Abstract
Protease inhibitors (PI) act by blocking human immunodeficiency virus (HIV) polyprotein processing, but there is no direct quantitative correlation between the degree of impairment of Gag processing and virion infectivity at low PI concentrations. To analyze the consequences of partial processing, virus particles were produced in the presence of limiting PI concentrations or by co-transfection of wild-type proviral plasmids with constructs carrying mutations in one or more cleavage sites. Low PI concentrations caused subtle changes in polyprotein processing associated with a pronounced reduction of particle infectivity. Dissection of individual stages of viral entry indicated a block in accumulation of reverse transcriptase products, whereas virus entry, enzymatic reverse transcriptase activity, and replication steps following reverse transcription were not affected. Co-expression of low amounts of partially processed forms of Gag together with wild-type HIV generally exerted a trans-dominant effect, which was most prominent for a construct carrying mutations at both cleavage sites flanking the CA domain. Interestingly, co-expression of low amounts of Gag mutated at the CA-SP1 cleavage site also affected processing activity at this site in the wild-type virus. The results indicate that low amounts (<5%) of Gag processing intermediates can display a trans-dominant effect on HIV particle maturation, with the maturation cleavage between CA and SP1 being of particular importance. These effects are likely to be important for the strong activity of PI at concentrations achieved in vivo and also bear relevance for the mechanism of action of the antiviral drug bevirimat.
Proteolytic maturation of the virus particle represents the final step in the replication cycle of human immunodeficiency virus (HIV).4 HIV particle formation is mainly driven by the structural polyprotein Gag, which assembles into incomplete spherical structures released from the plasma membrane of an infected cell. Concomitant with the release of these immature particles, the virus-encoded protease (PR) cleaves the main structural polyprotein Gag into its functional subunits matrix (MA), capsid (CA), nucleocapsid (NC), p6, and the two spacer peptides SP1 and SP2 (Fig. 1A). These cleavages lead to a rearrangement of the immature HIV Gag shell into the cone-shaped mature capsid, which is crucial for virion infectivity. Accordingly, drugs that inhibit the viral PR (protease inhibitors, PI) have been found to efficiently block HIV replication in vitro and in vivo and represent a keystone of modern antiretroviral therapy (reviewed in Ref. 1).
FIGURE 1.
A, protease cleavage sites in the HIV Gag and Gag-Pol precursor proteins. Processing at these sites occurs with different rates; sizes of the arrows indicate relative rates of cleavages within Gag (13). B, Gag-derived products from the indicated PR cleavage site mutants used in the experiments shown in Figs. 7 and 8. Gray boxes indicate the respective incompletely processed products.
It is straightforward to assume that inhibition of virion infectivity by PI is a direct consequence of the lack of sufficient amounts of mature and functional Gag and Gag-Pol subunits. This is likely to be the case for mutational inactivation of PR, high dose PI treatment, or mutation of all cleavage sites, respectively, for which loss of morphological maturation as well as significant reductions in fusogenicity and reverse transcriptase (RT) activity have been observed (2–6). However, early experiments on the effect of PI on HIV replication in tissue culture demonstrated a severe reduction of infectivity at PI concentrations that showed almost undetectable or minor effects on processing of the Gag precursor (7, 8). Accordingly, although the activity of PR is essential for HIV replication and inhibition of PR results in reduced infectivity in a dose-dependent manner, there is no direct quantitative correlation between the degree of impairment of Gag polyprotein processing and reduction of infectivity at low PI concentrations. Only half of the CA molecules present in an HIV particle are required to form the mature cone-shaped capsid (9, 10), indicating that the inhibitory effect is not caused by an insufficient number of subunits for the structural core. However, the complex process of dissociation and re-association reactions involved in HIV core rearrangement within the confined space of a viral particle requires a tightly controlled, ordered sequence of proteolytic processing events mediated by different rates of cleavage at the different processing sites (11–16). Subtle disturbances may be sufficient to interrupt this delicately balanced process and drive it toward a nonproductive end (7, 16). This is also illustrated by the antiviral potency of 3-O-[3′,3′-dimethylsuccinyl]-betulinic acid (bevirimat), which selectively interferes with the final maturation cleavage between CA and the adjacent spacer peptide SP1 by binding to the PR cleavage site in Gag (17). Again, severe reduction in virus titer is not correlated with a complete block in cleavage at this position but rather with a change in processing kinetics (18).
Thus, it is conceivable that the small amounts of incompletely processed Gag molecules generated by treatment with low doses of PI are sufficient to interfere with the highly ordered process of HIV morphological maturation and thereby block infectivity in a trans-dominant manner. It had indeed been shown for murine leukemia virus (MLV) that partially cleaved Gag molecules can interfere with virus infectivity when present in similar quantities as the mature proteins (19). Here we have tested the hypothesis that the effect of low doses of PI can be explained by the generation of very low proportions of partially processed Gag molecules using quantitative determination of the effect of a series of specific partially processed Gag molecules on HIV infectivity. These studies revealed a strong trans-dominant effect for most of the partially processed Gag derivatives tested, which was most prominent for a protein that included both the MA and NC domain, and showed that the inhibitory effect acts at the stage of reverse transcription without influencing proteolytic maturation or activity of reverse transcriptase (RT).
EXPERIMENTAL PROCEDURES
Plasmids
All HIV plasmids were based either on the proviral construct pNLC4-3 (20), which encodes the complete HIV-1 NL4-3 genome (21) under the control of the cytomegalovirus promoter, or on the subviral plasmid pCHIV (22), which expresses all NL4-3 proteins except Nef but cannot replicate because of the lack of long terminal repeat regions. Derivatives carrying fluorescent proteins fused to the C terminus of the MA domain of the Gag open reading frame (pCHIVeCFP and pCHIVeYFP) have been characterized previously (22, 23). pNLC4-3 derivatives carrying mutations at specific cleavage sites within Gag were cloned by exchange of restriction fragments based on the previously published plasmids CA6 (12), MA-CA, and MA/p6 (5, 24) (kindly provided by C. Aiken), and NC-SP2 (25) (kindly provided by D. Ott). pMM310, a vector expressing a BlaM.Vpr fusion protein, was a kind gift of N. Landau.
Tissue Culture and Virus Preparation
293T, HeLa, HeLaP4, and TZM-bl cells (26) were cultured in Dulbecco's modified Eagle's medium; C8166 cells and MT-4 cells were grown in RPMI 1640 medium. Both media were supplemented with 10% fetal calf serum, penicillin, streptomycin, 4 mm glutamine, and 10 mm Hepes. MT-4 cells were infected with HIV-1 NL4-3 by co-culture. For virus preparation, 293T cells were transfected with the indicated plasmids by standard procedures using calcium phosphate precipitation or polyethyleneimine, respectively. Tissue culture supernatants were harvested at 40–44 hours post transfection, filtered through 0.45-μm filters, and stored at −80 °C. Supernatants were used directly for immunoblotting, measurement of RT activity, and determination of virus infectivity. Particles were prepared from filtered supernatants by ultracentrifugation through a 20% (w/w) sucrose cushion. Virus amounts were determined by quantitating CA-containing proteins through enzyme-linked immunosorbent assay or quantitative immunoblotting, using purified recombinant CA as a standard.
Immunoblotting
Samples were separated by SDS-PAGE and transferred onto nitrocellulose by semi-dry blotting. Detection of viral proteins or quantitation of band intensities was performed either by standard ECL detection or by quantitative immunoblotting on a LiCor Odyssey system using secondary antibodies, protocols, and software (Odyssey version 2.0) provided by the manufacturer. Sheep polyclonal antiserum against HIV-1 CA was raised against recombinant CA. Rabbit polyclonal antisera were raised against purified recombinant proteins (MA, RT, and β-lactamase) or against synthetic peptides derived from the Gag sequence of NL4-3 (NC, p6). Goat anti-NC was a kind gift from L. Arthur and J. Lifson, NCI, Frederick, MD.
Functional Analysis of Virus Preparations
Single-round infectivity assays were performed in a 96-well plate format. 5 × 103 TZM-bl cells per well were plated, and infections were carried out on the following day by adding filtered medium from 293T producer cells, transfected with the indicated plasmids. At 44–48 h post infection, cells were lysed, and HIV Tat-driven luciferase activity was measured using the Steady-Glo assay (Promega) according to the manufacturer's recommendations. For TCID50 determination, serial 10-fold dilutions of virus stocks were used to infect C8166 cells in quadruplicate (96-well format, 2.5 × 104 cells per well). At 8 days post infection, wells containing infected cells were identified by the presence of syncytia, and the TCID50 was calculated according to Spearman-Kärber.
Virus entry into HeLaP4 cells was measured by fluorometry using a standard β-lactamase HIV fusion assay as described previously (22). RT activity was determined in supernatants from infected MT-4 cells using the RetroSys RT activity kit (Innovagen) according to the manufacturer's instructions. Samples were analyzed at three different dilutions with duplicate measurements each, and RT activity was calculated as a mean from all values obtained for one sample within the linear range of the measurement. Quantitation of early replication steps was carried out as described previously (27, 28). Briefly, NL4-3 Env or vesicular stomatitis virus G protein pseudotyped stocks of a three plasmid-based HIV-based GFP-expressing vector or of HIV-1 Env(−) GFP carrying BlaM.Vpr were prepared from transfected 293T cells. Particles were treated with DNase (Turbo-DNase, Ambion, Germany; 5 IU per 10 μl of concentrated virus stock) for 1 h at 37 °C to reduce the degree of plasmid contamination before MT-4 target cells were challenged with the vector. Measurements of entry efficiency and transduction efficiency were performed by flow cytometry, and amounts of late reverse transcription products were determined by quantitative PCR as described previously (27).
Electron Microscopy
MT-4 cells were infected by co-culture in the presence of the indicated concentrations of LPV, harvested at 24 h post infection, pelleted, and fixed with 2.5% glutaraldehyde. Post-fixation, embedding, and preparation for EM was performed as described in Ref. 29. For unbiased quantitative analysis of the morphology of budding structures and virus particles, embedded cell pellets were selected randomly for cutting, and sections were placed on grids, and a random starting point was determined at low magnification in grid squares containing sections. Grid squares were then scanned systematically at higher magnification, and digital images (pixel size 1.1 nm on the specimen level) were taken in a systematic fashion. About 200 viral structures were imaged and classified per sample.
Fluorescence Measurements
Fluorescently labeled particles were prepared from the supernatant of 293T cells transfected with plasmids pCHIV, pCHIVeYFP, and pCHIVeCFP at a molar ratio of 2:1:1 by ultracentrifugation through a 20% (w/w) sucrose cushion. Particle concentration was determined by enzyme-linked immunosorbent assay detection of p24, and particles were diluted in phosphate-buffered saline to ∼10 ng of p24/μl. Fluorescence measurements were carried out at 25 °C using an SLM Aminco AB-2 spectrofluorometer at excitation and emission wavelengths of 433 and 528 nm, respectively. Particle dissociation was initiated at t = 0 by the addition of Triton X-100 to a final concentration of 0.05%. Fluorescence intensity was recorded with a time resolution of 1 s−1. Volume corrected values were normalized to the fluorescence intensity measured before detergent addition.
RESULTS
Influence of Low Amounts of PI on HIV-1 Infectivity and Particle Morphology
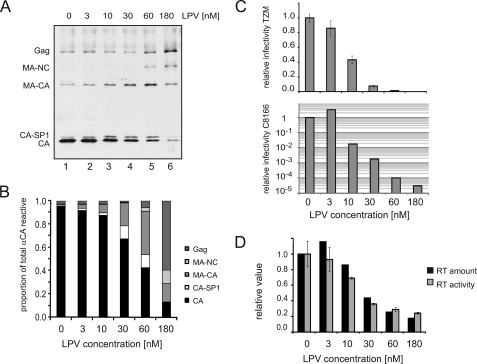
The initial experiments were aimed at obtaining a quantitative correlation between the relative degree of Gag processing at suboptimal concentrations of PI and the functional properties of the respective virions. Particles were purified from the tissue culture supernatant of MT-4 cells infected with HIV-1 NL4-3 grown at various suboptimal concentrations of the HIV PI lopinavir (LPV). Under these conditions, a final concentration of ∼2 μm LPV was required to accomplish complete inhibition of Gag processing. The degree of Gag processing in the particle preparations and the presence of the various partially processed intermediates was analyzed by immunoblotting, using polyclonal antisera raised against the different mature domains of HIV Gag and Pol (Fig. 2A and supplemental Fig. S1). Relative amounts of all Gag processing intermediates were determined by quantitative immunoblotting as shown in Fig. 2B. A pronounced effect on processing was observed at concentrations of 60 nm LPV and above, where <50% of completely cleaved CA was observed with a gradual shift to higher molecular mass intermediates. At lower LPV concentrations, however, only a slight increase of CA-SP1 and MA-CA was observed with >60% of fully processed CA at 30 nm and very little change at 10 nm LPV compared with virus produced in the absence of PI. Determination of relative virion infectivities from the same virus preparations (Fig. 2C), on the other hand, revealed a significant decrease in infectivity (>50% in single round infectivity and ∼60-fold when determined by end point titration on C8166 cells) at a concentration of 10 nm LPV.
FIGURE 2.
Effect of low concentrations of HIV PI on virus properties. HIV particles were prepared from NL4-3-infected MT-4 cells incubated at the indicated concentrations of LPV. A and B, influence on Gag processing. Particle lysates were separated by SDS-PAGE (15%; acrylamide/bisacrylamide, 200:1), and Gag-derived proteins were detected (A) and quantified (B) by quantitative immunoblot using polyclonal antiserum against CA. C, infectivity of virus preparations. Virus samples from A were tested for single round infectivity on TZM-bl indicator cells as well as for infectivity on C8166 cells by TCID50 titration. The graphs show relative infectivities normalized to the DMSO control (lane 1 in A) Error bars represent the standard deviation from three different dilutions. Note that relative titers on C8166 cells are plotted on a logarithmic scale although all other sales are linear. D, virus-associated reverse transcriptase. The amount of mature RT in particles (p66 and p51; black bars) in virus preparations from A was determined by quantitative immunoblotting. Particle-associated RT activity (gray bars) was measured in vitro using exogenous substrate. Error bars represent the standard deviation from three different dilutions.
Because inhibition of PR also affects processing of the Gag-Pol precursor and thereby the production of mature, functional RT, RT processing and functionality in the same virion preparations was tested in parallel. RT processing was analyzed by quantitative immunoblotting (Fig. 2D, black bars and supplemental Fig. S1), whereas particle-associated RT activity was determined by a standard in vitro RT assay using exogenously provided primer-template molecules (Fig. 2D, gray bars). Both analyses yielded consistent results. As expected, RT activity decreased at the higher concentrations of LPV, in correlation with the decrease of processed RT p66/p51 products. However, concentrations of up to 30 nm LPV, which strongly affected virus titer, resulted only in a minor decrease of RT processing or activity.
To correlate the effects on polyprotein processing and infectivity with potential changes in viral morphology, thin section EM of virus-producing MT-4 T-cells grown in the presence of different amounts of inhibitor was performed (Fig. 3). Virus-like structures at or near the plasma membrane of these cells were counted and morphologically classified into budding structures, immature spherical particles, particles with a mature conical core, as well as particles displaying aberrant morphologies (maturation defects, e.g. a diffuse or empty core, or an exocentric accumulation of electron dense material; examples can be found in Ref. 30). In agreement with earlier reports (7, 31), we observed that incomplete Gag processing significantly affected particle morphology. Quantitation of the different morphological classes revealed a gradual increase in budding structures at increasing PI concentrations (Fig. 3B), suggesting a delay in budding kinetics. Among the free particles, a gradual increase in aberrantly shaped free particles was observed at lower PI concentrations, although mostly immature particles were detected at concentrations of 100 nm LPV and above. Particles produced in the presence of 10 nm LPV, which showed only minor differences in Gag processing, already exhibited detectable differences in particle morphology compared with the untreated control (Fig. 3C). However, roughly 50% of free particles appeared morphologically mature at PI concentrations of 10 and 30 nm.
FIGURE 3.
Influence of low concentrations of LPV on particle morphology. HIV-1-infected MT-4 cells were grown in the presence of the indicated concentrations of LPV. Gag processing in particles released into the supernatant was determined by immunoblotting (data not shown). A, infected cells were pelleted, embedded in epoxy resin, and analyzed by thin section EM. Scale bars represent 200 nm. B, proportion of budding structures relative to total viral structures classified. Note that EM analysis is likely to underestimate the number of budding structures, because they may appear as free immature particles depending on their location with respect to the plane of sectioning. C, free virions were classified into immature particles (black bars), mature particles with conical capsid (light gray bars), and particles displaying morphological maturation defects (dark gray bars), and the percentage of particles in each class was calculated relative to the total amount of free particles analyzed. In total, 170–230 viral structures per condition were analyzed.
Effects of Partial Processing on Different Steps of HIV Replication
Complete inhibition of Gag processing by introducing a mutation into the active site of PR or by mutating all PR cleavage sites within Gag has been reported to reduce HIV entry efficiency by ∼10-fold. Furthermore, viruses containing various incompletely processed forms of Gag because of mutations of specific PR cleavage sites have been reported to display various degrees of fusion impairment (4–6). However, the effect of a partial inhibition of overall Gag processing on the efficiency of the fusion process had not been investigated. Thus, we determined the entry efficiency of viruses displaying various degrees of partial inhibition of processing using an established enzymatic assay for HIV-cell fusion using virus-like particles carrying a Vpr-β-lactamase (BlaM.Vpr) fusion protein (32). Susceptible cells were incubated with these particles and subsequently loaded with a fluorescent β-lactamase substrate. Upon virus-cell fusion, β-lactamase is delivered into the cytoplasm where it cleaves the substrate, leading to a shift in the fluorescence emission maximum. The ratio of blue to green fluorescence is taken as a measure of the extent of virus-cell fusion. Fig. 4, A and B, shows the results of a representative entry experiment carried out on HeLaP4 cells. BlaM.Vpr carrying particles raised in the presence of suboptimal concentrations of LPV did not display reduced fusion efficiency on HeLaP4 cells compared with the untreated control. Even particles generated in the presence of 100 nm or 2 μm LPV, where Gag processing was severely impaired as determined by immunoblot, fused with HeLaP4 cells with similar efficiency as particles produced in untreated cells (Fig. 4A). To determine whether the observed entry efficiency was influenced by delayed polyprotein processing (because of removal of PI upon incubation with target cells), we performed parallel experiments in the presence or absence of LPV during the entry phase (supplemental Fig. S2). Immunoblot analysis revealed that the incubation of particles at 37 °C in the absence of PI had only minor effects on Gag processing up to a concentration of 100 nm LPV. In the case of particles prepared in the presence of 2 μm LPV delayed processing of Gag and, possibly more importantly, of BlaM.Vpr was observed which could be blocked by adding 2 μm LPV during incubation periods of the entry assay (supplemental Fig. S2). Taken together, our results indicate, however, that severe inhibition of Gag polyprotein processing by PI does not interfere with HIV fusion capacity.
FIGURE 4.
Effect of suboptimal concentrations of LPV on early steps of HIV replication. A–C, effect on viral entry efficiency on HeLaP4 (A and B) and MT-4 (C) cells. Particles containing the BlaM-Vpr reporter protein, prepared in the presence of the indicated concentrations of LPV, were incubated in triplicate with HeLaP4 cells, and the amount of β-lactamase delivered to the cytoplasm was determined by fluorometry (A) as described previously (22). The plot shows mean values and standard deviation from the triplicate measurements. B, degree of Gag processing was analyzed by immunoblot against CA. C, entry efficiency in MT-4 cells. MT-4 target cells were infected with BlaM-Vpr loaded NL4-3 Env pseudotyped lentiviral vectors produced in the presence of the indicated concentrations of LPV for 4–6 h, washed, and stained overnight with CCF2/AM dye to determine viral entry efficiency. The proportion of cells in which entry had occurred was determined by flow cytometry. The figure shows mean values and standard deviation from triplicate samples from one representative experiment. D, correlation of virion infectivity and late RT products in MT-4 target cells. Late RT products were measured by quantitative PCR on day 1 post-challenge as described previously (27, 28), and the percentage of GFP-positive cells was measured by flow cytometry in the identical cultures on day 3 post-challenge. The graph shows levels of virion infectivity and late RT products of lentiviral vectors produced in the presence of different concentrations of LPV (3–100 nm) normalized to the untreated control from two independent experiments using vesicular stomatitis virus-G pseudotyped particles. Pearson's correlation coefficient R and the corresponding p value were calculated using GraphPad Prism Software.
To analyze the effect of partial processing on subsequent steps of HIV replication, we made use of a transduction system that allows the parallel determination of virus entry, reverse transcription, and protein expression on the same batch of transduced cells (27, 28). Viral vector particles were produced based on a three-plasmid vector system, which carried a BlaM.Vpr fusion protein for the detection of virus-cell fusion and packaged an RNA encoding enhanced GFP, thereby allowing the detection of transduced cells. MT-4 cells were incubated with these indicator particles, pseudotyped either with the vesicular stomatitis virus-G protein or with HIV-1 Env. At 6 h post-challenge, a sample of the cells was harvested and analyzed for viral entry by the β-lactamase fusion assay, determining the proportion of β-lactamase-positive cells by fluorescence-activated cell sorter analysis. Consistent with the results obtained on HeLaP4 cells, no significant effect of partial processing on viral entry efficiency was detected (Fig. 4C). At 24 h post-challenge, DNA was extracted from a second cell sample, and late reverse transcription products were quantitated by real time PCR. Furthermore, the amount of transduced GFP-expressing cells was quantitated at 72 h post-challenge by flow cytometric analysis for a 3rd aliquot taken from the same cultures. Even the lowest concentrations of LPV used resulted in significantly decreased production of RT products, as well as in a decrease in vector-mediated gene expression. The relative degree of inhibition of late reverse transcript formation showed a strong correlation (R = 0.97) with the relative proportion of GFP-expressing cells (Fig. 4D), indicating that the inhibition of viral infectivity occurred between virus-cell fusion and late reverse transcription, although earlier or later replication steps between attachment and viral gene expression did not appear to be affected.
Influence of Partial Processing on Gag Shell Stability
One possible mechanism that could explain the effect of partial Gag processing on early post-entry steps would be the stabilization of the Gag shell by incompletely processed precursors. Disturbances or kinetic changes in the poorly understood process of viral uncoating might interfere with reverse transcription and formation of a functional pre-integration complex. Although mature lentiviral capsids are inherently unstable in the absence of the viral envelope and their preparation requires very subtle treatment (33, 34), the immature Gag shell is considerably more stable, and immature particles lacking the lipid envelope can easily be prepared (12, 35–36). To determine the effect of incomplete processing on the physical stability of the Gag shell, we made use of a fluorescence resonance energy transfer (FRET)-based assay measuring particle dissociation. We have previously generated and characterized fluorescently labeled HIV derivatives carrying either enhanced GFP or other fluorescent proteins as an additional domain between the MA and CA moieties of Gag, and we observed that particles carrying a fluorescent label at 50% of the Gag molecules displayed wild-type properties (23). For FRET analyses, we prepared mixed particles from the supernatant of cells co-transfected with HIV derivatives expressing wild-type Gag, eCFP-labeled Gag, and eYFP-labeled Gag at a molar ratio of 2:1:1. These particles displayed a strong FRET signal because of the high density of Gag molecules. Treatment of mature particles with 0.05% Triton X-100 led to a rapid loss of the FRET signal, indicating MA shell dissociation (Fig. 5B and data not shown). In contrast, the FRET signal associated with immature particles was completely resistant to detergent treatment. This is in agreement with the significantly lower stability of myristoylated MA oligomers as compared with Gag derivatives containing additional domains C-terminal of MA (37). To investigate the effect of partial processing on particle shell dissociation, we generated eCFP/eYFP-labeled particles in the presence of various amounts of LPV and confirmed the degree of Gag processing by immunoblotting. Fluorescence measurements yielded comparable FRET intensities for the different preparations (ratio of emission 528:475 nm of ∼0.85 as compared with a value of 0.4 for particles labeled with eCFP only). Particle dissociation was initiated by the addition of 0.05% Triton X-100, disrupting the lipid envelope. In the case of completely processed particles (Fig. 5B, 0 nm LPV), this led to a rapid decay of the FRET signal to a value comparable with that of purely eCFP-labeled particles measured in the absence of detergent, indicating complete dissociation of labeled MA molecules. Generation of particles in the presence of 2 μm LPV yielded almost complete inhibition of Gag processing. Correspondingly, only a minor decrease in FRET intensity was observed upon detergent addition, indicating that the labeled Gag molecules remained associated. An intermediate effect was observed for particles generated in the presence of 100 nm LPV. In this case, a detectable FRET signal remained at the end of the reaction, indicating that dissociation into MA.eCFP and MA.eYFP containing monomers was incomplete. The dissociation properties of particles that had been generated in the presence of 2.5, 10, or 30 nm LPV did not show any measurable difference to completely processed particles in this assay, however, indicating that lattice stability because of incomplete Gag processing is not likely to be the reason for reduced infectivity at these concentrations.
FIGURE 5.
Influence of partial processing on MA layer stability. A, assay principle. Particles were labeled with a mixture of Gag-embedded eCFP (donor D) and eYFP (acceptor A). Upon proteolytic maturation, the labels remain fused to MA. Detergent disruption of the viral envelope results in dissociation of MA molecules and thereby a loss of the FRET signal, although unprocessed Gag molecules remain associated. B, eCFP/eYFP-labeled particles were prepared from transfected 293T cells grown in the presence of the indicated concentrations of LPV. Particles were incubated at 25 °C and excited at a wavelength of 433 nm. At t = 0, Triton X-100 was added to a final concentration of 0.05%, and emission intensity at 528 nm was recorded over time. Volume-corrected relative fluorescence intensities were normalized to the value recorded before detergent addition.
Influence of Low Concentrations of PI on NC Processing
The NC protein represents an integral part of the viral nucleoprotein complex, and it is thus conceivable that the inhibition of late reverse transcription by low concentrations of PI may at least in part be due to interference with the temporal control of NC cleavage. Furthermore, we had previously identified cleavage at the NC-SP2 processing site as a rate-limiting step in HIV PI resistance (38). Therefore, we analyzed the extent of cleavage at this site depending on LPV concentration by quantitative immunoblotting. As shown in Fig. 6A, processing at this site appeared to be particularly sensitive to inhibition by PI. A pronounced increase of unprocessed NC-SP2 was observed at a concentration of 12.5 nm LPV, which resulted only in minor changes in the processing pattern at upstream and downstream sites. Comparing NC-SP2 cleavage efficiency with particle infectivity at low LPV concentrations (Fig. 6, A and B) indicated that the relative amount of fully processed NC correlated with viral infectivity much better than the relative amount of fully processed CA.
FIGURE 6.
Interference of low concentrations of PI with cleavage at the NC-SP2 processing site. A, virus was prepared from the supernatant of HeLa cells grown in the presence of the indicated concentrations of LPV. Virus samples were separated by SDS-PAGE on 16.5% Tris-Tricine gels and analyzed by quantitative immunoblotting using the indicated antisera. The positions of NC-SP2 and NC as well as CA-SP1 and CA are marked. B, relative infectivities of the respective virus samples were determined by titration as described previously (47). Data represent mean values and standard deviations from triplicate measurements. The curve shows nonlinear regression to a standard dose-response equation.
Dominant Negative Effects of Small Proportions of Intermediate Processing Products
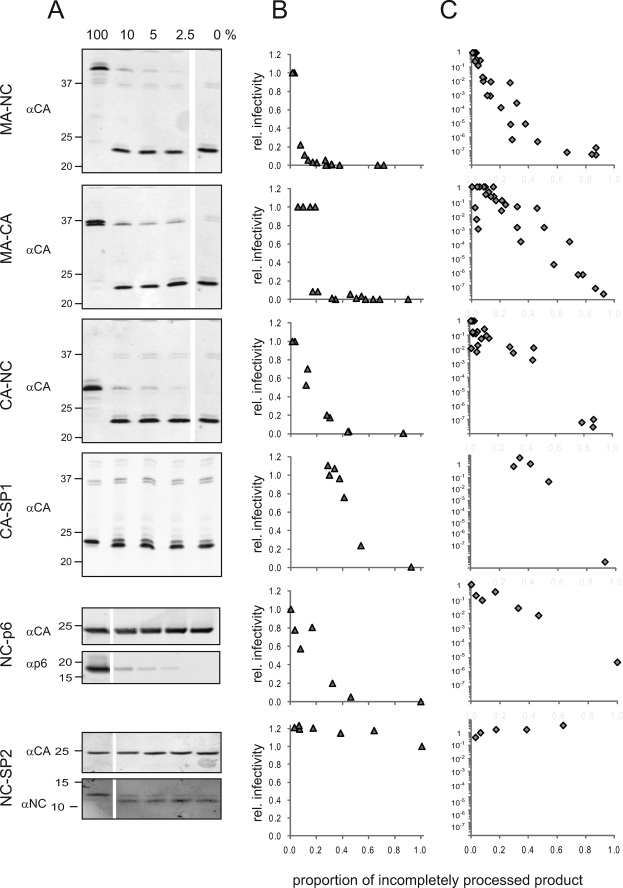
It is conceivable that the presence of small amounts of partially processed products during assembly or maturation may exhibit a trans-dominant negative effect on the formation of the mature, infectious particle. To investigate whether the incorporation of small amounts of specific partially processed intermediates affects HIV-1 infectivity, we carried out a systematic series of phenotypic mixing experiments. In these experiments, a wild-type proviral plasmid was co-transfected with varying amounts of isogenic variants carrying mutations at defined PR cleavage sites within Gag, as outlined in Fig. 1B. Mutated HIV derivatives selected for these analyses gave rise to the main processing intermediates observed upon partial inhibition of PR, namely MA-NC, MA-CA, CA-SP1, and NC-SP2, but also included other processing impaired constructs, leading to the generation of CA-NC and NC-p6. The mutated variants were added at concentrations between 2.5 and 50% of total DNA transfected, aiming in particular at the low proportions of partially cleaved products observed upon treatment with suboptimal concentrations of LPV (compare Fig. 2). The proportions of the respective partially processed products were determined by quantitative immunoblot. The respective Gag intermediates represented 1–3% of total Gag-derived products at the lowest concentration of the mutated variant and up to 95% when the mutated variant was transfected without wild-type plasmid.
Infectivity of phenotypically mixed particles was determined by end point titration on C8166 cells and by single round infection of TZM-bl indicator cells. It should be noted that the end point titration in this case also mainly records the first round of infection, at least when low amounts of mutant plasmid had been co-transfected for production of input virus. In these cases, phenotypically mixed particles will mostly carry wild-type genomes and thus give rise to the production of wild-type viruses in all successive rounds. As shown in Fig. 7, both infectivity measurements yielded consistent results. As reported previously, most mutated variants by themselves did display severely reduced or no detectable infectivity in these assays (12, 25). Only variant NC-SP2 showed no significant impairment of infectivity, in agreement with published results (25). When different amounts of mutated virus derivatives were co-transfected with wild-type HIV, all partially cleaved Gag proteins tested except for NC-SP2 displayed a dose-dependent, dominant negative effect on virus infectivity. However, the degree of inhibition varied between the variants. The strongest effect was obtained upon co-transfection of the MA-NC construct. Virion infectivity steeply declined at the lowest concentrations of the partially cleaved product analyzed; it can be estimated from the data that ∼5% of MA-NC relative to the total Gag-derived products are required for half-maximal inhibition of infectivity on TZM-bl cells (supplemental Fig. S3) or for an ∼20-fold drop in titer on C8166 cells, respectively. The relative effects of MA-CA, CA-NC, NC-p6, and CA-SP1 were similar to each other and somewhat less pronounced than that of MA-NC. Here, concentrations from 15 to 20% were required to obtain half-maximal inhibition on TZM-bl cells. Co-transfections with the fully infectious NC-SP2 variant at any molar ratio to wild type had no significant effect on infectivity in either readout system.
FIGURE 7.
Trans-dominant effect of very low amounts of specific intermediate cleavage products. 293T cells were co-transfected with a mixture of pNLC4-3 wild-type and the pNLC4-3 variants carrying mutations at specific PR cleavage sites (see scheme in Fig. 1B). At 44 hours post transfection, supernatants and cells were harvested for analysis. A, fraction of the respective incompletely processed forms relative to total Gag was determined by quantitative immunoblot using the indicated polyclonal antisera. The figure shows immunoblots of tissue culture supernatants from cells transfected with mixtures containing between 0 and 100% of the respective mutated plasmid, as indicated above the lanes. Positions of molecular mass standards are indicated at the left (in kDa). Infectivity of virus preparations was determined by single-round infection of TZM-bl cells (B) and TCID50 titration on C8166 cells (C), respectively. Infectivities per ng of CA were normalized to the value for the wild-type control and plotted against the fraction of total Gag represented by the indicated incompletely processed Gag derivative used for titration. Note that relative titers in C are plotted on a logarithmic scale, and infectivities on TZM-bl cells (B) are plotted on a linear scale.
To determine whether these effects could be correlated to morphological alterations, similar to those observed in the presence of suboptimal concentrations of LPV (Fig. 3), the morphology of the mixed particles was investigated by EM as described above, using a ratio of 1:10 of the mutated plasmid to pNLC4-3 resulting in a proportion of ∼5% (MA-NC and CA-NC) or ∼15% (MA-CA and NC-SP2) of the respective protein. This resulted in all cases in a modest decrease of the percentage of mature virions with a cone-shaped core and an increased proportion of particles carrying an eccentric core or displaying other morphological defects. However, in parallel infectivity analyses, there was no clear correlation between the relative infectivities of the particle preparations and the relative number of morphologically aberrant particles determined (data not shown).
Immunoblot analyses of particles from the phenotypic mixing experiments revealed an additional interesting effect of incompletely processed Gag molecules. Although the mutated virus derivatives MA-NC and CA-NC themselves did not contain detectable amounts of a CA-SP1 intermediate processing product, the ratio of mature CA to CA-SP1 in particles resulting from co-transfections of these variants with wild-type decreased significantly, directly proportional to the amount of MA-NC or CA-NC present (Fig. 8 and data not shown). We conclude that the unprocessed CA-SP1 must have been derived from the wild-type Gag molecules present in the co-transfected cells, suggesting that upon interaction of Gag derivatives carrying C-terminal extensions to CA with wild-type Gag the maturation- impaired molecules interfere with the processing of neighboring wild-type Gag molecules at this site. One may speculate that the CA-SP1 mutant itself has a similar effect, but this cannot be demonstrated unambiguously based on this type of co-expression experiment. The highly significant correlation of impairment of CA-SP1 processing with the presence of incompletely processed product was not observed upon co-expression of other incompletely cleaved Gag derivatives not spanning the CA-SP1 cleavage site, e.g. MA-CA (Fig. 8 and data not shown).
FIGURE 8.
Incompletely processed Gag molecules impair processing at the CA-SP1 cleavage site. Viral particles were purified from cells co-transfected with a mixture of pNLC4-3 and various relative amounts of pNLC4-3 variants mutated at the respective PR cleavage sites in Gag, which led to the production of MA-NC, CA-NC, or MA-CA, respectively. A, immunoblot detection of cleavage at the CA-SP1 processing site. Cells were co-transfected with a mixture of wild-type pNLC4-3 and between 0 and 100% of the indicated variants, as indicated above the lanes. Gag derivatives containing the CA domain were detected by quantitative immunoblot. B, proportion of CA cleaved at the CA-SP1 processing site was calculated from integrated band intensities from this and analogous experiments and plotted against the integrated band intensities determined for the respective incompletely processed product. Pearson's correlation coefficients R and p values were calculated using GraphPad Prism software.
DISCUSSION
HIV PI are highly successful antiviral drugs with low nanomolar IC50 values in tissue culture, yet little inhibition of Gag polyprotein processing is observed at low PI concentrations. This study aimed at resolving this discrepancy by systematically analyzing the effects of low concentrations of PI on particle morphology and various steps of virus entry and replication. We observed that low dose PI have no effect on the efficiency of HIV-1 entry and, consistent with a recent report (31), largely affect the process of reverse transcription but not the enzymatic activity of RT itself. Post-reverse transcription stages of replication contributed little if anything to the observed PI effect on virion infectivity. To shed light on the mechanism of inhibition, we generated phenotypically mixed particles consisting of wild-type Gag and different amounts of variant Gag polyproteins carrying mutations in one or several cleavage sites. All mutations except for that affecting the cleavage between NC and SP2 displayed a trans-dominant negative effect on HIV-1 infectivity. The trans-dominant effect was most pronounced for variants with mutations on both sides of the CA domain. These results suggest that minor amounts of incompletely processed intermediates may block formation of productive reverse transcription complexes thus explaining PI activity at concentrations where little effect on polyprotein processing is observed. Surprisingly, the effect of low PI concentrations on infectivity appeared to correlate best with reduced processing between NC and SP2, whereas mutation of this cleavage site or incorporation of mutated Gag molecules into phenotypically mixed particles had no effect on infectivity.
An inhibitory effect of low PI concentrations is likely to be also relevant for the mechanism of action of PI in anti-retroviral therapy. Mean effective serum concentrations for many PI are in the mid or high nanomolar range. Because PI in general show a high degree of protein binding (for LPV, up to 99% of the inhibitor are bound to serum protein), this corresponds to available protein-free inhibitor concentrations in the low nanomolar range (summarized in Ref. 39). Furthermore, drug levels in clinically relevant HIV reservoirs can differ from those reflected in the plasma concentration. This is likely to also have an impact on HIV PI resistance development. Its progression is directly dependent on the ability of the virus to replicate at low levels in body compartments where the concentration of inhibitor is low, whether due to poor penetration of the drug in the respective compartment or due to insufficient treatment compliance. Low level HIV replication in the presence of low PI concentration will lead to a gradual selection of viral variants with an increasing number of resistance-associated mutations. As these mutations accumulate, viral resistance will increase, enabling HIV to replicate in tissue compartments where drug levels are higher. Thus, the impact of low level PI concentration on the ability of HIV to replicate is of high clinical relevance in view of both the overall antiviral potency of these drugs in vivo and of the conditions that make selection of resistant viruses possible.
Low dose PI treatment led to inhibition of HIV infectivity in TZM-bl indicator cells at an IC50 of 9 nm and in C8166 T-cells at an IC50 of <5 nm. These concentrations caused only a minor change in the overall processing profile with >85% completely processed CA at 10 nm LPV. Host cell and assay-dependent differences in IC50 have been reported previously for PI, and the observed numbers in the low nanomolar range for LPV are consistent with other published reports (e.g. Refs. 31, 40). Increasing concentrations of PI exhibited a more dramatic effect on end point titers in C8166 cells than on single-round infectivity in TZM-bl cells. Although end point titration involves multiple rounds of replication, this cannot be caused by a cumulative effect of the PI because input virus is strongly diluted upon infection, and the final concentration of PI in the titration is below its inhibitory potency. Furthermore, a more dramatic inhibition of infectivity in C8166 cells (by several orders of magnitude) compared with TZM-bl reporter cells was also observed for viruses carrying small amounts of processing intermediates. Also in this case, the effect appears to be mostly due to the initial infection, because progeny virus will be largely wild type due to loss of the low proportion of mutated genomes. Thus, host cell-dependent differences need to be considered when comparing the effects of inhibitors or mutations, and TZM-bl reporter cells, although being very suitable for qualitative comparisons, may not always accurately quantify inhibitory effects.
Detectable changes in morphology were observed by EM analysis of HIV particles produced in the presence of low dose PI, but the proportion of particles with an apparent maturation defect was too low to completely account for the observed loss in infectivity. Furthermore, no clear correlation between relative infectivity and proportion of morphologically aberrant structures was detected by thin section EM when phenotypically mixed particles carrying low amounts of a specific incompletely processed polyprotein were analyzed (data not shown). Thus, minor amounts of intermediate cleavage products do not appear to block overall maturation as indicated by the presence of the cone-shaped core in most particles. More subtle alterations in virion morphology, which are not easily resolved by thin section EM of fixed samples, may be functionally relevant, however.
Dissection of the early steps in HIV replication indicated that impairment of the reverse transcription process is the major reason for the observed reduction in particle infectivity, although steps up to cytoplasmic entry and subsequent to reverse transcription do not significantly contribute. This observation is in contrast to results reported for MLV by Rulli et al. (19), who had investigated the functionality of MLV-derived vector particles containing 1:2 to 2:1 mixtures of wild-type Gag with Gag impaired for processing at p12-CA. These authors also detected a significant inhibitory effect of partially processed Gag molecules on reverse transcription; however, the relative effect on this step was found to be less pronounced than that on reporter gene expression from the transduced vector, indicating an inhibitory effect of partial maturation on replication steps following completion of reverse transcription. The reduced accumulation of RT products in our study was not caused by reduced activity of the enzyme, and it appears likely that the correct uncoating of the viral core is a prerequisite for formation of a productive reverse transcription complex. This hypothesis is consistent with the effect of various mutations in the CA domains of HIV and MLV on accumulation of reverse transcription products (19, 41–43), as well as with the observation that virus prepared in the presence of bevirimat is impaired in reverse transcription (18). The observation that NC-SP2 cleavage was most affected at low PI concentrations would suggest that fully processed NC is needed for establishing a functional RT complex, and failure to release NC from SP2 leads to impaired viral replication. This hypothesis is not supported by the finding that mutation of the NC-SP2 cleavage had no effect on viral infectivity (see Ref. 25 and this study), however. The apparent discrepancy could be due to the effects of low PI concentration on NC-SP2 cleavage correlating with reduced infectivity without any causal relevance. Alternatively, it may indicate that NC-SP2 processing becomes more relevant when PR activity is limiting, although not being essential at wild-type PR activity. This hypothesis is consistent with the effects of resistance-associated mutations in the NC-SP2 cleavage site of viral fitness and NC-SP2 processing (38).
The observation that incomplete processing of the Gag shell did not impair the efficiency of virus-cell fusion even at high PI concentrations was unexpected, because studies from several laboratories had revealed a clear dependence of Env-mediated fusion on Gag cleavage. Complete inhibition of polyprotein processing by mutation of the PR active site reduced HIV fusion efficiency determined in a fusion-from-without-assay by ∼10-fold (4). Similarly, impairment of Gag processing by mutation of all PR cleavage sites within Gag resulted in a roughly 10-fold reduction of particle fusogenicity measured by the β-lactamase fusion assay (5). Using atomic force microscopy, Kol et al. (44) found that immature particles displayed a 14-fold higher mechanical stiffness than mature particles, which again correlated with decreased fusion efficiency. In all cases the inhibitory effect was dependent on or mediated by the cytoplasm tic tail of Env. From such studies it has been proposed that the uncleaved Gag polyprotein imposes structural constraints on the mobility of the gp41 transmembrane glycoprotein on the particle surface, thereby interfering with structural rearrangements of Env required for fusion (4, 5). Here we show that LPV-mediated inhibition of processing was accompanied by clear effects on morphological maturation with almost exclusively immature particles at 2 μm LPV, but it did not result in any measurable decrease in fusion efficiency (Fig. 4). Importantly, in accordance with published data (5), we also observed a 10-fold drop in fusion efficiency when analyzing a variant mutated in all cleavage sites. It appears likely, therefore, that the presence of even a minor proportion of processed Gag molecules confers sufficient flexibility to allow for efficient particle fusion.
We were particularly intrigued by the finding that the presence of incompletely processed forms of Gag carrying extensions C-terminal of CA interfered with the processing of wild-type Gag molecules in a dose-dependent manner (Fig. 8). This affected specifically the cleavage between CA and the adjacent spacer peptide SP1, which represents a very late processing event in Gag maturation. Cleavage at this site is essential for formation of the mature conical core (12), and it is also this site whose cleavage is blocked by the action of the antiviral compound bevirimat (reviewed in Ref. 45). The observation that cleavage at CA-SP1 can be impaired by the presence of uncleavable Gag processing intermediates indicates that the finely tuned process of HIV proteolytic maturation may include an additional level of order; in addition to the well established facts that cleavage at the different PR processing sites occurs with different rates and that a defined temporal sequence of cleavage events at the different PR cleavage sites within Gag and Gag-Pol is important for successful completion of morphological maturation (11–16), this process may involve also a defined spatial order of PR cleavage of individual Gag molecules within the immature assembly. Based on the finding that the immature Gag shell is not a closed sphere, but rather represents an incompletely closed structure with an average degree of closure of ∼230°, we had recently speculated that PR may not cleave the individual Gag molecules in a random order but that proteolytic maturation of the Gag precursor may commence at the accessible rim of this open structure and proceed from there inward (46). This hypothesis would offer an attractive explanation for the observed interference of Gag molecules containing mutations at cleavage sites downstream of CA on the processing of the CA-SP1 cleavage site in wild-type molecules. If maturation of the Gag shell occurs in a defined spatial sequence determined by the architecture of the immature shell, the remaining incompletely processed Gag molecules interspersed throughout the particle structure might present a physical obstacle impairing access to PR cleavage sites on neighboring Gag molecules. Alternatively, Gag molecules comprising a noncleavable CA-SP1 region may act by stabilizing a regional Gag conformation refractive to PR processing at this site in the adjacent wild-type molecules, similar to what has been discussed as a potential mechanism of action of bevirimat (45). The observed trans-dominant negative effects of processing intermediates are likely to explain the potency of low dose PI and suggest that they act by interfering with accurate transformation of the incoming viral capsid into a productive RT complex.
Supplementary Material
Acknowledgments
We gratefully acknowledge the receipt of plasmids from N. Landau, Salk Institute, La Jolla, CA (pMM310); C. Aiken, Vanderbilt University, Nashville, TN (pNL4-3 MA-CA and MA-p6); and D. Ott, NCI, Frederick, MD (NC-SP2). We also thank L. Arthur and J. Lifson, NCI, Frederick, MD, for goat anti-NC antiserum and V. Bartonova for providing purified recombinant CA protein. Lopinavir was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health.
Note Added in Proof
While this manuscript was under review Lee et al. (Lee, S. K., Harris, J., and Swanstrom, R. (2009) J. Virol. 83, 8536–8543) reported a trans-dominant effect of one of the cleavage site mutants analyzed in this study (blocked for cleavage at the MA-CA processing site) on HIV infectivity. Consistent with our data on partial PI inhibition, these authors concluded that the negative effect of the MA-CA cleavage site mutant manifested itself before reverse transcription or at an early reverse transcription step.
This work was supported in part by European Union FP6 Grant LSHP-CT-2007-036793 (HIV PI resistance) and by Deutsche Forschungsgemeinschaft Grant MU885/4-2.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- HIV
- human immunodeficiency virus
- HIV-1
- human immunodeficiency virus, type 1
- PI
- protease inhibitor
- LPV
- lopinavir
- CA
- capsid
- MA
- matrix
- NC
- nucleocapsid
- PR
- protease
- RT
- reverse transcriptase
- FRET
- Fluorescence resonance energy transfer
- eCFP
- enhanced cyan fluorescent protein
- eYFP
- enhanced yellow fluorescent protein
- GFP
- green fluorescent protein
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- MLV
- murine leukemia virus.
REFERENCES
- 1.Anderson J., Schiffer C., Lee S. K., Swanstrom R. (2009) Handb Exp. Pharmacol. 189, 85–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr., Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J., et al. (1990) Nature 343, 90–92 [DOI] [PubMed] [Google Scholar]
- 3.Lambert D. M., Petteway S. R., Jr., McDanal C. E., Hart T. K., Leary J. J., Dreyer G. B., Meek T. D., Bugelski P. J., Bolognesi D. P., Metcalf B. W., et al. (1992) Antimicrob. Agents Chemother. 36, 982–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami T., Ablan S., Freed E. O., Tanaka Y. (2004) J. Virol. 78, 1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyma D. J., Jiang J., Shi J., Zhou J., Lineberger J. E., Miller M. D., Aiken C. (2004) J. Virol. 78, 3429–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J., Aiken C. (2006) Virology 346, 460–468 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan A. H., Zack J. A., Knigge M., Paul D. A., Kempf D. J., Norbeck D. W., Swanstrom R. (1993) J. Virol. 67, 4050–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kräusslich H. G. (1992) J. Virol. 66, 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briggs J. A., Simon M. N., Gross I., Kräusslich H. G., Fuller S. D., Vogt V. M., Johnson M. C. (2004) Nat. Struct. Mol. Biol. 11, 672–675 [DOI] [PubMed] [Google Scholar]
- 10.Lanman J., Lam T. T., Emmett M. R., Marshall A. G., Sakalian M., Prevelige P. E., Jr. (2004) Nat. Struct. Mol. Biol. 11, 676–677 [DOI] [PubMed] [Google Scholar]
- 11.Pettit S. C., Moody M. D., Wehbie R. S., Kaplan A. H., Nantermet P. V., Klein C. A., Swanstrom R. (1994) J. Virol. 68, 8017–8027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiegers K., Rutter G., Kottler H., Tessmer U., Hohenberg H., Kräusslich H. G. (1998) J. Virol. 72, 2846–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettit S. C., Henderson G. J., Schiffer C. A., Swanstrom R. (2002) J. Virol. 76, 10226–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettit S. C., Everitt L. E., Choudhury S., Dunn B. M., Kaplan A. H. (2004) J. Virol. 78, 8477–8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettit S. C., Lindquist J. N., Kaplan A. H., Swanstrom R. (2005) Retrovirology 2, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettit S. C., Clemente J. C., Jeung J. A., Dunn B. M., Kaplan A. H. (2005) J. Virol. 79, 10601–10607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F., Goila-Gaur R., Salzwedel K., Kilgore N. R., Reddick M., Matallana C., Castillo A., Zoumplis D., Martin D. E., Orenstein J. M., Allaway G. P., Freed E. O., Wild C. T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13555–13560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J., Yuan X., Dismuke D., Forshey B. M., Lundquist C., Lee K. H., Aiken C., Chen C. H. (2004) J. Virol. 78, 922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rulli S. J., Jr., Muriaux D., Nagashima K., Mirro J., Oshima M., Baumann J. G., Rein A. (2006) Virology 347, 364–371 [DOI] [PubMed] [Google Scholar]
- 20.Bohne J., Kräusslich H. G. (2004) FEBS Lett. 563, 113–118 [DOI] [PubMed] [Google Scholar]
- 21.Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. (1986) J. Virol. 59, 284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampe M., Briggs J. A., Endress T., Glass B., Riegelsberger S., Kräusslich H. G., Lamb D. C., Bräuchle C., Müller B. (2007) Virology 360, 92–104 [DOI] [PubMed] [Google Scholar]
- 23.Müller B., Daecke J., Fackler O. T., Dittmar M. T., Zentgraf H., Kräusslich H. G. (2004) J. Virol. 78, 10803–10813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyma D. J., Kotov A., Aiken C. (2000) J. Virol. 74, 9381–9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coren L. V., Thomas J. A., Chertova E., Sowder R. C., 2nd, Gagliardi T. D., Gorelick R. J., Ott D. E. (2007) J. Virol. 81, 10047–10054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X., Decker J. M., Liu H., Zhang Z., Arani R. B., Kilby J. M., Saag M. S., Wu X., Shaw G. M., Kappes J. C. (2002) Antimicrob. Agents Chemother. 46, 1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goffinet C., Michel N., Allespach I., Tervo H. M., Hermann V., Kräusslich H. G., Greene W. C., Keppler O. T. (2007) Retrovirology 4, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tervo H. M., Goffinet C., Keppler O. T. (2008) Retrovirology 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goffinet C., Allespach I., Homann S., Tervo H. M., Habermann A., Rupp D., Oberbremer L., Kern C., Tibroni N., Welsch S., Krijnse-Locker J., Banting G., Kräusslich H. G., Fackler O. T., Keppler O. T. (2009) Cell Host Microbe. 5, 285–297 [DOI] [PubMed] [Google Scholar]
- 30.Bartonova V., Igonet S., Sticht J., Glass B., Habermann A., Vaney M. C., Sehr P., Lewis J., Rey F. A., Kräusslich H. G. (2008) J. Biol. Chem. 283, 32024–32033 [DOI] [PubMed] [Google Scholar]
- 31.Moore M. D., Fu W., Soheilian F., Nagashima K., Ptak R. G., Pathak V. K., Hu W. S. (2008) Virology 379, 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavrois M., De Noronha C., Greene W. C. (2002) Nat. Biotechnol. 20, 1151–1154 [DOI] [PubMed] [Google Scholar]
- 33.Welker R., Hohenberg H., Tessmer U., Huckhagel C., Kräusslich H. G. (2000) J. Virol. 74, 1168–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Accola M. A., Ohagen A., Göttlinger H. G. (2000) J. Virol. 74, 6198–6202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J., Morrow C. D. (1993) Virology 194, 843–850 [DOI] [PubMed] [Google Scholar]
- 36.Rosé J. R., Babé L. M., Craik C. S. (1995) J. Virol. 69, 2751–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang C., Loeliger E., Luncsford P., Kinde I., Beckett D., Summers M. F. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dam E., Quercia R., Glass B., Descamps D., Launay O., Duval X., Kräusslich H. G., Hance A. J., Clavel F. (2009) PLoS pathogens 5, e1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Justesen U. S. (2008) Dan. Med. Bull. 55, 165–185 [PubMed] [Google Scholar]
- 40.Sham H. L., Kempf D. J., Molla A., Marsh K. C., Kumar G. N., Chen C. M., Kati W., Stewart K., Lal R., Hsu A., Betebenner D., Korneyeva M., Vasavanonda S., McDonald E., Saldivar A., Wideburg N., Chen X., Niu P., Park C., Jayanti V., Grabowski B., Granneman G. R., Sun E., Japour A. J., Leonard J. M., Plattner J. J., Norbeck D. W. (1998) Antimicrob. Agents Chemother. 42, 3218–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang S., Murakami T., Agresta B. E., Campbell S., Freed E. O., Levin J. G. (2001) J. Virol. 75, 9357–9366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forshey B. M., von Schwedler U., Sundquist W. I., Aiken C. (2002) J. Virol. 76, 5667–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang S., Murakami T., Cheng N., Steven A. C., Freed E. O., Levin J. G. (2003) J. Virol. 77, 12592–12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kol N., Shi Y., Tsvitov M., Barlam D., Shneck R. Z., Kay M. S., Rousso I. (2007) Biophys. J. 92, 1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salzwedel K., Martin D. E., Sakalian M. (2007) AIDS Rev. 9, 162–172 [PubMed] [Google Scholar]
- 46.Carlson L. A., Briggs J. A., Glass B., Riches J. D., Simon M. N., Johnson M. C., Müller B., Grünewald K., Kräusslich H. G. (2008) Cell Host Microbe. 4, 592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Race E., Dam E., Obry V., Paulous S., Clavel F. (1999) AIDS 13, 2061–2068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.