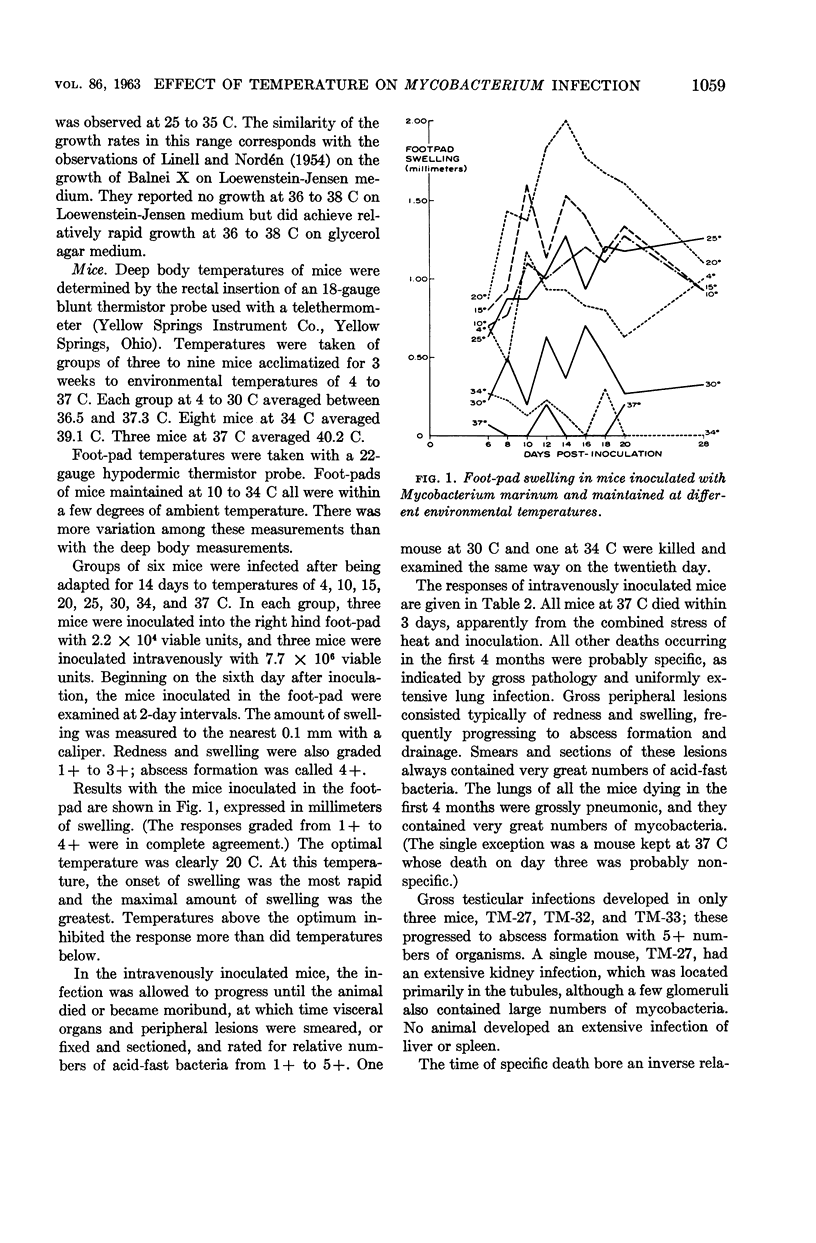
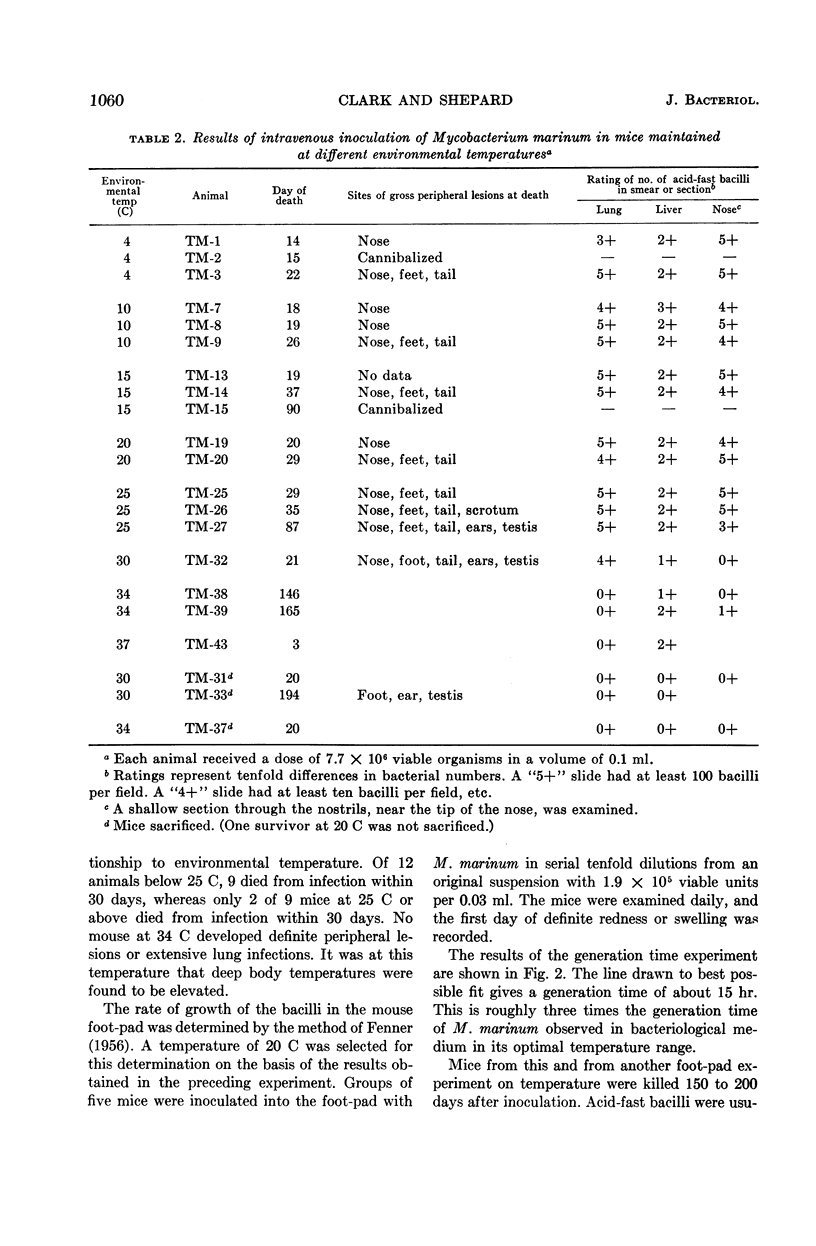
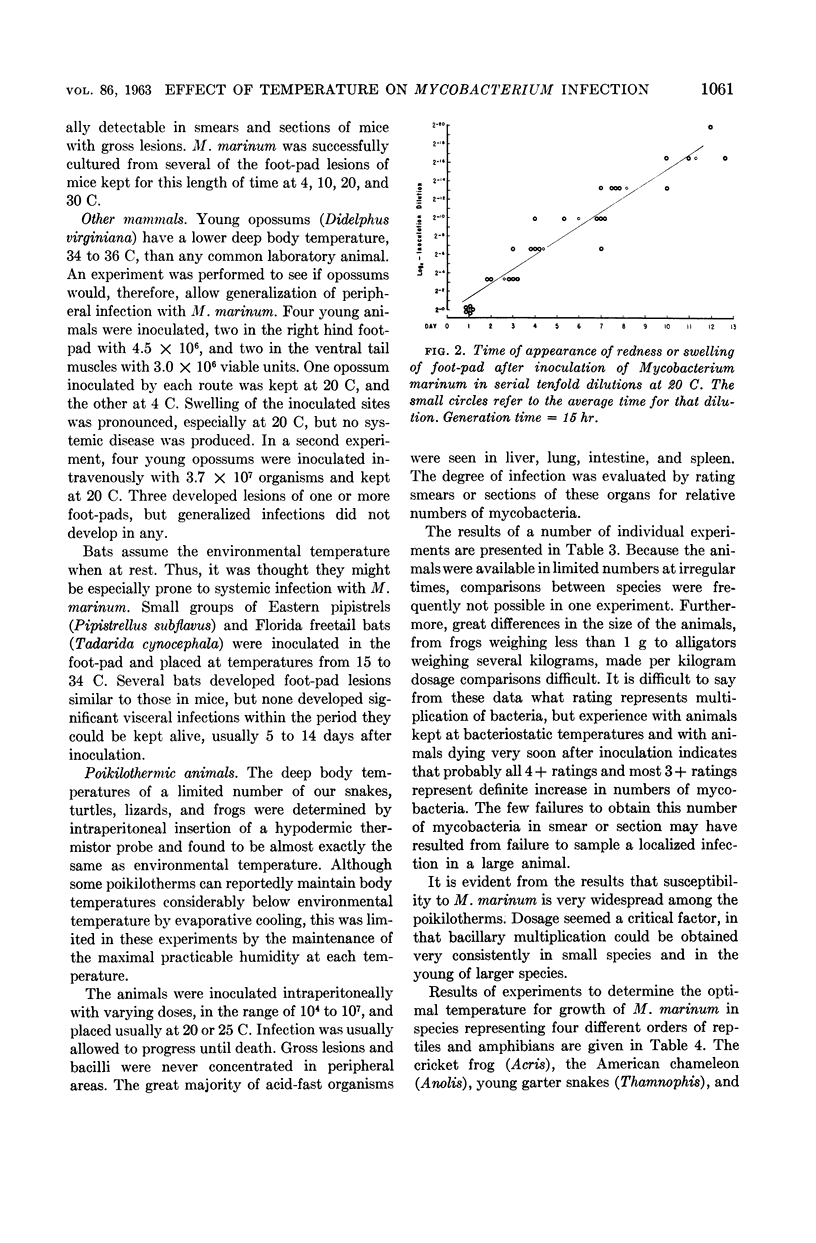
Abstract
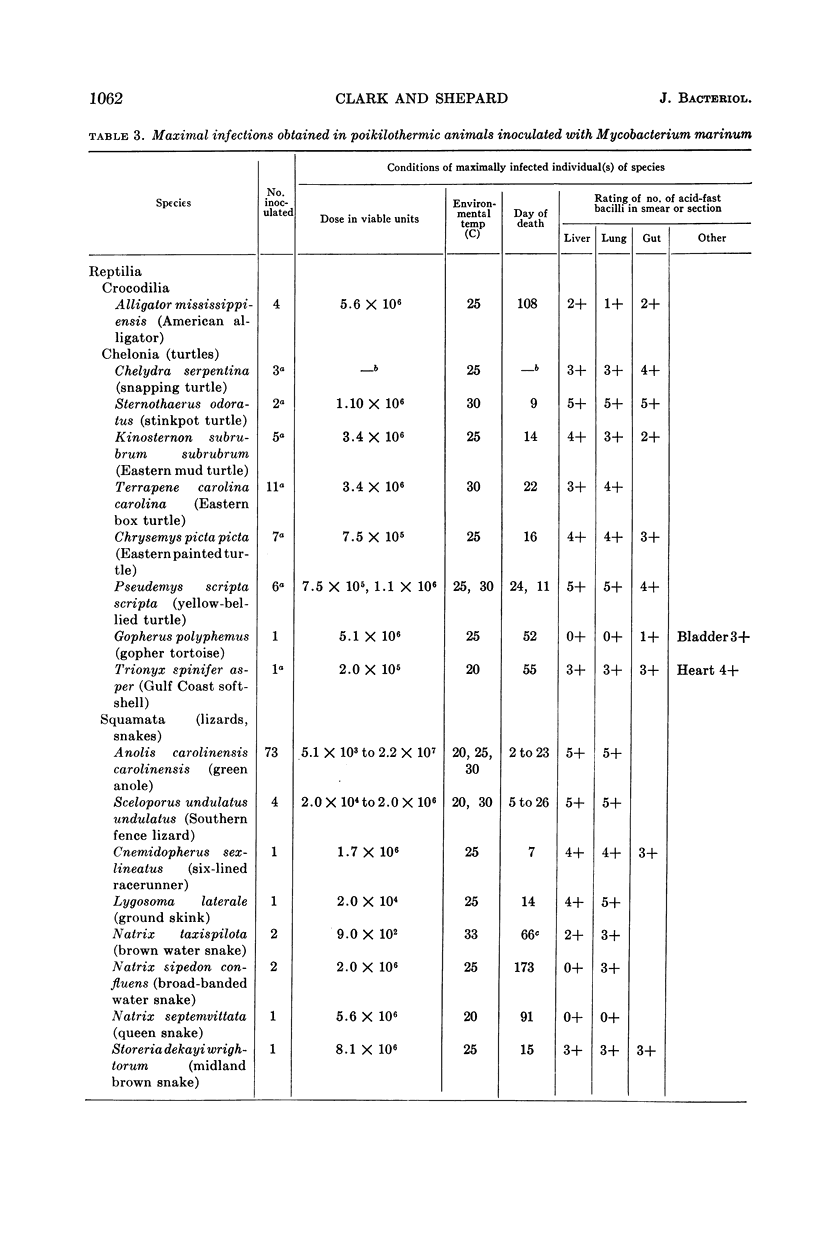
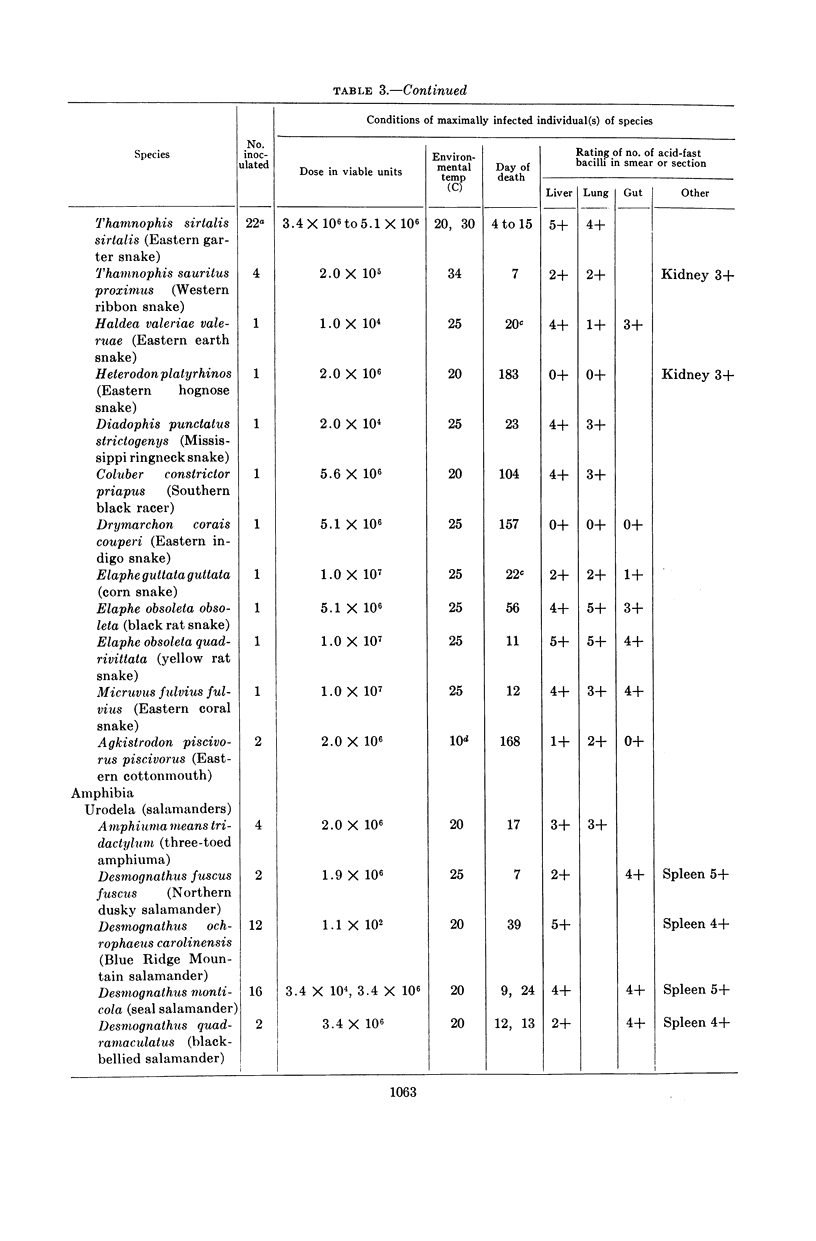
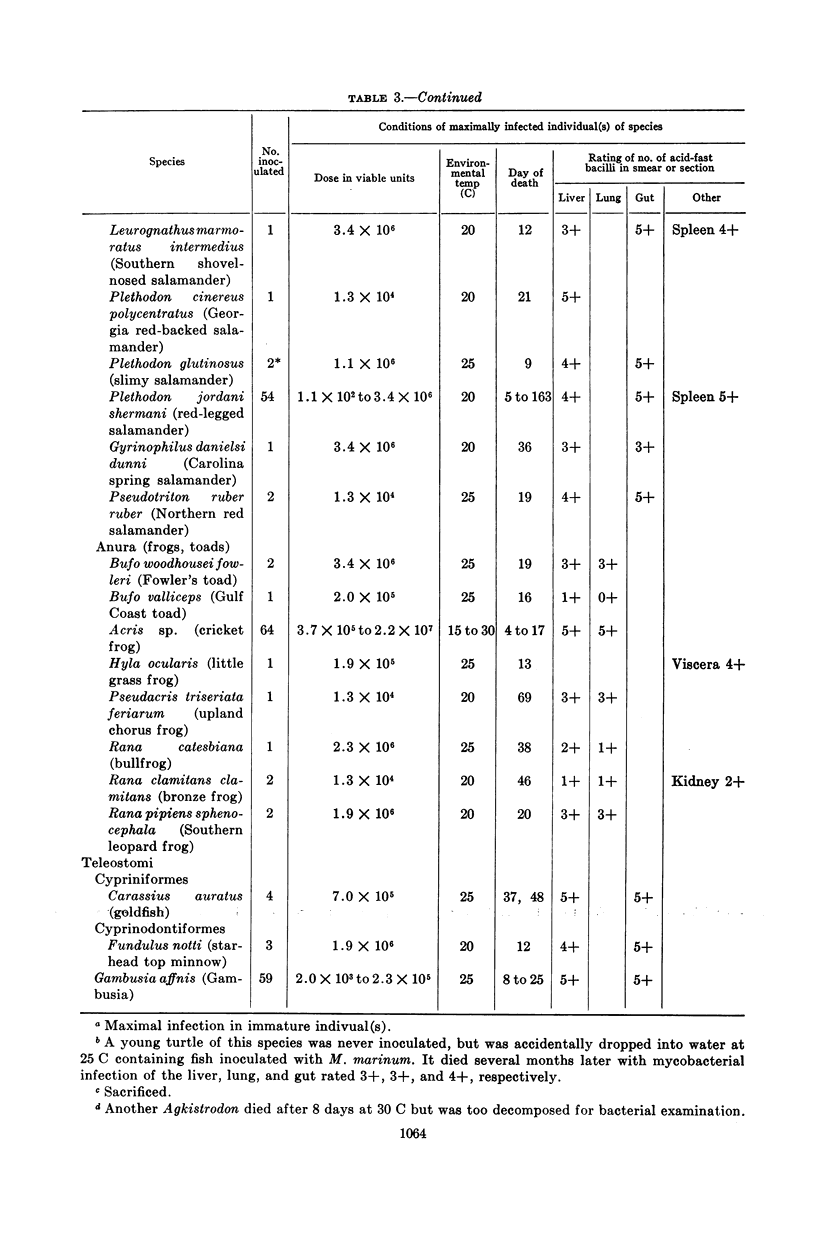
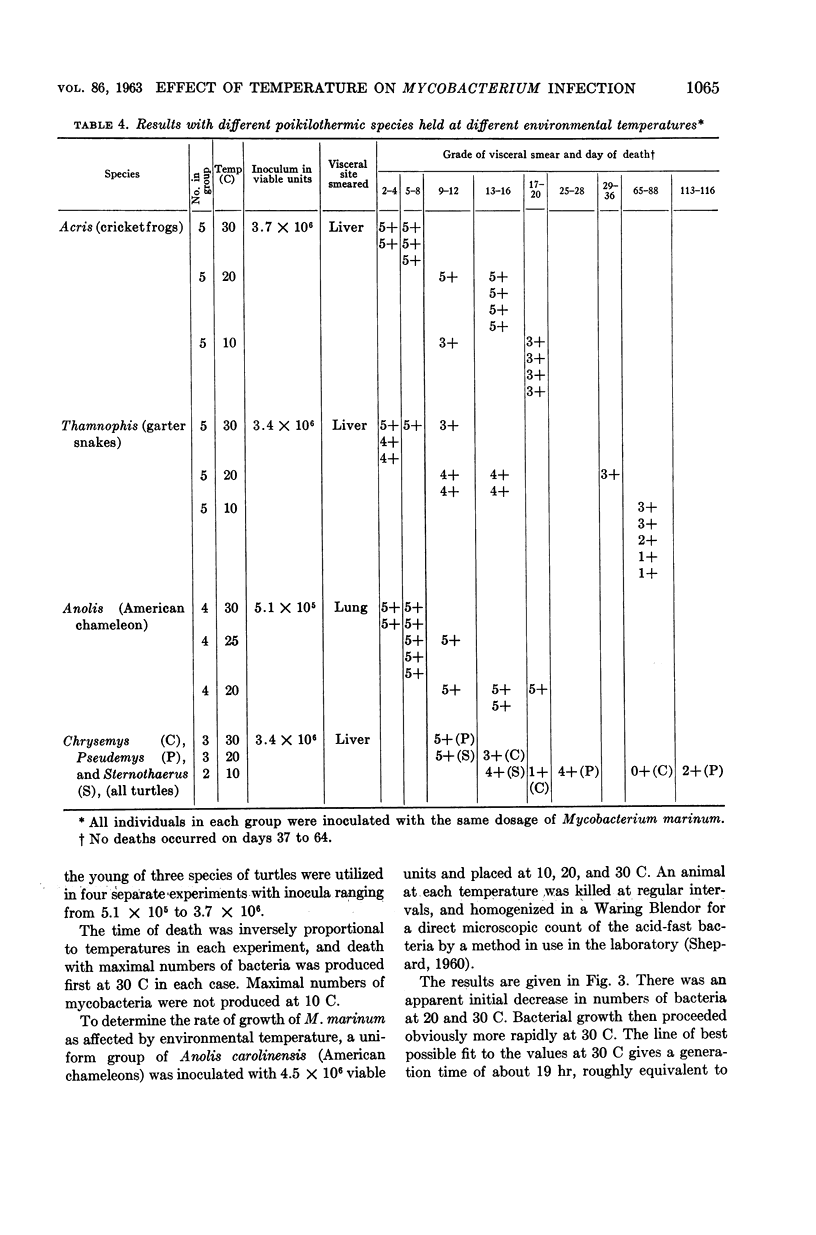
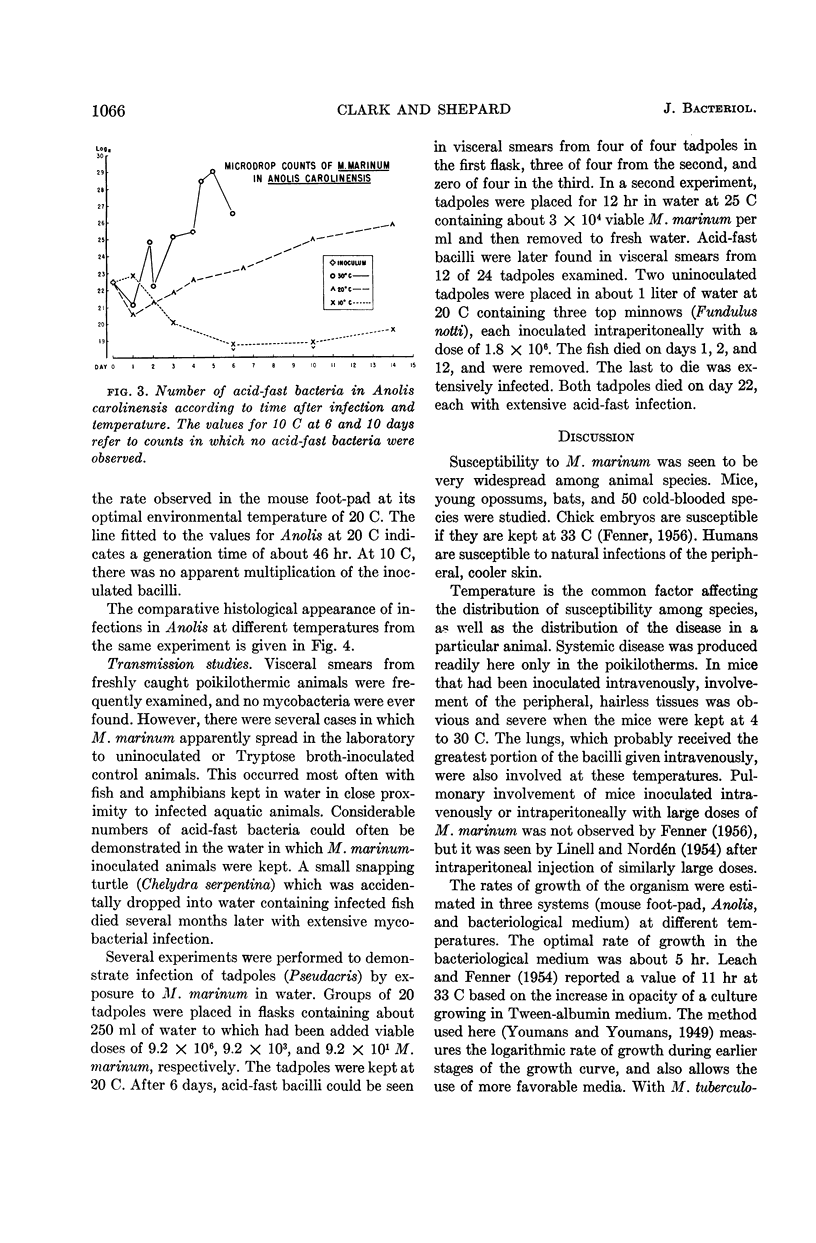
Clark, H Fred (Communicable Disease Center, Atlanta, Ga.), and Charles C. Shepard. Effect of environmental temperatures on infection with Mycobacterium marinum (Balnei) of mice and a number of poikilothermic species. J. Bacteriol. 86:1057–1069. 1963.—An exploration was made of the effect of environmental temperature on infections with Mycobacterium marinum of mice, young opossums, and bats, and of 50 species of poikilothermic animals. In artificial medium (7H9 broth) M. marinum grew most rapidly from 25 to 35 C, with generation times of 4 to 6 hr. At 37 C, the generation time was 14 hr; at 20 C, 20 hr; and, at 15 C and lower, little growth was observed. In mice, deep body temperatures were found to be 36.5 to 37.3 C at environmental temperatures of 4 to 30 C. At an environmental temperature of 34 C, they averaged 39.1 C; at 37 C they averaged 40.2 C. Foot-pad temperatures were within a few degrees of ambient temperatures from 10 to 34 C. In mouse foot-pad infections, the optimal environmental temperature for infection was 20 C, and the generation time of the infecting bacilli at this environmental temperature was about 15 hr. Intravenously inoculated mice developed peripheral infections of nose, feet, and tail at environmental temperatures of 4 to 30 C. At these temperatures, they had severe pneumonic involvement, and the mice at lower temperatures tended to succumb most rapidly to systemic infection. At 34 C, the intravenously infected mice did not develop peripheral infections and there was no pulmonary involvement. Young opossums, whose deep body temperatures are only 34 to 36 C, were inoculated in the foot-pad and intravenously. Foot-pad infection developed without systemic involvement. Bats, which assume environmental temperature when at rest, were inoculated in the foot-pad. Foot-pad infections were observed but no systemic disease. The bats could be maintained for only short periods, however. Poikilothermic animals were studied. Deep body temperatures were found to be nearly identical with ambient temperature. A total of 50 species of reptiles, amphibians, and fish were infected intraperitoneally in a number of experiments, as animals were available. Susceptibility to M. marinum was found throughout these species. There was no tendency to peripheral involvement. In experiments to determine the optimal environmental temperature for infection, cricket frogs (Acris), American chameleons (Anolis), young garter snakes (Thamnophis), and the young of three species of turtles were inoculated intraperitoneally. The optimal temperature for infection was found to be 30 C in each case, and infections at 20 C were definitely slower. The generation time of M. marinum in American chameleons at 30 C was about 19 hr; at 20 C, it was about 46 hr; and, at 10 C, the bacilli did not apparently multiply. Transmission studies revealed instances where infected animals shed M. marinum into the waters in which they were kept, and where animals became infected from water containing M. marinum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FENNER F. The pathogenic behavior of Mycobacterium ulcerans and Mycobacterium balnei in the mouse and the developing chick embryo. Am Rev Tuberc. 1956 May;73(5):650–673. doi: 10.1164/artpd.1956.73.5.650. [DOI] [PubMed] [Google Scholar]
- LEACH R. H., FENNER F. Studies on Mycobacterium ulcerans and Mycobacterium balnei. III. Growth in the semi-synthetic culture media of Dubos and drug sensitivity in vitro and in vivo. Aust J Exp Biol Med Sci. 1954 Dec;32(6):835–852. [PubMed] [Google Scholar]
- LINELL F., NORDEN A. Mycobacterium balnei, a new acid-fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc Scand Suppl. 1954;33:1–84. [PubMed] [Google Scholar]
- MOLLOHAN C. S., ROMER M. S. Public health significance of swimming pool granuloma. Am J Public Health Nations Health. 1961 Jun;51:883–891. doi: 10.2105/ajph.51.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. J Exp Med. 1957 Jan 1;105(1):39–48. doi: 10.1084/jem.105.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. A METHOD FOR THE DETERMINATION OF THE RATE OF GROWTH OF TUBERCLE BACILLI BY THE USE OF SMALL INOCULA. J Bacteriol. 1949 Aug;58(2):247–255. doi: 10.1128/jb.58.2.247-255.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZETTERGRAN L., ZETTERBERG B., MALMGREN B., KARTH B. Nya rön beträffande simhallssjukdomen (mycobacteriosis balnearea). Sven Lakartidn. 1952 Nov 28;49(48):2936–2948. [PubMed] [Google Scholar]