Abstract
Membrane fusion without lysis has been reconstituted with purified yeast vacuolar SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors), the SNARE chaperones Sec17p/Sec18p and the multifunctional HOPS complex, which includes a subunit of the SNARE-interactive Sec1-Munc18 family, and vacuolar lipids: phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidic acid (PA), cardiolipin (CL), ergosterol (ERG), diacylglycerol (DAG), and phosphatidylinositol 3-phosphate (PI3P). We now report that many of these lipids are required for rapid and efficient fusion of the reconstituted SNARE proteoliposomes in the presence of SNARE chaperones. Omission of either PE, PA, or PI3P from the complete set of lipids strongly reduces fusion, and PC, PE, PA, and PI3P constitute a minimal set of lipids for fusion. PA could neither be replaced by other lipids with small headgroups such as DAG or ERG nor by the acidic lipids PS or PI. PA is needed for full association of HOPS and Sec18p with proteoliposomes having a minimal set of lipids. Strikingly, PA and PE are as essential for SNARE complex assembly as for fusion, suggesting that these lipids facilitate functional interactions among SNAREs and SNARE chaperones.
Biological membrane fusion is the regulated rearrangement of the lipids in two apposed sealed membranes to form one bilayer while mixing lumenal contents without leakage or lysis. It is fundamental for intracellular vesicular traffic, cell growth and division, regulated secretion of hormones and other blood proteins, and neurotransmission and thus has attracted wide and sustained study (1, 2). Its fundamental mechanisms are conserved and employ a Rab-family GTPase, proteins which bind to the GTP-bound form of a Rab, termed its “effectors” (3), and SNARE3 (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins (4) with their attendant chaperones. SNAREs are integral or peripheral membrane proteins with characteristic heptad-repeat domains, which can associate in 4-helical coiled-coils (5), termed “cis-SNARE complexes,” if they are all anchored to the same membrane bilayer, or “trans-SNARE complexes” if they are anchored to apposed membranes.
Stable membrane proximity (docking) does not suffice for fusion. Studies in model systems have shown that fusion can be promoted by any of several agents, which promote bilayer rearrangement, such as diacylglycerol (6), high levels of calcium (7), viral-encoded fusion proteins (8, 9), or SNAREs (10, 11). These studies frequently employed liposomes or proteoliposomes of simple lipid composition, suggesting that fusion may not have stringent requirements of lipid head group species. However, each of these model fusion reactions is accompanied by substantial lysis (12–15), whereas the preservation of subcellular compartments is a hallmark of physiological membrane fusion.
We have studied membrane fusion with the vacuole (lysosome) of Saccharomyces cerevisiae (reviewed in Ref. 16). The fusion of isolated vacuoles requires the Rab Ypt7p, 4 SNAREs (Vam3p, Vti1p, Vam7p, and Nyv1p), the SNARE chaperones Sec17p (α-soluble N-ethylmaleimide-sensitive factor attachment protein)/Sec18p (N-ethylmaleimide-sensitive factor) and the hexameric HOPS complex (17), and key “regulatory” lipids including ERG, phosphoinositides, and DAG (18). HOPS interacts physically or functionally with each component of this fusion system. HOPS stably associates with Ypt7p in its GTP-bound state (19). One HOPS subunit, Vps33p, is a member of the Sec1-Munc18 family of SNARE-binding proteins, and HOPS exhibits direct affinity for SNAREs (17, 20–22) and proofreads correct vacuolar SNARE pairing (23). HOPS also has direct affinity for phosphoinositides (17). The SNAREs on isolated vacuoles are in cis-complexes, which are disassembled by Sec17p, Sec18p, and ATP (24). Docking requires Ypt7p (25) and HOPS (17). During docking, vacuoles are drawn against each other until each has a substantial membrane domain tightly apposed to the other. Each of the proteins (26) and lipids (18) required for fusion becomes enriched in a ring-shaped microdomain, the “vertex ring,” which surrounds the two tightly apposed membrane domains. Not only do the proteins depend on each other, in a cascade fashion, for vertex ring enrichment, and the lipids depend on each other for their vertex ring enrichment as well, but the lipids and proteins are mutually interdependent for their enrichment at this ring-shaped microdomain (18, 27). Fusion occurs around the ring, joining the two organelles. The fusion of vacuoles bearing physiological fusion constituents does not cause measurable organelle lysis, although fusion supported exclusively by higher levels of SNARE proteins is accompanied by massive lysis (28), in accord with model liposome studies (14). Thus fusion microdomain assembly and the coordinate action of SNAREs with other proteins and lipids to promote fusion without lysis are central topics in membrane fusion studies.
Reconstitution of fusion with pure components allows chemical definition of essential elements of this biologically important reaction. Although SNAREs can drive a slow fusion of PC/PS proteoliposomes (29), this was not stimulated by HOPS and Sec17p/Sec18p (30). SNARE proteoliposomes bearing all the vacuolar lipids (18, 31–33), PC, PE, PI, PS, CL, PA, ERG, DAG, PI3P, and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), showed rapid and efficient fusion that was fully dependent on Sec17p/Sec18p and HOPS (30). The omission of either DAG, ERG, or phosphoinositide from the liposomes caused a marked reduction in fusion (30). We now report that PE and PA are also necessary for rapid and efficient fusion, function in distinct manners, and are required for efficient assembly of newly formed SNARE complexes by the SNARE chaperones Sec17p/Sec18p and HOPS.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Three yeast vacuolar SNAREs, Vam3p, Vti1p, and Nyv1p, were produced in Escherichia coli Rosetta2 (Novagen) and purified as described previously (30).
For Vam7p (yeast vacuolar Qc-SNARE), the GST and linker sequences upstream of VAM7 were deleted from the expression vector for GST-Vam7p (18) by QuikChange mutagenesis kit (Stratagene) to purify full-length untagged Vam7p from inclusion bodies in E. coli Rosetta2(DE3)pLys (Novagen). After the induction at 37 °C for 3 h by 1 mm isopropyl 1-thio-β-d-galactopyranoside, cells from a 2-liter culture were harvested, suspended in 80 ml of buffer A (20 mm Hepes-NaOH (pH 8.0), 0.5 m NaCl) containing 1 mm phenylmethylsulfonyl fluoride and 1 μg/ml pepstatin, lysed by French press at 4 °C, and centrifuged (60-Ti, Beckman, 50,000 rpm, 30 min, 4 °C). Pellets were washed with 40 ml of buffer A containing 50 mm β-octylglucoside followed by 80 ml of buffer A, re-suspended with 20 ml of buffer A containing 50 mm dithiothreitol, 1 mm EDTA, and 6 m guanidine hydrochloride, and incubated at 37 °C for 1 h with shaking. The suspension was centrifuged (60-Ti, Beckman, 20,000 rpm, 30 min, 4 °C), and the supernatant was dialyzed against 2-liter portions of buffer A at 4 °C for 12 h, then 3 h, and centrifuged again (60-Ti, Beckman, 50,000 rpm, 30 min, 4 °C), yielding re-folded Vam7p in the supernatant.
Tobacco etch virus protease (23), affinity-purified antibodies against Vam3p, Vti1p, Vam7p, Nyv1p, Sec17p, Sec18p, and Vps33p (22), His6-Sec17p (34), His6-Sec18p (34), and HOPS-GST (23) were purified as described.
Preparation of SNARE Proteoliposomes
Reconstituted proteoliposomes (RPLs) were prepared with purified yeast vacuolar SNAREs as described (30). Detergent-mixed micellar solutions of lipids and SNAREs, including the inherently water-soluble Vam7p SNARE, were dialyzed to allow spontaneous proteoliposome formation and any assembly of SNAREs into cis-SNARE complexes (30). The RPLs were reconstituted with a variety of lipid compositions as shown in Table 1, and diC8-PI3P (Echelon) was the only phosphoinositide used in this study.
TABLE 1.
Lipid compositions of the SNARE RPLs used in this study
The percentages of POPC for each lipid composition are shown as percentage for donor RPLs/percentage for acceptor RPLs. Donor and acceptor RPL lipid mixes contain NBD-PE/rhodamine-PE (1.5% each) and dansyl-PE (1%), respectively.
| RPL lipid composition | Lipids |
|||||||
|---|---|---|---|---|---|---|---|---|
| POPC | POPE | SoyPI | ERG | DAG | POPS | POPA | CL | |
| %, mol/mol | ||||||||
| PC | 97/99 | |||||||
| PC/PE | 79/81 | 18 | ||||||
| PC/PE/PI | 61/63 | 18 | 18 | |||||
| PC/PE/PI/ERG/DAG | 52/54 | 18 | 18 | 8 | 1 | |||
| Complete | 44/46 | 18 | 18 | 8 | 1 | 4.4 | 2 | 1.6 |
| PC/PE/PS | 61/63 | 18 | 18 | |||||
| PC/PE/PA | 61/63 | 18 | 18 | |||||
| PC/PE/ERG | 61/63 | 18 | 18 | |||||
| PC/PE/DAG | 61/63 | 18 | 18 | |||||
| PC/PA | 79/81 | 18 | ||||||
| Complete minus PA | 46/48 | 18 | 18 | 8 | 1 | 4.4 | 1.6 | |
| Complete minus PE | 62/64 | 18 | 8 | 1 | 4.4 | 2 | 1.6 | |
Proteoliposome Lipid Mixing Assay
Lipid mixing between the SNARE-RPLs was assayed as described (30), with modifications. RPL reaction mixtures (RB150 (20 mm HEPES-NaOH (pH 7.4), 0.15 m NaCl, 10% glycerol) with 1 mm ATP, 2 mm MgCl2, 50 μm donor RPLs, 400 μm acceptor RPLs, and 90 μm diC8-PI3P) were prepared in black 384-well plates (#3676, Corning) on ice and incubated at 27 °C for 10 min in a SpectraMAX Gemini XPS plate reader (Molecular Devices) pre-equilibrated at 27 °C. After 10 min, His6-Sec17p (1.2 μm), His6-Sec18p (1.0 μm), HOPS-GST (55 nm), and Vam7p (600 nm) were added to the RPL reactions where indicated, followed by further 60-min incubation at 27 °C in the plate reader. During the lipid mixing reactions, NBD fluorescence was monitored. For calculating the ratios of NBD fluorescence, F/F0, the fluorescence just before the Sec17p/Sec18p/HOPS addition (at 0 min) was used for F0, except in Fig. 6, where the lowest values during the initial 10-min incubation were used for F0. Topology of lipid mixing (Fig. 2D) was analyzed by addition of sodium dithionite (Sigma) to lipid mixing reactions while monitoring NBD fluorescence, as described previously (30).
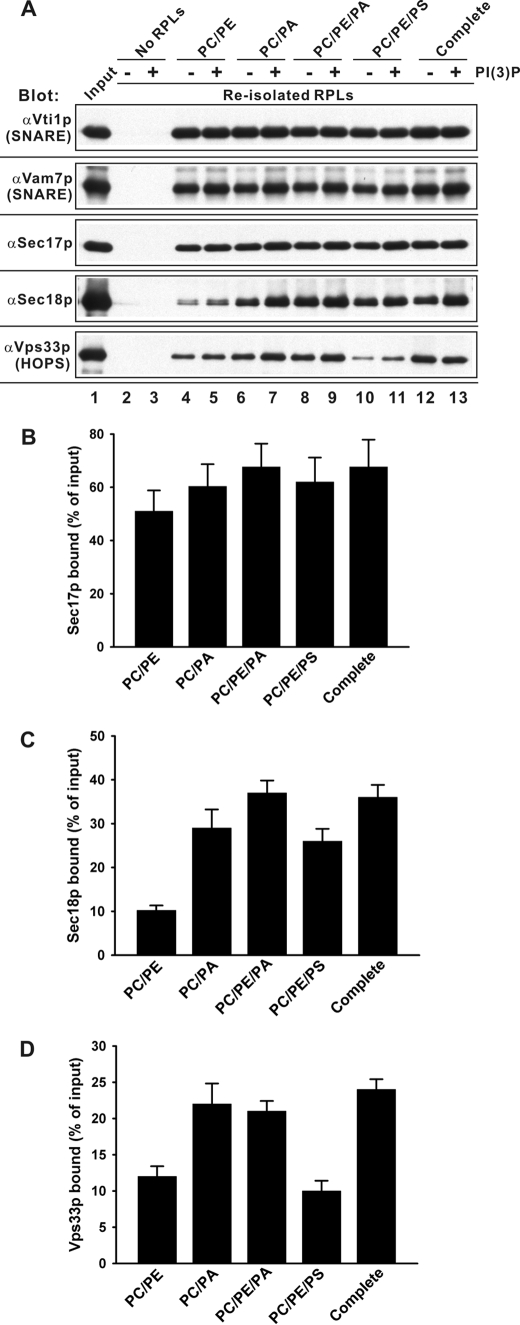
FIGURE 2.
Membrane association of soluble components with the RPLs. 4-SNARE RPLs with a variety of lipid compositions (PC/PE, PC/PA, PC/PE/PA, PC/PE/PS, and complete) were incubated with SNARE chaperones and diC8-PI3P as in Fig. 1 (27 °C for 30 min), then re-isolated by Histodenz-gradient ultracentrifugation. A, proteins that floated with the RPLs were analyzed by immunoblotting. B–D, mean values and standard deviations of RPL-bound Sec17p, Sec18p, and Vps33p in the presence of diC8-PI3P for the experiment described in A, performed in triplicate.
Flotation Assays of Liposome Association
4-SNARE RPL reactions (100 μl each, 450 μm donor RPLs) in RB150, with 1 mm ATP, 2 mm MgCl2, 90 μm diC8-PI3P, 1.2 μm His6-Sec17p, 1.0 μm His6-Sec18p, and 55 nm HOPS-GST were incubated at 27 °C for 30 min, placed on ice for 10 min, and mixed with 400 μl of 50% Histodenz (Sigma) in RB150 to a final Histodenz concentration of 40%, then transferred to 11 × 60 mm tubes, overlaid with 1.5 ml of 35% Histodenz in RB150, 2 ml of 30% Histodenz in RB150, and 200 μl of RB150, and centrifuged (SW60Ti, 55,000 rpm, 3 h, 4 °C). RPLs, which floated, were harvested in 400 μl from the top of the gradients and analyzed by SDS-PAGE and immunoblotting with affinity-purified antibodies against Vti1p, Vam7p, Sec17p, Sec18p, and Vps33p.
SNARE Complex Assembly Assay
RPL lipid mixing reactions (20 μl each), which had been prepared with Qabc-SNARE RPLs, R-SNARE RPLs, diC8-PI3P, and SNARE chaperones, as described above, were incubated at 27 °C for 40 min while monitoring NBD fluorescence, then transferred to 1.7-ml microcentrifuge tubes on ice and left for 10 min. The RPL reactions were mixed with αVam3p (5 μl each, 1.7 μm final), incubated on ice for 10 min, further mixed with 230 μl each of Protein A-Sepharose (Amersham Biosciences) in RB150 with 1% Triton X-100, and nutated at 4 °C for 30 min. The Protein A-Sepharose beads were washed with 500 μl each of RB150 with 1% Triton X-100 three times. Vam3p and Nyv1p bound to the Protein A-Sepharose beads were eluted from the beads with 5× SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting.
RESULTS
Membrane fusion can be assayed by the resultant lipid mixing. Two sets of liposomes are prepared, one bearing NBD-PE and sufficient rhodamine-PE to quench the NBD fluorescence and another set of liposomes that have neither of these fluorophores. Upon fusion, dilution of the fluorophores by lipid mixing relieves the quenching, and the resulting NBD fluorescence is a measure of fusion (35). Proteoliposomes, which bear the four vacuolar SNAREs, will fuse when given purified HOPS, Sec17p, Sec18p, and ATP if they are composed of vacuolar lipids (30). We express the fusion as F/Fo, the fluorescence F at each time normalized for the fluorescence immediately before the addition of soluble SNARE and SNARE chaperones, Fo. At the end of each incubation, detergent is added and the maximally dequenched NBD fluorescence, Fm, is measured (calculated values of [F − Fo/Fm − Fo] × 100% are presented in the figure legends). The vacuolar lipid composition is PC, PE, PI, PS, CL, PA, ERG, DAG, PI3P, and PI(4,5)P2 (18, 31–33). Fusion was shown to be depressed ∼50% by the omission of DAG and to be even more depressed by omission of ERG, PI(4,5)P2, or PI3P (30). We now report that PA and PE have important and distinct roles in fusion as well.
SNARE proteoliposomes were prepared by the dialysis method of detergent removal from mixed micellar solutions, which bore all four SNAREs, the R-SNARE Nyv1p alone, the two integral Qab-SNAREs Vti1p and Vam3p, or all three Qabc-SNAREs (supplemental Fig. S1, A–D) and lipid mixtures ranging in complexity from PC alone to the complete vacuolar lipid mixture except for phosphoinositides (Table 1); diC8-PI3P can be added independently (30) and will fulfill all the roles of phosphoinositides for fusion.4 In all cases, the level of reconstitution of SNAREs was unaffected by the lipid composition (supplemental Fig. S1). Nevertheless, as shown below, lipid composition has a profound effect on fusion.
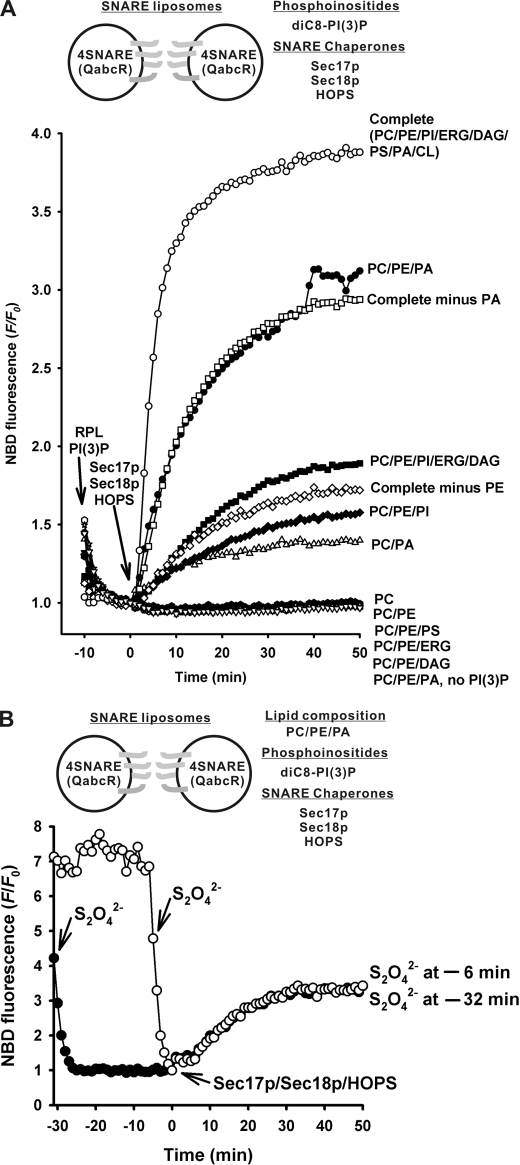
Proteoliposomes with all four vacuolar SNAREs and the complete vacuole lipid mixture were incubated with diC8-PI3P, then mixed with Sec17p, Sec18p, and HOPS to initiate rapid and complete fusion (Fig. 1A, open circles), as reported.4 Omission of three of the acidic lipids, PS, PA, and CL, caused a 14-fold reduction in the initial rate of fusion (filled squares), which was further reduced 2.3-fold by the omission of small headgroup lipids ERG and DAG (filled diamonds). Removal of PI from this mixture abolished fusion entirely; we therefore determined whether PI, or another lipid, would optimally complement PC and PE for fusion. PA was optimal for this function, and rapid fusion was seen with RPLs bearing PC, PE, and PA (filled circles). Other acidic (PS) or small headgroup (DAG and ERG) lipids could not substitute for PA at all. Thus PC, PE, PA, and PI3P are a minimal set of lipids for reconstitution of fusion; the single omission of any of the latter three of this set causes drastic loss of fusion (Fig. 1A). Were PA and PE only important for fusion with a minimal lipid composition, or did they also have an important role in the complex, complete vacuolar lipid mixture? The rapid initial rate of fusion characteristic of the complete lipid mixture (Fig. 1A, open circles) was diminished 2.9-fold by the single omission of PA and 9.8-fold by the single omission of PE. Although there is some redundancy to lipid function, optimal fusion requires both of these lipids.
FIGURE 1.
Minimal lipid composition required for SNARE- and SNARE chaperone-dependent membrane fusion. All reactions of RPL lipid mixing were assayed in RB150 with 4SNARE-RPLs (450 μm total lipids, 300–750 nm of each SNARE), ATP (1 mm), MgCl2 (2 mm), diC8-PI3P (90 μm), Sec17p (1.2 μm), Sec18p (1.0 μm), and HOPS (55 nm). Each panel shows representative data from at least three experiments. A, lipid composition regulates the fusion of SNARE proteoliposomes. Maximal dequenched fluorescence, Fm, was assayed at 50 min by addition of β-octylglucoside. The percent maximal dequenching, [F − Fo/Fm − Fo] × 100%, was 93% for RPLs of the complete lipid mix, but 71 and 31% when PA or PE had been omitted. It was 103% for RPLs bearing PC/PE/PA (supplemented with PI3P), but was 31% with PC/PE/PI/ERG/DAG, 28% with PC/PE/PI, and 24% with PC/PA. All others were ∼0%. B, the lipid mixing of 4-SNARE-RPLs with the minimal lipid composition, PC/PE/PA/PI3P, represents complete membrane fusion with little accompanying lysis. Topology analysis of the lipid mixing reactions was assayed in the presence of sodium dithionite (S2O42−) (30, 37). Sodium dithionite (4 mm in final) was added either 32 or 6 min before the Sec17p/Sec18p/HOPS addition and reduced the exposed NBD groups on the donor RPLs to inactivate their fluorescence. The sodium dithionite, which had been added 6 min before Sec17p/Sec18p/HOPS addition, was still active to reduce any accessible NBD groups, whereas dithionite, which had been added 32 min earlier, was not (30).
To determine whether the lipid mixing between proteoliposomes bearing four vacuolar SNAREs and PC, PE, PA, and PI3P in the presence of the SNARE chaperones Sec17p/Sec18p and HOPS represents authentic fusion between sealed membrane compartments or lysis and re-annealing, we exploited a published assay (30, 36, 37) that uses the membrane-impermeable reductant dithionite (S2O42−). The fluorescence donor proteoliposomes in our fusion reactions have NBD-PE and rhodamine-PE on both outer and inner monolayers. Exposure of these proteoliposomes to dithionite reduced the NBD-PE to N-7-amino-2,1,3-benzoxadiazol-4-yl-PE, destroying its fluorescence (Fig. 1B, filled circles, −32 to −26 min). The NBD-PE on the inner monolayer of these sealed proteoliposomes, though inaccessible to S2O42−, remained strongly quenched by rhodamine-PE. After 32 min, the S2O42− in this solution is fully oxidized and can no longer destroy the NBD fluorescence (30). Addition of the HOPS, Sec17p, and Sec18p SNARE-chaperones triggered lipid mixing. An identical mixture of proteoliposomes and diC8-PI3P was incubated in parallel but without S2O42−, then mixed with S2O42− only 6 min before the addition of SNARE chaperones (open circles). Although the S2O42− in this second sample was largely active at the time of chaperone-triggered lipid mixing (30), mixing-induced dequenching occurred at the same rate in each sample, showing that this lipid mixing was accompanied by maintenance of a sealed membrane, which excluded the S2O42− reductant.
We next determined whether PE or PA were required for the steady-state association of peripherally bound fusion proteins to the proteoliposomal membrane. 4-SNARE proteoliposomes were mixed with diC8-PI3P or control buffer, and then fusion was initiated with a mixture of the three SNARE chaperones (Sec17p, Sec18p, and HOPS). After 30 min, proteoliposomes were re-isolated by flotation and assayed by immunoblot for bound Vti1p (an integral membrane SNARE, as control) or peripheral membrane proteins Vam7p, Sec17p, Sec18p, and HOPS (Fig. 2). HOPS (detected via its Vps33p subunit), Sec18p, Sec17p, and Vam7p associated with proteoliposomes of PC, PE, and PA (lane 8, no PI3P; lane 9, with PI3P) to a similar extent as with proteoliposomes bearing the complete vacuolar mixture (lanes 12 and 13). Omission of PE from the minimal mixture had little effect on the association of peripheral membrane proteins (lanes 6 and 7), but omission of PA (lanes 4 and 5) or substitution of PS for PA (lanes 10 and 11) reduced the level of bound Sec18p and HOPS (Fig. 2, C and D). Fusion between 4-SNARE-RPLs was diminished by reduced Sec18p or HOPS (Fig. S2). Purified HOPS has direct affinity for protein-free liposomes bearing PA in addition to its affinity for protein-free liposomes bearing phosphoinositides (17).
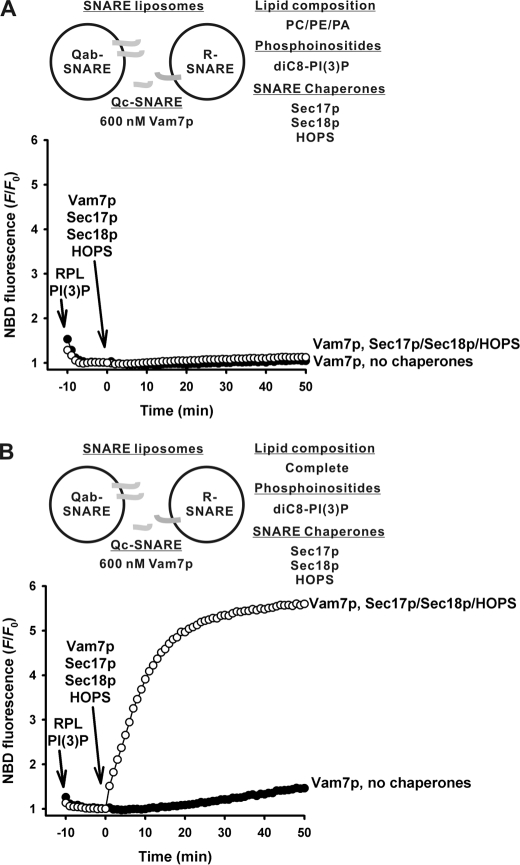
The requirements for PE and PA for fusion were even more strict when the proteoliposomes, which were assayed for fusion, bore separate SNARE populations (Fig. 3). With 3Q- and 1R-SNARE proteoliposomes, chaperone-dependent fusion was seen with PC/PE/PA proteoliposomes in the presence of diC8-PI3P (Fig. 3A), but omission of either PE (Fig. 3B) or PA (Fig. 3C), or substitution of PS for PA (Fig. 3D), abolished fusion. However, when the two integral membrane Qab-SNAREs were on the acceptor proteoliposomes and the soluble Qc-SNARE Vam7p was added along with SNARE chaperones (Fig. 4), even PC/PE/PA proteoliposomes, which were supplemented with PI3P, 600 nm Vam7p, and the SNARE chaperones, could not support fusion (Fig. 4A), although the full vacuolar lipid mix supported fusion well (Fig. 4B). Excess Vam7p (6 μm) could support mixing of lipids between PC/PE/PA SNARE proteoliposomes with PI3P, but this was independent of SNARE chaperones and showed little response to their addition (data not shown).
FIGURE 3.
The minimal lipids, PC/PE/PA/PI3P, are required and sufficient for SNARE chaperones to promote fusion between Qabc-SNARE RPLs and R-SNARE RPLs. A–E, lipid mixing between the RPLs bearing Qabc-SNAREs (400 μm total lipids, 270–670 nm each SNARE), and the RPLs bearing the R-SNARE (50 μm total lipids, 60 nm SNARE) were assayed in RB150, 1 mm ATP, and 2 mm MgCl2, and 90 μm diC8-PI3P, in the absence or in the presence of Sec17p (1.2 μm), Sec18p (1.0 μm), and HOPS (55 nm). The RPL lipid compositions used are PC/PE/PA (A), PC/PA (B), PC/PE (C), PC/PE/PS (D), and complete (E), where indicated. The percent maximal dequenching, [F − Fo/Fm − Fo] × 100%, in A was 59% with chaperones, 13% in the absence of chaperones and in E was 75% with SNARE chaperones, 26% in their absence. The experiment shown is representative of more than three experiments.
FIGURE 4.
The minimal lipids do not support fusion with Qab-SNARE RPLs, R-SNARE RPLs, and exogenous Qc-SNARE Vam7p. A and B, lipid mixing between RPLs bearing Qab-SNAREs (400 μm total lipids, 390–500 nm each SNARE) and RPLs bearing the R-SNARE (50 μm total lipids, 50–71 nm SNARE) were assayed in RB150, 1 mm ATP, 2 mm MgCl2, 90 μm diC8-PI3P, and 600 nm exogenous Vam7p, in the absence or presence of Sec17p (1.2 μm), Sec18p (1.0 μm), and HOPS (55 nm). The RPL lipid compositions used are PC/PE/PA (A) and complete (B), where indicated. Maximal dequenched fluorescence, Fm, was assayed at 50 min by addition of β-octylglucoside. The percent maximal dequenching, [F − Fo/Fm − Fo] × 100%, as defined in Fig. 1, was 86% when RPLs bore the vacuolar complete set of vacuolar lipids (B) and chaperones were added (open circles) but only 8% when the chaperones were omitted (filled circles). The experiment shown is representative of more than three experiments.
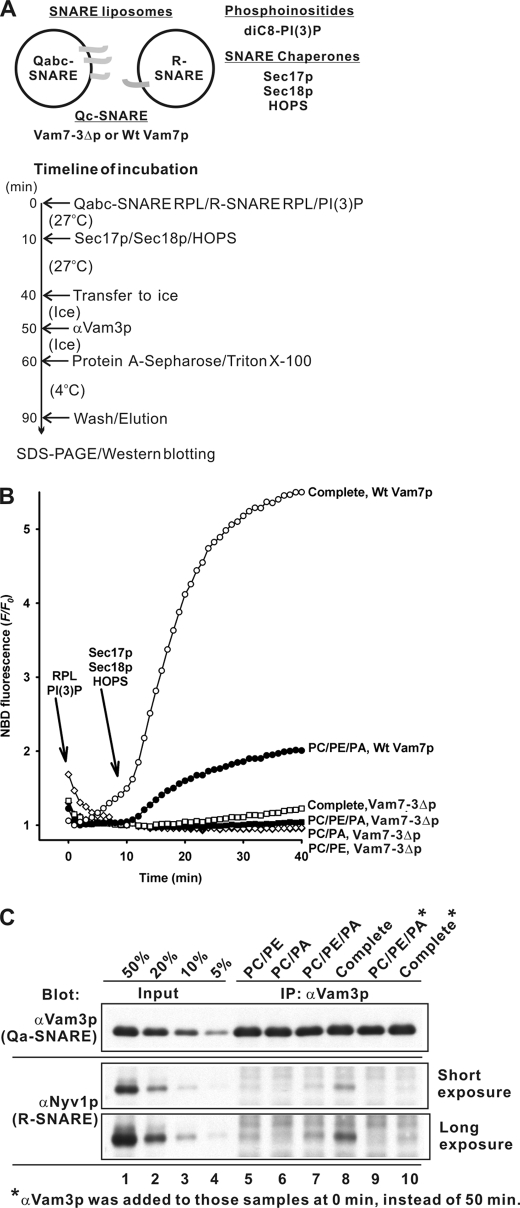
Trans-SNARE complex formation is required to initiate lipid mixing and membrane fusion. Specific “regulatory” lipids are important for SNARE enrichment in vacuolar fusion domains (18), but may also be required for the lipid rearrangements in each membrane leaflet, which accompany subreactions such as hemifusion and fusion completion. To assay the role of lipids in SNARE complex assembly, we employed proteoliposomes with either the single R-SNARE Nyv1p or with three Qabc-SNAREs (Fig. 5A), prepared with either wild-type Vam7p or with Vam7-3Δp, a C-terminally truncated form of Vam7p that has been shown to support SNARE pairing on vacuoles but to not support fusion (38). Proteoliposomes had either simple lipid compositions (PC/PE, PC/PA, or PC/PE/PA) or the complete vacuolar lipid mix. These proteoliposomes, with the Vam3p Qa-SNARE and the Nyv1p R-SNARE on separate vesicles, were mixed, supplemented with diC8-PI3P, and then given a mixture of the SNARE chaperones (Sec17p, Sec18p, and HOPS). After a 30-min incubation, proteoliposomes that bore Vam7-3Δp and had therefore exhibited arrested fusion were assayed for trans-SNARE complexes which included Vam3p and Nyv1p. The complete vacuolar lipid mixture supported the most robust fusion (Figs. 3 and 5B), and the association of Nyv1p with Vam3p reflected the formation of new SNARE complex (Fig. 5C, lane 8). Although <5% of RPLs bearing Vam7-3Δp underwent fusion (Fig. 5B), 20% of the Nyv1p had become associated with Vam3p (Fig. 5C), showing that this represented trans-SNARE complex formation. The three Q-SNARE and one R-SNARE proteoliposomes of the minimal PC/PE/PA plus diC8-PI3P composition, which had approximately one-fourth the rate of fusion as liposome pairs of the same SNAREs with vacuolar lipid composition (Fig. 5B, open versus filled circles), showed a similar reduction in new SNARE complex (Fig. 5C, lane 7 versus 8), whereas single omission of either PE or PA abolished both fusion and new SNARE complex formation (Figs. 3 and 5, B and C). Thus lipid composition is not only important for allowing the specific bilayer rearrangements during fusion, but for the regulation of trans-SNARE complex formation.
FIGURE 5.
Lipids regulate trans-SNARE complex assembly. RPLs with 3Q- and 1R-SNARE, using either wild-type Vam7p or Vam7-3Δp (38) where indicated, and of lipid compositions PC/PE, PC/PA, and PC/PE/PA, and complete vacuole lipid mix were used. A, experimental design of SNARE complex assembly assays with Qabc-SNARE RPLs and R-SNARE RPLs. B, proteoliposomes supplemented with diC8-PI3P, Sec17p, Sec18p, and HOPS as in Fig. 3 were assayed for lipid mixing. C, the Nyv1p bound to Vam3p during the RPL lipid mixing reactions with Vam7-3Δp in B was analyzed as outlined in A by co-immunoprecipitation, SDS-PAGE, and immunoblotting. Antibody to Vam3p blocks SNARE complex formation (lanes 9 and 10); thus the SNARE complex seen in lanes 7 and 8 had formed before detergent addition.
DISCUSSION
The specific lipids are as important for the homotypic fusion of vacuole membranes as the fusion proteins (18). Of the 10 known vacuolar lipid species (18, 31–33), 7 are implicated in the membrane fusion process. The need for 3- and 4-phosphoinositides, ERG, and DAG was first appreciated through studies of intact cells (39) and purified vacuoles (18, 33, 40–42), and this has been confirmed and extended to PE and PA through this study of fusion reconstituted with all-purified components. We consider these lipid requirements below and propose a model of their coordinated role with proteins for membrane fusion.
Exploiting a colorimetric assay of the fusion of purified vacuoles, specific roles for 3- and 4-phosphoinositides were inferred from assay inhibition by specific antibodies, ligands, and lipases (40, 42). Phosphoinositides were also implicated in vacuole fusion by a systematic screen of the library of yeast strains with deletions in nonessential genes (39). Vacuoles constantly undergo fusion and fission, regulated by the osmolarity of the growth medium; mutations that slow or block fusion but permit undiminished fission cause a vacuole morphology (vam) phenotype (43) of multiple small, unfused vacuoles. Deletion of nonessential genes in phosphoinositide metabolism gave a strong vam phenotype. What are the fusion functions of phosphoinositides? PI3P was shown to contribute to fusion by binding the PX domain of the Vam7p SNARE (42, 44), and PI(4,5)P2 may serve as a precursor for DAG through the action of phospholipase C (33). Phosphoinositides are also thought to contribute to fusion through their affinity for HOPS (17) and have multiple functions in fusion that are unrelated to the membrane association of peripheral fusion proteins.4 They become highly enriched in the vertex ring microdomain of docked vacuoles (18), along with ERG and DAG and fusion proteins, including the SNAREs, Ypt7p, and HOPS (26); the enrichment of each of these lipids and proteins is highly interdependent (18, 27).
ERG was first implicated in vacuole fusion by the aforementioned vam screen (39), in which strains bearing deletion of any of several nonessential genes of ERG biosynthesis showed a fragmented vacuole morphology. Biochemical studies (41) showed that the fusion of the isolated organelle was sensitive to ERG ligands or to modulation of sterol levels by β-methylcyclodextrin. Reconstituted proteoliposome fusion was diminished when ERG was omitted from the lipid mixture (30). Sterols are believed to stabilize microdomains by slowing the egress of enriched factors (45, 46), which might contribute to vacuole fusion; ERG enrichment in the fusion-active vertex ring microdomain of docked vacuoles is interdependent with the enrichment of other fusion lipids and proteins (18).
DAG, or phospholipase C, which generates DAG, can by itself trigger the fusion of protein-free liposomes (47, 48). Recombinant C1b domain, a DAG ligand, was used to show that DAG is important for fusion (33) and is enriched at vertex fusion microdomains (18). DAG only has a modest, 2-fold role in stimulating the fusion of reconstituted proteoliposomes with vacuolar SNAREs and lipids (30).
The advent of an authentic, lysis-free reconstituted fusion reaction with all pure components has allowed further study of the need for each lipid (30).5 We have now found that PA and PE have important roles in proteoliposome fusion. PA contributes to HOPS and Sec18p association with the proteoliposome membrane of a minimal lipid mix (PC/PE/PA/PI3P). HOPS has separable affinities for Ypt7p:GTP (19), Vam7p (17), Vam3p (49), and the assembled SNARE complex (22), as well as for phosphoinositides and PA (17), yet it is the product of these affinities that may together regulate HOPS association with the vacuole. PE is a nonbilayer lipid, and is required for rapid fusion of liposomes bearing either minimal or full vacuolar lipids (Fig. 1A). Further studies will be needed to determine whether PE contributes to fusion by promoting lipid rearrangements and nonbilayer phases. Because its omission blocks SNARE complex assembly (Fig. 5), it is unlikely to only function by promoting nonbilayer structure. It is also possible that PE might regulate the capacity of SNARE chaperones to act on the SNAREs.
Specific lipids are known to regulate other membrane fusion reactions. PI(4,5)P2 confers both positive and negative regulation on PC12 exocytosis (50). Phosphatidic acid has been implicated in many fusion systems, from SNARE-driven liposomal fusion (51) to Glut4 vesicle traffic to the plasma membrane (52), PC12 cell exocytosis, regulated by direct interactions of syntaxin 1A with phosphatidic acid (53), prospore membrane fusion in S. cerevisiae (54), and mitochondrial fusion (55). Sterol is also important for other membrane fusion events, whether at the plasma membrane (46, 56, 57) or in chemically defined SNARE-dependent proteoliposome fusion (58, 59). Most of these fusion studies have examined the role of one or another lipid, but not the entire set of lipids as studied here.
Although reconstituted liposomal fusion reactions are powerful for their relative chemical simplicity, they lack the inherent asymmetric distribution of lipids and proteins of the intact cellular membrane. In addition, biological membranes have far higher ratios of protein:lipid than are commonly achieved in reconstitutions. These considerations limit the ability to quantitatively compare the need for each lipid and protein for in vitro fusion of reconstituted proteoliposomes to that seen with isolated organelles.
We present a working model of how lipids and proteins cooperate for vacuole fusion. The first function of lipids is to contribute to the affinity of tethering and fusion factors, such as HOPS, Vam7p, and Sec18p, for the membrane. The second role of the lipids is to cooperate in establishing a fusion microdomain on the organelle. This microdomain may provide an optimal environment for SNARE assembly and remodeling by the SNARE chaperones Sec17p/Sec18p and HOPS. Finally, certain lipids with bulky headgroups (e.g. PI(4,5)P2) or with small headgroups (e.g. PE, PA, or DAG) may have specific roles on each face of the apposed bilayers as they pass through the hemifusion and fusion completion stages. Asymmetric reconstitutions may be needed to fully test this model.
Supplementary Material
Acknowledgments
We thank laboratory members for fruitful discussions and Amy Burfeind for expert assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-GM23377.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Mima, J., and Wickner, W. (2009) Proc. Natl. Acad. Sci. U.S.A., in press.
C. Stroupe, C. M. Hickey, J. Mima, A. S. Burfeind, and W. Wickner, submitted for publication.
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- PC
- phosphatidylcholine
- PI
- phosphatidylinositol
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- PA
- phosphatidic acid
- CL
- cardiolipin
- ERG
- ergosterol
- DAG
- diacylglycerol
- PI3P
- phosphatidylinositol 3-phosphate
- PI(4,5)P2
- phosphatidylinositol (4,5)-bisphosphate
- RPL
- reconstituted proteoliposome
- HOPS
- homotypic vacuole fusion and protein sorting complex
- GST
- glutathione S-transferase.
REFERENCES
- 1.Jahn R., Lang T., Südhof T. C. (2003) Cell 112, 519–533 [DOI] [PubMed] [Google Scholar]
- 2.Wickner W., Schekman R. (2008) Nat. Struct. Mol. Biol. 15, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosshans B. L., Ortiz D., Novick P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahn R., Scheller R. H. (2006) Nat. Rev. Mol. Cell Biol. 7, 631–643 [DOI] [PubMed] [Google Scholar]
- 5.Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. (1998) Nature 395, 347–353 [DOI] [PubMed] [Google Scholar]
- 6.Allan D., Thomas P., Michell R. H. (1978) Nature 276, 289–290 [DOI] [PubMed] [Google Scholar]
- 7.Ingolia T. D., Koshland D. E., Jr. (1978) J. Biol. Chem. 253, 3821–3829 [PubMed] [Google Scholar]
- 8.Melikyan G. B., Brener S. A., Ok D. C., Cohen F. S. (1997) J. Cell Biol. 136, 995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong R. T., Kushnir A. S., White J. M. (2000) J. Cell Biol. 151, 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 11.McNew J. A., Weber T., Parlati F., Johnston R. J., Melia T. J., Söllner T. H., Rothman J. E. (2000) J. Cell Biol. 150, 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess S. W., McIntosh T. J., Lentz B. R. (1992) Biochemistry 31, 2653–2661 [DOI] [PubMed] [Google Scholar]
- 13.Nickel W., Weber T., McNew J. A., Parlati F., Söllner T. H., Rothman J. E. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12571–12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennison S. M., Bowen M. E., Brunger A. T., Lentz B. R. (2006) Biophys. J. 90, 1661–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau W. L., Ege D. S., Lear J. D., Hammer D. A., DeGrado W. F. (2004) Biophys. J. 86, 272–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowicz C. W., Meiringer C. T., Ungermann C. (2008) Autophagy 4, 5–19 [DOI] [PubMed] [Google Scholar]
- 17.Stroupe C., Collins K. M., Fratti R. A., Wickner W. (2006) EMBO J. 25, 1579–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fratti R. A., Jun Y., Merz A. J., Margolis N., Wickner W. (2004) J. Cell Biol. 167, 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seals D. F., Eitzen G., Margolis N., Wickner W. T., Price A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price A., Seals D., Wickner W., Ungermann C. (2000) J. Cell Biol. 148, 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato T. K., Rehling P., Peterson M. R., Emr S. D. (2000) Mol. Cell 6, 661–671 [DOI] [PubMed] [Google Scholar]
- 22.Collins K. M., Thorngren N. L., Fratti R. A., Wickner W. T. (2005) EMBO J. 24, 1775–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starai V. J., Hickey C. M., Wickner W. (2008) Mol. Biol. Cell 19, 2500–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungermann C., Nichols B. J., Pelham H. R., Wickner W. (1998) J. Cell Biol. 140, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer A., Wickner W. (1997) J. Cell Biol. 136, 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Seeley E. S., Wickner W., Merz A. J. (2002) Cell 108, 357–369 [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Merz A. J., Collins K. M., Wickner W. (2003) J. Cell Biol. 160, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starai V. J., Jun Y., Wickner W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13551–13558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda R., McNew J. A., Weber T., Parlati F., Engel T., Nickel W., Rothman J. E., Söllner T. H. (2000) Nature 407, 198–202 [DOI] [PubMed] [Google Scholar]
- 30.Mima J., Hickey C. M., Xu H., Jun Y., Wickner W. (2008) EMBO J. 27, 2031–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinser E., Sperka-Gottlieb C. D., Fasch E. V., Kohlwein S. D., Paltauf F., Daum G. (1991) J. Bacteriol. 173, 2026–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneiter R., Brügger B., Sandhoff R., Zellnig G., Leber A., Lampl M., Athenstaedt K., Hrastnik C., Eder S., Daum G., Paltauf F., Wieland F. T., Kohlwein S. D. (1999) J. Cell Biol. 146, 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jun Y., Fratti R. A., Wickner W. (2004) J. Biol. Chem. 279, 53186–53195 [DOI] [PubMed] [Google Scholar]
- 34.Haas A., Wickner W. (1996) EMBO J. 15, 3296–3305 [PMC free article] [PubMed] [Google Scholar]
- 35.Struck D. K., Hoekstra D., Pagano R. E. (1981) Biochemistry 20, 4093–4099 [DOI] [PubMed] [Google Scholar]
- 36.McIntyre J. C., Sleight R. G. (1991) Biochemistry 30, 11819–11827 [DOI] [PubMed] [Google Scholar]
- 37.Meers P., Ali S., Erukulla R., Janoff A. S. (2000) Biochim. Biophys. Acta 1467, 227–243 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz M. L., Merz A. J. (2009) J. Cell Biol. 185, 535–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeley E. S., Kato M., Margolis N., Wickner W., Eitzen G. (2002) Mol. Biol. Cell 13, 782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer A., Scheglmann D., Dove S., Glatz A., Wickner W., Haas A. (2000) Mol. Biol. Cell 11, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato M., Wickner W. (2001) EMBO J. 20, 4035–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boeddinghaus C., Merz A. J., Laage R., Ungermann C. (2002) J. Cell Biol. 157, 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada Y., Ohsumi Y., Anraku Y. (1992) J. Biol. Chem. 267, 18665–18670 [PubMed] [Google Scholar]
- 44.Cheever M. L., Sato T. K., de Beer T., Kutateladze T. G., Emr S. D., Overduin M. (2001) Nat. Cell Biol. 3, 613–618 [DOI] [PubMed] [Google Scholar]
- 45.Valdez-Taubas J., Pelham H. R. (2003) Curr. Biol. 13, 1636–1640 [DOI] [PubMed] [Google Scholar]
- 46.Lang T., Bruns D., Wenzel D., Riedel D., Holroyd P., Thiele C., Jahn R. (2001) EMBO J. 20, 2202–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez-Migallón M. P., Aranda F. J., Gómez-Fernández J. C. (1995) Biophys. J. 68, 558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villar A. V., Alonso A., Goñi F. M. (2000) Biochemistry 39, 14012–14018 [DOI] [PubMed] [Google Scholar]
- 49.Dulubova I., Yamaguchi T., Wang Y., Südhof T. C., Rizo J. (2001) Nature Struct. Biol. 8, 258–264 [DOI] [PubMed] [Google Scholar]
- 50.James D. J., Khodthong C., Kowalchyk J. A., Martin T. F. (2008) J. Cell Biol. 182, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S., Wilson K. A., Rice-Stitt T., Neiman A. M., McNew J. A. (2007) Traffic 8, 1630–1643 [DOI] [PubMed] [Google Scholar]
- 52.Vicogne J., Vollenweider D., Smith J. R., Huang P., Frohman M. A., Pessin J. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14761–14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam A. D., Tryoen-Toth P., Tsai B., Vitale N., Stuenkel E. L. (2008) Mol. Biol. Cell 19, 485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakanishi H., Morishita M., Schwartz C. L., Coluccio A., Engebrecht J., Neiman A. M. (2006) J. Cell Sci. 119, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 55.Choi S. Y., Huang P., Jenkins G. M., Chan D. C., Schiller J., Frohman M. A. (2006) Nat. Cell Biol. 8, 1255–1262 [DOI] [PubMed] [Google Scholar]
- 56.Puri N., Roche P. A. (2006) Traffic 7, 1482–1494 [DOI] [PubMed] [Google Scholar]
- 57.Predescu S. A., Predescu D. N., Shimizu K., Klein I. K., Malik A. B. (2005) J. Biol. Chem. 280, 37130–37138 [DOI] [PubMed] [Google Scholar]
- 58.Chang J., Kim S. A., Lu X., Su Z., Kim S. K., Shin Y. K. (2009) Biophys. J. 96, 1839–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong J., Borbat P. P., Freed J. H., Shin Y. K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 5141–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.