Abstract
POSH (plenty of SH3) is a scaffold protein that has been shown to act as an E3 ubiquitin ligase. Here we report that POSH stimulates the ubiquitination of Kir1.1 (ROMK) and enhances the internalization of this potassium channel. Immunostaining reveals the expression of POSH in the renal cortical collecting duct. Immunoprecipitation of renal tissue lysate with ROMK antibody and glutathione S-transferase pulldown experiments demonstrated the association between ROMK and POSH. Moreover, immunoprecipitation of lysates of HEK293T cells transfected with ROMK1 or with constructs encoding the ROMK-N terminus or ROMK1-C-Terminus demonstrated that POSH binds to ROMK1 on its N terminus. To study the effect of POSH on ROMK1 channels, we measured potassium currents with electrophysiological methods in HEK293T cells and in oocytes transfected or injected with ROMK1 and POSH. POSH decreased potassium currents, and the inhibitory effect of POSH on ROMK channels was dose-dependent. Biotinylation assay further showed that POSH decreased surface expression of ROMK channels in HEK293T cells transfected with ROMK1 and POSH. The effect of POSH on ROMK1 channels was specific because POSH did not inhibit sodium current in oocytes injected with ENaC-α, β, and γ subunits. Moreover, POSH still decreased the potassium current in oocytes injected with a ROMK1 mutant (R1Δ373–378), in which a clathrin-dependent tyrosine-based internalization signal residing between amino acid residues 373 and 378 is deleted. However, the inhibitory effect of POSH on ROMK channels was absent in cells expressing with dominant negative dynamin and POSHΔRING, in which the RING domain was deleted. Expression of POSH also increased the ubiquitination of ROMK1, whereas expression of POSHΔRING diminished its ubiquitination in HEK293T cells. The notion that POSH may serve as an E3 ubiquitin ligase is also supported by in vitro ubiquitination assays in which adding POSH increased the ROMK ubiquitination. We conclude that POSH inhibits ROMK channels by enhancing dynamin-dependent and clathrin-independent endocytosis and by stimulating ubiquitination of ROMK channels.
ROMK channels (Kir1.1) are located in the apical membrane of the epithelial cells of the renal thick ascending limb (TAL)2 and the CCD, where they are responsible for potassium recycling across the apical membrane in the TAL and potassium secretion in the CCD (1, 2). The expression of ROMK channels in the plasma membrane in the CCD is regulated by a variety of factors including protein kinases and dietary potassium intake (3–9). For instance, with-no-lysine kinase 4 (WNK4) and Src family protein-tyrosine kinase (PTK) reduce the expression of ROMK channels in the plasma membrane by stimulating dynamin-dependent endocytosis (10, 11). Several studies have demonstrated that potassium restriction decreased, and high potassium intake increased, the ROMK channel expression in the apical membrane of CCD epithelial cells (12, 13). Although the mechanism by which dietary potassium intake regulates surface expression is not completely understood, one possible mechanism is through modulating the ubiquitination of ROMK channels. The role for ubiquitination in regulating channel surface expression and endocytosis is best demonstrated by the observation that NEDD-4, an E3 ligase that contains the HECT domain (homologous to E6-AP C-terminal), regulates the ubiquitination of epithelial sodium channels (ENaC) (14–16). It has been shown that Nedd4 binds to ENaC on a PY motif (XPPXY) and causes channel internalization (17). Nedd-4 has also been reported to be responsible for ubiquitination of channels other than ENaC (18–21). We have previously demonstrated that ROMK1 channels can be monoubiquitinated and ubiquitinated ROMK channels were subjected to endocytosis (22). However, because ROMK channels lack a PY motif, it is unlikely that Nedd4 regulates ROMK channels in this fashion. POSH is a RING (really interesting new gene)-containing scaffold protein and has been suggested to be an E3 ligase for Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate) and Herp (homocystein-induced ER protein), and it has been shown to play an obligate role in cellular production of the human immunodeficiency virus, type 1 virus (23–25). Thus, the aim of the present study is to test whether POSH may act as an E3 ubiquitin ligase for the ubiquitination of ROMK channels.
EXPERIMENTAL PROCEDURES
Tissue Preparation
Sprague-Dawley rats (6 weeks, either sex) were purchased from Taconic Farms (Germantown, NY). The rats were maintained on rat chow with free access to water. The animals (<90 g) were anesthetized through intraperitoneal injection of Nembutal (6 mg/100 g) and euthanized by cervical dislocation. After opening the abdomen, both kidneys were removed immediately. The animal use protocol was approved by Institutional Animal Care and Use Committee of New York Medical College. The renal cortex and the outer medulla were separated under a dissecting microscope and suspended in radioimmune precipitation assay buffer solution (1:8 ratio, w/v) containing 1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS. 10 μl of phenylmethylsulfonyl fluoride (10 mg/ml stock solution in isopropanol) and 10 μl of a mixture of protease inhibitors (Sigma) were added per ml of buffer at the time of lysis. The samples were homogenized on ice for 15 min with a mortar and pestle. The suspension was incubated at 4 °C for 1 h in the presence of DNase (5 μg/ml) followed by centrifugation at 1800 rpm for 10 min, and the resultant supernatant was collected. Protein concentrations were measured in duplicate using a Bio-Rad Dc protein assay kit.
Preparation of Xenopus Oocytes
Xenopus laevis females were obtained from NASCO (Fort Atkinson, WI). The method for obtaining oocytes has been described previously (26). Viable oocytes were selected for injection with different cRNA. The oocytes were incubated at 19 °C in a 66% Dulbecco's modified Eagle's medium/Ham's F-12 medium with freshly added 2.5 mm sodium pyruvate and 50 μg/ml gentamycin. Two electrode voltage clamp experiments were performed on days 1–2 after injection.
Two-electrode Whole Cell Voltage Clamping
A Warner oocyte clamp OC-725C was used to measure the whole cell potassium current. Voltage and current microelectrodes were filled with 1000 mm KCl and had a resistance of less than 2 mΩ. The current was recorded on a chart recorder (Gould TA240). To exclude leak currents, 2 mm Ba2+ was used to determine the Ba2+-sensitive K+ current.
Western Blot
Proteins in homogenates of renal tissue or in lysates of cultured cells were separated by electrophoresis on 8–10% SDS-polyacrylamide gels and transferred to immunoblot polyvinylidene difluoride membranes (Bio-Rad). The membrane was blocked with Odyssey blocking buffer and incubated with the primary antibody at 4 °C for 12 h. The membrane was washed four times for 5 min with PBS containing 0.1% Tween 20, followed by incubation with the secondary antibody for an additional 30 min. The membrane was then washed several times and scanned with the Odyssey infrared imaging system (LI-COR, Lincoln, NE) set to the wavelength 700–800 channel.
Confocal Microscopy
Surface fluorescence detected by confocal microscopy at the equatorial plane of oocytes expressing GFP-tagged ROMK correlates with channel activity and has been used by us to assess channel expression in the plasma membrane (27). Briefly, GFP fluorescence was excited at 488 nm with an argon laser beam and viewed with an inverted Olympus FV300 confocal system equipped with a 10× lens. We used Scion Image software (Scion Co., Frederick, MD) to determine the fluorescence intensity. All of the images were acquired and processed with identical parameters.
Immunocytochemistry
Kidneys were perfused with 50 ml of PBS containing heparin (40 unit/ml) followed by 200 ml of 4% paraformaldehyde and were fixed with 4% paraformaldehyde for 12 h. A Leica 1900 cryostat (Leica) was used to cut kidney slices, which were dried at 42 °C for 1 h. After washing with 1× PBS, the samples were permeablized with 0.4% Triton dissolved in 1× PBS buffer containing 1% bovine serum albumin and 0.1% lysine (pH 7.4) for 15 min. The kidney slices were blocked with 2% goat serum for 1 h at room temperature and then incubated with antibodies to POSH and AQP2 for 12 h at 4 °C. The slides were washed with PBS buffer, followed by incubation with a secondary antibody for 2 h at room temperature.
GST Pulldown Assay
The ROMK and POSH constructs were subcloned into pGEX4T-1 to produce the GST fusion proteins for GST pulldown assay. GST, GST-ROMK, and GST-POSH immobilized on glutathione-Sepharose beads were incubated with 10 μl of POSH or ROMK labeled with [35S]methionine in NETN buffer (20 mm Tris, pH 8.0, 100 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40), respectively. The beads were then washed five times with NETN buffer. Bound proteins were subjected to SDS-PAGE followed by autoradiography. POSH and ROMK labeled with [35S]methionine were prepared by in vitro translation that was performed using the TnT quick-coupled rabbit reticulocyte system (Promega) with plasmid DNA according to the manufacturer's instructions. In addition, no 35S-labeled ROMK1 was also used in some GST pulldown experiments.
Cell Culture and Transfection
HEK293T cells were cultured in modified Dulbecco's modified Eagle's medium with 10% fetal calf serum at 37 °C with 5% CO2. Before the day of transfection, the cells were plated into 35-mm cell culture dishes at 70% confluence. For each transfection experiment, 2 μg of plasmid DNA/dish was mixed with OPTI-MEM@1 culture medium (Invitrogen) containing 6 μl of TransIT-293 (Mirus, Madison, WI). 24 h post-transfection, the cells were lysed with cold radioimmune precipitation assay buffer (25 mm Tris-Cl, pH 7.4, 150 mm NaCl, 50 mm KCl, 1% SDS, 2 mm EDTA, 0.5% glycerol, 50 mm NaF with protein inhibitor and phosphotase I and II inhibitor mixture from Sigma) and rocked for 1 h at 4 °C, followed by centrifugation at 13,000 rpm for 30 min. The supernatants were stored at −80 °C until use. pRK5-Myc-POSH was the kind gift from Dr. Anrette Seg (Medical Research Council Laboratory for Molecular Cell Biology and Cell Biology Unit, University College, London, UK), Full-length POSH was subcloned into the mammalian expression vector pcDNA3 (Invitrogen), peGFPC1-R1 was made as previously described (11). All of the mutations were made by overlapping PCR, and the constructs were sequenced for confirmation. Primer sequences are available upon request.
In Vitro Ubiquitination Assay
To carry out in vitro ubiquitination assay, we used ROMK protein produced from the TnT quick coupled transcription/translation system (Promega) and His-tagged POSH. Briefly, a T7 promoter-driven vector with a ROMK insertion (1 μg) was mixed with 40 μl of TnT T7 quick master mix, including 1 μm methionine in a total volume of 50 μl and incubated at 37 °C for 90 min. The protein was quantitated by Western blot. His-tagged POSH protein was purified from HEK293T cells transfected with the pcDNA6Myc- His-POSH plasmid. The whole cell lysate was mixed with 50 μl of nickel-nitrilotriacetic acid magnetic agarose beads in the binding buffer (50 mm NaH2PO4, 300 mm NaCl, 10 mm imidazole, 0.05% Tween 20), rocked at room temperature for 1 h, and washed three times with the binding buffer. The His-tagged POSH was eluted with 50 mm NaH2PO4, 300 mm NaCl, 300 mm imidazole, 0.05% Tween 20 and stored at −80 °C. The in vitro ubiquitination assay was performed as follows: the 50-μl reaction mixture containing 40 mm Tris-HCl (pH 7.5), 5 mm MgCl, 2 mm ATP, 2 mm dithiothreitol, 200 μg of ubiquitin, 1 μg of UBE1, 5 μg of UbcH5a (these three reagents were from BostonBiochem, Cambridge, MA), 5 μl of quick coupled ROMK protein, 500 ng of purified His-POSH protein was incubated at 30 °C for 2 h. The reaction was terminated with 2× sample buffer and resolved by SDS-PAGE. Western blotting was performed with ubiquitin antibody and ROMK antibody. An in vivo ubiquitination assay was carried out in HEK293T cells. The cells were transfected with pEGFPC1-R1 alone or cotransfected with 1 or 2 μg of pcDNA3-POSH. One group of the cells were incubated with 20 μm MG132 for 2 h before being lysed with radioimmune precipitation assay buffer. 200 μg of total protein was used for immunoprecipitation followed by Western blot.
Electrophysiology Experiments
Within 24 h after transfection, the cells were treated with trypsin-containing medium (TrypleTM Ecpresscare) (Invitrogen) for 10 min to detach the cells. The cell suspension (0.2 ml in volume) was carefully removed to a 5 × 5-mm coverglass coated with polylysin followed by additional incubation for 30 min to allow the cells to adhere to the coverglass. The coverglass was transferred to a chamber (1 ml) mounted on the stage of a Nikon inverted microscope. For the perforated whole cell patch clamp experiments, the cells were incubated with a bath solution containing 138 mm KCl, 0.5 mm MgCl2, 1.5 mm CaCl2, and 10 mm HEPES (pH 7.4). The experiments were performed at room temperature. Fluorescence signal (an indication of positive transfection) was detected with an intensified video imaging system including SIT 68 camera (Long Island Industries, North Baltimore, NY). Borosilicate glass (1.7-mm OD) was used to make the patch clamp pipette, which was pulled with a Narishege electrode puller. The pipette had a resistance of 2–4 MΩ when filled with 138 mm KCl. The tip of the pipette was filled with pipette solution containing 138 mm KCl, 4 mm MgCl2, 1 mm CaCl2, 1 mm EGTA, and 5 mm HEPES (pH 7.4). Then the pipette was back-filled with amphotericin B (2 μg/0.1 ml) containing pipette solution. After forming a high resistance seal (>2 GΩ), the membrane capacitance was monitored until the whole cell patch configuration was formed. The cell membrane capacitance was measured and compensated. The potassium current was measured by an Axon 200A patch clamp amplifier. The current was low pass filtered at 1 KHz and digitized by an Axon interface (Digidata 1200), and data were stored in an IBM-compatible computer and were analyzed using the pClamp software system 7 (Axon).
Biotinylation Assay
HEK293T cells were transfected with pEGFPC1-ROMK and pcDNA3-POSH. 24 h after transfection the cells were washed twice with cold PBS and then incubated with 1 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Pierce) for 30 min at 4 °C. This reaction was then quenched by adding quench buffer, and the cells were washed at least two times with TBS buffer, lysed with cold radioimmune precipitation assay buffer, and subjected to pull down with NeutrAvidin Gel (Pierce).
Experimental Materials and Statistics
POSH, ubiquitin, nonimmune rabbit IgG, and AQP2 antibodies were purchased from Santa Cruz (Santa Cruz, CA), and ROMK antibody was obtained from Alomone (Jerusalem, Israel). FLAG and GFP antibodies were purchased from Sigma and Clontech (Mountain View, CA), respectively. The data are presented as the means ± S.E. We used the paired Student's t test or one-way analysis of variance to determine the statistical significance. If the p value is less than 0.05, the difference is considered to be significant.
RESULTS
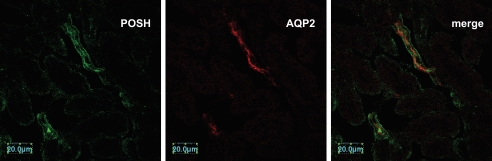
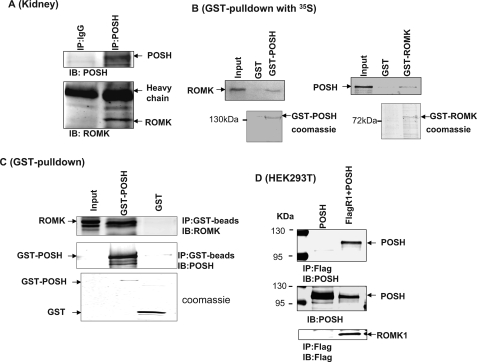
We first carried out a double immunofluorescence staining experiment to assess whether POSH is expressed in the renal CCD. We used AQP2 as a marker for the CCD. Fig. 1 is a confocal image demonstrating that POSH is expressed in AQP2-positive CCD tubule segments. Because POSH has been reported to be a scaffold protein (23, 25, 28), we examined whether POSH was able to interact with ROMK channels in the kidney. Tissue lysates harvested from renal cortex were immunoprecipitated with POSH antibody, and the immunoprecipitated proteins were resolved by electrophoresis followed by Western blotting with ROMK antibody. Fig. 2A is a Western blot showing that ROMK protein is immunoprecipitated with POSH. No ROMK coimmunoprecipitated with nonimmune IgG, which was used as a control. We have also performed GST pulldown experiments to examine the interaction between ROMK and POSH (Fig. 2B). GST-POSH fusion protein or GST-ROMK protein was bound to glutathione-Sepharose beads and incubated with either 10 μl of purified ROMK1 protein or POSH protein labeled with [35S]methionine in NETN buffer. After washing, the bound proteins were resolved by 10% SDS-PAGE followed by autoradiography. Fig. 2B shows that POSH was able to pull down ROMK1 protein (Fig. 2B, left panel) and that ROMK1 can also pull down POSH (right panel). Moreover, we have used Western blot to examine the interaction between POSH and ROMK1. Fig. 2C is a Western blot showing that GST-POSH pulled down ROMK. We then examined the interaction between POSH and ROMK1 in HEK293T cells transfected with FLAG-tagged ROMK1 and POSH or with POSH alone. Cell lysates were subjected to immunoprecipitation with FLAG antibody, and immunoprecipitated proteins were resolved by electrophoresis. Fig. 2D is a Western blot showing that POSH protein is immunoprecipitated with FLAG antibody in cells transfected with FLAG-tagged ROMK1 but not in cells transfected with POSH alone, although POSH was expressed under both conditions. Thus, these results indicate that POSH is associated with ROMK channels both in heterologous expression systems as well as in native renal tissue.
FIGURE 1.
Confocal images demonstrating the immunostaining of POSH and AQP2 in the cortex of the rat kidney.
FIGURE 2.
ROMK channels are associated with POSH. A, a Western blot demonstrating that ROMK channels are immunoprecipitated with POSH in the rat kidney. B, a GST pulldown experiment shows that GST-POSH fusion protein is able to pull down ROMK1 (left panel) and that GST-ROMK protein is also able to associate with POSH (right panel). GST-POSH or GST-ROMK1 was bound to glutathione-Sepharose beads and was incubated with either 10 μl of purified ROMK1 protein or POSH protein labeled with [35S]methionine in NETN buffer. After washing, the bound proteins were resolved by 10% SDS-PAGE followed by autoradiography. C, GST pulldown experiments showing the association between GST-POSH and ROMK1. D, a Western blot showing that POSH is specifically immunoprecipitated with FLAG-tagged ROMK1 in 293T cells transfected with FLAG-R1+POSH but not in cells transfected with POSH alone. Two bands on the left side of the gel are molecular size markers. IP, immunoprecipitation; IB, immunoblot.
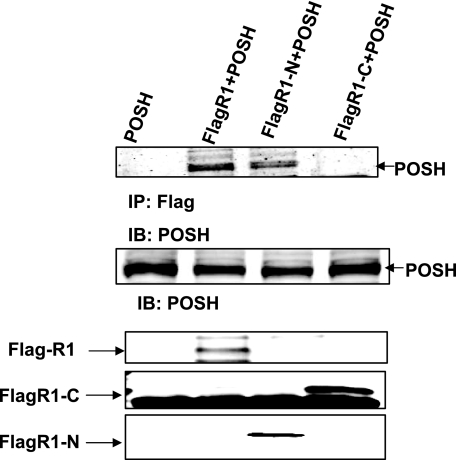
We then examined whether POSH binds to ROMK1 on its C or N terminus. Lysates of HEK293T cells transfected with POSH alone, FLAG-tagged ROMK1+POSH, FLAG-tagged ROMK1-N terminus (1–82)+POSH, and FLAG-tagged ROMK1-C terminus (181–392)+POSH were subjected to immunoprecipitation with a FLAG antibody, followed by Western blotting with the antibody directed against POSH. From inspection of Fig. 3, it is apparent that POSH is immunoprecipitated with FLAG antibody only in cells transfected with ROMK1 or ROMK1-N terminus but not in cells transfected with ROMK C terminus, suggesting that POSH associates with the N terminus of the ROMK1 protein.
FIGURE 3.
POSH associates with the N terminus of ROMK1. The top panel is a Western blot showing that POSH is immunoprecipitated with FLAG-tagged ROMK1 or FLAG-tagged ROMK1-N terminus (FLAGR1-N) but not with FLAG-tagged ROMK1 C terminus (FLAGR1-C). The middle panel shows the expression of POSH in 293T cells. The bottom three panels demonstrate the expression of FLAG-tagged ROMK1(R1), FLAGR1-C, and FLAGR1-N, respectively. IP, immunoprecipitation; IB, immunoblot.
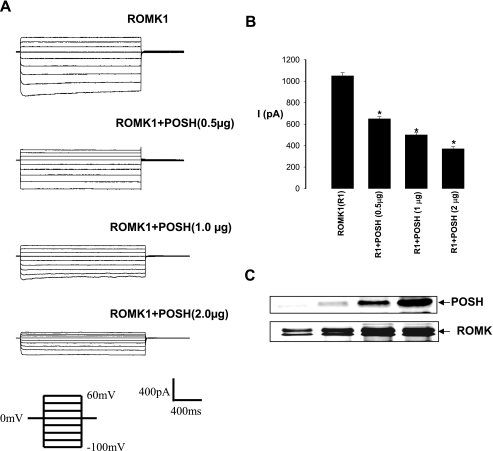
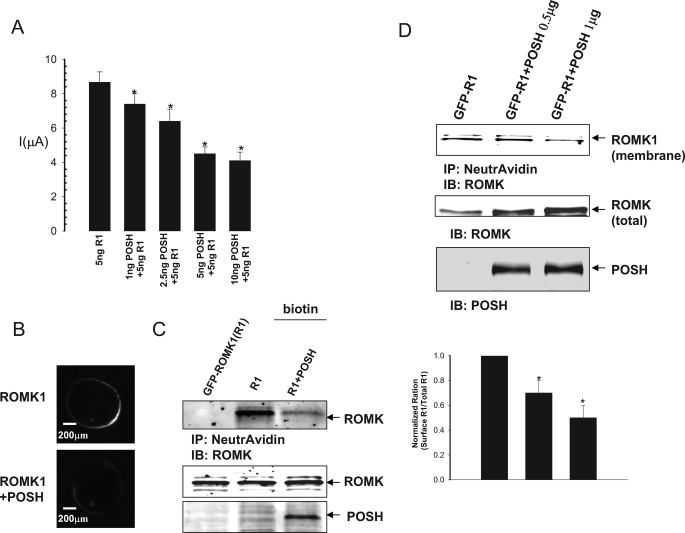
We next examined whether POSH regulates ROMK1 channel activity in both HEK293T cells transfected with GFP-ROMK1 and POSH at different concentration (Fig. 4) and Xenopus oocytes injected with different concentrations of POSH cRNA and 5 ng of ROMK1 (Fig. 5). Fig. 4A is a typical whole cell potassium current measured with the perforated whole cell patch recording in HEK293T cells transfected with GFPROMK1+POSH. It is apparent POSH decreased potassium current at a dose-dependent manner. Expression of POSH at 0.5, 1.0, and 2.0 μg decreased potassium currents at −100 mV cell membrane potential from the control value 1045 ± 20 to 600 ± 16, 500 ± 15, and 370 ± 15 pA (n = 4), respectively (Fig. 4B). The POSH-induced decrease in potassium currents was not due to changes in ROMK1 protein expression because the expression of POSH did not decrease the total ROMK1 protein level (Fig. 4C). We also examined the effect of POSH on ROMK channels in Xenopus oocytes injected with different concentrations of POSH cRNA and 5 ng of ROMK1 (Fig. 5). The results summarized in Fig. 5A demonstrate that POSH inhibited ROMK channel activity in a dose-dependent manner. Coinjection of 1, 2.5, 5, and 10 ng of POSH cRNA decreased potassium current in oocytes injected with 5 ng of ROMK1 cRNA by 12 ± 2, 25 ± 5, 45 ± 5, and 53 ± 7%, respectively. Injection of 10 ng of POSH also decreased the potassium current from 8.7 ± 0.6 to 4.1 ± 0.5 μA (n = 49). Confocal microscope experiments further showed that coinjection of 10 ng of POSH decreased the fluorescence intensity of ROMK localized to the membrane by 49 ± 6% (p < 0.05, n = 20) in oocytes injected with GFP-ROMK1 (Fig. 5B).
FIGURE 4.
POSH inhibits ROMK1 channels. A, effect of POSH on potassium currents measured with the perforated whole cell recording in HEK293 cells transfected with GFP-ROMK1 and POSH at different concentrations. B, a bar graph summarizes the results of the effect of POSH on ROMK1 channels (n = 4). C, a Western blot showing the expression of ROMK1 and POSH in HEK293 transfected with GFP-ROMK1 and POSH at different concentrations. The asterisk indicates significant different (p < 0.05) in comparing with the control (ROMK1 alone).
FIGURE 5.
Effect of POSH on ROMK1 channel activity and expression. A, effect of POSH on potassium current in oocytes injected with ROMK1 and POSH at different concentrations. The asterisks indicate a significant difference between control (without POSH) and POSH-injected oocytes. The experimental numbers are at least 11 for each condition. B, a confocal image showing the effect of POSH on the surface expression of GFP-ROMK1 in oocytes. C, POSH decreases the surface expression of ROMK1 channels in HEK293T cells transfected with POSH+GFP-ROMK1 or GFP-ROMK1 alone. Sulfo-NHS-SS biotin was used to label the surface ROMK1, and 50 μg of protein from cell lysates was used for the experiments. D, biotin labeling experiments showing the effect of increased POSH expression on the ROMK1 expression in plasma membranes of HEK293T cells. A bar graph summarizing the results from four experiments is shown on the bottom. IP, immunoprecipitation; IB, immunoblot.
The notion that POSH may decrease the surface expression of ROMK1 channels is also supported by experiments in which the biotin-labeling technique was used to examine the expression of ROMK1 in the plasma membrane of HEK293T cells transfected with GFP-ROMK1 and POSH. We labeled the surface ROMK1 with biotin 24 h after transfection and determined the total ROMK1 expression by Western blot. Fig. 5C is a Western blot from four experiments showing that the ROMK expression in plasma membrane decreased in cells transfected with 1 μg of POSH. Moreover, the effect of POSH on the surface expression is dose-dependent. Fig. 5D is a Western blot showing that expression of POSH at 0.5 and 1 μg decreased the surface ROMK expression by 25 ± 10 and 50 ± 10% (n = 4), respectively (Fig. 5D, bottom panel), calculated from the normalized ratio between the total ROMK1 and biotin-labeled ROMK1. Thus, results suggest that POSH inhibits ROMK1 channel activity by decreasing its surface expression.
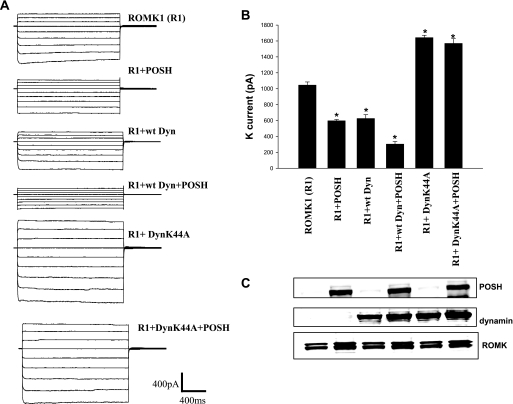
Decrease in surface expression of ROMK1 could be the result of either reducing delivery or increasing endocytosis. We next examined whether inhibiting endocytosis by expressing dominant negative dynamin (DynK44A) could abolish the effect of POSH on ROMK1 in HEK293T cells transfected with GFP-ROMK1(R1), R1+POSH, R1+wtDyn, R1+wtDyn+POSH, R1+DynK44A, and R1+DynK44A+POSH (Fig. 6). Fig. 6A is a whole cell potassium current measured with the perforated whole cell patch clamp recording showing that the inhibitory effect of POSH on ROMK1 channel was enhanced in 293T cells transfected with wt dynamin (control: 1090 ± 23, POSH: 612 ± 10, wt dynamin: 675 ± 28, wt dynamin+POSH:300 ± 18 pA) (n = 4; Fig. 6B). However, the inhibitory effect of POSH was completely abolished in 293T cells transfected with DynK44A (DynK44A, 1640 ± 17; DynK44A+POSH, 1570 ± 35 pA) (n = 4; Fig. 6B), although the ROMK protein levels among different groups were the same (Fig. 6C).
FIGURE 6.
POSH stimulates dynamin (Dyn)-dependent endocytosis of ROMK1. A, a whole cell potassium currents measured with the perforated whole cell patch recording in HEK293T cells transfected with GFP-ROMK1(R1), R1+POSH, R1+wtDyn, R1+wtDyn+POSH, R1+DynK44A, and R1+DynK44A+POSH (n = 4). The voltage protocol is the same as shown in Fig. 4 (from −100 to 60 mV). B, the potassium currents at −100 mV measured in different experiments are summarized in a bar graph. An asterisk indicates significant different (p < 0.05) in comparison with the control (ROMK1 alone). C, a Western blot shows the expression of corresponding proteins in all groups.
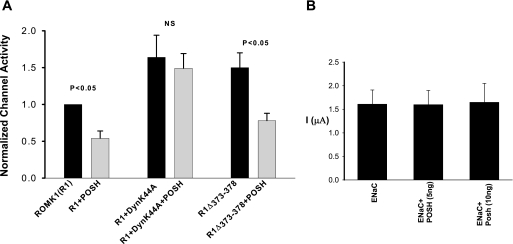
We have also examined the role of dynamin-dependent endocytosis in mediating the effect of POSH on ROMK1 channels in Xenopus oocytes injected with ROMK1, POSH, and DynK44A. The data summarized in Fig. 7A show that expression of DynK44A completely abolished the inhibitory effect of POSH on ROMK1 channels, suggesting that POSH-mediated inhibition of ROMK1 channels was at least in part the result of enhancing channel internalization. ROMK1 has a clathrin-dependent NPXY-like internalization domain in its C terminus (29) and WNK4 inhibits ROMK channels by stimulating clathrin-dependent endocytosis (10). Thus, we examined whether POSH also stimulates ROMK1 channel endocytosis by a clathrin-dependent mechanism. We examined the effect of POSH on potassium current in oocytes injected with a ROMK1 mutant, R1Δ373–378, in which the NPXY-like domain was deleted. From inspection of Fig. 7A, however, it is apparent that deleting the clathrin-binding motif failed to abolish the inhibitory effect of POSH on ROMK1 channels. POSH decreased ROMK channel activity by 47 ± 8% (n = 23) (Fig. 5A). This suggests that POSH inhibits ROMK1 channels by stimulating dynamin-dependent but clathrin-independent ROMK1 endocytosis. The effect of POSH on ROMK1 channels was specific, because POSH did not inhibit sodium current in oocytes injected with ENaC-α, β, and γ subunits (Fig. 7B).
FIGURE 7.
POSH stimulates the endocytosis of ROMK1. A, effect of POSH on potassium currents in oocytes injected with ROMK1 (R1)+POSH, R1+dominant negative dynamin (DynK44A) +POSH, and POSH+R1Δ373–378. B, effect of POSH on amiloride-sensitive sodium current in oocytes injected with ENaC (2 ng of α, 1 ng of β, and 1 ng of γ subunit).
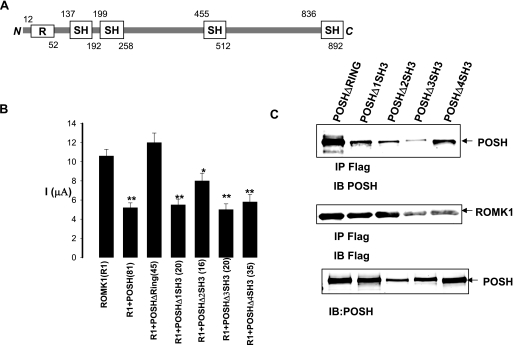
We then examined the structural requirements for the effects of POSH on ROMK1 channels in oocytes injected with ROMK1 and POSH or with truncated POSH, in which either the RING domain or each SH3 domain was deleted (Fig. 8A). We measured potassium current in the oocytes using a two-electrode voltage clamp. The data summarized in Fig. 6B demonstrate that deleting the RING domain completely abolished the inhibitory effect of POSH on ROMK1 channels (control, 10.2 ± 1 μA; POSHΔRING, 11.6 ± 1 μA). In contrast, the truncated POSH without its SH3 domains was still able to inhibit ROMK1 channels (Fig. 8B). Deleting the RING domain, however, did not affect the association between POSH and ROMK1. Fig. 8C is a Western blot showing that ROMK1 channels were still able to bind to all of the truncated POSH proteins. This suggests that the deletion of the RING domain abolished the effect of POSH by a mechanism other than through alteration of the association between ROMK1 and POSH.
FIGURE 8.
The RING domain is required for the effect of POSH. A, diagram shows a schematic structure of POSH. R, RING domain; SH, SH3 domain. The number above or below the corresponding domain indicates the deletion of amino acids in the constructs tested. B, effect of POSH and the truncated POSH on potassium current in oocytes injected with ROMK1 (R1), R1+POSH, and R1+truncated POSH. An asterisk indicates a significant difference in comparison with the control value (no POSH). **, p < 0.01; *, p < 0.05. The number in parentheses under each bar is the experimental number for each group. C, a Western blot demonstrating the association of ROMK1 and POSH or truncated POSH. IP, immunoprecipitation; IB, immunoblot.
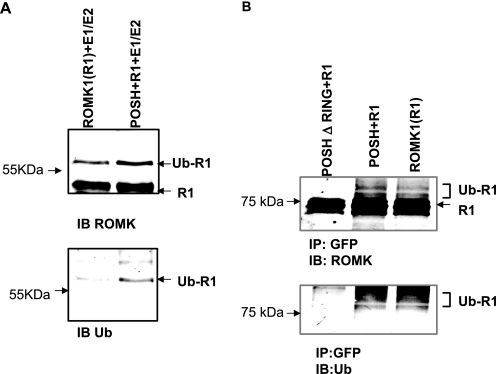
Proteins with RING domains have been shown to act as E3 ubiquitin ligases, and POSH has been reported to be an E3 ubiquitin ligase (24, 25). Thus, it is possible that POSH may be involved in mediating the ubiquitination of ROMK channels. This hypothesis was tested by examining whether POSH enhances ROMK1 ubiquitination using in vitro ubiquitin assay. ROMK proteins (generated with a TnT quick coupled transcription/translation system) were incubated with exogenous ubiquitin and E1 and E2 ubiquitin ligases (UbcH5a) in the presence or absence of His-tagged POSH. After the reaction, ROMK proteins were resolved by electrophoresis and Western blotted with either ROMK or ubiquitin antibodies. Fig. 9A is a Western blot showing that POSH increased the ubiquitination of ROMK1. We also examined the effect of POSH on ROMK1 ubiquitination in HEK293T cells transfected with GFP-ROMK1, GFP-ROMK1+POSH, and GFP-ROMK1+POSHΔ RING. Fig. 9B is a Western blot showing that the ubiquitinated ROMK1 channels were observed in both 293 cells transfected with ROMK1 or ROMK1+POSH but were diminished substantially in cells transfected with POSHΔRING. This suggests that the RING domain is essential for the POSH-mediated ubiquitination of ROMK1.
FIGURE 9.
POSH stimulates ubiquitination of ROMK. A, in vitro ubiquitination assay shows the effect of POSH on ROMK1 ubiquitination. ROMK proteins (generated with TnT quick coupled transcription/translation system) were incubated at 37 °C for 30 min with exogenous ubiquitin, ATP, E1 and E2 ubiquitin ligase (UbcH5a) in the presence or absence of His-tagged POSH. After the reaction ROMK proteins were resolved by electrophoresis and Western blotting with either ROMK or ubiquitin antibodies. B, a Western blot shows the effect of POSH or POSHΔRING on ROMK1 ubiquitination. 293T cells were transfected with ROMK1 (R1), R1+POSH, or R1+ POSHΔRING. MG132, an inhibitor of proteosome was added during experiments. IP, immunoprecipitation; IB, immunoblot.
DISCUSSION
ROMK channels are mainly expressed in the apical membrane of the renal TAL and CCD (30–32). ROMK channels are essential for potassium recycling across the apical membrane in the TAL. Because potassium recycling plays a key role in maintaining the function of sodium/potassium/chlorine cotransporter in the TAL, a defective gene product encoding ROMK channels causes type II Barter syndrome (33, 34). In the connecting tubule and CCD, ROMK channels are also responsible for potassium secretion under a normal potassium diet (35, 36). A large body of evidence has demonstrated that ROMK channel activity in the apical membrane of both the TAL and the CCD is regulated by dietary potassium intake; a high potassium intake stimulates, whereas a low potassium intake decreases, ROMK channel activity in the apical membrane (12, 37, 38). Moreover, a decrease in ROMK channel activity in response to a low potassium intake is at least partially the result of down-regulating the expression of ROMK channels in the plasma membrane (8, 13). In contrast, an increase in ROMK channel activity in response to a high potassium intake is also partially the result of augmenting ROMK expression in the plasma membrane (8, 38). Thus, regulating ROMK expression in plasma membranes through either insertion or internalization is an important way to control ROMK channel activity and renal potassium secretion.
Several protein kinases have been shown to play a role in regulating either internalization or insertion of ROMK channels: protein kinase A and serum-glucocorticoid-induced kinase (SGK) stimulate the insertion (3), whereas the Src family PTK and WNK4 increase ROMK endocytosis (10, 11). It has been reported that SGK1 phosphorylates the ROMK1 channel on serine residue 44 in the N terminus, thereby increasing the export of ROMK1 channel from the ER to plasma membrane (3). WNK4 decreases ROMK channel expression in the plasma membrane by stimulating a clathrin-dependent endocytosis (10). We have previously demonstrated that a low potassium intake stimulates the Src family PTK activity, which in turn increases the tyrosine phosphorylation of ROMK, thereby enhancing the endocytosis of ROMK channels (11, 12). Moreover, we have shown that the tetraspan protein CD63 is physically associated with ROMK channels and c-Src. The triple association is required for activation of c-Src (39).
In addition to protein kinases, we previously demonstrated that ROMK channel activity was also regulated by ubiquitination, because blocking ubiquitination of ROMK channels by deleting a lysine residue in the N terminus increased the surface expression of ROMK channels (22). Thus, regulation of ROMK ubiquitination could play an important role in modulating ROMK channel expression in the apical membrane of the TAL and CCD. The role of ubiquitination in regulating ion channel expression is best documented in experiments in which ubiquitination of ENaC regulates the surface expression of ENaC activity (14, 40). For ubiquitination of ENaC, Nedd-4, a HECT domain-containing E3 ligase, binds to a PY motif of ENaC and facilitates the transfer of ubiquitin to sodium channels (17). Consequently, ubiquitinated ENaC is internalized and degraded, thereby decreasing the surface expression of ENaC. If the ubiquitination of ENaC is defective, overexpression of ENaC causes excessive sodium absorption and hypertension (Liddle syndrome) (17, 41). In addition to ENaC, Nedd-4 E3 ligase has also been shown to facilitate the ubiquitination of KCNQ2/3 and KCNQ3/5 in the nervous system (20), voltage-gated sodium channels in neurons and myocytes (19), KCNQ1 in myocytes (18), and the chlorine channel, ClC-5, in proximal tubules (21).
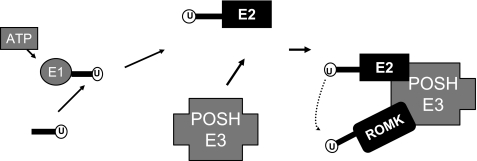
Although Nedd-4 is highly expressed in the CCD (42), it is unlikely that Nedd-4 is responsible for the ubiquitination of ROMK channels because they do not have a PY motif. Moreover, the observation that manipulation of Nedd-4 expression affected ENaC activity but had no effect on ROMK channel activity suggests that Nedd-4 was not the E3 ligase for ROMK channels (15). Several lines of evidence have strongly suggested that POSH could serve as an E3 ubiquitin ligase for ROMK channels: 1) POSH is expressed in the CCD and associated with ROMK channels; 2) expression of POSH decreased potassium current and surface expression of ROMK channels; and 3) adding exogenous POSH increased the ubiquitination of ROMK channels. Unlike HECT-containing E3 ligases such as Nedd-4, which transfers the activated ubiquitin to its target protein, POSH belongs to the RING domain-containing E3 ligase family, whose members act as scaffolds to bring the E2 ubiquitin ligase in close proximity to the target protein (43). The importance of the RING domain in mediating ROMK ubiquitination is also supported by the observation that deleting RING domain of POSH attenuated the ubiquitination of ROMK channels and abolished the inhibitory effect of POSH on ROMK channel activity. In contrast, deletion of each SH3 domain failed to abolish the effect of POSH on ROMK channel activity. Thus, POSH is an E3 ubiquitin protein ligase responsible for ubiquitinating ROMK channels. The notion that POSH is an E3 ligase has also been suggested by previous findings that POSH regulates ubiquitination of Herp and Hrs (24, 25). Fig. 10 is a scheme illustrating the role of POSH in mediating ubiquitination of ROMK channels. POSH is served as a scaffold protein associating with both ROMK1 channels and E2 ubiquitin ligase, thereby increasing the ubiquitination of ROMK channels.
FIGURE 10.
A scheme illustrating the proposed mechanism by which POSH induces the ubiquitination and hence the inhibition of ROMK channels.
In the present study we have also observed that expression of dominant negative dynamin abolished the inhibitory effect of POSH on ROMK channel activity. This suggests that POSH-induced inhibition of ROMK channels is the result of stimulating endocytosis. We speculate that POSH facilitates the ubiquitination of ROMK channels, thereby increasing endocytosis. Although we have demonstrated the role of POSH in mediating the ROMK ubiquitination, we did not explore whether POSH-mediated ubquitination of ROMK channels plays a role in response to a physiological stimulation such as high or low potassium intake. For instance, ENaC ubiquitination is controlled by aldosterone and SGK1, and increases in aldosterone levels or SGK1 activity inhibit the ubiquitination of ENaC by a mechanism involving blockade of the interaction of ENaC with Nedd-4 (44). Thus, we speculate that POSH-mediated ubiquitination of ROMK must also be regulated by factors such as hormones and dietary potassium intake. In this regard, it has been reported that tyrosine phosphorylation of Siah1 (homologues of the Drosophila seven-in-abscetia) enhances the association between POSH and Siah1 (45). ROMK channels are the substrate of Src family PTK, and increased tyrosine phosphorylation of ROMK has been shown to enhance the ROMK channel endocytosis (11). Hence, it is possible that Src family PTK may play an important role in regulating ROMK ubiquitination through modulating the association between ROMK and POSH. Further experiments are needed to explore the mechanism by which POSH-mediated ubiquitination of ROMK channels is regulated by factors such as PTK. We conclude that POSH is physically associated with ROMK on its N terminus and facilitates the ubiquitination of ROMK channels.
This work was supported, in whole or in part, by National Institutes of Health Grants DK54983, DK17433, and DK072614.
- TAL
- thick ascending limb
- E1
- ubiquitin-activating enzyme
- E2
- ubiquitin carrier protein
- E3
- ubiquitin-protein isopeptide ligase
- CCD
- cortical collecting duct
- GST
- glutathione S-transferase
- PTK
- protein-tyrosine kinase
- ENaC
- epithelial sodium channel
- PBS
- phosphate-buffered saline
- GFP
- green fluorescent protein
- wt
- wild type
- SGK
- serum-glucocorticoid-induced kinase.
REFERENCES
- 1.Hebert S. C., Desir G., Giebisch G., Wang W. (2005) Physiol. Rev. 85, 319–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. (1993) Nature 362, 31–38 [DOI] [PubMed] [Google Scholar]
- 3.Yoo D., Kim B. Y., Campo C., Nance L., King A., Maouyo D., Welling P. A. (2003) J. Biol. Chem. 278, 23066–23075 [DOI] [PubMed] [Google Scholar]
- 4.Ring A. M., Leng Q., Rinehart J., Wilson F. H., Kahle K. T., Hebert S. C., Lifton R. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4025–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He G., Wang H. R., Huang S. K., Huang C. L. (2007) J. Clin. Invest. 117, 1078–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazrak A., Liu Z., Huang C. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connell A. D., Leng Q., Dong K., MacGregor G. G., Giebisch G., Hebert S. C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9954–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin D. H., Sterling H., Yang B., Hebert S. C., Giebisch G., Wang W. H. (2004) Am. J. Physiol. Renal Physiol. 286, F881–F892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin D., Sterling H., Lerea K. M., Giebisch G., Wang W. H. (2002) J. Biol. Chem. 277, 44278–44284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahle K. T., Wilson F. H., Leng Q., Lalioti M. D., O'Connell A. D., Dong K., Rapson A. K., MacGregor G. G., Giebisch G., Hebert S. C., Lifton R. P. (2003) Nat. Genet. 35, 372–376 [DOI] [PubMed] [Google Scholar]
- 11.Sterling H., Lin D. H., Gu R. M., Dong K., Hebert S. C., Wang W. H. (2002) J. Biol. Chem. 277, 4317–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Y., Bloom P., Lin D., Gu R., Wang W. H. (2001) Am. J. Physiol. Renal Physiol. 281, F206–F212 [DOI] [PubMed] [Google Scholar]
- 13.Chu P. Y., Quigley R., Babich V., Huang C. L. (2003) Am. J. Physiol. Renal Physiol. 285, F1179–F1187 [DOI] [PubMed] [Google Scholar]
- 14.Staub O., Abriel H., Plant P., Ishikawa T., Kanelis V., Saleki R., Horisberger J. D., Schild L., Rotin D. (2000) Kidney Int. 57, 809–815 [DOI] [PubMed] [Google Scholar]
- 15.Konstas A. A., Shearwin-Whyatt L. M., Fotia A. B., Degger B., Riccardi D., Cook D. I., Korbmacher C., Kumar S. (2002) J. Biol. Chem. 277, 29406–29416 [DOI] [PubMed] [Google Scholar]
- 16.Staub O., Gautschi I., Ishikawa T., Breitschopf K., Ciechanover A., Schild L., Rotin D. (1997) EMBO J. 16, 6325–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staub O., Dho S., Henry P., Correa J., Ishikawa T., McGlade J., Rotin D. (1996) EMBO J. 15, 2371–2380 [PMC free article] [PubMed] [Google Scholar]
- 18.Jespersen T., Membrez M., Nicolas C. S., Pitard B., Staub O., Olesen S. P., Baró I., Abriel H. (2007) Cardiovasc. Res. 74, 64–74 [DOI] [PubMed] [Google Scholar]
- 19.Rougier J. S., van Bemmelen M. X., Bruce M. C., Jespersen T., Gavillet B., Apothéloz F., Cordonier S., Staub O., Rotin D., Abriel H. (2005) Am. J. Physiol. Cell Physiol. 288, C692–C701 [DOI] [PubMed] [Google Scholar]
- 20.Ekberg J., Schuetz F., Boase N. A., Conroy S. J., Manning J., Kumar S., Poronnik P., Adams D. J. (2007) J. Biol. Chem. 282, 12135–12142 [DOI] [PubMed] [Google Scholar]
- 21.Hryciw D. H., Ekberg J., Lee A., Lensink I. L., Kumar S., Guggino W. B., Cook D. I., Pollock C. A., Poronnik P. (2004) J. Biol. Chem. 279, 54996–55007 [DOI] [PubMed] [Google Scholar]
- 22.Lin D. H., Sterling H., Wang Z., Babilonia E., Yang B., Dong K., Hebert S. C., Giebisch G., Wang W. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4306–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alroy I., Tuvia S., Greener T., Gordon D., Barr H. M., Taglicht D., Mandil-Levin R., Ben-Avraham D., Konforty D., Nir A., Levius O., Bicoviski V., Dori M., Cohen S., Yaar L., Erez O., Propheta-Meiran O., Koskas M., Caspi-Bachar E., Alchanati I., Sela-Brown A., Moskowitz H., Tessmer U., Schubert U., Reiss Y. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim G. H., Park E., Kong Y. Y., Han J. K. (2006) Cell Signal. 18, 553–563 [DOI] [PubMed] [Google Scholar]
- 25.Tuvia S., Taglicht D., Erez O., Alroy I., Alchanati I., Bicoviski V., Dori-Bachash M., Ben-Avraham D., Reiss Y. (2007) J. Cell Biol. 177, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macica C. M., Yang Y. H., Hebert S. C., Wang W. H. (1996) Am. J. Physiol. Renal Physiol. 271, F588–F594 [DOI] [PubMed] [Google Scholar]
- 27.Moral Z., Dong K., Wei Y., Sterling H., Deng H., Ali S., Gu R., Huang X. Y., Hebert S. C., Giebisch G., Wang W. H. (2001) J. Biol. Chem. 276, 7156–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z., Kukekov N. V., Greene L. A. (2003) EMBO J. 22, 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng W. Z., Babich V., Ortega B., Quigley R., White S. J., Welling P. A., Huang C. L. (2002) Am. J. Physiol. Renal Physiol. 283, F630–F639 [DOI] [PubMed] [Google Scholar]
- 30.Boim M. A., Ho K., Shuck M. E., Bienkowski M. J., Block J. H., Slightom J. L., Yang Y., Brenner B. M., Hebert S. C. (1995) Am. J. Physiol. Renal Physiol. 268, F1132–F1140 [DOI] [PubMed] [Google Scholar]
- 31.Mennitt P. A., Wade J. B., Ecelbarger C. A., Palmer L. G., Frindt G. (1997) J. Am. Soc. Nephrol. 8, 1823–1830 [DOI] [PubMed] [Google Scholar]
- 32.Kohda Y., Ding W., Phan E., Housini I., Wang J., Star R. A., Huang C. L. (1998) Kidney Int. 54, 1214–1223 [DOI] [PubMed] [Google Scholar]
- 33.Simon D. B., Karet F. E., Rodriguez-Soriano J., Hamdan J. H., DiPietro A., Trachtman H., Sanjad S. A., Lifton R. P. (1996) Nat. Genet. 14, 152–156 [DOI] [PubMed] [Google Scholar]
- 34.Lu M., Wang T., Yan Q., Yang X., Dong K., Knepper M. A., Wang W., Giebisch G., Shull G. E., Hebert S. C. (2002) J. Biol. Chem. 277, 37881–37887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer L. G. (1999) Am. J. Physiol. Renal Physiol. 277, F821–F825 [DOI] [PubMed] [Google Scholar]
- 36.Wang W., Hebert S. C., Giebisch G. (1997) Annu. Rev. Physiol. 59, 413–436 [DOI] [PubMed] [Google Scholar]
- 37.Babilonia E., Wei Y., Sterling H., Kaminski P., Wolin M., Wang W. H. (2005) J. Biol. Chem. 280, 10790–10796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer L. G., Antonian L., Frindt G. (1994) J. Gen. Physiol. 104, 693–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin D., Kamsteeg E. J., Zhang Y., Jin Y., Sterling H., Yue P., Roos M., Duffield A., Spencer J., Caplan M., Wang W. H. (2008) J. Biol. Chem. 283, 7674–7681 [DOI] [PubMed] [Google Scholar]
- 40.Staub O., Rotin D. (2006) Physiol. Rev. 86, 669–707 [DOI] [PubMed] [Google Scholar]
- 41.Kellenberger S., Gautschi I., Rossier B. C., Schild L. (1998) J. Clin. Invest. 101, 2741–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staub O., Yeger H., Plant P. J., Kim H., Ernst S. A., Rotin D. (1997) Am. J. Physiol. Cell Physiol. 272, C1871–C1880 [DOI] [PubMed] [Google Scholar]
- 43.Pickart C. M. (2004) Cell 116, 181–190 [DOI] [PubMed] [Google Scholar]
- 44.Snyder P. M., Olson D. R., Thomas B. C. (2002) J. Biol. Chem. 277, 5–8 [DOI] [PubMed] [Google Scholar]
- 45.Xu Z., Sproul A., Wang W., Kukekov N., Greene L. A. (2006) J. Biol. Chem. 281, 303–312 [DOI] [PubMed] [Google Scholar]