Abstract
The NCX1 (sodium-calcium exchanger) is up-regulated in human heart failure and in many animal models of heart failure. The potential benefits and risks of therapeutically blocking NCX1 in heart failure and during ischemia-reperfusion are being actively investigated. In this study, we demonstrate that prolonged administration of the NCX1 inhibitor KB-R7943 resulted in the up-regulation of Ncx1 gene expression in both isolated adult cardiomyocytes and intact mouse hearts. Ncx1 up-regulation is mediated by the activation of p38. Importantly, p38 is not activated by KB-R7943 treatment in heart tubes from Ncx1−/− mice at 9.5 days postcoitum but is activated in heart tubes from Ncx1+/+ mice. p38 activation does not appear to be in response to changes in cytosolic calcium concentration, [Ca2+]i. Interestingly, chronic KB-R7943 treatment in mice leads to the formation of an NCX1-p38 complex. Our study demonstrates for the first time that the electrogenic sarcolemma membrane cardiac NCX1 can act as a regulator of “activity-dependent signal transduction” leading to changes in gene expression.
The cardiac NCX1 (Na+-Ca2+ exchanger) plays a critical role in maintaining the balance of Ca2+ flux across the sarcolemmal membrane in excitation-contraction coupling (1). Cardiac muscle contracts in response to the rise in [Ca2+]i, which is released from the sarcoplasmic reticulum (SR)5 and from influx across the sarcolemma through voltage-sensitive channels. SR Ca2+-ATPase recycles Ca2+ from the cytosol into the lumen of the SR, and NCX1 mediates the movement of [Ca2+]i across the sarcolemma to the extracellular space. NCX1 transports ∼28% of the cytosolic Ca2+ during a contraction-relaxation cycle in large animals and humans, with 70% being reaccumulated in the SR (via SR Ca2+-ATPase) (2–4). Alterations in any of the activities associated with this complex process causes a corresponding change in the amount of Ca2+ released from the SR, and the resulting force of cardiac contraction.
The exchanger is regulated at the transcriptional level in animal models of cardiac hypertrophy (5, 6) and ischemia and failure (7–12). Importantly, both NCX1 mRNA and protein levels are significantly up-regulated in human end-stage heart failure (13–16). The diastolic performance of failing human myocardium correlates inversely with protein levels of NCX1 (17), and up-regulation of Ncx1 alone contributes directly to impaired SR loading and contractile dysfunction (18, 19). Ventricular tachycardia, a precursor to ventricular fibrillation and a major cause of sudden death in heart failure, has also been linked to up-regulation of NCX1. NCX1 up-regulation results in a greater potential for delayed after depolarizations, which are major initiators of ventricular tachycardia (9, 20). In addition, Ca2+ loading, which is one of the major causes of myocardial damage following ischemia-reperfusion, is mediated via reverse mode NCX1. All three benzyloxyphenyl NCX inhibitors, KB-R7943, SN-6, and SEA-0400, have been reported to confer some cardioprotective effects against ischemia-reperfusion injury and heart failure. Both KB-R7943 and the more potent and selective but less available SEA-0400 have been utilized in many in vitro and in vivo studies to analyze NCX1 function and its role in ischemia-reperfusion and heart failure (21–26).
Although KB-R7943, SN-6, and SEA-0400 have been utilized in a variety of animal and cell models, most studies have focused only on the acute effects on INCX1 and Ca2+ homeostasis. In this study, we focus our attention on what occurs during chronic NCX1 inhibition, both in the heart and in isolated adult cardiomyocytes. Prolonged blockade of L-type calcium channels, β-adrenergic receptors, or the Na+/H+ exchanger results in their up-regulation (27, 28). In light of what occurs with chronic inhibition of several cardiac channels, receptors, and exchangers (29), we investigated whether chronic inhibition of NCX1 activity had any effect on the expression of Ncx1 in cardiomyocytes. This is an important consideration given the potential of NCX1 inhibition as part of a future therapeutic approach. The results of our study show that chronic inhibition of NCX1 by KB-R7943 results in Ncx1 gene up-regulation via a p38-activated pathway. We demonstrate that the increase in expression is directly mediated by the interaction of NCX1 with p38.
EXPERIMENTAL PROCEDURES
Adult Cardiomyocyte Cell Culture
Adult feline cardiomyocytes were isolated via a hanging heart preparation using enzymatic digestion and cultured by the protocols approved by the Institutional Animal Care and Use Committee as described previously (30). The cardiomyocytes were plated on culture trays that were coated with laminin at an initial plating density of 7.5 × 104 cells/ml. After overnight incubation, the cardiomyocytes were rinsed and maintained in serum-free media.
Adenovirus Cell Infection
Cardiomyocytes were infected on day 1 in culture by adding titered adenovirus to the culture medium at different multiplicities of infection (m.o.i.). After an infection of 8 h, the media were changed, and a second adenovirus was added if the experiment called for more than one virus. When more than one adenoviral construct was used to infect cells, additional experiments were carried out to ensure there was no competition for infection between the constructs at the m.o.i. used. Adult cardiomyocytes infected with m.o.i. of 1 resulted in infection of and gene transfer to more than 85% of the plated cells as determined by analysis of GFP expression.
Preparation of Cell and Ventricular Tissue Lysates
Cells were treated with NCX1 inhibitors (10 μm KB-R7943 or 10 μm SN-6 from Tocris Bioscience) 24 h after adenovirus infection. For pathway inhibition studies, cells were pretreated with the PKC inhibitor bisindolylmaleimide (2.5 μm), the CaMKII inhibitor KN-93 (10 μm), the PLC inhibitor U73122 (2 μm), the phosphatidylinositol 3-kinase inhibitor LY294002 (10 μm), or the cAMP-dependent protein kinase inhibitor H-89 (10 μm). All the above inhibitors are from Calbiochem. 30 min after addition of the inhibitor, cells were treated with either KB-R7943 or SN-6 for 48 h. Following treatment, cells were washed twice in sterile-filtered cold PBS. The cells were then lysed in Triton lysis buffer (20 mm Tris, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm β-glycerol, 2.5 mm sodium pyrophosphate, 1% Triton X-100) for Western blot analysis and co-immunoprecipitation studies or in Reporter Assay Buffer for luciferase assay (Promega, Madison WI). Phosphatase and protease inhibitors were added to the buffers (1:100 dilutions of phosphatase inhibitor mixtures I and II and protease inhibitor mixture from Sigma). The cells were then incubated on ice for 15 min, and insoluble material was pelleted by centrifugation in a tabletop microcentrifuge at 4 °C.
Embryonic Heart Tube Isolation and Culture
Whole E9.5 embryos were harvested; the heart tubes were micro-dissected, and the embryos were genotyped by PCR as described previously (31). Individual isolated hearts were placed into 24-well tissue culture plates (Falcon) and washed three times in 4 °C phosphate-buffered saline (PBS). PBS was replaced with Dulbecco's modified Eagle's medium, and the hearts were equilibrated at 32 °C for 5 min. The hearts were then transferred to Dulbecco's modified Eagle's medium containing either 10 μm KB-R7943 or vehicle (DMSO) alone at 32 °C for 20 min. After treatment, the heart tubes were homogenized in lysis buffer.
Western Blot Analysis
Protein concentrations were determined by the Bio-Rad protein assay. Cell lysates were subjected to SDS-PAGE, and Western blotting was performed with the appropriate antibodies. Antibodies to NCX1 were obtained from Swant, Bellinzona, Switzerland. Antibodies to CaMKII, P-CaMKII, p38, P-p38, P-JNK, JNK, P-ERK, and ERK were obtained from Cell Signaling Technology. The proteins were visualized by enhanced chemiluminescence.
Co-immunoprecipitation Studies
Cell lysates were prepared as described above. For immunoprecipitation of p38 or NCX1, 2 μg of antibody was added to 500 μg of precleared lysate, and complex formation was carried out at 4 °C overnight. The protein-antibody complexes were recovered using protein A/G-agarose (Santa Cruz Biotechnology). The immunoprecipitates were washed four times in immune precipitation buffer, eluted by boiling for 5 min in 2× SDS sample buffer, and analyzed by Western blot.
Luciferase and GFP Assay
To determine luciferase activity in adult cardiomyocytes or heart tissue, 5 μl of cell or tissue lysate was added to 50 μl of luciferin mixture (Promega, Madison, WI). Light emission was measured using an Auto Lumat LB 953 luminometer. To measure GFP fluorescence, 50 μl of crude lysate was added to 100 μl of PBS, and the samples were read in a fluorometer at the excitation wavelength of 488 nm and emission of 510 nm. Luciferase readings were normalized by GFP expression.
RNA Isolation and Northern Analysis
Total RNA was isolated from adult cardiomyocytes with TRIzol (Invitrogen). Total RNA was electrophoresed on a 1% agarose-formaldehyde gel and transferred to a Duralon membrane to which it was then UV-cross-linked. The membrane was blocked and then hybridized to [32P]dCTP-labeled PCR probes corresponding to a 2257–3185-bp segment of the feline Ncx1 cDNA sequence. Membranes were then washed to a stringency of 1× SSC, 0.1% SDS at 42 °C.
Statistical Analysis
The results were presented as means ± S.E. The data were evaluated for significance by performing ANOVA with p values < 0.05 considered to be significant.
Electrophysiological Recording and Analysis
Isolated single ventricular cells with clear striations were superfused with HEPES-buffered Tyrode's solution (35 °C) and whole-cell patch clamped to a holding potential of −40 mV. The extracellular solution was immediately changed to a nominally calcium-free blocker solution containing 150 mm NaCl, 2 mm BaCl2, 2 mm CsCl, 0.01 mm verapamil, 0.05 mm ouabain, 4 mm MgCl2, and 5.5 mm dextrose. Patch pipettes contained 20 mm NaCl, 20 mm tetraethylammonium-Cl, 50 mm EGTA, 21.6 mm Ca2+, 40 mm HEPES, 10 mm MgATP, 3 mm MgCl2, and 5 mm Tris2 creatine phosphate, pH 7.4, with CsOH (free [Ca2+]pip = 47 nm (WinMAXC, C. Patton, Stanford University)). Reverse exchanger current was activated (τ ≈2 s) by changing the perfusate from 0 to 4 mm Ca2+ (4 to 0 mm Mg2+). Voltage pulses (175 ms, −80 to 60 mV in 20-mV steps) were applied at 0.5-s intervals to obtain a complete current-voltage relationship, before, during, and after each calcium application. The exchanger current at each potential was taken as the difference between the mean currents during the last 30 ms of the test and control pulses.
RESULTS
Chronic Inhibition of NCX1 Activity Results in Up-regulation of Ncx1 Expression
We first examined whether prolonged KB-R7943 treatment had any effect on the regulation of Ncx1 gene expression in adult cardiomyocytes. We utilized an adenoviral construct of the full-length Ncx1 promoter that contains 1831 bp of the promoter and the entire first exon fused to the luciferase reporter gene. The 1831Ncx1 construct has been previously shown to be sufficient for cardiac restricted expression that is up-regulated in response to α-adrenergic stimulation in both adult and neonatal cardiomyocytes (32–34). Adult cardiomyocytes infected with the 1831Ncx1 adenovirus were treated with 10 μm KB-R7943 for 48 h. Inhibition of reverse mode NCX1 activity with KB-R7943 treatment resulted in a 3-fold up-regulation of the 1831Ncx1 promoter-driven luciferase expression when compared with control (Fig. 1A). Importantly, Fig. 1B demonstrates that there is also an increase in endogenous Ncx1 message levels after 48 h of KB-R7943 treatment of adult cardiomyocytes. To determine whether the increase in Ncx1 expression was because of the inhibition of NCX1 activity and not the result of a nonspecific or generalized stress response of the cardiomyocyte to KB-R7943, we examined the expression levels of atrial natriuretic factor, a protein that is often up-regulated in response to cardiac stress. Fig. 1C shows that although the atrial natriuretic factor promoter-luciferase reporter construct is highly responsive to stimulation with the α-adrenergic agonist phenylephrine, there is no increase in luciferase expression in response to KB-R7943 treatment. Importantly, KB-R7943 treatment does not adversely affect resting adult cardiomyocytes. There is no discernable difference in cell morphology or other evidence of cell stress or cell death even after 72 h of KB-R7943 treatment (data not shown).
FIGURE 1.
KB-R7943 activates Ncx1 gene expression. A, isolated adult cardiomyocytes were infected with adenoviruses expressing the 1831Ncx1 promoter luciferase reporter construct (m.o.i. of 1.5). Cells were treated with NCX1 inhibitor KB-R7943 (KB-R) for 48 h. Cells were lysed in reporter buffer, and luciferase activity was determined. The values are expressed as relative luciferase units normalized to GFP levels (RLU/GFP). *, p < 0.05 when compared with control as determined by ANOVA (n = 6). B, isolated adult cardiomyocytes were treated with KB-R for 48 h, and then total RNA was isolated by TRIzol method. Northern blot analysis was performed for Ncx1 using PCR probes corresponding to 2257–3185 bp of the feline Ncx1 DNA sequence. C, adult cardiomyocytes were infected with adenoviruses expressing the ANF promoter luciferase reporter construct (m.o.i. of 5). Cells were treated with or without the α-adrenergic agonist 10 μm phenylephrine (PE) and KB-R for 48 h. Cells were lysed in reporter buffer, and luciferase activity was determined. The values are expressed as relative luciferase units normalized to GFP levels (RLU/GFP). *, p < 0.01 when compared with control as determined by ANOVA (n = 4).
To determine whether the KB-R7943 mediated up-regulation of Ncx1 promoter activity occurred in vivo as well as in vitro, we utilized an 1831Ncx1 promoter reporter mouse model. Our previous studies have shown that transgenic expression of the 1831Ncx1 promoter luciferase reporter construct follows the same cardiac restricted pattern of endogenous Ncx1 promoter expression and is up-regulated to the same extent as the endogenous Ncx1 transcript in response to pressure overload (34, 35). The mice were injected twice daily with KB-R7943 at a dosage of 10 mg/kg body weight. After 2 days, the mice were sacrificed and the hearts extracted for analysis. We observed an ∼2-fold increase in expression of 1831Ncx1 promoter-driven luciferase expression in mice injected with KB-R7943 when compared with mice injected with vehicle only (Fig. 2A). After 24 h of KB-R7943 treatment there is a slight but nonsignificant increase in NCX1 protein (data not shown). Importantly, Western blotting showed that endogenous NCX1 protein levels were significantly increased in mice after 48 h of KB-R7943 treatment when compared with nontreated controls (Fig. 2B).
FIGURE 2.
Chronic inhibition of NCX1 by KB-R7943 in vivo up-regulates Ncx1 gene expression. A, KB-R (10 mg/kg) or vehicle (control) was injected subcutaneously into 3-month-old 1831 Ncx1 promoter-luciferase reporter mice every 12 h. After 48 h the mice were euthanized; hearts were harvested in Promega reporter lysis buffer, and luciferase activity was determined. All animal experimentation was performed in accordance with National Institutes of Health Guidelines, and protocols were approved by the Animal Care Committee of the Medical University of South Carolina. The values are expressed as relative luciferase units normalized to GFP levels (RLU/GFP). *, p < 0.05 when compared with control as determined by ANOVA (n = 6). B, KB-R (10 mg/kg) or vehicle (control (C)) was injected into six, 3-month-old FVB mice every 12 h for 48 h. After 48 h the mice were euthanized; hearts were harvested and processed for Western blotting. Similar data were obtained in three independent experiments.
Up-regulation of Ncx1 by KB-R7943 Treatment Is Mediated by Activation of p38
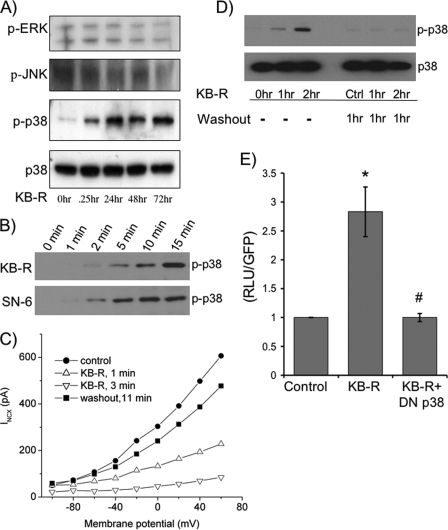
Because earlier studies in our laboratory have demonstrated a role for MAPKs in modulating exchanger expression, we examined whether they were activated by KB-R7943 treatment (36). Adult cardiomyocytes were treated with KB-R7943 at various times and then lysed, and the phosphorylation states of JNK, ERK, and p38 MAPK were analyzed by Western blot. There is no activation of ERK or JNK detected in response to KB-R7943 at any time point (Fig. 3A). However, p38 is activated as early as 5 min after treatment (Fig. 3B), and this activation persists for up to 72 h (Fig. 3A). Importantly, treatment of adult cardiomyocytes with a second NCX1 inhibitor, SN-6 (37), also results in activation of p38.
FIGURE 3.
Inhibition of NCX1 by KB-R7943 activates p38. A, lysates from cells treated with 10 μm KB-R at different time points were subjected to SDS-PAGE followed by Western blotting to determine the activation of MAPKs by probing with phosphorylation-specific antibodies to ERK (p-ERK), JNK (p-JNK), and p38 (p-p38). The data are representative of six independent experiments. B, cells were treated with KB-R or another NCX1 inhibitor, SN-6, for 1–15 min. Cells were lysed and subjected to SDS-PAGE followed by Western blotting to determine the activation by phosphorylation of p38 (p-p38). The Western blot is representative of four experiments. C, representative current-voltage relationship for the exchanger 1 min before application of KB-R (filled circles), 1 min after the application of 10 μm KB-R (open triangles), 3 min after KB-R (inverted open triangles), and 11 min after beginning washout (filled squares). The data are representative of five experiments. D, cardiomyocytes were treated with 10 μm KB-R for 1 and 2 h and washed out for 1 h, and then cells were lysed and subjected to SDS-PAGE followed by Western blotting to determine the activation of p38. Ctrl, control. E, isolated adult cardiomyocytes were sequentially co-infected with the 1831Ncx1 promoter luciferase reporter adenovirus (m.o.i. of 1.5) followed after 8 h with the dominant negative p38 adenovirus. Cells were treated with or without 10 μm KB-R for 48 h. Cells were lysed in reporter buffer and luciferase activity was determined relative to GFP levels. *, p < 0.05 when compared with control; #, p < 0.05 when compared with KB-R stimulated cells as determined by ANOVA (n = 6).
The activation of p38 closely follows the kinetics of INCX inhibition by KB-R7943. In patch clamp experiments, 10 μm KB-R7943 rapidly and reversibly blocked over 90% of the calcium-activated exchanger current without altering the shape of the current-voltage relationship (Fig. 3C). In five independent experiments the mean current at 0 mV was 271 ± 20 pA at 1 min before application of KB-R7943, 51 ± 21 pA at 1 min after application, and 24 ± 7 pA at 3 min after application. Recovery after washout was slower and was incomplete because of time-dependent rundown, but the current returned to 247 ± 27 pA within 11 min after removal of KB-R7943. As would be expected, activation of p38 returns to control levels within 60 min after KB-R7943 washout from the cells (Fig. 3D). Importantly, up-regulation of 1831Ncx1 reporter gene expression in response to KB-R7943 is attenuated by co-expression of a dominant-negative p38 construct (Fig. 3E). These data support the conclusion that the up-regulation of Ncx1 by KB-R7943 treatment is mediated by activation of p38.
Activation of p38 by KB-R7943 Treatment Is Mediated by NCX1
KB-R7943 has been used extensively to investigate the molecular mechanisms of NCX1 activity and its role in heart disease. A potential caveat of these data is the sole reliance on KB-R7943 to implicate a NCX1-dependent mechanism. Unfortunately, KB-R7943 has been shown to partially inhibit L-type ICa, INa, the inward rectifying IK, and delayed rectifier IK at concentrations used for NCX1 inhibition (22). Therefore, KB-R7943-mediated activation of p38 and up-regulation of Ncx1 could be due to inhibition of any of these currents or by other unknown off-target effects. To determine whether activation of p38 and the resulting Ncx1 up-regulation in response to KB-R7943 treatment was specifically due to inhibition of the exchanger rather than an off-target effect of the drug, we examined the effects of KB-R7943 in Ncx1 knock-out mice. This allowed us to directly compare the activation of p38 in the presence or absence of exchanger expression. Ncx1 null mice have relatively normal heart morphogenesis but lack a heartbeat and only survive to 11.0 days postcoitum (31). Therefore, in these experiments, heart tubes were isolated from the embryos of Ncx1−/− and wild type Ncx1+/+ littermates at 9.5 days postcoitum, prior to the appearance of any morphological abnormalities. The tubes were treated with either vehicle alone (DMSO) or KB-R7943 for 20 min. The tubes were then homogenized in Laemmli buffer and analyzed by Western blot for p38 activation. As seen in Fig. 4A, heart tubes from wild type mice (Ncx1+/+) shows p38 activation in response to treatment with KB-R7943, whereas total p38 levels remained unchanged, just as was observed in adult cardiomyocytes. Alternatively, heart tubes from Ncx1−/− mice had no p38 activation in response to KB-R7943, even though the amount of total p38 was comparable with that of the Ncx1+/+ mice. This experiment directly demonstrates that KB-R7943 is acting via NCX1 to activate p38.
FIGURE 4.
Activation of p38 by KB-R7943 treatment is mediated by NCX1 complexing with p38. A, heart tubes were isolated from the embryos of Ncx1−/− mice and wild type Ncx1+/+ littermates at days postcoitum 9.5. Depending on the number of embryos available for each experiment, equal numbers of heart tubes were individually treated with either vehicle alone (DMSO) or KB-R for 20 min. The Ncx1−/− or Ncx1+/+ heart tubes from each treatment were combined for homogenization in Laemmli buffer and then analyzed by Western blot for p-p38. Left panel, Ncx1−/− or Ncx1+/+ heart tubes treated with vehicle or KB-R. Right panel, Ncx1−/− or Ncx1+/+ heart tubes treated with KB-R. B, isolated adult cardiomyocytes were pretreated with one of the following inhibitors: (i) PKC inhibitor bisindolylmaleimide (BIM; 2.5 μm); (ii) CaMKII inhibitor KN-93 (10 μm), (iii) PLC inhibitor U73122 (2 μm); or (iv) phosphatidylinositol 3-kinase inhibitor LY294002 (10 μm). 30 min after addition of the inhibitor, cells were treated with KB-R for 30 min, then lysed, and subjected to SDS-PAGE followed by Western blotting with anti p-p38 to determine the activation of p38. C, cell lysates from adult cardiomyocytes, treated with KB-R7943, or untreated (control), were immunoprecipitated (I.P.) with anti-p38 antibody or rabbit IgG as negative control. The resulting co-immunoprecipitates were analyzed by Western blotting with anti-NCX1 antibody. Similar data were obtained in three independent experiments.
KB-R7943 Treatment Does Not Activate p38 via Changes in [Ca2+]i
We next began to examine the possible mechanisms behind KB-R7943-mediated p38 activation. Inhibition of reverse-mode exchanger activity could possibly result in the activation of signaling pathways in one of two ways: 1) inhibition of NCX1 could affect cytosolic or localized [Ca2+]i, which activates a signaling cascade; or 2) inhibition of NCX1 activity might induce a change in exchanger conformation that could mediate activation of signaling pathways through direct interaction of the exchanger with an associated cofactor.
There are still uncertainties on how blocking NCX1 affects resting [Ca2+]i. One recent work demonstrated that resting [Ca2+]i was unaffected by treatment of adult canine cardiomyocytes with 1 μm SEA-0400 (38). However, a second work using mouse and pig cardiomyocytes (21) reported that 1 μm SEA-0400 treatment resulted in a slight increase in diastolic [Ca2+]i. In our feline cardiomyocytes, 10 μm KB-R7943 treatment had no apparent affect resting [Ca2+]i (data not shown). This did not eliminate the possibility of localized [Ca2+]i changes that might activate pathways mediating phosphorylation of p38. Therefore, we examined whether any of the well known signaling factors activated by changes in localized [Ca2+]i were activated by KB-R7943 treatment and whether they were required for p38 activation.
The calcium-sensing receptor has been shown to be an important downstream target of Ca2+ in the heart (39). In cardiomyocytes calcium-sensing receptor activates PLC, which in turn hydrolyzes the membrane phospholipid, phosphatidylinositol 4,5-bisphosphate, to generate diacylglycerol and inositol 1,4,5-triphosphate. Diacylglycerol and inositol 1,4,5-triphosphate then mediate the activation of PKC and intracellular Ca2+ mobilization, respectively, resulting in increased intracellular Ca2+ levels and activation of ERK1/2, PKC, and CaMKII (40). We found that inhibition of PKC with bisindolylmaleimide, inhibition of CaMKII with KN-93, inhibition of PLC with U-73122, or inhibition of phosphatidylinositol 3-kinase with LY294002 did not prevent activation of p38 with KB-R7943 treatment (Fig. 4B). In addition, calcium-sensing receptor activation results in rapid ERK1/2 phosphorylation. ERK1/2 is not activated between 1 and 15 min following KB-R7943 treatment (data not shown), and Fig. 3A reveals no ERK1/2 activation at later time points. Therefore, none of the classic pathways activated by changes in [Ca2+]i play a role in KB-R7943-stimulated p38 activation.
NCX1 Is in Complex with p38
All three benzyloxyphenyl NCX1 inhibitors, KB-R7943, SN-6, and SEA-0400, inhibit NCX1 activity in a [Na+]i-dependent manner (21, 41). The potency of each of these drugs is coupled to the rate of [Na+]i-dependent inactivation (I1), and these compounds may stabilize NCX1 molecules in the I1 confirmation or favor the rate of I1 formation (24, 37). Therefore, we examined the second possibility that NCX1 in the I1 conformation could initiate signaling through its direct interaction or cross-talk with transmembrane receptors or in association with a scaffold complex containing signaling factors. We began by testing whether p38 directly complexes with NCX1. In these experiments adult mice were treated twice daily with KB-R7943 (10 mg/kg) or vehicle. After 24 h, the mice were sacrificed and the hearts extracted and solubilized in 1% Triton X-100 for analysis. Ventricular lysate supernatant was immunoprecipitated with p38 antibody, and the resulting co-immunoprecipitates were analyzed by Western blot for NCX1. Equivalent levels of p38 were immunoprecipitated for each treatment. Importantly, NCX1 was efficiently co-immunoprecipitated with the anti-p38 antibody only in mice that were treated with KB-R7943. In contrast to observations at later time points, KB-R7943 treatment for only 24 h does not result in an increase in NCX1 protein (data not shown). Therefore, we can rule out an increase in NCX1 levels as being a factor for its co-immunoprecipitation with p38. Hence, inhibition of NCX1 with KB-R7943 results in the association p38 with NCX1.
DISCUSSION
Previous studies have demonstrated that NCX1 inhibitors can act as positive inotropic drugs for the treatment of ischemia-reperfusion injury and congestive heart failure (21, 23, 41, 42). The potential for modulation of NCX1 activity to correct the impaired contractile properties seen in diseased cardiomyocytes makes it an extremely attractive target for therapeutic intervention. However, these studies primarily focus on acute treatment with NCX1 inhibitors. Therefore, we endeavored to examine the effects that might result from chronic exposure to KB-R7943.
The results of our studies impart compelling insight into the regulation of NCX1 function and expression. We demonstrate for the first time that cardiac NCX1 expression is increased at both the transcriptional and protein levels in response to chronic inhibition of NCX1 activity with KB-R7943. The level of up-regulation is similar to what we have observed with pressure overload hypertrophy (35). Heterozygous NCX1 transgenic overexpression results in transcript levels manyfold above that of control levels, but NCX1 protein and activity is only 2-fold above nontransgenic level (43). Even the homozygous NCX1 overexpressors have only a 3-fold increase in NCX1 activity (44). Therefore, we would speculate that even with greatly extended KB-R7943 treatments, the up-regulation would plateau at or just above the levels we observed after 2 days of treatment.
Even with homozygous NCX1 overexpression, there is only a 3-fold increase in NCX1 activity (44). Although this finding was an unexpected result, it is not an unprecedented occurrence. Chronic inhibition of the Na+/H+ exchanger by the selective inhibitor, cariporide, results in an increase in the number and activity of functional exchangers in the cell (28). It has also been reported that chronic treatment of rat cardiomyocytes with the L-type channel inhibitor nifedipine also results in an increase in the number of channels present in cardiac membranes (27). Chronic administration of anti-arrhythmic drugs, which inhibit the fast Na+ channel, results in the up-regulation of Na+ channel expression in cardiomyocytes (45). Furthermore, it has long been understood that inhibition of β-adrenergic receptor activity leads to increases in the density of β-adrenergic receptors present in the cell (29). Interestingly, the withdrawal of β-blockers has been found to greatly increase the risk of unstable angina, myocardial infarction, and sudden death (46, 47).
Because NCX1 plays a crucial role in calcium handling in the cardiomyocyte, it is an adaptive response of the cell to attempt to counteract diminished exchanger activity by increasing exchanger expression. However, the findings presented here, showing that NCX1 levels reach a new set point with chronic inhibition, is an important consideration for the therapeutic modulation of NCX1 activity. Therefore, because the number of exchangers is increased by inhibition, the withdrawal of the therapy could theoretically make the heart even more susceptible to arrhythmia.
Which signal mediates Ncx1 up-regulation as a result of prolonged in vivo NCX1 inhibition? Treatment of adult cardiomyocytes with either KB-R7943 or SN-6 resulted in the rapid and prolonged activation of p38. We determined not only that up-regulation of Ncx1 expression by KB-R7943 is dependent on p38 activation, but we also demonstrated that the activation of p38 by KB-R7943 treatment is dependent on the inhibition of NCX1. Importantly, our studies utilizing heart tubes from Ncx1 null mice allowed us to prove that the absence of NCX1 prevented the activation of p38 in response to KB-R7943 treatment. What then mediates p38 activation? Even though inhibition of NCX1 activity might affect [Ca2+]i, which could be a possible mechanism for activation of signal transduction, there is no change in [Ca2+]i in resting (nonstimulated) cardiomyocytes with chronic KB-R7943 treatment. Although we did not eliminate the possibility of localized [Ca2+]i changes that might activate pathways mediating phosphorylation of p38, we clearly demonstrate that none of the well known signaling factors that would be expected to be activated by changes in localized [Ca2+]i were required for p38 activation.
Our data demonstrate that p38 directly complexes with NCX1 in the cardiomyocytes of mice treated with KB-R7943. We suggest that KB-R7943 and SN-6 may stabilize NCX1 molecules in the I1 confirmation or favor the rate of I1 formation (24, 37). NCX1 in the I1 conformation could initiate signaling by direct interaction or cross-talk with transmembrane receptors or in association with a scaffold complex containing signaling factors, resulting in p38 activation. The anti-p38 antibody used for the co-immunoprecipitation studies recognizes an epitope on the C terminus. It is interesting that we do not see NCX1 associated with p38 when we used the phospho-p38 antibody that recognizes an epitope around the Thr-180 and Tyr-182 region for immunoprecipitation. We speculate that either the phospho-p38 antibody may disrupt the NCX1-p38 complex or, once phosphorylated, p38 disassociates from the complex. The reciprocal co-immunoprecipitation of p38 with R3F1, the anti-NCX1 antibody, did not work. R3F1 recognizes two intracellular epitopes (amino acids 560–629 and 649–705) located on the large cytoplasmic loop (48). Again, we speculate that the binding of R3F1 to the large intracellular loop disrupts the interaction of p38 with NCX1.
Interestingly, components of the p38 pathway have been demonstrated to act as effectors of the hypertrophic response (49). p38 has been shown to mediate the up-regulation of A- and B-type natriuretic peptides and α-skeletal actin (50), the down-regulation of SERCA2 (51), and prolongation of decay of the contractile Ca2+ transient. Therefore, it appears that p38 activation may be a very important common mediator in the regulation of several gene products, including those that directly modulate calcium homeostasis. Importantly, we have demonstrated that p38 activation mediates Ncx1 up-regulation in response to α-adrenergic stimulation in adult cardiomyocytes. Furthermore, we show that the p38-stimulated up-regulation is mediated via the −80 CArG box on the Ncx1 promoter (36).
Although this is the first time NCX1 has been identified as an active participant in signal transduction processes in the cardiomyocyte, the potential for ion transporters to possess signaling capabilities is not an unprecedented finding. A relevant example of this is seen in the response of the Na+/K+-ATPase to ouabain (52, 53). The results of studies conducted in rat cardiomyocytes show that treatment of cells with ouabain at concentrations that partially inhibit Na+/K+-ATPase activity results in activation of multiple signal transduction pathways, including an increase in tyrosine phosphorylation and activation of the ERK/MAPK cascade. This effect is also seen when the Na+/K+-ATPase is inhibited by lowering extracellular K+ (52). Although the exact mechanism of Na+/K+-ATPase-mediated signal transduction is unclear, Ras, PKC, and a complex including Src and the epidermal growth factor receptor have been shown to be involved (54). Neurons are the best known example of a cell type where channel activity directly regulates the signaling mechanisms linking neuronal activity to changes in gene expression (55). Neuronal activity triggers the opening of L-type voltage-gated calcium channels, N-methyl-d-aspartic acid- or α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid-gated calcium channels, which in turn activate signaling pathways that initiate activity-dependent patterns of gene transcription. Many studies have begun to identify the specific signaling pathways linking specific channels on the synapse surface to the circuitry that translates stimuli into altered patterns of gene expression in the nuclei of the neuron (56).
In conclusion, our study demonstrates for the first time that, in the heart, NCX1 can act as a regulator of activity-dependent transcription. The data presented here reveals that the Na+-Ca2+ exchanger, in addition to transporting Ca2+ out of the cytosol, also participates in signal transduction through its membership in a signaling complex. In response to inhibition of NCX1 transport activity, p38 forms a complex with the exchanger, activating signal transduction cascades, which result in changes in Ncx1 gene expression. NCX1 is essential for maintaining calcium homeostasis in adult cardiomyocytes. Up-regulation of NCX1 during cardiac hypertrophy, ischemia-reperfusion, and failure can be considered a compensatory adaptation to improve contractile function. However, this compensation comes with various consequences, including limiting SR loading and an increased risk of arrhythmias (9). Because of these negative consequences, NCX1 inhibition could be a beneficial therapeutic strategy that has been proposed in ischemia-reperfusion, as well as in cardiac hypertrophy and failure (21, 23, 24, 41). However, the regulation of both NCX1 activity and expression is incompletely understood, and the effects of NCX1 targeted therapy especially in light of this study are currently quite unpredictable.
Acknowledgment
We thank Ben Addy for excellent technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 HL085098 (to S. J. C.) and Grants R01 HL066223 and P01 HL48788, Project 3 (to D. R. M.). This work was also supported by The Riley Children's Foundation, Indiana University Department of Pediatrics/ Cardiology.
- SR
- sarcoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- MAPK
- mitogen-activated protein kinase
- GFP
- green fluorescent protein
- RLU
- relative luciferase unit
- ANOVA
- analysis of variance
- PBS
- phosphate-buffered saline
- m.o.i.
- multiplicity of infection
- PKC
- protein kinase C
- CaMKII
- Ca2+/ calmodulin-dependent protein kinase
- PLC
- phospholipase C.
REFERENCES
- 1.Bers D. M. (2002) Nature 415, 198–205 [DOI] [PubMed] [Google Scholar]
- 2.Bassani R. A., Bers D. M. (1994) J. Mol. Cell. Cardiol. 26, 1335–1347 [DOI] [PubMed] [Google Scholar]
- 3.Bers D. M., Bridge J. H. (1989) Circ. Res. 65, 334–342 [DOI] [PubMed] [Google Scholar]
- 4.Bers D. M., Lederer W. J., Berlin J. R. (1990) Am. J. Physiol. 258, C944–C954 [DOI] [PubMed] [Google Scholar]
- 5.Kent R. L., Rozich J. D., McCollam P. L., McDermott D. E., Thacker U. F., Menick D. R., McDermott P. J., Cooper G., 4th (1993) Am. J. Physiol. 265, H1024–H1029 [DOI] [PubMed] [Google Scholar]
- 6.Menick D. R., Barnes K. V., Thacker U. F., Dawson M. M., McDermott D. E., Rozich J. D., Kent R. L., Cooper G., 4th (1996) Ann. N.Y. Acad. Sci. 779, 489–501 [DOI] [PubMed] [Google Scholar]
- 7.Studer R., Reinecke H., Vetter R., Holtz J., Drexler H. (1997) Basic Res. Cardiol. 92, 53–58 [DOI] [PubMed] [Google Scholar]
- 8.Hobai I. A., O'Rourke B. (2000) Circ. Res. 87, 690–698 [DOI] [PubMed] [Google Scholar]
- 9.Pogwizd S. M., Schlotthauer K., Li L., Yuan W., Bers D. M. (2001) Circ. Res. 88, 1159–1167 [DOI] [PubMed] [Google Scholar]
- 10.Sipido K. R., Volders P. G., Vos M. A., Verdonck F. (2002) Cardiovasc. Res. 53, 782–805 [DOI] [PubMed] [Google Scholar]
- 11.Ahmmed G. U., Dong P. H., Song G., Ball N. A., Xu Y., Walsh R. A., Chiamvimonvat N. (2000) Circ. Res. 86, 558–570 [DOI] [PubMed] [Google Scholar]
- 12.Litwin S. E., Bridge J. H. (1997) Circ. Res. 81, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G., Reinecke H., Studer R., Meyer M., Pieske B., Holtz J., Holubarsch C., Posival H., Just H., Drexler H. (1994) Circ. Res. 75, 434–442 [DOI] [PubMed] [Google Scholar]
- 14.Hasenfuss G., Meyer M., Schillinger W., Preuss M., Pieske B., Just H. (1997) Basic Res. Cardiol. 92, 87–93 [DOI] [PubMed] [Google Scholar]
- 15.Hasenfuss G., Reinecke H., Studer R., Pieske B., Meyer M., Drexler H., Just H. (1996) Basic Res. Cardiol 91, 17–22 [DOI] [PubMed] [Google Scholar]
- 16.Studer R., Reinecke H., Bilger J., Eschenhagen T., Böhm M., Hasenfuss G., Just H., Holtz J., Drexler H. (1994) Circ. Res. 75, 443–453 [DOI] [PubMed] [Google Scholar]
- 17.Hasenfuss G., Schillinger W., Lehnart S. E., Preuss M., Pieske B., Maier L. S., Prestle J., Minami K., Just H. (1999) Circulation 99, 641–648 [DOI] [PubMed] [Google Scholar]
- 18.Schillinger W., Janssen P. M., Emami S., Henderson S. A., Ross R. S., Teucher N., Zeitz O., Philipson K. D., Prestle J., Hasenfuss G. (2000) Circ. Res. 87, 581–587 [DOI] [PubMed] [Google Scholar]
- 19.Ranu H. K., Terracciano C. M., Davia K., Bernobich E., Chaudhri B., Robinson S. E., Bin Kang Z., Hajjar R. J., MacLeod K. T., Harding S. E. (2002) J. Mol. Cell. Cardiol. 34, 389–400 [DOI] [PubMed] [Google Scholar]
- 20.Pogwizd S. M., Qi M., Yuan W., Samarel A. M., Bers D. M. (1999) Circ. Res. 85, 1009–1019 [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir S., Bito V., Holemans P., Vinet L., Mercadier J. J., Varro A., Sipido K. R. (2008) Circ. Res. 102, 1398–1405 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H., Nishimaru K., Aikawa T., Hirayama W., Tanaka Y., Shigenobu K. (2002) Br. J. Pharmacol. 135, 1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobai I. A., Maack C., O'Rourke B. (2004) Circ. Res. 95, 292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C., Dhalla N. S., Hryshko L. V. (2005) Can. J. Cardiol. 21, 509–516 [PubMed] [Google Scholar]
- 25.Satoh H., Ginsburg K. S., Qing K., Terada H., Hayashi H., Bers D. M. (2000) Circulation 101, 1441–1446 [DOI] [PubMed] [Google Scholar]
- 26.Nagy Z. A., Virág L., Tóth A., Biliczki P., Acsai K., Bányász T., Nánási P., Papp J. G., Varró A. (2004) Br. J. Pharmacol. 143, 827–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan P. E., Aiello E. A., Chiappe de Cingolani G. E., Mattiazzi A. R., Cingolani H. E. (1999) J. Mol. Cell. Cardiol. 31, 1873–1883 [DOI] [PubMed] [Google Scholar]
- 28.Camilión de Hurtado M. C., Ennis I. L., Pérez N. G., Chiappe de Cingolani G. E., Morgan P., Cingolani H. E. (2002) J. Mol. Cell. Cardiol. 34, 1539–1547 [DOI] [PubMed] [Google Scholar]
- 29.Aarons R. D., Nies A. S., Gal J., Hegstrand L. R., Molinoff P. B. (1980) J. Clin. Invest. 65, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent R. L., Mann D. L., Urabe Y., Hisano R., Hewett K. W., Loughnane M., Cooper G., 4th (1989) Am. J. Physiol. 257, H1717–H1727 [DOI] [PubMed] [Google Scholar]
- 31.Koushik S. V., Wang J., Rogers R., Moskophidis D., Lambert N. A., Creazzo T. L., Conway S. J. (2001) FASEB J. 15, 1209–1211 [DOI] [PubMed] [Google Scholar]
- 32.Cheng G., Hagen T. P., Dawson M. L., Barnes K. V., Menick D. R. (1999) J. Biol. Chem. 274, 12819–12826 [DOI] [PubMed] [Google Scholar]
- 33.Xu Q., Yu L., Liu L., Cheung C. F., Li X., Yee S. P., Yang X. J., Wu Z. (2002) Mol. Biol. Cell 13, 1940–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L., Renaud L., Müller J. G., Baicu C. F., Bonnema D. D., Zhou H., Kappler C. S., Kubalak S. W., Zile M. R., Conway S. J., Menick D. R. (2006) J. Biol. Chem. 281, 34430–34440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller J. G., Isomatsu Y., Koushik S. V., O'Quinn M., Xu L., Kappler C. S., Hapke E., Zile M. R., Conway S. J., Menick D. R. (2002) Circ. Res. 90, 158–164 [DOI] [PubMed] [Google Scholar]
- 36.Xu L., Kappler C. S., Menick D. R. (2005) J. Mol. Cell. Cardiol. 38, 735–743 [DOI] [PubMed] [Google Scholar]
- 37.Niu C. F., Watanabe Y., Ono K., Iwamoto T., Yamashita K., Satoh H., Urushida T., Hayashi H., Kimura J. (2007) Eur. J. Pharmacol. 573, 161–169 [DOI] [PubMed] [Google Scholar]
- 38.Birinyi P., Tóth A., Jóna I., Acsai K., Almássy J., Nagy N., Prorok J., Gherasim I., Papp Z., Hertelendi Z., Szentandrássy N., Bányász T., Fülöp F., Papp J. G., Varró A., Nánási P. P., Magyar J. (2008) Cardiovasc. Res. 78, 476–484 [DOI] [PubMed] [Google Scholar]
- 39.Smajilovic S., Tfelt-Hansen J. (2007) Cardiovasc. Res. 75, 457–467 [DOI] [PubMed] [Google Scholar]
- 40.Tfelt-Hansen J., Hansen J. L., Smajilovic S., Terwilliger E. F., Haunso S., Sheikh S. P. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H1165–H1171 [DOI] [PubMed] [Google Scholar]
- 41.O'Rourke B. (2008) Circ. Res. 102, 1301–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDonald A. C., Howlett S. E. (2008) Eur. J. Pharmacol. 580, 214–223 [DOI] [PubMed] [Google Scholar]
- 43.Adachi-Akahane S., Lu L., Li Z., Frank J. S., Philipson K. D., Morad M. (1997) J. Gen. Physiol. 109, 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuter H., Han T., Motter C., Philipson K. D., Goldhaber J. I. (2004) J. Physiol. 554, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang J. X., Li Y., Leaf A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 2724–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller R. R., Olson H. G., Amsterdam E. A., Mason D. T. (1975) N. Engl. J. Med. 293, 416–418 [DOI] [PubMed] [Google Scholar]
- 47.Diaz R. G., Somberg J. C., Freeman E., Levitt B. (1973) Lancet 1, 1068. [DOI] [PubMed] [Google Scholar]
- 48.Porzig H., Li Z., Nicoll D. A., Philipson K. D. (1993) Am. J. Physiol. 265, C748–C756 [DOI] [PubMed] [Google Scholar]
- 49.Liang Q., Molkentin J. D. (2003) J. Mol. Cell. Cardiol. 35, 1385–1394 [DOI] [PubMed] [Google Scholar]
- 50.Zechner D., Thuerauf D. J., Hanford D. S., McDonough P. M., Glembotski C. C. (1997) J. Cell Biol. 139, 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews C., Ho P. D., Dillmann W. H., Glembotski C. C., McDonough P. M. (2003) Cardiovasc. Res. 59, 46–56 [DOI] [PubMed] [Google Scholar]
- 52.Haas M., Askari A., Xie Z. (2000) J. Biol. Chem. 275, 27832–27837 [DOI] [PubMed] [Google Scholar]
- 53.Tian J., Gong X., Xie Z. (2001) Am. J. Physiol. Heart Circ. Physiol. 281, H1899–H1907 [DOI] [PubMed] [Google Scholar]
- 54.Mohammadi K., Kometiani P., Xie Z., Askari A. (2001) J. Biol. Chem. 276, 42050–42056 [DOI] [PubMed] [Google Scholar]
- 55.Cohen S., Greenberg M. E. (2008) Annu. Rev. Cell Dev. Biol. 24, 183–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flavell S. W., Greenberg M. E. (2008) Annu. Rev. Neurosci. 31, 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]