protein
- DBD
- DNA binding domain
- GFP
- green fluorescent protein
- HUVEC
- human umbilical vein endothelial cells
- IκBα
- inhibitor of κBα
- IKK
- IκBα kinase
- IL
- interleukin
- ko
- knock-out
- luc
- luciferase
- MEF
- mouse embryonic fibroblasts
- MSK1
- mitogen- and stress-activated protein kinase 1
- NES
- nuclear export signal
- NFAT
- nuclear factor of activated T cells
- NLS
- nuclear localization signal
- siRNA
- small interfering RNA
- TAB
- transforming growth factor-activated protein kinase 1-binding protein
- TAK1
- transforming growth factor β-activated kinase 1
- TNFα
- tumor necrosis factor α
- TRAF
- tumor necrosis factor receptor-associated factor
- TZF
- tandem zinc-finger
- WT
- wild type.
REFERENCES
- 1.Lim R. W., Varnum B. C., Herschman H. R. (1987) Oncogene 1, 263–270 [PubMed] [Google Scholar]
- 2.Ma Q., Herschman H. R. (1991) Oncogene 6, 1277–1278 [PubMed] [Google Scholar]
- 3.Varnum B. C., Lim R. W., Sukhatme V. P., Herschman H. R. (1989) Oncogene 4, 119–120 [PubMed] [Google Scholar]
- 4.Heximer S. P., Cristillo A. D., Russell L., Forsdyke D. R. (1998) DNA Cell Biol. 17, 249–263 [DOI] [PubMed] [Google Scholar]
- 5.Heximer S. P., Forsdyke D. R. (1993) DNA Cell Biol. 12, 73–88 [DOI] [PubMed] [Google Scholar]
- 6.Kaneda N., Oshima M., Chung S. Y., Guroff G. (1992) Gene 118, 289–291 [DOI] [PubMed] [Google Scholar]
- 7.Lai W. S., Stumpo D. J., Blackshear P. J. (1990) J. Biol. Chem. 265, 16556–16563 [PubMed] [Google Scholar]
- 8.DuBois R. N., McLane M. W., Ryder K., Lau L. F., Nathans D. (1990) J. Biol. Chem. 265, 19185–19191 [PubMed] [Google Scholar]
- 9.Gomperts M., Pascall J. C., Brown K. D. (1990) Oncogene 5, 1081–1083 [PubMed] [Google Scholar]
- 10.Taylor G. A., Thompson M. J., Lai W. S., Blackshear P. J. (1996) Mol. Endocrinol. 10, 140–146 [DOI] [PubMed] [Google Scholar]
- 11.Carballo E., Lai W. S., Blackshear P. J. (1998) Science 281, 1001–1005 [DOI] [PubMed] [Google Scholar]
- 12.Carballo E., Lai W. S., Blackshear P. J. (2000) Blood 95, 1891–1899 [PubMed] [Google Scholar]
- 13.Worthington M. T., Pelo J. W., Sachedina M. A., Applegate J. L., Arseneau K. O., Pizarro T. T. (2002) J. Biol. Chem. 277, 48558–48564 [DOI] [PubMed] [Google Scholar]
- 14.Lai W. S., Carballo E., Strum J. R., Kennington E. A., Phillips R. S., Blackshear P. J. (1999) Mol. Cell. Biol. 19, 4311–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hau H. H., Walsh R. J., Ogilvie R. L., Williams D. A., Reilly C. S., Bohjanen P. R. (2007) J. Cell. Biochem. 100, 1477–1492 [DOI] [PubMed] [Google Scholar]
- 16.Lykke-Andersen J., Wagner E. (2005) Genes Dev. 19, 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrestensen C. A., Schroeder M. J., Shabanowitz J., Hunt D. F., Pelo J. W., Worthington M. T., Sturgill T. W. (2004) J. Biol. Chem. 279, 10176–10184 [DOI] [PubMed] [Google Scholar]
- 18.Hitti E., Iakovleva T., Brook M., Deppenmeier S., Gruber A. D., Radzioch D., Clark A. R., Blackshear P. J., Kotlyarov A., Gaestel M. (2006) Mol. Cell. Biol. 26, 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoecklin G., Stubbs T., Kedersha N., Wax S., Rigby W. F., Blackwell T. K., Anderson P. (2004) EMBO J. 23, 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutler B., Kruys V. (1995) J. Cardiovasc. Pharmacol. 25, Suppl 2, S1–S8 [DOI] [PubMed] [Google Scholar]
- 21.Taylor G. A., Carballo E., Lee D. M., Lai W. S., Thompson M. J., Patel D. D., Schenkman D. I., Gilkeson G. S., Broxmeyer H. E., Haynes B. F., Blackshear P. J. (1996) Immunity 4, 445–454 [DOI] [PubMed] [Google Scholar]
- 22.Carrick D. M., Chulada P., Donn R., Fabris M., McNicholl J., Whitworth W., Blackshear P. J. (2006) J. Autoimmun. 26, 182–196 [DOI] [PubMed] [Google Scholar]
- 23.Sauer I., Schaljo B., Vogl C., Gattermeier I., Kolbe T., Müller M., Blackshear P. J., Kovarik P. (2006) Blood 107, 4790–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoecklin G., Tenenbaum S. A., Mayo T., Chittur S. V., George A. D., Baroni T. E., Blackshear P. J., Anderson P. (2008) J. Biol. Chem. 283, 11689–11699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu H., Xiong J., Goeddel D. V. (1995) Cell 81, 495–504 [DOI] [PubMed] [Google Scholar]
- 26.Devin A., Cook A., Lin Y., Rodriguez Y., Kelliher M., Liu Z. (2000) Immunity 12, 419–429 [DOI] [PubMed] [Google Scholar]
- 27.Fagerlund R., Kinnunen L., Köhler M., Julkunen I., Melén K. (2005) J. Biol. Chem. 280, 15942–15951 [DOI] [PubMed] [Google Scholar]
- 28.Moroianu J. (1999) J. Cell. Biochem. Suppl.32–33, 76–83 [DOI] [PubMed] [Google Scholar]
- 29.Rout M. P., Aitchison J. D. (2001) J. Biol. Chem. 276, 16593–16596 [DOI] [PubMed] [Google Scholar]
- 30.Campbell K. J., Perkins N. D. (2004) Biochem. Soc. Trans. 32, 1087–1089 [DOI] [PubMed] [Google Scholar]
- 31.Vanden Berghe W., De Bosscher K., Boone E., Plaisance S., Haegeman G. (1999) J. Biol. Chem. 274, 32091–32098 [DOI] [PubMed] [Google Scholar]
- 32.Harant H., de Martin R., Andrew P. J., Foglar E., Dittrich C., Lindley I. J. (1996) J. Biol. Chem. 271, 26954–26961 [DOI] [PubMed] [Google Scholar]
- 33.Hofer-Warbinek R., Schmid J. A., Stehlik C., Binder B. R., Lipp J., de Martin R. (2000) J. Biol. Chem. 275, 22064–22068 [DOI] [PubMed] [Google Scholar]
- 34.Schabbauer G., Schweighofer B., Mechtcheriakova D., Lucerna M., Binder B. R., Hofer E. (2007) Thromb. Haemost. 97, 988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz M. L., dos Santos Silva M. A., Baeuerle P. A. (1995) J. Biol. Chem. 270, 15576–15584 [DOI] [PubMed] [Google Scholar]
- 36.Harhaj E. W., Sun S. C. (1999) Mol. Cell. Biol. 19, 7088–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W. J., Wojta J., Binder B. R. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 465–474 [DOI] [PubMed] [Google Scholar]
- 38.Chen C. A., Okayama H. (1988) BioTechniques 6, 632–638 [PubMed] [Google Scholar]
- 39.Baker A., Saltik M., Lehrmann H., Killisch I., Mautner V., Lamm G., Christofori G., Cotten M. (1997) Gene Ther. 4, 773–782 [DOI] [PubMed] [Google Scholar]
- 40.Solan N. J., Miyoshi H., Carmona E. M., Bren G. D., Paya C. V. (2002) J. Biol. Chem. 277, 1405–1418 [DOI] [PubMed] [Google Scholar]
- 41.Lai W. S., Blackshear P. J. (2001) J. Biol. Chem. 276, 23144–23154 [DOI] [PubMed] [Google Scholar]
- 42.Lai W. S., Kennington E. A., Blackshear P. J. (2002) J. Biol. Chem. 277, 9606–9613 [DOI] [PubMed] [Google Scholar]
- 43.Lai W. S., Parker J. S., Grissom S. F., Stumpo D. J., Blackshear P. J. (2006) Mol. Cell. Biol. 26, 9196–9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai W. S., Carballo E., Thorn J. M., Kennington E. A., Blackshear P. J. (2000) J. Biol. Chem. 275, 17827–17837 [DOI] [PubMed] [Google Scholar]
- 45.Birbach A., Gold P., Binder B. R., Hofer E., de Martin R., Schmid J. A. (2002) J. Biol. Chem. 277, 10842–10851 [DOI] [PubMed] [Google Scholar]
- 46.Blank V., Kourilsky P., Israël A. (1991) EMBO J. 10, 4159–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilmore T. D., Temin H. M. (1988) J. Virol. 62, 703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q., Falsey R. R., Gaitonde S., Sotello V., Kislin K., Martinez J. D. (2007) Oncogene 26, 7885–7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winzen R., Thakur B. K., Dittrich-Breiholz O., Shah M., Redich N., Dhamija S., Kracht M., Holtmann H. (2007) Mol. Cell. Biol. 27, 8388–8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishmael F. T., Fang X., Galdiero M. R., Atasoy U., Rigby W. F., Gorospe M., Cheadle C., Stellato C. (2008) J. Immunol. 180, 8342–8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xylourgidis N., Roth P., Sabri N., Tsarouhas V., Samakovlis C. (2006) J. Cell Sci. 119, 4409–4419 [DOI] [PubMed] [Google Scholar]
- 52.Carman J. A., Nadler S. G. (2004) Biochem. Biophys. Res. Commun. 315, 445–449 [DOI] [PubMed] [Google Scholar]
- 53.Emmons J., Townley-Tilson W. H., Deleault K. M., Skinner S. J., Gross R. H., Whitfield M. L., Brooks S. A. (2008) RNA 14, 888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.