Abstract
Activation of fibroblast growth factor (FGF) signaling is initiated by a multiprotein complex formation between FGF, FGF receptor (FGFR), and heparan sulfate proteoglycan on the cell membrane. Cross-talk with other factors could affect this complex assembly and modulate the biological response of cells to FGF. We have previously demonstrated that anosmin-1, a glycosylated extracellular matrix protein, interacts with the FGFR1 signaling complex and enhances its activity in an IIIc isoform-specific and HS-dependent manner. The molecular mechanism of anosmin-1 action on FGFR1 signaling, however, remains unknown. Here, we show that anosmin-1 directly binds to FGFR1 with high affinity. This interaction involves domains in the N terminus of anosmin-1 (cysteine-rich region, whey acidic protein-like domain and the first fibronectin type III domain) and the D2–D3 extracellular domains of FGFR1. In contrast, anosmin-1 binds to FGFR2IIIc with much lower affinity and displays negligible binding to FGFR3IIIc. We also show that FGFR1-bound anosmin-1, although capable of binding to FGF2 alone, cannot bind to a FGF2·heparin complex, thus preventing FGFR1·FGF2·heparin complex formation. By contrast, heparin-bound anosmin-1 binds to pre-formed FGF2·FGFR1 complex, generating an anosmin-1·FGFR1·FGF2·heparin complex. Furthermore, a functional interaction between anosmin-1 and the FGFR1 signaling complex is demonstrated by immunofluorescence co-localization and Transwell migration assays where anosmin-1 was shown to induce opposing effects during chemotaxis of human neuronal cells. Our study provides molecular and cellular evidence for a modulatory action of anosmin-1 on FGFR1 signaling, whereby binding of anosmin-1 to FGFR1 and heparin can play a dual role in assembly and activity of the ternary FGFR1·FGF2·heparin complex.
FGF5 signaling plays an important role in a wide range of fundamental biological responses (1–3). Both FGF and FGFR bind to heparan sulfate (HS) and heparin, a highly sulfated type of HS produced in connective tissue mast cells. Heparan sulfate proteoglycans (HSPG) are the cell surface co-receptors essential for the formation of functional FGF·FGFR signaling complex (4, 5). There are four structurally related FGFRs (FGFR1–4), which consist of an extracellular ligand-binding region containing three immunoglobulin (Ig)-like domains (D1–D3), a single transmembrane domain, and a cytoplasmic domain with protein-tyrosine kinase catalytic activity. The 22 members of the FGF family bind to the interface formed by the D2/D3 domains and the linker between these domains (6, 7), whereas a conserved positively charged region in D2 serves as the HS binding site (8). An unusual stretch of seven to eight acidic residues designated as the “acid box” is present in the linker connecting D1 and D2. Alternative splicing events occur to generate various isoforms, including a truncated receptor lacking D1 and the D1–D2 linker or a full-length receptor that differs in the second half of D3, designated as IIIb and IIIc isoforms (5). Two crystal structures have been proposed to demonstrate how the FGF·FGFR·heparin complex is assembled (9, 10). Recent evidence suggests that both may be biologically relevant (11, 12).
The diversity of FGF signaling pathways and consequent biological functions require that activation of FGFR should be tightly regulated. Such regulation can occur either at the level of the extracellular receptor-ligand complex assembly or via intracellular modulation of downstream effectors (13). Extracellular regulation mainly involves the interaction between each component of the FGF·FGFR·HS signaling complex. For example, FGF8 is shown to bind mostly to the FGFR IIIc isoforms, whereas FGF7 acts as the preferential ligand for the FGFR2 IIIb isoform (13, 14). Sequence specificity, length, and sulfation patterns of HS are also important regulators of the FGF·FGFR interaction (15, 16).
Cell surface proteins other than FGFs and HSPGs participate in FGFR signaling regulation. FLRT3 (a member of the fibronectin-leucine-rich transmembrane protein family) promotes FGF signaling and interacts with FGFR1 and FGFR4 via its extracellular fibronectin type III (FnIII) domain (17). Sef (similar expression to fgf genes) functions as an antagonist of FGF signaling in zebrafish. The two FnIII regions of Sef are essential for its function and interaction with FGFR1 and FGFR2 (18). Neuronal cell adhesion molecule (NCAM), N-cadherin, and L1 have also been identified as functionally relevant in FGFR-mediated neurite outgrowth (19–22). The FnIII domains of NCAM bind to the D2 and D3 domains of FGFR1 (19) and FGFR2 (23) to induce ligand-independent receptor phosphorylation.
Anosmin-1, an extracellular matrix-associated glycosylated protein, appears to be a novel member of the extracellular FGFR signaling modulators (24, 25). Loss-of-function mutations of anosmin-1 and FGFR1 are associated with Kallmann syndrome (KS), underlying X-linked, and autosomal dominant/recessive inheritance mode, respectively (26–28). KS is a human developmental genetic disorder characterized by loss of sense of smell (anosmia) caused by abnormal olfactory bulb development and delayed, even arrested puberty caused by disrupted migration of the gonadotropin-releasing hormone (GnRH)-secreting neuron. We previously reported that anosmin-1 acts as an FGFR1IIIc isoform-specific co-ligand, which enhances signaling activity. In human embryonic GnRH olfactory neuroblast FNC-B4 cells, anosmin-1 induced neurite outgrowth and cytoskeletal rearrangements through FGFR1-dependent mechanisms involving p42/44 and p38 mitogen-activated protein kinases and Cdc42/Rac1 activation (25). A functional interaction is also demonstrable between anosmin-1 and FGFR1 in optic nerve oligodendrocyte precursor development (24). Structurally, anosmin-1 comprises an N-terminal cysteine-rich domain (CR) and a whey acidic protein-like (WAP) domain, followed by four tandem FnIII repeats and a C-terminal histidine rich region (Fig. 1a). Current evidence suggests that anosmin-1 functions by affecting FGF2-induced activation of FGFR1 signaling rather than by directly stimulating the receptor. However, the precise molecular mechanism of this interaction remains unclear.
FIGURE 1.
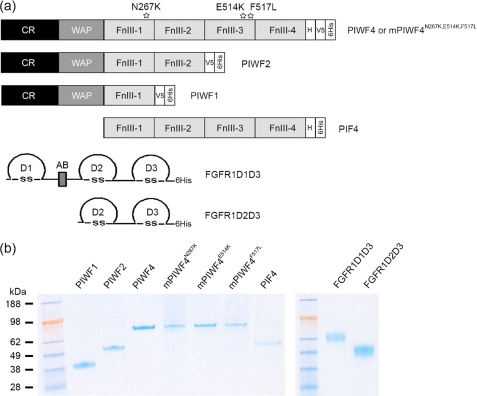
Generation of recombinant anosmin-1, anosmin-1 mutants, FGFR1D1D3, and FGFR1D2D3 proteins. a, the schematic structures of recombinant proteins of anosmin-1 and FGFR1. Each domain in the wild type (PIWF4), point mutants (mPIWF4N267K, mPIWF4E514K, and mPIWF4F517L), and truncated (PIWF1, PIWF2, and PIF4) anosmin-1 protein analogues are represented by a shaded rectangle. V5 and 6His epitopes at the C terminus are represented by a clear rectangle. Each immunoglobulin-like domain in the full ectodomain (FGFR1D1D3) and truncated form (FGFR1D2D3) of FGFR1 is represented by a half circle. The acid box (AB) is represented by a filled rectangle. H, histidine-rich region. b, 0.5–1 μg of purified recombinant proteins are loaded in each lane and visualized by colloidal blue staining. Molecular mass markers in kilodaltons are shown on the left.
We now report for the first time that anosmin-1 directly binds to FGFR1 using surface plasmon resonance (SPR), chemical cross-linking, and immunofluorescence co-localization studies in living cells. This interaction occurs between the N-terminal CR, WAP, and the first FnIII domain of anosmin-1 and D2 and D3 ectodomains of FGFR1. Moreover, SPR studies using sequential injections and Transwell migration assays in immortalized FNC-B4-hTERT cells suggest that anosmin-1 can have opposing effects in the formation and activation of the FGF2·FGFR1·heparin complex depending on the order of their binding interactions with anosmin-1.
EXPERIMENTAL PROCEDURES
Generation of Recombinant Protein Anosmin-1
We have previously described the generation of wild-type anosmin-1 (PIWF4) and its truncated forms comprising the CR and WAP domain followed by one or two FnIII repeats (designated as PIWF1 or PIWF2 (Fig. 1a)) in Drosophila S2 cells (29). To generate the PIF4 construct containing only the four FnIII domains, the corresponding cDNA coding sequence was amplified by PCR using the 5′-CCCGGATCCACTCTGTACAAAGGTGTCCCCC-3′ forward oligonucleotide primer with a 5′ BamHI restriction site and the 5′-CCCTCTAGATGGAGAAGGCTTGTAATGATGT-3′ reverse primer with a 3′ XbaI site. The amplified cDNA fragment was subcloned into a modified Drosophila expression vector PMT/BiP/6His in which the V5 epitope was removed from the original PMT/BiP/V5/6His vector (Invitrogen). Drosophila secretory signal is present at the 5′-end and the His6 epitope at the 3′-end. A QuikChange site-directed mutagenesis kit (Stratagene) was used to introduce the single amino acid substitution of N267K in the first FnIII domain and E514K and F517L substitution in the third FnIII domain of PIWF4, creating the three mutant constructs mPIWF4N267K, mPIWF4E514K, and mPIWF4F517L (Fig. 1a). All constructs were confirmed by DNA sequencing before being transfected into S2 cells, and recombinant proteins were generated according to previously described protocols (29). The schematic representation and purity of various recombinant anosmin-1 analogues are shown in Fig. 1.
Generation of Recombinant FGFR1D1D3 and FGFR1D2D3 Proteins
pCEP-Pu FGFR1 constructs encoding the D1–D3 or the D2–D3 ectodomains of human FGFR1 IIIc isoform (SwissProt entry P11362) have been described previously (30). For generation of FGFR1 ectodomain proteins (D1–D3 and D2–D3), 293-EBNA cells were cultured in Dulbecco's modified Eagle's medium/F-12 (Invitrogen) supplemented with 10% fetal calf serum and 250 μg/ml G418. After transfection with pCEP-Pu FGFR1 expression constructs, which are maintained episomally in these cells, stable cell lines were obtained by selection in medium containing 1 μg/ml puromycin (Sigma). Secreted recombinant proteins in the conditioned medium were purified using TALON metal affinity resins (Clontech). Purity of the proteins was confirmed by SDS-PAGE and colloidal blue staining (Invitrogen), and quantification of eluted proteins was measured by Bradford assay (Bio-Rad). The schematic domain structure and purity of recombinant FGFR1 D1D3 and D2D3 are shown in Fig. 1.
SPR Analysis of Anosmin-1 and FGFR Interactions
Binding analysis was performed using a BIAcore 3000 SPR-based biosensor (BIAcore AB) to quantify kinetic parameters for the interaction between anosmin-1 and recombinant FGFR1D1D3 and FGFR1D2D3 proteins. PIWF4 was immobilized onto a research grade CM4 chip (Biosensor AB, Uppsala, Sweden), whereas the truncated and mutated variants PIWF1, PIWF2, PIF4, mPIWF4N267K, mPIWF4E514K, and mPIWF4F517L were coupled onto research grade CM5 chips (Biosensor AB, Uppsala, Sweden). The CM4 chip is similar to sensor Chip CM5 but with a lower degree of carboxymethylation, which can improve sensitivity for certain interactions. FGFR1D1D3, FGFR1D2D3, FGFR2c, and FGFR3c (the recombinant FGFR2c and FGFR3c ectodomain proteins were generous gifts from Alan Brown (University of Cambridge)) were used as soluble analytes for the binding assays. Immobilization of anosmin-1 to the chips was through its primary amino groups using N-ethyl-N-(dimethyaminopropyl)carbodiimide/N-hydroxysuccinimide according to a standard amine-coupling protocol. Carboxymethyl groups on the chip surface were first activated using an injection pulse of 50 μl (flow rate, 5 μl/min) of an equimolar mix of N-ethyl-N-(dimethyaminopropyl)carbodiimide and N-hydroxysuccinimide (final concentration, 0.05 m; mixed immediately prior to injection). Following activation with N-ethyl-N-(dimethyaminopropyl)carbodiimide/N-hydroxysuccinimide, immobilization of anosmin-1 was achieved by applying diluted anosmin-1 (150–300 μg/ml) in HBS-EP buffer (0.01 m HEPES, 0.15 m NaCl, 3.4 mm EDTA, 0.005% polysorbate 20 (v/v)), pH 7.4) onto the activated chip surface. Excess unreacted sites on the sensor surface were deactivated with a 40-μl injection of 1 m ethanolamine. Successful immobilization was confirmed by the observation of a 3000–6000 response unit (RU) increase for most anosmin-1 constructs and 600-RU increase for PIF4. Three flow cells were coupled, and one flow cell contained a blank sensor chip serving as a reference surface.
Different concentrations of analytes (FGFR1D1D3, FGFR1D2D3, FGFR2c, and FGFR3c) in HBS-EP buffer were injected over the anosmin-1 sensor chip at a flow rate of 20 μl/min. At the end of each sample injection (240 s), HBS-EP buffer was passed over the sensor surface to monitor the dissociation phase. Following 240 s of dissociation, the sensor surface was fully regenerated by injection of 50 μl of 2 m NaCl in 100 mm sodium acetate buffer (pH 4). The bulk shift due to changes in refractive index was measured using a reference surface and was subtracted from the binding signal to correct for nonspecific signals. Sensorgrams and kinetic parameters generated were analyzed using BIA Evaluation software version 3.0.
SPR Analysis of Effects of Anosmin-1 on FGF2·FGFR1·Heparin Assembly by Sequential Injection
To investigate the role of anosmin-1 on the formation of the FGF2·FGFR1·heparin complex, SPR analysis was performed by sequential injections of FGFR1, FGF2, and heparin over a PIWF4-coupled chip. All experiments were carried out at 25 °C in HBS-EP buffer at a flow rate of 20 μl/min with the sample injection of 240s. In one experiment, FGFR1 was first injected over PIWF4 to form a PIWF4·FGFR1 complex on the sensor chip surface. FGFR1, either at a constant (250 nm) or at various concentrations, was first injected over PIWF4 at constant HBS-EP buffer flow in the dissociation phase to remove unbound FGFR1, leaving bound FGFR1 on the chip surface. This was then followed by the second injections of FGF2 at a constant (50 nm) or at various concentrations, heparin alone or preincubated FGF2·heparin at specified concentrations. To further analyze the effect of heparin on PIWF4·FGF2·FGFR1 complex assembly, various concentrations (0, 0.1, 1, and 10 μg/ml) of heparin were injected after the first injection of 250 nm FGFR1 and the second injection of 50 nm FGF2 over the PIWF4-coupled chip. The time lag within each injection was due to limitations in the BIAcore instrument. In another experiment, 10 μg/ml heparin was first injected over PIWF4-coupled chip resulting in heparin·PIWF4 complex on the chip surface, followed by subsequent second injections of 50 nm FGFR1, 50 nm FGF2 alone or preincubated FGFR1·FGF2 or FGFR1·FGF2·heparin complex.
Cross-linking Studies of Interaction between Anosmin-1 and FGFR1
Bis-sulfosuccinimidyl suberate (BS3) cross-linking experiments were carried out in a volume of 100 μl in phosphate-buffered saline. The mixture of PIWF4 and FGFR1D1D3 was incubated for 1 h at room temperature, and cross-linking of complex was initiated by adding BS3 (Pierce) to a final concentration of 0.01 mg/ml concentration, incubated for a further 15 min, and then quenched by adding 20 mm Tris, pH 7.5.
The photoactive cross-linking assay was carried out by using PIWF1 labeled with heterobifunctional cross-linker Sulfo-SANPAH containing an amine-reactive N-hydroxysuccinimide ester and a photoactivatable nitrophenyl azide. To label PIWF1, 10-fold molar excess of Sulfo-SANPAH (Pierce) was mixed with 3.25 μm PIWF1 and incubated at room temperature for 1 h. Non-reacted cross-linker was removed by dialysis overnight at 4 °C. Various amounts (0, 5, 10, and 15 μl) of Sulfo-SANPAH-labeled PIWF1 was mixed with 233 nm FGFR1 D1D3 in a volume of 15 μl for 1 h at room temperature. The mixture was then placed on ice, and photolysis was performed with a UV light source that irradiates at 365 nm at a distance of 5 cm for 15 min. The photoactivated samples were heat-denatured at 95 °C for 5 min prior to SDS-PAGE electrophoresis.
All cross-linked samples were then resolved by a 4–12% SDS-PAGE and analyzed by polyclonal anti-FGFR1 (H-76, Santa Cruz Biotechnology), monoclonal anti-V5 (Invitrogen), and anti-anosmin-1 polyclonal antibody raised against recombinant protein PIWF1 as previously described (29).
Generation of COS7 Cells Co-expressing FGFR1 and Anosmin-1-GFP
To generate the full-length FGFR1 IIIc expression construct with a C-terminal 3xMyc tag, the coding sequence was amplified by PCR primers introducing BamHI (5′-end) and SalI (3′-end) restriction sites and subcloned into pCMV-3Tag-9 vector (Stratagene). Anosmin-1-GFP was generated by PCR amplification of the full-length human KAL1 gene coding sequences, including the signal peptide, by using primers that introduced SalI (5′-end) and BamHI (3′-end) restriction sites. The digested PCR product was ligated to pEGFP-N1 vector to create a C-terminal green fluorescence protein (enhanced GFP). COS7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin in a 5% CO2 at 37 °C. FUGENE reagent (Roche Applied Science) was used for all transfections according to the manufacturer's protocol. To establish stably transfected anosmin-1-GFP linage, the transfected COS7 cells were selected in G418 (600 μg/ml)-containing medium for 2 weeks before being analyzed by immunofluorescence. To confirm whether the exogenous constructs were expressing the respective proteins, total cell lysates of the transfected COS7 cells were prepared in lysis buffer (1% Triton X-100, 50 mm Tris-HCl at pH 8.0, 150 mm NaCl) containing 1% aprotinin, 100 μg/ml phenylmethylsulfonyl fluoride, 10 mm sodium fluoride, 1 mm sodium orthovanadate, and 1 mm dithiothreitol, separated on SDS-PAGE, transferred onto nitrocellulose membrane, and probed with monoclonal antibodies against Myc-epitope (9E10) and green fluorescence protein (3E1), both from Cancer Research UK. Secondary antibody was peroxidase-labeled horse anti-mouse IgG (Vector Laboratories).
Immunofluorescence
The COS7 cells were plated on a coverslip in a 24-well culture plate 24–30 h after transfection. Once the cells were attached, depending on the experiment, the culture medium was changed to serum-free medium for 12 h before stimulation with 25 ng/ml FGF2 for 20 min. Cells were fixed in chilled 4% formaldehyde on ice, washed in phosphate-buffered saline and 10 mm ammonium chloride, and permeabilized with 0.05% Triton X-100. For staining of cell surface proteins, the permeabilization step was omitted. After blocking with 3% bovine serum albumin in phosphate-buffered saline, cells were incubated with rabbit polyclonal FGFR1 antibodies (C-15 or H-76, both from Santa Cruz Biotechnology), which were subsequently detected by goat anti-rabbit IgG conjugated with Alexa Fluor 555 (Invitrogen Molecular Probes). Hoechst (Invitrogen) was used for nuclear staining. Coverslips were mounted on slides using ProLong Gold Antifade (Invitrogen). Each experiment was repeated a minimum of three times, and the most representative images from random fields were shown. Confocal microscopy was performed using an Axiovert 200M/LSM 510 Meta laser-scanning confocal microscope (Zeiss, UK). All images were taken under a Zeiss Plan-Apochromat 40 × 1.3 numerical aperture oil-immersion objective and pinhole setting at 1.00 Airey unit. Twelve-bit single directional images were collected to peak by separate excitation of enhanced GFP, Alexa Fluor 555, and Hoechst using the 488, 543, and 405 nm lasers, respectively. All images shown are single confocal sections.
Telomerase-mediated Immortalization of FNC-B4 Cells
A primary neuroblast culture, FNC-B4, originated from human fetal olfactory epithelium, has been previously described (31). To establish an immortal derivative of FNC-B4 (designated as FNC-B4-hTERT), cells grown in F-12 Coon's modification medium supplemented with antibiotics and 10% fetal bovine serum were sequentially transduced with two different replication-defective retroviral vectors. First, pWXL-Neo-Eco, an amphotropic retroviral construct containing the mouse basic amino acid transporter (ecotropic receptor) and the neomycin resistance marker, was transfected into AM12 amphotropic retrovirus packaging cell line. After 48 h, the live virus stock was harvested to infect the FNC-B4 cells in the presence of Polybrene (4 μg/ml). After selection with G418 (400 μg/ml), the ecotropic receptor expressing FNC-B4 cells were further infected with pBabe-puro-hTERT, an ecotrophic retroviral vector containing the catalytic subunit of human telomerase and puromycin resistance, which had been produced in BOSC293 packaging cell line. After selection with puromycin (1 μg/ml), resistant colonies were pooled and regularly passaged until the cells overcame the cellular senescence and continued to grow, as compared with the pBabe-puro empty vector-infected cells, which ceased to proliferate (supplemental Fig. S1).
Cell Migration Assay
Migration assays were performed on a 24-well Transwell with 8.0-μm polycarbonate membrane (Corning Costar), which was coated with 0.2 mg/ml gelatin in phosphate-buffered saline. The lower compartment was loaded with either SFM, FGF2 (1 nm), PIWF4 alone (1, 10, or 50 nm), or 10 nm PIWF4 in the presence of 20 μm SU5402. In the top compartment, serum-starved FNC-B4-hTERT cells (5 × 104 cells in 200 μl of SFM) were plated and incubated at 37 °C in 5% CO2 for 4 h before quantification of the migrated cells. To generate HS-deficient FNC-B4-hTERT cells, 30 mm sodium chlorate was added in the culture medium throughout all processes. For the PIWF4·heparin functional interaction study (Fig. 9c), the lower compartment was loaded with SFM, 1 μg/ml heparin, 10 nm PIWF4 alone, or 10 nm PIWF4 plus varying concentrations of heparin, whereas the sodium chlorate-treated cells were loaded onto the Transwell insert. For the PIWF4/FGFR1 functional interaction study (Fig. 9d), sodium chlorate-treated cells were preincubated with SFM, 10 nm PIWF4, or 10 nm PIWF4 plus 10 nm FGFR1D1D3. After 1-h incubation, cells were gently spun down, resuspended in SFM and loaded onto the top chamber, while the mixture of 10 nm PIWF4 and 200 ng/ml heparin was loaded into the lower compartment. For this experiment, cells were incubated for 20 h to allow significant migration. Migrated cells on the bottom of the membrane were fixed in 96% methanol, stained with hematoxylin solution (Sigma), and mounted onto the glass slides. Chemotaxis was quantified by counting five random fields per membrane using a 20× objective. The average values for each membrane were expressed as number of cells per high power field. All experiments were performed at least twice in duplicates.
FIGURE 9.
Opposing effects of anosmin-1 on FGFR1-mediated cell migration. FNC-B4-hTERT cells are plated in the top chamber of Transwells and allowed to migrate for 4 h toward the lower chamber containing different chemoattractants (SFM, FGF2, PIWF4, and PIWF4 plus SU5402) at varying concentrations. A representative microscopic image of migrated cells after staining under each condition is shown in a and the quantification of the migrated cells is shown in b. **, p < 0.01 compared with SFM control. c, sodium chlorate (SC)-treated cells were plated on the top chamber and similarly allowed to migrate toward SFM, heparin, or PIWF4 plus heparin at the indicated concentrations. The quantification of migrated cells is shown. **, p < 0.01; *, p < 0.05, in comparison to 10 nm PIWF4 treatment alone. d, SC-treated cells were preincubated with SFM, PIWF4, or PIWF4 plus FGFR1D1D3 for 1 h and then resuspended in SFM before being loaded in the top chamber of Transwell. A mixture of 10 nm PIWF4 and 200 ng/ml heparin was introduced into the lower chamber. The numbers of migrated cells after 20 h were quantified. **, p < 0.01 compared with PIWF4 preincubation. All values are shown as mean ± S.D. calculated from at least two separate experiments performed in duplicates.
RESULTS
Determination of Direct Binding of Anosmin-1 to FGFR1
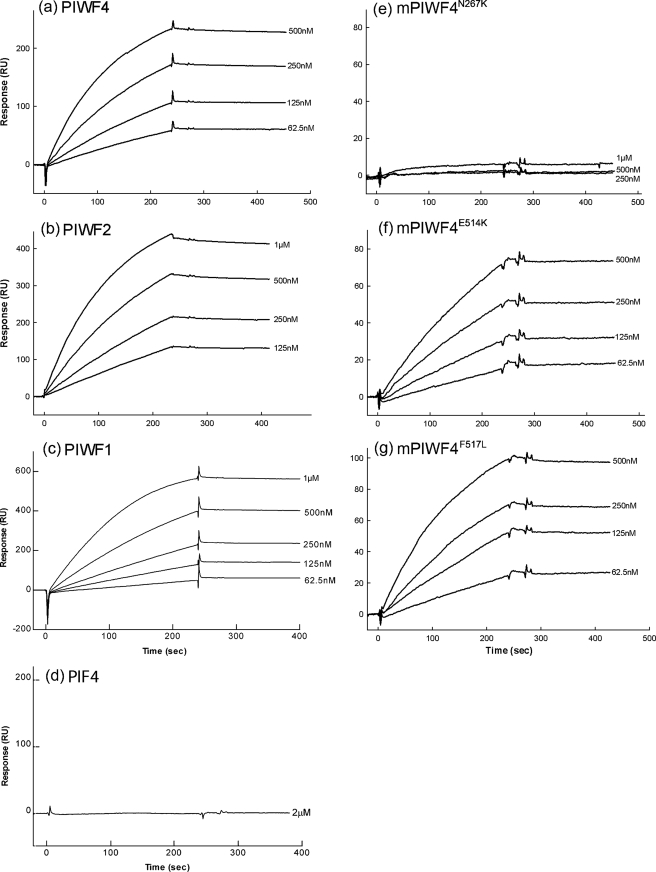
Direct interaction of anosmin-1 and FGFR1 was first detected using SPR analysis. In this study, the recombinant wild-type full-length anosmin-1 and its various mutant analogues were immobilized individually on the sensor chip surface, through their native amine groups generating comparable coupling response unit (RU) increases. Soluble FGFR1 D1D3 and D2D3 proteins were injected at serial dilutions. Representative SPR sensorgrams for each experiment are shown (Figs. 2 and 3). The results demonstrate that full-length anosmin-1 (PIWF4) can directly bind to FGFR1 with a relatively high association rate and very low dissociation rate generating a nanomolar range (<10 nm) dissociation constant (Kd), which indicates high binding affinity between the two proteins (Fig. 2a and Table 1). Similar binding affinities were observed not only with the FGFR1D1D3 containing the three extracellular Ig domains, but also with FGFR1D2D3 with only two Ig domains, missing the first Ig domain (D1) and the acid box located in the D1–D2 linker region (Fig. 3a and Table 1). This indicates that FGFR1 binding to anosmin-1 is mainly dependent on its second (D2) and third (D3) Ig domains. Interestingly, however, an approximate 3-fold increase of the Kd values was obtained in the binding of PIWF4 with FGFR1D2D3 (∼2 nm) as compared with that with FGFR1D1D3 (∼7 nm). One interpretation of these data could be that the acid box together with the D1 domain of FGFR1 may play a regulatory role in the interaction with anosmin-1 by partially blocking the anosmin-1 binding site within the D2–D3 domains, a scenario reminiscent of the intramolecular autoinhibition by these domains.
FIGURE 2.
BIAcore analysis of anosmin-1 binding to FGFR1D1D3. Soluble FGFR1D1D3 at varying concentrations was injected over PIWF4 (a), PIWF2 (b), PIWF1 (c), PIF4 (d), mPIWF4N267K (e), mPIWF4E514K (f), and mPIWF4F517L (g) coupled sensor chips.
FIGURE 3.
BIAcore analysis of anosmin-1 binding to FGFR1D2D3. Soluble FGFR1D2D3 at varying concentrations was injected over PIWF4 (a), PIWF2 (b), PIWF1 (c), PIF4 (d), mPIWF4N267K (e), mPIWF4E514K (f), and mPIWF4F517L (g) coupled sensor chips.
TABLE 1.
BIAcore analysis of anosmin-1 binding to FGFR1
| Soluble analyte | Coupled reagent | kon | koff | Kd |
|---|---|---|---|---|
| m−1s−1 | s−1 | nm | ||
| FGFR1 | PIWF4 | 1.01 ± 0.18 × 104 | 7.68 ± 0.98 × 10−5 | 7.64 ± 0.68 |
| D1D3 | PIWF2 | 8.82 ± 3.71 × 103 | 1.01 ± 0.59 × 10−4 | 11.93 ± 8.05 |
| PIWF1 | 4.07 ± 1.32 × 103 | 1.50 ± 0.87 × 10−4 | 35.10 ± 8.57 | |
| PIF4 | NBa | NB | NB | |
| mPIWF4N267K | NB | NB | NB | |
| mPIWF4E514K | 4.75 ± 3.03 × 103 | 6.64 ± 4.35 × 10−5 | 15.08 ± 7.53 | |
| mPIWF4F517L | 5.28 ± 2.48 × 103 | 2.36 ± 1.20 × 10−4 | 48.63 ± 19.86 | |
| FGFR1 | PIWF4 | 3.92 ± 0.07 × 104 | 1.04 ± 0.37 × 10−4 | 2.66 ± 0.96 |
| D2D3 | PIWF2 | 2.24 ± 0.09 × 104 | 2.30 ± 2.27 × 10−4 | 3.79 ± 2.69 |
| PIWF1 | 6.84 ± 0.60 × 103 | 1.10 ± 0.37 × 10−4 | 16.20 ± 6.18 | |
| PIF4 | NB | NB | NB | |
| mPIWF4N267K | NB | NB | NB | |
| mPIWF4E514K | 8.25 ± 3.61 × 103 | 1.99 ± 0.66 × 10−4 | 26.70 ± 10.62 | |
| mPIWF4F517L | 6.80 ± 1.90 × 103 | 3.48 ± 1.12 × 10−4 | 50.80 ± 4.08 | |
NB, negligible binding.
Identification of Domain-specific Interaction of Anosmin-1 with FGFR1
To further determine the specific domain(s) of anosmin-1 binding to FGFR1, we generated C-terminal truncated mutants PIWF1 and PIWF2, encompassing the N-terminal CR and WAP followed by one or two FnIII domains, respectively (Fig. 1b). SPR analyses using immobilized PIWF1 or PIWF2 clearly demonstrate that both are still capable of binding to FGFR1D1D3 and FGFR1D2D3 (b and c of Figs. 2 and 3). As seen with PIWF4, they both bind to FGFR1D2D3 with slightly higher affinity than to FGFR1D1D3. The only difference was that PIWF2 generated similar Kd values to the full-length counterpart, whereas PIWF1 showed an approximate 5-fold lower binding affinity (Table 1). These observations suggested that the N-terminal CR, WAP, and the first FnIII domains of anosmin-1 (i.e. PIWF1) are sufficient for FGFR1 binding and the additional FnIII domains are dispensable in this interaction.
Because there is growing evidence showing that FnIII domains in NCAM and L1 are involved in direct FGFR1 binding (19, 20), we sought to determine whether the N-terminal region (CR and WAP) of anosmin-1 was required for the FGFR1 binding by testing the PIF4 construct, which contains only the four FnIII domains. In addition, we introduced three point mutations (N267K, E514K, and F517L) into the full-length PIWF4 to investigate the effects of KS-related missense mutations in the FnIII domains. When SPR assays were conducted using these mutant anosmin-1 proteins, PIF4 showed no apparent binding with the two forms of FGFR1 ectodomains (Figs. 2d and 3d). N267K substitution in the first FnIII domain also leads to a complete loss of FGFR1 binding. By contrast, E514K and F517L substitutions in the third FnIII domain still retained nanomolar range binding affinity, albeit 2- to 6-fold reduced, to both FGFR1D1D3 and D2D3 (Table 1 and e–g of Figs. 2 and 3). Taken together, these data suggest that the first, but not the third, FnIII domain is critical for FGFR1 binding and the N-terminal CR and WAP domains are also required for FGFR1 interaction.
Determination of Anosmin-1 Binding to FGFR2c and FGFR3c
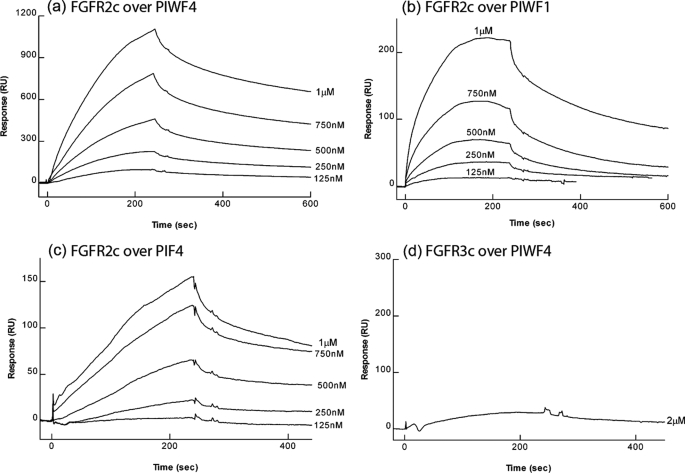
Our study so far has been focused on interactions with FGFR1, based on the fact that anosmin-1 showed FGFR1IIIc specific activity in a BaF3 cell system (25) and a point mutation presumably affecting the FGFR1IIIc-FGF8b interaction has been implicated with KS cases (32). It is unknown, however, whether anosmin-1 has the capacity to interact with other FGF receptors. Thus, we further examined the binding affinity of anosmin-1 with FGFR2IIIc and FGFR3IIIc by SPR (Fig. 4). Soluble FGFR2IIIc D1D3 protein injected over the PIWF4-coupled sensor chips generated a more than 7-fold higher Kd value compared with that of FGFR1IIIc D1D3 (57.63 nm versus 7.64 nm), indicating that anosmin-1 preferentially binds to FGFR1 over FGFR2 (Tables 1 and 2). By contrast, anosmin-1 showed negligible binding to FGFR3c (Fig. 4d). Interestingly, PIF4, which showed no binding to FGFR1, demonstrated some weak binding to FGFR2c, albeit with significant decrease of affinity with a Kd increasing from 57.63 nm to 380.15 nm, when compared with PIWF4 (Table 2). This again indicates a requirement of the N-terminal CR and WAP domains for optimal FGFR binding.
FIGURE 4.
BIAcore analysis of anosmin-1 binding to FGFR2c and FGFR3c. Soluble FGFR2IIIcD1D3 at varying concentrations was injected over PIWF4 (a), PIWF1 (b), and PIF4 (c), and soluble FGFR3IIIcD1D3 was injected over PIWF4 (d).
TABLE 2.
BIAcore analysis of anosmin-1 binding to FGFR2c and FGFR3c
Association rate constants (kon), dissociation rate constants (koff), and apparent dissociation constants (Kd) for soluble FGFR1D1D3, FGFR1D2D3, FGFR2c, and FGFR3c binding to immobilized anosmin-1. Values are shown as mean ± S.D. derived from duplicate measurements in at least two separate experiments.
| Soluble analyte | Coupled reagent | Kon | koff | Kd |
|---|---|---|---|---|
| m−1s−1 | s−1 | nm | ||
| FGFR2c | PIWF4 | 2.93 ± 2.46 × 104 | 1.39 ± 0.93 × 10−3 | 57.63 ± 28.21 |
| PIWF1 | 2.58 ± 1.44 × 104 | 1.52 ± 0.63 × 10−3 | 69.22 ± 34.62 | |
| PIF4 | 4.24 ± 1.53 × 103 | 1.64 ± 0.68 × 10−3 | 380.15 ± 99.67 | |
| FGFR3c | PIWF4 | NBa | NB | NB |
NB, negligible binding.
Cross-linking Study on the Interaction of Anosmin-1 with FGFR1
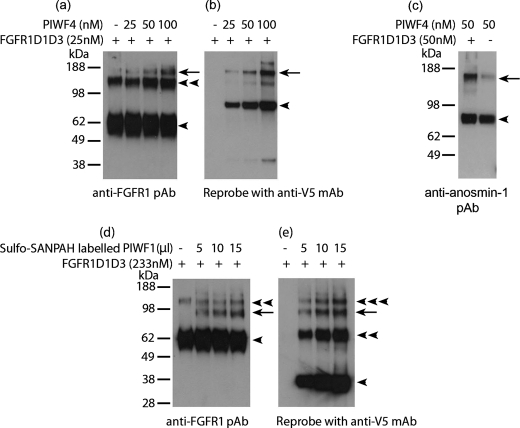
In the SPR assay, recombinant anosmin-1 analogues were immobilized, and thus the interaction detected is in an immobilized phase. To investigate whether anosmin-1 and FGFR1 can interact in the solution state, we conducted two types of chemical cross-linking experiments, either by adding BS3 cross-linker directly to the reaction solution, or by coupling anosmin-1 with photoactive Sulfo-SANPAH cross-linker. In the BS3 cross-linking study where 25 nm FGFR1D1D3 was incubated with increasing amount of PIWF4, three bands were detected by anti-FGFR1 (H-76) antibody. The bands corresponding to ∼60 and ∼120 kDa represent the monomeric and homodimeric forms of FGFR1D1D3, respectively, as they are present even in the absence of PIWF4. A higher band migrating with a molecular mass of ∼150 kDa, however, started to appear with increasing intensity only when increased amount of PIWF4 was added to the reaction. This band was thought to represent the PIWF4·FGFR1D1D3 complex (∼90 kDa of PIWF4 and ∼60 kDa of FGFR1D1D3) (Fig. 5a). To confirm this finding, the same blot was reprobed with anti-V5 antibody, which detected two bands: one at ∼90 kDa, which corresponds to PIWF4, and another band at ∼150 kDa, which overlaps with the band previously identified by the FGFR1 antibody, indicating that this band indeed contains the cross-linked PIWF4·FGFR1D1D3 complex (Fig. 5b). We further used a polyclonal anti-anosmin-1 antibody to confirm complex formation. Again, incubation of an equal amount (50 nm) of PIWF4 and FGFR1D1D3 resulted in the appearance of a band of ∼150 kDa. The very faint background band of the same size present even in the absence of D1D3 indicates a self-dimerized PIWF4 (Fig. 5c). To eliminate the possibility that anosmin-1 protein in solution may stick to random proteins nonspecifically, the cross-linking experiments were repeated using an irrelevant recombinant protein, Robo, which also contains two Ig domains. No apparent band corresponding to the PIWF4·Robo complex was observed (data not shown), confirming the specific interaction between PIWF4 and FGFR1D1D3.
FIGURE 5.
Cross-linking study on the interaction of anosmin-1 with FGFR1D1D3. a and b, PIWF4 at the indicated concentrations was incubated with 25 nm FGFR1D1D3, and the complexes formed were cross-linked with BS3, resolved by 4–12% SDS-PAGE, and identified with anti-FGFR1 polyclonal antibody (a) or anti-V5 monoclonal Ab (b). c, 50 nm PIWF4 with or without 50 nm FGFR1D1D3 were cross-linked with BS3, and the cross-linked complex was analyzed by anti-anosmin-1 polyclonal Ab. d and e, PIWF1 labeled with photoactivatable heterobifunctional cross-linker Sulfo-SANPAH was incubated with 233 nm FGFR1D1D3. The cross-linked complex was detected by anti-FGFR1 pAb (d) or anti-V5 mAb (e). ◀, monomer; ◀◀, dimer; ◀◀◀, trimer; ←, cross-linked anosmin-1·FGFR1D1D3 complex.
For the photoactive cross-linking assay, PIWF1 was first labeled with Sulfo-SANPAH cross-linker, which is then activated by photolysis after UV light exposure. Western blot analysis of the cross-linked proteins with anti-FGFR1 antibody detected an emerging band of ∼98 kDa, equivalent to the combined molecular mass of PIWF1 (∼38 kDa) and FGFR1D1D3 (∼60 kDa), whose intensity increased in proportion to the amount of PIWF1 added (Fig. 5d). Again, when this blot was reprobed with anti-V5 antibody, the presence of PIWF1 was confirmed in this band (Fig. 5e). Moreover, it was found that PIWF1 could self-dimerize, ranging from monomeric to trimeric forms in contrast to the full-length PIWF4, mostly present in monomeric form. Data from these cross-linking assays also indicate that anosmin-1·FGFR1 complex is formed with 1:1 stoichiometry.
Co-recruitment of Anosmin-1 and FGFR1 to the Peripheral Plasma Membrane
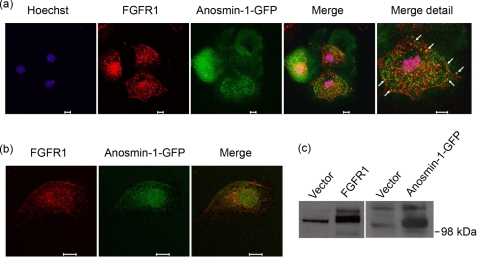
We have previously reported that anosmin-1 can be co-immunoprecipitated with FGFR1 (25). Our current studies showing direct binding between recombinant anosmin-1 analogues and FGFR1 ectodomains further support this observation. To demonstrate whether the physical interactions between these two proteins also occur in the presence of intact extracellular matrix in living cells, we employed confocal laser scanning microscopy to visualize the subcellular localization of these proteins after immunofluorescence staining. When COS7 cells transfected with full-length FGFR1 expression construct were immunostained with polyclonal anti-FGFR1 antibody (C-15), the signal was detected as a punctate pattern at the plasma membrane and in cytoplasmic endosomal vesicles. FGFR1 staining was also detected in and around the cell nucleus (Fig. 6a), similar to previous reports (22, 33). When cells transfected with anosmin-1-GFP construct were examined, it was evident that GFP-tagged anosmin-1 protein was secreted into surrounding medium, scattering to adjacent cells (Fig. 6a). Anosmin-1-GFP also showed a punctate pattern on the cell periphery and within the cytoplasm, indicating accumulation along the plasma membrane as well as within the endosomal vesicles, as expected from an extracellular matrix-associated secretory protein, and also consistent with a previous report (34). When FGFR1 and anosmin-1-GFP images were overlaid, there was considerable overlap of the FGFR1 staining with the GFP (marked with arrows in Fig. 6a), compatible with FGFR1·anosmin-1 co-localization in cells. No signal was observed in untransfected cells (in the case of anosmin-1-GFP) or when primary antibodies were omitted (in the case of FGFR1) (data not shown). Western blotting of lysates from the transfected COS7 cells indicated that the exogenous constructs were expressing the respective proteins of expected size (Fig. 6c).
FIGURE 6.
FGFR1 co-localizes with anosmin-1 in COS7 cells. a, COS7 cells were co-transfected transiently with constructs expressing FGFR1–3xMyc and anosmin-1-GFP. The cells were fixed on the coverslips before incubation with polyclonal FGFR1 antibody (C-15) raised against C terminus of human FGFR1, which is subsequently labeled by anti-rabbit secondary antibody conjugated with Alexa Fluor 555 (red). Anosmin-1 is detected by GFP (green). Hoechst staining shows the nucleus (blue). Scale bars represent 10 μm. Co-localization of FGFR1 and anosmin-1 is evidenced by overlapping fluorescence signals (yellow), as indicated with arrows in the merged image. b, COS7 cells were stably transfected with anosmin-1-GFP by G418 selection. Selected cells were plated on coverslips, serum-starved overnight, and stimulated with FGF2 (25 ng/ml) for 20 min to induce cell surface receptor recruitment. After fixing, unpermeabilized cells were analyzed for localization of endogenous FGFR1 and anosmin-1 using polyclonal FGFR1 antibody (H-76) and GFP, respectively. FGFR1 H-76 antibody, raised against the ectodomain of human FGFR1, was detected by anti-rabbit secondary antibody conjugated with Alexa Fluor 555. The merged image indicates overlapping fluorescence signals (yellow) of the endogenous FGFR1 ectodomain (red) and stably transfected anosmin-1 (green) on the cell surface. Scale bars represent 10 μm. c, Western blotting analysis to confirm expression of respective tagged proteins in these cells. Exogenous FGFR1 was probed with anti-Myc antibody (9E10) to distinguish it from endogenous protein, although FGFR1 antibody was used for all immunofluorescence staining. Anosmin-1 expression was detected with anti-GFP antibody (3E1).
It is possible that forced expression of exogenous genes at high levels may lead to abnormal protein localization. To address this possibility, we next examined the interaction of the endogenous FGFR1 protein in cells that are stably transfected solely with anosmin-1-GFP construct, because stable transfection leads to a moderate, more physiologically relevant level of protein expression. These modifications, however, also resulted in decreased immunofluorescence signal intensity detected by the confocal microscopy (data not shown). To enhance the visualization of FGFR1 and anosmin-1 interaction on the cell surface, we stained the cells without permeabilization, thereby helping to preserve plasma membrane integrity. In addition, we used an FGFR1 ectodomain-specific antibody (H-76) in the serum-starved cells after stimulation with FGF2, which allowed better detection of the extracellular domains of FGFR1 on the cell surface (Fig. 6b). In these cells, the majority of the endogenous FGFR1 detected on the plasma membrane co-localized with the anosmin-1-GFP. This result is consistent with observations from the initial experiments using overexpression constructs. Together, these data support the notion that anosmin-1 directly interacts with FGFR1 in living cells under physiological conditions.
Effect of Anosmin-1 on FGF2·FGFR1·Heparin Assembly Using Sequential Injection Analysis
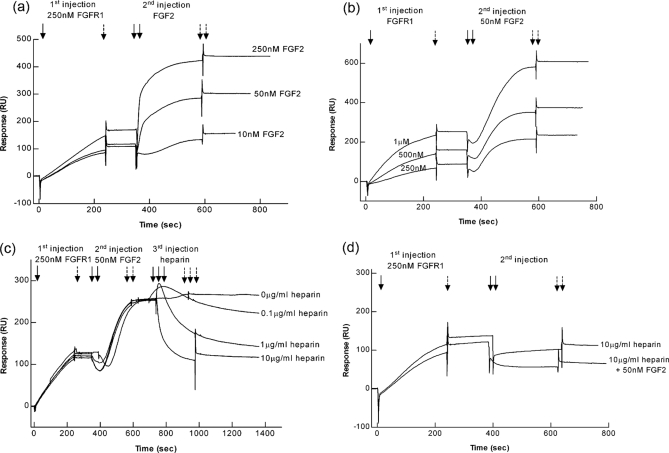
Having documented the direct interaction between anosmin-1 and FGFR1 using multiple complementary approaches, we sought to further determine whether complex formation between anosmin-1 and FGFR1 would affect the subsequent interaction with FGF2 and heparin, and thus conducted a series of SPR experiments using a sequential injection protocol. We first injected FGFR1D1D3 over a PIWF4-coupled sensor surface, followed by a second injection with FGF2 at varying concentrations. Thus PIWF4-bound FGFR1 would be expected to remain on the sensor surface and unbound FGFR1 washed away by HBS-EP buffer passing over the sensor surface. As shown in Fig. 7a, FGF2 did bind to PIWF4·FGFR1 complex in a dose-dependent manner. This interaction was reproducibly observed in independent sequential injection experiments where varying concentrations of FGFR1 (first injection) and a constant concentration of FGF2 (second injection) were used (Fig. 7b). However, when FGF2 was injected over PIWF4-coupled surface without the prior injection of FGFR1, there was no change in RU, indicating that FGF2 could not bind to PIWF4 alone (supplemental Fig. S2). These data suggested that binding of PIWF4 to FGFR1 did not impair the ability of FGFR1 to bind to FGF2 and that, although PIWF4 does not bind to FGF2, PIWF4·FGF2·FGFR1 complex could be formed on a pre-existing PIWF4·FGFR1 complex. Because a functional FGFR1 signaling complex would still require HS, we then used a third injection with heparin at varying concentrations to see if heparin could affect this PIWF4·FGF2·FGFR1 complex. The results, however, showed that heparin stripped FGF2 off from the PIWF4·FGF2·FGFR1 complex in a dose-dependent manner. At 10 μg/ml heparin, the RU dropped from ∼260 to ∼120 RU, which is equivalent to the level of PIWF4·FGFR1 complex, rather than to the initial baseline, indicating that stripping of FGF2 from PIWF4· FGF2·FGFR1 complex did not disrupt the PIWF4·FGFR1 interaction (Fig. 7c). This observation was supported by the fact that when 10 μg/ml heparin was injected directly over PIWF4·FGFR1-bound surface, it did not change the RU level significantly. Furthermore, when FGF2 was preincubated with heparin, it could not further bind to PIWF4·FGFR1 (Fig. 7d). Taken together, these data show that PIWF4-bound FGFR1 still retains the ability to bind FGF2, but cannot lead to subsequent FGF2·FGFR1·heparin complex formation.
FIGURE 7.
Effect of anosmin-1 binding to FGFR1 on the FGF2·FGFR1·heparin complex assembly by SPR using sequential injections. a, 250 nm FGFR1was first injected over a PIWF4-coupled sensor chip, followed by the second injection of FGF2 at various concentrations. b, FGFR1 at various concentrations was first injected with the second injection of 50 nm FGF2. c, 250 nm FGFR1 was first injected, followed by the second injection of 50 nm FGF2, and the third injection of heparin at various concentrations. d, 250 nm FGFR1 was first injected over a PIWF4-coupled sensor chip, followed by the injection of 10 μg/ml heparin and the preincubated FGF2 with heparin, respectively. single solid arrow, first start of injection; single dashed arrow, first stop of injection; double solid arrows, second start of injection; double dashed arrows, second stop of injection; triple solid arrows, third start of injection; and triple dashed arrows, third stop of injection.
We have previously demonstrated that anosmin-1 binds to heparin with a Kd around 2 nm (29), a binding affinity comparable to the anosmin-1·FGFR1 interaction in the present study. We therefore asked whether heparin-bound anosmin-1 could affect the interaction of FGFR1 and FGF2·FGFR1·heparin complex formation. To address this, 10 μg/ml heparin was first injected over PIWF4- coupled sensor chip allowing heparin·PIWF4 complex formation on the surface, followed by the injection of either FGFR1, FGF2 alone, or preincubated FGF2·FGFR1 or FGF2·FGFR1·heparin. There was no RU change when FGFR1 (50 nm) was injected (Fig. 8), even at higher concentrations (data not shown), indicating that heparin-bound anosmin-1 lost the ability to bind FGFR1, such that the anosmin-1·FGFR1· heparin ternary complex could not be assembled. Notably, when FGF2 was injected, it reduced the RU almost to basal level, equivalent to the pre-heparin injection state, suggesting that FGF2 could strip off heparin from anosmin-1, consistent with the observation that heparin can competitively displace FGF2 from anosmin-1·FGFR1·FGF2 complex (Fig. 7c). However, a significant increase of RU was observed when preincubated FGFR1·FGF2 was injected (Fig. 8), suggesting that the anosmin-1·heparin interaction could favor subsequent binding to pre-existing binary FGF2·FGFR1 complex, allowing anosmin-1·FGF2·FGFR1·heparin complex formation. Taken together, these data demonstrate that the order of anosmin-1 interaction with FGFR1 and heparin can lead to differential effects on the ternary FGFR1·FGF2·heparin complex formation.
FIGURE 8.
Effect of anosmin-1 binding to heparin on FGF2·FGFR1·heparin complex assembly by SPR using sequential injections. 10 μg/ml heparin was first injected over a PIWF4-coupled sensor chip, followed by the second injection of 50 nm FGF2, 50 nm FGFR1D1D3, and preincubated FGF2·FGFR1D1D3, FGF2·FGFR1D1D3·heparin at the indicated concentrations, respectively. single solid arrow, first start of injection; single dashed arrow, first stop of injection; double solid arrows, second start of injection; double dashed arrows, second stop of injection; triple solid arrows, third start of injection; and triple dashed arrows, third stop of injection.
Interactions of Anosmin-1 with FGFR1 and HS Play Opposing Roles in the Migration of FNC-B4-hTERT Cells
Our findings from the sequential injection experiments suggest that heparin-bound anosmin-1 facilitates functional FGFR1 signaling complex formation, while anosmin-1-bound FGFR1 cannot. To investigate whether this dual role of anosmin-1 on FGF2·FGFR1·HS complex formation can influence the biological activity of FGFR1 in living cells, we have employed immortalized human olfactory GnRH neuroblasts (FNC-B4-hTERT) to perform Transwell migration assays.
The primary FNC-B4 cells, originally derived from human fetal olfactory epithelium (31) have provided a useful model system to study olfactory GnRH neurogenesis, and we have previously reported that both anosmin-1 and FGF2 can induce neurite outgrowth in these cells (25). By using a retroviral vector expressing the catalytic subunit of human telomerase (hTERT), we have established an immortal derivative line of FNC-B4 cells (supplemental Fig. S1a). Our analyses indicate that the immortalized cells still maintain the molecular and cellular characteristics of the original GnRH neuroblasts, show normal DNA damage checkpoint control (data not shown), and, most importantly, express endogenous FGF2 and FGFR1 IIIc (supplemental Fig. S1b). These FNC-B4-hTERT cells, therefore, represent an ideal system to functionally confirm our biochemical data and have been used in Transwell migration assays as described under “Experimental Procedures.”
The FNC-B4-hTERT cells plated in the upper chamber of the Transwell showed minimal migration toward the SFM. When PIWF4 (1 and 10 nm) was loaded in the lower chamber, however, cells exhibited a significant 3- to 6-fold increase in migration (p < 0.01), compared with SFM. The dose-dependent chemotactic migration induced by PIWF4 most likely involves FGFR1·FGF2 signaling activity, because FGFR1 antagonist SU5402 could abolish it and exogenous FGF2 can trigger similar migration of these cells (Fig. 9, a and b). In contrast, when a higher concentration (50 nm) of PIWF4 was loaded, the cell migration was decreased to a basal level (Fig. 9b), suggesting that PIWF4 can mediate opposing effects on FGFR1 signaling activity depending on the level of expression.
To establish the role of HS in this phenomenon, cells were treated with sodium chlorate, which prevents endogenous HS sulfation during biosynthesis, without affecting the expression of FGF2 and FGFR1. As shown in Fig. 9c, HS-deficient cells no longer exhibited chemotactic migration toward PIWF4, and heparin alone did not enhance migration of these cells, indicating that the presence of both PIWF4 and HS are required to induce migration. As expected, chemotactic migration was restored when PIWF4 and heparin were loaded together in the lower chamber, in a heparin dose-dependent manner. These observations are consistent with the notion that the anosmin-1·HS complex may facilitate the formation of a functional FGFR1 signaling complex on the surface of these cells, inducing FGFR1-mediated chemotactic migration.
Next, we preincubated the HS-deficient cells with 10 nm PIWF4 to allow the binding of anosmin-1 to the endogenous FGFR1 on the cell surface, which may then further recruit endogenous FGF2 to form a PIWF4·FGF2·FGFR1 complex (Fig. 7, a and b). This progression of complex formation is expected to occur preferentially in these cells, because FGF2 cannot bind to anosmin-1 directly without first interacting with FGFR1 (supplemental Fig. S2b). Then, after 1-h preincubation, the cells were washed and allowed to migrate toward the lower chamber containing PIWF4 and heparin. The results showed that preincubation with PIWF4, but not SFM, led to a 50% reduction in cell migration, and this inhibitory activity of PIWF4 could be reversed by addition of FGFR1 D1D3 recombinant protein in the preincubation mixture, which competitively inhibits PIWF4 binding to the FGFR1 on the cell surface (Fig. 9d). These data are consistent with the notion that PIWF4-bound FGFR1 cannot further engage FGF2 to form a functional FGF2· FGFR1·HS complex (Fig. 7, c and d), thus anosmin-1 blocks the activation of FGFR1 signaling complex.
Taken together, these findings enable us to conclude that the order of interactions of anosmin-1 with FGFR1 and HS can result in opposing cellular responses and the multiprotein binding interactions observed in our biochemical studies also occur in physiologically relevant cell systems.
DISCUSSION
We previously reported that anosmin-1 could modulate FGFR1 signaling involving p42/44 and p38 mitogen-activated protein kinases and Cdc42/Rac1 activation, in the process of neurite outgrowth and cytoskeletal rearrangements. In heterologous BaF3 lymphoblast cells, anosmin-1 enhanced FGF2 signaling specifically through FGFR1 IIIc isoform in a HS-dependent manner (25). It has been speculated that anosmin-1 affects FGFR1 signaling by regulating FGF2·FGFR1·heparin signaling complex formation. The present study provides the first demonstration of high affinity direct binding between anosmin-1 and FGFR1; we further propose a molecular mechanism whereby binding of anosmin-1 to FGFR1 and heparin exhibits different effects on ternary FGFR1·FGF2·heparin complex formation.
Anosmin-1 binding to FGFR1 is characterized by rapid association followed by a very slow dissociation pattern, giving a dissociation constant Kd of 7 nm. The preferred FGF receptor for anosmin-1 binding is FGFR1, because a much lower binding affinity was observed for FGFR2c, with negligible binding for FGFR3c, which makes the preferential binding order: FGFR1>FGFR2≫FGFR3. The difference in FGFR binding provides the explanation for the specificity of anosmin-1 in BaF3 cell proliferation, in which anosmin-1 functions only on cells expressing FGFR1c but not on FGFR2c and FGFR3c isoforms. This finding also brings important new insights into the mechanism of anosmin-1 action; high affinity binding of anosmin-1 to the respective receptor is both necessary and sufficient to ensure anosmin-1 activity on the receptor. It should be noted that, although SPR studies have the limitation that they are carried out in the absence of extracellular matrix, we have also demonstrated here equivalent interactions in functional cell assays (Figs. 6 and 9), thus supporting the biochemical data.
Fibronectin type III domains in NCAM, L1 and XFLRT3 play important roles in FGFR1 binding. In anosmin-1, at least one of the FGFR1 binding sites is likely located in the first FnIII domain because the N-terminal fraction of anosmin-1 with the single first FnIII domain retains FGFR1 binding capacity, whereas the N267K mutation in the first FnIII domain, but not the other two substitutions in the third FnIII domain, leads to loss of FGFR1 binding. According to the x-ray scattering and constrained modeling, the four FnIII domains of anosmin-1 have an elongated and flexible inter-domain arrangement (35); hence the dimensional orientation of first FnIII domain would not be affected by the other three FnIII domains. As observed in the homology modeling (36), N267K was expected to cause either disruption of protein folding or the loss of activity, by introducing an additional basic residue near a large basic surface. Notably, N-terminal truncated anosmin-1 containing only the four FnIII domains completely loses the capacity to bind to FGFR1IIIc and shows a 7-fold decreased binding affinity to FGFR2IIIc. These results raise the possibility that the N-terminal CR and WAP domains of anosmin-1 may assist the first FnIII domain to adopt an optimal orientation for FGFR1 binding. This idea is supported by the fact that the surface of the WAP domain is acidic in charge and a large basic patch is present on the first FnIII domain surface (36), which makes it possible for these neighboring domains to engage in electrostatic interactions. The functional interdependence between the CR/WAP domain and the FnIII repeats has been previously documented by in vivo studies; in both Caenorhabditis elegans and Drosophila, it has been reported that the removal of the FnIII domains or the equivalent substitution of N267K caused anosmin-1 protein to completely lose its biological activity and cell adhesion property (37, 38), and the additional phenotype induced by the N267K equivalent substitution in Drosophila was suppressed by a WAP domain mutation (37). Moreover, we have previously reported that PIWF1 is sufficient to induce neurite outgrowth in human olfactory GnRH neuroblast culture, but a point mutation in the WAP domain (C172R) abolishes this activity (25).
In terms of the FGFR, the acid box has currently been identified as a motif required for the interaction with N-cadherin and all the major NCAM isoforms (22). However, we have shown in this study that FGFR1 lacking D1 and the acid box binds anosmin-1, with 3-fold higher affinity, indicating that the anosmin-1 binding motif is located within D2/D3 domains and the linker between these domains. Even though these areas are also required for FGF ligand binding, the binding motif for FGF2 and anosmin-1 does not seem to be completely overlapping because the SPR sequential injection demonstrated that FGF2 is still capable of binding to the pre-formed anosmin-1·FGFR1 complex. It has been proposed that D1 and acid box have autoinhibition activity by potentially interfering with the ligand·HS binding sites in D2 and D3. Deletion of D1 and acid box is known to enhance the binding efficiency between FGFs and FGFR1 (39). This phenomenon was also observed with anosmin-1, suggesting that D1 and the acid box may exert a modulatory activity over anosmin-1 interactions with D2/D3.
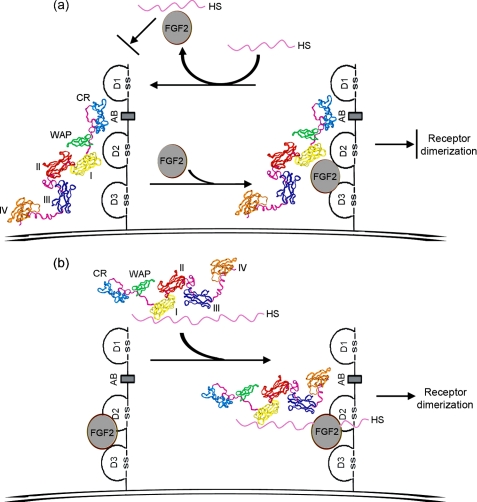
HSPG is essential for the stability of FGF·FGFR·HS ternary complex (5). The initial binary complex formation is proposed to occur, either as FGF·FGFR or FGF·HS. In the first scenario, FGF·FGFR pairing is further stabilized by HS, as proposed in the 2:2:2 FGF2·FGFR1c·heparin ternary complex model (40). In this case, the preformed FGF·FGFR complex seeks distinct sulfation patterns of HS of certain length, which may differ according to individual FGFs or FGFRs (41). In another scenario, HS first induces oligomerization of FGF molecules and subsequently enables FGFR dimerization, as proposed in the 2:2:1 FGF1·FGFR2c·heparin ternary complex model (9). These two binary FGF·FGFR and FGF·HS complexes may co-exist as the driving force for ternary signaling complex assembly, which can be regulated by the different length and sulfation patterns of heparin·HS saccharides (11). Here we have shown that heparin-bound anosmin-1 facilitates FGF2·FGFR1 as the preferred fundamental binary complex, resulting in anosmin-1·FGF2· FGFR1·heparin complex formation. We have also observed that short heparin fragment-bound anosmin-1 loses binding capability with FGF2·FGFR1,6 suggesting that the modulatory effect of anosmin-1 on ternary signaling complex formation is heparin length-dependent. In contrast, anosmin-1-bound FGFR1 cannot interact with a FGF2·heparin complex. Although anosmin-1-bound FGFR1 can still bind to FGF2, this is insufficiently stable for subsequent FGF2·FGFR1·heparin complex generation, because heparin rapidly dissociates FGF2 from it. Therefore, we envisage that anosmin-1 can play differential roles during ternary FGF2·FGFR1·heparin complex assembly depending on whether it is bound to FGFR1 or heparin. These mechanisms, depicted in Fig. 10, are novel and distinct from the molecular mechanism used by other FGFR signaling modulators known so far. For example, it has been proposed that cell adhesion molecules cluster upon homophilic binding, to induce co-clustering of the FGFR, leading to receptor activation and biological activity (42). Antiangiogenic forms of antithrombin block FGF2-induced angiogenesis by competing with FGF2 for binding to heparin to prevent FGF2·FGFR1·heparin complex formation (43).
FIGURE 10.
Putative model for the dual role of anosmin-1 on FGF2·FGFR1·HS complex formation. a, a diagram of model whereby anosmin-1 binding to FGFR1 inhibits FGF2·FGFR1·HS complex formation. FGF2 is able to bind to pre-formed anosmin-1·FGFR1 complex to form anosmin-1·FGF2·FGFR1 complex, but HS can strip FGF2 off this ternary complex. Binary complex of FGF2·HS cannot bind to anosmin-1-bound FGFR1. Thus, FGF2·FGFR1·HS signaling complex would not form. b, a schematic representation of the manner in which anosmin-1 binding to HS is capable of facilitating FGF2·FGFR1·HS complex formation. HS-bound anosmin-1 preferentially binds to pre-formed FGF2·FGFR1 pair, resulting in anosmin-1·FGF2·FGFR1·HS complex formation. The domain structure of anosmin-1 is based on the model proposed in Ref. 35. The cell membrane is shown as a double line. CR, cysteine-rich region; WAP, whey acidic protein-like domain; and I–IV, fibronectin-like type III 1–4.
Anosmin-1 and FGFR1 are known to be involved in GnRH neuron migration and neurite outgrowth (25, 34, 44, 45). We now report that anosmin-1 can function as a chemoattractant inducing migration of immortalized FNC-B4-hTERT cells via an FGFR1- and HS-dependent mechanism (Fig. 9). HS-bound anosmin-1 will preferentially associate with pre-existing FGF2·FGFR1 pairs to facilitate FGF2·FGFR1·HS signaling complex formation on target cells, enabling the FGFR1-mediated cell migration as shown here. In this situation, the role of anosmin-1 could be to present the appropriate HS to the complex, and if so, the endogenous HS context will be crucial in determining the anosmin-1-mediated responses. However, under the conditions where anosmin-1 level is high, HS-unbound anosmin-1 could diffuse freely and bind to the FGF receptor on the host and neighboring cells, subsequently resulting in inhibitory effect on FGFR signaling complex formation. We have tested this scenario in our Transwell migration assays where endogenous HS synthesis is artificially blocked to create an extracellular matrix environment where anosmin-1 is mostly free (HS-unbound) and allowed to bind to FGFR1 on the cell surface. Our results confirmed that anosmin-1 can inhibit FGFR1-mediated cell migration under these conditions. Notably, anosmin-1 has been previously reported to inhibit the FGFR1·FGF2-induced cell migration in mouse oligodendrocyte precursor cells (24); however, the molecular mechanism for this phenomenon was hitherto unknown.
The diffusible characteristic of anosmin-1 and its dual activities on FGFR1 signaling complex make it likely that anosmin-1 will act as a guidance cue with combined chemoattractant and chemorepellant properties to direct cell migration and axon targeting. This idea is supported by our current studies where anosmin-1 promotes GnRH neuroblast migration at lower concentration but inhibits at higher concentration. The binding capacity of HSPG and the expression levels of anosmin-1 may determine these opposing activities by shifting anosmin-1 either into an FGFR1-bound or an HS-bound state. This may provide one explanation as to why FGFR1 mutations cause KS with a wide spectrum of reproductive phenotype (46). Confirming how individual FGFR1 mutations affect interaction with anosmin-1 may also offer further clues. Our current study showed that the two KS-related mutations of anosmin-1, E514K and F517L, resulted in decreased binding affinity to FGFR1, which in combination with the potentially altered protein stability/expression in vivo may contribute to the phenotype of KS. FGF8 has been currently identified as one of six KS genes (47, 48). It is reasonable to assume that anosmin-1 can regulate FGF8·FGFR1·HS signaling complex formation in a manner similar to that proposed here.
Supplementary Material
Acknowledgments
We are grateful for Ruby Quartey-Papafio for her kind help with SPR experiments. We are thankful for Dr. Shane Minogue at the Centre for imaging, University College London Medical School, Royal Free Campus, for his expert advice in the use of confocal microscope and immunofluorescence. We are very grateful for the generous donation of the recombinant FGFR2 and FGFR3 ectodomain proteins by Alan Brown (University of Cambridge). We also thank Dr. Federico Carafoli (Imperial College London) for the FGFR1 D1–D3 and D2–D3 expression constructs and technical assistance in protein purification. The retroviral vectors used in cell immortalization were generous gifts from Dr. Gordon Peters (Cancer Research UK, London Research Institute). Dr. David Ornitz (Washington University, St. Louis, MO) has kindly provided the FGFR1 IIIc cDNA construct.
This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/F007167/1.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Y. Hu, S. E. Guimond, P. Travers, S. Cadman, E. Hohenester, J. E. Turnbull, S.-H. Kim, and P.-M. Bouloux, unpublished observations.
- FGF
- fibroblast growth factor
- FGFR
- fibroblast growth factor receptor
- HSPG
- heparan sulfate proteoglycan
- D1–D3
- immunoglobulin (Ig)-like domains 1–3
- AB
- acid box
- NCAM
- neuronal cell adhesion molecule
- CR
- cysteine-rich domain
- WAP
- whey acidic protein-like domain
- FnIII
- fibronectin type III
- KS
- Kallmann syndrome
- GnRH
- gonadotropin-releasing hormone
- SPR
- surface plasmon resonance
- GPI
- glycosylphosphatidylinositol
- hTERT
- human telomerase
- BS3
- bis-sulfosuccinimidyl suberate
- SFM
- serum-free medium
- Sulfo-SANPAH
- sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate.
REFERENCES
- 1.Detillieux K. A., Sheikh F., Kardami E., Cattini P. A. (2003) Cardiovasc. Res. 57, 8–19 [DOI] [PubMed] [Google Scholar]
- 2.Freeman K. W., Gangula R. D., Welm B. E., Ozen M., Foster B. A., Rosen J. M., Ittmann M., Greenberg N. M., Spencer D. M. (2003) Cancer Res. 63, 6237–6243 [PubMed] [Google Scholar]
- 3.Ornitz D. M. (2005) Cytokine Growth Factor Rev. 16, 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eswarakumar V. P., Lax I., Schlessinger J. (2005) Cytokine Growth Factor Rev. 16, 139–149 [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi M., Olsen S. K., Ibrahimi O. A. (2005) Cytokine Growth Factor Rev. 16, 107–137 [DOI] [PubMed] [Google Scholar]
- 6.Plotnikov A. N., Schlessinger J., Hubbard S. R., Mohammadi M. (1999) Cell 98, 641–650 [DOI] [PubMed] [Google Scholar]
- 7.Plotnikov A. N., Hubbard S. R., Schlessinger J., Mohammadi M. (2000) Cell 101, 413–424 [DOI] [PubMed] [Google Scholar]
- 8.Kan M., Wang F., Xu J., Crabb J. W., Hou J., McKeehan W. L. (1993) Science 259, 1918–1921 [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini L., Burke D. F., von, Delft F., Mulloy B., Blundell T. L. (2000) Nature 407, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 10.Schlessinger J., Plotnikov A. N., Ibrahimi O. A., Eliseenkova A. V., Yeh B. K., Yayon A., Linhardt R. J., Mohammadi M. (2000) Mol. Cell 6, 743–750 [DOI] [PubMed] [Google Scholar]
- 11.Goodger S. J., Robinson C. J., Murphy K. J., Gasiunas N., Harmer N. J., Blundell T. L., Pye D. A., Gallagher J. T. (2008) J. Biol. Chem. 283, 13001–13008 [DOI] [PubMed] [Google Scholar]
- 12.Harmer N. J., Ilag L. L., Mulloy B., Pellegrini L., Robinson C. V., Blundell T. L. (2004) J. Mol. Biol. 339, 821–834 [DOI] [PubMed] [Google Scholar]
- 13.Kim S. H., Hu Y., Cadman S., Bouloux P. (2008) J. Neuroendocrinol. 20, 141–163 [DOI] [PubMed] [Google Scholar]
- 14.Miki T., Fleming T. P., Bottaro D. P., Rubin J. S., Ron D., Aaronson S. A. (1991) Science 251, 72–75 [DOI] [PubMed] [Google Scholar]
- 15.Guimond S. E., Turnbull J. E. (1999) Curr. Biol. 9, 1343–1346 [DOI] [PubMed] [Google Scholar]
- 16.Ostrovsky O., Berman B., Gallagher J., Mulloy B., Fernig D. G., Delehedde M., Ron D. (2002) J. Biol. Chem. 277, 2444–2453 [DOI] [PubMed] [Google Scholar]
- 17.Böttcher R. T., Pollet N., Delius H., Niehrs C. (2004) Nat. Cell Biol. 6, 38–44 [DOI] [PubMed] [Google Scholar]
- 18.Tsang M., Friesel R., Kudoh T., Dawid I. B. (2002) Nat. Cell Biol. 4, 165–169 [DOI] [PubMed] [Google Scholar]
- 19.Kiselyov V. V., Skladchikova G., Hinsby A. M., Jensen P. H., Kulahin N., Soroka V., Pedersen N., Tsetlin V., Poulsen F. M., Berezin V., Bock E. (2003) Structure 11, 691–701 [DOI] [PubMed] [Google Scholar]
- 20.Kulahin N., Li S., Hinsby A., Kiselyov V., Berezin V., Bock E. (2008) Mol. Cell Neurosci. 37, 528–536 [DOI] [PubMed] [Google Scholar]
- 21.Saffell J. L., Williams E. J., Mason I. J., Walsh F. S., Doherty P. (1997) Neuron 18, 231–242 [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Heras E., Howell F. V., Williams G., Doherty P. (2006) J. Biol. Chem. 281, 35208–35216 [DOI] [PubMed] [Google Scholar]
- 23.Christensen C., Lauridsen J. B., Berezin V., Bock E., Kiselyov V. V. (2006) FEBS Lett. 580, 3386–3390 [DOI] [PubMed] [Google Scholar]
- 24.Bribián A., Barallobre M. J., Soussi-Yanicostas N., de Castro F. (2006) Mol. Cell Neurosci. 33, 2–14 [DOI] [PubMed] [Google Scholar]
- 25.González-Martínez D., Kim S. H., Hu Y., Guimond S., Schofield J., Winyard P., Vannelli G. B., Turnbull J., Bouloux P. M. (2004) J. Neurosci. 24, 10384–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodé C., Levilliers J., Dupont J. M., De, Paepe A., Le, Dû N., Soussi-Yanicostas N., Coimbra R. S., Delmaghani S., Compain-Nouaille S., Baverel F., Pêcheux C., Le, Tessier D., Cruaud C., Delpech M., Speleman F., Vermeulen S., Amalfitano A., Bachelot Y., Bouchard P., Cabrol S., Carel J. C., Delemarre-van de, Waal H., Goulet-Salmon B., Kottler M. L., Richard O., Sanchez-Franco F., Saura R., Young J., Petit C., Hardelin J. P. (2003) Nat. Genet. 33, 463–465 [DOI] [PubMed] [Google Scholar]
- 27.Franco B., Guioli S., Pragliola A., Incerti B., Bardoni B., Tonlorenzi R., Carrozzo R., Maestrini E., Pieretti M., Taillon-Miller P., Brown C., Willard H., Lawrence C., Persico G., Camerino G., Ballabio A. (1991) Nature 353, 529–536 [DOI] [PubMed] [Google Scholar]
- 28.Legouis R., Hardelin J. P., Levilliers J., Claverie J. M., Compain S., Wunderle V., Millasseau P., Le Paslier D., Cohen D., Caterina D., Bougueleret L., Delemarre-Van de Waal H., Lutfalla G., Weissenbach J., Petit C. (1991) Cell 67, 423–435 [DOI] [PubMed] [Google Scholar]
- 29.Hu Y., González-Martínez D., Kim S. H., Bouloux P. M. (2004) Biochem. J. 384, 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carafoli F., Saffell J. L., Hohenester E. (2008) J. Mol. Biol. 377, 524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vannelli G. B., Ensoli F., Zonefrati R., Kubota Y., Arcangeli A., Becchetti A., Camici G., Barni T., Thiele C. J., Balboni G. C. (1995) J. Neurosci. 15, 4382–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitteloud N., Quinton R., Pearce S., Raivio T., Acierno J., Dwyer A., Plummer L., Hughes V., Seminara S., Cheng Y. Z., Li W. P., Maccoll G., Eliseenkova A. V., Olsen S. K., Ibrahimi O. A., Hayes F. J., Boepple P., Hall J. E., Bouloux P., Mohammadi M., Crowley W. (2007) J. Clin. Invest. 117, 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandilands E., Akbarzadeh S., Vecchione A., McEwan D. G., Frame M. C., Heath J. K. (2007) EMBO Rep. 8, 1162–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cariboni A., Pimpinelli F., Colamarino S., Zaninetti R., Piccolella M., Rumio C., Piva F., Rugarli E. I., Maggi R. (2004) Hum. Mol. Genet. 13, 2781–2791 [DOI] [PubMed] [Google Scholar]
- 35.Hu Y., Sun Z., Eaton J. T., Bouloux P. M., Perkins S. J. (2005) J. Mol. Biol. 350, 553–570 [DOI] [PubMed] [Google Scholar]
- 36.Robertson A., MacColl G. S., Nash J. A., Boehm M. K., Perkins S. J., Bouloux P. M. (2001) Biochem. J. 357, 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrenacci D., Grimaldi M. R., Panetta V., Riano E., Rugarli E. I., Graziani F. (2006) BMC. Genet. 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bülow H. E., Berry K. L., Topper L. H., Peles E., Hobert O. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen S. K., Ibrahimi O. A., Raucci A., Zhang F., Eliseenkova A. V., Yayon A., Basilico C., Linhardt R. J., Schlessinger J., Mohammadi M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammadi M., Olsen S. K., Goetz R. (2005) Curr. Opin. Struct. Biol. 15, 506–516 [DOI] [PubMed] [Google Scholar]
- 41.Allen B. L., Rapraeger A. C. (2003) J. Cell Biol. 163, 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty P., Williams G., Williams E. J. (2000) Mol. Cell Neurosci. 16, 283–295 [DOI] [PubMed] [Google Scholar]
- 43.Zhang W., Swanson R., Xiong Y., Richard B., Olson S. T. (2006) J. Biol. Chem. 281, 37302–37310 [DOI] [PubMed] [Google Scholar]
- 44.Gill J. C., Moenter S. M., Tsai P. S. (2004) Endocrinology 145, 3830–3839 [DOI] [PubMed] [Google Scholar]
- 45.Tsai P. S., Moenter S. M., Postigo H. R., El, Majdoubi M., Pak T. R., Gill J. C., Paruthiyil S., Werner S., Weiner R. I. (2005) Mol. Endocrinol. 19, 225–236 [DOI] [PubMed] [Google Scholar]
- 46.Pitteloud N., Meysing A., Quinton R., Acierno J. S., Jr., Dwyer A. A., Plummer L., Fliers E., Boepple P., Hayes F., Seminara S., Hughes V. A., Ma J., Bouloux P., Mohammadi M., Crowley W. F., Jr. (2006) Mol. Cell. Endocrinol. 254–255, 60–69 [DOI] [PubMed] [Google Scholar]
- 47.Chung W. C., Moyle S. S., Tsai P. S. (2008) Endocrinology 149, 4997–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardelin J. P., Dodé C. (2008) Sex Dev. 2, 181–193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.