Abstract
Newly synthesized peroxisomal matrix proteins are targeted to the organelle by PEX5, the peroxisomal cycling receptor. Over the last few years, valuable data on the mechanism of this process have been obtained using a PEX5-centered in vitro system. The data gathered until now suggest that cytosolic PEX5·cargo protein complexes dock at the peroxisomal docking/translocation machinery, where PEX5 becomes subsequently inserted in an ATP-independent manner. This PEX5 species is then monoubiquitinated at a conserved cysteine residue, a mandatory modification for the next step of the pathway, the ATP-dependent dislocation of the ubiquitin-PEX5 conjugate back into the cytosol. Finally, the ubiquitin moiety is removed, yielding free PEX5. Despite its usefulness, there are many unsolved mechanistic aspects that cannot be addressed with this in vitro system and that call for a cargo protein-centered perspective instead. Here we describe a robust peroxisomal in vitro import system that provides this perspective. The data obtained with it suggest that translocation of a cargo protein across the peroxisomal membrane, including its release into the organelle matrix, occurs prior to PEX5 ubiquitination.
Peroxisomal matrix proteins are synthesized in cytosolic ribosomes and post-translationally targeted to the organelle (1, 2). The vast majority of proteins destined to this compartment possess the so-called peroxisomal targeting sequence type 1 (PTS1),3 a short domain present at their extreme C termini and frequently ending with the sequence SKL (3, 4). A small number of matrix proteins lack this domain and contain instead a PTS2, a degenerated nonapeptide with the sequence (R/K)(L/V/I)X5(H/Q)(L/A) present at their N termini (5, 6). In contrast to the PTS1, which is not cleaved upon peroxisomal import, the PTS2 signal is proteolytically removed in the peroxisomal matrix of many organisms by a peroxisomal processing peptidase (1, 7, 8).
In mammals and many other organisms, both PTS1-containing and PTS2-containing proteins are targeted to the organelle by PEX5, the peroxisomal cycling receptor (9–12). PTS1 proteins interact directly with the C-terminal half of PEX5, a region comprising seven tetratricopeptide repeats arranged into a ring-like structure, whereas the PEX5-PTS2 interaction is bridged by the adaptor protein PEX7 (13–19). This adaptor protein interacts with a small region within the largely unfolded N-terminal half of PEX5 (17, 18, 20). Interestingly, not all proteins derived from the mammalian PEX5 gene have the capacity to bind PEX7. This is due to alternative splicing of the PEX5 transcript yielding two major mRNAs, one encoding the so-called large isoform of PEX5 (PEX5L) and the other coding for the small PEX5 isoform (PEX5S). PEX5S lacks a 37-amino-acid region that is involved in the PEX7 interaction, and so it is incompetent in the peroxisomal targeting of PTS2 proteins (16–18).
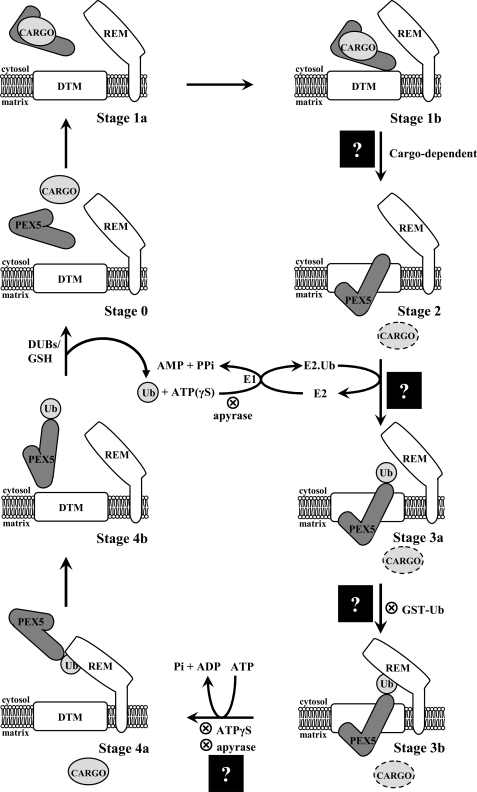
In recent years, valuable data on the mechanistic details of the PEX5-mediated protein import pathway in mammals have been obtained using a PEX5-centered in vitro system (21–23). In this system, an organelle suspension (e.g. a postnuclear supernatant) is incubated with 35S-labeled PEX5 under different conditions, and the behavior of the radiolabeled protein is monitored. The data gathered until now together with information coming from protein-protein interaction studies (see Ref. 24 and references cited therein) and cell biology experiments (17, 18, 25, 26) support the following pathway (Fig. 1) (see Ref. 27 for a recent review). First, cytosolic PEX5 (stage 0) binds newly synthesized peroxisomal matrix proteins in the cytosol. The PEX5·cargo protein complex (stage 1) then docks at the peroxisomal docking/translocation machinery (DTM), a membrane-embedded protein complex comprising PEX13, PEX14, and the RING finger proteins PEX2, PEX10, and PEX12 (28, 29). This interaction ultimately results in the ATP-independent but cargo protein-dependent insertion of PEX5 into the DTM (stage 2), an essentially irreversible step (30–32). The DTM-embedded PEX5 is then monoubiquitinated at a conserved cysteine residue (stage 3) (33, 34). This is a mandatory modification for the next step of the pathway, the ATP-dependent dislocation of monoubiquitinated PEX5 (Ub·PEX5) by the receptor export module, a protein complex containing PEX1 and PEX6, two members of the AAA protein family (ATPases associated with diverse cellular activities family), and PEX26, a peroxisomal membrane protein (21, 34, 35). Finally, ubiquitin is removed from the cytosolic Ub·PEX5 conjugate (stage 4), probably by a combination of enzymatic and non-enzymatic mechanisms (23) yielding free PEX5 (stage 0). A new protein transport cycle then starts.
FIGURE 1.
The PEX5-mediated peroxisomal protein import pathway. There are five major stages in this protein sorting pathway (numbered 0–4). Substages (a and b) are mostly of conceptual nature. The different stages have been characterized with a PEX5-centered in vitro system, applying several strategies that block (⊗) the pathway at different steps. Stage 0, cytosolic cargo-free PEX5. Stage 1, cytosolic PEX5·cargo protein complex. Stage 2, PEX5 embedded in the peroxisomal DTM. Stage 3, DTM-embedded monoubiquitinated PEX5. Stage 4, cytosolic monoubiquitinated PEX5. Insertion of PEX5 into the DTM is cargo protein-dependent. Monoubiquitination of stage 2 PEX5 yielding stage 3 PEX5 requires activated ubiquitin. Activation of ubiquitin by the ubiquitin-activating enzyme (E1) is an ATP-dependent process; E2 indicates ubiquitin carrier protein. Note that this activation, which involves the synthesis of the acyl phosphate AMP-ubiquitin anhydride and the release of pyrophosphate, can also be achieved with ATPγS (a sulfur atom at a non-bridge position of the γ-phosphate of ATP does not affect the reaction (62)). The ubiquitin analogue GST·Ub is also used efficiently by the ubiquitin-conjugating cascade acting on PEX5. However, this stage 3 species is no longer a substrate for the receptor export module (REM), presumably because of the bulkiness of GST·Ub. Apyrase hydrolyzes ATP and thus blocks PEX5 both at stage 2 and stage 3b levels. Note that if apyrase is added to the in vitro assays before PEX5 (or ΔC1PEX5L), the receptor will not proceed to stage 3a. Stage 4 PEX5 is deubiquitinated, yielding stage 0 PEX5 in a process that probably involves deubiquitinating enzymes (DUBs) and GSH. The step at which cargo proteins are translocated across the peroxisomal membrane (black squares with a question mark) is the topic of this work. Mammalian PEX7 is not represented in the model because it is presently unknown whether it is retained at the DTM together with PEX5L or translocated across the peroxisomal membrane together with the cargo protein, as proposed for the yeast protein (63).
Ironically, although aiming at describing the mechanism of protein translocation across the peroxisomal membrane, the model described above actually contains very few data regarding the cargo proteins themselves. Two obvious events still missing in this model regard the steps where the cargo protein is 1) moved from the cytosolic side of the peroxisomal membrane into the DTM and 2) released from the DTM into the peroxisomal matrix. Based on the observation that PEX5 at the stage 2 level exposes the majority of its mass into the peroxisomal matrix (22) and on the finding that insertion of PEX5 into the DTM is cargo protein-dependent (31), we previously proposed that cargoes are translocated across the organelle membrane at the stage 1-to-stage 2 transition (36). However, the fact remains that no direct evidence supporting this idea is presently available. Regarding the release of the cargo protein from the DTM, there are virtually no data, only some hypotheses (27, 33).
A classical in vitro import system where a 35S-labeled cargo protein is incubated with an organelle suspension is, of course, a first choice strategy to address this type of mechanistic question. However, besides a few pioneering studies performed many years ago (37–39), such a strategy was never very popular among researchers in the peroxisomal field. There are several reasons explaining why this approach was abandoned (see Ref. 40), but in essence its main problem is its low yield, a strong limitation when drawing the line between specific and unspecific phenomena.
In this work, we describe a simple procedure that dramatically improves this cargo-centered in vitro system. We show that when a rat liver postnuclear supernatant is fortified with selected recombinant proteins, a robust amount of a 35S-labeled PTS2-containing protein can be specifically imported into peroxisomes. The data obtained allowed us to map the step of protein translocation across the peroxisomal membrane into the PEX5 cycling pathway.
EXPERIMENTAL PROCEDURES
Recombinant Proteins
The recombinant large isoform of human PEX5 (PEX5L) (41), a protein comprising the first 324 amino acid residues of PEX5L (ΔC1PEX5L) (42), PEX5L containing the missense mutation N526K (PEX5L(N526K); (43)), and the GST·ubiquitin fusion protein (GST·Ub (34)) were obtained as described previously. For the production of a histidine-tagged recombinant protein comprising the first 287 amino acid residues of the small isoform of human PEX5 (ΔC1PEX5S), the plasmid pGEM4-PEX5S (43) was used as the template in a PCR reaction with the primers 5′-GCGAACTGCATATGGCAATGCGGGAGCTGG-3′ and 5′-GCGCGGATCCTCATTAGTACCCCTTATCATAGGTAGCTG-3′. The resulting PCR product was digested with NdeI and BamHI and cloned into the NdeI/BamHI sites of pET28c vector (Promega). This protein was then expressed in the Escherichia coli strain BL21 and purified using HIS-SelectTM nickel affinity gel (Sigma).
Synthesis of Radiolabeled Proteins
35S-Labeled PEX5L(C11K) was synthesized as described before (23). The cDNA encoding full-length human thiolase precursor (clone IMAGE ID 3860150, from imaGenes) was amplified by PCR using the primers 5′-GCGAAGCTTGCCACCATGCAGAGGCTGCAGGTA-3′ and 5′-GCGCGAATTCTCAGTTCCCAGGGTATTCAA-3′, designed according to the published sequence (44). A cDNA encoding the amino acid sequence of mature thiolase preceded by a methionine (ΔPTS2 thiolase) was obtained by PCR using the primers 5′-GAATACGAAGCTTGCCACCATGCTGAGCGGTGCC-3′ and 5′-GCGCGAATTCTCAGTTCCCAGGGTATTCAA-3′. Both cDNAs were cloned into the HindIII and the EcoRI sites of pGEM4 (Promega). 35S-Labeled proteins were synthesized using the TnT® T7 quick coupled transcription/translation kit (Promega) in the presence of [35S]methionine (specific activity >1000 Ci/mmol; PerkinElmer Life Sciences) following the standard conditions of the manufacturer. The amounts of 35S-labeled proteins obtained with this kit were not determined, but according to the manufacturer, yields of 3–6 ng/μl are common.
In Vitro Import Reactions
Rat liver PNS was prepared in SEM buffer (0.25 m sucrose, 20 mm MOPS-KOH, pH 7.4, 1 mm EDTA-NaOH, pH 7.4, 2 μg/ml N-(trans-epoxysuccinyl)-l-leucine 4-guanidinobutylamide (E-64)) as described before (22). In a typical import reaction, 400 μg of rat liver PNS protein and 1 μl of a rabbit reticulocyte lysate containing 35S-labeled prethiolase or ΔPTS2 thiolase were used. Incubation was for 45 min at 37 °C in 100 μl of import buffer (0.25 m sucrose, 50 mm KCl, 20 mm MOPS-KOH, pH 7.4, 3 mm MgCl2, 20 μm methionine, 2 μg/ml E-64, and 2 mm GSH, pH 7.2) containing 3 mm ATP. GSH increases the amounts of 35S-labeled prethiolase acquiring a protease-resistant status. Recombinant PEX5 proteins (2 and 4 ng/μl for ΔC1PEX5 versions and full-length proteins, respectively; ∼55 nm final concentrations), GST·Ub, or bovine ubiquitin (10 μm) were added to some reactions, as indicated. The ionophores valinomycin (added from a 1 mm stock solution in ethanol), Fluka calcium ionophore II (0.5 mm in ethanol), and carbonylcyanide m-chlorophenylhydrazone (1 mm in ethanol) were used at 10, 5, and 10 μm, respectively. In the experiments aiming at determining whether or not import of prethiolase requires hydrolysis of cytosolic ATP, both the 35S-labeled protein and the PNS were pretreated with ATP as follows. Rabbit reticulocyte lysates were incubated with 40 μg of rat liver cytosolic protein prepared according to Grou et al. (42) in the presence or absence of 200 ng of ΔC1PEX5L in 10 μl of import buffer containing 0.3 mm ATP (quantities per μl of lysate) at 37 °C for 10 min. At the end of the incubation, the energetic status of the solution was either reinforced by adding ATP to 3 mm or changed by adding either ATPγS (3 mm final concentration) or apyrase (20 units/ml final concentration). Incubation proceeded for an additional 10 min. PNS at 8 μg/μl in import buffer was incubated for 5 min at 37 °C in the presence of 0.3 mm ATP. Then, 50-μl aliquots of this suspension were added to 40 μl of import buffer containing ATP (6.75 mm) or ATPγS (6.75 mm) or apyrase (45 units/ml) and either bovine ubiquitin or GST·Ub (22.5 μm each), as specified in the legends for Figs. 4 and 5. After 3 min at 37 °C, 10 μl of the energetically matching rabbit reticulocyte lysate solutions were added, and the mixtures were incubated for an additional 45 min. Protease treatment of import reactions was performed on ice for 45 min using 400 μg/ml trypsin (final concentration). After inactivation of the protease with 500 μg/ml phenylmethylsulfonyl fluoride for 2 min on ice, the organelle suspensions were diluted to 500 μl with SEMK (SEM buffer containing 50 mm KCl) and isolated by centrifugation (11,300 × g, 20 min at 4 °C). The samples were then subjected to SDS-PAGE and transferred to nitrocellulose, and the radioactive proteins were detected by autoradiography. All of the in vitro import experiments reported in this work were performed at least five times.
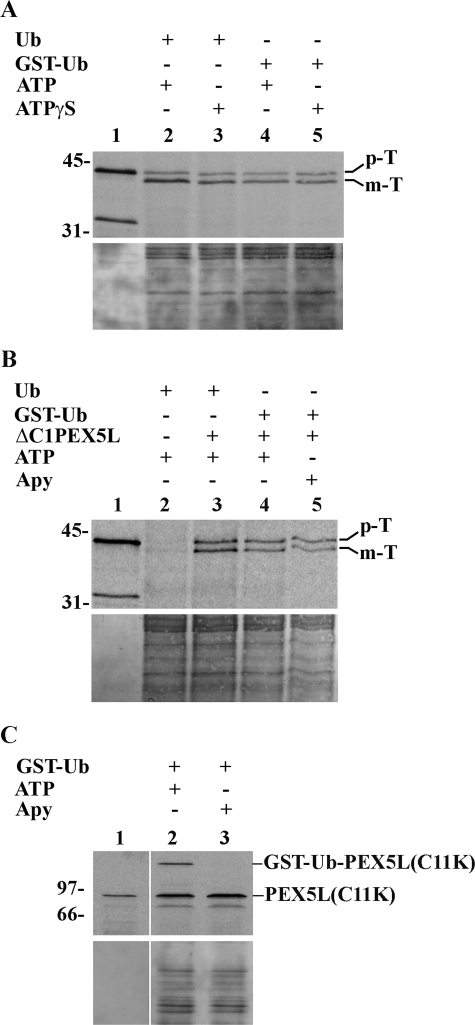
FIGURE 4.
Import of prethiolase into peroxisomes does not require hydrolysis of cytosolic ATP. A, import reactions assembled with components pretreated with 0.3 mm ATP (see “Experimental Procedures”) and supplemented with either bovine Ub or GST·Ub were performed in the presence of 3 mm ATP or 3 mm ATPγS, as indicated. After trypsin treatment, the organelles were isolated by centrifugation and analyzed as described in the legend for Fig. 2. The autoradiograph (upper panel) and the corresponding Ponceau S-stained membrane (lower panel) are shown. Lane 1, 10% of 35S-labeled prethiolase solution used in each lane. B, a rat liver PNS was pretreated with 0.3 mm ATP for 5 min and divided into four equal aliquots (lanes 2–5). The first and second aliquot (lanes 2 and 3, respectively) received bovine ubiquitin and 3 mm ATP; the third aliquot received GST·Ub and 3 mm ATP (lane 4); and the fourth received GST·Ub and apyrase (Apy). After 3 min at 37 °C, the first aliquot received 35S-labeled prethiolase preincubated with ATP in the absence of ΔC1PEX5L, whereas the second and third aliquots received 35S-labeled prethiolase preincubated with ATP in the presence of ΔC1PEX5L (lanes 3 and 4). The fourth aliquot received 35S-labeled prethiolase preincubated with ATP plus ΔC1PEX5L and treated with apyrase (see “Experimental Procedures” for details). After 45 min at 37 °C, the samples were treated with trypsin and processed as described above. Lane 1, 10% of the 35S-labeled prethiolase used in the import reactions. The autoradiograph (upper panel) and the corresponding Ponceau S-stained membrane (lower panel) are shown. p-T and m-T, precursor and mature forms of thiolase, respectively. C, in vitro assays using 35S-labeled PEX5L(C11K). The experimental conditions used in lanes 2 and 3 were exactly the ones used in lanes 4 and 5 of the experiment shown in B, respectively, with the exception that recombinant ΔC1PEX5L was omitted because it strongly competes with the radiolabeled protein for the DTM. At the end of the 45-min incubation at 37 °C, the organelles were sedimented and processed for SDS-PAGE. Note that PEX5L(C11K) is as functional as normal PEX5L in these assays (23). It was used here for practical reasons because the GST·Ub·PEX5L(C11K) conjugate, unlike the GST·Ub·PEX5L, is not destroyed by prolonged incubation in the presence of GSH and can be analyzed under normal SDS-PAGE conditions (23). The complete absence of GST·Ub·PEX5L(C11K) in lane 3 indicates that the amount of apyrase used in these assays efficiently depletes ATP from the reactions. Lane 1, 10% of 35S-labeled PEX5L(C11K) used in each reaction. Numbers to the left indicate the molecular masses of protein standards in kDa.
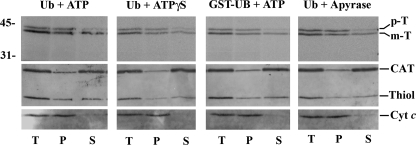
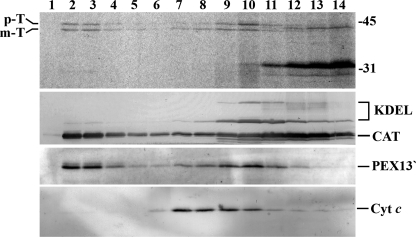
FIGURE 5.
Release of in vitro imported 35S-labeled thiolase into the peroxisomal matrix occurs before stage 3. Trypsin-treated organelles from in vitro import reactions, containing the indicated combinations of bovine Ub, GST·Ub, ATP, ATPγS, or apyrase and performed as described in legend for Fig. 4, were disrupted by sonication in a low ionic strength buffer and divided into two halves. One-half (samples T) was kept on ice, whereas the other was subjected to ultracentrifugation to separate membranes (samples P) from soluble proteins (samples S). Equivalent portions of samples T, P, and S were subjected to SDS-PAGE and blotted onto a nitrocellulose. The membrane was first exposed to an x-ray film to detect the 35S-labeled protein (top panel) and afterward probed with the following antisera: anti-thiolase (Thiol); anti-catalase (CAT), and anti-cytochrome c (Cyt c). p-T and m-T, precursor and mature forms of thiolase, respectively. Numbers to the left indicate the molecular masses of protein standards in kDa.
Miscellaneous
For the density gradient centrifugation analysis, a 4-fold scale-up of the standard import reaction was used. After trypsin treatment and inactivation of the protease, the complete import mixture was diluted to 1.5 ml with SEM buffer and analyzed by Nycodenz step gradient centrifugation exactly as described before (45). Sonication of protease treated organelles in SEM buffer containing 1 μg/μl phenylmethylsulfonyl fluoride and 1:500 (v/v) mammalian protease inhibitors mixture (Sigma) and ultracentrifugation was done as described previously (46). The anti-thiolase and anti-PEX13 antibodies were prepared as described (47, 48). The antibodies directed to catalase (catalogue number RDI-CATALASEabr; Research Diagnostics, Inc.), KDEL (catalogue number ab12223; Abcam), and cytochrome c (catalogue number 556433; BD Pharmingen) were purchased. Rabbit and mouse antibodies were detected on Western blots using alkaline phosphatase-conjugated anti-rabbit and anti-mouse antibodies (Sigma).
RESULTS AND DISCUSSION
The intrinsic fragility of peroxisomes and their high content in PTS1-containing proteins (the PTS1 is not cleaved upon import (1)) have hampered the development of a robust PTS1-centered in vitro import system. The main problem stems from the fact that peroxisome suspensions, be it a postnuclear supernatant or a purified organelle fraction, always contain soluble PTS1 proteins that have leaked from the organelles during tissue homogenization, organelle purification, or even simple manipulation (38, 49). The presence of these proteins in in vitro import reactions (a few hundreds of nanograms in our PNS-based assay; inferred from the data in Ref. 31) means that an in vitro synthesized 35S-labeled PTS1-containing reporter protein (3–6 ng/μl; see “Experimental Procedures”) has little chance of binding endogenous PEX5 (∼30 ng (46)). A partial solution to this problem is to supplement import reactions with recombinant PEX5. However, even with this modification, the in vitro import yield for a PTS1 reporter is still modest, probably because the addition of the recombinant protein to the reaction also increases the concentration of PEX5·endogenous PTS1 protein complexes now creating a competition problem at the DTM.4
Mammalian PTS2 proteins are much less abundant than PTS1 proteins (50–52). More importantly, the PTS2 peptide is proteolytically removed upon import (1, 7). This property, besides providing one extra criterion to assess in vitro import, implies that a 35S-labeled PTS2-containing reporter protein will not face much competition from endogenous proteins in the interaction with endogenous PEX7. However, PEX7·PTS2 cargo protein complexes are transported to the peroxisome also by PEX5L, and thus, at least one of the problems stated above, the competition with PEX5·PTS1 protein complexes at the DTM level, also applies to this class of peroxisomal proteins. A solution to this problem comes from the observation that some mutant versions of PEX5L (but not PEX5S), unable to bind efficiently PTS1-containing proteins, are still competent in targeting PTS2 proteins to the peroxisome in vivo (9, 17). Thus, the addition of a PEX5L recombinant protein with these properties to an in vitro import assay programmed with a PTS2 reporter protein would not lead to an increase in the concentration of PEX5·endogenous PTS1 protein complexes. We note that PEX5L mutant proteins possessing the required characteristics also seem to have the capacity to enter the DTM in a cargo protein-independent process (31, 43). However, the results presented below indicate that the stimulatory effect obtained with these proteins is much larger than their inhibitory action.
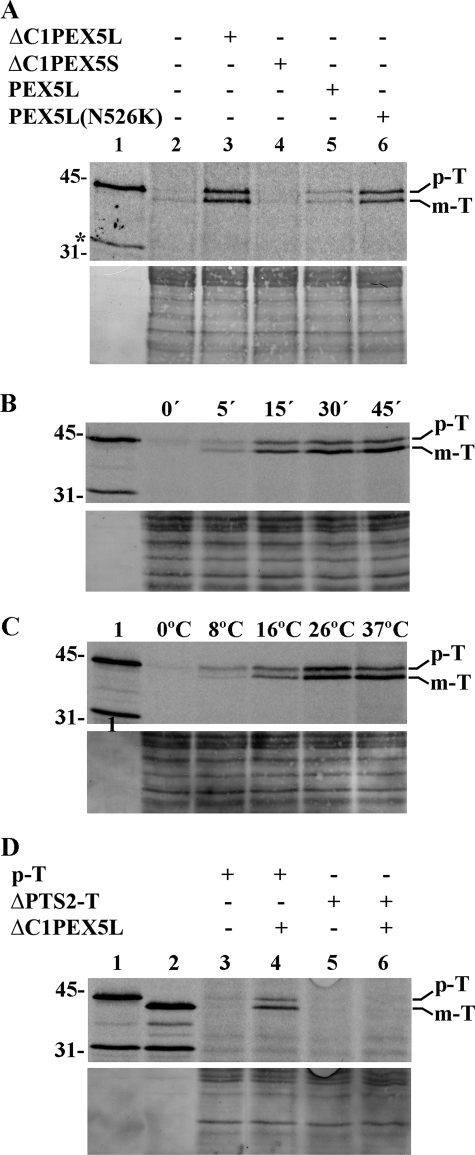
An in vitro import reaction consists of incubating a 35S-labeled reporter protein with an organelle suspension under physiological conditions. At the end of the incubation, a large amount of a highly active protease is added to the reaction to degrade non-imported 35S-labeled protein. Finally, after inactivation of the protease, the organelles are pelleted and analyzed by SDS-PAGE and autoradiography. Fig. 2 shows the results of such an assay using 35S-labeled prethiolase as the PTS2 reporter protein. When the system is used in its basic configuration (i.e. no recombinant proteins added), the amount of protease-resistant 35S-labeled protein that can be detected in organelle pellets oscillates between none (the most frequent result) and almost none (lane 2), suggesting that no significant amount of the reporter protein reaches the interior of peroxisomes under these conditions. Supplementation of the import reaction with recombinant PEX5L consistently results in a small amount of protease-resistant 35S-labeled protein being sedimented with the organelles (lane 5). About half of the protease-resistant protein migrates on SDS-PAGE exactly as mature thiolase (Fig. 2B), suggesting that 35S-labeled prethiolase did reach the correct compartment. However, the yield of this process is extremely low (less than 1% of the input; data not shown). A dramatic improvement was obtained when a previously titrated amount of ΔC1PEX5L, a recombinant protein comprising amino acid residues 1–324 of PEX5L and unable to bind PTS1 proteins efficiently, was included in the import buffer (lane 3). The stimulatory effect of ΔC1PEX5L on prethiolase import is clearly dependent on the PEX7-binding domain it contains because negligible amounts of protease-protected 35S-labeled protein were obtained in import reactions supplemented with ΔC1PEX5S (lane 4; see the Introduction). Essentially the same stimulatory effect was obtained with PEX5L(N526K) (lane 6). The N526K missense mutation abolishes the capacity of PEX5L to bind PTS1 proteins efficiently (53); its PEX7 binding activity, nevertheless, remains unaffected (9).
FIGURE 2.
A robust amount of 35S-labeled prethiolase acquires a protease-protected and organelle-associated status in in vitro import reactions fortified with ΔC1PEX5L or PEX5L(N526K). A, a rat liver PNS fraction was incubated with 35S-labeled prethiolase in import buffer containing ATP in the absence (lane 2) or presence of recombinant ΔC1PEX5L (lane 3), ΔC1PEX5S (lane 4), PEX5L (lane 5), or PEX5L(N526K) (lane 6). After trypsin treatment, the organelles were isolated by centrifugation, subjected to SDS-PAGE, and blotted onto a nitrocellulose membrane. The Ponceau S-stained membrane (lower panel) and its autoradiograph (upper panel) are shown. Lane 1, 10% of the reticulocyte lysate containing 35S-labeled prethiolase used in each reaction. B, time dependence of the ΔC1PEX5L-mediated 35S-labeled prethiolase import. Aliquots from a 5-fold standard import reaction were withdrawn at the indicated time points and processed as described in A. The Ponceau S-stained membrane (lower panel) and its autoradiograph (upper panel) are shown. Lane 1, 10% of the reticulocyte lysate containing 35S-labeled prethiolase used in each lane. C, import of 35S-labeled prethiolase is temperature-dependent. A PNS pretreated with 0.3 mm ATP for 5 min at 37 °C (see “Experimental Procedures”) was used in import reactions containing ATP and recombinant ΔC1PEX5L. After 45 min at the indicated temperatures, the samples were processed as described in A. The Ponceau S-stained membrane (lower panel) and its autoradiograph (upper panel) are shown. Lane 1, 10% of the reticulocyte lysate containing 35S-labeled prethiolase used in each reaction. D, 35S-labeled thiolase lacking its PTS2 does not acquire a protease-resistant/organelle-associated status when incubated with ΔC1PEX5L-supplemented rat liver PNS. Standard import reactions supplemented with ΔC1PEX5L (lanes 4 and 6) or lacking this recombinant protein (lanes 3 and 5) were programmed with 35S-labeled prethiolase (lanes 3 and 4) or 35S-labeled thiolase lacking the PTS2 (ΔPTS2-T; lanes 5 and 6). At the end of the incubation, the samples were processed as described in A. The Ponceau S-stained membrane (lower panel) and its autoradiograph (upper panel) are shown. Lane 1, 35S-labeled prethiolase (10% of the input used in lanes 3 and 4); lane 2, 35S-labeled thiolase lacking the PTS2 (10% of the input used in lanes 5 and 6). p-T and m-T, precursor and mature forms of thiolase, respectively. The asterisk marks a radiolabeled band produced by the in vitro translation kit in an unspecific manner. The numbers at the left indicate the molecular masses of the applied standards in kDa.
ΔC1PEX5L-mediated import of prethiolase is time- and temperature-dependent (Fig. 2, B and C, respectively) and requires the PTS2 present in the reporter protein. Indeed, as shown in Fig. 2D, a 35S-labeled protein corresponding to mature thiolase does not acquire a protease-resistant status when subjected to in vitro assays in the presence of the recombinant protein (lane 6).
To determine whether the protease-resistant 35S-labeled reporter protein is indeed inside peroxisomes, a complete protease-treated import reaction (i.e. organelles plus soluble phase) was loaded onto a discontinuous Nycodenz gradient and centrifuged. The gradient was then fractionated, and equivalent volumes of each fraction were analyzed by SDS-PAGE and Western blot/autoradiography. As shown in Fig. 3, PEX13, an intrinsic peroxisomal membrane protein, displays a dual behavior in these gradients (see also Ref. 45). One fraction is recovered in fractions 2 and 3 of the gradient (the 30–45% (w/v) Nycodenz interface) and represents highly pure peroxisomes; the other peaks at fractions 9–10 and represents peroxisomes/peroxisomal vesicles of lower density (22, 45). Importantly, this is also the gradient distribution profile displayed by 35S-labeled prethiolase and mature thiolase. Thus, the 35S-labeled reporter protein was imported into peroxisomes.
FIGURE 3.
35S-Labeled prethiolase is specifically imported into peroxisomes. A ΔC1PEX5L-supplemented PNS fraction was incubated with 35S-labeled prethiolase in import buffer containing ATP for 45 min. After trypsin treatment and inactivation of the protease, the complete import mixture was diluted with SEM buffer and subjected to Nycodenz gradient centrifugation. The gradient was then fractionated from the bottom (lane 1) to the top (lane 14), and equal aliquots from each fraction were subjected to SDS-PAGE and Western blotting. The nitrocellulose membrane was exposed to an x-ray film to detect the 35S-labeled protein (top panel) and afterward probed with the following antisera: anti-KDEL (KDEL; recognizes GRP72 and GRP98, two endoplasmic reticulum proteins), anti-cytochrome c (Cyt c; a mitochondrial marker), anti-catalase (CAT; a peroxisomal enzyme), and anti-PEX13. This last serum recognizes on trypsin-treated peroxisomes a 30-kDa fragment of PEX13 (PEX13′) (46). Note that catalase remaining at the top of the gradient (lanes 11–14) results from leakage of peroxisomes during preparation of PNS fractions. p-T and m-T, precursor and mature forms of thiolase, respectively. The numbers at the right indicate the molecular masses of the applied standards in kDa.
Having validated this PTS2-centered in vitro import assay, we then asked whether or not translocation of prethiolase across the organelle membrane requires cytosolic ATP hydrolysis. Besides providing valuable information on the energetics of this process, an answer to this question should also allow us to link the translocation step of the cargo protein to a specific stage of the PEX5 cycling pathway (Fig. 1).
The classical way to address the energetics of a given process is to compare the efficiency at which it occurs in the presence of ATP with that obtained in the absence of ATP or in the presence of poorly hydrolyzable ATP analogues (e.g. ATPγS). There are, however, several properties of the experimental system used here that must be taken into consideration when performing this type of experiment. First, most of the DTM in PNS fractions is occupied by endogenous PEX5. In the absence of ATP or in the presence of ATPγS, endogenous PEX5 is not released from this machinery, and so extremely low levels of import will be obtained if these conditions are used from the very beginning of the experiment (30) (data not shown). To avoid this problem, PNS fractions were always incubated in import buffer containing 0.3 mm ATP for 5 min at 37 °C before changing the energetic status of the reactions (by adding apyrase or ATPγS; see below) or before reinforcing it with more ATP and before adding the 35S-labeled reporter protein. Second, during the incubation period, the soluble PEX5/ΔC1PEX5L species will associate with the DTM. However, in the absence of ATP or in the presence of ATPγS, these molecules will no longer be dislocated from the DTM, and thus, the import capacity of peroxisomes will decrease over time. To control this variable, a large amount of the ubiquitin analogue GST·Ub was included in some import reactions. GST·Ub is efficiently used by the ubiquitin-conjugating cascade acting on peroxisomal PEX5 or ΔC1PEX5L. However, GST·Ub·PEX5 or GST·Ub·ΔC1PEX5L conjugates formed at the DTM are not substrates for the ATP-dependent receptor export module (42). Thus, in the presence of GST·Ub, the rates at which the amounts of available/free DTM decrease over time in reactions containing ATP, ATPγS, or apyrase will be the same because in all cases, the DTM-embedded PEX5/ΔC1PEX5L species will not be dislocated. Finally, when a protein is synthesized in an in vitro translation system, there is no warranty that the final product corresponds to a homogeneous, monodisperse protein. Often, a considerable fraction of that protein is bound by ATP-dependent chaperones (54). Although this aspect was not addressed in detail here, the 35S-labeled reporter protein used in the experiments described below was preincubated with a rat liver cytosolic fraction containing the ΔC1PEX5L recombinant protein in the presence of 0.3 mm ATP for 10 min at 37 °C so that any interferences from putative ATP-dependent steps occurring upstream of the cargo protein-receptor interaction could be minimized. The energetic status of this solution was then changed or reinforced as described above before starting the import assay.
Two different strategies were used to determine whether or not translocation of prethiolase across the peroxisomal membrane requires hydrolysis of cytosolic ATP. In the first, the import efficiencies of prethiolase obtained with ATP-pretreated components in reactions supplemented with bovine ubiquitin or GST·Ub and containing 3 mm ATP or 3 mm ATPγS were compared. As shown in Fig. 4A, whereas in the presence of ubiquitin the amount of protease-resistant reporter protein obtained in the presence of ATP is larger than the one obtained in the presence of ATPγS (lanes 2 and 3, respectively), no significant difference is observed in reactions containing GST·Ub (compare lanes 4 and 5). Thus, under conditions where dislocation of PEX5/ΔC1PEX5L is blocked, a vast excess of ATPγS over ATP has no effect on the amount of reporter protein acquiring a protease-resistant status.
In the second strategy, we compared the import efficiencies of prethiolase obtained with ATP-pretreated components in reactions supplemented with GST·Ub and ATP or apyrase, an enzyme that degrades NTPs (55). As shown in Fig. 4B, the amount of protease-protected 35S-labeled protein is not significantly affected by the presence of apyrase in the reaction (compare lanes 4 and 5). The control experiment shown in Fig. 4C confirms that the amount of apyrase used in these assays efficiently removes ATP from these reactions because conjugation of GST·Ub to PEX5L(C11K), an ATP-dependent step, (23), no longer occurs.
Interestingly, the fractions of prethiolase undergoing processing in these reactions are all very similar, suggesting that at least the N terminus of the reporter protein reaches the matrix of the peroxisome in a process that requires neither dislocation of the ubiquitinated receptor from the DTM (reactions containing ATPγS plus ubiquitin or ATP plus GST·Ub; Fig. 1) nor even the ubiquitination of the receptor (reaction containing apyrase). Actually, the data shown in Fig. 5 suggest that the same is true for the complete polypeptide chain of a major fraction of mature 35S-labeled thiolase. In this experiment, protease-treated organelles from in vitro import assays performed in the presence of ubiquitin plus ATP (all steps of the PEX5 cycling pathway can occur; Fig. 1), ubiquitin plus ATPγS (the stage 3b-to-stage 4a transition is blocked), GST·Ub plus ATP (the stage 3a-to-stage 3b transition is blocked), or ubiquitin plus apyrase (the stage 2-to-stage 3a transition is blocked) were disrupted by sonication in a low ionic strength buffer and subjected to ultracentrifugation to separate soluble proteins from membrane-associated ones. The conditions employed resulted in an almost complete extraction of catalase from the organelles (Fig. 5, lanes S), whereas mitochondrial cytochrome c, a peripheral membrane protein, remained in the membrane pellets (lanes P). Endogenous rat liver thiolase displayed a dual behavior with comparable amounts of the protein appearing in the membrane and soluble fractions. Importantly, a similar behavior was observed for in vitro imported mature thiolase, suggesting that this fraction of the protease-resistant reporter protein reached its final location, i.e. the matrix of the organelle. Interestingly, the majority of prethiolase (but not all) was found in the membrane pellets. Whether this species represents a population associated with some intraperoxisomal membrane-bound or high molecular mass protein (e.g. the peroxisomal Lon protease; see Ref. 56) or even an intraperoxisomal off-pathway species (e.g. a misfolded pool) remains to be determined. The increasing accumulation of protease-protected prethiolase in our assays (Fig. 2B), an abnormal behavior for a true (productive) intermediate, could support either possibility.
Taken together, these experiments strongly suggest that translocation of 35S-labeled prethiolase across the peroxisomal membrane, as assessed by its protease-resistant status, its processing into the mature form, and its release into the peroxisomal matrix, occurs before stage 3a (Fig. 1). Although this finding provides for the first time direct evidence supporting the idea that the PEX5-mediated insertion of a cargo protein into the DTM occurs at the stage 1b-to-stage 2 transition (see the Introduction), the observation that release of the cargo protein into the peroxisomal matrix also occurs before stage 3a was unexpected. Apparently, neither ubiquitination of the receptor (33) nor the ATP-dependent dislocation of the receptor from the DTM (27) contributes to the cargo release event. What then triggers release of the cargo protein from the DTM? Although the peroxisomal membrane is freely permeable to small solutes (57, 58) and, in agreement with this property, the ΔC1PEX5L-mediated import of prethiolase is completely insensitive to proton, potassium, or calcium ionophores (data not shown), there is still a possibility that the ionic composition of the peroxisomal matrix is somewhat different from the one found in the cytosol due to a Donnan effect (59). If true, the triggering mechanism for the cargo release step could involve an ion-induced (e.g. pH-induced) conformational alteration of the cargo-binding site in DTM-embedded receptors (see Ref. 60). Alternatively, and perhaps more plausibly, a still unidentified peroxisomal protein, belonging or not to the DTM, could trigger this event. We are currently addressing this hypothesis.
Recently, Miyata et al. (61) proposed that translocation of proteins across the peroxisomal membrane requires hydrolysis of ATP. The evidence supporting that conclusion comes from in vitro import assays where both the postnuclear supernatants and the 35S-labeled reporter proteins were supplemented from time 0 of the experiments with either ATP or a non-hydrolyzable ATP analogue. For the reasons mentioned above, this type of experimental design is poised to reveal differences in protein import efficiencies. Nevertheless, it is interesting to note that even in the presence of a non-hydrolyzable ATP analogue, considerable amounts of protease-resistant reporter proteins were observed in some of those experiments (e.g. Figs. 4D and 5D in Ref. 61), although it remains unclear whether the reported phenomena is or is not PEX5-dependent.
Here we describe a strategy to study protein translocation across the mammalian peroxisomal membrane using an in vitro import system. In its basic configuration (i.e. no recombinant proteins added), this system has been used to unveil several mechanistic aspects of the PEX5-mediated import pathway using as a reporter protein PEX5 itself. The low abundance of endogenous PEX5 and the presence of import-competent PTS1-containing cargo proteins in the organelle suspensions explain the success of the PEX5-centered in vitro system. Unfortunately, these are also the reasons why it has been so difficult to develop a robust cargo-centered in vitro import system. By supplementing import reactions with recombinant PEX5 proteins, this problem was, at least partially, solved. Naturally, the configuration of these assays can still be changed. For instance, by supplementing the import reactions with a recombinant PTS2-containing protein and one of the recombinant PEX5L mutant versions used in this work, it should be possible to develop a robust PEX7-centered in vitro system.
Supplementary Material
The work was supported by grants from Fundação para a Ciência e Tecnologia (PTDC program) and Fundo Europeu de Desenvolvimento Regional, Portugal and by the European Union VI Framework program Grant LSHG-CT-2004-512018, Peroxisomes in Health and Disease.
This article was selected as a Paper of the Week.
I. S. Alencastre, T. A. Rodrigues, C. P. Grou, M. Fransen, C. Sá-Miranda, and J. E. Azevedo, unpublished results.
- PTS1
- peroxisomal targeting sequence type 1
- PTS2
- peroxisomal targeting sequence type 2
- DTM
- docking/translocation machinery
- Ub
- ubiquitin
- Ub·PEX5
- monoubiquitinated PEX5 species
- GST·Ub
- glutathione S-transferase-ubiquitin
- PNS
- postnuclear supernatant
- GSH
- glutathione
- ATPγS
- adenosine 5-O-(thiotriphosphate)
- MOPS
- 4-morpholinepropanesulfonic acid.
REFERENCES
- 1.Purdue P. E., Lazarow P. B. (2001) Annu. Rev. Cell Dev. Biol. 17, 701–752 [DOI] [PubMed] [Google Scholar]
- 2.Brown L. A., Baker A. (2008) Mol. Membr. Biol. 25, 363–375 [DOI] [PubMed] [Google Scholar]
- 3.Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. (1989) J. Cell Biol. 108, 1657–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocard C., Hartig A. (2006) Biochim. Biophys. Acta 1763, 1565–1573 [DOI] [PubMed] [Google Scholar]
- 5.Swinkels B. W., Gould S. J., Bodnar A. G., Rachubinski R. A., Subramani S. (1991) EMBO J. 10, 3255–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarow P. B. (2006) Biochim. Biophys. Acta 1763, 1599–1604 [DOI] [PubMed] [Google Scholar]
- 7.Kurochkin I. V., Mizuno Y., Konagaya A., Sakaki Y., Schönbach C., Okazaki Y. (2007) EMBO J. 26, 835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helm M., Lück C., Prestele J., Hierl G., Huesgen P. F., Fröhlich T., Arnold G. J., Adamska I., Görg A., Lottspeich F., Gietl C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11501–11506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braverman N., Dodt G., Gould S. J., Valle D. (1998) Hum. Mol. Genet. 7, 1195–1205 [DOI] [PubMed] [Google Scholar]
- 10.Otera H., Okumoto K., Tateishi K., Ikoma Y., Matsuda E., Nishimura M., Tsukamoto T., Osumi T., Ohashi K., Higuchi O., Fujiki Y. (1998) Mol. Cell. Biol. 18, 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodward A. W., Bartel B. (2005) Mol. Biol. Cell 16, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galland N., Demeure F., Hannaert V., Verplaetse E., Vertommen D., Van der Smissen P., Courtoy P. J., Michels P. A. (2007) Biochim. Biophys. Acta 1773, 521–535 [DOI] [PubMed] [Google Scholar]
- 13.Gatto G. J., Jr., Geisbrecht B. V., Gould S. J., Berg J. M. (2000) Proteins 38, 241–246 [DOI] [PubMed] [Google Scholar]
- 14.Brocard C., Kragler F., Simon M. M., Schuster T., Hartig A. (1994) Biochem. Biophys. Res. Commun. 204, 1016–1022 [DOI] [PubMed] [Google Scholar]
- 15.Dodt G., Braverman N., Wong C., Moser A., Moser H. W., Watkins P., Valle D., Gould S. J. (1995) Nat. Genet. 9, 115–125 [DOI] [PubMed] [Google Scholar]
- 16.Matsumura T., Otera H., Fujiki Y. (2000) J. Biol. Chem. 275, 21715–21721 [DOI] [PubMed] [Google Scholar]
- 17.Dodt G., Warren D., Becker E., Rehling P., Gould S. J. (2001) J. Biol. Chem. 276, 41769–41781 [DOI] [PubMed] [Google Scholar]
- 18.Otera H., Setoguchi K., Hamasaki M., Kumashiro T., Shimizu N., Fujiki Y. (2002) Mol. Cell. Biol. 22, 1639–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams C., Distel B. (2006) Biochim. Biophys. Acta 1763, 1585–1591 [DOI] [PubMed] [Google Scholar]
- 20.Carvalho A. F., Costa-Rodrigues J., Correia I., Costa Pessoa J., Faria T. Q., Martins C. L., Fransen M., Sá-Miranda C., Azevedo J. E. (2006) J. Mol. Biol. 356, 864–875 [DOI] [PubMed] [Google Scholar]
- 21.Miyata N., Fujiki Y. (2005) Mol. Cell. Biol. 25, 10822–10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouveia A. M., Guimaraes C. P., Oliveira M. E., Reguenga C., Sa-Miranda C., Azevedo J. E. (2003) J. Biol. Chem. 278, 226–232 [DOI] [PubMed] [Google Scholar]
- 23.Grou C. P., Carvalho A. F., Pinto M. P., Huybrechts S. J., Sá-Miranda C., Fransen M., Azevedo J. E. (2009) J. Biol. Chem. 284, 10504–10513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransen M., Brees C., Ghys K., Amery L., Mannaerts G. P., Ladant D., Van Veldhoven P. P. (2002) Mol. Cell Proteomics 1, 243–252 [DOI] [PubMed] [Google Scholar]
- 25.Dodt G., Gould S. J. (1996) J. Cell Biol. 135, 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dammai V., Subramani S. (2001) Cell 105, 187–196 [DOI] [PubMed] [Google Scholar]
- 27.Grou C. P., Carvalho A. F., Pinto M. P., Alencastre I. S., Rodrigues T. A., Freitas M. O., Francisco T., Sá-Miranda C., Azevedo J. E. (2009) Cell Mol. Life Sci. 66, 254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reguenga C., Oliveira M. E., Gouveia A. M., Sá-Miranda C., Azevedo J. E. (2001) J. Biol. Chem. 276, 29935–29942 [DOI] [PubMed] [Google Scholar]
- 29.Agne B., Meindl N. M., Niederhoff K., Einwächter H., Rehling P., Sickmann A., Meyer H. E., Girzalsky W., Kunau W. H. (2003) Mol. Cell 11, 635–646 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira M. E., Gouveia A. M., Pinto R. A., Sá-Miranda C., Azevedo J. E. (2003) J. Biol. Chem. 278, 39483–39488 [DOI] [PubMed] [Google Scholar]
- 31.Gouveia A. M., Guimarães C. P., Oliveira M. E., Sá-Miranda C., Azevedo J. E. (2003) J. Biol. Chem. 278, 4389–4392 [DOI] [PubMed] [Google Scholar]
- 32.Costa-Rodrigues J., Carvalho A. F., Gouveia A. M., Fransen M., Sá-Miranda C., Azevedo J. E. (2004) J. Biol. Chem. 279, 46573–46579 [DOI] [PubMed] [Google Scholar]
- 33.Williams C., van den Berg M., Sprenger R. R., Distel B. (2007) J. Biol. Chem. 282, 22534–22543 [DOI] [PubMed] [Google Scholar]
- 34.Carvalho A. F., Pinto M. P., Grou C. P., Alencastre I. S., Fransen M., Sá-Miranda C., Azevedo J. E. (2007) J. Biol. Chem. 282, 31267–31272 [DOI] [PubMed] [Google Scholar]
- 35.Platta H. W., Grunau S., Rosenkranz K., Girzalsky W., Erdmann R. (2005) Nat. Cell Biol. 7, 817–822 [DOI] [PubMed] [Google Scholar]
- 36.Azevedo J. E., Costa-Rodrigues J., Guimarães C. P., Oliveira M. E., Sã-Miranda C. (2004) Cell Biochem. Biophys. 41, 451–468 [DOI] [PubMed] [Google Scholar]
- 37.Miura S., Miyazawa S., Osumi T., Hashimoto T., Fujiki Y. (1994) J. Biochem. 115, 1064–1068 [DOI] [PubMed] [Google Scholar]
- 38.Imanaka T., Small G. M., Lazarow P. B. (1987) J. Cell Biol. 105, 2915–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiki Y., Lazarow P. B. (1985) J. Biol. Chem. 260, 5603–5609 [PubMed] [Google Scholar]
- 40.Walton P. A., Gould S. J., Feramisco J. R., Subramani S. (1992) Mol. Cell. Biol. 12, 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa-Rodrigues J., Carvalho A. F., Fransen M., Hambruch E., Schliebs W., Sá-Miranda C., Azevedo J. E. (2005) J. Biol. Chem. 280, 24404–24411 [DOI] [PubMed] [Google Scholar]
- 42.Grou C. P., Carvalho A. F., Pinto M. P., Wiese S., Piechura H., Meyer H. E., Warscheid B., Sá-Miranda C., Azevedo J. E. (2008) J. Biol. Chem. 283, 14190–14197 [DOI] [PubMed] [Google Scholar]
- 43.Carvalho A. F., Grou C. P., Pinto M. P., Alencastre I. S., Costa-Rodrigues J., Fransen M., Sá-Miranda C., Azevedo J. E. (2007) Biochim. Biophys. Acta 1773, 1141–1148 [DOI] [PubMed] [Google Scholar]
- 44.Bout A., Teunissen Y., Hashimoto T., Benne R., Tager J. M. (1988) Nucleic Acids Res. 16, 10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto M. P., Grou C. P., Alencastre I. S., Oliveira M. E., Sá-Miranda C., Fransen M., Azevedo J. E. (2006) J. Biol. Chem. 281, 34492–34502 [DOI] [PubMed] [Google Scholar]
- 46.Gouveia A. M., Reguenga C., Oliveira M. E., Sa-Miranda C., Azevedo J. E. (2000) J. Biol. Chem. 275, 32444–32451 [DOI] [PubMed] [Google Scholar]
- 47.Antonenkov V. D., Van Veldhoven P. P., Waelkens E., Mannaerts G. P. (1997) J. Biol. Chem. 272, 26023–26031 [DOI] [PubMed] [Google Scholar]
- 48.Fransen M., Wylin T., Brees C., Mannaerts G. P., Van Veldhoven P. P. (2001) Mol. Cell. Biol. 21, 4413–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexson S. E., Fujiki Y., Shio H., Lazarow P. B. (1985) J. Cell Biol. 101, 294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kikuchi M., Hatano N., Yokota S., Shimozawa N., Imanaka T., Taniguchi H. (2004) J. Biol. Chem. 279, 421–428 [DOI] [PubMed] [Google Scholar]
- 51.Wiese S., Gronemeyer T., Ofman R., Kunze M., Grou C. P., Almeida J. A., Eisenacher M., Stephan C., Hayen H., Schollenberger L., Korosec T., Waterham H. R., Schliebs W., Erdmann R., Berger J., Meyer H. E., Just W., Azevedo J. E., Wanders R. J., Warscheid B. (2007) Mol. Cell Proteomics 6, 2045–2057 [DOI] [PubMed] [Google Scholar]
- 52.Islinger M., Lüers G. H., Li K. W., Loos M., Völkl A. (2007) J. Biol. Chem. 282, 23055–23069 [DOI] [PubMed] [Google Scholar]
- 53.Gatto G. J., Jr., Geisbrecht B. V., Gould S. J., Berg J. M. (2000) Nat. Struct. Biol. 7, 1091–1095 [DOI] [PubMed] [Google Scholar]
- 54.Kruse M., Brunke M., Escher A., Szalay A. A., Tropschug M., Zimmermann R. (1995) J. Biol. Chem. 270, 2588–2594 [DOI] [PubMed] [Google Scholar]
- 55.Agah A., Aghajan M., Mashayekhi F., Amini S., Davis R. W., Plummer J. D., Ronaghi M., Griffin P. B. (2004) Nucleic Acids Res. 32, e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omi S., Nakata R., Okamura-Ikeda K., Konishi H., Taniguchi H. (2008) J. Biochem. 143, 649–660 [DOI] [PubMed] [Google Scholar]
- 57.Van Veldhoven P., Debeer L. J., Mannaerts G. P. (1983) Biochem. J. 210, 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rokka A., Antonenkov V. D., Soininen R., Immonen H. L., Pirilä P. L., Bergmann U., Sormunen R. T., Weckström M., Benz R., Hiltunen J. K. (2009) PLoS ONE 4, e5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonenkov V. D., Hiltunen J. K. (2006) Biochim. Biophys. Acta 1763, 1697–1706 [DOI] [PubMed] [Google Scholar]
- 60.Wang D., Visser N. V., Veenhuis M., van der Klei I. J. (2003) J. Biol. Chem. 278, 43340–43345 [DOI] [PubMed] [Google Scholar]
- 61.Miyata N., Hosoi K., Mukai S., Fujiki Y. (2009) Biochim. Biophys. Acta 1793, 860–870 [DOI] [PubMed] [Google Scholar]
- 62.Haas A. L., Warms J. V., Rose I. A. (1983) Biochemistry 22, 4388–4394 [DOI] [PubMed] [Google Scholar]
- 63.Nair D. M., Purdue P. E., Lazarow P. B. (2004) J. Cell Biol. 167, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.