Abstract
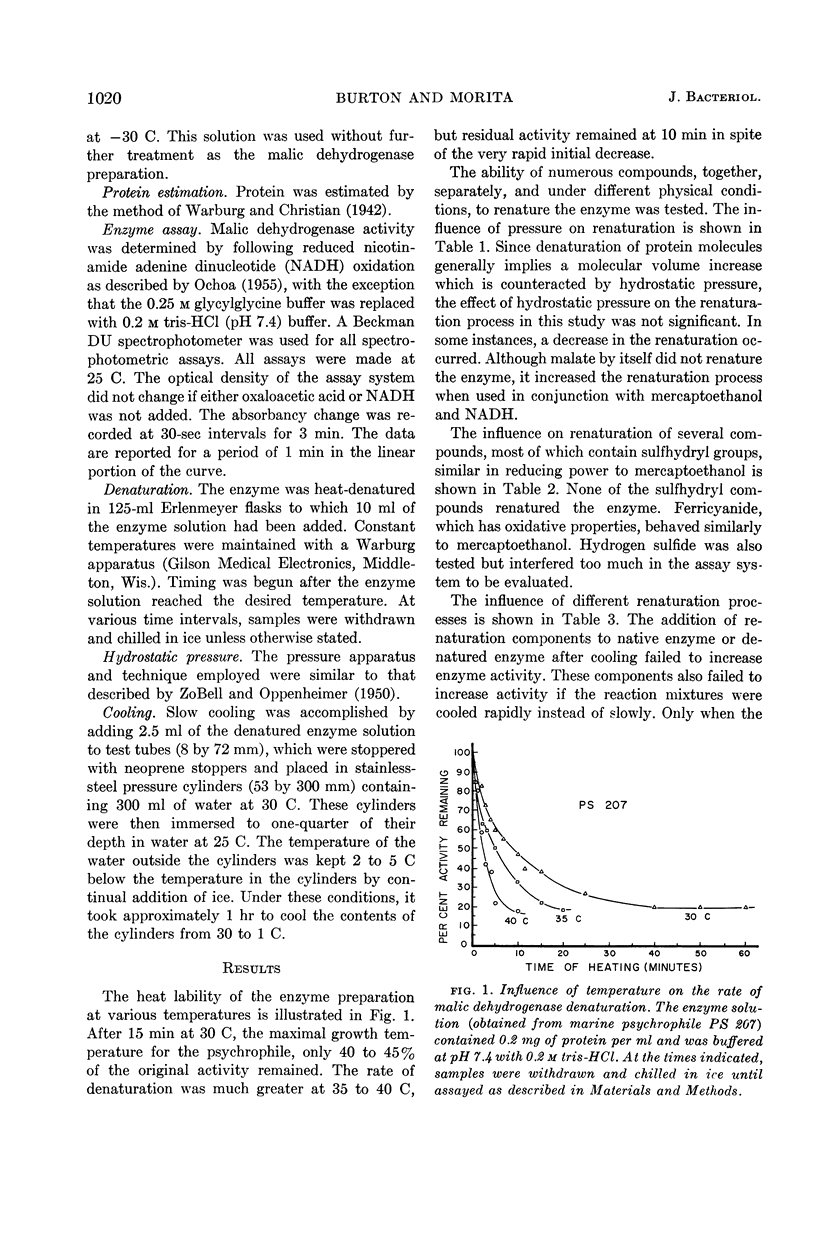
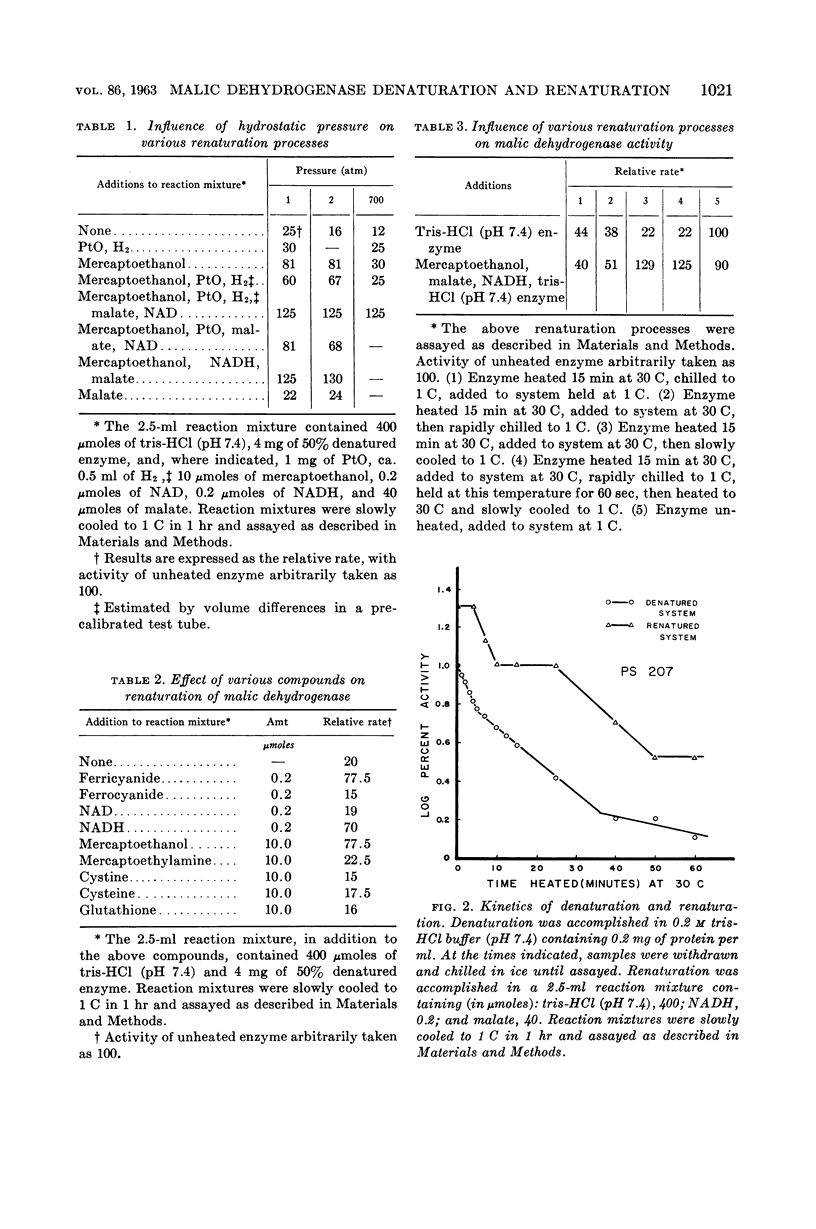
Burton, Sheril D. (Oregon State University, Corvallis), and Richard Y. Morita. Denaturation and renaturation of malic dehydrogenase in a cell-free extract from a marine psychrophile. J. Bacteriol. 86:1019–1024. 1963.—Malic dehydrogenase from a marine psychrophilic vibrio (PS 207) was found to be heat-sensitive at 30 C, the maximal growth temperature for the organism. Initial denaturation was reversible, with maximal renaturation occurring when the denatured enzyme was slowly cooled in the presence of mercaptoethanol, reduced nicotinamide adenine dinucleotide, and malate. No renaturation occurred when these compounds were added after slow cooling, or when the renaturation mixture was rapidly cooled. Mercaptoethylamine, cysteine, glutathione, or mercaptoacetic acid could not replace mercaptoethanol. The kinetics of denaturation and renaturation suggest the presence of several malic isozymes each with different heat labilities, or that these processes are occurring in several distinct steps.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards O. F., Rettger L. F. Relation of Certain Respiratory Enzymes to the Maximum Growth Temperatures of Bacteria. J Bacteriol. 1937 Nov;34(5):489–515. doi: 10.1128/jb.34.5.489-515.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGEN P. O., ROSE A. H. A psychrophilic Cryptoccus. Can J Microbiol. 1961 Jun;7:287–294. doi: 10.1139/m61-035. [DOI] [PubMed] [Google Scholar]
- HAGEN P. O., ROSE A. H. Studies on the biochemical basis of the low maximum temperature in a psychrophilic cryptococcus. J Gen Microbiol. 1962 Jan;27:89–99. doi: 10.1099/00221287-27-1-89. [DOI] [PubMed] [Google Scholar]
- INGRAHAM J. L., BAILEY G. F. Comparative study of effect of temperature on metabolism of psychrophilic and mesophilic bacteria. J Bacteriol. 1959 May;77(5):609–613. doi: 10.1128/jb.77.5.609-613.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOYCE B. K., GRISOLIA S. Variations in malic dehydrogenase activity, with lyophilization, dialysis, and conditions of incubation. J Biol Chem. 1961 Mar;236:725–729. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- MORITA R. Y., BURTON S. D. INFLUENCE OF MODERATE TEMPERATURE ON GROWTH AND MALIC DEHYDROGENASE ACTIVITY OF A MARINE PSYCHROPHILE. J Bacteriol. 1963 Nov;86:1025–1029. doi: 10.1128/jb.86.5.1025-1029.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. O., Villee C. A. Malic Dehyrogenases in Sea Urchin Eggs. Science. 1962 Oct 26;138(3539):508–509. doi: 10.1126/science.138.3539.508. [DOI] [PubMed] [Google Scholar]
- UPADHYAY J., STOKES R. L. Temperature-sensitive formic hydrogenlyase in a psychrophilic bacterium. J Bacteriol. 1963 Jan;85:177–185. doi: 10.1128/jb.85.1.177-185.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGGERT B., VILLEE C. A. Lactic and malic dehydrogenases in human fetal tissues. Science. 1962 Oct 26;138(3539):509–510. doi: 10.1126/science.138.3539.509. [DOI] [PubMed] [Google Scholar]
- ZOBELL C. E., OPPENHEIMER C. H. Some effects of hydrostatic pressure on the multiplication and morphology of marine bacteria. J Bacteriol. 1950 Dec;60(6):771–781. doi: 10.1128/jb.60.6.771-781.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]



