Abstract
Collapsin response mediator protein 2 (CRMP2) is an intracellular protein that mediates signaling of Semaphorin3A (Sema3A), a repulsive axon guidance molecule. Fyn, a Src-type tyrosine kinase, is involved in the Sema3A signaling. However, the relationship between CRMP2 and Fyn in this signaling pathway is still unknown. In our research, we demonstrated that Fyn phosphorylated CRMP2 at Tyr32 residues in HEK293T cells. Immunohistochemical analysis using a phospho-specific antibody at Tyr32 of CRMP showed that Tyr32-phosphorylated CRMP was abundant in the nervous system, including dorsal root ganglion neurons, the molecular and Purkinje cell layer of adult cerebellum, and hippocampal fimbria. Overexpression of a nonphosphorylated mutant (Tyr32 to Phe32) of CRMP2 in dorsal root ganglion neurons interfered with Sema3A-induced growth cone collapse response. These results suggest that Fyn-dependent phosphorylation of CRMP2 at Tyr32 is involved in Sema3A signaling.
Collapsin response mediator proteins (CRMPs)4 have been identified as intracellular proteins that mediate Semaphorin3A (Sema3A) signaling in the nervous system (1). CRMP2 is one of the five members of the CRMP family. CRMPs also mediate signal transduction of NT3, Ephrin, and Reelin (2–4). CRMPs interact with several intracellular molecules, including tubulin, Numb, kinesin1, and Sra1 (5–8). CRMPs are involved in axon guidance, axonal elongation, cell migration, synapse maturation, and the generation of neuronal polarity (1, 2, 4, 5).
CRMP family proteins are known to be the major phosphoproteins in the developing brain (1, 9). CRMP2 is phosphorylated by several Ser/Thr kinases, such as Rho kinase, cyclin-dependent kinase 5 (Cdk5), and glycogen synthase kinase 3β (GSK3β) (2, 10–13). The phosphorylation sites of CRMP2 by these kinases are clustered in the C terminus and have already been identified. Rho kinase phosphorylates CRMP2 at Thr555 (10). Cdk5 phosphorylates CRMP2 at Ser522, and this phosphorylation is essential for sequential phosphorylations by GSK3β at Ser518, Thr514, and Thr509 (2, 11–13). These phosphorylations disrupt the interaction of CRMP2 with tubulin or Numb (2, 3, 13). The sequential phosphorylation of CRMP2 by Cdk5 and GSK3β is an essential step in Sema3A signaling (11, 13). Furthermore, the neurofibrillary tangles in the brains of people with Alzheimer disease contain hyperphosphorylated CRMP2 at Thr509, Ser518, and Ser522 (14, 15).
CRMPs are also substrates of several tyrosine kinases. The phosphorylation of CRMP2 by Fes/Fps and Fer has been shown to be involved in Sema3A signaling (16, 17). Phosphorylation of CRMP2 at Tyr479 by a Src family tyrosine kinase Yes regulates CXCL12-induced T lymphocyte migration (18). We reported previously that Fyn is involved in Sema3A signaling (19). Fyn associates with PlexinA2, one of the components of the Sema3A receptor complex. Fyn also activates Cdk5 through the phosphorylation at Tyr15 of Cdk5 (19). In dorsal root ganglion (DRG) neurons from fyn-deficient mice, Sema3A-induced growth cone collapse response is attenuated compared with control mice (19). Furthermore, we recently found that Fyn phosphorylates CRMP1 and that this phosphorylation is involved in Reelin signaling (4). Although it has been shown that CRMP2 is involved in Sema3A signaling (1, 11, 13), the relationship between Fyn and CRMP2 in Sema3A signaling and the tyrosine phosphorylation site(s) of CRMPs remain unknown.
Here, we show that Fyn phosphorylates CRMP2 at Tyr32. Using a phospho-specific antibody against Tyr32, we determined that the residue is phosphorylated in vivo. A nonphosphorylated mutant CRMP2Y32F inhibits Sema3A-induced growth cone collapse. These results indicate that tyrosine phosphorylation by Fyn at Tyr32 is involved in Sema3A signaling.
EXPERIMENTAL PROCEDURES
Antibodies and Chemicals
Antibodies used were: anti-Myc antibody (9E10, Sigma), anti-phosphotyrosine antibodies (PY99, Santa Cruz Biotechnology, and 4G10, Cell Signaling Technology), anti-Fyn antibody (3) (Fyn, Santa Cruz Biotechnology), anti-GFP antibody (B2, Santa Cruz Biotechnology), anti-NeuN (Chemicon), anti-Tuj1 (COVANCE), anti-calbindin-D-28K antibody (Sigma), Alexa Fluor 488-labeled goat anti-rabbit IgG antibody and Alex Fluor 568-labeled goat anti-mouse IgG1 and IgG2a antibodies (Invitrogen). Anti-CRMP2 monoclonal antibody (C4G) was kindly provided by Dr. Yasuo Ihara (Doshisha University). Anti-pCRMP (Y32), the rabbit polyclonal antibody against CRMP2 phosphorylated at Tyr32, was raised against synthetic phosphopeptide (CNDDQSFpYADIYME: amino acids 26–38 plus cysteine for conjugation). The antiserum and preimmune serum were purified with protein A beads. The purified preimmune serum was used as negative control for antiserum. TO-PRO-3 iodide (Invitrogen) was used for nuclei staining.
Plasmid Construction
Rat CRMP2 mutants were constructed by PCR-based mutagenesis. Each mutant was generated with two primers as follows: CRMP2Y32F (5′-atctacatggaagatgggttgat-3′ and 5′-atctgcaaagaaggactggtcatcattcac-3′), CRMP2Y36F (5′-atcttcatggaagatgggttgatcaag-3′ and 5′-atctgcatagaaggactggtcatcattcac-3′), CRMP2Y182F (5′-tcttcgaagtactgagcgtgatccg-3′ and 5′-acttcgaagatctgggaatccgtcag-3′), and CRMP2Y479F (5′-gactggctgagctgaggggggtccct-3′ and 5′-gactccttgccttgatgcgtttgaacacaaagtcagggaa-3′). The mutated codons (Tyr to Phe) are bold. The underlines show a new generated restriction site to distinguish the mutants from wild-type (wt) (Y32F and Y36F:EcoRV, Y182F:Csp45I, and Y479F:SalI). PCR was performed using Myc-tagged CRMP2 as the template DNA and 5′-phosphorylated primers. After finishing the reaction, the template DNA was restricted by DpnI. The PCR products were self-ligated. In the Y182F mutant, the DpnI-digested PCR fragment was restricted by Csp45I and self-ligated. CRMP2Y32F/Y36F was generated using CRMP2Y36F with the CRMP2Y32F primer set. CRMP2Y32F/Y182F was generated using the CRMP2Y182F mutant as the template and the Y32F primer set. CRMP2 deletion mutants and N-terminal GST fusion vectors of CRMP2wt and the Y32F mutant were also constructed by PCR. GFP-Fes expression vector was kindly provided by Dr. Yanagi (Tokyo University of Pharmacy and Life Science).
Cell Culture and Immunoprecipitation
HEK293T cells were seeded at 5 × 105 cells/6-cm dish. After 2 days, the cells were transfected with 1 μg of expression vectors. After 1–2 days of incubation, the cells were lysed in Nonidet P-40 buffer (20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% Nonidet P-40, 1 mm EDTA, 50 mm NaF, 20 mm sodium pyrophosphate, 1 mm Na3VO4, 50 μm p-amidinophenylmethanesulfonyl fluoride, 0.1 unit/ml aprotinin, and 50 μm leupeptin). The lysates were incubated with anti-Myc antibody for 2 h on ice and then incubated with protein G beads for 2 h at 4 °C. After washing three times with the Nonidet P-40 buffer, the samples were analyzed by immunoblotting with various antibodies.
In Vitro Kinase Assay of Fyn
GST fusion proteins (Fyn and N-terminal mutants of CRMP2wt and CRMP2Y32F) were expressed in the Escherichia coli BL21 strain and purified. An 18-μl (5-μg) sample of CRMP2wt or Y32F N-terminal fragment and 2 μl (1 μg) of Fyn were mixed with 10 μl of 4× reaction buffer (100 mm HEPES-NaOH (pH 7.2), 125 mm MgCl2, 25 mm MnCl2, 2 mm EGTA, 0.25 mm Na3VO4, 2 mm dithiothreitol). The kinase reaction was initiated by the addition of 10 μl of an ATP mixture (75 mm MnCl2, 0.5 mm ATP). After incubation for 1 h at 30 °C, the reaction was stopped by the addition of SDS-PAGE sample buffer. The proteins were resolved by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibody (4G10) (1/2500) or anti-pCRMP (Y32) antibody (1/1000).
Sema3A-induced Phosphorylation of CRMP2 in COS-7 Cells
COS-7 cells were seeded at 1 × 106 cells/10-cm dish. The next day, the cells were transfected with Neuropilin1 (NRP1), PlexinA2, Fyn, and CRMP2-Myc, incubated for 4 h at 37 °C, and then replated at 5 × 105 cells per 10-cm dish. After 24 h, the transfected cells were serum-starved for 4 h. The cells were lysed in the Nonidet P-40 buffer at the indicated time after the application of 3 nm Sema3A. The lysates were resolved by SDS-PAGE and immunoblotted with anti-pCRMP (Y32) antibody (1/1000). The same membrane was reblotted with anti-Myc antibody.
Recombinant Herpes Simplex Virus Preparations, Infection, and Growth Cone Collapse Assay
Recombinant herpes simplex virus preparations and infections of chick embryonic day 7 (E7) DRG explants were performed as described previously (19). Growth cone collapse assays using chick DRGs were performed with purified recombinant chick Sema3A (collapsin-His6) as described previously (19).
Immunohistochemistry
The E13.5 C57BL6 mouse embryos were fixed with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) for 24 h at 4 °C. In E18.5 and adult mice, we performed perfusion fixation. After 24 h, the tissues were replaced in a 20% sucrose solution in phosphate-buffered saline for 24 h and thereafter in OCT compound. Sections were cut with a cryostat (16 μm). The sections were permeabilized by 0.1% Triton X-100 in phosphate-buffered saline and blocked by 0.1% Triton X-100 and 2% goat serum in phosphate-buffered saline. The anti-pCRMP (Y32) antibody (1/200) and preimmune control serum (1/200) were used with anti-NeuN (1/200), anti-Tuj1 (1/500), or anti-calbindin (1/500) antibody. Then, Alexa Fluor 488-labeled goat anti rabbit IgG and Alexa Fluor 568-labeled goat anti-mouse IgG1 or IgG2a antibody (1/1000) was used as a second antibody. Nuclei were stained by TO-PRO-3. Finally, they were analyzed using Leica confocal microscope SP5.
RESULTS
Fyn Phosphorylates CRMP Family Proteins in HEK293T Cells
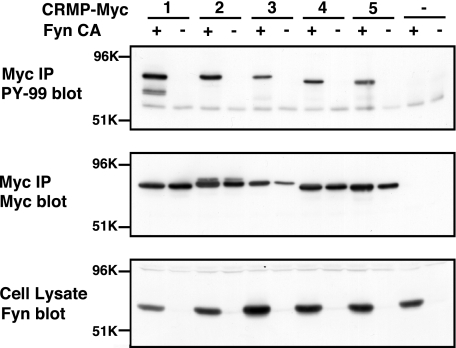
We reported previously that CRMP1 was phosphorylated by Fyn and this phosphorylation was involved in Reelin signaling (4). We first examined whether other CRMP family members are phosphorylated by Fyn. Each Myc-tagged CRMP family member proteins (CRMP1–5) was expressed with or without constitutively active Fyn (FynCA) in HEK293T cells and immunoprecipitated with anti-Myc antibody. The immunoprecipitates were immunoblotted with anti-phosphotyrosine antibody. All CRMP family members were phosphorylated by Fyn in HEK293T cells (Fig. 1, top). The tyrosine-phosphorylated CRMPs did not exhibit mobility shift on immunoblotting (Fig. 1, middle).
FIGURE 1.
CRMP family proteins are phosphorylated by Fyn in HEK293T cells. Each CRMP family member was transfected into HEK293T cells with or without FynCA. Myc-epitope tagged CRMPs were immunoprecipitated (IP) with anti-Myc antibody and immunoblotted with anti-phosphotyrosine antibody (top). The same membrane was reprobed by anti-Myc antibody (middle). The expression of FynCA in the cell lysates was confirmed by the immunoblotting with anti-Fyn antibody (bottom). All members of the CRMP family (CRMP1–5) are tyrosine-phosphorylated in the presence of Fyn (top).
N-terminal Region of CRMP2 Contains the Phosphorylation Site(s)
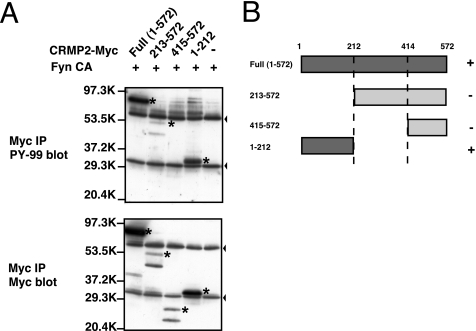
To determine the physiological significance of tyrosine phosphorylation of the CRMP family, we tried to identify the phosphorylation site(s). We constructed three partial fragments of CRMP2: CRMP2-(1–212), CRMP2-(213–572), and CRMP2-(415–572). These CRMP2 fragments and FynCA were coexpressed in HEK293T cells. Among these mutants, the N-terminal fragment CRMP2-(1–212) was highly phosphorylated (Fig. 2). Another two fragments, CRMP2-(213–572) and CRMP2-(415–572), were also moderately phosphorylated. These findings suggest that N-terminal region CRMP2-(1–212) contains major phosphorylation site(s).
FIGURE 2.
CRMP2 is phosphorylated by Fyn in the region of 1–212. A, identification of the region of CRMP2 phosphorylated by Fyn. CRMP2 full-length or CRMP2 partial fragment 1–212, 231–572, or 415–572 was transfected into HEK293T cells with FynCA. The fragments were immunoprecipitated (IP) and subjected to immunoblotting with anti-phosphotyrosine antibody (PY-99) or anti-Myc antibody. The N-terminal region of CRMP2-(1–212) was well phosphorylated by Fyn. Asterisks indicate phosphorylated or intact bands of CRMP2. Arrowheads indicate nonspecific bands. The two bands in Myc blot of CRMP2-(213–572) and CRMP2-(415–572) may be caused by proteolysis. B, summary of the results shown in A.
Tyr32 Is the Major Phosphorylation Site of CRMP2 by Fyn and Fes Tyrosine Kinases
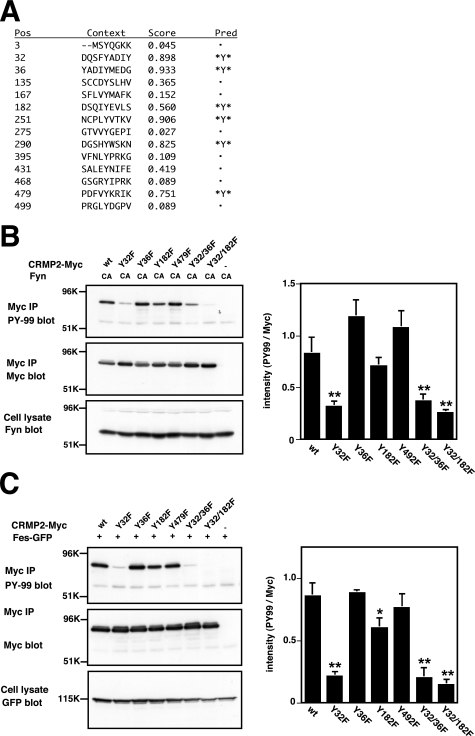
To elucidate the phosphorylated tyrosine residue(s) in CRMP2, we used a Web-based program, NetPhos (20), which predicts phosphorylation sites for several kinases. Three tyrosine residues, Tyr32, Tyr36, and Tyr182, were predicted as the phosphorylation sites in the N-terminal region (amino acids 1–212) of CRMP2 (Fig. 3A). In the C-terminal region (amino acids 213–572), three additional residues, Tyr251, Tyr290, and Tyr479, were predicted. Tyr479 of CRMP4 has been proposed previously as a phosphorylation site for tyrosine kinases (9), and Tyr479 of CRMP2 is phosphorylated by Src family tyrosine kinase Yes (18). We thus generated six point mutants of CRMP2, CRMP2Y32F, Y36F, Y182F, Y479F, Y32F/Y36F, and Y32F/Y182F. The Tyr (Y) residues in these mutants were replaced by Phe (F). These mutants were coexpressed with FynCA in HEK293T cells. Among them, the CRMP2Y32F mutant showed an ∼60% reduction in phosphorylation level compared with the wt (Fig. 3B). The remaining signal may be due to additional minor phosphorylation site(s). The phosphorylation of CRMP2 by Fes tyrosine kinase was reduced in Y32F mutants (Fig. 3C). The mutation of Tyr36 or Tyr479 did not alter the level of tyrosine phosphorylation, thereby suggesting that these residues are not major phosphorylation sites for Fyn or Fes.
FIGURE 3.
Tyr32 is a common phosphorylation site of CRMP2 by Fyn and Fes tyrosine kinases. A, prediction of the phosphorylation sites of rat CRMP2 using the Web-based program NetPhos (20). This program proposes the tyrosine phosphorylation sites of CRMP2 at Tyr32, Tyr36, Tyr182, Tyr251, Tyr290, and Tyr479. B, Tyr32 is the phosphorylation site of Fyn. The nonphosphorylated mutants, CRMP2Y32F, Y36F, Y182F, Y479F, Y32F/Y36F, and Y32F/Y182F were generated by PCR-based mutagenesis. These mutants or CRMP2wt were cotransfected with FynCA into HEK293T cells. The cell lysates were immunoprecipitated (IP) with anti-Myc antibody. The samples were separated by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibody (PY-99). The same membrane was reprobed with anti-Myc antibody. To confirm expression of FynCA, the cell lysates were immunoblotted with anti-Fyn antibody. The phosphorylation level of the CRMP2Y32F mutant was decreased compared with that of wt. The quantitative data include mean values ± S.E. for n = 3. **, p < 0.01, significantly different from CRMP2wt using analysis of variance. C, Fes phosphorylates CRMP2 at Tyr32 and Tyr182. The mutants or CRMP2wt was cotransfected with Fes-GFP into HEK293T cells. The cell lysates were immunoprecipitated with anti-Myc antibody. The samples were separated by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibody (PY-99). The same membrane was reprobed with anti-Myc antibody. To confirm expression of Fes-GFP, the cell lysates were immunoblotted with anti-GFP antibody. The quantitative data include mean values ± S.E. for n = 3. *, p < 0.05 and **, p < 0.01, significantly different from CRMP2wt using analysis of variance.
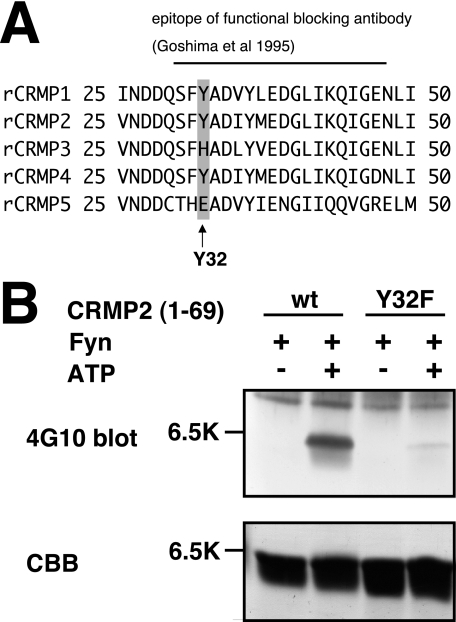
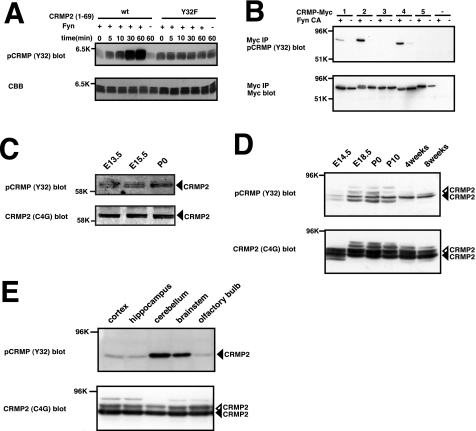
Introduction of an anti-CRMP2 antibody against amino acid residues 30–48 into DRG neurons blocks Sema3A-induced growth cone collapse response (1). Because the epitope contains Tyr32, we focused on Tyr32 phosphorylation of CRMP2 (Fig. 4A). We performed an in vitro kinase assay using purified CRMP2 and Fyn to prove the direct phosphorylation by Fyn. To minimize the effect of other phosphorylation sites besides Tyr32, we constructed another mutant, GST-tagged CRMP2-(1–69). This mutant and Fyn were expressed and purified from E. coli. We performed an in vitro kinase assay using these purified proteins. As shown in Fig. 4, CRMP2wt-(1–69) was phosphorylated by Fyn. The phosphorylation of CRMP2Y32F-(1–69) was barely detectable. These results indicate that Tyr32 is one of the major phosphorylation sites in CRMP2 by Fyn (Fig. 4B).
FIGURE 4.
In vitro phosphorylation of CRMP2wt-(1–69) and CRMP2Y32F-(1–69). A, alignment of amino acid sequences of rat CRMP1–5. Sequence of rat CRMP1–5, amino acids 25–50. Black line indicates that the epitope of the functional blocking antibody (1) Tyr32 is included in this region. B, in vitro phosphorylation of CRMP2wt-(1–69) or the CRMP2Y32F-(1–69) mutant. In vitro kinase assay was performed using purified Fyn and CRMP2wt-(1–69) or CRMP2Y32F-(1–69) with or without ATP (0.5 mm). Each reaction mixture was subjected to SDS-PAGE and immunoblot analysis with anti-phosphotyrosine antibody (4G10). The same samples were separated and stained by Coomassie Brilliant Blue (CBB) to show that relatively similar amounts of CRMP2 were loaded.
Distribution of Phosphorylated CRMP at Tyr32 in Vivo
For further analysis of the function of Tyr32 phosphorylation in vivo, we prepared a rabbit polyclonal antibody, anti-pCRMP (Y32), that recognizes the phosphorylated CRMP2 at Tyr32. To examine the specificity of this antibody, we performed an in vitro kinase assay and immunoblot analysis with anti-pCRMP (Y32) antibody. A time-dependent increase in the phosphorylation of CRMP2wt-(1–69) at Tyr32 was detected with this antibody. However, the phosphorylation of the Y32F mutant was not detectable (Fig. 5A). Next, we examined whether this antibody cross-reacts with other CRMP family members. The amino acid sequence of CRMP2 antigen (amino acids 25–39) is identical to that of CRMP4 (Fig. 4A). As expected, this antibody reacted with the phosphorylated forms of CRMP2 and CRMP4 (Fig. 5B). This antibody also weakly cross-reacted with phosphorylated CRMP1 (Fig. 5B). Thus, pCRMP (Y32) antibody reacts with the tyrosine-phosphorylated form of CRMP2 or CRMP4 and reacts to a lesser extent with that of CRMP1. This result suggests that Fyn also phosphorylates CRMP1 and CRMP4 at Tyr32. We confirmed that tyrosine phosphorylation of the CRMP4Y32F mutant by Fyn was markedly reduced. These results indicate that Fyn phosphorylates CRMP4 at Tyr32 (data not shown).
FIGURE 5.
Tissue distribution of phosphorylated CRMP at Tyr32. A, specificity of phospho-specific antibody for CRMP2. The specificity of anti-pCRMP (Y32) antibody was examined with immunoblot analysis. Purified CRMP2wt-(1–69) or CRMP2Y32F-(1–69) was subjected to a phosphorylation assay using Fyn, separated by SDS-PAGE, and immunoblotted with anti-pCRMP (Y32) antibody. This antibody recognizes phosphorylated CRMP2 but not nonphosphorylated CRMP2. The phosphorylation levels of CRMP2 increase in a time-dependent manner. The same samples were separated by SDS-PAGE and stained with Coomassie Brilliant Blue (CBB) to show the amount of CRMP2 loaded. B, phosphorylation of CRMP1, CRMP2, and CRMP4 at Tyr32 by Fyn in HEK293T cells. HEK293T cells were transfected with CRMP1–5 alone or together with FynCA. The cell lysates were immunoprecipitated (IP) with anti-Myc antibody and then immunoblotted with anti-pCRMP (Y32) antibody and Myc antibody. This anti-pCRMP (Y32) antibody recognizes the phosphorylated form of CRMP2, CRMP4, and weakly CRMP1. C, developmental changes in the levels of the phosphorylated CRMP2 at Tyr32 in spinal cord. Spinal cord lysates (10 μg) at several developmental stages were immunoblotted with anti-pCRMP (Y32) antibody and anti-CRMP2 antibody (C4G). Black arrowheads indicate CRMP2. The multiple bands may be due to cross-reaction of the anti-pCRMP (Y32) antibody against the phosphorylated form of CRMP2 and CRMP4. D, developmental changes in the levels of the phosphorylated CRMP2 at Tyr32 in brain. Whole brain lysates (20 μg) at several developmental stages were immunoblotted with anti-pCRMP (Y32) antibody and anti-CRMP2 antibody (C4G). Black arrowheads indicate CRMP2. Open arrowheads indicate Ser/Thr-phosphorylated form of CRMP2. E, lysates (20 μg) of the cortex, hippocampus, cerebellum, brain stem, and olfactory bulb of adult mouse were immunoblotted with anti-pCRMP (Y32) antibody. The level of phosphorylated CRMP at Tyr32 was highest in the cerebellum. Black arrowheads indicate CRMP2. Open arrowhead indicates Ser/Thr-phosphorylated form of CRMP2.
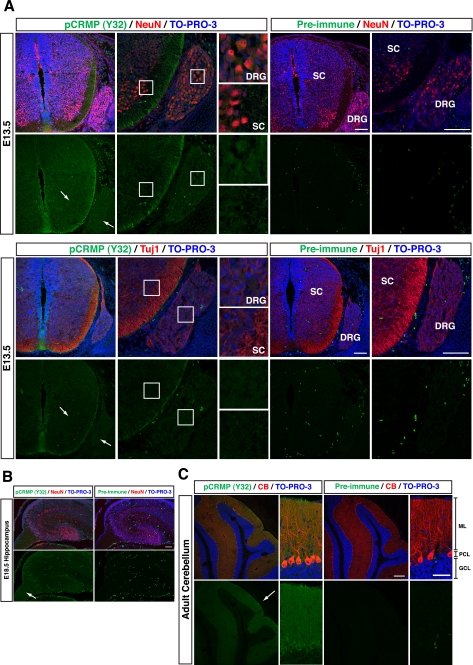
Using this anti-pCRMP (Y32) antibody, we examined the variation of phosphorylation levels of Tyr32 in developmental stages of the mouse spinal cord and brain. The spinal cord and brain lysates from several developmental stages were immunoblotted with anti-pCRMP (Y32) antibody. In the spinal cord, the tyrosine phosphorylation was observed from E13.5 to postnatal day 0. In the brain, the tyrosine phosphorylation at Tyr32 was observed at E18.5 and continued to adulthood (Fig. 5, C and D). Among the adult brain regions that were tested, the highest level of phosphorylated CRMP at Tyr32 was detected in the cerebellum (Fig. 5E). Immunohistochemical analysis at E13.5 sections around the spinal cord revealed that white matter of the spinal cord and DRG were stained with anti-pCRMP (Y32) antibody (Fig. 6A). Anti-pCRMP (Y32) antibody signals were colocalized with the antibody signals of anti-NeuN or Tuj1 neuronal markers. The level of phosphorylated CRMP2 at Tyr32 was also high in the fimbria of hippocampus at E18.5, which is a prominent band of white matter (Fig. 6B). In the adult brain, the highest level was seen in the molecular layer and the Purkinje cell layer of cerebellum (Fig. 6C). We also stained E13.5 sections with anti-CRMP2 antibody and found that the pattern of the immunofluorescence signals was similar to that obtained with anti-pCRMP (Y32) antibody (data not shown).
FIGURE 6.
Imunohistochemical analysis with anti-pCRMP (Y32) antibody. A, immunohistochemistry with anti-pCRMP (Y32) antibody (green), anti-NeuN antibody or anti-Tuj1 antibody, a marker for neurons (red), in the coronal sections around spinal cord and DRG at E13.5. TO-PRO-3 iodide was used for nuclei staining (blue). Preimmunized serum was used for the negative control. Arrows show DRG and spinal cord. Scale bars, 100 μm. SC, spinal cord. B, immunohistochemistry with anti-pCRMP (Y32) antibody (green) with anti-NeuN antibody (red) in the sagittal sections of hippocampus at E18.5. TO-PRO-3 iodide was used for nuclei staining (blue). The arrow shows hippocampal fimbria. Scale bar, 100 μm. C, immunohistochemistry with anti-pCRMP (Y32) antibody (green) with anti-calbindin (CB) antibody, a marker for Purkinje cells (red), in the sagittal sections of the cerebellum at 4-week-old wt mouse. TO-PRO-3 iodide was used for nuclei staining (blue). Low magnification and high magnification images are shown. The highest level of the phosphorylation was seen in the Purkinje cell layer and molecular layer (arrow). ML, molecular layer; PCL, Purkinje cell layer; GCL, granule cell layer. Scale bars, 200 μm (low magnification) and 50 μm (high magnification).
Phosphorylation of CRMP2 at Tyr32 Is Involved in Sema3A Signaling
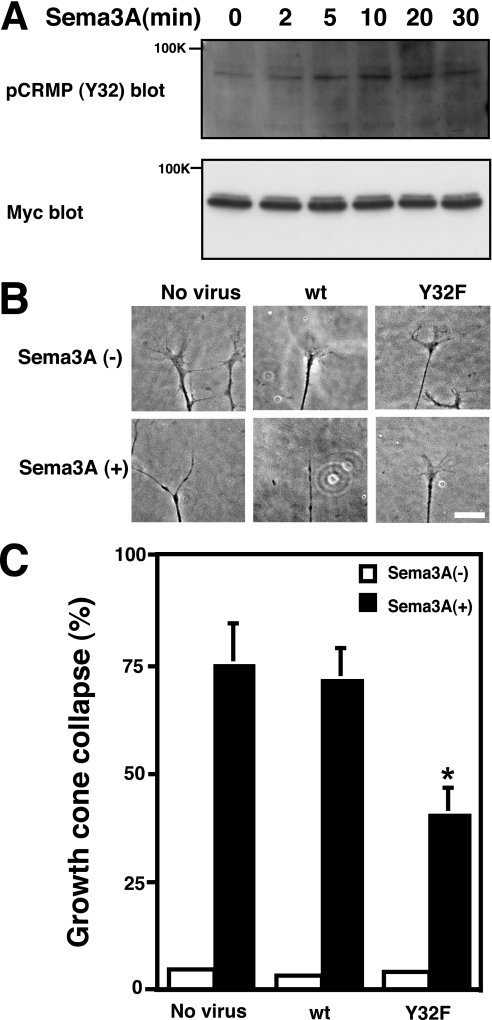
To examine whether Tyr32 phosphorylation of CRMP2 is involved in Sema3A signaling, we examined whether Sema3A induces phosphorylation of CRMP2 at Tyr32 in COS-7 cells expressing PlexinA2/NRP1, Fyn, and CRMP2. We found that the stimulation induced the phosphorylation of CRMP2 at Tyr32 (Fig. 7A). To determine the physiological significance of Tyr32 phosphorylation of CRMP2 in Sema3A signaling, we introduced the nonphosphorylated mutant CRMP2Y32F in DRG neurons using herpes simplex virus gene transfer. Overexpression of the CRMP2Y32F mutant significantly suppressed Sema3A-induced growth cone collapse response, whereas that of CRMP2wt did not show any effects (Fig. 7, B and C).
FIGURE 7.
Sema3A-induced CRMP2 phosphorylation at Tyr32 and suppression of Sema3A-induced growth cone collapse by introduction of the nonphosphorylated mutant. A, COS-7 cells were transfected with PlexinA2, NRP1, Fyn, and CRMP2-Myc. After being stimulated with 3 nm Sema3A, the cell lysates were immunoblotted with anti-pCRMP (Y32) antibody and anti-Myc antibody. A typical immunoblot analysis is shown. B, chick E7 DRG neurons were infected with recombinant herpes simplex virus preparations directing the expression of CRMP2wt or the CRMP2Y32F mutant. Expression of CRMP2wt did not alter Sema3A-induced growth cone collapse, whereas expression of CRMP2Y32F suppressed the Sema3A response. Scale bar, 20 μm. C, rate of growth cone collapse of CRMP2wt- or CRMP2Y32F-expressing DRG neurons stimulated with Sema3A (0.5 nm). Data include mean values ± S.E. for n = 9–13. *, p < 0.01, significantly different from no virus control using analysis of variance.
DISCUSSION
Tyrosine Phosphorylation Site of CRMP2 Is Tyr32
In this study, we determined that the phosphorylation site of CRMP2 by Fyn and Fes is Tyr32. In the CRMP2Y32F mutant, the phosphorylation of CRMP2 by Fyn was markedly suppressed (Fig. 3). The remaining phosphorylation in CRMP2Y32F suggests the presence of another minor phosphorylation site(s). Our data showing that the CRMP2Y32F mutant suppressed Sema3A-induced growth cone collapse (Fig. 7) support the involvement of phosphorylation in Sema3A signaling.
Based on crystal structure analyses of CRMP1 and CRMP2, the N-terminal region (amino acids 15–69) forms a small β-sheet that is relatively flexible (21, 22). When the residues 49, 50, 52, 53, 55, and 56 are substituted with Ala in CRMP1, the CRMP1 mutant behaves as the constitutively active form in COS-7 cell contraction assay (21). Deo et al. (21) conclude that the N-terminal β-sheet domain acts as negative regulator of basal CRMP activity. Anti-CRMP2 antibody blocks Sema3A-induced growth cone collapse for antigen amino acids 30–48 but not 475–491 (1). Because Tyr32 is included in the negative regulator domain, the phosphorylation at Tyr32 may act as a trigger for CRMP2 activation. The C-terminal region of CRMP2 has the Ser/Thr phosphorylation sites that are phosphorylated by Cdk5 (Ser522), GSK3β (Ser518, Thr514, and Thr509), and Rho kinase (Thr555) (2, 10–13). Some of these phosphorylations are involved in Sema3A signaling. The N-terminal region and the C-terminal region may cooperate and regulate the activation of CRMP2.
Fes has also been shown to be a mediator of Sema3A signaling (16). Fes associates with and phosphorylates the CRMP2·CRMP5 complex. In COS-7 cells, Fes mediates Sema3A-induced cell contraction and CRMP2·CRMP5 phosphorylation (16). Introduction of a kinase-negative mutant of Fes to DRG neurons suppresses Sema3A-induced growth cone collapse (16). In the DRG neurons of fyn-deficient mice, Sema3A-induced growth cone collapse is attenuated (19). Here, we demonstrate that Fyn and Fes phosphorylate CRMP2 at Tyr32 (Fig. 3). Fyn and Fes may be involved in Sema3A signaling through Tyr32 phosphorylation of CRMP2. Fer, a Fes homolog, also phosphorylates CRMP2 and mediates Sema3A signaling (17). Thus, the related kinase, Fyn, Fes, and Fer mediate Sema3A signaling through phosphorylation of CRMP2 at Tyr32. Each of these kinases may have distinct roles in mediating a wide variety of biological actions of Sema3A at different developmental stages and brain regions.
What is the role of the tyrosine phosphorylation of CRMPs in Sema3A signaling? One possibility is that CRMP2 may be recruited to Cdk5 or adaptor proteins through its tyrosine phosphorylation. Several proteins have a phosphotyrosine-binding domain, such as a SH2 domain. A candidate is α2-chimaerin. α2-Chimaerin has an SH2 domain and Rac GTPase-activating protein domain and is also involved in Sema3A signaling (11). The GTPase-activating protein inactive mutant of α2-chimaerin and SH2 domain mutant of α2-chimaerin inhibit Sema3A-induced growth cone collapse (11). Furthermore, α2-chimaerin associates with CRMP2 after orthovanadate treatment in N1E-115 cells. α2-Chimaerin also interacts with the Cdk5·p35 complex (11). α2-Chimaerin may associate with CRMP2 phosphorylated at Tyr32 and recruit CRMP2 to the Cdk5·p35 complex. CRMP1–4 can accelerate Sema3A-induced COS-7 cell contraction (21). Although CRMP3 can enhance Sema3A-induced COS-7 cell contraction, neither Tyr32 nor Ser/Thr phosphorylation sites in the C terminus are conserved in CRMP3. This fact raises the possibility that CRMP3 may mediate the signal via another phosphorylation site(s) and/or by a phosphorylation-independent mechanism.
In Vivo Role of Tyrosine Phosphorylation of CRMPs
What is the in vivo role of the tyrosine phosphorylation of CRMPs? One apparent role is the intracellular signaling of axon guidance. Our data showing that the CRMP2Y32F mutant suppressed Sema3A-induced growth cone collapse (Fig. 7, B and C) support involvement of the phosphorylation in the Sema3A signaling. The Tyr32-phosphorylated CRMPs are abundant in spinal cord, DRG, and fimbria of the hippocampus, which is the axon bundle structure (Fig. 6B). This also supports the involvement of Tyr32 phosphorylation in axon guidance molecule signaling. CRMP2 is expressed in oligodendrocyte and neurons. Sema3A-induced branching of oligodendrocytes is blocked by anti-CRMP2 antibody (23). In addition, Fyn has been implicated in myelination (24). CRMP2 may play a role in oligodendrocyte development. The highest level of phosphorylation at Tyr32 is also observed in the adult mice cerebellum (Fig. 5E). The biological role of the phosphorylation in this region is currently unknown. The immunostaining of pCRMP (Y32) was also abundant in axon-bundled structures (Fig. 6B). This may reflect concentrated, tyrosine-phosphorylated CRMP2 in the axonal cytoplasm. It has been shown that CRMP2 is involved in axonal transport of tubulin dimers (5). If the staining reflects the former case, it raises the possibility that the tyrosine phosphorylation may be involved in the regulation of axonal transport. In fact, Sema3A facilitates axonal transport, and this facilitation is blocked by a tyrosine kinase inhibitor and is attenuated in fyn-deficient mouse DRG (25).
Tyrosine Phosphorylation of CRMP2 and Alzheimer Disease
Both Tau and CRMP2 have Ser/Thr phosphorylation sites around a tubulin-binding domain (5). It has been shown that hyperphosphorylated Tau and CRMP2 accumulate in neurofibrillary tangles in brains affected with Alzheimer disease (13, 14, 26). Tau and CRMP2 also have a tyrosine phosphorylation site in the N termini (27) (Fig. 3). Tau is phosphorylated by Fyn at Tyr18 (26), and this property is quite similar to that of CRMP2. Taken together, the phosphorylation on Tyr32 of CRMP2 may have some relevance to the pathogenesis of Alzheimer disease.
In conclusion, the current data demonstrate that CRMP2 is phosphorylated at Tyr32 and this phosphorylation mediates signal transduction of Sema3A. Further work is necessary to determine the physiological significance of CRMP2 phosphorylation at Tyr32.
Acknowledgments
We thank the staff of the Research Resources Center in RIKEN Brain Science Institute for generation of anti-phosphorylated antibody of CRMP at Tyr32. We also thank Dr. Masaharu Ogawa (RIKEN Brain Science Institute) for advice on mouse phenotypic analysis. We acknowledge Dr. Yasuo Ihara (Doshisya University) and Dr. Shigeru Yanagi (Tokyo University of Pharmacy and Life Science) for the gifts of materials. We are grateful to Erin O'Donnell for checking the grammar of this manuscript.
This work was supported by grants-in-aid for scientific research on priority areas and targeted proteins research program from the Ministry of Education, Culture, Sports, Science, and Technology (to Y. G.) and Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency (to Y. G.).
- CRMP
- collapsin response mediator protein
- Cdk5
- cyclin-dependent kinase 5
- DRG
- dorsal root ganglion
- E
- embryonic day
- FynCA
- constitutively active Fyn
- GFP
- green fluorescent protein
- GSK3β
- glycogen synthase kinase 3β
- Sema3A
- Semaphorin3A
- SH2
- Src homology 2
- wt
- wild type.
REFERENCES
- 1.Goshima Y., Nakamura F., Strittmatter P., Strittmatter S. M. (1995) Nature 376, 509–514 [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. (2005) Cell 120, 137–149 [DOI] [PubMed] [Google Scholar]
- 3.Arimura N., Ménager C., Kawano Y., Yoshimura T., Kawabata S., Hattori A., Fukata Y., Amano M., Goshima Y., Inagaki M., Morone N., Usukura J., Kaibuchi K. (2005) Mol. Cell. Biol. 25, 9973–9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita N., Uchida Y., Ohshima T., Hirai S., Nakamura F., Taniguchi M., Mikoshiba K., Honnorat J., Kolattukudy P., Thomasset N., Takei K., Takahashi T., Goshima Y. (2006) J. Neurosci. 26, 13357–13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukata Y., Itoh T. J., Kimura T., Ménager C., Nishimura T., Shiromizu T., Watanabe H., Inagaki N., Iwamatsu A., Hotani H., Kaibuchi K. (2002) Nat. Cell Biol. 4, 583–591 [DOI] [PubMed] [Google Scholar]
- 6.Nishimura T., Fukata Y., Kato K., Yamaguchi T., Matsuura Y., Kamiguchi H., Kaibuchi K. (2003) Nat. Cell Biol. 5, 819–826 [DOI] [PubMed] [Google Scholar]
- 7.Kimura T., Arimura N., Fukata Y., Watanabe H., Iwamatsu A., Kaibuchi K. (2005) J. Neurochem. 93, 1371–1382 [DOI] [PubMed] [Google Scholar]
- 8.Kawano Y., Yoshimura T., Tsuboi D., Kawabata S., Kaneko-Kawano T., Shirataki H., Takenawa T., Kaibuchi K. (2005) Mol. Cell. Biol. 25, 9920–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minturn J. E., Fryer H. J., Geschwind D. H., Hockfield S. (1995) J. Neurosci. 15, 6757–6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arimura N., Inagaki N., Chihara K., Ménager C., Nakamura N., Amano M., Iwamatsu A., Goshima Y., Kaibuchi K. (2000) J. Biol. Chem. 275, 23973–23980 [DOI] [PubMed] [Google Scholar]
- 11.Brown M., Jacobs T., Eickholt B., Ferrari G., Teo M., Monfries C., Qi R. Z., Leung T., Lim L., Hall C. (2004) J. Neurosci. 24, 8994–9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole A. R., Knebel A., Morrice N. A., Robertson L. A., Irving A. J., Connolly C. N., Sutherland C. (2004) J. Biol. Chem. 279, 50176–50180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida Y., Ohshima T., Sasaki Y., Suzuki H., Yanai S., Yamashita N., Nakamura F., Takei K., Ihara Y., Mikoshiba K., Kolattukudy P., Honnorat J., Goshima Y. (2005) Genes Cells 10, 165–179 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida H., Watanabe A., Ihara Y. (1998) J. Biol. Chem. 273, 9761–9768 [DOI] [PubMed] [Google Scholar]
- 15.Gu Y., Ihara Y. (2000) J. Biol. Chem. 275, 17917–17920 [DOI] [PubMed] [Google Scholar]
- 16.Mitsui N., Inatome R., Takahashi S., Goshima Y., Yamamura H., Yanagi S. (2002) EMBO J. 21, 3274–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapovalova Z., Tabunshchyk K., Greer P. A. (2007) BMC Dev. Biol. 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varrin-Doyer M., Vincent P., Cavagna S., Auvergnon N., Noraz N., Rogemond V., Honnorat J., Moradi-Améli M., Giraudon P. (2009) J. Biol. Chem. 284, 13265–13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki Y., Cheng C., Uchida Y., Nakajima O., Ohshima T., Yagi T., Taniguchi M., Nakayama T., Kishida R., Kudo Y., Ohno S., Nakamura F., Goshima Y. (2002) Neuron 35, 907–920 [DOI] [PubMed] [Google Scholar]
- 20.Blom N., Gammeltoft S., Brunak S. (1999) J. Mol. Biol. 294, 1351–1362 [DOI] [PubMed] [Google Scholar]
- 21.Deo R. C., Schmidt E. F., Elhabazi A., Togashi H., Burley S. K., Strittmatter S. M. (2004) EMBO J. 23, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenmark P., Ogg D., Flodin S., Flores A., Kotenyova T., Nyman T., Nordlund P., Kursula P. (2007) J. Neurochem. 101, 906–917 [DOI] [PubMed] [Google Scholar]
- 23.Ricard D., Rogemond V., Charrier E., Aguera M., Bagnard D., Belin M. F., Thomasset N., Honnorat J. (2001) J. Neurosci. 21, 7203–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umemori H., Sato S., Yagi T., Aizawa S., Yamamoto T. (1994) Nature 367, 572–576 [DOI] [PubMed] [Google Scholar]
- 25.Li C., Sasaki Y., Takei K., Yamamoto H., Shouji M., Sugiyama Y., Kawakami T., Nakamura F., Yagi T., Ohshima T., Goshima Y. (2004) J. Neurosci. 24, 6161–6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee V. M., Goedert M., Trojanowski J.Q. (2001) Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 27.Lee G., Thangavel R., Sharma V. M., Litersky J. M., Bhaskar K., Fang S. M., Do L. H., Andreadis A., Van Hoesen G., Ksiezaki-Reding H. (2004) J. Neurosci. 24, 2304–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]