Abstract
The α-synuclein immunopositive and chaotrope-insoluble material from human brains with Lewy body pathology was analyzed by mass spectrometry. From the proteinase K-cleavable peripheral fraction of Lewy bodies, which was densely cross-linked by γ-glutamyl-ϵ-lysine bonds between HspB1 and ubiquitin in a pattern similar to neurofibrillary tangles (Nemes, Z., Devreese, B., Steinert, P. M., Van Beeumen, J., and Fésüs, L. (2004) FASEB J. 18, 1135–1137), 53 proteins were identified. In the core of Lewy bodies only α-synuclein was found, and it contained a low amount of intramolecular cross-links between Gln-99 and Lys-58. In vitro cross-linking of α-synuclein by transglutaminases 1–3 and 5 produced a heterogeneous population of variably cross-linked α-synucleins in solution, which inhibited the aggregation of the protein into amyloid. However, in the presence of phosphatidylserine-rich membranes and micromolar calcium concentrations, the cross-linking by transglutaminases 1, 2, and 5 showed specificity toward the utilization of Gln-99 and Lys-58. As shown by thioflavin T fluorescence monitoring, the formation of this cross-link accelerated the aggregation of native α-synuclein. Chemical cross-linking of residues 58–99 triggered amyloid formation, whereas such bonding of residues 99 to 10 was inhibitory. Our findings reveal the pivotal role of membrane attachment and transglutaminase-mediated intermolecular cross-linking for the propagative misfolding and aggregation of α-synuclein.
α-Synuclein is a widely expressed neuronal protein, which can form amyloid deposits under pathological conditions. The extracellularly deposited α-synuclein, or the central segment of it, is known as the “non-Aβ component” of amyloid and is involved in the formation of senile plaques in Alzheimer disease (AD).2
The intraneuronal aggregation of α-synuclein leads to the formation of Lewy bodies. These inclusions were initially thought to hallmark the degenerating dopaminergic neurons in Parkinson disease; however, they are abundant in cortical neurons and basal ganglia in distinct neurodegenerative dementias, such as AD and Lewy body dementia (LBD), and are often found in neurons of elderly people without any clinical record of parkinsonism or dementia (1). The ultrastructure of a Lewy body reveals a compact amyloid core surrounded by a fuzzy halo that is vaguely abridged from the neuronal cytoplasm. Lewy bodies (LB) are strongly immunopositive for α-synuclein and ubiquitin. They may also contain many other components (such as synphilin-1, Dorfin, or Parkin), which have not yet been investigated in detail (see Ref. 2 and references therein).
α-Synuclein is a small protein of 140 amino acids and consists of an N-terminal lipid-binding amphipathic domain, a C-terminal acidic tail, and amyloid-forming repetitive sequences in between. α-Synuclein is almost unstructured as a monomer in aqueous solution, but it undergoes a conformational change to an α-helical structure upon association with negatively charged membranes or β-pleated sheet conformation upon aggregation to amyloid (see Ref. 3 for references therein). Because of its natively disordered conformation, the protein readily aggregates with itself and co-aggregates with other proteins, a property that may be necessary for LB formation and its cytopathic effects. Given that α-synuclein does not assemble into persistent aggregates under normal conditions of a living neuron, it is critical to identify triggers of propagative α-synuclein misfolding, as these may result in a complex pathological sequelae leading to LB formation and neuron loss.
Transglutaminases (TGs) are a family of proteins that catalyze the exchange of the γ-(carbox)amido group with a different amine. If the amine is provided by the ϵ-amino group of a protein-bound lysine, TGs cross-link proteins via γ-glutamyl-ϵ-lysine (GGEL) isopeptide cross-links (see Ref. 4 for references therein). Four of the nine human TGs (TG1–3 and -5) are expressed in the brain (5, 6). The most investigated TG2 is present in two splice variants, the shorter of which results from an intronic read-through and was shown to associate with Alzheimer neurons (7). A previous report (8) from our laboratories identified α-synuclein cross-linked via its Gln-99 moiety to ubiquitin Lys-48 in ubiquitylated intracellular aggregates from AD specimens.
Using an anti-GGEL antibody, two earlier reports showed the presence of GGEL cross-links in α-synuclein aggregates from Lewy bodies. However, the precise role and the manner of synuclein cross-linking and its relevance to the pathogenic process have not been revealed (9, 10). The in vitro and cellular models yielded conflicting conclusions; both pro- and anti-aggregation roles have been proposed (10–12), and also the lack of α-synuclein cross-linking in a transfection study was also reported (13).
A former in vitro analysis identified Gln-79 and Lys-80 as TG2-substrate residues (14), and a recent study (15) found that guinea pig TG2 preferentially forms cross-links in soluble α-synuclein between Gln-79 and Lys-60, Gln-99 and Lys-10, Gln-109 and Lys-32 or Lys-34, and also Gln-109 and Lys-96. This study also analyzed the cross-linking of α-synuclein by soluble TG2 in a phosphatidylglycerol membrane-bound state and found that the cross-linking pattern is dramatically affected by the membrane binding of the substrate inasmuch as it reduced the residues amenable for cross-linking to one, without identifying where this particular cross-link was.
In this study, we demonstrate that a small fraction of α-synuclein in the aggregated core of LB is intramolecularly cross-linked, and we show by in vitro model experiments how LB can be nucleated by GGEL cross-links in a membrane-dependent fashion. Our findings suggest that the formation of LB is triggered by TG-mediated intramolecular cross-linking, and this may be an early decisive step in the biogenesis of LB.
EXPERIMENTAL PROCEDURES
Human Specimens
LBD cases (two males and one female, mean age 76 ± 5 years), AD cases (three females, mean age 83 ± 7 years), and control cases (three males and two females, mean age 78 ± 4 years) were obtained from the Institute of Pathology, University of Debrecen Medical and Health Sciences Center. Control brain tissue was obtained from patients whose medical records had no hints of neurological or psychiatric problems. The diagnosis of probable LBD and AD was made according to clinical records and consensus neuropathologic criteria (16). Brain tissue was harvested 9–16 h post-mortem with the permission of the Regional Board for Research Ethics. Tissue blocks were taken from the left hippocampus and neocortex (medial frontal cortex).
Isolation of α-Synuclein Immunoreactive, Chaotrope-insoluble Protein Particles
Chaotrope-insoluble material from tissue blocks was obtained by repeated extractions with Tripure reagent (phenolic guanidine HSCN, Roche Applied Science) using a Teflon-head homogenizer and repeated centrifugations at 30,000 × g. The insoluble material was boiled in Tris-Cl, pH 8.0, containing 2% SDS and 100 mm dithiothreitol, washed in water, and reacted with 0.1 m iodoacetamide for 1 h at room temperature and then washed with water and pelleted as before.
The remaining particulate material was washed with PBS containing 5 mm EDTA-Na and resuspended in 2 ml of PBS with 1% bovine serum albumin and shaken overnight with 1:50 diluted rabbit anti-α-synuclein antiserum (Sigma) at 4 °C. Particles were cleared from unbound antiserum by washing with PBS/bovine serum albumin buffer and then reacted with iron microbead-coupled goat anti-rabbit immunoglobulins (Miltenyi Biotec, Bergisch Gladbach, Germany). Immunoreactive particles were captured, washed, and eluted on a Macs LS column (Miltenyi Biotec) according to the manufacturer's protocol. Eluted particles were stripped of bound antibodies by two extractions with hot Tris-Cl, pH 8.0, containing 2% SDS and 100 mm dithiothreitol, and washed with water. Ubiquitin affinity separation was done exactly as published (8).
Determination of the Relative Abundance of GGEL Cross- links
The amount of amino acids was assessed from an aliquot of the samples by complete acid hydrolysis (6 n HCl, 110 °C overnight) followed by amino acid analysis using a Beckman 6300 amino acid analyzer. Total brain tissue proteins were purified after TriPure lysis and merging TriPure-soluble and insoluble fractions. Sample proteins were supplemented with ∼ 0.1 pmol of GGEL (Sigma) as internal (light) standard, subjected to limited acid hydrolysis (0.5 n trifluoroacetic acid) at 95 °C for 16 h), dried, and then digested with proteinase K (Roche Applied Science, 20 μg/ml in 10 mm HEPES-Na, pH 8.0, and 1 mm CaCl2) in water containing 20% H218O (Aldrich) at 56 °C overnight. After inactivating the protease at 95 °C, the peptides were further digested by the addition of a mixture of carboxypeptidase A, Y, and leucine-aminopeptidase (all from Roche Applied Science, 5 μg/ml each at 37 °C for 16 h), and the GGEL content was determined by derivatizing the samples with 9-fluorenylmethyl-succinimidyl carbonate, separated by high pressure liquid chromatography on a C18 column, and analyzed by liquid chromatography/mass spectrometry (Q-TRAP4000, Applied Biosystems, Foster City, CA) monitoring of the ratio of masses at 719/721/723 atomic mass units as published (17).
Digestion, Enrichment, and Sequencing of LB and in Vitro Cross-linked α-Synuclein
20–30 μg of affinity-isolated particles from brain tissue were shaken overnight in 70% formic acid containing 0.1% CNBr, dried in vacuum, and digested first with 50 units/μg bacterial alkaline phosphatase (Sigma type III) in 0.1 m ammonium bicarbonate buffer (ABB) at 37 °C for 60 min, then overnight with 1:20 weight modified trypsin (Roche Applied Science). The undissolved particulate material was collected by centrifugation at 30,000 × g. The supernatants were dried, dissolved in PBS containing 0.5% bovine serum albumin and 0.5% octyl glycoside, and incubated overnight with 20 μg of mouse anti-GGEL immunoglobulin 81D4 (Covalab, Lyon, France) at 4 °C. Subsequently, 0.5 mg of protein A-Sepharose was added to the samples and washed twice with centrifugation and resuspension in PBS. Finally the pellets were suspended in 0.1% trifluoroacetic acid to release the bound GGEL peptides from the antibody and spun through a Microcon YM-10 membrane. In-gel digestion of Coomassie-stained gel slices was preceded by a 20-min wash with ABB in 50% acetonitrile and a 10-min 75% acetonitrile wash. Then 4 μg of modified trypsin (Roche Applied Science) was added, and proteolysis was carried out two times overnight at 37 °C. In some experiment a third round of digestion with 2 μg of Glu-C endoproteinase (Sigma) was applied. The peptide fragments were analyzed by liquid chromatography/electrospray ionization-tandem mass spectrometry using an Ultimate MicroLC System (Dionex-LC Packings, Sunnyvale, CA) coupled to the Q-Trap tandem mass spectrometer. In some experiments samples were mixed with sinapinic acid, dried on steel source plates, and analyzed on a MALDI TOF/TOF 4700 instrument (Applied Biosystems). Read sequences were searched against the NCBI nonredundant data base using the Mascot algorithm. Nonhuman and hypothetical proteins, keratins, were ignored.
Proteinase Digestion of the Proteinase-resistant LB Fractions
The fraction of LB remaining after tryptic or proteinase K digestions was dissolved in 100 μl of 6 m guanidine HCl containing 1 mg/ml egg albumin, heated at 95 °C for 5 min, desalted, and digested with 4 μg of trypsin as above.
Immunoelectron Microscopy
Particulate material, occasionally digested with 10 mg/ml proteinase K overnight as above, was blocked with 10% normal swine serum in PBS for 30 min in suspension and then stained with rabbit anti-α-synuclein (1:200; Sigma). Primary antibodies were detected with 1 nm gold-conjugated goat anti-rabbit immunoglobulins (1:50; Amersham Biosciences), fixed in 2% glutaraldehyde in PBS, and intensified using a silver enhancement kit (Amersham Biosciences). After washing with ABB, particles were dried, resuspended in 1% uranyl acetate in 70% ethanol, and then embedded in Lowicryl. Ultrathin sections were cut (∼70 nm) with a diamond knife, collected on copper mesh grids, and viewed using a Hitachi 7100 electron microscope at ×20,000 magnification. Adobe Photoshop (Adobe, San Jose, CA) was used to adjust brightness and contrast and to construct the final images.
Cloning, Expression, and Purification of Recombinant Proteins
α-Synuclein cDNA was amplified from a human brain large insert cDNA library (Clontech) and was ligated into pET-3b (Novagen, Darmstadt, Germany). K58C/Q99C and K10C/Q99C double mutants were created by site-directed mutagenesis using the QuikChange kit and protocol (Stratagene, La Jolla, CA). α-Synuclein cDNAs were expressed in BL21(DE3) Escherichia coli and purified from the lysates according to Conway et al. (18), with the exception that HiTrapQ columns (Amersham Biosciences) were used instead of DEAE columns. TG1–3 and -5 were expressed in insect cells and purified as published (5, 19–22).
In Vitro Cross-linking Experiments
Activity of the TG preparations was normalized to 0.005 ΔOD/min by the plated enzyme-linked immunosorbent assay method of Slaughter et al. (23). TG3 was activated by dispase as before (24). This was followed by the incubation of 4 μg (or 100 μg in high concentration experiments) of α-synuclein with the TG of interest in 400 μl of 50 mm Tris-Cl, pH 8.5, 2 mm dithiothreitol with or without 1 mm CaCl2 for 30 min at 37 °C. The products were either immediately digested with trypsin or analyzed by SDS-PAGE followed by immunoblotting with mouse anti-α-synuclein antibody (Sigma) or separated by SDS-PAGE, stained with Coomassie, digested in-gel with trypsin, and desalted with PerfectPure C-18 tips (Eppendorf, Hamburg, Germany).
Preparation of Lipid Vesicles (LV)
Solution mixtures (55:15:30) of palmitoyl-oleyl-phosphatidylcholine, palmitoyl-oleyl-phosphatidylserine (PS), and cholesterol (all from Sigma) were made in chloroform/methanol (95:5). The solvent was evaporated and then resuspended by vortexing in 0.5 ml of a buffer containing 50 mm Tris-Cl, pH 8.0, 100 mm NaCl, 3 mm NaN3, 5 mm dithiothreitol, and 200 mm sucrose and dounced for 15 min through a 200-nm polycarbonate membrane using a LiposoFast pump (Sigma). Stock mixtures were made in 0.5 ml and contained 50 μmol of total lipids.
Protein-LV Association Assays
Mixtures contained 10 pmol of protein and variable amounts of LV. Binding of proteins to LV was measured by spinning 20–40% of 0.5-ml mixtures of α-synuclein and LV through a Microcon YM-100 or TG and LV through a Centrisart 300-kDa membrane and comparing the concentration of proteins in the ultrafiltrates to LV-free controls using the Bradford protein assay method. Free calcium ion concentrations at 1 mm were buffered with (5 mm) Ca2+ citrate, 138 and 32 μm with Ca2+ nitrilotriacetic acid, and 2.3 μm with Ca2+ 1,2-bis(2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetraacetic acid (Calbiochem) and calculated using MaxC program (25). The membrane fusion of LV preparations in calcium-containing buffer was monitored by the measurement of light scattering (absorbance) at 360 nm. A decrease of light scattering (resulting from vesicle membrane fusion) was less than 10% of the initial absorbance in protein-free buffer over 24 h at 37 °C with any calcium buffer used and less than 4% when 10 μg/ml α-synuclein and TG was added to the reaction mixtures, respectively.
Cross-linking in the Presence of LV
Mixtures contained 10 μmol of LV lipids, 50 mm Tris-Cl, pH 8.0, 3 mm NaN3, 5 mm dithiothreitol, 0.1 m sucrose, and 4 μg of α-synuclein and 5 mΔOD/min TG in a 400-μl volume. Cross-linking was initiated by the addition of 5 mm calcium buffer, and then reaction mixtures were incubated for 16–24 h at 37 °C. In some experiments 4 mm p-nitrophenyl acetate was used as a TG substrate, and hydrolysis was detected by photometry at 405 nm in a plate reader.
Aggregation Experiments
Lyophilized α-synuclein was dissolved in 6 m guanidine HCl to make a stock solution at 50 mg/ml. Guanidine HCl was removed and buffer switched to 50 mm Tris-Cl, pH 7.6, 5 mm dithiothreitol and either 1 mm CaCl2 or 0.5 mm EDTA by passing through a Sephadex-25 column. 2 mg of α-synuclein solution was inoculated with 50 μg of control or treated synuclein in a 2.8-ml volume and incubated at 37 °C for 10 days. Inoculating α-synuclein samples were delipidized by solvent extraction. Thioflavin T binding analysis of α-synuclein was performed by diluting 50 times a 20-μl aliquot of the sample solutions at each incubation time with 10 mm Tris, pH 8.0, and 10 μm thioflavin T, respectively, and fluorescence was measured after mixing and standing for 20 min at room temperature (22 °C) by an M4 spectrofluorimeter (Zeiss, Göttingen, Germany). The excitation and emission wavelengths were 440 and 480 nm, respectively, and bandwidths of excitation/emission lights were 8:8 nm.
Cross-linking of K58C/Q99C and K10C/Q99C Mutant α-Synucleins
Intramolecularly cross-linked proteins were made by adding 5 mm bis-maleimidoethane (Pierce) to a 0.2 mg/ml solution of mutant α-synuclein, freshly gel-filtered from dithiothreitol-containing buffer in 50 mm Veronal-Na, pH 6.5, and incubating at room temperature. for 4 h. Intramolecularly cross-linked proteins were purified by preparative SDS-PAGE and masses were verified by MALDI-mass spectrometry.
RESULTS
Similar Recovery of GGEL Cross-links from Brain Tissue by Ubiquitin and α-Synuclein Affinity Isolations
Previously we developed a technique to isolate insoluble macro-polymeric protein particles from Alzheimer diseased brains using ubiquitin affinity purification. Because the particles featured similar antigens to neurofibrillary tangles, we claimed that the ubiquitin immunopositive material may correspond to those. Here we applied a similar approach to Lewy body dementia using α-synuclein as bait. However, α-synuclein is a component of different intra- and extracellular aggregates, such as senile plaques. Therefore, we first compared Alzheimer diseased and LBD brain tissue with age-matched controls, using either anti-ubiquitin or anti-synuclein affinity methods to see if the isolated materials were similar and differently or specifically cross-linked.
GGEL content was measured using protease digestion, 18O labeling, and comparison with internal standards in mass analysis. The overall frequency of GGEL in brain cortex varied between 42 ± 23/109 and 572 ± 117/109 amino acids in the specimens, where the age-matched not-demented control was the least cross-linked, and Alzheimer parietal cortex was the most cross-linked. Cross-linking of LBD specimens was consistently intermediate between the Alzheimer and the control group of samples. Statistically significant (p < 0.05) differences between the LBD versus both control and Alzheimer groups were measured only in case of tissues from the cortex (Fig. 1A). Further on, hippocampal tissue specimens were used as cross-linking was the highest here in the case of LBD. During the chaotrope, detergent and thiol extractions 97.2–98.1% of total amino acids and 23.4–37.6% of GGEL were lost. Affinity isolation of the insoluble fractions with anti-ubiquitin antibodies (applied in excess) pulled down 74–81%, whereas anti-α-synuclein antibodies recovered 55–63% of residual GGEL content from either sample. A repeated extraction with the same antisera recovered 1.2–1.6% of the previous protein mass both in case of anti-ubiquitin and anti-α-synuclein antibodies. Re-extraction of proteins from LBD hippocampi using the other primary antibody yielded 2.8–3.5% when anti-ubiquitin pre-cleared LB were probed with anti-α-synuclein and 13.8 ± 6.5% when anti-synuclein isolation was followed by anti-ubiquitin (Fig. 1B).
FIGURE 1.
γ-Glutamyl-ϵ-lysine cross-links in brain fractions accumulate in α-synuclein immunopositive chaotrope-insoluble inclusions/Lewy bodies. The relative abundance of GGEL was determined by enzymatic hydrolysis of sample proteins in 18O-water and comparison with light internal GGEL standard by mass spectrometry. GGEL was related to total amino acid content. A, GGEL accumulation associates with regions affected by degenerative changes in human brain specimen affected by Lewy body dementia (LBD), Alzheimer disease, or in age-matched controls (CTRL). Over 50% of total tissue GGEL content was recovered by either anti-ubiquitin or anti-α-synuclein affinity isolation from chaotrope-, detergent-, and thiol-insoluble brain proteins (B), indicating that most of the cross-linked inclusions expose both antigens in AD and LBD. Lewy bodies were exhaustively digested with proteinase K. Residual proteins remaining after digestions were dissolved in guanidine HCl and co-precipitated with a different protein (egg albumin) to disrupt β-sheet amyloid arrays between proteins. The GGEL density in proteinase K-resistant fractions is by an order of magnitude lower than in the proteinase-digestible part (C). Bars represent the means of duplicate determinations from three different donors. Asterisks and hatch marks indicate significant (p < 0.05) differences from the corresponding controls and AD samples, respectively.
These data indicate that the major fraction of the isolated particulate material must be double immunopositive for ubiquitin and α-synuclein and thus originates from intracellular deposits. Thereafter this material is referred to as LB.
Protease-degradable and Highly Cross-linked Part of Lewy Bodies
The frequency of GGEL cross-links of LB was 1122 ± 261/106, as determined by rating the GGEL content released by proteinase K digestions to the amino acids liberated by total acid hydrolysis from the corresponding aliquots. When, in place of acidolysis, the amino acids were hydrolyzed by enzymatic catalysis (or a sequence of limited acid hydrolysis or CNBr cleavage followed by exhausting endo- and ectoproteinase digestions), the abundance of GGEL was significantly higher (3280 ± 494/106) indicating that the bulk of the protein mass did not disintegrate to amino acid monomers, and that the digestion failed to release further material when fresh aliquots of proteinase K were added. The protease-susceptible fraction of LB contained 43 ± 5.2% of total LB amino acids and 92–95% of cross-links (Fig. 1C). These data showed that LB are inhomogeneous in terms of protease susceptibility and cross-linking.
Peripheral “Halo” and Dense “Core” Fractions of Lewy Bodies Can Be Revealed by Immunoelectron Microscopy
Ultrastructural analysis of the LB particles showed that whole particles consist of a dense central core and a loose electron-lucid bulky external layer, which hereafter we refer to as the halo zone. The halo zone vanished when LBs were digested with proteinase K (Fig. 2).
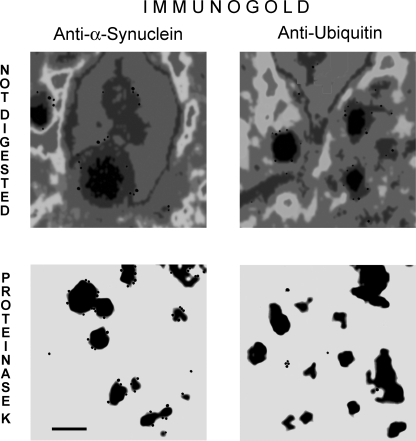
FIGURE 2.
Halo of Lewy bodies is fuzzy and contains ubiquitin, whereas the core consists of compacted α-synuclein. Transmission electron microscopic images of Lewy bodies without (top panel) and after proteinase K digestion (bottom panel). Lewy bodies were affinity-isolated with anti-α-synuclein antiserum from brain cortex and immunostained with anti-ubiquitin and anti-synuclein primary and gold particle-labeled secondary antibodies and counterstained with uranyl acetate before embedding. Bar, 0.25 μm.
Immunogold labeling revealed the presence of α-synuclein immunoreactivity in both LB compartments. Ubiquitin immunoreactivity was confined to the halo zone and was effectively removed by proteinase K digestion. This finding suggests that the inhomogeneity of LBs in terms of composition and cross-links may rather result from a different composition of LB core and periphery rather than from the dispersion of α-synuclein droplets within a meshwork of variably cross-linked and diverse cellular proteins.
Halo Zone of LBs Is Built from Various Proteins
Proteomic analysis of tryptic peptides obtained from the proteinase-digestible halo of LB from LBD hippocampi was performed. Using ion-exchange and reverse phase fractionation (“2D-LC”) followed by tandem mass spectrometry sequencing, the data allowed the identification of 53 proteins from tryptic LB peptides (Table 1), 41 of which were identified by multiple unique peptides. Of the 53, 47 proteins had no previously known association with LB. Functional class distribution of these 53 proteins was 23% cytoskeletal or structural, 19% energy and metabolism, 6% intracellular trafficking or synaptic, 17% signaling or protein modification, 17% ubiquitin-proteasome system, stress response or chaperon, and 17% unknown or other. Expected proteins such as α-synuclein, ubiquitin, and TG2 were identified in our proteomic screen, confirming the ability of this approach to identify LB-associated proteins.
TABLE 1.
Tryptic peptides identified by electrospray ionization/tandem mass spectrometry from the peripheral (directly digestible) zone of Lewy bodies
“Multiple entries” indicate that the sequences are comon in many related sequences belonging to redundant entries, splice variants, or close homologues of the same type of protein.
Cross-linked Proteins in LB Halo Zone
Using affinity separation for the GGEL isopeptide link, seven cross-linked sequences were identified from the protease-susceptible fraction of LB (Table 2.). These sequences included ubiquitin Lys-29 and Lys-48, Parkin Lys-49, HspB1 Gln-31 and Gln-190, and also α-synuclein Gln-99. The most abundant of these were the ubiquitin Lys-29-HSPB1 Gln-31 cross-bridges. Although a random cross-linking between substrate lysines and glutamines would predict the presence of HspB1 Gln-31-ubiquitin Lys-48 and also HspB1 Gln-31-Parkin Lys-49 cross-links, such peptide pairs were not found, similar to previous works with AD specimens (8).
TABLE 2.
Sequence assignments of cross-linked tryptic peptide pairs from the complex, protease-digestible halo, and the α-synuclein amyloid-containing core of Lewy bodies
Cross-linked peptides were affinity-isolated from tryptic digests and sequenced using liquid chromatography/tandem mass spectrometry. Boldface residues are forming the γ-glutamyl-ϵlysine isopeptide cross-links. A sequence deconvolution of pair 8 is shown in Fig. 3.
Proteinase K-resistant LB Core
The residual fraction of LBs after proteinase K digestion made up 57.1 ± 3.7% of total LB amino acid mass. This material was completely soluble in chaotropic salts, such as guanidine hydrochloride and guanidine isothiocyanate, i.e. agents used to separate LBs from unpolymerized cellular proteins. This particulate material showed no apparent immunoreactivity to either ubiquitin or GGEL isopeptide bonds (data not shown) but was strongly immunopositive for α-synuclein (Fig. 2). Electron density of the protease-resistant material was similar to that of LB cores and lacked the electron-lucid halo. The strong intermolecular interaction between the LB core α-synuclein molecules was suspended by chaotrope lysis and neutralization through mixing with an indifferent protein. Mass sequencing of LB core proteins identified solely α-synuclein. This apparent contradiction that α-synuclein aggregates were first not dissolved by chaotropic salts unless the halo was digested away might be explained by wrapping of α-synuclein amyloid (the core) into a bag of loosely packed, though densely cross-linked, protein meshwork.
GGEL Cross-links in LB Core
The frequency of GGEL cross-links in proteinase K-resistant LB fractions was 188 ± 11/106 amino acids (Fig. 1). As this fraction was built from virtually pure α-synuclein protein, this frequency corresponds to 1 cross-link in ∼500 lysines or 1 cross-link for ∼40 α-synuclein molecules. Sequencing of cross-linked tryptic peptides from LB core proteins showed that the cross-link was positioned between Lys-58 and Gln-99 (Fig. 3A). MALDI mass analysis of α-synuclein from LB and sequences obtained from peptides with missed cleavages indicated that cross-links may connect residues within one α-synuclein sequence (Fig. 3B).
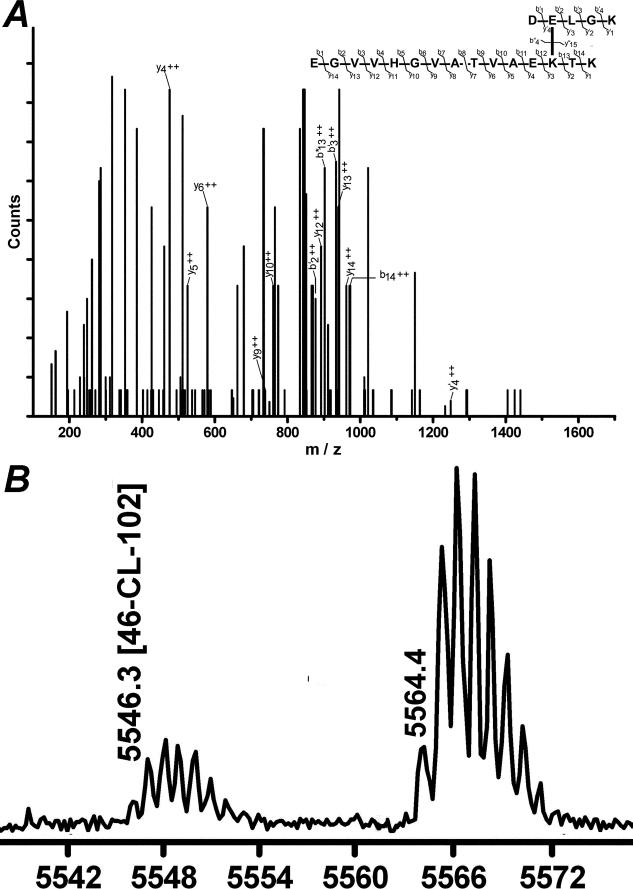
FIGURE 3.
α-Synuclein from Lewy bodies is internally cross-linked between Gln-99 and Lys-58. Ion nomenclature and Q-Trap mass spectrum of the tryptic peptide pair with γ-glutamyl-ϵ-lysine cross-link between Gln-99 and Lys-58 of α-synuclein (A). Only the fragment ion masses harboring the cross-link are labeled. Asterisk marks fragments 18 mass units below the expected mass (water loss). B, MALDI mass analysis of trypsin-digested α-synuclein features a peak of 5546 Da (monoisotopic, M + H+) comprising internally cross-linked residues 49–102 of α-synuclein, which cannot form from two intermolecularly cross-linked proteins. The peak at 5564 Da is a mixture of multiple isobaric sequences resulting from Gln deamidation of the (not cross-linked) 49–102 α-synuclein peptide and hydrolytic cleavage of the same cross-linked sequence between Lys-58 and Gln-99 residues.
Cross-linking of α-Synuclein by Soluble Transglutaminases in Vitro
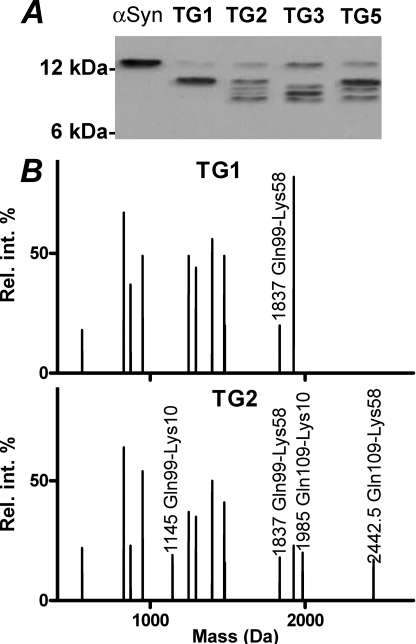
Human TG1–3 and -5 were expressed in insect cells and were reacted with α-synuclein to analyze substrate specificity and comparison with cross-linking patterns seen in LB. All the enzymes resulted in a promiscuous and apparently random utilization of most α-synuclein Gln and Lys residues, with exceptions for Gln-134, -62, and -24 and Lys-12, -22, -45, -60, and -95, which were not used (Fig. 4). This promiscuity of residue utilization corresponds to a randomly coiling, intrinsically disordered conformation of α-synuclein in solution (26). High α-synuclein concentrations yielded a higher amount of intermolecular cross-links, which showed a poorly separating mixture of dimers and higher molecular weight oligomers, whereas intramolecular cross-linking created a dispersion of α-synuclein mobility below the band of the native α-synuclein on SDS-PAGE (Fig. 4). Cross-linked residues were identified by sequencing in-gel trypsinized bands. Remarkably, only the first lysines were reactive from the X-Lys-Thr-Lys-X repeat tracts, and Gln-62 was not accessible for cross-linking for either of the TGs.
FIGURE 4.
A, transglutaminase 1–3 and 5 elicit a promiscuous cross-linking of α-synuclein (α-Syn) in solution, as evidenced by the formation of cross-linked oligomers not fully separated by size on SDS-PAGE. 250 μg/ml α-synuclein was exhaustively reacted with recombinant human TGs in solution. Electrophoretic separation of 1 μg/lane transglutaminase-modified α-synuclein was visualized by immunoblotting. Low (10 μg/ml) α-synuclein concentrations favor intramolecular cross-linking, as shown by the dispersion of α-synuclein electrophoretic mobility toward faster moving products (B) and multiple cross-linked glutamine and lysine residues identified in the transglutaminated products by mass sequencing after digestion with trypsin and Glu-C endoprotease (C). Mass peaks were sequenced by electrospray ionization-tandem mass spectrometry and shown in a deconvoluted format. Only transglutaminated product peaks are labeled. The cross-linked residues are listed in D. Residues utilized for cross-linking are shown in superscript, and the neglected ones are shown in subscript. Single letter code for amino acids is used.
α-Synuclein and TG Binding to Membranes
To simulate biological conditions, the interaction of α-synuclein with lipid membranes was analyzed. Previous reports using 1:1 lipid mixtures of PS and phosphatidylcholine found a large variability in binding capacities ranging from 185 to 2000 lipid molecules per bound α-synuclein (27, 28). The dispersion of binding strengths might have resulted from different experimental conditions and variable membrane fluidity at ambient temperatures. We compared the binding of α-synuclein to LV with fixed (15%) PS content at room temperature (22 °C) and 37 °C. As compared with room temperature, α-synuclein binding was found to increase largely at 37 °C. Remarkably, heating LV/α-synuclein mixtures briefly to 37 °C maximized binding even after cooling back to lower temperatures. This indicated that membrane fluidity is needed to anneal interacting PS molecules below the adhering protein (Fig. 5A).
FIGURE 5.
Binding of α-synuclein and transglutaminases to LV. 10 pmol of α-synuclein was added to varying amounts of LV from 15% phosphatidylserine, 55% phosphatidylcholine, and 30% cholesterol at room temperature (solid circles) and 37 °C (open circles). The not membrane-bound fraction of protein was measured from centrifugal ultrafiltrate. A, warming LV/α-synuclein mixtures to 37 °C (triangles) results in increased protein binding, which is preserved at the lower temperature thereafter. This phenomenon indicates that membrane fluidity is needed to fit interacting PS and α-synuclein residues together. B, calcium ions increase α-synuclein association to PS-containing LV. The ratio of synuclein/PS (mol/mol) is plotted; error bars show 95% confidence intervals. C, binding of TG1 to LV is independent of calcium concentration, whereas TG2 and TG5 associate with LV in a calcium ion-dependent manner. In the presence of LV, micromolar calcium ion concentrations elicit detectable TG activation for TG2 and TG5 (D); however, in solution these concentrations do not activate them (inset in D). The patterning of columns showing different free Ca2+ concentrations is the same as on C. TG1 is fully active at the lowest tested calcium concentration, whereas TG3 does not associate with LV and neither is its activity affected by LV. TG activity was measured by assaying p-nitrophenyl acetate hydrolysis by photometry. Points indicate means of five independent assays.
When membrane fluidity was assured by the addition of cholesterol and incubation at 37 °C, ∼26 exposed PS molecules were sufficient to bind an α-synuclein. Next, we studied the effect of calcium ions on binding of α-synuclein to LV. The addition of calcium ions to the medium increased the affinity of α-synuclein to LV and reduced the number of required PS molecules to bind an α-synuclein to ∼11 at 1 mm Ca2+ and ∼15 at 2.3 μm free Ca2+ (Fig. 5B).
Next, we studied the binding of TGs to LV and the effect of membrane binding on enzyme activity. In the absence of calcium ions, LV did not show detectable binding to either TG, except for TG1, which is bound to membranes through fatty acylation (29). The addition of (buffered) calcium ions, however, leads to the adsorption of TG2 and TG5, but not TG3, onto LV (Fig. 5C) and resulted in the appearance of latent TG activity even at Ca2+ concentrations, which were by 2 magnitudes of order below the free calcium requirements typically used and required by soluble TGs (Fig. 5D).
Cross-linking of α-Synuclein in the Presence of Membranes
The addition of LV radically interfered with the residue selectivity of TG1 and restricted the promiscuous utilization of glutamines and lysines to intramolecular cross-linking between Gln-99 and Lys-58. Under conditions when α-synuclein was fully adsorbed to membranes, and free calcium concentration was high enough to ascertain full TG activity (1 mm), the presence of LV also restricted the residue utilization of TG2 (Fig. 6), as well as that of TG3 and TG5 (mass data not shown). Intramolecular cross-linking between α-synuclein Lys-58 and Gln-99 was abundant, but cross-links between Gln-109 and Lys-10, Gln-109 and Lys-58, and Gln-99 and Lys-10 were also formed. Only one intramolecular cross-link was formed per α-synuclein, in agreement with previous findings (15).
FIGURE 6.
Addition of LV restricts substrate residue utilization in α-synuclein for TGs. α-Synuclein (α-Syn) was cross-linked by transglutaminases in the presence of LV and calcium buffers. A, 2 μg of α-synuclein per lane was detected by immunoblotting. In case of TG1, which is membrane-bound by default, only Gln-99 and Lys-58 were utilized. The orienting effect of acidic membranes in the presence of 1 mm calcium concentration reduced the complexity of α-synuclein products generated by TG2, TG3, and TG5, as shown by the separation of discrete bands on SDS-PAGE and mass fingerprint of TG-treated α-synuclein fragmented in-gel with CNBr and trypsin and analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry (B). Mass peaks are shown in deconvoluted format. TG3 and TG5 produced fragments corresponding to the pattern of TG2 and are therefore not shown. Under these conditions α-synuclein is fully membrane-bound, whereas TG2 is present as active-soluble and membrane-bound state.
Cross-linking of α-Synuclein by LV-associated TGs
We tested if the Ca2+-dependent binding of TG2 and TG5 could restrict residue utilization similarly to membrane binding of TG1 through fatty acylation. Whereas cross-linking at 32 μm free Ca2+ generated multiple cross-linked products, similar to 1 mm Ca2+, at 2.3 μm free Ca2+, TG2 and TG5 created solely Gln-99–Lys-58 cross-links, and alternatively bonded products were not detected (Fig. 7). This suggests that product heterogeneity is not excluded only by the membrane adsorption (and possibly acquiring a membrane-bound helical configuration) of α-synuclein if active TGs can access its exposed Gln and Lys residues from many directions. Only if the activating effect of the LV membrane and probably membrane-presented calcium ions restrict TG activity to the plane of the membrane surface will soluble TGs cross-link as if they were fixed to the membrane by a lipid anchor.
FIGURE 7.
Membrane association of α-synuclein and TGs direct residue specificity to Gln-99 to Lys-58 cross-linking and eliminates the production of variably cross-linked α-synuclein products. Micromolar calcium levels are locally accumulated on the surface of PS-containing LV and activate TG2 and TG5 in a membrane-dependent manner. Under low calcium conditions the soluble TG is not activated. Contrasted to 32 μm Ca2+, at 2.3 μm Ca2+ concentrations, TG2 and TG5 shows specificity toward utilization of Gln-99 and Lys-58 for cross-linking. 1 μg of α-synuclein (α-Syn) per lane was detected by SDS-PAGE immunoblotting. Cross-linked residue positions were detected as on Fig. 6.
Effects of Cross-linking on α-Synuclein Aggregation to Amyloid
The rate of amyloid formation, i.e. propagative β-pleated sheet forming aggregation process, was assayed by measuring the increase of fluorescence quantum efficiency of thioflavin T dye in the presence of a minor part of α-synuclein products, which were cross-linked by TG. As the frequency of GGEL isopeptide cross-links was ∼1 cross-link in 40 α-synuclein molecules of LB core, TG-modified synuclein was added to a 40-fold excess of native α-synuclein freshly dialyzed from 6 m guanidine HCl. The addition of α-synuclein cross-linked by soluble TGs strongly inhibited α-synuclein aggregation, in agreement with previous findings (12, 15, 30). The addition of Lys-58–Gln-99 intramolecularly cross-linked α-synuclein resulted in an earlier appearance of the aggregation signal, which was not affected by chaotrope interventions (boiling, guanidine HCl) of the cross-linked proteins (Fig. 8A).
FIGURE 8.
Effect of cross-linked α-synuclein on amyloid formation. The aggregation of unmodified α-synuclein was monitored by recording thioflavin T (ThT) fluorescence after addition of 2.5% α-synuclein incubated exhaustively with TGs. A, heterogeneously cross-linked α-synuclein produced by TG2 in solution (●) or in the presence of LV and 32 μm Ca2+ (▲) inhibits aggregation to amyloid, whereas homogeneously cross-linked α-synuclein produced by either TG1 (♦) or TG2 on LV at 2.3 μm Ca2+ (○) does not block fibril assembly. Inset in B, K58C/Q99C (lane 1) and K10C/Q99C (lane 3) α-synuclein mutants were cross-linked between their two cysteine residues (lane 2, 58–99; lane 4, 10–99 cross-linked) by bis-maleimidoethane. The site of cross-linking resulted in a different electrophoretic mobility of the isomeric products on 4–20% SDS-polyacrylamide gel. The 58–99 cross-linked protein shows seeding effect and accelerates the assembly of α-synuclein into amyloid (B), whereas the 10–99 cross-linked protein blocks amyloid growth in a dose-dependent manner (C). These effects are detectable and dose-dependent in the presence of the other type of cross-linked product (D). Points show the means of a representative experiment performed in triplicates.
Seeding and Blocking Effects of Chemical Cross-linking Depending on Cross-link Position
Wild type α-synuclein contains no cysteines, and thus introduction of two cysteines to replace TG-reactive Gln and Lys residues offered a simple model to replace transglutamination and to obtain an intramolecularly cross-linked protein. Although the formation of intramolecular disulfide bridges is a spontaneous process in a slightly oxidizing milieu, oxidized K58C/Q99C α-synuclein did not enhance amyloid formation, possibly due to disulfide exchange and formation of inter-α-synuclein disulfide bridges. Therefore, the cysteines of mutant α-synuclein were cross-linked chemically through a short bis-maleimidoethane spacer, which does not isomerize in solution and could be purified by SDS-PAGE under reducing conditions. The addition of the 58–99 chemically cross-linked mutant enhanced α-synuclein aggregation into amyloid even at a 1:400 ratio to wild type synuclein. This effect was also inhibited by the addition of randomly TG2-cross-linked α-synuclein. The similarly cross-linked K10C/Q99C α-synuclein blocked aggregate growth in a dose-dependent manner. Remarkably, the addition of this latter product decreased peak fluorescence, whereas the addition of 58–99 bonded synuclein accelerated aggregation. We tested if the pro- or contra-aggregation effect was dominant over the other. The addition of both cross-linked isomers showed that the seeding effect of 58–99 was detectable also in the presence of the blocking 10–99 or soluble TG-treated α-synuclein products (Fig. 8).
DISCUSSION
The key event of Lewy Body formation is the appearance of a specific and propagating α-synuclein conformation, when this natively unfolded protein acquires a structure that recruits other molecules to stick to the end of the protofibril by forming β-pleated sheets between its repeat domains. Such amyloid-forming chain reactions are common in the extracellular compartment; though apart from prions, inside the cytoplasm α-synuclein is the only known wild type human protein that behaves like that. Because the abundance of α-synuclein is not too high and other sticky proteins (such as heat shock proteins) can block the process (31), the initial formation of LB requires some kind of catalytic effect or loss of blocking or clearance mechanisms (32).
Here LB were found to be first insoluble in chaotropic agents, which are capable of disrupting noncovalent and disulfide bond interactions, but amenable to proteinase attack. In the α-synuclein amyloid core of LB, the cohesion of the proteins must have relied on homophile secondary interactions, which could not have survived the disruption and lysis by chaotropic salts applied during the isolation process, unless a cross-linked protein bag protected the proteins from diffusing away from one another. After this surrounding meshwork was digested away, the amyloid became readily soluble in chaotropic salts. This suggests that α-synuclein first may aggregate with itself and form amyloid that grows (“one-dimensional crystal”) as long as it can remain hidden from different proteins such as chaperones and ubiquitinating enzymes, which deposit onto the surface of amyloid as a surrounding and cross-linked layer afterward.
Previous data from our laboratories revealed a similar pattern for the covalent cross-linking of the outer sheath of ubiquitin-immunopositive insoluble aggregates from Alzheimer diseased brains and age-matched controls (8). These were regarded as neurofibrillary tangles, although they may have been a mixture of LBs, tangles, and possibly other aggregates (such as granulo-vacuoles, Hirano bodies), because ubiquitin and α-synuclein are ubiquitous components in several types of intramolecular inclusions. The restricted number of cross-linked sequences indicated that covalent isopeptide cross-linking targets generic, aggregated sequence-independent proteins, a finding largely corroborated by the current data. It is also noteworthy to mention that the proteomic analysis of Alzheimer neurofibrillary tangles identified a remarkably similar set of proteins to the ones found in the LB halo (33), which again suggests unspecific cellular response pathways to protein aggregation.
The role of TG-mediated cross-linking in the aggregation of α-synuclein has intrigued researchers because cross-links were detected in LBs by anti-GGEL immunohistochemistry and immunoblotting techniques (9, 10, 14, 34). The overexpression of TG2 favored α-synuclein aggregation in cell cultures (10); nevertheless, a direct and unregulated cross-linking of α-synuclein blocked aggregation (12, 15, 30). This apparent conflict is reconciled and clarified by our findings, which show that α-synuclein from LB core contains sub-stoichiometric amounts of Lys-58–Gln-99 cross-links between residues of a single primary sequence and thus form a hairpin loop within the molecule. The abundance of transglutaminated α-synuclein is less than 1 cross-link in 40 α-synucleins. This low rate of cross-linking is unlikely to deplete cellular α-synuclein pools, as suggested in Ref. 9. Moreover, such infrequent cross-linking may have modest impact on α-synuclein solubility and persistence, once it has aggregated into amyloid.
Our findings suggest that low amounts of Gln-99–Lys-58 cross-linked synuclein might act as an inoculating seed for amyloid-type aggregation of unstructured soluble α-synuclein chains. Intramolecular cross-linking of α-synuclein trigger the amyloid fibril formation of native α-synuclein, whereas heterogenic mixtures of differently cross-linked proteins block it, as shown also by previous reports (12, 15, 30). This observation is in accord with recent models of α-synuclein fibrillarization, which predicted that the core region of the protein (∼35–105 positions) stack to one another as hairpin-like folds of five short (7–9-residue long) β-pleated sheet forming tracts, where Lys-58 and Gln-99 would be located to the flexible loops connecting these sheets (35). Even the 61–95 α-synuclein sequence or shorter tracts of it may form powerful β-amyloid aggregates (36). As we showed here with chemically cross-linked α-synuclein, cross-linking variable or different residues can mismatch and disrupt annealing, whereas a precise hairpin cross-linking of the randomly coiling backbone can accelerate matching and assembly of complementary hydrogen bonding partners within and between the loops.
The cytoplasmic surface of membranes is a unique micro-compartment for many catalytic processes. The calcium ions coming from extracellular or vesicular pools are accumulated by the phosphatidylserine-rich inner membrane surface, and thus the cytosolic face of membranes serves as calcium stores that can promptly regulate calcium-dependent enzymes as exemplified by the processes of synaptic vesicle trafficking, docking, and fusion in presynaptic nerve termini or dendritic spine movements in glutamatergic synapses (37, 38). α-Synuclein is a synaptic vesicle-associated protein that variably associates to, and dissociates from, the outer surface of vesicles, a process presumably regulated by the master regulator of synaptic vesicle turnover, i.e. calcium signaling. Transglutaminases need to bind multiple calcium ions for activation (39) and to attain active conformation at calcium concentrations that do not occur in the gross cytoplasm of living cells other than in sub-membranous calcium pools, and even there only for microseconds.
In our model the accumulation of calcium ions by phosphatidylserine-rich membranes lowered the calcium ion requirements for soluble TGs by magnitudes or order, possibly both by calcium and substrate presentation effects. This effect has been noted earlier for TG1 (22) but not for membrane-bound soluble TGs. This indicates that the effect of membrane coordination is not unique to TG1 but also affects soluble cytosolic TG isoenzymes, the activities of which were thus far thought to be latent under the low calcium conditions occurring in living cells (40).
Although TG2 is held to be the most expressed TG in neurons, this belief is largely due to the fact that the other TG congeners have not yet been investigated in detail, and redundantly expressed TGs may exert overlapping activities with TG2 (4). The binding of both α-synuclein and transglutaminase in the presence of calcium ions was sufficient to direct the broad residue specificity of TG1, TG2, and TG5 to the same substrate residues in α-synuclein and block alternative sites for cross-linking. The orienting effect of membrane attachment might explain why TG2, an enzyme ubiquitously present in cells both in cytosolic and acylated membrane-attached forms (41, 42), favored α-synuclein inclusion formation in cell culture (10) but blocked aggregation in vitro (30).
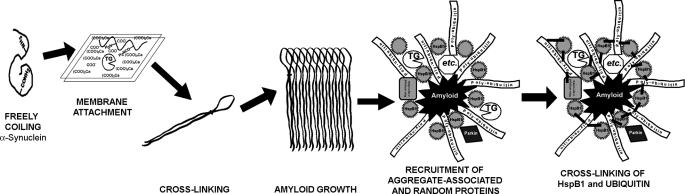
Our findings reveal that intramolecular cross-linking by membrane-bound trans-glutaminase activity promotes the nucleation of α-synuclein amyloid (proto)fibrils, and the cross-linking of ubiquitin and HspB1 on the surface of these inclusions is a generic secondary process that encapsulates Lewy bodies in vivo (Fig. 9). What the cell may gain from the formation of structured Lewy bodies still needs to be clarified.
FIGURE 9.
Scheme for LB biogenesis. The propagative β-sheet hairpin formation is favored by restricting α-synuclein random coiling with membrane association and intramolecular cross-linking. The growth of fibrils can proceed as long as other proteins, such as chaperones clog the growing ends. Attachment of (poly)ubiquitin chains, and chaperones (such as HspB1) block amyloid expansion. A covalent meshwork by cross-linking small heat shock proteins with ubiquitin encapsulates the unclearable aggregate.
Acknowledgments
We are indebted to Peter Jarnik for the invaluable assistance with electron microscopy and to Gonzales Van Driessche for amino acid analyses.
This work was supported in part by the Hungarian Scientific Research Fund OTKA TS 044798, the Hungarian Ministry of Health Grant ETT115/03, the Hungarian Ministry of Education Grant OM00427/04, the Flemish-Hungarian Intergovernmental Research Grants B33/04 and B/06093, the Fund for Scientific Research/Flanders G0422.98, and European Union Grants MRTN-CT-2006-036032, MRTN-CT-2006-035624, and LSHB-CT-2007-037730.
- AD
- Alzheimer disease
- ABB
- ammonium-bicarbonate buffer
- GGEL
- γ-glutamyl-ϵ-lysine
- LB
- Lewy bodies
- LBD
- Lewy body dementia
- PS
- phosphatidylserine
- LV
- lipid vesicles
- TG
- transglutaminase
- PBS
- phosphate-buffered saline
- MALDI-TOF
- matrix-assisted laser desorption ionization time-of-flight.
REFERENCES
- 1.Colosimo C., Hughes A. J., Kilford L., Lees A. J. (2003) J. Neurol. Neurosurg. Psychiatry 74, 852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto M., Kawahara K., Bar-On P., Rockenstein E., Crews L., Masliah E. (2004) J. Mol. Neurosci. 24, 343–352 [DOI] [PubMed] [Google Scholar]
- 3.Beyer K. (2007) Cell Biochem. Biophys. 47, 285–299 [DOI] [PubMed] [Google Scholar]
- 4.Fesus L., Piacentini M. (2002) Trends Biochem. Sci. 27, 534–539 [DOI] [PubMed] [Google Scholar]
- 5.Candi E., Paradisi A., Terrinoni A., Pietroni V., Oddi S., Cadot B., Jogini V., Meiyappan M., Clardy J., Finazzi-Agro A., Melino G. (2004) Biochem. J. 381, 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S. Y., Grant P., Lee J. H., Pant H. C., Steinert P. M. (1999) J. Biol. Chem. 274, 30715–30721 [DOI] [PubMed] [Google Scholar]
- 7.Citron B. A., SantaCruz K. S., Davies P. J., Festoff B. W. (2001) J. Biol. Chem. 276, 3295–3301 [DOI] [PubMed] [Google Scholar]
- 8.Nemes Z., Devreese B., Steinert P. M., Van Beeumen J., Fésüs L. (2004) FASEB J. 18, 1135–1137 [DOI] [PubMed] [Google Scholar]
- 9.Andringa G., Lam K. Y., Chegary M., Wang X., Chase T. N., Bennett M. C. (2004) FASEB J. 18, 932–934 [DOI] [PubMed] [Google Scholar]
- 10.Junn E., Ronchetti R. D., Quezado M. M., Kim S. Y., Mouradian M. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2047–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H. J., Lee S. J. (2002) J. Biol. Chem. 277, 48976–48983 [DOI] [PubMed] [Google Scholar]
- 12.Segers-Nolten I. M., Wilhelmus M. M., Veldhuis G., van Rooijen B. D., Drukarch B., Subramaniam V. (2008) Protein Sci. 17, 1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh M. D., Park C. H., Kim S. S., Kil M. O., Lee G. H., Johnson G. V., Chun W. (2004) Arch. Pharm. Res. 27, 850–856 [DOI] [PubMed] [Google Scholar]
- 14.Jensen P. H., Sørensen E. S., Petersen T. E., Gliemann J., Rasmussen L. K. (1995) Biochem. J. 310, 91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid A. W., Chiappe D., Pignat V., Grimminger V., Hang I., Moniatte M., Lashuel H. A. (2009) J. Biol. Chem. 284, 13128–13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeith I. G., Dickson D. W., Lowe J., Emre M., O'Brien J. T., Feldman H., Cummings J., Duda J. E., Lippa C., Perry E. K., Aarsland D., Arai H., Ballard C. G., Boeve B., Burn D. J., Costa D., Del Ser T., Dubois B., Galasko D., Gauthier S., Goetz C. G., Gomez-Tortosa E., Halliday G., Hansen L. A., Hardy J., Iwatsubo T., Kalaria R. N., Kaufer D., Kenny R. A., Korczyn A., Kosaka K., Lee V. M., Lees A., Litvan I., Londos E., Lopez O. L., Minoshima S., Mizuno Y., Molina J. A., Mukaetova-Ladinska E. B., Pasquier F., Perry R. H., Schulz J. B., Trojanowski J. Q., Yamada M. (2005) Neurology 65, 1863–1872 [DOI] [PubMed] [Google Scholar]
- 17.Nemes Z., Petrovski G., Fésüs L. (2005) Anal. Biochem. 342, 1–10 [DOI] [PubMed] [Google Scholar]
- 18.Conway K. A., Harper J. D., Lansbury P. T., Jr. (2000) Biochemistry 39, 2552–2563 [DOI] [PubMed] [Google Scholar]
- 19.Candi E., Melino G., Lahm A., Ceci R., Rossi A., Kim I. G., Ciani B., Steinert P. M. (1998) J. Biol. Chem. 273, 13693–13702 [DOI] [PubMed] [Google Scholar]
- 20.Candi E., Tarcsa E., Idler W. W., Kartasova T., Marekov L. N., Steinert P. M. (1999) J. Biol. Chem. 274, 7226–7237 [DOI] [PubMed] [Google Scholar]
- 21.Kim H. C., Nemes Z., Idler W. W., Hyde C. C., Steinert P. M., Ahvazi B. (2001) J. Struct. Biol. 135, 73–77 [DOI] [PubMed] [Google Scholar]
- 22.Nemes Z., Marekov L. N., Steinert P. M. (1999) J. Biol. Chem. 274, 11013–11021 [DOI] [PubMed] [Google Scholar]
- 23.Slaughter T. F., Achyuthan K. E., Lai T. S., Greenberg C. S. (1992) Anal. Biochem. 205, 166–171 [DOI] [PubMed] [Google Scholar]
- 24.Candi E., Melino G., Mei G., Tarcsa E., Chung S. I., Marekov L. N., Steinert P. M. (1995) J. Biol. Chem. 270, 26382–26390 [DOI] [PubMed] [Google Scholar]
- 25.Bers D. M., Patton C. W., Nuccitelli R. (1994) Methods Cell Biol. 40, 3–29 [DOI] [PubMed] [Google Scholar]
- 26.Csosz E., Bagossi P., Nagy Z., Dosztanyi Z., Simon I., Fesus L. (2008) J. Mol. Biol. 383, 390–402 [DOI] [PubMed] [Google Scholar]
- 27.Jo E., McLaurin J., Yip C. M., St George-Hyslop P., Fraser P. E. (2000) J. Biol. Chem. 275, 34328–34334 [DOI] [PubMed] [Google Scholar]
- 28.Rhoades E., Ramlall T. F., Webb W. W., Eliezer D. (2006) Biophys. J. 90, 4692–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinert P. M., Kim S. Y., Chung S. I., Marekov L. N. (1996) J. Biol. Chem. 271, 26242–26250 [DOI] [PubMed] [Google Scholar]
- 30.Konno T., Morii T., Hirata A., Sato S., Oiki S., Ikura K. (2005) Biochemistry 44, 2072–2079 [DOI] [PubMed] [Google Scholar]
- 31.Klucken J., Shin Y., Masliah E., Hyman B. T., McLean P. J. (2004) J. Biol. Chem. 279, 25497–25502 [DOI] [PubMed] [Google Scholar]
- 32.Beyer K., Ariza A. (2007) J. Neuropathol. Exp. Neurol. 66, 965–974 [DOI] [PubMed] [Google Scholar]
- 33.Wang Q., Woltjer R. L., Cimino P. J., Pan C., Montine K. S., Zhang J., Montine T. J. (2005) FASEB J. 19, 869–871 [DOI] [PubMed] [Google Scholar]
- 34.Muma N. A. (2007) J. Neuropathol. Exp. Neurol. 66, 258–263 [DOI] [PubMed] [Google Scholar]
- 35.Vilar M., Chou H. T., Lührs T., Maji S. K., Riek-Loher D., Verel R., Manning G., Stahlberg H., Riek R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8637–8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giasson B. I., Murray I. V., Trojanowski J. Q., Lee V. M. (2001) J. Biol. Chem. 276, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 37.Flavell S. W., Greenberg M. E. (2008) Annu. Rev. Neurosci. 31, 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neher E., Sakaba T. (2008) Neuron 59, 861–872 [DOI] [PubMed] [Google Scholar]
- 39.Casadio R., Polverini E., Mariani P., Spinozzi F., Carsughi F., Fontana A., Polverino de Laureto P., Matteucci G., Bergamini C. M. (1999) Eur. J. Biochem. 262, 672–679 [DOI] [PubMed] [Google Scholar]
- 40.Smethurst P. A., Griffin M. (1996) Biochem. J. 313, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harsfalvi J., Arato G., Fesus L. (1987) Biochim. Biophys. Acta 923, 42–45 [DOI] [PubMed] [Google Scholar]
- 42.Signorini M., Caselli L., Lanzara V., Ferrari C., Melandri P., Bergamini C. M. (1996) Biol. Chem. Hoppe-Seyler 377, 167–173 [DOI] [PubMed] [Google Scholar]