Abstract
Actin filament assembly in nonmuscle cells is regulated by the actin polymerization machinery, including the Arp2/3 complex and formins. However, little is known about the regulation of actin assembly in muscle cells, where straight actin filaments are organized into the contractile unit sarcomere. Here, we show that Fhod3, a myocardial formin that localizes to thin actin filaments in a striated pattern, regulates sarcomere organization in cardiomyocytes. RNA interference-mediated depletion of Fhod3 results in a marked reduction in filamentous actin and disruption of the sarcomeric structure. These defects are rescued by expression of wild-type Fhod3 but not by that of mutant proteins carrying amino acid substitution for conserved residues for actin assembly. These findings suggest that actin dynamics regulated by Fhod3 are critical for sarcomere organization in striated muscle cells.
In striated muscle, thin actin filaments and thick filaments of myosin are highly organized to form myofibrils (1) (Fig. 1A). During myofibrillogenesis, actin cytoskeleton undergoes dynamic remodeling to produce uniform lengths of straight filaments packaged in the sarcomere, a contractile unit of myofibrils (2–4). In nascent sarcomeres, a filamentous actin-containing structure, referred to as the Z-body or I-Z-I structure, emerges as a precursor of the Z-line that anchors actin filaments. Subsequent alignment of the precursors leads to formation of a striated pattern of the Z-line, and myosin filaments are incorporated between Z-lines. Finally, the M-line that serves as an anchoring site for myosin filaments becomes visible; the appearance is accompanied by alignment of the unanchored end of actin filaments (5). Thus, the mature distribution pattern of actin filaments is constructed at the final step in myofibril assembly, indicating that actin filaments continue to develop throughout myofibrillogenesis. However, the regulation of actin dynamics in this process has remained poorly understood. In nonmuscle cells, organization of actin cytoskeleton is achieved by two major actin nucleating-polymerizing systems, formins and the Arp2/3 complex, with the former producing long straight actin filaments and the latter producing branched actin network (6, 7). Because an unbranched straight actin filament is the major form in striated muscle cells, it is possible that a formin family protein serves as the key regulator of actin dynamics in myofibrils.
FIGURE 1.
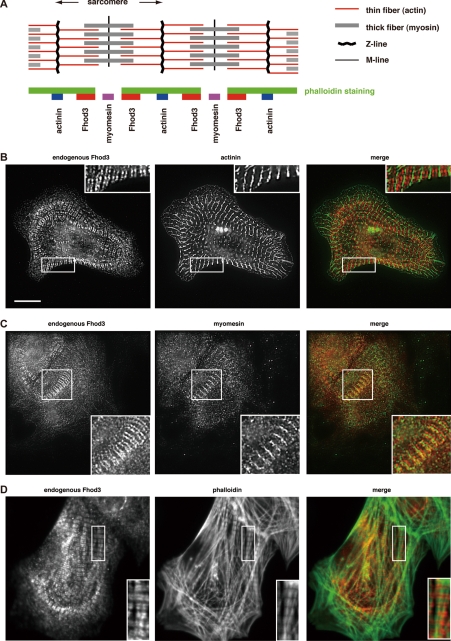
Localization of Fhod3 in cultured rat cardiomyocytes. A, shown is a representation of the sarcomere structure (upper panel) and relative localization of Fhod3 and other sarcomeric proteins from B–D (lower panel). B–D, neonatal rat cardiomyocytes were subjected to immunofluorescent double staining for endogenous Fhod3 (red) and α-actinin (green) (B), myomesin (green) (C), or phalloidin (green) (D). For Fhod3 staining, the anti-Fhod3-(650–802) polyclonal antibodies were used. Scale bar, 10 μm.
Formins are characterized by the presence of two conserved regions, the formin homology 1 and 2 domains (FH1 and FH2 domains, respectively)2 (8, 9). The FH2 domain associates with the barbed end of an actin filament and promotes actin nucleation and polymerization. The FH2 domain continues to associate with the barbed end during polymerization; this processive association protects the growing barbed end from capping proteins that inhibit actin elongation. The FH1 domain, located N-terminally to the FH2 domain, accelerates the FH2-mediated actin elongation via recruiting profilin complexed with an actin monomer. Through cooperation of the FH1 and FH2 domains, formins produce long straight actin filaments even in the presence of capping proteins. Here, we focused on the role of the mammalian formin Fhod3 (previously designated as Fhos2L), which is expressed predominantly in the heart (10), in actin assembly in myofibrils.
EXPERIMENTAL PROCEDURES
Construction of Recombinant Plasmids and Recombinant Adenoviruses
The cDNA fragments encoding mouse Fhod3 of 1578 amino acids and its spliced variant Fhod3S with deletion of amino acids 401–551 (designated as Fhos2L and Fhod2S, respectively, in our previous paper (10)) and human Fhod1 were prepared as described previously (10, 11). The cDNA encoding mouse Fhod3-ΔN (amino acids 931–1578) was constructed by PCR using the cDNA encoding mouse Fhod3. The cDNA encoding mDia1-FH1FH2 (amino acids 549–1175) was cloned by PCR using the expressed sequence tag clone MGC:86169 as a template. The cDNA for human profilin IIa was cloned by PCR using human brain cDNAs from the Human Multiple Tissue cDNA Panel (BD Biosciences) as a template. Mutations leading to the indicated amino acid substitutions were introduced by PCR-mediated sited-directed mutagenesis.
The DNA fragments were ligated to pEGFP-C1 (Clontech) or pEF-BOS for expression in HeLa cells as an N-terminally green fluorescent protein (GFP)-tagged protein or for expression in HEK-293F cells as an N-terminally FLAG-tagged protein, respectively. For expression of Fhod3 and their mutants as HA-tagged proteins in primary cultures of neonatal rat cardiomyocytes, the adenoviruses encoding HA-tagged mouse Fhod3 and their mutants were constructed by the Adeno-XTM Expression System (Clontech) according to the manufacturer's instructions. All constructs were sequenced for confirmation of their identities.
Antibodies
Affinity-purified rabbit polyclonal antibodies specific for Fhod3 (anti-Fhod3-(650–802), anti-Fhod3-(873–974), and anti-Fhod3-(C-20)) were prepared as described previously (10). Monoclonal antibodies were purchased from commercial sources as indicated: clone EA-53 against α-actinin (Sigma); clone 3-48 against cardiac myosin heavy chain (Abcam); clone Alpha-Sr-1 against sarcomeric actin (Dako); clone 16B12 against HA (Covance). Goat polyclonal antibodies against myomesin-1 (C-16) were purchased from Santa Cruz Biotechnology.
Cells and Transfection
Primary cultures of cardiac myocytes were prepared from ventricles of neonatal Sprague-Dawley rats according to the method of Simpson (12) with minor modifications (13). Briefly, hearts were isolated from 1-day-old postnatal Sprague-Dawley rats, trisected, and then incubated with trypsin (50 μg/ml, Worthington TRLS) in Hanks' balanced saline solution buffer (137 mm NaCl, 5.3 mm KCl, 0.34 mm Na2HPO4, 0.44 mm KH2PO4, 4.2 mm NaHCO3, and 5.6 mm glucose) overnight at 4 °C. The next day, they were digested with collagenase type II (300 units/ml, Worthington CLS2) on a shaking instrument (140 rpm) for 20 min at 37 °C. Cells were preplated for 70 min into 100-mm culture dishes in DMEM supplemented with 10% FCS to reduce the number of nonmyocytes. Cells that were not attached to the dishes were plated and cultured in DMEM with 10% FCS. The purity of myocytes in the culture was >80%, which was estimated by immunofluorescent staining with an anti-sarcomeric actin antibody.
Transfection of cardiomyocytes with the adenovirus encoding Fhod3 was performed 24 h after plating. After incubation with the adenovirus for 60 min, cells were cultured in DMEM with 10% FCS for another 48 h and used for the experiments. In the case of sequential transfection with the adenovirus and siRNA, cells were transfected with siRNA using Lipofectamine 2000 (Invitrogen) immediately after incubation with the adenovirus for 60 min. After the sequential transfection, the cells were cultured for 48 h and used for the experiments.
HeLa cells were cultured in DMEM supplemented with 10% FCS. HeLa cells were transfected with plasmids using Lipofectamine and cultured for 3 h. After the addition of DMEM containing 10% FCS, cells were cultured for another 13 h.
Fixation and Immunofluorescence Staining
Cells were fixed in 100% methanol for 10 min at −20 °C and blocked with phosphate-buffered saline (137 mm NaCl, 2.68 mm KCl, 8.1 mm Na2HPO4, and 1.47 mm KH2PO4, pH 7.4) containing 3% bovine serum albumin for 60 min. In the case of phalloidin staining, cells were fixed in 3.7% formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 4 min, and blocked with phosphate-buffered saline containing 3% bovine serum albumin for 60 min. Indirect immunofluorescence analysis was performed using anti-Fhod3, anti-α-actinin, anti-myomesin, and anti-sarcomeric actin antibodies as primary antibodies, and Alexa Fluor 488-labeled anti-rabbit, anti-mouse, and anti-goat antibodies (Invitrogen) were used as secondary antibodies. For F-actin staining, Texas Red-X phalloidin (Invitrogen) was used. Images were taken with a microscope (Axiovert 200; Carl Zeiss MicroImaging) coupled to a camera (Axiocam HRm; Carl Zeiss MicroImaging), with the exception of those shown in Fig. 1, B and C, and supplemental Fig. S1, A and B, which were acquired and processed by deconvolution using a DeltaVision RT imaging system (Applied Precision).
RNA Interference (RNAi) for Knock Down of Fhod3
Double-stranded siRNAs targeting Fhod3 were synthesized as a 25-nucleotide modified synthetic RNA (StealthTM RNAi; Invitrogen). The sequences were as follows: Fhod3-1(3212) (sense), 5′-CGGUAAUUUAUUGGCUUCUCCUGUG-3′; Fhod3-1(3212) (antisense), 5′-CACAGGAGAAGCCAAUAAAUUACCG-3′; Fhod3-2(3429) (sense), 5′-GAGCACCUGUUUGAGUCCAAGUCUA-3′; Fhod3-2(3429) (antisense), 5′-UAGACUUGGACUCAAACAGGUGCUC-3′; Fhod3-3(U1) (sense), 5′-CGCAUUGACAUGGAUCUCCAGAAAU-3′; Fhod3-3(U1) (antisense), 5′-AUUUCUGGAGAUCCAUGUCAAUGCG-3′; Fhod3-4(U3) (sense), 5′-UCUCCAGAAAUCUCCUGCAUGUAUU-3′; and Fhod3-4(U3) (antisense), 5′-AAUACAUGCAGGAGAUUUCUGGAGA-3′. Fhod3-1 and Fhod3-2 were designed for a coding region, and Fhod3-3 and Fhod3-4 were for a 3′-untranslated region of Fhod3. As a negative control for Fhod3 siRNAs, Low GC Duplex of StealthTM RNAi negative control duplexes (Invitrogen) were used. Transfection of cardiomyocytes with siRNA was performed using Lipofectamine 2000, according to the manufacturer's protocol. After transfection, cells were cultured for 48 h and used for the experiments.
Western Blot Analysis
Cells were broken with a lysis buffer (10% glycerol, 135 mm NaCl, 5 mm EDTA, and 20 mm Hepes, pH 7.4) containing 1% Triton X-100. The lysates were applied to SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was probed with the anti-Fhod3 polyclonal antibodies or anti-HA monoclonal antibody.
Protein Expression and Purification
For preparation of Fhod3 and mDia1 proteins, FreeStyle HEK-293F cells (Invitrogen) were transfected with an expression vector encoding the respective cDNAs. The transfected cells were broken with the lysis buffer, and the lysate was precipitated with an anti-FLAG antibody (M2)-conjugated agarose (Sigma). Proteins eluted with FLAG peptide (200 μg/ml) in X buffer (2 mm MgCl2, 100 mm KCl, 0.1 mm CaCl2, 5 mm EGTA, 1 mm dithiothreitol, and 10 mm Hepes, pH 7.9) were used for an actin polymerization assay as described below. Human profilin IIa were expressed in Escherichia coli as a glutathione S-transferase fusion protein, purified by glutathione-Sepharose 4B (Amersham Biosciences), and cleaved with PreScission protease (Amersham Biosciences). Spectrin-actin seeds were isolated from erythrocyte ghosts by the method of Lin and Lin (14).
Actin Polymerization Assay
Pyrene-actin polymerization assays were performed in X buffer as described previously (15). Briefly, G-actin (10% pyrene-labeled) was prepared in G buffer (5 mm Tris-HCl, pH 8.0, 0.2 mm CaCl2, 0.2 mm ATP, 0.2 mm dithiothreitol) and centrifuged at 100,000 × g for 60 min at 4 °C for the removal of residual F-actin. Polymerization reactions were performed in 100 μl of X buffer containing 2 μm actin (10% pyrene-labeled) or 0.5 μm actin (20% pyrene-labeled), and 25 nm formin proteins. All reaction components except the actin were mixed in X buffer, and the reaction was started by the addition of actin. Fluorescence changes (excitation wavelength of 365 nm; emission wavelength of 430 nm) were measured using the microplate reader FlexStation3 (Molecular Devices).
RESULTS
We have shown previously that, in the fetal rat heart, Fhod3 distributes in a striated pattern (10). To investigate the detailed localization of Fhod3 with respect to other sarcomeric proteins, we double stained neonatal rat cardiomyocytes for Fhod3 and the Z-line marker α-actinin. Immunofluorescence staining with the anti-Fhod3-(650–802) antibodies demonstrated that cardiac muscle Fhod3 localizes to a pair of narrow bands between the α-actinin-containing Z-lines (Fig. 1B). This periodic localization was confirmed with another two anti-Fhod3 polyclonal antibodies: the anti-Fhod3-(C-20) and anti-Fhod3-(873–974) (supplemental Fig. S1A). Exogenously expressed Fhod3 also localized as paired narrow bands between the Z-lines (supplemental Fig. S1B). We next investigated the localization of Fhod3 with respect to the M-line, located at the center of a sarcomere. Double staining for Fhod3 and the sarcomeric M-line protein myomesin (16) revealed that the M-line was localized in a narrower gap of Fhod3 bands (Fig. 1C). Double staining of F-actin and Fhod3 revealed that endogenous Fhod3 (Fig. 1D) as well as exogenous Fhod3 (supplemental Fig. S1C) localizes at both edges of the phalloidin-stained thin filaments. Thus, Fhod3 in cardiac myocytes is located on thin actin filaments, particularly accumulated near the middle of the sarcomere (Fig. 1A).
In more than half of cells overexpressing Fhod3, striated myofibrils did not extend throughout the cell but underwent a structural transition to actin filament bundles that lacked normal striated pattern of α-actinin, although central areas of the cell showed well developed myofibril organization (supplemental Fig. S2A). These characteristic bundles are also observed in cells overexpressing Fhod3S, a short splice variant expressed predominantly in the kidney and brain (10) (supplemental Fig. S2, B and C), supporting the idea that Fhod3 plays a role in sarcomere organization.
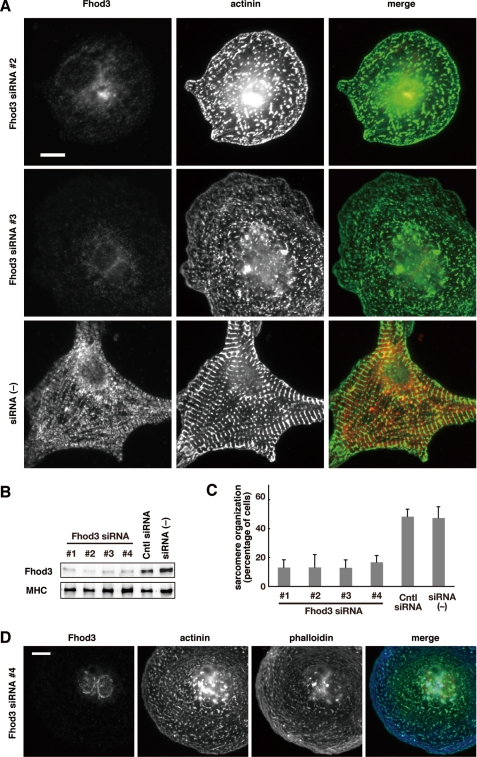
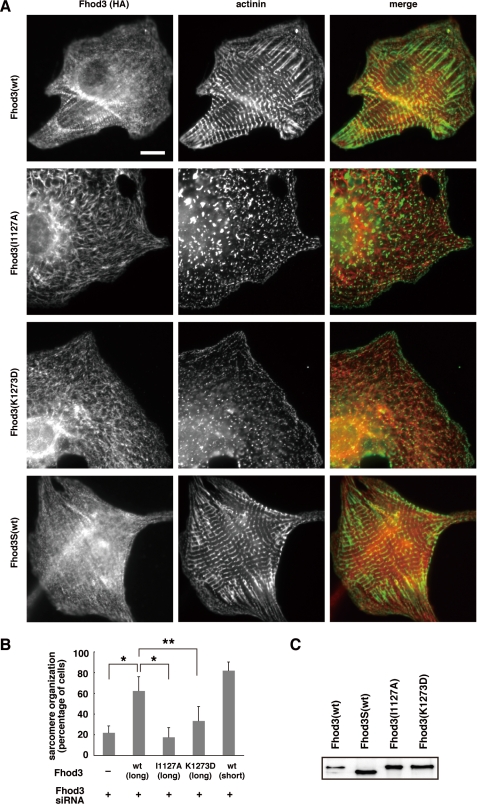
To investigate the role of Fhod3 in sarcomeric organization, we knocked down Fhod3 in rat cardiomyocytes using four distinct double-stranded siRNAs (siRNAs 1–4). Transfection of cardiomyocytes with these siRNAs led to a significant decrease in Fhod3 at the protein level (Fig. 2, A and B). The effect of these siRNAs appeared to be specific to Fhod3 because the amount of myosin heavy chain, a sarcomeric protein, was not affected (Fig. 2B). In siRNA-treated cells, sarcomere organization was disrupted: the Z-band marker α-actinin concentrated in a punctated manner, which is in marked contrast with the striated pattern of α-actinin expression in control cells (Fig. 2, A and C, and supplemental Fig. S3). Depletion of Fhod3 also resulted in drastic decrease of phalloidin staining, indicating that actin filament formation per se is blocked (Fig. 2D). Although control cardiac myocytes had a flattened star-shaped morphology with longitudinal myofibrils along the edge of cells, Fhod3-depleted cardiomyocytes showed a rounded cell shape. Thus, depletion of Fhod3 induced a drastic change of myocardial cytoskeleton. To confirm the involvement of Fhod3 in sarcomere organization, we sequentially transfected the cardiomyocytes with Fhod3 siRNA 3, targeted to a 3′-untranslated region, and with the adenovirus encoding the wild-type Fhod3 protein, which lacks the 3′-untranslated region, and thus mRNA derived from the cDNA was expected to be unaffected by the siRNA. Expression of RNAi-resistant wild-type Fhod3 sufficiently rescued the effect of Fhod3 siRNA on sarcomere organization (Fig. 3, A and B), confirming that Fhod3 is required for sarcomere organization.
FIGURE 2.
Fhod3 knock down disrupted sarcomere assembly in rat cardiomyocytes. A, cardiomyocytes were transfected with Fhod3-specific siRNAs 2 and 3 and cultured for 48 h. Cells were fixed and double stained with the antibodies against anti-Fhod3-(650–802) (red) and α-actinin (green). Scale bar, 10 μm. Images for cells transfected with Fhod3-specific siRNAs 1 and 4 are shown in supplemental Fig. S3. B, the protein level of Fhod3 was determined by immunoblot analysis using anti-Fhod3-(873–974). As a control, a monoclonal antibody against the cardiac myosin heavy chain (MHC) was used. C, cells with well developed sarcomere organization were counted, and the percentages from four independent transfections are expressed as the means ± S.D.; cells with organized sarcomere are defined as ones displaying at least five repeated α-actinin bands with a width more than 2 μm by α-actinin staining. D, cardiomyocytes transfected with Fhod-specific siRNA 4 were fixed and stained with the antibodies against anti-Fhod3-(650–802) (red) and α-actinin (green) and with phalloidin (blue). Scale bar, 10 μm.
FIGURE 3.
Sarcomere disorganization caused by Fhod3 knock down is restored by expression of the wild-type (wt) Fhod3 but not by that of mutant proteins defective in the actin assembly activity. A, cardiomyocytes were sequentially transfected with the adenovirus encoding HA-tagged wild-type or mutant Fhod3 and with Fhod3 siRNA 4 and cultured for 48 h. Cells were fixed and double stained with the antibodies against anti-HA (red) and α-actinin (green). Scale bar, 10 μm. B, cells with well developed sarcomere organization were counted, and the percentages from five independent transfections are expressed as the means ± S.D. as in Fig. 2C. *, p < 0.001; **, p < 0.005, Welch's t test. C, the protein level of exogenously expressed HA-tagged Fhod3 was determined by immunoblot analysis using the anti-HA antibody.
We next tested whether the Fhod3-mediated sarcomere organization requires its actin assembly activity. Fhod3-ΔN, a truncated fragment expected to function as an active form because of a lack of the intramolecular interaction mediated by the N-terminal region (10), markedly induced formation of stress fibers in HeLa cells (Fig. 4A). It has been shown that substitution of Ala for Ile1431 or Asp for Lys1601 in the FH2 domain of the yeast formin Bni1p abolishes the actin-nucleating activity (17, 18). The corresponding substitution in the Fhod3 FH2 domain, i.e. I1127A or K1273D, resulted in a loss of stress fiber formation (Fig. 4, A and B), indicating that these substitutions abrogate the actin assembly activity of Fhod3. In contrast to the wild-type Fhod3, Fhod3-(I1127A) or Fhod3-(K1273D) in an RNAi-resistant form failed to rescue the formation of the striated pattern of α-actinin (Fig. 3, A and B), although these mutant proteins were fully expressed in the siRNA-treated cells (Fig. 3C). Similarly, F-actin organization in a striated pattern was not restored by expression of Fhod3-(I1127A) or Fhod3-(K1273D) in cells depleted of endogenous Fhod3 (supplemental Fig. S4). These findings indicate that the Fhod3 FH2-mediated actin assembly is critical for sarcomere organization in myofibrils.
FIGURE 4.
Actin assembly activity of Fhod3. A, HeLa cells were transfected with plasmids encoding the wild-type Fhod3-ΔN or mutant Fhod3-ΔN carrying the I1127A or K1273D substitution. Cells were fixed followed by visualization by GFP fluorescence (green) or phalloidin staining (red). Scale bar, 20 μm. B, quantitative analysis of stress fiber formation by expression of the wild-type Fhod3-ΔN or mutant Fhod3-ΔN protein carrying the I1127A or K1273D substitution. Cells showing parallel actin fibers aligned with the long axis throughout the cell (as in A) were counted, and the percentages are expressed as the mean ± S.D.
Finally, we tested the ability of Fhod3 proteins purified (supplemental Fig. S5) to assemble actin in vitro. As shown in Fig. 5A, Fhod3-ΔN inhibited spontaneous assembly of actin. The inhibition was attenuated by substitution of alanine for Ile1127 in Fhod3. We also investigated the effect of profilin, an actin-monomer-binding protein that accelerates actin elongation from barded ends by delivering actin monomers to the FH1 domain of several formins (19, 20). Even in the presence of profilin, Fhod3 did not accelerate but rather inhibited actin assembly, although mDia1 effectively did (Fig. 5B). To know the mechanism whereby Fhod3 inhibits actin assembly, we monitored seed-dependent actin assembly in the presence of 0.5 μm G-actin (Fig. 5C). Under the conditions, actin elongation is allowed only from the barbed end. The wild-type Fhod3 reduced the elongation rate from barbed ends, although it was marginally affected by Fhod3-(I1127A). Thus, Fhod3 likely associates the barbed end of actin filaments and regulates the elongation of actin filaments.
FIGURE 5.
Effect of Fhod3 on in vitro actin assembly. A, effect of Fhod3 on spontaneous assembly of actin filaments. 10% pyrene-labeled actin (2 μm) was incubated in the presence of Fhod3-ΔN-(wt), Fhod3-ΔN-(I1127A), or mDia1-FH1FH2 at a concentration of 25 nm. Actin polymerization was monitored by fluorescence enhancement of pyrene-labeled actin. B, effect of profilin on Fhod3-mediated actin assembly. Actin (2 μm) was incubated with 25 nm Fhod3-ΔN-(wt), Fhod3-ΔN-(I1127A), or mDia1-FH1FH2 in the presence of profilin (2 μm). C, effect of Fhod3 on the barbed end assembly. 20% pyrene-labeled actin (0.5 μm) was incubated with 25 nm Fhod3-ΔN-(wt) or Fhod3-ΔN-(I1127A) in the presence of spectrin-actin seeds (5 μg/ml).
DISCUSSION
As shown in the present study, Fhod3 in cardiomyocytes localizes to thin actin filaments, particularly to areas near the middle of the sarcomere. Because actin filaments in mature myofibrils direct their pointed ends toward the M-line at the center of the sarcomere, Fhod3 appears to associate with actin filaments near the pointed end (Fig. 1A). On the other hand, it seems likely that Fhod3 functions at the barbed end of actin filaments in a manner similar to that of other formins: the residues Ile1431 and Lys1601 in the FH2 domain of Bni1p are critical for binding to the barbed-end side of an actin molecule (18), and conserved in the Fhod3 FH2 domain (10). As expected, Fhod3 regulates the elongation rate from the barbed end (Fig. 5). Thus, Fhod3 likely functions by associating directly with the barbed end of actin filaments.
The region where Fhod3 localizes in cells containing the sarcomere formed (i.e. near the center of the sarcomere; between the Z-band and M-line in the sarcomere) appears to be different from the site where Fhod3 has function to form actin filaments. The present study demonstrates that the Fhod3S, a short spliced variant that lacks 151 amino acids in the N-terminal region (10), is not periodically localized in contrast to Fhod3 (Fig. 3A and supplemental Fig. S4), suggesting that the N-terminal region rather than the FH2 domain is responsible for the localization of Fhod3 near the center of the sarcomere. In Fhod3-depleted cardiomyocytes, Fhod3S fully rescues the periodic accumulation of α-actinin (Fig. 3B). Fhod3S also promotes F-actin formation, but in a much less striated pattern (supplemental Figs. S2C and S4). Thus, the periodic localization of Fhod3 appears to be involved in adequate sarcomere organization.
It is also possible that the barbed ends with which Fhod3 associates are close to the central region of the sarcomere. Quantitative estimation of fluorescent-actin subunit exchange in living muscle cells has revealed that actin dynamics predominate at the pointed ends near the center of the sarcomere (21). If short filaments capped at their barbed ends by Fhod3 are incorporated to the pointed end of thin filaments through end-to-end annealing, Fhod3-associated barbed ends might be present near the middle of the sarcomere. In this context, it should be noted that actin filaments nucleated by the yeast formin Cdc12p anneal end-to-end in the presence of tropomyosin, whose vertebrate ortholog is a major component of the thin filaments in myofibrils (22). Future studies are awaited to elucidate the mechanism for localization of Fhod3.
The present study shows that Fhod3 plays a crucial role in the sarcomere organization of cardiomyocytes, which requires its actin assembly activity. It is well known that, in mature myofibrils, both pointed and barbed ends of the thin filaments are capped and stabilized: the barbed ends by CapZ and the pointed ends by tropomodulin (1). These two capping proteins have been shown to be essential for proper organization of the sarcomere (23, 24). On the other hand, actin polymerization per se is indispensable for myofibrillogenesis (25). For effective addition of actin monomers to both ends during myofibrillogenesis, protection of the ends from capping proteins seems to be required. Because formins and capping proteins compete for the barbed end of actin filaments (26, 27), the cardiac formin Fhod3 may protect the barbed end for actin assembly in sarcomere formation.
Surprisingly, Fhod3 does not promote in vitro actin polymerization with purified actin in the presence or absence of profilin and/or preformed actin seeds (Fig. 5). These findings suggest that Fhod3 functions together with factors absent in the in vitro system to regulate organization of actin filaments. In addition to the effects on actin filaments, Fhod3 might also contribute to sarcomere formation through activation of the serum response factor presumably via depletion of the monomeric actin pool (28–30); Fhod3 and Fhod1 induce serum response factor activation in a reporter assay.3
Recently, Drosophila melanogaster SALS (31) and mammalian leiomodin (32) have been proposed as regulators of actin polymerization at pointed ends in muscle cells. Fhod3 may regulate actin assembly and turnover in sarcomeres in combination with these pointed-end regulators. The present findings not only give information on a physiological role of Fhod3 in cardiomyocytes but also provide insights into the regulation of actin dynamics during myofibrillogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Masayoshi Yoshida (Kyushu University) for advice on primary cultures of neonatal rat cardiomyocytes; Drs. Naoki Watanabe (Kyoto University), Chiharu Higashida (Kyoto University), and Shigeki Morita (Saga University) for helpful discussion and encouragement; Yohko Kage (Kyushu University), Natsuko Morinaga (Kyushu University), Namiko Kubo (Kyushu University), Masumi Otsu (Kyushu University), and Masafumi Sasaki (Kyushu University) for technical assistance; and Minako Nishino (Kyushu University) for secretarial assistance. We also appreciate the technical support from the Research Support Center, Kyushu University Graduate School of Medical Sciences.
This work was supported in part by Grants-in-aid for Scientific Research and Targeted Proteins Research Program (TPRP) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by Kyushu University Interdisciplinary Programs in Education and Projects in Research Development, by the Takeda Science Foundation, and by CREST of Japan Science and Technology Agency.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
R. Takeya and H. Sumimoto, unpublished observations.
- FH1 and FH2
- formin homology 1 and 2, respectively
- DMEM
- Dulbecco's modified Eagle's medium
- FCS
- fetal calf serum
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- siRNA
- small interfering RNA.
REFERENCES
- 1.Clark K. A., McElhinny A. S., Beckerle M. C., Gregorio C. C. (2002) Annu. Rev. Cell Dev. Biol. 18, 637–706 [DOI] [PubMed] [Google Scholar]
- 2.Gregorio C. C., Antin P. B. (2000) Trends Cell Biol. 10, 355–362 [DOI] [PubMed] [Google Scholar]
- 3.Sanger J. W., Kang S., Siebrands C. C., Freeman N., Du A., Wang J., Stout A. L., Sanger J. M. (2005) J. Muscle Res. Cell. Motil. 26, 343–354 [DOI] [PubMed] [Google Scholar]
- 4.Littlefield R. S., Fowler V. M. (2008) Semin. Cell Dev. Biol. 19, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markwald R. R. (1973) J. Mol. Cell. Cardiol. 5, 341–350 [DOI] [PubMed] [Google Scholar]
- 6.Pollard T. D. (2007) Annu. Rev. Biophys. Biomol. Struct. 36, 451–477 [DOI] [PubMed] [Google Scholar]
- 7.Chhabra E. S., Higgs H. N. (2007) Nat. Cell Biol. 9, 1110–1121 [DOI] [PubMed] [Google Scholar]
- 8.Kovar D. R. (2006) Curr. Opin. Cell Biol. 18, 11–17 [DOI] [PubMed] [Google Scholar]
- 9.Goode B. L., Eck M. J. (2007) Annu. Rev. Biochem. 76, 593–627 [DOI] [PubMed] [Google Scholar]
- 10.Kanaya H., Takeya R., Takeuchi K., Watanabe N., Jing N., Sumimoto H. (2005) Genes Cells 10, 665–678 [DOI] [PubMed] [Google Scholar]
- 11.Takeya R., Sumimoto H. (2003) J. Cell Sci. 116, 4567–4575 [DOI] [PubMed] [Google Scholar]
- 12.Simpson P. (1985) Circ. Res. 56, 884–894 [DOI] [PubMed] [Google Scholar]
- 13.Suematsu N., Tsutsui H., Wen J., Kang D., Ikeuchi M., Ide T., Hayashidani S., Shiomi T., Kubota T., Hamasaki N., Takeshita A. (2003) Circulation 107, 1418–1423 [DOI] [PubMed] [Google Scholar]
- 14.Lin D. C., Lin S. (1980) Anal. Biochem. 103, 316–322 [DOI] [PubMed] [Google Scholar]
- 15.Suetsugu S., Miki H., Takenawa T. (2001) J. Biol. Chem. 276, 33175–33180 [DOI] [PubMed] [Google Scholar]
- 16.Grove B. K., Kurer V., Lehner C., Doetschman T. C., Perriard J. C., Eppenberger H. M. (1984) J. Cell Biol. 98, 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Moseley J. B., Sagot I., Poy F., Pellman D., Goode B. L., Eck M. J. (2004) Cell 116, 711–723 [DOI] [PubMed] [Google Scholar]
- 18.Otomo T., Tomchick D. R., Otomo C., Panchal S. C., Machius M., Rosen M. K. (2005) Nature 433, 488–494 [DOI] [PubMed] [Google Scholar]
- 19.Kovar D. R., Kuhn J. R., Tichy A. L., Pollard T. D. (2003) J. Cell Biol. 161, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D., Carlier M. F. (2004) Cell 119, 419–429 [DOI] [PubMed] [Google Scholar]
- 21.Littlefield R., Almenar-Queralt A., Fowler V. M. (2001) Nat. Cell Biol. 3, 544–551 [DOI] [PubMed] [Google Scholar]
- 22.Skau C. T., Neidt E. M., Kovar D. R. (2009) Mol. Biol. Cell. 20, 2160–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer D. A., Hug C., Cooper J. A. (1995) J. Cell Biol. 128, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregorio C. C., Weber A., Bondad M., Pennise C. R., Fowler V. M. (1995) Nature 377, 83–86 [DOI] [PubMed] [Google Scholar]
- 25.Manasek F. J., Burnside B., Stroman J. (1972) Proc. Natl. Acad. Sci. U.S.A. 69, 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zigmond S. H., Evangelista M., Boone C., Yang C., Dar A. C., Sicheri F., Forkey J., Pring M. (2003) Curr. Biol. 13, 1820–1823 [DOI] [PubMed] [Google Scholar]
- 27.Harris E. S., Li F., Higgs H. N. (2004) J. Biol. Chem. 279, 20076–20087 [DOI] [PubMed] [Google Scholar]
- 28.Sotiropoulos A., Gineitis D., Copeland J., Treisman R. (1999) Cell 98, 159–169 [DOI] [PubMed] [Google Scholar]
- 29.Copeland J. W., Treisman R. (2002) Mol. Biol. Cell 13, 4088–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westendorf J. J. (2001) J. Biol. Chem. 276, 46453–46459 [DOI] [PubMed] [Google Scholar]
- 31.Bai J., Hartwig J. H., Perrimon N. (2007) Dev. Cell 13, 828–842 [DOI] [PubMed] [Google Scholar]
- 32.Chereau D., Boczkowska M., Skwarek-Maruszewska A., Fujiwara I., Hayes D. B., Rebowski G., Lappalainen P., Pollard T. D., Dominguez R. (2008) Science 320, 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.