Abstract
In yeast, trehalose is synthesized by a multimeric enzymatic complex: TPS1 encodes trehalose 6-phosphate synthase, which belongs to a complex that is composed of at least three other subunits, including trehalose 6-phosphate phosphatase Tps2 and the redundant regulatory subunits Tps3 and Tsl1. The product of the TPS1 gene plays an essential role in the control of the glycolytic pathway by restricting the influx of glucose into glycolysis. In this paper, we investigated whether the trehalose synthesis pathway could be involved in the control of the other energy-generating pathway: oxidative phosphorylation. We show that the different mutants of the trehalose synthesis pathway (tps1Δ, tps2Δ, and tps1,2Δ) exhibit modulation in the amount of respiratory chains, in terms of cytochrome content and maximal respiratory activity. Furthermore, these variations in mitochondrial enzymatic content are positively linked to the intracellular concentration in cAMP that is modulated by Tps1p through hexokinase2. This is the first time that a pathway involved in sugar storage, i.e. trehalose, is shown to regulate the mitochondrial enzymatic content.
The control of glycolysis in the yeast Saccharomyces cerevisiae has been extensively studied. First, allosteric regulation of the irreversible steps catalyzed by phosphofructokinase (1), pyruvate kinase (review in Ref. 2), and fructose-1,6-bisphosphatase (1) has been proposed, even though the overexpression of these key enzymes does not increase the glycolytic flux (3). Other mechanisms of control have been proposed such as futile cycle activity (4) and an inhibitory effect of ATP (5). Indeed, it seems likely that the regulation of glycolysis is a complex process involving different hierarchical events leading from gene expression to the metabolic fluxes via protein levels, enzyme activities, and metabolite effects (6, 7). Among these actors, the product of the TPS1 gene has been shown to play an essential role in the control of the glycolytic pathway by restricting the influx of glucose into glycolysis (8). TPS1 encodes trehalose 6-phosphate (Tre6P)3 synthase (9–12). This enzyme is part of a multimeric protein complex composed of at least three other subunits, i.e. Tre6P phosphatase encoded by TPS2 (13) and the redundant regulatory subunits Tps3 and Tsl1 (14).
A particularly intriguing finding is that tps1Δ mutants are defective not only for Tre6P synthesis but also for growth on glucose or related rapidly fermented sugars (8, 11, 15). This may be explained by an uncontrolled influx of glucose into the glycolytic pathway. This phenomenon is characterized by hyperaccumulation of glucose 6-phosphate, fructose 6-phosphate, and fructose 1,6-bisphosphate (Fru1,6bP) (8, 16–18) and depletion of ATP, Pi, and all intermediates of glycolysis downstream of glyceraldehyde-3-phosphate dehydrogenase (19). Several mutations have been described that suppress the growth defect of tps1Δ mutants apparently by reducing sugar influx into glycolysis (16, 20) or by diverting the excess sugar phosphate into glycerol synthesis through overexpression of the GPD1-encoded NAD-dependent glycerol-3-phosphate dehydrogenase (17, 21). Reconstitution of ethanolic fermentation in permeabilized yeast spheroplasts indicated that in addition to Tre6P, the Tps1 protein itself also seems to play a role in restricting glucose influx into glycolysis (22).
Whatever the mechanism by which the multimeric complex involved in trehalose synthesis controls glycolytic flux in yeast, such a regulation is associated with modification of the cellular content of sugar phosphates. Moreover, in a recent paper, we have shown that in yeast, low physiological concentrations of glucose 6-phosphate and fructose 6-phosphate slightly (20%) stimulate the respiratory flux and that this effect was strongly antagonized by Fru1,6bP (18). On the other hand, Fru1,6bP by itself is able to inhibit mitochondrial respiration only in mitochondria isolated from a Crabtree-positive strain. Taken together, these results indicate that besides the thermodynamic link between glycolysis and mitochondrial respiration (i.e. the cytosolic ATP/ADP and NADH/NAD+ ratio), a kinetic control of oxidative phosphorylation activity is exerted by the level of glycolytic sugar phosphates (18, 23). This raises the question of a possible direct or indirect regulation of oxidative phosphorylation by the trehalose synthesis pathway.
EXPERIMENTAL PROCEDURES
Cell Culture
The S. cerevisiae strains used in this work were the following: W303-1A, Mata leu2-3.112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 GAL SUC; tps1Δ, YSH648 (Mata leu2-3.112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 tps1::TRP1 GAL SUC; tps2Δ, YSH672 (Mata leu2-3.112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 tps2::LEU2 GAL SUC; tps1Δ tps2Δ, YSH652 (Mata leu2-3.112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 tps1::TRP1 tps2::LEU2 GAL SUC; hxk2Δ, YSH310 (Mata leu2-3.112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 hxk2Δ::LEU2 GAL SUC; tps1Δ hxk2Δ, YSH312 (Mata leu2-3.112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 tps1Δ::TRP1 hxk2Δ::LEU2 GAL SUC; and tps2Δ hxk2Δ, W303–1A (Mata hxk2::LEU2 tps2::HIS3. The cells were grown in a shaking incubator at 28 °C for one night in complete medium (YP, pH 5.5), containing 1% yeast extract, 0.1% potassium phosphate, 0.12% ammonium sulfate supplemented with 2% galactose (YPGal2%), or 2% lactate (YPLac2%) as a carbon source. The cells were harvested in the middle of the logarithmic growth phase at an optical density of 4.
Cellular Respiratory Rate
Oxygen consumption was measured at 28 °C in a 2-ml thermostatically controlled chamber equipped with a Clark oxygen electrode (Gilson) giving an on-line display of rate values.
The cells were incubated in the growing medium (YPGal2% or YPLac2%, spontaneous respiration) or the growing medium supplemented with 107 mm ethanol and 5 μm carbonyl cyanide m-chlorophenylhydrazone (uncoupled respiratory rate).
Cytochrome Content Measurements
Yeast cells were harvested, washed twice with distilled water, and concentrated to 20 mg of dry weight/ml. Cytochrome content was determined by using a dual beam spectrophotometer (Amico DW2000); a differential spectrum was performed between a cell oxidized state (in the presence of 1 μl of H2O2) and a cell reduced state (in the presence of dithionite). Calculations of cytochrome c + c1 and cytochrome b contents were performed using an extinction coefficient of 18,000 m−1 cm−1 for the wavelength pairs 550–540 and 561–575 nm, respectively (24). The calculation of cytochrome a + a3 contents was performed using an extinction coefficient of 12,000 m−1 cm−1 for the 603–630-nm interval (25).
Tre6P and cAMP Determination
Yeast cells grown on either YPGal2% or YPLac2% were harvested in exponential growth phase (optical density of 4). After the addition of 7% perchloric acid and glass beads, the cells were broken by mixing the test tube vigorously for 90 s on a vortex mixer. The yeast extract was transferred to a microcentrifuge tube, and the pH was adjusted to 6.8 with KOMO (0.3 m KOH, 2 m MOPS).
Tre6P was determined in cell extracts using a modification of the protocol described in Ref. 13. Briefly, the samples were loaded onto a CarboPac PA-1 column equilibrated with a solution containing 50 mm NaAc and 100 mm NaOH. The samples were eluted by increasing NaAc concentrations up to 400 mm over 15 min at constant NaOH concentration. Determination of cAMP in cell extracts was performed as described previously (26) using a cAMP Assay kit including a standard (Amersham Biosciences).
Statistical Analysis
The results are expressed as the means ± S.E. Statistical analysis was carried out using nonparametric Mann-Whitney test for all results. Prism software (GraphPad, San Diego, CA) was used for all tests. A p value of less than 0.05 was considered significant; n denotes the number of experiments.
RESULTS
Mutants in the Trehalose Pathway Exhibit Modifications in the Respiratory Chain Content
We assessed spontaneous respiratory rate as well as substrate-related respiratory rate in wild type, tps1Δ, tps2Δ, and tps1Δ tps2Δ cells (Table 1). The spontaneous respiratory rate is decreased in the tps1Δ cells, the tps2Δ cells exhibit a respiratory rate higher than the wild type, whereas in the double mutant tps1Δ tps2Δ, the respiratory rate is comparable with that in the tps1Δ cells. Although glucose addition has only a very slight effect on the tps2Δ respiratory rate, it drastically inhibits oxygen consumption in both the tps1Δ and tps1Δtps2Δ cells. In this condition, ethanol addition did not restore the respiratory rate in these mutants, confirming that glucose induced inhibition of respiratory rate is not due to a lack of substrate availability. For all four strains, respiratory rate with ethanol alone as substrate is slightly increased compared with spontaneous respiratory rate (Table 1). The maximal respiratory rate (i.e. in the presence of ethanol and uncoupler) is lower in tps1Δ cells and higher in tps2Δ cells compared with the wild type, whereas in the double mutant the maximal velocity is almost identical to the wild type one.
TABLE 1.
Cellular oxygen consumption rate of wild type and mutant strains
The respiratory rates of wild type and the three mutant strains were measured in growth conditions on YPGal2% when the optical density was ∼4 (spontaneous respiration). When added, glucose was 15 mm, ethanol was 107 mm, and CCCP was 5 μm. the values are the means ± S.E. of 10 measurements. nat O, nanoatoms of oxygen.
| Respiratory rate |
||||
|---|---|---|---|---|
| Wild type | tps1Δ | tps2Δ | tps1Δ tps2Δ | |
| nat O·min−1·mg−1 DW | ||||
| None (spontaneous) | 145 ± 7 | 111 ± 7a | 180 ± 8a | 105 ± 6a |
| Glucose | 144 ± 7 | 39 ± 4b | 151 ± 4 | 30 ± 5b |
| Ethanol + glucose | 166 ± 10 | 36 ± 4b | 174 ± 9 | 34 ± 3b |
| Ethanol | 167 ± 9 | 126 ± 10a | 187 ± 8 | 132 ± 7a |
| Ethanol + CCCP | 267 ± 16 | 193 ± 14a | 341 ± 24a | 251 ± 9 |
a The differences between wild type and mutant strains are significant at p < 0.05.
b The differences between wild type and mutant strains are significant at p < 0.001.
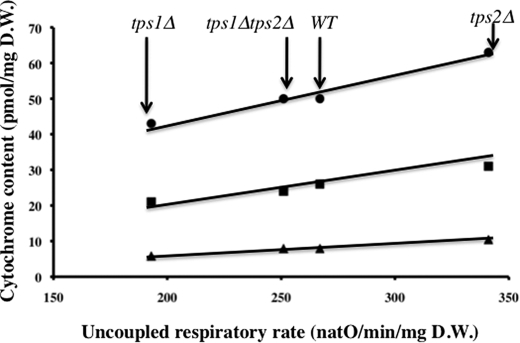
The cellular uncoupled respiratory rate is generally considered as an assessment of the total amount of respiratory chain components within the cell (27). To confirm that the amount of respiratory chain components was modified in the three mutant strains studied, we measured the cytochrome content of cells grown on galactose. Table 2 shows that cytochrome content in the four strains varies like the maximal respiratory rate, i.e. it is lower in tps1Δ cells and higher in tps2Δ cells compared with the wild type, whereas in the double mutant, it is almost identical to that in the wild type. Furthermore, Fig. 1 shows that there is a linear relationship between the amount of any of the cytochromes of the respiratory chain and the maximal respiratory rate in the different strains.
TABLE 2.
Cytochrome content of wild type, tps1Δ, tps2Δ, and tps1Δ tps2Δ mutant cells grown on YPGal2% medium
Cytochrome content was measured in cells harvested at an optical density of 4. The values are the averages ± S.E. of eight experiments made with different cultures.
| Cytochrome content |
||||
|---|---|---|---|---|
| Wild type | tps1Δ | tps2Δ | tps1Δ tps2Δ | |
| pmol·mg−1 DW | ||||
| c + c1 | 50 ± 4 | 43 ± 4 | 63 ± 5 | 50 ± 4 |
| b | 26 ± 2 | 21 ± 2 | 31 ± 4 | 24 ± 2 |
| a + a3 | 7.9 ± 0.5 | 5.8 ± 0.4a | 10.4 ± 0.6a | 7.9 ± 0.5 |
a The differences between wild type and mutant strains were significant at p < 0.05.
FIGURE 1.
Linear relationship between the amount of cytochromes and the uncoupled respiratory rate for cells grown in YPGal 2%. The cells were harvested in the middle of the logarithmic growth phase (optical density of 4). Uncoupled respiration was measured in the growing medium (YPGal2%) supplemented with 107 mm ethanol and 5 μm CCCP. Cytochrome content was assessed as described under “Experimental Procedures.” The strains used are indicated on the figure. ●, c + c1; ■, b; ▲, a + a3. The values are the averages of eight experiments made with different cultures. WT, wild type. natO, nanoatoms of oxygen.
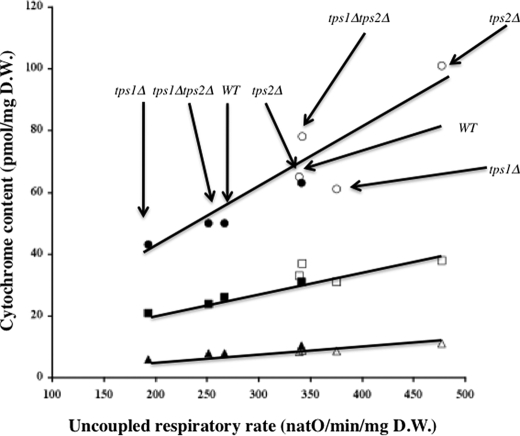
The results presented above concern cells grown on galactose medium, which induces a respiro-fermentative metabolism. To further study the influence of the trehalose pathway on mitochondrial metabolism in conditions that avoid any effect of these mutations on glycolysis, we switched to a purely respiratory growth substrate, i.e. lactate. As expected and compared with that on galactose (Table 1), the spontaneous respiratory rate is higher except for the tps2Δ mutant, for which the rate is comparable with the one measured with galactose (Table 3). The spontaneous respiratory rate is pretty much the same for all four strains because the difference between the double mutant and the other strains is not significant. In contrast, the uncoupled respiratory rate is strongly increased in the tps2Δ strain, whereas the other strains exhibit comparable rates. In accordance with this result, the cytochrome content is increased in the tps2Δ strain, whereas it is comparable in the other strains. For each strain, it is worth noting that the cytochrome content is increased when cells are grown on lactate (compare Tables 2 and 3). However, whatever the carbon source and the strain, we observed a good correlation between the amount of each of the cytochromes and the uncoupled respiratory rate (Fig. 2). The differences observed show that in the absence of Tps1 there is a decrease in the amount of respiratory chain components (on galactose medium) within the cells, whereas in the absence of Tps2 there is an increase in the cytochrome content of the respiratory chain (on both media).
TABLE 3.
Respiration rate of cells of wild type and mutant strains grown on YPLac2% medium
The respiratory rate of cells of the wild type and the three mutant strains was measured in cells harvested at an optical density of 4 (spontaneous respiration). When added, as indicated in the table, ethanol was 107 mm, and CCCP was 5 μm. Cytochrome content was measured as described under “Experimental Procedures.” The values are the means ± S.E. of seven measurements made with different cultures. natO, nanoatoms of oxygen.
| Respiratory rate |
||||
|---|---|---|---|---|
| Wild type | tps1Δ | tps2Δ | tps1Δ tps2Δ | |
| natO·min−1·mg−1 DW | ||||
| None (spontaneous) | 176 ± 20 | 173 ± 6 | 170 ± 19 | 143 ± 20 |
| Ethanol | 203 ± 24 | 204 ± 8 | 243 ± 41 | 195 ± 11 |
| Ethanol + CCCP | 339 ± 36 | 375 ± 33 | 477 ± 22a | 342 ± 21 |
| c + c1 | 65 ± 3 | 61 ± 8 | 101 ± 15a | 78 ± 11 |
| b | 33 ± 6 | 31 ± 8 | 38 ± 11 | 37 ± 6 |
| a + a3 | 8.5 ± 0.8 | 8.6 ± 0.7 | 11.2 ± 0.7a | 8.9 ± 0.7 |
a The differences between wild type and mutant strains were significant at p < 0.05.
FIGURE 2.
Linear relationship between the amount of cytochromes and the uncoupled respiratory rate in both YPGal2% and YPLac2%. The cells were grown on YPGal2% or YPLac2% and harvested in the middle of the logarithmic growth phase (optical density of 4). Uncoupled respiration was measured in the growing medium supplemented with 107 mm ethanol and 5 μm CCCP. Cytochrome content was assessed as described under “Experimental Procedures.” The strains used are indicated on the figure. ●, c + c1, YPGal2%l ■, b, YPGal2%; ▲, a + a3, YPGal2%; ○, c + c1, YPLac2%; □, b, YPLac2%; △, a + a3, YPLac2%. The values are the means of at least three measurements made with different cultures. WT, wild type. natO, nanoatoms of oxygen.
The Effect of the Mutations in the Trehalose Synthesis Pathway on Mitochondrial Enzymatic Content Is Mediated by the cAMP Level
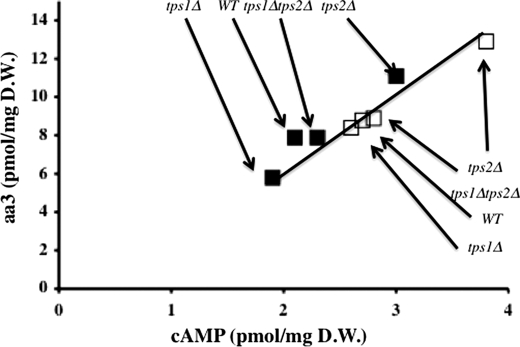
We have previously shown that the mitochondrial enzymatic content is modulated through the activity of the Ras/cAMP-dependent protein kinase/cAMP pathway in such a way that overactivation of this pathway increases, whereas underactivation of this pathway decreases the mitochondrial enzymatic content (28–30). We thus investigated whether this pathway was involved in the presently observed regulation. Because the activity of cAMP-dependent protein kinase is largely dependent on the cAMP level in the cell (31), we assessed cAMP content under our different experimental conditions. Fig. 3 shows that there is a clear positive correlation between the cytochrome a + a3 content and the cellular cAMP amount, whatever the strain and the growth medium. This strongly suggests that the effect of the mutations in the trehalose synthesis pathway on mitochondrial enzymatic content is mediated by the cAMP level.
FIGURE 3.
Relationship between cytochrome a + a3 and cAMP content. Cytochrome a + a3 and cAMP content were assessed as described under “Experimental Procedures.” The strains used are indicated on the figure. ■, cells cultured in YPGal2%; □, cells cultured in YPLac2%. The values are the means of at least three measurements made with different cultures. WT, wild type.
Cellular cAMP Content Modulation Goes through Hxk2
Inactivation of Tps1 causes a specific growth defect in the presence of glucose in the medium. This was suggested to be due to the absence of Tre6P inhibition on hexokinase. However, overexpression of Tre6P-insensitive hexokinase from Schizosaccharomyces pombe in a wild type strain does not affect growth on glucose (32). Thus, Tre6P inhibition of hexokinase does not seem to be the major mechanism or at least not the only mechanism by which Tps1 controls the influx of glucose into glycolysis. Possibly, Tps1 and Hxk2 interact with each other to regulate hexokinase activity and thus glucose influx into glycolysis. To determine whether the regulation of the mitochondrial enzymatic content through cAMP in the Tps mutants was related to the regulation of hexokinase, we investigated the consequences of such mutations in hexokinase 2-deleted strains. Table 4 shows that the cytochrome content of the mutant strains is not significantly different from that of the wild type cells. Moreover, spontaneous respiration, uncoupled respiration, and cAMP content are also similar in all three hexokinase-deleted strains. This shows that Hxk2 is required for all effects caused by the mutations in the trehalose pathway and that thus the influence of the tps1 and tps2 deletions on the cAMP level is likely to go through hexokinase.
TABLE 4.
Role of Hxk2 on the modulation of respiratory rates and cytochrome content in the Tps mutants
The respiratory rate of cells of the wild type and the three mutant strains was measured during growth on YPGal2% at an optical density of 4 (spontaneous respiration). When added, as indicated in the table (uncoupled JO2), the ethanol concentration was 107 mm, and CCCP was 5 μm. Cytochrome and cAMP contents were measured as described under “Experimental Procedures.” The values are the means ± S.E. of five measurements made with different cultures. natO, nanoatoms of oxygen.
| Parameters | Wild type | tps1Δ hxk2Δ | tps2Δ hxk2Δ | hxk2Δ |
|---|---|---|---|---|
| Spontaneous JO2(natO/min/mg DW) | 139 ± 11 | 156 ± 7 | 153 ± 6 | 155 ± 9 |
| Uncoupled JO2(natO/min/mg DW) | 282 ± 24 | 339 ± 18 | 340 ± 25 | 356 ± 24 |
| cAMP (pmol/mg DW) | 2.2 ± 0.1 | 2.4 ± 0.1 | 2.6 ± 0.1 | 2.4 ± 0.2 |
| c + c1 (pmol/mg DW) | 63 ± 5 | 78 ± 5 | 77 ± 6 | 74 ± 4 |
| b (pmol/mg DW) | 32 ± 3 | 42 ± 4 | 42 ± 4 | 39 ± 3 |
| a + a3 (pmol/mg DW) | 7.7 ± 0.7 | 9.0 ± 1.0 | 8.8 ± 0.9 | 9.5 ± 1.1 |
The growth defect in the presence of glucose in the tps1Δ mutant is suggested to be linked to the absence of Tre6P inhibition of hexokinase. To further understand how the deletions in TPS1 and TPS2 are linked to changes in the amount of respiratory chain components within the cells, we investigated whether there was a relationship between the amount of cytochromes and the amount of Tre6P, the concentration of which is known to be affected in these mutants. To evaluate this hypothesis, we modulated the amount of Tre6P in the tps2Δ mutant by increasing the growth temperature. It is well known that in this condition the Tre6P concentration increases very strongly (33). Table 5 shows that, in a tps2Δ mutant, an increase in temperature induces a clear increase in Tre6P, cAMP, and also mitochondrial enzymatic content. These increases are abolished when HXK2 is deleted in the tps2Δ strain. As expected, there is almost no Tre6P in the tps1Δ strain, and mitochondrial enzymatic content is related to the cAMP content. However, it can be hypothesized that Tre6P is the intermediary between the deletions (tps1Δ and tps2Δ) and the influence of hexokinase2 on the cAMP concentration. Furthermore, Tre6P was assessed in most of our experimental conditions, and even though in a tps2Δ strain there is an increase in Tre6P concomitant with an increase in mitochondrial enzymatic content, there is no relationship between mitochondrial enzymatic content and Tre6P concentration in the other strains (data not shown). This rules out a possible direct regulation of mitochondrial enzymatic content by Tre6P.
TABLE 5.
Effect of high temperature on respiratory rate and cytochrome content in tps2Δ strains
The respiratory rate of cells of the wild type and the three mutant strains was measured during growth on YPGal2% at 35 °C at an optical density of 4 (spontaneous respiration). When added, as indicated in the table (uncoupled JO2), the ethanol concentration was 107 mm, and CCCP was 5 μm. The cytochrome, Tre6P, and cAMP contents were measured as described under “Experimental Procedures.” The values are the means ± S.E. of seven measurements made with different cultures. natO, nanoatoms of oxygen.
| Parameters | Wild type | tps2Δ | tps2Δ hxk2Δ | tps1Δ |
|---|---|---|---|---|
| Spontaneous JO2(natO/min/mg DW) | 187 ± 10 | 207 ± 9 | 118 ± 7a | 131 ± 11b |
| Uncoupled JO2(natO/min/mg DW) | 257 ± 16 | 364 ± 12a | 206 ± 8 | 202 ± 27 |
| cAMP (pmol/mg DW) | 1.9 ± 0.2 | 4.6 ± 0.2a | 1.5 ± 0.2 | 1.4 ± 0.4 |
| c + c1 (pmol/mg DW) | 44 ± 2 | 53 ± 5 | 41 ± 2 | 40 ± 4 |
| b (pmol/mg DW) | 27 ± 3 | 31 ± 4 | 25 ± 2 | 24 ± 3 |
| a + a3 (pmol/mg DW) | 6.0 ± 0.7 | 11 ± 1.3b | 4.2 ± 0.5 | 5 ± 1.8 |
| Tre6P (nmol/mg DW) | 3.1 ± 0.5 | 22.2 ± 7.3b | 3.3 ± 0.4 | 0.8 ± 0.4b |
a The differences between wild type and mutant strains were significant at p < 0.001.
b The differences between wild type and mutant strains were significant at p < 0.05.
In yeast, the cAMP intracellular concentration depends on the respective activities of Cyr1 (adenylate cyclase) and of the phosphodiesterases Pde1 and Pde2. Pde2 is the high affinity phosphodiesterase. When the PDE2 gene was overexpressed, the cAMP content in the wild type strain was slightly decreased, as were cytochrome content and respiratory activities (Table 6). Moreover, the increase in cAMP and cytochrome content as well as the respiratory rate in tps2Δ cells was completely suppressed by overexpression of PDE2, indicating that the effects of this mutation on the electron transport chain machinery and activity go through the cAMP content.
TABLE 6.
Effect of PDE2 overexpression on respiratory rate and cytochrome content
The respiratory rate of cells of the wild type and the tps2Δ strain with or without PDE2 overexpression was measured during growth on YPGal2% at an optical density of 4 (spontaneous respiration). When added, as indicated in the table (uncoupled JO2), the ethanol concentration was 107 mm, and CCCP was 5 μm. The cytochrome and cAMP contents were measured as described under “Experimental Procedures.” The values are the means ± S.E. of nine measurements made with different cultures.
| Parameters | Wild type | Wild type pPDE2 | tps2Δ | tps2ΔpPDE2 |
|---|---|---|---|---|
| Spontaneous JO2(natO/min/mg DW) | 142 ± 8 | 108 ± 6a | 181 ± 7a | 146 ± 6 |
| Uncoupled JO2(natO/min/mg DW) | 293 ± 10 | 213 ± 9a | 354 ± 11a | 235 ± 8a |
| cAMP (pmol/mg DW) | 2.3 ± 0.08 | 1.9 ± 0.08a | 2.8 ± 0.15a | 2.3 ± 0.14 |
| c + c1 (pmol/mg DW) | 44 ± 4 | 34 ± 5 | 54 ± 4 | 43 ± 6 |
| b (pmol/mg DW) | 35 ± 5 | 24 ± 3 | 38 ± 3 | 30 ± 4 |
| a + a3 (pmol/mg DW) | 8 ± 0.6 | 5.8 ± 0.4a | 10.1 ± 0.4a | 7.1 ± 0.5 |
a The differences between wild type and mutant strains significant at p < 0.05.
DISCUSSION
The main goal of this work was to investigate whether the trehalose synthesis pathway is involved in the regulation of oxidative phosphorylation activity in yeast mitochondria. It is now well established that Tps1 plays a major role in the control of glycolytic flux by restricting glucose influx at the level of glucose phosphorylation. Thus, in a tps1Δ mutant that is not able to grow on glucose (8, 11, 15), glucose addition to cells growing on galactose or on nonfermentable carbon sources induces a large increase in sugar phosphates and particularly in fructose 1,6-bisphosphate (8, 16–18). Because fructose 1,6-bisphosphate is a potent inhibitor of respiration in Crabtree-positive yeast strains, such a huge increase in this compound must induce a strong inhibition of mitochondrial oxidative phosphorylation (18). Thus, through the control of glycolytic intermediate content, the trehalose synthesis pathway may be involved in the kinetic regulation of oxidative phosphorylation. This made the question arise of whether Tps1 would participate in the regulation of respiration also under conditions permissive for its growth and thus in a way independent of the dramatic metabolic changes associated with the specific growth defect of the tps1Δ mutant on rapidly fermented sugars like sucrose, fructose, and glucose.
When growing on galactose or on a nonfermentable carbon source (i.e. lactate), the different mutants in the trehalose pathway (tps1Δ, tps2Δ, and tps1Δ tps2Δ) are not much affected in their growth characteristics compared with the wild type strain (34). However, as evidenced by cytochrome content, these mutations do modulate the quantity of respiratory chain components also under these permissive growth conditions. The quantity decreased or increased in the absence of Tps1 or Tps2, respectively. There is a good correlation between maximal respiratory capacity (measured in the presence of ethanol and uncoupler) and the cytochrome content whatever the strain considered (Fig. 2). The Ras-cAMP cascade in yeast has been extensively studied (35–37). By using different mutants in the RAS/cAMP signaling pathway, we have shown that underactivation or overactivation of this signaling pathway leads to a decrease or an increase in the content of all the cytochromes and in maximal respiratory capacity, respectively (28). As in the present study, these changes in cytochrome content correlated with the variation in respiratory capacity of the cells under different conditions. A direct involvement of cAMP in the regulation of cellular mitochondrial content has been shown by making use of the OL556 strain (38), in which the high affinity cAMP phosphodiesterase (Pde2) and Cdc25 are inactivated. Consequently, this strain is sensitive to exogenous cAMP, and addition of cAMP in the culture medium induced all the mitochondrial characteristics typically seen in mutants with an overactive RAS-cAMP protein kinase A pathway (29). Moreover, we showed that the yeast protein kinase Tpk3p is specifically involved in the regulation of mitochondrial enzymatic content during the transition phase to a reduced cell growth rate in response to substrate limitation (30).
The results presented here are in agreement with previous data indicating a major role for the Ras-cAMP protein kinase A pathway in the control of mitochondrial respiratory content in yeast cells. One of the main interesting results is the strong linear relationship between mitochondrial cytochromes and cAMP content observed in all the mutants studied (Fig. 3 and Tables 4 and 6). A strong correlation is also present between cytochrome content and respiratory capacity and of course as a consequence respiratory capacity and cAMP content. Thus, all the effects of the mutations in the trehalose synthesis pathway on the mitochondrial respiratory content and its activity go through modulation of the cAMP content. Hence, this paper reveals the existence of a new signaling pathway, leading from the trehalose synthesis pathway to the mitochondrial composition. We have clearly shown that the TPS1 gene product modulates cAMP concentration through hexokinase 2. It is worth noting that Tre6P synthase, Tps1, is involved in both the regulation of glycolytic flux and control of mitochondrial respiratory content through hexokinase 2. Glycolysis and mitochondrial activity through oxidative phosphorylation are the cellular “energetic” pathways that convert redox potential in phosphate potential. It thus makes great physiological sense that both pathways have common regulatory mechanisms. Glycolysis control by Tps1 is well documented, and this work shows that Tps1 is also involved in the regulation of mitochondrial biogenesis through Hxk2 and cAMP in the yeast S. cerevisiae. There is a great rationale to this regulation: when the glycolysis flux is inhibited through hexokinase 2/tps1 interaction, mitochondrial biogenesis is increased, thus allowing a balance in ATP synthesis between glycolysis and oxidative phosphorylation.
This work was supported by Agence Nationale pour la Recherche 05-BLAN-0339-01 and the Conseil Regional d'Aquitaine.
- Tre6P
- trehalose 6-phosphate
- Fru1,6bP
- fructose 1,6-bisphosphate
- MOPS
- 4-morpholinepropanesulfonic acid
- CCCP
- cyanide m-chlorophenylhydrazone
- DW
- dry weight.
REFERENCES
- 1.Estévez A. M., Heinisch J. J., Aragón J. J. (1995) FEBS Lett. 374, 100–104 [DOI] [PubMed] [Google Scholar]
- 2.Gancedo C., Serrano R. (1989) in The Yeasts, pp. 205–260, Academic Press, London [Google Scholar]
- 3.Schaaff I., Heinisch J., Zimmermann F. K. (1989) Yeast 5, 285–290 [DOI] [PubMed] [Google Scholar]
- 4.Navas M. A., Cerdán S., Gancedo J. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson C., Nilsson A., Blomberg A., Gustafsson L. (1997) J. Bacteriol. 179, 7243–7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossell S., van der Weijden C. C., Kruckeberg A. L., Bakker B. M., Westerhoff H. V. (2005) FEMS Yeast Res. 5, 611–619 [DOI] [PubMed] [Google Scholar]
- 7.Daran-Lapujade P., Rossell S., van Gulik W. M., Luttik M. A., de Groot M. J., Slijper M., Heck A. J., Daran J. M., de Winde J. H., Westerhoff H. V., Pronk J. T., Bakker B. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15753–15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thevelein J. M., Hohmann S. (1995) Trends Biochem. Sci. 20, 3–10 [DOI] [PubMed] [Google Scholar]
- 9.Van Aelst L., Hohmann S., Bulaya B., de Koning W., Sierkstra L., Neves M. J., Luyten K., Alijo R., Ramos J., Coccetti P. (1993) Mol. Microbiol. 8, 927–943 [DOI] [PubMed] [Google Scholar]
- 10.Vuorio O. E., Kalkkinen N., Londesborough J. (1993) Eur. J. Biochem. 216, 849–861 [DOI] [PubMed] [Google Scholar]
- 11.González M. I., Stucka R., Blázquez M. A., Feldmann H., Gancedo C. (1992) Yeast 8, 183–192 [DOI] [PubMed] [Google Scholar]
- 12.Bell W., Klaassen P., Ohnacker M., Boller T., Herweijer M., Schoppink P., Van der Zee P., Wiemken A. (1992) Eur. J. Biochem. 209, 951–959 [DOI] [PubMed] [Google Scholar]
- 13.De Virgilio C., Bürckert N., Bell W., Jenö P., Boller T., Wiemken A. (1993) Eur. J. Biochem. 212, 315–323 [DOI] [PubMed] [Google Scholar]
- 14.Bell W., Sun W., Hohmann S., Wera S., Reinders A., De Virgilio C., Wiemken A., Thevelein J. M. (1998) J. Biol. Chem. 273, 33311–33319 [DOI] [PubMed] [Google Scholar]
- 15.Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. (1979) Biochemistry 18, 4487–4499 [DOI] [PubMed] [Google Scholar]
- 16.Hohmann S., Neves M. J., de Koning W., Alijo R., Ramos J., Thevelein J. M. (1993) Curr. Genet. 23, 281–289 [DOI] [PubMed] [Google Scholar]
- 17.Van Aelst L., Hohmann S., Zimmermann F. K., Jans A. W., Thevelein J. M. (1991) EMBO J. 10, 2095–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz-Ruiz R., Avéret N., Araiza D., Pinson B., Uribe-Carvajal S., Devin A., Rigoulet M. (2008) J. Biol. Chem. 283, 26948–26955 [DOI] [PubMed] [Google Scholar]
- 19.Hohmann S., Bell W., Neves M. J., Valckx D., Thevelein J. M. (1996) Mol. Microbiol. 20, 981–991 [DOI] [PubMed] [Google Scholar]
- 20.Blázquez M. A., Gancedo C. (1994) Curr. Genet. 25, 89–94 [DOI] [PubMed] [Google Scholar]
- 21.Luyten K., Albertyn J., Skibbe W. F., Prior B. A., Ramos J., Thevelein J. M., Hohmann S. (1995) EMBO J. 14, 1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noubhani A., Bunoust O., Rigoulet M., Thevelein J. M. (2000) Eur. J. Biochem. 267, 4566–4576 [DOI] [PubMed] [Google Scholar]
- 23.Beauvoit B., Rigoulet M., Bunoust O., Raffard G., Canioni P., Guérin B. (1993) Eur. J. Biochem. 214, 163–172 [DOI] [PubMed] [Google Scholar]
- 24.Onishi T., Kröger A., Heldt H. W., Pfaff E., Klingenberg M. (1967) Eur. J. Biochem. 1, 301–311 [DOI] [PubMed] [Google Scholar]
- 25.Vanneste W. H. (1966) Biochemistry 5, 838–848 [DOI] [PubMed] [Google Scholar]
- 26.Thevelein J. M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., den Hollander J. A., Jans A. W. (1987) J. Gen. Microbiol. 133, 2191–2196 [DOI] [PubMed] [Google Scholar]
- 27.Dejean L., Beauvoit B., Guérin B., Rigoulet M. (2000) Biochim. Biophys. Acta 1457, 45–56 [DOI] [PubMed] [Google Scholar]
- 28.Dejean L., Beauvoit B., Bunoust O., Guérin B., Rigoulet M. (2002) Biochem. Biophys. Res. Commun. 293, 1383–1388 [DOI] [PubMed] [Google Scholar]
- 29.Dejean L., Beauvoit B., Alonso A. P., Bunoust O., Guérin B., Rigoulet M. (2002) Biochim. Biophys. Acta 1554, 159–169 [DOI] [PubMed] [Google Scholar]
- 30.Chevtzoff C., Vallortigara J., Avéret N., Rigoulet M., Devin A. (2005) Biochim. Biophys. Acta 1706, 117–125 [DOI] [PubMed] [Google Scholar]
- 31.Portela P., Van Dijck P., Thevelein J. M., Moreno S. (2003) FEMS Yeast Res. 3, 119–126 [DOI] [PubMed] [Google Scholar]
- 32.Bonini B. M., Van Dijck P., Thevelein J. M. (2003) Biochim. Biophys. Acta 1606, 83–93 [DOI] [PubMed] [Google Scholar]
- 33.van Vaeck C., Wera S., van Dijck P., Thevelein J. M. (2001) Biochem. J. 353, 157–162 [PMC free article] [PubMed] [Google Scholar]
- 34.Devin A., Dejean L., Beauvoit B., Chevtzoff C., Avéret N., Bunoust O., Rigoulet M. (2006) J. Biol. Chem. 281, 26779–26784 [DOI] [PubMed] [Google Scholar]
- 35.Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. (1985) Cell 40, 27–36 [DOI] [PubMed] [Google Scholar]
- 36.Toda T., Cameron S., Sass P., Zoller M., Scott J. D., McMullen B., Hurwitz M., Krebs E. G., Wigler M. (1987) Mol. Cell. Biol. 7, 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toda T., Cameron S., Sass P., Zoller M., Wigler M. (1987) Cell 50, 277–287 [DOI] [PubMed] [Google Scholar]
- 38.Boy-Marcotte E., Tadi D., Perrot M., Boucherie H., Jacquet M. (1996) Microbiology 142, 459–467 [DOI] [PubMed] [Google Scholar]