Abstract
The pleiotropic cytokines, transforming growth factor β1 (TGFβ1), and tumor necrosis factor (TNF) play critical roles in tissue homeostasis in response to injury and are implicated in multiple human diseases and cancer. We reported that the loss of Timp3 (tissue inhibitor of metalloproteinase 3) leads to abnormal TNF signaling and cardiovascular function. Here we show that parallel deregulation of TGFβ1 and TNF signaling in Timp3−/− mice amplifies their cross-talk at the onset of cardiac response to mechanical stress (pressure overload), resulting in fibrosis and early heart failure. Microarray analysis showed a distinct gene expression profile in Timp3−/− hearts, highlighting activation of TGFβ1 signaling as a potential mechanism underlying fibrosis. Neonatal cardiomyocyte-cardiofibroblast co-cultures were established to measure fibrogenic response to agonists known to be induced following mechanical stress in vivo. A stronger response occurred in neonatal Timp3−/− co-cultures, as determined by increased Smad signaling and collagen expression, due to increased TNF processing and precocious proteolytic maturation of TGFβ1 to its active form. The relationship between TGFβ1 and TNF was dissected using genetic and pharmacological manipulations. Timp3−/−/Tnf−/− mice had lower TGFβ1 than Timp3−/−, and anti-TGFβ1 antibody (1D11) negated the abnormal TNF response, indicating their reciprocal stimulatory effects, with each manipulation abolishing fibrosis and improving heart function. Thus, TIMP3 is a common innate regulator of TGFβ1 and TNF in tissue response to injury. The matrix-bound TIMP3 balances the anti-inflammatory and proinflammatory processes toward constructive tissue remodeling.
Tissue repair requires the coordinated response between cellular and stromal compartments, involving regulated cytokine release, inflammation, cellular turnover, and structural remodeling, in order to restore organ function. The consequence of an inadequate remodeling program is reflected as necrosis, hyperplasia, and fibrosis, which are the hallmarks of multiple human diseases. Tissue fibrosis is the outcome of excessive and disorganized deposition of extracellular matrix (ECM)3 proteins, resulting in disruption of normal tissue architecture and homeostasis that contributes to organ dysfunction. Myocardial fibrosis is the underlying cause of diastolic heart failure and a complicating factor in multiple heart disorders (1–3). Although cell surface-bound and soluble matrix metalloproteinases (MMPs) along with their natural tissue inhibitors (tissue inhibitors of metalloproteinase) constitute an important system for regulating ECM turnover (4), inflammation is emerging as an important co-contributor to fibrosis (5, 6). Recent studies have linked specific subsets of metalloproteinases to inflammatory processes through their ability to cleave a wide variety of ECM-bound and cell surface cytokines (7, 8). Common regulators of inflammatory cytokines and fibrogenic ligands may be the key to building an adequate tissue response, and their identification can lead to developing new strategies against tissue fibrosis.
TGFβ1 is considered the major regulator of fibroblast response during normal ECM homeostasis as well as during the pathogenesis of fibrosis (9). TGFβ1-activated fibroblasts, characterized by the expression of α-smooth muscle actin, are the main source of collagen biosynthesis in the myocardium (10). TGFβ1 can trigger the differentiation of cardiac fibroblasts to activated myofibroblasts that synthesize collagen types I and III (11, 12). It also regulates the proteolytic systems responsible for ECM turnover (13). Secreted TGFβ1 is sequestered and concentrated in the ECM by specific binding proteins, which render it biologically inactive (14). Enhanced TGFβ1 signaling can result from defective matrix-binding due to fibrillin mutations as observed in Marfan syndrome or by increased release from the ECM through excessive proteolysis (7, 15, 16). As such, MMP2, -9, and -14 have been suggested to activate latent TGFβ1 directly, via cleavage and release of latency-associated peptide (7, 15). MMPs and TGFβ1 also play a role in fibroblast migration (17). Altogether, protease activity and TGFβ1 may cooperate at several levels to generate tissue fibrosis.
TGFβ1 and TNF are two multifunctional cytokines, and each inhibits the signal transduction pathways activated by the other cytokine (18, 19). During fibrosis, both compete for p300, a transcriptional cofactor that can positively or negatively modulate collagen synthesis to orient the fibrotic response (20). TNF has been shown to negatively regulate TGFβ1 signaling through its central effector molecule NF-κB, which activates the inhibitory Smad7 (21, 22), providing another means of cross-talk between these two cytokines. Both TGFβ1 and TNF exist as proproteins anchored to ECM and cell surface, respectively (8, 14). Therefore, another level of control involves the proteolytic cleavage of TNF on the cell surface and of TGFβ1 anchored to the ECM, to become soluble effector proteins. Overall, an adequate interplay of these opposing pathways is critical for a coordinated cellular stress response, which depends in part on their extracellular bioavailability.
Timp3−/− mice have an abnormal inflammatory response due to excessive TNF signaling (23, 24). Timp3−/− hearts are more susceptible to mechanical stress induced by constriction of the aorta (aortic banding (AB)) and exhibit accelerated dilated cardiomyopathy with an early onset of heart failure, due to increased ADAM17 (a disintegrin and metalloproteinase 17)-mediated TNF processing and enhanced MMP-mediated ECM degradation (25). Here we show that after aortic banding, Timp3−/− as well as Timp3+/− mice develop marked myocardial fibrosis offering a model to study the role of deregulated cytokine signaling in fibrosis. Using microarrays, a primary neonatal cardiomyocyte-cardiofibroblast co-culture system, and genetic and pharmacological manipulations in mice, we demonstrate the importance of TGFβ-TNF co-activation and cross-talk. Our study highlights the unique ability of TIMP3 to co-regulate these cytokines in the heart.
EXPERIMENTAL PROCEDURES
In Vivo Biomechanical Stress and Cardiac Function Measurement
Timp3−/− mice were described previously (26). Eight-week-old C57Bl/6 wild type (WT) and Timp3−/− mice were subjected to pressure overload by constriction of aorta to generate a pressure gradient of 40–50 mm Hg, which would impose a pressure overload and mechanical stress on the heart. This procedure was performed in a blinded manner, and the consistency of aortic constriction was comparable across genotypes. Cardiac function was monitored by echocardiography as before (25). Diastolic dysfunction was assessed by examining the transmitral Doppler flow profile. Sham-operated mice from each group served as controls. For TGFβ1 inhibition, TGFβ1-neutralizing antibody (catalog number 1D11; Genzyme Corp.) was administered intraperitoneally every other day starting 1 week prior to aortic banding, at 5 mg/kg as described (27). Tissue for protein and RNA analyses was flash-frozen and stored at −80 °C.
Microarray Analysis
Microarray experiments were performed using Affymetrix mouse gene chips 430A 2.0 (Affymetrix, Inc., Santa Clara, CA) containing 22600 probe sets. Five micrograms of RNA were pooled from three different mice for each condition, cRNA-biotinylated, and hybridized to Affymetrix chips. One chip was used for each condition representing sham, 6 h, 1 week, and 3 weeks, for both WT and Timp3-deficient mice. Data were analyzed using GeneSpring software version 7.3 (Agilent Technologies Inc.) and normalized by the GCRMA (GC robust multiarray analysis) method. Probe sets were filtered based on a minimum threshold of 50. We performed time series analysis in which WT and Timp3−/− data sets were separately analyzed with their corresponding sham as the base line. Genotype analysis was also undertaken in which WT and Timp3−/− conditions were directly compared at each time point, using WT sham as the base line. In both approaches, genes with more than 2-fold change were considered significant. Further, in order to identify co-regulated genes with smaller but consistent changes, gene set analysis was performed using the GAzer tool, which is based on parametric analysis of gene set enrichment with -fold change values. Parametric analysis of gene set enrichment with -fold change values compares two conditions and calculates statistics, as well as the Z-score to indicate the significant gene sets. These gene sets included gene ontology categories, pathways (KEGG and GenMAPP), and chromosomal locations and InterPro domains. A minimal number of 10 genes/set was defined as the cut-off threshold, and p < 0.01 as significant statistical value. GAzer is implemented in R language (available on the World Wide Web) and uses MySQL as a data base management system. This program is wrapped by JAVA. Ingenuity software was also used.
Cardiomyocyte and Cardiofibroblast Cultures
Neonatal mouse cardiac ventricular myocytes and fibroblasts were isolated by modifying a protocol used for neonatal rat cardiomyocytes (28). Briefly, hearts were excised from 1–2-day-old WT or Timp3−/− neonatal mice, minced, and digested in 0.1% trypsin in calcium-free Hanks' buffer (with HEPES) by serial digestion. Cardiofibroblasts were separated from cardiomyocytes by differential adhesion. Cells were plated for 40–45 min (37 °C, 5% CO2), during which time fibroblasts adhere to the plate but not the cardiomyocytes. Cardiomyocytes were collected and cultured separately in the presence of bromodeoxyuridine to prevent further proliferation of any residual fibroblasts. After 24 h, cardiomyocytes are easily identified as they begin to contract and exhibit a striated structure (supplemental movies). Fibroblasts were passaged once to eliminate any myocytes that did adhere during the incubation. Cardiomyocytes do not survive the passaging process; hence, the surviving cells are primarily fibroblasts. Whereas fibroblasts were cultured in Dulbecco's modified Eagle's medium/Ham/F-12 medium plus 15% fetal bovine serum for 36 h and then cultured with serum-free medium overnight prior to treatments, cardiomyocytes were cultured at confluence in parallel, in Dulbecco's modified Eagle's medium/F-12 plus 15% fetal bovine serum for 24 h, followed by 24 h in serum-free medium prior to treatments. The purity of each culture was confirmed by immunostaining and additionally by TaqMan RNA expression of selected cell-specific markers (cardiomyocytes, α-sarcomeric actin; cardiofibroblasts, vimetin; endothelial cells, CD31; smooth muscle cells, calponin) as shown in supplemental Fig. S1. To generate co-cultures of cadiomyocyte-cardiofibroblast, fibroblasts were trypsinized 2 days after isolation and added to the myocyte cultures in serum-free medium overnight before treatments. Each 35-mm co-culture dish contained 7 × 105 myocytes and 1 × 106 fibroblasts. Cultures were treated with angiotensin II (Ang II; 1 μm), phenylephrine (PE; 1 μm), or vehicle for 24 h or with 10 ng/ml recombinant TGFβ1 or TNF for 1 h. Additionally, all treatments were performed in serum-free medium containing 0.1 mm bromodeoxyuridine to prevent cell proliferation in response to the agonists (28) in order to eliminate alteration in cell numbers as a variable in our comparative studies on WT versus Timp3−/− genotypes.
The few published reports that exist on primary heart cell cultures have utilized a range of Ang II concentrations (0.01–10 μm) (29, 30). We therefore tested WT monocultures with 1 and 10 μm Ang II and found that although the latter concentration resulted in a significant induction of both collagens I and III by fibroblasts, it also generated noticeable cell death. No cell death was evident with 1 μm Ang II, indicating this to be a more suitable concentration of Ang II for the primary cultures.
Collagen Quantification in Fixed Adult Mouse Hearts
Ten-micrometer sections from three different levels of fixed hearts (from apex to base) were stained with Picro-Sirius Red (PSR). Collagen content was quantified in the left ventricle of the PSR-stained sections by taking image stacks of eight slices using a two-photon microscope. The collagen-to-volume fraction was calculated for each slice using Image ProPlus software, and the cumulative data were expressed as a percentage of total left ventricular mass. This measurement was performed on eight separate areas of each section, and five hearts were analyzed per group.
Biochemical Analyses
Cleaved TGFβ1 and total and phosphorylated Smad2/3 were detected by Western blotting of cell lysates from cultured myocytes and fibroblasts (antibodies from Cell Signaling). Cleaved TGFβ1 was measured by ELISA (R&D Systems) of culture media and tissue homogenate of sham operated or aortic banded hearts. Total gelatinase and collagenase activities were performed using the EnzCheck assay as before (25). Formalin-fixed hearts were stained with trichrome and PSR or specific antibodies for phosphorylated Smad2/3 and α-smooth muscle actin. Fibrosis was quantified in PSR-stained hearts, using two-photon confocal microscopy (Zeiss LSM 510 META NLO) and Image ProPlus software (25).
RNA levels were quantified by real time TaqMan reverse transcription-PCR with 18 S used as an endogenous control, as described (31, 32). All RNA samples were treated to remove the genomic DNA, using a “DNA-free kit” (Ambion) and then reverse-transcribed to generate cDNA. For each gene, a standard curve was generated using known concentrations of mouse brain cDNA (0.625, 1.25, 2.5, 5, 10, and 20 μg) as a function of cycle threshold (CT). The standard curve of [cDNA]brain as a function of CT is fit to a linear regression, Y = aX + b, where Y represents CT, a is slope of the standard curve, and X is [cDNA]experimental sample. The SDS2.2 software (integral to the ABI7900 real time machine) fits the CT values for the experimental samples in this formula and generates values for cDNA levels. Subsequently, these values are normalized by our internal control, 18 S, and the values are expressed as relative expression. All samples were run in triplicates in 384-well plates. Primers and probes are listed in Table 1.
TABLE 1.
TaqMan primer and probe sequences for mouse genes
Probes were labeled with 6-carboxyfluorescein (FAM) and 6-carboxy-N,N,N′,N′-tetramethylrhodamine (TAMRA).
| Gene | Sequence |
|---|---|
| TIMP1 | |
| Forward primer | 5′-CATGGAAAGCCTCTGTGGATATG-3′ |
| Reverse primer | 5′-AAGCTGCAGGCACTGATGTG-3′ |
| Probe | 5′-FAM-CTCATCACGGGCCGCCTAAGGAAC-TAMRA-3′ |
| MMP2 | |
| Forward primer | 5′-AACTACGATGATGACCGGAAGTG-3′ |
| Reverse primer | 5′-TGGCATGGCCGAACTCA-3′ |
| Probe | 5′-FAM-TCTGTCCTGACCAAGGATATAGCCTATTCCTCG-TAMRA-3′ |
| MMP8 | |
| Forward primer | 5′-GATTCAGAAGAAACGTGGACTCAA-3′ |
| Reverse primer | 5′-CATCAAGGCACCAGGATCAGT-3′ |
| Probe | 5′-FAM-CATGAATTTGGACATTCTTTGGGACTCTCTCAC-TAMRA-3′ |
| MMP9 | |
| Forward primer | 5′-CGAACTTCGACACTGACAAGAAGT-3′ |
| Reverse primer | 5′-GCACGCTGGAATGATCTAAGC-3′ |
| Probe | 5′-FAM-TCTGTCCAGACCAAGGGTACAGCCTGTTC-TAMRA-3′ |
| MMP13 | |
| Forward primer | 5′-GGGCTCTGAATGGTTATGACATTC-3′ |
| Reverse primer | 5′-AGCGCTCAGTCTCTTCACCTCTT-3′ |
| Probe | 5′-AAGGTTATCCCAGAAAAATATCTGACCTGGGATTC-3′ |
| MMP14 (MT1-MMP) | |
| Forward primer | 5′-AGGAGACAGAGGTGATCATCATTG-3′ |
| Reverse primer | 5′-GTCCCATGGCGTCTGAAGA-3′ |
| Probe | 5′-FAM-CCTGCCGGTACTACTGCTGCTCCTG-TAMRA-3′ |
| TNFα | |
| Forward primer | 5′-ACAAGGCTGCCCCGACTAC-3′ |
| Reverse primer | 5′-TTTCTCCTGGTATGAGATAGCAAATC-3′ |
| Probe | 5′-FAM-TGCTCCTCACCCACACCGTCAGC-TAMRA-3′ |
| TGFß1 | |
| Forward primer | 5′-CCTGCAAGACCATCGACATG-3′ |
| Reverse primer | 5′-ACAGGATCTGGCCACGGAT-3′ |
| Probe | 5′-FAM-CTGGTGAAACGGAAGCGCATCGAA-TAMRA-3′ |
| Pro-col I-α1 | |
| Forward primer | 5′-CTTCACCTACAGCACCCTTGTG-3′ |
| Reverse primer | 5′-TGACTGTCTTGCCCCAAGTTC-3′ |
| Probe | 5′-FAM-CTGCACGAGTCACACC-TAMRA-3′ |
| Pro-col III-α1 | |
| Forward primer | 5′-TGTCCTTTGCGATGACATAATCTG-3′ |
| Reverse primer | 5′-AATGGGATCTCTGGGTTGGG-3′ |
| Probe | 5′-FAM-ATGAGGAGCCACTAGACT-TAMRA-3′ |
Statistical Analysis
Comparisons among groups were performed by analysis of variance followed by multiple comparison testing (Student-Neuman Keuls). Values are reported as mean ± S.E. Statistical significance is recognized at p < 0.05.
RESULTS
Extensive Myocardial Fibrosis in Timp3−/− and Timp3+/− Mice
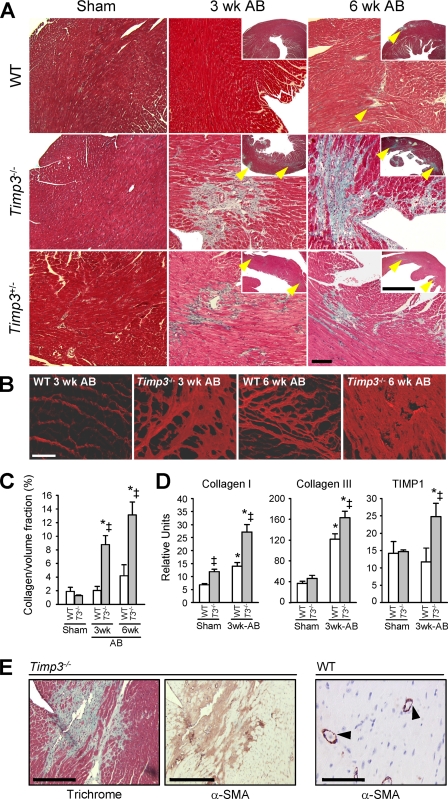
We previously reported the rapid development of severe dilated cardiomyopathy and early heart failure in Timp3−/− mice following aortic constriction (AB) (25). In this study, we investigated how TIMP3 deficiency affects the process of myocardial remodeling. In WT hearts, the collagen fibers surrounding the myocytes thicken due to collagen fiber accumulation starting at 6 weeks after AB. In contrast, fibrosis was detected by 1 week (data not shown) and was prevalent in Timp3−/−-AB hearts, with excess collagen accumulation at multiple locations replacing the cardiomyocytes by 3 weeks and becoming more extensive by 6 weeks, as revealed by trichrome staining (Fig. 1A). Interstitial and perivascular fibrosis were seen in the left ventricular walls. PSR staining and confocal microscopy further highlighted the disarray of accumulated fibrillar collagen (Fig. 1B), and histomorphometric measurement of the collagen/volume fraction showed significantly higher collagen content in Timp3−/−-AB hearts (Fig. 1C). Expression of predominant collagens in the myocardial matrix (types I and III) and a marker of myocardial fibrosis TIMP1 (33) were significantly increased in Timp3−/−-AB hearts (Fig. 1D); collagen type I was also higher in Timp3−/− sham heart compared with WT.
FIGURE 1.
Excessive interstitial fibrosis in aortic banded Timp3-deficient hearts. A, trichrome staining of sham-operated or AB WT, Timp3−/− and Timp3+/− hearts. The arrowheads indicate the areas of fibrosis. Scale bar, 100 μm. B, fibrillar collagen was visualized by PSR staining and confocal microscopy in WT and Timp3−/− hearts at 3 and 6 weeks (wk) post-AB. Scale bar, 50 μm. C, collagen content is presented as collagen volume fraction calculated from PSR-stained sections. D, TaqMan quantification of RNA level of collagen type I, type III, and TIMP1 normalized to 18 S in WT and Timp3−/− hearts in sham and at 3 weeks post-aortic banding. E, trichrome and α-smooth muscle actin (α-SMA) staining show co-localization of collagen deposit (in blue) and fibroblast activation (in brown), respectively, into fibrotic areas in Timp3−/− hearts after 3 weeks post-AB. In contrast, WT tissue shows a limited α-smooth muscle actin immunostaining around blood vessels, as shown by arrows, and absence of tissue fibrosis. Scale bar, 400 μm. *, p < 0.05 compared with the corresponding sham groups; ‡, p < 0.05 compared with WT-AB.
Most intriguingly, we found that Timp3 heterozygous mice, in which Timp3 expression is reduced by 50%, also showed abundant fibrosis at 3 and 6 weeks post-AB (Fig. 1A and supplemental Fig. S2). Comparison of heart function among WT, Timp3+/−, and Timp3−/− mice (supplemental Fig. S3) revealed that the loss of one Timp3 allele is sufficient to accelerate the development of dilated cardiomyopathy. Activated fibroblasts are generally responsible for producing collagens (34). Immunostaining of cardiac tissue sections showed that α-smooth muscle actin-positive fibroblasts were far more abundant and localized to fibrotic lesions in Timp3−/−-AB tissue. As expected, α-smooth muscle actin was also seen in vessel walls (Fig. 1E). Overall, Timp3−/− mice offered a novel model to study the molecular mechanisms of myocardial fibrosis in response to injury.
Microarray Analysis Reveals Temporal Shifts in Pro- and Anti-inflammatory Pathways in Timp3−/− Hearts
WT mice are in the compensatory stage of disease at 3 weeks and do not develop heart failure until about 15 weeks after aortic banding, whereas Timp3−/− mice decompensate to severe left ventricular dilation and dysfunction by 3 weeks, showing overt signs of heart failure by 6 weeks (supplemental Fig. S3) (25). We used Affymetrix mouse gene chip 430A 2.0 to map the global gene expression in WT and Timp3−/− left ventricle tissue at 6 h and at 1 and 3 weeks post-AB (n = 3 mice/group/genotype). The base-line control was provided by three independent sham-operated mice of each genotype. In WT hearts, ∼400 genes were altered by >2-fold at 6 h and 3 weeks, with a greater number being altered at 1 week (1380 genes), whereas Timp3−/− myocardium displayed a difference in 564 genes at 1 week but >1000 genes at both 6 h and 3 weeks post-AB.
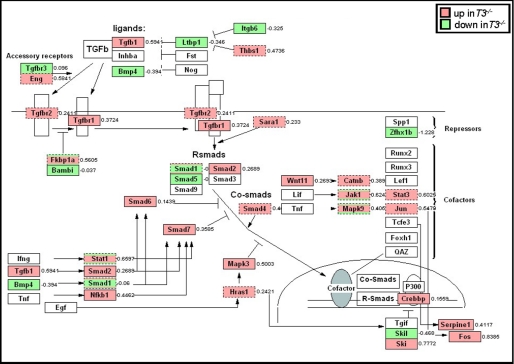
We then used data mining software programs (Ingenuity, GeneMAPP, and Gazer) to identify statistically different gene ontology groups and signaling pathways induced by aortic banding. Genes that were either significantly altered by >2-fold or functionally co-regulated, showing a smaller change, were further considered. Time series analysis using Ingenuity showed elevated gene expression of several molecules at 6 h in Timp3−/− hearts (supplemental Fig. S4), which are known to participate in proinflammatory signaling (35, 36). Consistent with this, we have previously demonstrated the critical role of increased TNF bioactivity in Timp3−/−-AB myocardium (25). Gene set enrichment analysis via Gazer revealed an aberration of the anti-inflammatory TGFβ1 signaling in Timp3−/− mice. Up-regulation of the TGFβ1 signaling pathway, normally observed at 3 weeks in WT mice, was seen earlier at 6 h in Timp3−/− hearts (Fig. 2) (data not shown). Together, microarray data led us to hypothesize that the early and concurrent activation of pro- and anti-inflammatory cytokines may underlie the excessive fibrosis in Timp3−/− myocardium.
FIGURE 2.
Up-regulation of TGFβ signaling in Timp3−/−-AB hearts. Gene set enrichment analysis indicated TGFβ signaling pathway to be significantly up-regulated at 6 h after aortic banding. This was visualized by GenMAPP. Genes labeled in red were up-regulated, whereas those labeled in green were down-regulated in Timp3−/− hearts.
Timp3 Deficiency Enhances Fibrogenic Response That Requires Cardiomyocyte-Cardiofibroblast Interaction
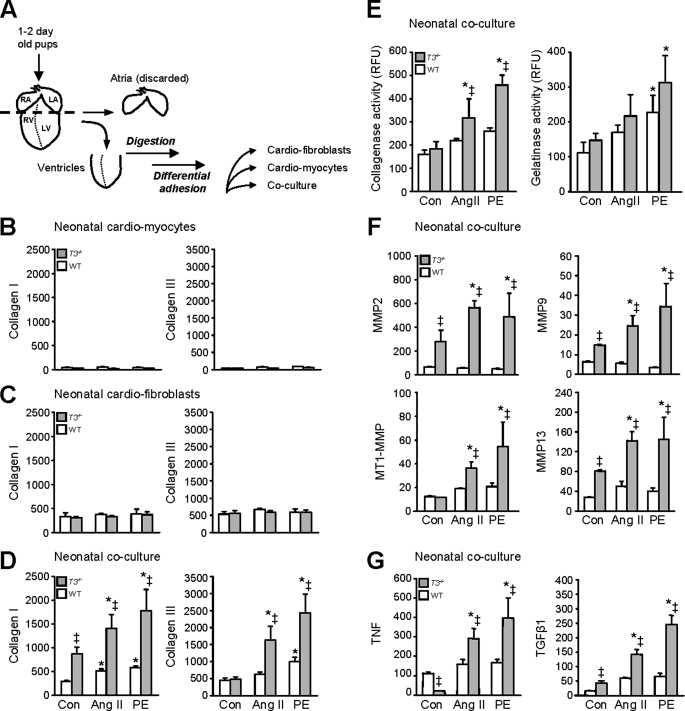
To examine if the TIMP3-deficient cardiac cells possess the capacity to generate a stronger fibrogenic response and to study the relationship between TIMP3 and cytokines during fibrosis under more controlled conditions, we generated cell cultures from neonatal hearts. Cardiomyocytes and cardiofibroblasts were prepared separately from neonatal (1–2-day-old) ventricles of WT or Timp3−/− mice (Fig. 3A) and treated with Ang II or PE for 24 h. Ang II is a well known humoral agonist critical in pathophysiology of fibrosis in heart diseases, including biomechanical stress or pressure overload (37, 38). PE is a pharmacological agonist of the α-adrenergic receptors. Stimulation of α-adrenergic receptors by catecholamines is a fundamental trigger in myocardial fibrosis and heart failure (39, 40). Collagen type I and III RNA expression was used as the readout of the fibrogenic response. The monocultures of cardiomyocytes (Fig. 3B) and cardiofibroblasts (Fig. 3C) of both genotypes did not show increased collagen expression in response to 1 μm PE or Ang II. Since myocytes and fibroblasts co-exist within the myocardium, and TIMP3 is a matrix-bound metalloproteinase inhibitor, we reasoned that co-cultures of these cell types may more closely mimic the in vivo scenario. Neonatal co-cultures of cardiomyocyte-cardiofibroblast were thus generated from WT or Timp3−/− mice, and these exhibited syncytial contraction (supplemental movies). Stimulation of WT co-cultures with Ang II or PE resulted in a small but significant induction of collagens I and III (Fig. 3D). Remarkably, TIMP3-deficient co-cultures that expressed more collagen I at base line responded with a greater increase in collagen I and III expression to these agonists. The fibrogenic response depended on cardiomyocyte-cardiofibroblast interaction and was significantly heightened by the loss of TIMP3.
FIGURE 3.
Timp3 deficiency enhances the fibrogenic response, which requires cardiomyocyte-cardiofibroblast interaction. A, schematic for isolation of neonatal mouse ventricular myocytes, fibroblasts, or their co-culture. Differential adhesion generates purified single cultures (see “Experimental Procedures”) that are verified by cell-specific markers (supplemental Fig. 1). All cultures were treated with Ang II (1 μm) or PE (1 μm) for 24 h. Collagen type I and type III RNA levels were measured by qPCR in the indicated mono or co-culture setting (B–D). Total collagenase and gelatinase activities are expressed as relative fluorescent units (RFU) (E). Shown are qPCR measurements of RNA for MMP2, -9, and -13 and MT1-MMP (F) and for TNF and TGFβ1 (G) in the neonatal co-cultures. RNA values were normalized to 18 S and are expressed as relative units as described under “Experimental Procedures.” *, p < 0.05 compared with the corresponding control cultures; ‡, p < 0.05 compared with WT. RA, right auricle; LA, left auricle; RV, right ventricle; LV, left ventricle. Con, control.
We next tested whether the co-cultures showed characteristics of in vivo AB hearts. Specifically, Timp3−/−-AB hearts express higher RNA levels of interstitial MMPs than WT hearts and display greater gelatinase and collagenase activities (25). Collagenase activity was significantly higher in Timp3−/− co-cultures following treatment with PE or Ang II compared with WT co-cultures (Fig. 3E). qPCR measurements showed higher levels of MMP2, MMP9, MMP13, and MMP14 (MT1-MMP) that are known to degrade interstitial collagen in Timp3−/− co-cultures (Fig. 3F). Higher MMP2, MMP9, and MMP13 expression was also noted at base line in these co-cultures. We then determined the cytokine production in this culture system. Timp3−/− co-cultures showed base-line perturbations in TNF and TGFβ1 expression compared with WT, with both cytokines displaying far greater expression in response to the two fibrogenic agonists (Fig. 3G). Overall, the co-culture system allowed direct interaction and paracrine signaling between cardiomyocytes and cardiofibroblasts and recapitulated several critical features of fibrotic myocardial tissue, including expression of cytokines, metalloproteinases, and matrix molecules. In this setting, Timp3−/− cardiac cells possessed a general propensity for expressing molecules related to tissue remodeling, and the observed powerful fibrogenic response was associated with significantly higher co-expression of the two antagonistic cytokines, TNF and TGFβ1.
Increased TGFβ1 Processing and Smad Signaling in Timp3−/− Co-cultures
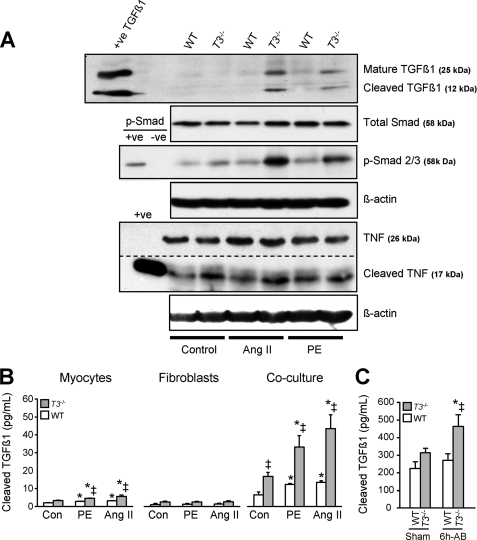
Having confirmed that co-cultures mimic the MMP, cytokine, and collagen production after fibrogenic stimuli as observed in Timp3−/− heart in vivo, we determined whether these co-cultures allowed increased TGFβ1 processing and signaling. Western blotting of cell lysates showed higher levels of mature full-length TGFβ1 in agonist-treated Timp3−/− co-cultures compared with WT co-cultures, consistent with increased TGFβ1 expression. In addition, we observed higher levels of cleaved TGFβ1 fragment, as well as phosphorylated Smad2/3, indicating greater activation of the TGFβ1 signaling pathway in agonist-treated Timp3−/− co-cultures (Fig. 4A). In parallel to increased TGFβ1 cleavage, we observed increased TNF conversion from its cell surface-bound 26-kDa form to the soluble 17-kDa form (Fig. 4A), as we had expected from our previous in vivo study (25). We also measured the levels of TGFβ1 released into the medium of mono- or co-cultures using an ELISA that recognizes the active cleaved TGFβ1 fragment. Stimulation with PE or Ang II caused a small but statistically significant increase of active TGFβ1 in cardiomyocyte cultures (Fig. 4B), whereas cardiomyocyte-cardiofibroblast co-cultures resulted in far greater TGFβ1 release into the medium at base line and after all treatments. Notably, Timp3−/− co-cultures had a 3-fold higher TGFβ1 level than observed in WT cells (Fig. 4B). Overall, these data provide direct evidence of increased processing of the two critical ligands, TGFβ1 and TNF, as a result of TIMP3 deficiency.
FIGURE 4.
Enhanced proteolytic processing of TGFβ1 and TNF, and activation of Smad signaling in Timp3−/− cultures. A, detection of mature and cleaved TGFβ1, total and phosphorylated Smad2/3, full-length and cleaved TNF, and the corresponding β-actin as the loading control, in Ang II (1 μm)-treated or PE (1 μm)-treated (24 h) co-cultures of the indicated genotypes. The dotted line indicates different exposures of the same blot. B, ELISA was performed to measure active TGFβ1 protein released into the conditioned media of neonatal cardiomyocytes, cardiofibroblasts, or co-cultures. C, in vivo levels of cleaved TGFβ1 protein measured by ELISA in tissue homogenates of WT and Timp3−/− hearts, 6 h after aortic banding. *, p < 0.05 compared with the corresponding sham groups; ‡, p < 0.05 compared with WT-AB.
We questioned whether TGFβ1 levels also differed between WT and Timp3−/− hearts in vivo following aortic banding. Measurement of TGFβ1 using ELISA on myocardial tissue homogenates showed significantly higher levels in Timp3−/− hearts at 6 h post-AB compared with WT hearts (Fig. 4C). This confirmed that TGFβ1 cleavage is an early in vivo event that temporally coincides with the increased processing of TNF, as we have previously reported in this disease model (25).
TGFβ1 Blocking Antibody Prevents Fibrosis in Timp3−/− Hearts
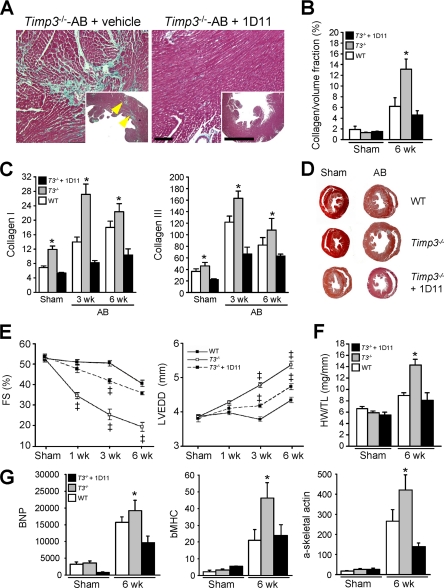
To investigate whether TGFβ1 is responsible for myocardial fibrosis in Timp3−/− mice, we utilized 1D11, a TGFβ1-neutralizing antibody that blocks TGFβ signaling in vitro and in vivo (41, 42). Administration of 1D11 in a model of unilateral urethral obstruction significantly prevented renal fibrosis in both mice (43) and rats (44). Aortic banded Timp3−/− mice treated with 1D11 (5 mg/kg, every 2 days, intraperitoneally) over 6 weeks showed a complete absence of fibrosis, as assessed by trichrome staining (Fig. 5A), collagen/volume fraction (Fig. 5B), and production of collagen types I and III (Fig. 5C); this treatment also reduced collagens type I and III expression in Timp3−/− sham hearts. Heart structure-function analysis showed that blocking TGFβ1 significantly improved the severe dilated cardiomyopathy otherwise seen in Timp3−/−-AB mice (Fig. 5, D and E). Specifically, left ventricular dilation was markedly improved, as was the fractional shortening, a measure of systolic function. In addition, the marked hypertrophy observed in TIMP3−/−-AB hearts (25) was significantly reduced, as measured by heart weight-to-tibial length ratio and by expression of molecular markers of hypertrophy (Fig. 5, F and G). Given that myocardial fibrosis is also closely linked to diastolic dysfunction, we examined diastolic function by the transmitral Doppler filling pattern. The E/AC ratio (corrected for heart rate) and E-wave deceleration rate were reduced in Timp3−/−-AB compared with WT-AB mice, suggesting diastolic dysfunction in the former group. Consistent with the absence of interstitial fibrosis described above, the E/AC ratio and E-wave deceleration rate were found to be normal in 1D11-treated Timp3−/−-AB mice (Table 2). Thus, blocking the TGFβ1 pathway by 1D11 prevented the development of fibrosis and diastolic dysfunction while significantly improving LV dilation and systolic dysfunction in Timp3−/−-AB mice.
FIGURE 5.
Treatment with TGFβ-neutralizing antibody (1D11) prevents fibrosis and improves heart function in aortic banded Timp3−/− mice. A, trichrome staining of Timp3−/− hearts after 6 weeks (wk) post-AB, with or without 1D11 treatment. Scale bar, 100 μm. B, collagen volume fraction as a measure of myocardial fibrosis. C, qPCR of RNA for collagen types I and III, normalized to 18 S. D, transverse cross-sections of sham and aortic banded hearts 6 weeks after AB. E, heart function measurements performed as fractional shortening (FS) and LV end-diastolic dimension (LVEDD) in sham and 1, 3, and 6 weeks after aortic banding. F, heart weight-to-tibial length ratio (HW/TL). G, qPCR of RNA for molecular markers of hypertrophy, brain natriuretic peptide (BNP), β-myosin heavy chain (β-MHC), and α-skeletal actin in sham or aortic banded WT, Timp3−/−, and 1D11-treated hearts after 6 weeks. *, p < 0.05 compared with the 1D11-treated group. ‡, p < 0.05 compared with WT-AB.
TABLE 2.
Echocardiography parameters in AB Timp3−/− mice treated with 1D11, TGFβ−blocking antibody
AB, aortic banding; HR (beats/min), heart rate; LVEDD, LVESD (mm), left ventricular end diastolic and systolic dimension, respectively; LVEDI (mm/mg), left ventricular end diastolic index (= LVEDD/body weight); FS, fractional shortening (= LVEDD − LVESD)/LVEDD × 100%; VCFc (circumference/s), velocity of circumferential shortening corrected for HR = FS/ETc; PAVc (cm/s), peak aortic velocity corrected for HR.
| 3 weeks post-AB |
6 weeks post-AB |
|||||
|---|---|---|---|---|---|---|
| WT | Timp3−/− + vehicle | Timp3−/− + 1D11 | WT | Timp3−/− + vehicle | Timp3−/− + 1D11 | |
| n | 8 | 8 | 8 | 8 | 8 | 8 |
| HR (beats/min) | 535 ± 7 | 544 ± 9 | 554 ± 10 | 542 ± 9 | 537 ± 15 | 551 ± 12 |
| PWT (mm) | 0.84 ± 0.05 | 0.82 ± 0.03 | 0.63 ± 0.04a | 0.8 ± 0.04 | 0.89 ± 0.05 | 0.75 ± 0.03a |
| LVEDD (mm) | 3.77 ± 0.1a | 4.8 ± 0.1 | 4.08 ± 0.09a | 4.35 ± 0.08a | 5.41 ± 0.12 | 4.74 ± 0.05 |
| LVESD (mm) | 1.78 ± 0.1a | 3.56 ± 0.2 | 2.3 ± 0.1a | 2.6 ± 0.09a | 4.35 ± 0.24 | 3.015 ± 0.06a |
| FS (%) | 52.8 ± 1.3a | 25.8 ± 2.2 | 42.9 ± 1.2a | 40.2 ± 1.7a | 19.6 ± 2.2 | 36.2 ± 0.72a |
| VCFc (circumference/s) | 9.78 ± 0.3a | 4.62 ± 0.5 | 8.71 ± 0.4a | 7.83 ± 0.27a | 3.92 ± 0.3 | 7.12 ± 0.32a |
| PAVc (cm/s) | 95.7 ± 2.3a | 70.9 ± 2.1 | 99.1 ± 1.2a | 84.9 ± 2.4a | 66.3 ± 3.1 | 97.5 ± 1.80a |
| E/Ac ratio | 2.52 ± 0.31a | 1.78 ± 0.33 | 2.45 ± 0.27a | 2.13 ± 0.13a | 1.43 ± 0.33 | 2.08 ± 0.15a |
| EDR (cm/s2) | 2.68 ± 0.23a | 2.01 ± 0.17 | 2.59 ± 0.21a | 2.44 ± 0.11a | 1.98 ± 0.09 | 2.29 ± 0.20a |
p < 0.05 compared with Timp3−/−-AB.
Ligand Cross-talk between TGFβ1 and TNF in Timp3−/− Hearts
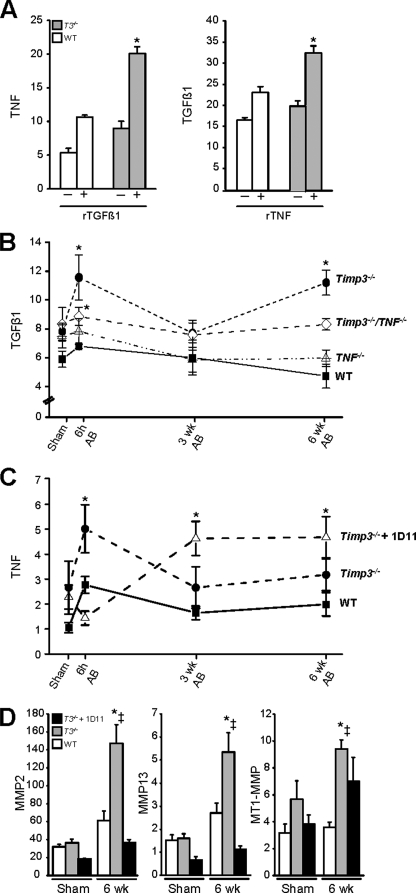
We have previously found that ADAM17, TNF, and MMP activities are increased in Timp3−/−-AB mice and that the combined inhibition of these pathways (achieved by TNF deletion plus a synthetic MMP inhibitor) prevents dilated cardiomyopathy and early heart failure in Timp3−/−-AB mice (25). However, in the current study, treatment with a TGFβ1 blocker alone was sufficient to inhibit fibrosis and significantly improve the cardiac structure and function. This striking improvement prompted us to study the relationship between TGFβ1 and TNF further. We tested whether each cytokine directly affected the expression of the other by treating WT or Timp3−/− co-cultures with recombinant TNF or recombinant TGFβ1 protein. Each cytokine induced the production of the other within 1 h of stimulation, although this increase was statistically significant only in Timp3−/− co-cultures (Fig. 6A).
FIGURE 6.
Dissecting TGFβ1-TNF link through genetic and biochemical manipulations. A, RNA expression of TNF and TGFβ in cardiomyocyte-cardiofibroblast cultures derived from WT and Timp3−/− hearts treated with recombinant TGFβ1 (10 ng/ml) or recombinant TNF (10 ng/ml) for 1 h. Changes in TGFβ1 expression as measured by qPCR in mouse hearts of the indicated genotypes after aortic banding (B) and in TNF RNA in Timp3−/−-AB hearts treated with or without 1D11 (C). D, qPCR measurement of RNA for MMP2, MMP13, and MT1-MMP at 6 weeks (wk) post-AB in 1D11-treated Timp3−/− mice compared with untreated Timp3−/− and WT mice. *, p < 0.05 compared with their corresponding sham; ‡, p < 0.05 compared with WT-AB.
To determine whether these cytokines reciprocally affected each other in vivo, we compared TGFβ1 expression in WT, Timp3−/−, Tnf−/−, and Timp3−/−/Tnf−/− mice and TNF expression in WT, Timp3−/−, and 1D11-treated Timp3−/− mice at 6 h and 3 and 6 weeks post-AB as well as in sham groups. Alterations in TGFβ1 expression after aortic banding were similar in our two control groups, WT and Tnf−/−. TGFβ1 RNA was significantly elevated in Timp3−/− hearts compared with WT, consistent with the microarray and co-culture data. However, the increase in TGFβ1 at 6 h was significantly lower in Timp3−/−/Tnf−/− mice compared with Timp3−/− mice, demonstrating that the lack of TNF signaling interferes with the rise in TGFβ1 RNA level (Fig. 6B). Conversely, the dependence of TNF production on TGFβ1 was far greater, since 1D11 pretreatment for 1 week completely prevented the increase in TNF normally seen in Timp3−/− mice at 6 h post-AB (Fig. 6C). TNF levels did eventually rise by 3 and 6 weeks in 1D11-treated Timp3−/−-AB mice, although the expression of MMP2 and MMP13 (but not MT1-MMP/MMP14) remained low at 6 weeks (Fig. 6D), and dilated cardiomyopathy remained improved (Fig. 5). Overall, TGFβ1 and TNF induce the expression of one another in co-cultures, and the loss of TIMP3 amplifies these responses. Blocking each of these cytokines in vivo greatly reduces the induction of the other, prevents fibrosis, and improves heart function. These data indicate an important role of TIMP3 in co-regulating TGFβ1 and TNF.
DISCUSSION
Aortic constriction is a well established experimental model to generate cardiac pressure overload (mechanical stress), representing the condition in patients with hypertension or aortic stenosis. Such tissue perturbation must invoke a coordinated response between cellular and stromal compartments through the interplay of cytokines that are regulated both in time and space. We show that TIMP3 is a common stromal regulator of critical proinflammatory (TNF) and anti-inflammatory (TGFβ1) cytokines involved in this process, and its loss initiates a distinct gene expression program after aortic banding. The concurrent increase of these two cytokines occurs at the levels of RNA expression and protein maturation involving proteolytic cleavage, leading to their concomitant bioavailability as seen through the activation of their signal transduction pathways in TIMP3 null mice. Their reciprocal induction generates an aberrant cross-talk responsible for the unconstructive tissue remodeling, which culminates in myocardial fibrosis, dysfunction, and heart failure.
Microarray analyses to map changes in global gene expression suggested an early involvement of TGFβ1 in Timp3−/− mice after aortic banding. To test directly the hypothesis that TGFβ1 is responsible for fibrosis in TIMP3-deficient mice, we established a well controlled in vitro system of primary neonatal murine cardiomyocytes and cardiofibroblasts that were cultured separately or in combination. This system mimics the cellular environment and in vivo responses for expression of cytokines, MMPs, and matrix molecules. We demonstrate that the cardiomyocyte-cardiofibroblast interaction is required for a strong fibrogenic response and that Timp3−/− co-cultures have the capacity to generate a more potent fibrogenic response compared with WT co-cultures in response to agonists. Using rat neonatal co-cultures, another group has shown the importance of fibroblast-myocyte interaction for Ang II-induced collagen production (30). Furthermore, we show that co-cultures are far more efficient at TGFβ production and release. The molecular mechanism underlying the severe phenotype associated with TIMP3 deficiency includes increased transcription as well as proteolytic processing of TGFβ1 and TNF, heightened Smad2/3 phosphorylation, and up-regulation of specific metalloproteinases capable of cleaving interstitial collagens. Additionally, the co-culture setting reveals base-line perturbations in expression of collagen I, MMPs, and cytokines, indicating altered propensity toward matrix remodeling upon loss of TIMP3.
Since a neonatal culture system bears limitations, we confirmed the role of TGFβ in the severe myocardial fibrosis in Timp3−/− hearts in vivo. The removal of TNF through the use of Tnf−/− mice or blocking TGFβ1 by 1D11 antibody allowed us to dissect the importance of each cytokine. TGFβ1 was still somewhat induced in Timp3−/−/Tnf−/− mice, whereas TGFβ1-neutralizing antibody profoundly suppressed TNF induction after aortic banding, indicating that the TGFβ1 effects may supersede those of TNF. Although TNF levels rise at later points in these mice, the MMP expressions remain suppressed, with the cardiomyopathy remaining significantly improved at 3 and 6 weeks post-AB. This could suggest that the initial rise in TNF (in the presence of TGFβ) in Timp3−/− heart is the key event in triggering the disease initiation and progression and involves MMP elevation. These data collectively show that severity of the cardiac phenotype resulting from the loss of TIMP3 is due to its simultaneous impact on two opposing cytokines, one anti-inflammatory and the other proinflammatory. It disrupts their time of action, the magnitude of increase, and co-regulation, resulting in maladaptive tissue remodeling that leads to fibrosis.
TGFβ1 is a key regulator of inflammation and fibrosis and is itself regulated at several levels. TGFβ1 null mice develop a multifocal inflammatory disorder and die shortly after weaning (45, 46), whereas TGFβ1 overexpression results in kidney, liver, and lung fibrosis (47, 48). In humans, TGFβ levels are increased in patients with fibrotic kidney disease, idiopathic pulmonary fibrosis, and hepatic fibrosis (49). Plasma levels of TGFβ1 are twice as high in patients who have developed idiopathic dilated cardiomyopathy compared with controls (50). TGFβ1 polymorphisms affect its level of expression and can play a role in predisposition to fibrotic disease (49, 51). For instance, a polymorphism that increases TGFβ1 production has been linked to the development of fibrotic lung disease (52), and one that correlates with elevated circulating levels of TGFβ1 is associated with dilated cardiomyopathy (53, 54). The current study reveals yet another level of regulation for the ECM-bound TGFβ1. Its bioavailability is controlled by the proteolytic release mediated by TIMP3-sensitive metalloproteinase(s), with the loss of TIMP3 resulting in myocardial fibrosis, diastolic dysfunction, and dilated cardiomyopathy. Interruption of the TGFβ pathway by an anti-TGFβ antibody (1D11) prevented myocardial fibrosis. Echocardiographic assessment of diastolic function mirrored the changes in systolic function; the diastolic filling parameters were more affected in Timp3−/−-AB mice but exhibited a marked improvement following TGFβ blockade. The improvement in diastolic dysfunction in these mice could be partly due to improved systolic function. These results are consistent with a pivotal role of maladaptive cytokine signaling in mediating systolic and diastolic dysfunctions.
Myocardial fibrosis causes reduced compliance, compromised LV filling, and diastolic heart failure (55–57). Fibrosis is a characteristic of hypertensive, hypertrophic, and dilated cardiomyopathy. A recent study reported the occurrence of interstitial fibrosis in dilated left ventricles even when no evidence of ischemic heart disease was observed (58). Further, TIMP3 levels are significantly reduced in patients with dilated cardiomyopathy and heart failure (59), and as we report here, even the loss of a single TIMP3 allele in mice leads to myocardial fibrosis. Thus, TIMP3 can be a powerful therapeutic candidate in strategies aimed at blocking myocardial fibrosis at early stages of heart disease.
Supplementary Material
Acknowledgments
We thank Aditya Murthy and Dr. Paul Waterhouse for critical review of the manuscript.
This work was supported by funding from the Heart and Stroke Foundation (to R. K.) and a Tailored Advanced Collaborative Training in Cardiovascular Sciences fellowship (to Z. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Movies 1–3.
- ECM
- extracellular matrix
- Ang II
- angiotensin II
- PE
- phenylephrine
- TGFβ
- transforming growth factor β
- TNF
- tumor necrosis factor
- MMP
- matrix metalloproteinase
- AB
- aortic banding
- WT
- wild type
- PSR
- Picro-Sirius Red
- ELISA
- enzyme-linked immunosorbent assay
- CT
- cycle threshold
- qPCR
- quantitative PCR.
REFERENCES
- 1.Zile M. R., Baicu C. F., Gaasch W. H. (2004) N. Engl. J. Med. 350, 1953–1959 [DOI] [PubMed] [Google Scholar]
- 2.Katz A. M., Zile M. R. (2006) Circulation 113, 1922–1925 [DOI] [PubMed] [Google Scholar]
- 3.Herpel E., Pritsch M., Koch A., Dengler T. J., Schirmacher P., Schnabel P. A. (2006) Histopathology 48, 736–747 [DOI] [PubMed] [Google Scholar]
- 4.Spinale F. G. (2007) Physiol. Rev. 87, 1285–1342 [DOI] [PubMed] [Google Scholar]
- 5.Stramer B. M., Mori R., Martin P. (2007) J. Invest. Dermatol. 127, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto H., Grahovac G., Zeisberg M., Kalluri R. (2007) Diabetes 56, 1825–1833 [DOI] [PubMed] [Google Scholar]
- 7.Yu Q., Stamenkovic I. (2000) Genes Dev. 14, 163–176 [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy G., Murthy A., Khokha R. (2008) Trends Immunol. 29, 75–82 [DOI] [PubMed] [Google Scholar]
- 9.Gauldie J., Bonniaud P., Sime P., Ask K., Kolb M. (2007) Biochem. Soc. Trans. 35, 661–664 [DOI] [PubMed] [Google Scholar]
- 10.Stawowy P., Kallisch H., Veinot J. P., Kilimnik A., Prichett W., Goetze S., Seidah N. G., Chrétien M., Fleck E., Graf K. (2004) Circulation 109, 770–776 [DOI] [PubMed] [Google Scholar]
- 11.Petrov V. V., Fagard R. H., Lijnen P. J. (2002) Hypertension 39, 258–263 [DOI] [PubMed] [Google Scholar]
- 12.Asano Y., Ihn H., Yamane K., Kubo M., Tamaki K. (2004) J. Clin. Invest. 113, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overall C. M., Wrana J. L., Sodek J. (1991) J. Biol. Chem. 266, 14064–14071 [PubMed] [Google Scholar]
- 14.Hyytiäinen M., Penttinen C., Keski-Oja J. (2004) Crit. Rev. Clin. Lab. Sci. 41, 233–264 [DOI] [PubMed] [Google Scholar]
- 15.Mu D., Cambier S., Fjellbirkeland L., Baron J. L., Munger J. S., Kawakatsu H., Sheppard D., Broaddus V. C., Nishimura S. L. (2002) J. Cell Biol. 157, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins G. (2008) Int. J. Biochem. Cell Biol. 40, 1068–1078 [DOI] [PubMed] [Google Scholar]
- 17.Stawowy P., Margeta C., Kallisch H., Seidah N. G., Chrétien M., Fleck E., Graf K. (2004) Cardiovasc. Res. 63, 87–97 [DOI] [PubMed] [Google Scholar]
- 18.Inagaki Y., Truter S., Tanaka S., Di Liberto M., Ramirez F. (1995) J. Biol. Chem. 270, 3353–3358 [DOI] [PubMed] [Google Scholar]
- 19.Bitzer M., von Gersdorff G., Liang D., Dominguez-Rosales A., Beg A. A., Rojkind M., Böttinger E. P. (2000) Genes Dev. 14, 187–197 [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh A. K., Varga J. (2007) J. Cell. Physiol. 213, 663–671 [DOI] [PubMed] [Google Scholar]
- 21.Roman-Blas J. A., Stokes D. G., Jimenez S. A. (2007) Osteoarthritis Cartilage 15, 1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg M. T., Han Y. P., Yan C., Shaw M. C., Garner W. L. (2007) J. Invest. Dermatol. 127, 2645–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed F. F., Smookler D. S., Taylor S. E., Fingleton B., Kassiri Z., Sanchez O. H., English J. L., Matrisian L. M., Au B., Yeh W. C., Khokha R. (2004) Nat. Genet. 36, 969–977 [DOI] [PubMed] [Google Scholar]
- 24.Smookler D. S., Mohammed F. F., Kassiri Z., Duncan G. S., Mak T. W., Khokha R. (2006) J. Immunol. 176, 721–725 [DOI] [PubMed] [Google Scholar]
- 25.Kassiri Z., Oudit G. Y., Sanchez O., Dawood F., Mohammed F. F., Nuttall R. K., Edwards D. R., Liu P. P., Backx P. H., Khokha R. (2005) Circ. Res. 97, 380–390 [DOI] [PubMed] [Google Scholar]
- 26.Leco K. J., Waterhouse P., Sanchez O. H., Gowing K. L., Poole A. R., Wakeham A., Mak T. W., Khokha R. (2001) J. Clin. Invest. 108, 817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavoie P., Robitaille G., Agharazii M., Ledbetter S., Lebel M., Larivière R. (2005) J. Hypertens. 23, 1895–1903 [DOI] [PubMed] [Google Scholar]
- 28.Kassiri Z., Zobel C., Nguyen T. T., Molkentin J. D., Backx P. H. (2002) Circ. Res. 90, 578–585 [DOI] [PubMed] [Google Scholar]
- 29.Ju H., Dixon I. M. (1996) Mol. Cell Biochem. 163, 231–237 [DOI] [PubMed] [Google Scholar]
- 30.Pathak M., Sarkar S., Vellaichamy E., Sen S. (2001) Hypertension 37, 833–840 [DOI] [PubMed] [Google Scholar]
- 31.Nuttall R. K., Sampieri C. L., Pennington C. J., Gill S. E., Schultz G. A., Edwards D. R. (2004) FEBS Lett. 563, 129–134 [DOI] [PubMed] [Google Scholar]
- 32.Young D. A., Phillips B. W., Lundy C., Nuttall R. K., Hogan A., Schultz G. A., Leco K. J., Clark I. M., Edwards D. R. (2002) Biochem. J. 364, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swiderski R. E., Dencoff J. E., Floerchinger C. S., Shapiro S. D., Hunninghake G. W. (1998) Am. J. Pathol. 152, 821–828 [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkranz S. (2004) Cardiovasc. Res. 63, 423–432 [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal B. B. (2003) Nat. Rev. Immunol. 3, 745–756 [DOI] [PubMed] [Google Scholar]
- 36.O'Shea J. J., Murray P. J. (2008) Immunity 28, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aras O., Dilsizian V. (2008) Curr. Cardiol. Rep. 10, 128–134 [DOI] [PubMed] [Google Scholar]
- 38.Billet S., Aguilar F., Baudry C., Clauser E. (2008) Kidney Int. 74, 1379–1384 [DOI] [PubMed] [Google Scholar]
- 39.Osadchii O. E. (2007) Heart Fail. Rev. 12, 66–86 [DOI] [PubMed] [Google Scholar]
- 40.Swynghedauw B. (1999) Physiol. Rev. 79, 215–262 [DOI] [PubMed] [Google Scholar]
- 41.Graham C. H., Lysiak J. J., McCrae K. R., Lala P. K. (1992) Biol. Reprod. 46, 561–572 [DOI] [PubMed] [Google Scholar]
- 42.Jester J. V., Barry-Lane P. A., Petroll W. M., Olsen D. R., Cavanagh H. D. (1997) Cornea 16, 177–187 [PubMed] [Google Scholar]
- 43.Yu L., Border W. A., Anderson I., McCourt M., Huang Y., Noble N. A. (2004) Kidney Int. 66, 1774–1784 [DOI] [PubMed] [Google Scholar]
- 44.El Chaar M., Chen J., Seshan S. V., Jha S., Richardson I., Ledbetter S. R., Vaughan E. D., Jr., Poppas D. P., Felsen D. (2007) Am. J. Physiol. Renal Physiol. 292, F1291–F1301 [DOI] [PubMed] [Google Scholar]
- 45.Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. (1992) Nature 359, 693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopp J. B., Factor V. M., Mozes M., Nagy P., Sanderson N., Böttinger E. P., Klotman P. E., Thorgeirsson S. S. (1996) Lab. Invest. 74, 991–1003 [PubMed] [Google Scholar]
- 48.Sanderson N., Factor V., Nagy P., Kopp J., Kondaiah P., Wakefield L., Roberts A. B., Sporn M. B., Thorgeirsson S. S. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2572–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blobe G. C., Schiemann W. P., Lodish H. F. (2000) N. Engl. J. Med. 342, 1350–1358 [DOI] [PubMed] [Google Scholar]
- 50.Kühl U., Noutsias M., Schultheiss H. P. (1995) Eur. Heart J. 16, Suppl. O, 100–106 [DOI] [PubMed] [Google Scholar]
- 51.Li B., Khanna A., Sharma V., Singh T., Suthanthiran M., August P. (1999) Hypertension 33, 271–275 [DOI] [PubMed] [Google Scholar]
- 52.Awad M. R., El-Gamel A., Hasleton P., Turner D. M., Sinnott P. J., Hutchinson I. V. (1998) Transplantation 66, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 53.Holweg C. T., Baan C. C., Niesters H. G., Vantrimpont P. J., Mulder P. G., Maat A. P., Weimar W., Balk A. H. (2001) J. Heart Lung Transplant. 20, 979–984 [DOI] [PubMed] [Google Scholar]
- 54.Yamada Y., Miyauchi A., Goto J., Takagi Y., Okuizumi H., Kanematsu M., Hase M., Takai H., Harada A., Ikeda K. (1998) J. Bone Miner. Res. 13, 1569–1576 [DOI] [PubMed] [Google Scholar]
- 55.Mandinov L., Eberli F. R., Seiler C., Hess O. M. (2000) Cardiovasc. Res. 45, 813–825 [DOI] [PubMed] [Google Scholar]
- 56.Senni M., Redfield M. M. (2001) J. Am. Coll. Cardiol. 38, 1277–1282 [DOI] [PubMed] [Google Scholar]
- 57.Hogg K., Swedberg K., McMurray J. (2004) J. Am. Coll. Cardiol. 43, 317–327 [DOI] [PubMed] [Google Scholar]
- 58.Brooks A., Schinde V., Bateman A. C., Gallagher P. J. (2003) Heart 89, 1255–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y. Y., Feldman A. M., Sun Y., McTiernan C. F. (1998) Circulation 98, 1728–1734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.