Abstract
During the mitotic cell cycle, Geminin can act both as a promoter and inhibitor of initiation of DNA replication. As a promoter, Geminin stabilizes Cdt1 and facilitates its accumulation leading to the assembly of the pre-replication complex on DNA. As an inhibitor, Geminin prevents Cdt1 from loading the mini-chromosome maintenance complex onto pre-replication complexes in late S, G2, and M phases. Here we show that during meiosis Geminin functions as a stabilizer of Cdt1 promoting its accumulation for the early division cycles of the embryo. Depletion of Geminin in Xenopus immature oocytes leads to a decrease of Cdt1 protein levels during maturation and after activation of these oocytes. Injection of exogenous recombinant Geminin into the depleted oocytes rescues Cdt1 levels demonstrating that Geminin stabilizes Cdt1 during meiosis and after fertilization. Furthermore, Geminin-depleted oocytes did not replicate their DNA after meiosis I indicating that Geminin does not act as an inhibitor of initiation of DNA replication between meiosis I and meiosis II.
In eukaryotes, initiation of DNA replication involves the formation and activation of the pre-replication complex (pre-RC)3 at the origins of replication. Pre-RCs are formed by the sequential binding of the origin recognition complex components, Cdc6, Cdt1, and mini-chromosome maintenance complex (MCM 2–7) proteins, to DNA. After loading the MCM complex, the pre-RCs are activated by S phase kinases (Dbf4-dependent kinase and Cdks) to initiate DNA replication (1). Replication of DNA, limited to only once per cell cycle, is critical to maintain genomic stability. Redundant mechanisms exist to ensure that DNA replication is tightly regulated during the cell cycle (1, 2). A small protein named Geminin has been shown to play a significant role in such regulatory mechanisms during mitosis (2–6). Geminin, a multifunctional 25-kDa protein, was first identified in a screen for proteins degraded during mitosis in Xenopus laevis egg extracts (7). Geminin is present in higher eukaryotes, but its presence in yeast has not yet been reported (7–10). Geminin plays a major role in regulating the function of Cdt1, one of the pre-RC components (8, 11–13). Numerous studies suggest that in higher eukaryotes the interaction between Geminin and Cdt1 is pivotal to restrict DNA replication to only once per cell cycle (6, 14–22). Furthermore, in Xenopus egg extracts, the Geminin/Cdt1 ratio seems to control the assembly of pre-RCs at replication origins and to determine whether the origins are licensed or not (23). The positive and negative roles of Geminin in origin licensing and DNA replication are made possible by their temporal separation during the cell cycle. Pre-RC formation occurs during late M and early G1 phase, whereas pre-RC inhibition occurs from late S to mid M phase.
As a positive regulator of DNA replication, Geminin has been shown to stabilize Cdt1. In human osteosarcoma cells, silencing of GEMININ expression limits CDT1 accumulation during mitosis and therefore the formation of pre-RCs in the subsequent cell cycle. This stabilizing effect is the result of a direct interaction between CDT1 and GEMININ preventing CDT1 ubiquitination and degradation (13). Similar findings were also recently observed in normal human cells and various cancer cells (24). However, in both human normal and tumor cells, the low level of CDT1, generated by the absence of GEMININ, did not always prevent cellular proliferation or re-replication of the genome (5, 24, 25). Therefore, one might question the importance of the role of GEMININ in stabilizing CDT1 in human cells. Beyond its role as a stabilizer of Cdt1 levels, Geminin has also been shown to participate directly in the formation of pre-RCs in Xenopus egg extracts. A complex between Cdt1 and Geminin binds to chromatin and supports pre-RC assembly. However, the recruitment of additional Geminin molecules to this complex on the chromatin blocks further pre-RC formation. These results indicate that the stoichiometry of Cdt1 and Geminin in this complex regulates its activity as a promoter or inhibitor of pre-RC assembly and DNA replication (23, 26). Several mechanisms have been shown to modulate the Geminin/Cdt1 balance on the chromatin. In Xenopus the binding of Cdt1 to the MCM9 protein seems to block the recruitment of an excess of Geminin to the chromatin and therefore favors pre-RC assembly (27). Similarly, the inactivation of Geminin by either ubiquitination or degradation also has a positive effect on pre-RC assembly (8, 11, 28–30). On the other hand, the replication-dependent degradation of Cdt1 has the opposite effect and prevents refiring of replication origins during S and G2 phases of the mitotic cell cycle (18, 20, 31).
Although the role of Geminin during mitosis has been extensively studied, not much is known about its function during meiosis. The expression pattern of Geminin during oocyte maturation is unclear. The presence of Geminin in immature stage VI Xenopus oocytes is controversial, but the protein is fully expressed in mature oocytes arrested in metaphase of meiosis II (7, 32). To form haploid gametes, DNA replication has to be inhibited between meiosis I (MI) and meiosis II (MII). In Xenopus oocytes, cyclin B-dependent kinase 1 (Cdk1) also known as maturation-promoting factor (MPF) plays a role in preventing DNA replication between the two meiotic divisions (33–36). Inhibition of Cdk1 activity between MI and MII leads to the formation of interphase nucleus and DNA replication. However, the role of Geminin in preventing DNA replication between meiotic divisions has not been tested so far. Finally, the possibility that Geminin stabilizes Cdt1 during meiosis and ensures its accumulation for the early embryonic divisions has not been formally examined.
Here we show that the levels of Geminin and Cdt1 proteins increase significantly during meiosis in Xenopus oocytes and that the primary role of geminin is to promote the accumulation of Cdt1 and not to repress DNA replication between meiosis I and meiosis II. Depletion of Geminin in Xenopus immature oocytes does not lead to DNA replication after the first meiotic division but to a decrease in Cdt1 stability during the maturation and activation of these oocytes. Rescue of Cdt1 levels in these Geminin-depleted oocytes is achieved by injection of exogenous recombinant Geminin protein confirming the role of Geminin as a stabilizer of Cdt1 during meiosis and the early embryonic division cycles. These results provide further support for the idea that Geminin functions universally in stabilizing Cdt1. Although the stabilizing role of Geminin might not be its most important function in somatic cells, we show here that stabilizing Cdt1 is a dominant function for Geminin in Xenopus oocytes undergoing meiosis. This stabilizing role of Geminin is essential for the stockpiling of Cdt1 before fertilization that is required to sustain the rapid divisions of the early embryo.
EXPERIMENTAL PROCEDURES
Xenopus Oocyte Isolation and Microinjection
Stage VI oocytes were obtained from Xenopus ovary fragments treated with 2 mg/ml collagenase to remove follicle cells as described previously (37). Microinjection of stage VI oocytes was performed in the region between the animal and vegetal hemispheres. Oocytes were injected with a 50-nl solution containing 75 ng of either Geminin sense or antisense oligonucleotides (oligos) and incubated for 4 h in modified Barth's saline (MBS: 88 mm NaCl, 1 mm KCl, 0.91 mm CaCl2, 0.33 mm Ca(NO3)2, 0.82 mm MgSO4, 2.4 mm NaHCO3, 10 mm HEPES, pH 7.5). The sequences of the phosphorothioate-modified antisense and sense oligos were described previously (38). To induce maturation, oocytes were incubated with 20 μg/ml progesterone in MBS. Oocyte activation was triggered by calcium injection (200 nm) 4 h after GVBD and an incubation in 0.1× MMR (0.5 mm HEPES, pH 7.8, 10 mm NaCl, 0.2 mm KCl, 0.1 mm MgSO4, 0.2 mm CaCl2, 0.01 mm EDTA).
Western Analysis
For Western blot analysis, 10 oocytes were homogenized in 100 μl of extract buffer (20 mm EGTA, 15 mm MgCl2, 1 mm dithiothreitol, 80 mm β-glycerophosphate, and 10 μg ml−1 each of aprotinin, leupeptin, and pepstatin A). Phosphatase inhibitor, β-glycerophosphate, was added to the extract buffer when oocytes undergoing maturation were collected. Extracts were centrifuged at 20,000 × g at 4 °C for 10 min, and an equivalent to two or three oocytes was analyzed by SDS-PAGE and transferred onto nitrocellulose membrane.
Recombinant Protein Production and Antibodies
Geminin and Geminin del-H proteins were expressed as described previously (7). Polyclonal antibodies against Xenopus Mcm4, Cdt1, and Geminin were raised in rabbits using Escherichia coli recombinant full-length proteins as antigens (37, 39). Rabbit sera were affinity-purified by column chromatography against immobilized proteins as described previously (40). The cyclin B1 antibody was a kind gift from Dr. M. Doree, and the Cdc27 antibody was purchased from BD Biosciences.
H1 Kinase and Replication Assays
Both assays were carried out as described previously (37).
RESULTS
Geminin and Cdt1 Levels Increase during Meiotic Maturation
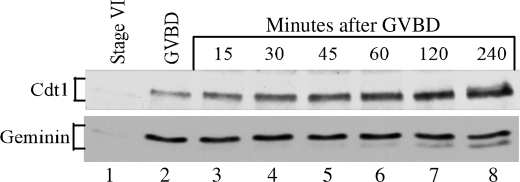
The level of Geminin and Cdt1 in Xenopus oocytes before and during the maturation process was determined by Western blot analysis. Following progesterone treatment, the successful re-entry of oocytes into meiosis was judged by the appearance of a white spot on their animal pole, corresponding to the GVBD or nuclear breakdown. Samples were collected at different times after GVBD. Our results indicate that the levels of Geminin and Cdt1 were extremely low in stage VI oocytes arrested in prophase of meiosis I (Fig. 1, lane 1), but they increased significantly during the maturation process (Fig. 1, lanes 2–8). The Geminin protein appeared at first as a single band whose maximum expression was reached at the time of GVBD and remained constant thereafter. A second Geminin band with faster mobility appeared later during meiosis II. The ratio of the two Geminin bands varied significantly between oocytes (compare Figs. 1, 2A, and Fig. 4A), but the upper band was always the predominant one. Although these two bands were bona fide Geminin bands, sensitive to Geminin antisense oligonucleotide treatment (as shown in Fig. 3B), their origin is currently unknown. They do not appear to be phosphorylated isoforms as they were unaffected by alkaline phosphatase treatment (data not shown). Contrary to Geminin, Cdt1 expression increased more gradually during maturation and reached its maximum in matured oocytes arrested in metaphase of meiosis II (Fig. 1, lane 8). These findings indicate the following: 1) both Xenopus Geminin and Cdt1 proteins accumulate during oocyte maturation; and 2) Geminin expression precedes Cdt1 expression suggesting that Geminin might facilitate Cdt1 accumulation by stabilizing it.
FIGURE 1.
Geminin and Cdt1 protein levels increase during meiotic maturation. Stage VI or immature oocytes were induced to undergo maturation by exposure to progesterone. Samples were collected at the indicated times and analyzed for Geminin and Cdt1 expression by immunoblot analysis.
FIGURE 2.
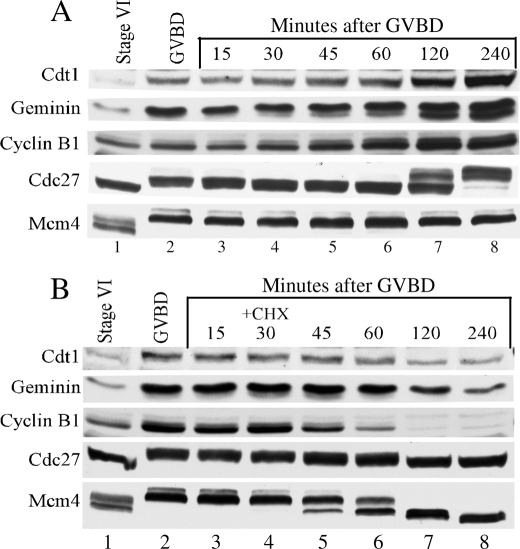
Cycloheximide treatment decreases Geminin and Cdt1 levels. Western blot analysis of Cdc27, cyclin B1, Geminin, and Cdt1 proteins at the indicated times during meiotic maturation. A, in the absence of CHX; B, in the presence of CHX. The Mcm4 protein was probed as a loading control.
FIGURE 4.
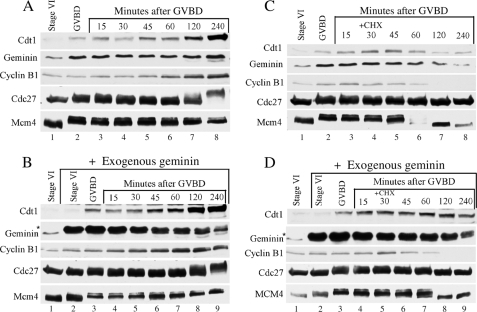
Geminin stabilizes Cdt1 during maturation. Western analysis of Cdc27, cyclin B1, Geminin, and Cdt1 proteins at the indicated times. A, during meiotic maturation. B, in the presence of exogenous Geminin during maturation. C, in the presence of CHX. D, in the presence of CHX and exogenous Geminin. The Mcm4 protein served as a loading control. Asterisk indicates exogenous Geminin.
FIGURE 3.
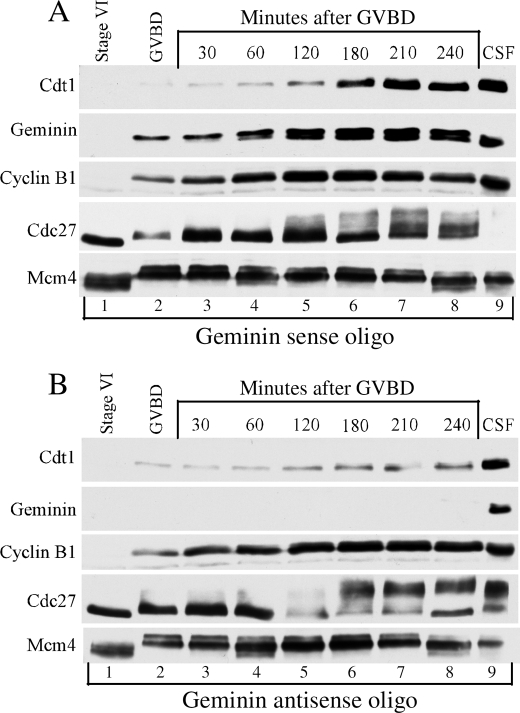
Absence of Geminin results in a decrease of Cdt1 levels during maturation. Immunoblot analysis of Cdc27, cyclin B1, Geminin, and Cdt1 during meiotic maturation is at the indicated times in Geminin sense oligo-injected oocytes (A). B, Geminin antisense oligo-injected oocytes. The Mcm4 protein is a loading control.
Inhibition of Protein Synthesis during Maturation Leads to a Simultaneous Decrease in Geminin and Cdt1 Levels
To investigate the potential role of Geminin as a stabilizing factor of Cdt1 during maturation, we first blocked protein synthesis midway through maturation and determined the effect on Geminin and Cdt1 levels. Addition of protein synthesis inhibitor (cycloheximide) was done at a time when Geminin level was close to its maximum, and a significant and measurable amount of Cdt1 was present (i.e. 30 min after GVBD). Variation in the level of Geminin and Cdt1 proteins after cycloheximide (CHX) treatment was followed by Western blot analysis (Fig. 2B). The amount of Geminin and Cdt1 proteins accumulated during the normal maturation process was also monitored as controls (Fig. 2A). The levels of the Xenopus Mcm4, Cdc27, and cyclin B1 proteins were also followed as they represent good markers of the progression through maturation and the effectiveness of the CHX treatment. Cyclin B1 levels gradually increased during the maturation and culminated in mature oocytes (Fig. 2A, lanes 2–8). This observed increase in cyclin B1 is known to participate in the maintenance of MPF kinase activity during maturation and to result in the phosphorylation of proteins such as Cdc27 and Mcm4. Other than the changes in their phosphorylation status, the Cdc27 and Mcm4 proteins remained at a constant level during meiosis (Fig. 2A, lanes 1–8). The Cdc27 protein is the core subunit of the anaphase-promoting complex (APC/C) that controls cyclin B degradation in Xenopus oocytes. Cdc27 phosphorylation reflects APC/C activity. During maturation Cdc27 is phosphorylated at the time of GVBD, and its level of phosphorylation is even higher in the mature oocyte (Fig. 2, lanes 7 and 8) (41). As described in the previous experiment, Cdt1 and Geminin levels increased during maturation (Fig. 2A). In the presence of CHX, cyclin B1 levels decreased rapidly indicating that protein synthesis was blocked and that most likely cyclin B1 was degraded by the APC/C (Fig. 2B, lanes 5–8). The decrease in cyclin B1 affected the MPF activity and led to the dephosphorylation of the Mcm4 and Cdc27 proteins. However, the amount of the Mcm4 and Cdc27 proteins remained constant after CHX addition, indicating that these proteins are relatively stable and that their accumulation occurred mainly in oocytes arrested in prophase of meiosis I (Fig. 2, A and B, lane 1,). In contrast, the level of Geminin and Cdt1 proteins decreased simultaneously in the absence of protein synthesis but not as fast as the cyclin B1 levels. This result suggests that under these conditions, Cdt1 and Geminin were degraded and that Cdt1 stability might be affected by Geminin stability.
Specific Ablation of Geminin during Oocyte Maturation Leads to a Decrease in Cdt1 Level
To further verify that Cdt1 stability was specifically dependent of Geminin, we used an antisense oligonucleotide approach to selectively inhibit Geminin expression during maturation and to measure the effect on Cdt1 level. Oocytes were injected with either Geminin sense or antisense oligonucleotides and induced to undergo maturation. Samples were collected at different times after GVBD and analyzed for protein expression by Western blot analysis. In oocytes injected with sense oligonucleotides, cyclin B1 and Cdc27 protein profiles showed the normal maturation pattern that we previously described (Fig. 3A, lanes 1–8). Geminin and Cdt1 protein levels also increased during maturation suggesting that injection of sense oligonucleotides did not affect their accumulation or the maturation process (Fig. 3A, lanes 1–8).
On the other hand, injection of antisense oligonucleotides inhibited Geminin expression to undetectable levels during maturation (Fig. 3B, lanes 1–8). Regardless of the Geminin ablation, cyclin B1 and Cdc27 profiles were not affected by the injection of antisense oligonucleotides suggesting that Geminin is not required for MPF activity and the maturation process (compare Fig. 3, A and B, lanes 1–8). Interestingly, in oocytes injected with antisense oligonucleotides, the accumulation of Cdt1 was significantly reduced (compare Fig. 3, A and B, lanes 1–8). Absence of Geminin affected the accumulation of Cdt1 during meiosis II and not Cdt1 accumulation during meiosis I (compare lanes 2–4 and 5–8 in Fig. 3, A and B). This finding suggests that the absence of geminin is most likely to increase the degradation rate of Cdt1 rather than decrease its rate of synthesis. Indeed, when the same experiment was repeated in the presence of the protein synthesis inhibitor CHX, Cdt1 levels during meiosis II were further decreased in the absence of Geminin (see supplemental Fig. 1). This result indicates that geminin protects Cdt1 against degradation during meiosis II. The instability of Cdt1 in Geminin-deficient oocytes treated with CHX further revealed that Cdt1 degradation is independent of Cdk as CHX prevents cyclin accumulation and Cdk activity.
Exogenous Geminin Stabilizes Cdt1 during Maturation in CHX-treated Oocyte
Next we wanted to perform a rescue experiment and test the ability of exogenous Geminin to protect Cdt1 from degradation in oocytes that have low or no Geminin during maturation. First, we tried this experiment in stage VI oocytes injected with Geminin antisense oligonucleotides that, as demonstrated in the previous experiment, were depleted for Geminin. However, this experiment required a second injection with exogenous Geminin protein at the beginning of the maturation process, and for unknown reason, oocytes quickly started to degenerate. In an effort to limit the number of injections and decrease the level of endogenous Geminin, we again used cycloheximide to block protein synthesis midway through the maturation process. Stage VI oocytes were induced to undergo maturation and were injected with an excess of recombinant nondegradable Geminin at the time of GVBD, and 30 min later, oocytes were treated with or without CHX. As a control, the same experiment was performed with oocytes injected with buffer instead of recombinant Geminin. Oocytes were collected at the indicated times and analyzed for protein expression by Western blot analysis (Fig. 4). In the absence of cycloheximide treatment, an excess of exogenous Geminin did not affect the maturation process as indicated by the Mcm4 and Cdc27 phosphorylation profile (Fig. 4, A and B, lanes 1–8). The excess of exogenous Geminin had no effect on Cdt1 accumulation during meiosis I but promoted a faster accumulation of Cdt1 during meiosis II (compare Fig. 4, A, lanes 7 and 8, and B, lanes 8 and 9). This finding reinforces the idea that Geminin does not affect the rate of Cdt1 synthesis but protects Cdt1 against degradation during meiosis II. When CHX was added 30 min after GVBD, protein synthesis was blocked, and cyclin B1 was degraded both in the presence or the absence of exogenous Geminin in oocytes (Fig. 4, C, lanes 7 and 8, and D, lanes 8 and 9). As observed previously in Fig. 2B, CHX treatment of control oocytes (injected with buffer) led to the simultaneous decrease of the endogenous level of Geminin and Cdt1 proteins (Fig. 4C, lanes 7 and 8). On the other hand, injection of excess recombinant Geminin into CHX-treated oocytes prevented the decrease in the level of endogenous Cdt1. Although Cdt1 synthesis was inhibited, Cdt1 levels in oocytes remained constant indicating that excess Geminin did protect Cdt1 against degradation (Fig. 4D, lanes 5–9).
Absence of Geminin Does Not Affect Meiotic Progression or the Induction of DNA Replication between Meiosis I and Meiosis II
One hallmark of meiosis is the prevention of DNA replication between meiosis I and meiosis II to produce haploid gametes. Earlier studies have established the importance of maintaining an intermediate level of MPF activity between MI and MII to suppress DNA replication (33–36). In the following experiments we addressed the contribution of Geminin, a well known mitotic inhibitor of pre-RC assembly, in the prevention of DNA replication between MI and MII.
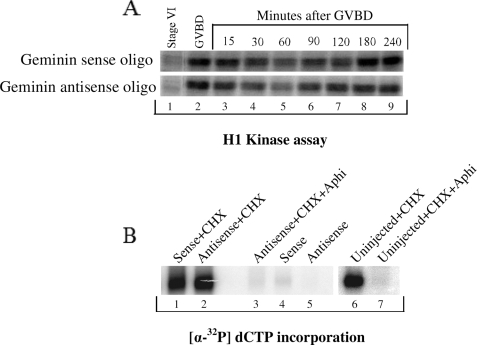
First we determined whether the ablation of Geminin expression affected MPF activity during meiosis. Immature Xenopus oocytes were injected with Geminin sense or antisense oligonucleotides and induced to undergo maturation. Samples were collected at different times during maturation, and the level of MPF activity was measured as the ability of the oocyte extract to phosphorylate in vitro the histone H1 protein. As shown in Fig. 5A, the fluctuation of H1 kinase activity during meiosis was similar in the presence or absence of Geminin. Most notably, an intermediate level of H1 kinase activity was maintained between MI and MII independent of the presence or absence of Geminin (see Fig. 5A, lane 5, upper and lower panels). Overall, this finding indicates that the absence of Geminin does not affect MPF activity during meiosis and is therefore unlikely to disturb meiotic progression. This result also explains our finding in Fig. 3 where ablation of Geminin did not affect the Cdk1-dependent phosphorylation of Cdc27 and Mcm4 during maturation.
FIGURE 5.
Depletion of Geminin does not induce DNA replication between MI and MII. Geminin sense or antisense oligo-injected stage VI oocytes were stimulated with progesterone to enter maturation, and samples were collected at the indicated times. A, to measure the MPF activity via H1 kinase assay, 10 oocytes were homogenized and centrifuged, and the supernatant was supplemented with histone-1 and [γ-32P]ATP and incubated for 45 min at room temperature. Phosphorylation of histone H1 was followed by SDS-PAGE and PhosphorImager analysis. B, for replication assay, Geminin-deficient or control (sense injected or uninjected) oocytes were injected with [α-32P]dCTP at the time of GVBD either in the presence or absence of CHX and/or aphidicolin. Replicated DNA was analyzed 4 h after GVBD.
To determine whether Geminin has a role in preventing DNA replication between MI and MII, immature Xenopus oocytes were injected with Geminin sense or antisense oligonucleotides and induced to undergo maturation. At the time of GVBD, oocytes were further injected with [α-32P]dCTP and incubated in presence or absence of cycloheximide. Addition of CHX 30 min after GVBD has been shown previously to prevent cyclin B1 synthesis, MPF activity at the end of meiosis I, and progression into meiosis II. Instead oocytes exiting meiosis I reform nuclei and replicate their DNA. Analysis of chromosomal DNA indicated that Geminin-depleted oocytes were unable to replicate their DNA after meiosis I (Fig. 5B, lane 5) and proceeded normally into meiosis II as shown in Fig. 5A. The only time oocytes were able to replicate their DNA after meiosis I was when MPF activity was inhibited by CHX irrespective of the presence or absence of Geminin (Fig. 5B, lanes 1, 2, and 7). These results clearly establish that Geminin is not required to prevent DNA replication between MI and MII. The block to replicate during meiosis is only controlled by the intermediate level of Cdk1 activity that exists between the two meiotic divisions (as shown in Fig. 5A, lane 5).
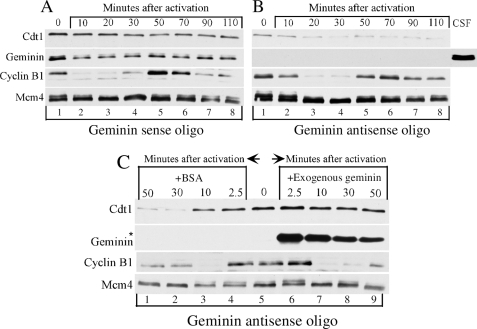
Geminin Is Required for Cdt1 Stabilization after Fertilization
Our results so far indicate that the main role of Geminin during oocyte maturation is to favor Cdt1 accumulation most likely in preparation for the early embryonic replication cycles that follow fertilization. Although Geminin is known to be partially degraded at the time of fertilization, how this affects Cdt1 levels is unclear. In addition, how Geminin-mediated stabilization of Cdt1 during maturation impacts Cdt1 levels after fertilization is currently unknown. We addressed these two questions in the next set of experiments. We used calcium chloride injection into mature oocytes to activate them and mimic the molecular events occurring during fertilization. First, we looked at the activation of oocytes that were matured either in the presence or absence of Geminin as they were injected with Geminin sense or antisense oligonucleotides, respectively, before maturation. The Cdt1 and Geminin levels were followed by Western blot analysis. The oscillation in the level of cyclin B1 and the phosphorylation of the Mcm4 protein were also followed as they are good markers of the activation process and the first embryonic cell cycle. Cyclin B1 showed its characteristic destruction after activation (Fig. 6, A and B, compare lanes 1 and 2) and then re-synthesis in M phase (Fig. 6, A and B, lanes 5 and 6) before its loss at the exit of the first cycle (Fig. 6, A and B, lane 7). Accordingly the Mcm4 protein underwent dephosphorylation after activation and S phase (Fig. 6, A and B, lanes 2–4) and became hyperphosphorylated during the next mitotic phase (Fig. 6, A and B, lanes 5 and 6). Consistent with previous reports (19, 28, 42), we found that in control mature oocytes (injected with Geminin sense oligonucleotides), Geminin was partially degraded at the time of activation, and the levels remained stable during the remainder of the cell cycle (Fig. 6A). Meanwhile, the levels of Cdt1 were not affected at the time of activation (Fig. 6A) indicating that unlike Geminin and cyclin B1, Cdt1 is not a target of APC/C during this transition. However, when oocytes matured in the absence of Geminin (by injection with antisense oligonucleotides) were activated, the level of Cdt1 was not only low to start with but decreased gradually over the first cycle (Fig. 6B). This decrease was insensitive to aphidicolin (data not shown) and thus was not the result of Cdt1 degradation associated with DNA replication during the first S phase. This result rather suggests that the presence of Geminin is required for stabilizing newly synthesized Cdt1 protein and for maintaining its level after activation. To further confirm our observation, we performed a rescue experiment in which exogenous Geminin was injected into oocytes containing no endogenous Geminin right before their activation (Fig. 6C). An excess of exogenous Geminin stabilized the Cdt1 levels after activation. Cdt1 and Geminin protein levels were also monitored during the first five cycles of developing embryos (see supplemental Fig. 2). In these embryos Cdt1 accumulates mostly during mitosis in parallel with Geminin further strengthening the idea that Geminin is also required for Cdt1 accumulation during the early embryonic cell cycles.
FIGURE 6.
Geminin stabilizes Cdt1 after activation. Geminin sense or antisense oligos injected in in vitro matured oocytes were injected with CaCl2. At the indicated times samples were collected and analyzed for cyclin B1, Geminin, and Cdt1 expression by Western analysis. CSF is a positive control; Mcm4 is a loading control, and the asterisk indicates exogenous Geminin. Zero time point corresponds to mature oocyte (240 min after GVBD). A and B, activation in the absence of exogenous Geminin. C, activation in the presence of exogenous Geminin. Experiments carried out in A and B are from the same batch of oocytes, whereas that of C is on a different batch of oocytes. BSA, bovine serum albumin.
DISCUSSION
Our data show that the presence of Geminin during meiosis and after fertilization is required for the proper accumulation of the licensing factor Cdt1. It is well established that the rapid embryonic divisions following fertilization require the accumulation of numerous maternal factors during oogenesis. Such factors include proteins like Cdt1 that will support initiation of DNA replication after fertilization. Large amounts of these factors are accumulated in Xenopus oocytes as the first 12 embryonic cell divisions rely almost exclusively on such maternal inheritance. Xenopus oocytes maturing without Geminin accumulate only a small amount of Cdt1 that almost completely disappears after the first embryonic cell cycle. It is unlikely that such Cdt1 levels would be able to support the early development of Xenopus embryos. Indeed a previous study reported the difficulty of obtaining Geminin-deficient Xenopus embryos following the injection of Geminin antisense oligonucleotides before meiosis (38). Geminin depletion in embryos was obtained by injection of antisense oligonucleotides at the two-cell stage after maternal accumulation of Geminin during meiosis. Under such conditions the levels of Geminin decreased significantly but remained detectable until the seventh division cycle of the embryo before disappearing completely when the embryo arrested after the midblastula transition. This slow disappearance of Geminin might explain why these embryos managed to develop until the midblastula transition.
Although Geminin is known to be an inhibitor of Cdt1 function, our data show that Geminin is not involved in the suppression of DNA replication between meiosis I and meiosis II. Absence of Geminin neither alters the overall meiotic progression nor pushes the oocytes to enter S phase after completion of meiosis I. Therefore, we conclude that the major role of Geminin during meiosis is to stabilize Cdt1 and favor its accumulation for the early embryonic division cycles after fertilization. We believe that this stabilizing role of Geminin remains predominant during the early division cycles following fertilization until zygotic gene transcription occurs and gap phases appear in a more typical somatic cell cycle. Thereafter, the major function of Geminin becomes the prevention of re-replication of the genome during S and G2 phases of the cell cycle. The idea that the positive role of Geminin is most important during meiosis and the early embryonic divisions but not in somatic cells is supported by previous studies showing the following: 1) no re-replication occurs in Geminin-deficient Xenopus embryos before midblastula transition (38); and 2) Geminin depletion in some mammalian cells does not prevent proliferation or re-replication despite a decrease in Cdt1 stability (5, 24, 25).
Accumulation of a cellular protein is determined by the balance between its rate of synthesis and degradation. Our data indicate that extremely low levels of Cdt1 are present in stage VI oocytes that are arrested in prophase of meiosis I. These low levels of Cdt1 were not affected by the injection of an excess of exogenous Geminin into stage VI oocytes, indicating that at this stage the level of Cdt1 might not be the result of a lack of stabilization by Geminin but rather be limited by its rate of synthesis. However, as both proteins appear to be localized in different cellular compartments (the nucleus for Cdt1 and cytoplasm for Geminin) the lack of stabilization of Cdt1 by Geminin cannot be completely excluded (32). Numerous mRNAs accumulated in Xenopus oocytes are translationally silent (43). We believe this could be the case for Cdt1 mRNA as its 3′UTR contains consensus sequences that are necessary to mediate translational repression. Two cytoplasmic polyadenylation elements (CPEs) and one hexanucleotide (AAUAAA) were identified in the Cdt1 3′UTR sequence as listed in the GenBankTM data base, suggesting that Cdt1 expression is likely translationally regulated during meiosis. Two CPEs and a single hexanucleotide sequence were also found within the 3′UTR of Geminin indicating that the low level of Geminin observed in immature oocytes might also result from a translational repression mechanism. De-repression of the Cdt1 and Geminin mRNAs occurred during maturation, but accumulation of Geminin protein preceded the accumulation of Cdt1 protein. This observation could in part be explained by the fact that the CPE and hexanucleotide sequences are organized differently in the Geminin and Cdt1 3′UTRs. The two CPEs in the Geminin 3′UTR are spaced further apart, but one is found in close proximity of the hexanucleotide sequence. A similar combination has previously been shown to weaken translational repression and facilitate translational activation and therefore could explain why Geminin synthesis precedes the one of Cdt1 during oocyte maturation (44).
The absence of Geminin had little effect on Cdt1 accumulation during meiosis I but had a strong destabilizing effect during meiosis II. The decrease in Cdt1 observed is specific to the lack of Geminin expression as it can be reversed by the injection of excess recombinant Geminin in the maturing oocytes. Our results indicate that the presence or absence of Geminin is unlikely to affect Cdt1 synthesis rate during meiosis but rather affects Cdt1 degradation rate during meiosis II. No similar stabilization effect of Geminin was observed early during meiosis I. Two reasons could explain why low levels of Cdt1 still accumulated in the absence of Geminin during meiosis I. First, other factors or post-translational modifications could stabilize Cdt1 and prevent its degradation during meiosis I. The Mcm9 protein has been shown to interact with Cdt1 and form a rather stable complex (27). Although the Mcm9 protein is present in mature oocytes, its expression in stage VI oocytes or during the maturation process is currently unknown. Upon examination of the Mcm9 3′UTR, we found no consensus sequence that would suggest that MCM9 expression is translationally regulated during meiosis. Therefore, it would not be surprising to find MCM9, as the other members of the MCM family, already expressed in immature oocytes arrested in prophase of meiosis I (37). Finally, the low levels of Cdt1 observed during meiosis I might simply be the result of a rate of Cdt1 degradation that is lower than its rate of synthesis.
In human cells GEMININ protects CDT1 from proteasome degradation by inhibiting its ubiquitination. It is likely that the same mechanism is involved in the protective effect of Geminin during meiosis. However, at this point we have no evidence that absence of Geminin during meiosis would lead to Cdt1 ubiquitination. In addition, the specific ubiquitin ligase involved in the degradation of Cdt1 in the absence of Geminin either during meiosis or mitosis is currently unknown.
Recent studies have shown that several ubiquitin ligases can mediate Cdt1 degradation in Xenopus. Cullin4-based ubiquitin ligase is responsible for the degradation of Cdt1 associated with DNA replication and after DNA damage (22, 45–47). In mammalian cells, Cdk2 has also been shown to target Cdt1 destruction via the SCFSkp2 ubiquitin ligase (48–50). None of these ubiquitin ligases are known to be active and to mediate degradation of specific factors during meiosis. In addition, Cdt1 degradation in oocytes is independent of Cdk activity and therefore unlikely mediated by SCFSkp2. The activity of two other ubiquitin ligases has been reported during the meiotic cell cycle in different species. In Xenopus, the SCFβ-TrCP ligase is active during meiosis and targets the destruction of the CPEB and Ringo proteins (51, 52). The involvement of SCFβ-TrCP in Cdt1 degradation during meiosis is unlikely as Xenopus Cdt1 does not contain the canonical DSGXXS motif that is present in many substrates of this ubiquitin ligase. The other well established ubiquitin ligase involved in the control of meiosis is the APC/C. The APC/C exists in two forms defined by the positive regulators Cdc20 or Cdh1 that are associated with it. In Xenopus oocytes, the APCCdc20 promotes the complete degradation of cyclin B and the partial Geminin degradation at the time of fertilization (7, 41). We observed no degradation of Cdt1 at the time of activation or fertilization, suggesting that Cdt1 is not the target of APCCdc20 during this transition. However, the resilience of the Cdt1 protein and a subset of the Geminin population at the time of activation could indicate that the Cdt1-Geminin complex is not a target of APCCdc20, and only free Geminin and Cdt1 are readily degraded by this pathway. Furthermore, one could imagine that APCCdc20 is responsible for Cdt1 degradation during meiosis II in Geminin-depleted oocytes as its activity is turned on and off during meiosis II arrest (53). Recent studies in mouse oocytes and Drosophila eggs have highlighted the role of APCCdh1 in regulating prophase I arrest and progression through meiosis and more generally the idea that both APC complexes are required for the proper execution of meiosis (54). Although APCCdh1 is expressed at a relatively low level in Xenopus oocytes, its role in the destruction of specific proteins during meiosis is controversial and largely unknown (55, 56). Two lines of evidence suggested that the degradation of Cdt1 in the absence of Geminin could be mediated by APCCdh1. First, a recent study demonstrated that Cdt1 is an APCCdh1 substrate (57) and that the Xenopus Cdt1 protein contains not only generic APC destruction boxes (D-box, RXXL) but also a putative Cdh1 recognition motif known as KEN-box. Second, the slow degradation of Cdt1 observed after fertilization in the absence of Geminin occurs at the time when the KEN-box substrates of APCCdh1 are degraded in mouse oocytes exiting meiosis II and after extrusion of the second polar body (58). It will therefore be interesting to determine whether APCCdh1 is involved in the degradation of Cdt1 that we observed in Xenopus oocytes undergoing meiosis in the absence of Geminin despite the fact that its activity is inhibited by a high level of MPF activity.
Supplementary Material
Acknowledgments
We thank Dr. Curt Pfarr for help with reading and preparation of the manuscript. Y. N. thanks Dr. Clinton Macdonald and Dr. Daniel Webster for careful reading of the manuscript and also thanks Dr. H. G. Bagaria for insightful discussions throughout this study and for helping with the manuscript preparation. We thank Tania Silva and Bettina Khan for technical support.
This work was supported, in whole or in part, by a National Institutes of Health grant (to M. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental.
- pre-RC
- pre-replication complex
- Cdk
- cyclin-dependent kinase
- CHX
- cycloheximide
- APC/C
- anaphase promoting complex
- CPE
- cytoplasmic polyadenylation element
- GVBD
- germinal vesicle breakdown
- UTR
- untranslated region
- MCM
- mini-chromosome maintenance complex
- MPF
- maturation-promoting factor
- H1
- histone 1
- CPE
- cytoplasmic polyadenylation element
- MI
- meiosis I
- MII
- meiosis II
- oligo
- oligonucleotide.
REFERENCES
- 1.Bell S. P., Dutta A. (2002) Annu. Rev. Biochem. 71, 333–374 [DOI] [PubMed] [Google Scholar]
- 2.Saxena S., Dutta A. (2003) Cell Cycle 2, 283–286 [PubMed] [Google Scholar]
- 3.Lygerou Z., Nurse P. (2000) Science 290, 2271–2273 [DOI] [PubMed] [Google Scholar]
- 4.Madine M., Laskey R. (2001) Nat. Cell Biol. 3, E49–E50 [DOI] [PubMed] [Google Scholar]
- 5.Melixetian M., Helin K. (2004) Cell Cycle 3, 1002–1004 [PubMed] [Google Scholar]
- 6.Saxena S., Dutta A. (2005) Mutat. Res. 569, 111–121 [DOI] [PubMed] [Google Scholar]
- 7.McGarry T. J., Kirschner M. W. (1998) Cell 93, 1043–1053 [DOI] [PubMed] [Google Scholar]
- 8.Quinn L. M., Herr A., McGarry T. J., Richardson H. (2001) Genes Dev. 15, 2741–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagi K., Mizuno T., You Z., Hanaoka F. (2002) J. Biol. Chem. 277, 40871–40880 [DOI] [PubMed] [Google Scholar]
- 10.Yanagi K., Mizuno T., Tsuyama T., Tada S., Iida Y., Sugimoto A., Eki T., Enomoto T., Hanaoka F. (2005) J. Biol. Chem. 280, 19689–19694 [DOI] [PubMed] [Google Scholar]
- 11.Wohlschlegel J. A., Dwyer B. T., Dhar S. K., Cvetic C., Walter J. C., Dutta A. (2000) Science 290, 2309–2312 [DOI] [PubMed] [Google Scholar]
- 12.Tada S., Li A., Maiorano D., Méchali M., Blow J. J. (2001) Nat. Cell Biol. 3, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballabeni A., Melixetian M., Zamponi R., Masiero L., Marinoni F., Helin K. (2004) EMBO J. 23, 3122–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaziri C., Saxena S., Jeon Y., Lee C., Murata K., Machida Y., Wagle N., Hwang D. S., Dutta A. (2003) Mol. Cell 11, 997–1008 [DOI] [PubMed] [Google Scholar]
- 15.Nishitani H., Lygerou Z., Nishimoto T. (2004) J. Biol. Chem. 279, 30807–30816 [DOI] [PubMed] [Google Scholar]
- 16.Gopalakrishnan V., Simancek P., Houchens C., Snaith H. A., Frattini M. G., Sazer S., Kelly T. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13114–13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomer M., May N. R., Aggarwal B. D., Kwok G., Calvi B. R. (2004) Development 131, 4807–4818 [DOI] [PubMed] [Google Scholar]
- 18.Arias E. E., Walter J. C. (2005) Genes Dev. 19, 114–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li A., Blow J. J. (2005) EMBO J. 24, 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiorano D., Krasinska L., Lutzmann M., Mechali M. (2005) Curr. Biol. 15, 146–153 [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K., Takisawa H., Kubota Y. (2005) Genes Cells 10, 63–73 [DOI] [PubMed] [Google Scholar]
- 22.Zhong W., Feng H., Santiago F. E., Kipreos E. T. (2003) Nature 423, 885–889 [DOI] [PubMed] [Google Scholar]
- 23.Lutzmann M., Maiorano D., Méchali M. (2006) EMBO J. 25, 5764–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W., Depamphilis M. L. (2009) Cancer Res. 69, 4870–4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machida Y. J., Dutta A. (2007) Genes Dev. 21, 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xouri G., Squire A., Dimaki M., Geverts B., Verveer P. J., Taraviras S., Nishitani H., Houtsmuller A. B., Bastiaens P. I., Lygerou Z. (2007) EMBO J. 26, 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutzmann M., Méchali M. (2008) Mol. Cell 31, 190–200 [DOI] [PubMed] [Google Scholar]
- 28.Li A., Blow J. J. (2004) Nat. Cell Biol. 6, 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li A., Blow J. J. (2004) Cell Cycle 3, 443–445 [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgson B., Li A., Tada S., Blow J. J. (2002) Curr. Biol. 12, 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda D. Y., Parvin J. D., Dutta A. (2005) J. Biol. Chem. 280, 23416–23423 [DOI] [PubMed] [Google Scholar]
- 32.Lemaître J. M., Bocquet S., Méchali M. (2002) Nature 419, 718–722 [DOI] [PubMed] [Google Scholar]
- 33.Ohsumi K., Sawada W., Kishimoto T. (1994) J. Cell Sci. 107, 3005–3013 [DOI] [PubMed] [Google Scholar]
- 34.Furuno N., Nishizawa M., Okazaki K., Tanaka H., Iwashita J., Nakajo N., Ogawa Y., Sagata N. (1994) EMBO J. 13, 2399–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwabuchi M., Ohsumi K., Yamamoto T. M., Sawada W., Kishimoto T. (2000) EMBO J. 19, 4513–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajo N., Yoshitome S., Iwashita J., Iida M., Uto K., Ueno S., Okamoto K., Sagata N. (2000) Genes Dev. 14, 328–338 [PMC free article] [PubMed] [Google Scholar]
- 37.Whitmire E., Khan B., Coué M. (2002) Nature 419, 722–725 [DOI] [PubMed] [Google Scholar]
- 38.McGarry T. J. (2002) Mol. Biol. Cell 13, 3662–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereverzeva I., Whitmire E., Khan B., Coué M. (2000) Mol. Cell. Biol. 20, 3667–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coué M., Kearsey S. E., Méchali M. (1996) EMBO J. 15, 1085–1097 [PMC free article] [PubMed] [Google Scholar]
- 41.Hochegger H., Klotzbücher A., Kirk J., Howell M., le Guellec K., Fletcher K., Duncan T., Sohail M., Hunt T. (2001) Development 128, 3795–3807 [DOI] [PubMed] [Google Scholar]
- 42.Auziol C., Méchali M., Maiorano D. (2007) Biochem. Biophys. Res. Commun. 361, 276–280 [DOI] [PubMed] [Google Scholar]
- 43.Radford H. E., Meijer H. A., de Moor C. H. (2008) Biochim. Biophys. Acta 1779, 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piqué M., López J. M., Foissac S., Guigó R., Méndez R. (2008) Cell 132, 434–448 [DOI] [PubMed] [Google Scholar]
- 45.Hu J., McCall C. M., Ohta T., Xiong Y. (2004) Nat. Cell Biol. 6, 1003–1009 [DOI] [PubMed] [Google Scholar]
- 46.Higa L. A., Mihaylov I. S., Banks D. P., Zheng J., Zhang H. (2003) Nat. Cell Biol. 5, 1008–1015 [DOI] [PubMed] [Google Scholar]
- 47.Kim Y., Kipreos E. T. (2007) Cell Div. 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Zhao Q., Liao R., Sun P., Wu X. (2003) J. Biol. Chem. 278, 30854–30858 [DOI] [PubMed] [Google Scholar]
- 49.Liu E., Li X., Yan F., Zhao Q., Wu X. (2004) J. Biol. Chem. 279, 17283–17288 [DOI] [PubMed] [Google Scholar]
- 50.Nishitani H., Sugimoto N., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K. I., Nakayama K., Fujita M., Lygerou Z., Nishimoto T. (2006) EMBO J. 25, 1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez G. J., Vögtlin A., Castro A., Ferby I., Salvagiotto G., Ronai Z., Lorca T., Nebreda A. R. (2006) Nat. Cell Biol. 8, 1084–1094 [DOI] [PubMed] [Google Scholar]
- 52.Setoyama D., Yamashita M., Sagata N. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18001–18006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto T. M., Iwabuchi M., Ohsumi K., Kishimoto T. (2005) Dev. Biol. 279, 345–355 [DOI] [PubMed] [Google Scholar]
- 54.Marangos P., Verschuren E. W., Chen R., Jackson P. K., Carroll J. (2007) J. Cell Biol. 176, 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papin C., Rouget C., Lorca T., Castro A., Mandart E. (2004) Dev. Biol. 272, 66–75 [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y., Ching Y. P., Ng R. W., Jin D. Y. (2002) Biochem. Biophys. Res. Commun. 294, 120–126 [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto N., Kitabayashi I., Osano S., Tatsumi Y., Yugawa T., Narisawa-Saito M., Matsukage A., Kiyono T., Fujita M. (2008) Mol. Biol. Cell 19, 1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang H. Y., Levasseur M., Jones K. T. (2004) J. Cell Sci. 117, 6289–6296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.