Abstract
Apolipoprotein E-containing lipoproteins (LpE) are generated in the central nervous system by glial cells, primarily astrocytes, and are recognized as key players in lipid metabolism and transport in the brain. We previously reported that LpE protect retinal ganglion neurons from apoptosis induced by withdrawal of trophic additives (Hayashi, H., Campenot, R. B., Vance, D. E., and Vance, J. E. (2007) J. Neurosci. 27, 1933–1941). LpE bind to low density lipoprotein receptor-related protein-1 and initiate a signaling pathway that involves activation of protein kinase Cδ and inhibition of the pro-apoptotic glycogen synthase kinase-3β. We now show that uptake of LpE is not required for the neuroprotection. Experiments with inhibitors of phospholipase Cγ1 and RNAi knockdown studies demonstrate that activation of phospholipase Cγ1 is required for the anti-apoptotic signaling pathway induced by LpE. In addition, the protein phosphatase-2B, calcineurin, is involved in a neuronal death pathway induced by removal of trophic additives, and LpE inhibit calcineurin activation. LpE also attenuate neuronal death caused by oxidative stress. Moreover, physiologically relevant apoE3-containing lipoproteins generated by apoE3 knock-in mouse astrocytes more effectively protect neurons from apoptosis than do apoE4-containing lipoproteins. Because inheritance of the apoE4 allele is the strongest known genetic risk factor for Alzheimer disease, the reduced neuroprotection afforded by apoE4-containing LpE might contribute to the neurodegeneration characteristic of this disease.
The lipoprotein composition of cerebrospinal fluid differs from that of plasma because the blood-brain barrier prevents the movement of lipoproteins from the peripheral circulation into the central nervous system (CNS)3 (1). The CNS contains a distinct population of lipoprotein particles that are generated within the CNS and are thought to play important roles in the metabolism and transport of lipids within the brain. These lipoproteins are the size and density of plasma high density lipoproteins and contain apolipoprotein (apo) E and apoJ as their major protein constituents (2–5). The apoE-containing lipoproteins (LpE) in the CNS are generated by non-neuronal glial cells, primarily astrocytes (5). Astrocytes are thought to provide nutrient support for neurons by delivering lipoproteins to neurons for axonal growth (6) and synaptogenesis (7). Interest in the function of apoE in the nervous system has blossomed recently because after nerve injury the synthesis of apoE dramatically increases (by up to 150-fold) (6, 8). In addition, inheritance of the ϵ4 allele of apoE instead of the more common ϵ3 allele is the strongest genetic risk factor known for development of late-onset Alzheimer disease (9, 10). Furthermore, apoE3-containing lipoproteins have been reported to stimulate axon growth more efficiently than those containing apoE4 (11, 12). Thus, it has been proposed that LpE assist in repairing neurons after injury.
Our laboratory has reported that astrocyte-derived LpE stimulate axon extension of retinal ganglion cells (RGCs; CNS neurons) by binding to a neuronal receptor of the low density lipoprotein receptor family on axons (13). Neurons in the CNS express several receptors of this superfamily for which apoE is a ligand (2, 14, 15). Some of these receptors can function both in the endocytosis of ligands (16) and in signaling pathways that are required for normal brain development (17, 18). Recently, we demonstrated that glia-derived LpE strikingly protect cultured RGCs from apoptosis induced by withdrawal of trophic additives (19). The prevention of neuronal apoptosis was promoted by LpE binding to the multifunctional low density lipoprotein receptor-related protein-1 (LRP1) whereupon a signaling pathway was initiated that involved activation of protein kinase Cδ and inactivation of the pro-apoptotic kinase, glycogen synthase kinase-3β (19).
The aim of the present study was to dissect further the mechanism by which LpE protect RGC from apoptosis. We demonstrate that uptake of LpE is not required for the prevention of apoptosis. Furthermore, a signaling pathway induced upon binding of LpE to LRP1 requires the action of phospholipase Cγ1 upstream of protein kinase Cδ. Our data also show that glial LpE containing apoE3 are more protective against apoptosis than are apoE4-containing lipoproteins.
EXPERIMENTAL PROCEDURES
Materials
FK506, deltamethrin, cytochalasin D, and U73122 were purchased from Sigma. Two-day-old Sprague-Dawley rats were used for isolation of glial cells. Colonies of mice lacking endogenous apoE but expressing human apoE3 or human apoE4 (B6.Cg-Tg(GFAP-APOE3)37Hol Apoetm1Unc/J and B6.Cg-Tg(GFAP-APOE4)1Hol Apoetm1Unc/J, respectively) were established at the University of Alberta from breeding pairs of mice obtained from The Jackson Laboratory (Bar Harbor, ME).
RGCs
RGCs were cultured according to the method of Barres et al. (20) with minor modifications. Briefly, retinae were isolated from 2-day-old Sprague-Dawley rats and digested with papain (16.5 units/ml) for 15 min at 37 °C. The retinae were triturated in phosphate-buffered saline containing 0.15% (w/v) trypsin inhibitor (Roche Applied Science) and 0.15% (w/v) bovine serum albumin (Sigma). The retinal cell suspension was incubated with anti-macrophage antiserum (Accurate Chemical and Scientific Corp., Westbury, NY) for 10 min at room temperature. The suspension was centrifuged for 5 min at 200 × g, after which cells were resuspended in phosphate-buffered saline containing 1% (w/v) trypsin inhibitor and 1% (w/v) bovine serum albumin. The preparation was centrifuged then resuspended in phosphate-buffered saline containing 0.02% bovine serum albumin and 5 μg/ml insulin. The cells were incubated on a panning plate (150-mm Petri dish) coated with goat anti-rabbit IgG (Pierce) for 30–40 min at room temperature. Nonadherent cells were filtered through a 20-μm Nitex filter (SEFAR America, Ltd., Kansas City, MO) and incubated for 45–60 min on a second panning plate (100-mm Petri dish) coated with goat anti-mouse IgMμ (Pierce) and mouse anti-Thy1.1 antibodies secreted from T11D7e2 cells (American Type Culture Collection, Manassas, VA). The plate was washed with phosphate-buffered saline, then adherent cells (the RGCs) were released by treatment with 0.125% (w/v) trypsin for 8 min at 37 °C. The neuronal suspension was mixed with 30% fetal bovine serum in Neurobasal medium (Invitrogen) and centrifuged at 200 × g for 5 min. The neurons were resuspended in basal medium containing trophic additives. Basal medium contains glutamine (1 mm), insulin (5 μg/ml), N-acetylcysteine (5 μg/ml), progesterone (62 ng/ml), putrescine (16 μg/ml), sodium selenite (40 ng/ml), bovine serum albumin (0.1 mg/ml), triiodothyronine (40 ng/ml), transferrin (0.1 mg/ml), and sodium pyruvate (1 mm) in Neurobasal medium. Trophic additives are defined as forskolin (10 μm, Sigma), brain-derived neurotrophic factor (50 ng/ml, PreproTech Inc., Rocky Hill, NJ), ciliary neurotrophic factor (50 ng/ml, PreproTech), basic fibroblast growth factor (50 ng/ml, PreproTech), and 2% B27 supplement (Invitrogen). The RGCs were plated on 96-well plates coated with poly-d-lysine (Sigma) and laminin (Sigma) (5000 cells/well).
Isolation and Culture of Cortical Glia
Glial cells were isolated from cerebral cortices of 2-day-old rats or mice according to the method of Gong et al. (21). Glia were cultured in Dulbecco's-modified Eagle's medium containing 10% fetal bovine serum. The cultures were highly enriched in astrocytes (13) (see Fig. 1).
FIGURE 1.
Immunocytochemical analysis of cultures of cortical glia and LpE secretion. Glial cells were stained with anti-glial fibrillary acidic protein antibodies (red, marker of astrocytes), anti-Iba1 antibodies (green, marker of microglia), and Hoechst 33258 (blue, nuclear staining) (A), anti-CNPase (2′,3′-cyclic nucleotide 3′-phosphodiesterase) antibodies (green, marker of oligodendrocytes) and Hoechst 33258 (blue, marker of nuclei) (B), and anti-βIII tubulin antibodies (green, marker of neurons) and Hoechst 33258 (blue, marker of nuclei) (C). Size bar, 100 μm. D, Coomassie Blue staining of LpE. Proteins from glia-conditioned medium (GCM) and the LpE fraction (LP) (2 μg protein/lane) were separated by polyacrylamide gel electrophoresis, and the gel was stained with Coomassie Blue G250. M, molecular weight markers. Data are from one experiment representative of three independent experiments with similar results.
Isolation of LpE from Glia-conditioned Medium
Glial cells were washed 3 times with phosphate-buffered saline and cultured for 3 days in basal medium (as used for culture of RGCs) lacking trophic additives. The conditioned medium was collected and centrifuged at room temperature for 10 min at 1000 × g. The supernatant, designated “glia-conditioned medium,” was subjected to ultracentrifugation on a discontinuous sucrose density gradient consisting of sucrose solutions of densities 1.30, 1.20, 1.10, and 1.006 g/ml at 4 °C for 48 h at 160,000 × g in a Beckman SW40-Ti rotor (13). Fractions were collected from the tops of the tubes and immunoblotted for apoE and apoJ, as described below. Fractions of densities 1.07–1.12 g/ml were combined and concentrated on an Amicon Ultra filter, 100-kDa molecular mass cut-off (Millipore, Bedford, MA). The amount of LpE used in the experiments was normalized to equal amounts of cholesterol (1 μg/ml) as measured by gas-liquid chromatography (13).
Immunoblotting
Proteins in RGCs and isolated lipoproteins were dissolved by boiling the samples for 5 min in buffer containing 62.5 mm Tris-HCl, 10% glycerol, 2% SDS, and 5% β-mercaptoethanol. Proteins were separated by electrophoresis on polyacrylamide gels containing 0.1% SDS, then transferred to polyvinylidene difluoride membranes. The membranes were incubated with 5% nonfat milk in 10 mm Tris-buffered saline containing 0.1% (v/v) Tween 20 for 1 h at room temperature, then incubated overnight at 4 °C with primary antibodies in 10 mm Tris-buffered saline containing 0.1% Tween 20 and 5% bovine serum albumin. Subsequently, membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (Pierce), goat anti-mouse IgG (Pierce), or mouse anti-goat IgG (Pierce) secondary antibodies for 1 h at room temperature. Immunoreactive proteins were visualized by enhanced chemiluminescence (GE Healthcare) or Super Signal West Dura (Pierce). The following primary antibodies were used: rabbit anti-bovine phospholipase Cγ1 (sc-81, dilution 1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human calcineurin (ab1695, dilution 1:1,000, Millipore), mouse anti-β-actin (a5441, dilution 1:10,000, Sigma), goat anti-human apoE (k74190g, dilution 1:5,000, Biodesign, Saco, ME), and goat anti-human apoJ (600–101-198, dilution 1:2,500, Rockland Immunochemicals, Inc., Gilbertsville, PA).
Induction and Detection of Apoptosis
Apoptosis was induced in RGCs by withdrawal of trophic additives (forskolin, brain-derived neurotrophic factor, ciliary neurotrophic factor, basic fibroblast growth factor, and B27 supplement) from the culture medium (13, 19). The RGCs were washed 3 times with basal medium lacking trophic additives (150 μl/well) then incubated for 24 h in the same medium. For some experiments apoptosis was induced by the addition of 25 μm H2O2 with trophic additives containing B27 supplement AO depleted of antioxidants (Invitrogen) instead of B27 supplement for 24 h. The number of apoptotic neurons was determined by nuclear staining of fixed cells with Hoechst 33258 (1 μg/ml, Invitrogen) (19). Fluorescent images were observed using a Leica DM IRE fluorescence microscope (Leica Microsystems, Bannockburn, IL). Fragmented or shrunken nuclei were counted as apoptotic. Data are the averages ± S.E. of a number of apoptotic nuclei as a percentage of total number of neurons. More than 300 neurons in each group were counted.
Preparation of Texas Red-labeled LpE
LpE were labeled with a FluoReporter Texas Red-X Protein labeling kit (Invitrogen) according to manufacturer's instructions. Briefly, 20 μl of 1 m NaHCO3 were added to 200 μl of LpE suspension in phosphate-buffered saline, then mixed with Texas Red dye solution (1.92 μl). The mixture was stirred for 1 h at room temperature in the dark. The Texas Red-labeled LpE were washed twice with phosphate-buffered saline using an Amicon Ultra filter (100-kDa molecular mass cut-off; Millipore).
RNA Silencing of Phospholipase Cγ1
Negative control small interfering RNA (siRNA) (AllStars Negative siRNA) and two siRNAs specific for phospholipase Cγ1 (catalogue numbers S101961939 and S1011961946) were purchased from Qiagen (Mississauga, ON, Canada). RGCs that had been cultured for 3–4 days were transfected with these siRNAs (20 pmol/well) using GeneSilencer siRNA Transfection Reagent (Gene Therapy Systems, San Diego, CA) according to the manufacturer's instructions. In brief, the siRNA and GeneSilencer Reagent were mixed for 10 min at room temperature then incubated with RGCs for 24 h. The medium was changed to fresh medium for an additional 24 h or for 48 h for immunoblotting experiments and assessment of apoptosis.
Lipid Composition of Glial Lipoproteins
Lipids were extracted (13, 22) from lipoproteins (200 μg protein) and analyzed for the mass of unesterified cholesterol and phospholipids (μg/mg protein) by gas-liquid chromatography (19).
Immunocytochemistry of Glia
Glial cells isolated from cerebral cortices were washed 3 times with phosphate-buffered saline, then fixed with 4% paraformaldehyde for 20 min. The cells were permeabilized in 0.2% Triton X-100 for 15 min at 4 °C and blocked with 5% goat serum in phosphate-buffered saline for 1 h. The cells were incubated for 2 h at room temperature with mouse anti-bovine glial fibrillary acidic protein (dilution 1:500, BD Biosciences), rabbit anti-Iba1 (dilution 1:500, Wako, Osaka, Japan), mouse anti-human 2′,3′-cyclic nucleotide 3′-phosphodiesterase (dilution 1:250, Abcam, Cambridge, MA), or mouse anti-rat βIII tubulin (dilution 1:500, Abcam) in phosphate-buffered saline containing 2% bovine serum albumin. The cells were washed 3 times with phosphate-buffered saline, then incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (dilution 1:200, Invitrogen) and Alexa Fluor 594-conjugated goat anti-mouse IgG (dilution 1:200, Invitrogen) for 1 h at room temperature. For assessment of cell survival, glia were incubated with Hoechst 33258 (1 μg/ml, Invitrogen) for 15 min at room temperature, washed 3 times with phosphate-buffered saline, then mounted with Vectashield (Vector Laboratories, Inc., Burlingame, CA). Photographs were taken using an Olympus IX71 microscope (Tokyo, Japan). The different types of glia were assessed by quantifying the number of stained cells as a percentage of total number of cells. More than 400 cells were counted.
RESULTS
Internalization of Glia-derived LpE Is Not Required for Protection against Apoptosis
We have previously demonstrated that glia-derived LpE activate an anti-apoptotic signaling pathway in RGCs upon binding to the low density LRP1 (19). Because LRP1 can internalize LpE via endocytosis and can also act as a signaling receptor (17, 18), we determined whether or not lipoprotein uptake is required for the neuroprotection. Glial cells were isolated from the cerebral cortex of 2-day-old rats (21) and cultured for 3 days in the absence of serum. These cultures contained 82.5% astrocytes, 16% microglia, 0.5% oligodendrocytes, and 1% neurons according to staining with fluorescence-labeled antibodies to marker proteins: glial fibrillary acidic protein (astrocytes), Iba1 (microglia), 2′,3′-cyclic nucleotide 3′-phosphodiesterase (oligodendrocytes), and βIII tubulin (neurons) (Figs. 1, A–C).
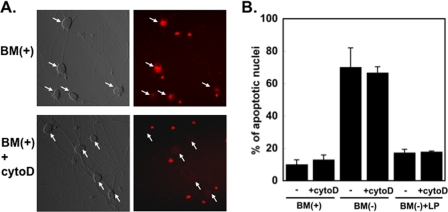
The glia-conditioned medium was collected, and lipoproteins (density 1.07–1.12 g/ml) were isolated by sucrose density gradient ultracentrifugation (13, 19). The fractions containing apoE were identified by immunoblotting, then combined and concentrated using a centrifuge filter. The concentrated fraction was designated as LpE. Proteins in glia-conditioned medium and in the LpE-rich fraction were stained with Coomassie Blue (Fig. 1D). ApoE secreted by the glia was highly enriched in the LpE fraction. The LpE were fluorescence-labeled with Texas Red and subsequently incubated with RGCs in the presence or absence of 20 μm cytochalasin D, an agent that induces actin depolymerization and blocks endocytosis (24). After 3 h, the uptake of fluorescent LpE by RGCs was visualized by fluorescence microscopy. Cytochalasin D markedly reduced the number of fluorescence-positive cells (from 69.0 to 8.0%; Fig. 2A). Fluorescent LpE also non-specifically stained cell debris, resulting in the intensely stained small red dots visible in Fig. 2A. To determine whether inhibition of LpE uptake by cytochalasin D abolished the protective effect of LpE, we assessed the number of apoptotic nuclei in RGCs after 24 h by Hoechst staining (19). Cells with shrunken and fragmented nuclei were counted as apoptotic, and the number of apoptotic neurons is expressed as the percentage of total number of neurons. We have previously examined apoptosis of RGC under these conditions by both Hoechst staining and by staining with annexin V-fluorescein/propidium iodide (19). As in our previous studies (19), removal of trophic additives from the culture medium markedly increased the percentage of apoptotic nuclei (from ∼8 to ∼70%) (Fig. 2B). LpE almost completely prevented the neuronal death caused by removal of trophic additives. The presence of cytochalasin D did not reduce the ability of LpE to protect the neurons from apoptosis (Fig. 2B) despite the finding (Fig. 2A) that cytochalasin D effectively blocked the uptake of Texas Red-labeled LpE. These experiments indicate that the internalization of glia-derived LpE is not required for LpE to exert their neuroprotective effect.
FIGURE 2.
Inhibition of LpE uptake does not eliminate the neuroprotective effect of LpE. A, RGCs were incubated for 2 h in the presence of trophic additives (BM(+)) ± 20 μm cytochalasin D (cytoD), after which Texas Red-labeled LpE were added for 3 h in the presence (lower panels) or absence (upper panels) of cytochalasin D. Neuronal uptake of LpE was visualized by fluorescence microscopy and is shown by diffuse red staining in cell bodies (arrows); the intensely stained small red dots represent cell debris that binds Texas Red-labeled LpE. B, RGCs were incubated with trophic additives (BM(+)) for 2 h with or without cytochalasin D (20 μm). The incubation was continued in the presence (BM(+)) or absence (BM(−)) of trophic additives for an additional 24 h with or without cytochalasin D and in the presence or absence of apoE-containing lipoproteins (LP) as indicated. Apoptosis was assessed by Hoechst staining. Data are means ± S.E. from three independent experiments.
LpE Attenuate Neuronal Death Caused by Oxidative Stress
To determine whether neuronal death induced by other interventions could also be prevented by LpE, we exposed RGCs to oxidative stress by incubation of the cells for 24 h with hydrogen peroxide (25 μm). To ensure a minimal antioxidant content of the culture medium, we used B27 supplement that had been depleted of antioxidants (Invitrogen catalog #10889) rather than the standard B27 supplement used in the other experiments. Neuronal survival was equivalent in the presence of either formulation of B27 supplement (>90%) (Fig. 3). Upon exposure of RGCs to H2O2 for 24 h, ∼80% of the neurons exhibited apoptotic nuclei according to Hoechst staining, and glial LpE significantly reduced neuronal death (Fig. 3). Thus, LpE protect neurons from apoptosis that is induced not only by withdrawal of trophic additives but also from death caused by oxidative stress. The magnitude of LpE-mediated protection from H2O2-induced apoptosis was less than for experiments in which trophic factors were removed (Fig. 2B). One potential explanation for this observation is that oxidative injury caused by H2O2 might partially disable the neuroprotective function of LRP1. H2O2 is known to oxidize lipids in lipoproteins, thereby partially masking the protective effect of LpE, and/or oxidized lipoproteins might themselves cause neuronal injury (25). In contrast to the results with H2O2, exposure of RGCs to tumor necrosis factor-α, an inflammatory mediator, for up to 48 h at concentrations of up to 100 ng/ml did not cause significant neuronal death. Nor did LpE affect survival of RGCs exposed to this agent (data not shown).
FIGURE 3.

LpE attenuate apoptosis induced by oxidative stress. RGCs were incubated for 24 h with trophic additives, including either B27 supplement (BM(+)) or B27 supplement depleted of antioxidants (BM(+)-AO). Some neurons, as indicated, were exposed to 25 μm H2O2 with or without glial lipoproteins (LP) for an additional 24 h, and apoptosis was assessed by Hoechst staining. Data are the means ± S.E. from five independent experiments. *, p < 0.05 for BM(+)-AO+H2O2 versus BM(+)-AO+2O2+LP. Statistical analysis was performed using one-way analysis of variance followed by Bonferroni's multiple comparison.
Calcineurin Participates in Neuronal Apoptosis Induced by Withdrawal of Trophic Additives
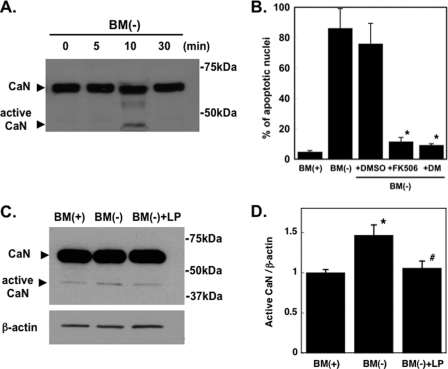
In our previous studies we started to define the anti-apoptotic signaling pathway(s) induced in RGCs upon binding of LpE to LRP1. We found that when trophic additives were removed from the culture medium of RGCs, phosphatidylserine became exposed on the cell surface of RGCs, and pro-apoptotic caspases were activated (19). An objective of the present study was to define in more detail the apoptotic pathway(s) that is/are induced in RGCs upon withdrawal of trophic additives. It has been reported that cleavage of the autoinhibitory domain of the protein phosphatase 2B, calcineurin, is enhanced in some chronic neurodegenerative diseases such as glaucoma (26). Moreover, the cleaved, active, form of calcineurin leads to apoptosis via a mitochondrial apoptotic pathway (27–30). We, therefore, examined the role of calcineurin in neuronal apoptosis caused by removal of trophic additives. A rapid (within ∼10 min) and transient cleavage of calcineurin to its active form (∼45 kDa) occurred upon withdrawal of trophic additives (Fig. 4A). Withdrawal of trophic additives from RGCs also caused pronounced neuronal death (Fig. 4B) to an extent (∼80%) similar to that shown in Fig. 2B. To determine whether calcineurin participated in the apoptotic pathway initiated by removal of trophic additives, we tested whether or not calcineurin inhibitors prevented apoptosis of RGCs. The calcineurin inhibitors FK506 (1 μm) (31) and deltamethrin (1 μm) (32, 33) were dissolved in dimethyl sulfoxide and given to the neurons. Control cells were given an equivalent amount of dimethyl sulfoxide (0.4 μl/ml) without inhibitor. Both calcineurin inhibitors prevented apoptosis of RGCs (Fig. 4B), indicating that activation of calcineurin is involved in an apoptotic pathway that is induced upon withdrawal of trophic additives. Furthermore, the amount of active calcineurin in RGCs cultured without trophic additives was reduced by LpE to the same level as in RGCs cultured in the presence of trophic additives (Figs. 4, C and D). Thus, LpE inhibit calcineurin activation, and inhibition of calcineurin prevents neuronal death. Consequently, both the calcineurin pathway and the glycogen synthase kinase-3β apoptotic pathway are inhibited by LpE.
FIGURE 4.
Involvement of calcineurin in apoptosis of RGCs. A, RGCs were incubated without trophic additives (BM(−)) for up to 30 min. Equal amounts of protein (25 μg) were loaded in each lane of the gel. Calcineurin (CaN) was detected by immunoblotting. Full-length calcineurin is shown at ∼60 kDa; the arrowhead shows the cleaved, active form of calcineurin (∼45 kDa). Data are representative of three independent experiments with similar results. B, RGCs were incubated for 24 h in the presence (BM(+)) or absence (BM(−)) of trophic additives. The calcineurin inhibitors FK506 (1 μm) and deltamethacin (DM) (1 μm) were dissolved in dimethyl sulfoxide (DMSO) and added to indicated cultures. DMSO indicates RGCs that were given an equivalent amount (0.4 μl/ml) of DMSO without inhibitor. Apoptosis was detected by Hoechst staining. Data are the means ± S.E. from three independent experiments. *, p < 0.005 for BM(−)+DMSO versus BM(−)+FK506 or BM(−)+DM. C, immunoblotting of active (45 kDa) form of calcineurin and β-actin in RGC after 10 min. RGCs were harvested after incubation for 10 min with BM(+), BM(−), or BM(−) with LpE. Neuronal proteins were separated by SDS-polyacrylamide gel electrophoresis. Equal amounts of protein (10 μg) were applied to each lane of the gel. Calcineurin was detected by immunoblotting; immunoblotting of β-actin was used as a loading control. Data are from one experiment representative of four experiments with similar results. D, densitometric quantification of amounts of active calcineurin (45 kDa form) relative to β-actin from immunoblotting experiments in C. Data are the ratios of active calcineurin:β-actin and are the means ± S.E. from four independent experiments. *, p < 0.05 for BM(+) versus BM(−). #, p < 0.05 for BM(−) versus BM(−)+LP. Statistical analysis was performed using one-way analysis of variance followed by Bonferroni's multiple comparison.
Phospholipase Cγ1 Contributes to the Neuroprotection Elicited by LpE
Activation of protein kinase Cδ occurs during an anti-apoptotic signaling pathway that is activated when LpE binds to LRP1 (19). Because diacylglycerols activate protein kinase C (34), we hypothesized that generation of diacylglycerols, via hydrolysis of phosphatidylinositol 4,5-bisphosphate by phospholipase C, might contribute to the neuroprotective effect of glial LpE. Removal of trophic additives from RGCs caused extensive neuronal death which was prevented by LpE (Fig. 5A; also Fig. 2B). The addition of the phospholipase C inhibitor, U73122 (5 μm), to trophic additive-deprived neurons completely abrogated the anti-apoptotic effect of LpE. Importantly, U73122 did not cause the death of RGCs that were provided with trophic additives (Fig. 5A). Thus, phospholipase Cγ1 appears to be involved in the LpE-mediated protection of RGCs from apoptosis.
FIGURE 5.
Phospholipase Cγ1 participates in the anti-apoptotic pathway induced by LpE. A, RGCs were cultured for 24 h in the presence (BM(+)) or absence (BM(−)) of trophic additives. ApoE-containing lipoproteins (LP) prevented apoptosis of neurons cultured in BM(−). An inhibitor of phospholipase C (U73122, 5 μm) was added to some neurons, and apoptosis was assessed by Hoechst staining. Data are the means ± S.E. from four independent experiments. *, p < 0.05 for BM(−)+LP versus BM(−)+LP+U73122. B–E, RNA silencing of phospholipase Cγ1 (PLCγ1). B, RGCs were cultured for 3–4 days, then transfected with 20 pmol of either negative control siRNA (Neg) or one of two siRNAs (designated #1 and #2) specific for PLCγ1. RGCs were harvested after 48 and 72 h, and neuronal proteins were separated by polyacrylamide gel electrophoresis. Equal amounts (25 μg) of protein were applied to each lane of the gel. PLCγ1 was detected by immunoblotting; immunoblotting of β-actin was used as a loading control. Data are from one experiment representative of three experiments with similar results. C and D, densitometric quantification of amounts of PLCγ1 at 48 h (C) and 72 h (D) relative to β-actin from the experiments shown in B. Data are the ratios of the amounts of PLCγ1:β-actin and are means ± S.E. from three independent experiments. *, p < 0.05 for Neg versus PLCγ1 siRNA #1. #, p < 0.01 for Neg versus PLCγ1 siRNA #2. E, RNA silencing of phospholipase Cγ1 blocks the anti-apoptotic effect of LpE. RGCs were cultured for 3–4 days then transfected with 20 pmol of either a negative control siRNA (Neg) or one of two siRNAs (#1 and #2) specific for phospholipase Cγ1. After 3 days, the RGCs were incubated for 24 h in medium containing (BM(+)) or lacking (BM(−)), trophic additives. Some neurons were given apoE-containing lipoproteins, as indicated. The percentage of neurons with apoptotic nuclei was determined by Hoechst staining. Data are the means ± S.E. of three independent experiments. *, p < 0.0001 for Neg versus #1 or #2. Statistical analysis was performed using one-way analysis of variance followed by Bonferroni's multiple comparison.
As an additional indication of the importance of phospholipase Cγ1 in the LpE-mediated survival pathway, we reduced the expression of phospholipase Cγ1 by RNA silencing. RGCs were cultured for 3–4 days, then transfected with either a control siRNA or one of two different siRNAs targeted to phospholipase Cγ1. The RGCs were cultured for 48 or 72 h after which immunoblotting was performed with anti-phospholipase Cγ1 antibodies. Neither of the phospholipase Cγ1 siRNAs reduced the amount of phospholipase Cγ1 protein significantly after 48 h (Fig. 5B). However, 72 h after transfection with either phospholipase Cγ1-specific siRNA, the amount of phospholipase Cγ1 was significantly reduced (Fig. 5B). In contrast, none of the siRNAs reduced the amount of the control protein β-actin (Fig. 5B). Quantification of immunoblots from three independent transfections with each siRNA construct (Figs. 5, C and D) revealed that 72 h after transfection the amount of phospholipase Cγ1 protein was reduced by 35–50%.
To determine whether or not reduction in phospholipase Cγ1 increased neuronal apoptosis, the siRNA-silenced RGCs were cultured with or without trophic additives and with or without glial LpE. Compared with control siRNA, neither phospholipase Cγ1 siRNA increased the number of apoptotic nuclei in RGCs cultured in the presence of trophic additives (Fig. 5E). As expected, however, removal of trophic additives from each of the three types of transfected neurons increased the percentage of apoptotic nuclei (from ∼20% to ∼70%) (Fig. 5E). Although LpE prevented apoptosis of neurons transfected with control siRNA, the neuroprotection by LpE was abolished in neurons in which phospholipase Cγ1 expression was reduced (Fig. 5E). These experiments demonstrate that phospholipase Cγ1 provides a neuroprotective signal in the anti-apoptotic pathway initiated by LpE. Because of the established role of phospholipase C in activation of protein kinase C (34), these data suggest that phospholipase Cγ1 acts upstream of protein kinase C in this anti-apoptotic pathway.
ApoE4-containing Lipoproteins Are Less Neuroprotective than ApoE3-containing Lipoproteins
In light of many reports that apoE4 is detrimental for neuronal functions (35–37), we compared the anti-apoptotic activity of glial LpE that contained either human apoE3 or human apoE4. We collected LpE secreted by astrocytes isolated from mice that lacked endogenous mouse apoE but expressed either human apoE3 or apoE4. According to immunoblotting, LpE isolated from astrocyte-conditioned medium contained approximately equal amounts of apoE and also similar amounts of apoJ (Fig. 6). We also analyzed the lipid content of the two types of glial LpE. Although phospholipids comprise the major lipid component of LpE, cholesterol is also abundant (Table 1). The lipid content (μg/mg protein) of apoE3- and apoE4-containing lipoproteins was indistinguishable and was also similar to that of rat glial LpE, although mouse LpE contained more phospholipid relative to cholesterol than did rat LpE (Table 1). The isolated lipoproteins that contained apoE3 or apoE4 were supplied to RGCs from which trophic additives had been withdrawn; control cultures were provided with trophic additives but were not given LpE. The apoE3-containing lipoproteins effectively protected the RGCs from apoptosis, whereas the apoE4-containing lipoproteins were significantly less protective (Fig. 6B).
FIGURE 6.
ApoE4-containing LpE less effectively protect RGCs against apoptosis than do apoE3-containing LpE. Cortical glia were isolated from mice lacking endogenous apoE but expressing human apoE3 or human apoE4. LpE were isolated from conditioned medium. A, equal amounts of LpE protein (10 μg) were separated by polyacrylamide gel electrophoresis and immunoblotted with antibodies directed against apoE and apoJ. The experiment was repeated two additional times with similar results. B, LpE secreted by mouse glia expressing human apoE3 or apoE4 were given to RGCs for 24 h in medium lacking trophic additives (BM(−)). Control cultures were supplied with trophic additives (BM(+)). The number of apoptotic neurons was determined by Hoechst staining and is given as percentage of total number of neurons. Data are the means ± S.E. from four independent experiments. *, p < 0.005 for BM(−) versus BM(−) + apoE3 and for BM(−) + apoE4. #, p < 0.001 for apoE3 versus apoE4. Statistical analysis was performed using one-way analysis of variance followed by Bonferroni's multiple comparison.
TABLE 1.
LpE were collected from conditioned medium of glia isolated from 2-day-old rats and apoE knock-out mice that expressed human apoE3 (E3 mouse) or apoE4 (E4 mouse) (41)
Lipids were extracted from 200 μg of protein that contained equal amounts of apoE3 and apoE4 according to immunoblotting; see Fig. 6. The mass of cholesterol and phospholipids was quantified by gas-liquid chromatography. Data are the means ± S.E. from duplicate analyses of three independent lipoprotein preparations.
| Source of LpE | Phospholipid | Cholesterol |
|---|---|---|
| μg/mg of protein | ||
| Rat | 21.7 ± 1.8 | 7.3 ± 1.1 |
| E3 mouse | 19.2 ± 3.4 | 2.5 ± 0.3 |
| E4 mouse | 15.8 ± 2.7 | 2.6 ± 0.4 |
DISCUSSION
Neuronal apoptosis is a characteristic of neurodegenerative disorders such as Alzheimer disease, Parkinson disease, and Niemann-Pick Type C disease. In the present study we provide novel information on mechanisms by which LpE protect neurons from apoptosis induced by removal of trophic additives. Consistent with our previous observation that LpE promote survival of RGCs (19), neuronal and behavioral defects have been observed in apoE knock-out mice, particularly after injury (38) and during aging (39, 40). Thus, the LpE-induced anti-apoptotic signaling pathway that we have identified might protect CNS neurons from apoptosis in some neurodegenerative disorders. Levels of neurotrophic factors such as brain-derived neurotrophic factor are decreased in the brains of individuals with Alzheimer disease and Parkinson disease (41, 42). Interestingly, the neuroprotective action of LpE in RGC is not restricted to apoptosis caused by withdrawal of trophic additives as our studies show that neuronal death caused by oxidative stress (exposure to H2O2) is also attenuated by LpE.
The presence of apoE on the lipoproteins is necessary for their anti-apoptotic properties. Moreover, association of apoE with lipid is required for initiation of the anti-apoptotic signaling pathway, presumably by maintaining an appropriate receptor binding conformation of the protein (43). Our data show that cholesterol is not required in LpE for the neuroprotection because reconstituted LpE completely lacking cholesterol effectively prevent apoptosis of RGCs (19). In addition to receptors of the low density lipoprotein receptor superfamily acting as endocytosis receptors (16) several of these receptors (for example, the apoE receptor-2 and the very low density lipoprotein receptor (44)) have been implicated in signaling pathways in the brain, particularly during development. An important conclusion from the present study is that the ability of LpE to protect RGCs from apoptosis is independent of internalization of the lipoprotein particles. This finding is consistent with the idea that the major anti-apoptotic pathway induced by LpE is a signaling pathway initiated by binding of LpE to LRP1 on the cell surface.
Involvement of Phospholipase Cγ1 in Protection of RGCs from Apoptosis
LpE bind to LRP1, a multifunctional receptor of the low density lipoprotein receptor superfamily, that is expressed in RGCs. Subsequently, a signaling cascade is initiated in which protein kinase Cδ is activated and the pro-apoptotic kinase, glycogen synthase kinase-3β, is phosphorylated and inactivated. Many studies have implicated glycogen synthase kinase-3β as a key contributor to neuronal apoptosis (45–48). Inhibition of glycogen synthase kinase-3β reduces tauropathy (49) as well as production of the Aβ peptide (50, 51), suggesting that inhibition of glycogen synthase kinase-3β is a potential therapeutic target for treatment of neurodegenerative disorders. Our current data demonstrate that the binding of LpE to LRP1 activates phospholipase Cγ1, suggesting that this lipase acts upstream of protein kinase Cδ in the anti-apoptotic signaling cascade initiated by LpE. Phospholipase Cγ1 produces diacylglycerols and inositol 1,4,5-trisphosphate upon hydrolysis of phosphatidylinositol 4,5-bisphosphate (34). Diacylglycerols activate members of the protein kinase C family, such as protein kinase Cδ. We used inhibitors of phospholipase Cγ1 as well as RNA silencing to demonstrate that phospholipase Cγ1 is required for the LpE-mediated protection of RGCs from apoptosis. Although the amount of phospholipase Cγ1 protein was reduced by only 35–50% in the RNA knockdown experiments, the LpE-mediated neuroprotection was completely abolished, highlighting the importance of this phospholipase in protecting RGCs from apoptosis. Several other reports have demonstrated that phospholipase Cγ1 is a component of signaling pathways that increase neuronal survival. For example, phospholipase Cγ1 has been implicated in signaling pathways induced by neurotrophins such as nerve growth factor and brain-derived neurotrophic factor (for review, see Ref. 52) as well as vascular endothelial growth factor (for review, see Ref. 53).
Involvement of Calcineurin in Neuronal Apoptosis
We previously showed that withdrawal of trophic additives caused exposure of phosphatidylserine on the surface of RGCs and that caspases were involved in this apoptotic pathway (19). We now show that withdrawal of trophic support from RGCs activates calcineurin, a protein phosphatase 2B. We also demonstrate that calcineurin inhibitors prevent neuronal death. Thus, calcineurin appears to play a role in the apoptotic pathway(s) induced by removal of trophic additives from RGCs. Activation of calcineurin has been reported to induce the dephosphorylation of BAD (a pro-apoptotic member of the Bcl-2 family) and activate caspase-3, thereby causing apoptosis in several types of cells including neurons (27–29). Moreover, FK506 and cyclosporine (inhibitors of calcineurin A) block mitochondria-mediated apoptosis in primary cortical neurons (54, 55) and glutamate-induced apoptosis in astrocytes (56). These data suggest that calcineurin plays an important role in apoptosis in the CNS. We now show that LpE attenuate the activation of calcineurin that occurs upon withdrawal of trophic additives from RGCs, although we cannot distinguish between calcineurin and glycogen synthase kinase-3β acting in the same or independent apoptotic pathways (Fig. 7). Further details of the link between activation of LRP1 and attenuation of calcineurin activation remain to be established. However, we speculate that reduction in neuronal calcium levels, induced by activation of LRP1, inhibits calcineurin cleavage (57, 58). Moreover, the calcium-dependent cysteine protease, calpain, cleaves full-length calcineurin to the constitutively active form (45 kDa) (59). Thus, a reduction in intracellular calcium levels induced by activation of LRP1 might prevent apoptosis by inhibiting calcineurin cleavage.
FIGURE 7.
Proposed pathway for protection of RGCs from apoptosis by apoE-containing lipoproteins. Withdrawal of trophic additives from RGCs promotes apoptosis which can be prevented by glia-derived apoE-containing lipoproteins (LP). When LP bind to LRP1, phospholipase Cγ1 (PLCγ1) is activated and hydrolyzes phosphatidylinositol bisphosphate (PIP2) to diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG activates protein kinase Cδ (PKCδ) which, directly or indirectly, phosphorylates and inactivates the pro-apoptotic kinase, glycogen synthase kinase 3β (GSK3β). In addition, withdrawal of trophic additives from RGCs induces cleavage/activation of calcineurin and activation of caspases, resulting in apoptosis within 24 h. Thus, withdrawal of trophic additives causes neuronal death via a GSK3β-dependent signaling pathway as well as via a calcineurin-dependent pathway, and both pathways are inhibited by LP.
We conclude that LpE protect neurons from death mediated by both the calcineurin pathway and the glycogen synthase kinase-3β pathway (Fig. 7). Our observations provide additional support for the idea that calcineurin-mediated apoptosis can be a component in nerve injuries and neurodegenerative diseases (23, 28, 60–62).
ApoE Isoforms and Neuronal Apoptosis
Inheritance of the ϵ4, rather than the more common ϵ3 allele of apoE is the strongest known genetic risk factor for development of Alzheimer disease (9). We, therefore, compared the ability of physiologically relevant apoE3- and apoE4-containing lipoproteins to protect RGCs from apoptosis induced by withdrawal of trophic additives. LpE secreted by astrocytes isolated from mice expressing human apoE3 or apoE4, but lacking endogenous mouse apoE, were given to RGCs. The apoE3-containing lipoproteins protected RGCs from apoptosis, whereas the apoE4-containing lipoproteins were significantly less protective (Fig. 6). To determine whether the difference in protection provided by the two types of LpE were because of gross differences in lipid composition of the LpE, we measured the cholesterol and phospholipid content of the LpE and found no significant differences between apoE3- and apoE4-containing LpE. These observations are consistent with our previous observation that reconstituted high density lipoprotein particles that contained only phospholipids and recombinant apoE4 were less neuroprotective for RGCs than were reconstituted apoE3-containing particles (19). We speculate that the differential protection afforded by apoE3- and apoE4-containing glial lipoproteins might contribute to the extensive neurodegeneration that occurs in late-onset Alzheimer disease patients that express apoE4.
In conclusion, we have demonstrated that LpE protect neurons from apoptosis via a signaling mechanism that does not require internalization of the LpE particles. The signaling pathway that occurs upon the binding of LpE to LRP1 on RGCs involves activation of phospholipase Cγ1 and subsequent activation of protein kinase Cδ. This kinase directly or indirectly phosphorylates and inactivates glycogen synthase kinase-3β and prevents apoptosis, as proposed in the model depicted in Fig. 7. Furthermore, withdrawal of trophic additives from RGCs activates a pro-apoptotic pathway that involves calcineurin; this activation is also prevented by LpE (Fig. 7). Finally, we show that physiologically relevant, astrocyte-derived apoE4-containing lipoproteins are less able to protect neurons from apoptosis than are apoE3-containing lipoproteins.
Acknowledgments
We thank Russell Watts, Priscilla Gao, Audric Moses, and Yuko Eguchi for excellent technical assistance.
This work was supported by the Canadian Institutes for Health Research and in part by Grant-in-aid for Young Scientists (Start-up) 20890174 and the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sport, Science and Technology, Japan.
- CNS
- central nervous system
- apo
- apolipoprotein
- LpE
- apoE-containing lipoproteins
- LRP
- low density lipoprotein receptor-related protein
- RGC
- retinal ganglion cell
- siRNA
- small interfering RNA.
REFERENCES
- 1.Dietschy J. M., Turley S. D. (2004) J. Lipid Res. 45, 1375–1397 [DOI] [PubMed] [Google Scholar]
- 2.Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. (1989) J. Clin. Invest. 83, 1015–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch S., Donarski N., Goetze K., Kreckel M., Stuerenburg H. J., Buhmann C., Beisiegel U. (2001) J. Lipid Res. 42, 1143–1151 [PubMed] [Google Scholar]
- 4.DeMattos R. B., Rudel L. L., Williams D. L. (2001) J. Lipid Res. 42, 976–987 [PubMed] [Google Scholar]
- 5.Han X. (2004) Cell. Mol. Life Sci. 61, 1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahley R. W. (1988) Science 240, 622–630 [DOI] [PubMed] [Google Scholar]
- 7.Mauch D. H., Nägler K., Schumacher S., Göritz C., Müller E. C., Otto A., Pfrieger F. W. (2001) Science 294, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 8.Ignatius M. J., Gebicke-Härter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 1125–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. (1993) Science 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 10.Strittmatter W. J., Roses A. D. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 4725–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathan B. P., Bellosta S., Sanan D. A., Weisgraber K. H., Mahley R. W., Pitas R. E. (1994) Science 264, 850–852 [DOI] [PubMed] [Google Scholar]
- 12.DeMattos R. B., Curtiss L. K., Williams D. L. (1998) J. Biol. Chem. 273, 4206–4212 [DOI] [PubMed] [Google Scholar]
- 13.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. (2004) J. Biol. Chem. 279, 14009–14015 [DOI] [PubMed] [Google Scholar]
- 14.Posse de Chaves E. I., Rusiñol A. E., Vance D. E., Campenot R. B., Vance J. E. (1997) J. Biol. Chem. 272, 30766–30773 [DOI] [PubMed] [Google Scholar]
- 15.Zhuo M., Holtzman D. M., Li Y., Osaka H., DeMaro J., Jacquin M., Bu G. (2000) J. Neurosci. 20, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown M. S., Goldstein J. L. (1976) Science 191, 150–154 [DOI] [PubMed] [Google Scholar]
- 17.Trommsdorff M., Borg J. P., Margolis B., Herz J. (1998) J. Biol. Chem. 273, 33556–33560 [DOI] [PubMed] [Google Scholar]
- 18.Herz J., Strickland D. K. (2001) J. Clin. Invest. 108, 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. (2007) J. Neurosci. 27, 1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barres B. A., Silverstein B. E., Corey D. P., Chun L. L. Y. (1988) Neuron 1, 791–803 [DOI] [PubMed] [Google Scholar]
- 21.Gong J. S., Kobayashi M., Hayashi H., Zou K., Sawamura N., Fujita S. C., Yanagisawa K., Michikawa M. (2002) J. Biol. Chem. 277, 29919–29926 [DOI] [PubMed] [Google Scholar]
- 22.Folch J., Lees M., Sloane-Stanley G. H. (1957) J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 23.Agostinho P., Lopes J. P., Velez Z., Oliveira C. R. (2008) Neurochem. Int. 52, 1226–1233 [DOI] [PubMed] [Google Scholar]
- 24.Szaszi K., Paulsen A., Szabo E. Z., Numata M., Grinstein S., Orlowski J. (2002) J. Biol. Chem. 277, 42623–42632 [DOI] [PubMed] [Google Scholar]
- 25.Ellman P. I., Smith R. L., Girotti M. E., Thompson P. W., Peeler B. B., Kern J. A., Kron I. L. (2008) J. Am. Coll. Surg. 206, 993–999 [DOI] [PubMed] [Google Scholar]
- 26.Huang W., Fileta J. B., Dobberfuhl A., Filippopolous T., Guo Y., Kwon G., Grosskreutz C. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H. G., Pathan N., Ethell I. M., Krajewski S., Yamaguchi Y., Shibasaki F., McKeon F., Bobo T., Franke T. F., Reed J. C. (1999) Science 284, 339–343 [DOI] [PubMed] [Google Scholar]
- 28.Springer J. E., Azbill R. D., Nottingham S. A., Kennedy S. E. (2000) J. Neurosci. 20, 7246–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cserepes J., Szentpétery Z., Seres L., Ozvegy-Laczka C., Langmann T., Schmitz G., Glavinas H., Klein I., Homolya L., Váradi A., Sarkadi B., Elkind N. B. (2004) Biochem. Biophys. Res. Commun. 320, 860–867 [DOI] [PubMed] [Google Scholar]
- 30.Bi X., Liao G. (2007) Autophagy 3, 646–648 [DOI] [PubMed] [Google Scholar]
- 31.Griffith J. P., Kim J. L., Kim E. E., Sintchak M. D., Thomson J. A., Fitzgibbon M. J., Fleming M. A., Caron P. R., Hsiao K., Navia M. A. (1995) Cell 82, 507–522 [DOI] [PubMed] [Google Scholar]
- 32.Enan E., Matsumura F. (1992) Biochem. Pharmacol. 43, 1777–1784 [DOI] [PubMed] [Google Scholar]
- 33.Wu Z. Z., Chen S. R., Pan H. L. (2006) Neuroscience 141, 407–419 [DOI] [PubMed] [Google Scholar]
- 34.Nishizuka Y. (1986) Science 233, 305–312 [DOI] [PubMed] [Google Scholar]
- 35.Jordán J., Galindo M. F., Miller R. J., Reardon C. A., Getz G. S., LaDu M. J. (1998) J. Neurosci. 18, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahley R. W., Weisgraber K. H., Huang Y. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5644–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belinson H., Dolev I., Michaelson D. M. (2007) Neurobiol. Aging 28, 689–692; discussion 704–706 [DOI] [PubMed] [Google Scholar]
- 38.Sheng H., Laskowitz D. T., Mackensen G. B., Kudo M., Pearlstein R. D., Warner D. S. (1999) Stroke 30, 1118–1124 [DOI] [PubMed] [Google Scholar]
- 39.Masliah E., Mallory M., Ge N., Alford M., Veinbergs I., Roses A. D. (1995) Exp. Neurol. 136, 107–122 [DOI] [PubMed] [Google Scholar]
- 40.Krzywkowski P., Ghribi O., Gagné J., Chabot C., Kar S., Rochford J., Massicotte G., Poirier J. (1999) Neuroscience 92, 1273–1286 [DOI] [PubMed] [Google Scholar]
- 41.Siegel G. J., Chauhan N. B. (2000) Brain Res. Brain Res. Rev. 33, 199–227 [DOI] [PubMed] [Google Scholar]
- 42.Murer M. G., Yan Q., Raisman-Vozari R. (2001) Prog. Neurobiol. 63, 71–124 [DOI] [PubMed] [Google Scholar]
- 43.Ruiz J., Kouiavskaia D., Migliorini M., Robinson S., Saenko E. L., Gorlatova N., Li D., Lawrence D., Hyman B. T., Weisgraber K. H., Strickland D. K. (2005) J. Lipid Res. 46, 1721–1731 [DOI] [PubMed] [Google Scholar]
- 44.Herz J. (2001) Neuron 29, 571–581 [DOI] [PubMed] [Google Scholar]
- 45.Pap M., Cooper G. M. (1998) J. Biol. Chem. 273, 19929–19932 [DOI] [PubMed] [Google Scholar]
- 46.Linseman D. A., Butts B. D., Precht T. A., Phelps R. A., Le S. S., Laessig T. A., Bouchard R. J., Florez-McClure M. L., Heidenreich K. A. (2004) J. Neurosci. 24, 9993–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jope R. S., Johnson G. V. (2004) Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 48.Mora A., Sabio G., González-Polo R. A., Cuenda A., Alessi D. R., Alonso J. C., Fuentes J. M., Soler G., Centeno F. (2001) J. Neurochem. 78, 199–206 [DOI] [PubMed] [Google Scholar]
- 49.Noble W., Planel E., Zehr C., Olm V., Meyerson J., Suleman F., Gaynor K., Wang L., LaFrancois J., Feinstein B., Burns M., Krishnamurthy P., Wen Y., Bhat R., Lewis J., Dickson D., Duff K. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6990–6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X., Sato S., Murayama O., Murayama M., Park J. M., Yamaguchi H., Takashima A. (2002) Neurosci. Lett. 321, 61–64 [DOI] [PubMed] [Google Scholar]
- 51.Su Y., Ryder J., Li B., Wu X., Fox N., Solenberg P., Brune K., Paul S., Zhou Y., Liu F., Ni B. (2004) Biochemistry 43, 6899–6908 [DOI] [PubMed] [Google Scholar]
- 52.Patapoutian A., Reichardt L. F. (2001) Curr. Opin. Neurobiol. 11, 272–280 [DOI] [PubMed] [Google Scholar]
- 53.Zachary I. (2005) Neurosignals 14, 207–221 [DOI] [PubMed] [Google Scholar]
- 54.Shou Y., Li L., Prabhakaran K., Borowitz J. L., Isom G. E. (2004) Biochem. J. 379, 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almeida S., Domingues A., Rodrigues L., Oliveira C. R., Rego A. C. (2004) Neurobiol. Dis. 17, 435–444 [DOI] [PubMed] [Google Scholar]
- 56.Szydlowska K., Zawadzka M., Kaminska B. (2006) J. Neurochem. 99, 965–975 [DOI] [PubMed] [Google Scholar]
- 57.Qiu Z., Strickland D. K., Hyman B. T., Rebeck G. W. (2002) J. Biol. Chem. 277, 14458–14466 [DOI] [PubMed] [Google Scholar]
- 58.Albanesi D., Mansilla M. C., de Mendoza D. (2004) J. Bacteriol. 186, 2655–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shioda N., Moriguchi S., Shirasaki Y., Fukunaga K. (2006) J. Neurochem. 98, 310–320 [DOI] [PubMed] [Google Scholar]
- 60.Poulter M. O., Payne K. B., Steiner J. P. (2004) Neuroscience 128, 1–6 [DOI] [PubMed] [Google Scholar]
- 61.Norris C. M., Kadish I., Blalock E. M., Chen K. C., Thibault V., Porter N. M., Landfield P. W., Kraner S. D. (2005) J. Neurosci. 25, 4649–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito J., Zhang L. Y., Asai M., Yokoyama S. (1999) J. Neurochem. 72, 2362–2369 [DOI] [PubMed] [Google Scholar]