Abstract
NUCB1 (nucleobindin 1) is a Golgi-localized soluble protein with a signal peptide and multiple functional domains. We reported recently that NUCB1 is a negative regulator of the unfolded protein response that activates various endoplasmic reticulum (ER)-originating signaling pathways. In that report, we also showed that Golgi localization of NUCB1 was essential to regulate the unfolded protein response. However, the localization mechanism of NUCB1 is still unknown. Here, we report that the proline residue at the +2-position (Pro+2) from the signal peptide cleavage site is the determinant of NUCB1 protein export from the ER and subsequent transport to the Golgi. Fusion of the N-terminal amino acids 1–35 peptide region, including both signal peptide (amino acids 1–26) and Pro+2, was sufficient for enhanced green fluorescent protein to localize in the Golgi, whereas single amino acid mutation of Pro+2 resulted in defective export from the ER without affecting the protein maturation process. Furthermore, we demonstrated that Pro+2 was important for the enhanced green fluorescent protein fusion protein to concentrate at a transport vesicle formation site within the ER, often termed the ER exit site. Interestingly, such a Pro+2 has also been functionally conserved in other Golgi-localized soluble proteins, Cab45 (Ca2+-binding protein of 45 kDa), reticulocalbin 1, and calumenin. Our findings indicate that Pro+2 can function as a novel ER export signal of some Golgi proteins.
NUCB1 (nucleobindin 1), also known as calnuc, was first identified as a soluble secretory 55-kDa protein (461 amino acids) in lupus-prone mice with the lymphoproliferation (lpr) mutation (1). NUCB1 has also been shown to be secreted in culture supernatant of a murine B cell line established from the mice (2). Later studies also demonstrated that NUCB1 is expressed ubiquitously and localizes in the Golgi apparatus of intact cells (3, 4). NUCB1 contains multiple putative functional domains, including an N-terminal endoplasmic reticulum (ER)2 signal peptide, a DNA binding site, a leucine zipper domain, two EF-hand Ca2+-binding sites, a nuclear localization signal, and G-protein-binding and cyclooxygenase-binding domains (1, 5, 6). Consistently, NUCB1 has been reported to function in various cellular processes, including osteogenesis, inflammation, autoimmunity, intracellular signaling, and cancer (6–10).
Newly synthesized, premature NUCB1 protein is first targeted into the ER via its N-terminal ER signal peptide. After removal of the signal peptide in the ER, a mature NUCB1 protein is transported to the Golgi apparatus and then secreted to the extracellular matrix (11). NUCB1 in the Golgi pool is probably involved in establishing the agonist-mobilizable Golgi Ca2+ store (3). Furthermore, the Golgi-localized NUCB1 regulates the unfolded protein response, which is a cellular stress response that triggers various events, such as ER-resident molecular chaperone induction, translational repression, and apoptosis under ER stress conditions (12). On the other hand, extracellular NUCB1 has been suggested to serve as a modulator of matrix maturation in bone, based on the observations that NUCB1 is secreted by osteoblasts and osteocytes and can, indeed, be detected in the osteoid extracellular matrix (7, 13). Thus, Golgi transport and subsequent secretion of NUCB1 seem to be important to exert the protein's activity, but little is known about its transport regulation mechanism.
In eukaryotic cells, a tremendous variety of soluble and membrane cargo proteins are packaged into transport vesicles at the ER. Vesicle formation on the ER membrane begins with the assembly of a coat protein complex II (COPII) (14). This COPII coat consists of Sar1, Sec23-Sec24, and Sec13-Sec31 complexes that are sequentially recruited to the ER membrane. Sar1 is a small GTPase that regulates coat assembly and disassembly. To assemble the COPII coat, Sar1-GTP transiently associates with an export cargo protein and then binds to Sec23-Sec24, which in turn attracts Sec13-Sec31 (14). Polymerization of the formed COPII coat, which occurs at the so-called ER exit site (ERES), triggers transport vesicle budding on the ER membrane (14, 15). Then the vesicles fuse with the VTC compartment (vesiculo-tubular clusters, also called ERGIC) that mediates further protein transport to the Golgi apparatus. Cargo proteins are then carried to their final destinations, such as organelle, cell surface membrane, and extracellular matrix (14).
Recent studies reveal that some transmembrane cargoes contain specific motifs to be selectively concentrated in the transport vesicle within the ER. This sorting motif is called the ER export signal. Representative ER export signals are the diacidic motif (DXE), dihydrophobic (LL) motif, and diaromatic motif (FF, YY) that have been found in vesicular stomatitis virus glycoprotein, ERGIC-53, and Emp46p, respectively (16–18). These export signals are present in the cytoplasmic region of the cargo proteins and mediate their interaction with the COPII complex at the outer side of the ER membrane, resulting in concentration in the newly formed budding vesicle. On the other hand, the soluble type of cargo proteins require their cargo receptor to be sorted into the COPII vesicle, because they cannot interact directly with COPII complex, since these proteins have no cytoplasmic region. Although recent studies have reported the existence of the cargo receptor and functional ER export signals found in some soluble cargo proteins, little is known about many other cargo receptors and the export signals of soluble cargo proteins (19, 20).
Here, we report that Pro28, which is located at the +2-position (Pro+2) from the signal peptide cleavage site of the NUCB1 protein, is a determinant of its export from the ER. In fact, single amino acid substitution (P28A) led to predominant ER distribution and reduced the secretion of NUCB1 without affecting its maturation process in the ER. We also demonstrated that Pro+2 is required for concentration at the ERES. It is important to note that Pro+2 was also conserved functionally in other proteins. Our results indicate that Pro+2 can function as a new ER export signal.
EXPERIMENTAL PROCEDURES
Antibodies
Rabbit polyclonal anti-NUCB1 antibody was raised against a synthetic peptide corresponding to C-terminal CEKKLLERLPEVEVPQHL of the human NUCB1 protein (12). The following commercially available antibodies were used: mouse monoclonal anti-KDEL (StressGen; Victoria, Canada), anti-Golgin-97 (Invitrogen), anti-ATF6 (activation transcription factor 6) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-FLAG M2, anti-β-actin (Sigma), anti-V5 (Sigma), and horseradish peroxidase-conjugated anti-rabbit or -mouse IgG (GE Healthcare).
Cell Culture and Treatment
Cells were maintained in RPMI 1640 medium (for HT1080) or Dulbecco's modified Eagle's medium (for 293T) supplemented with 10% heat-inactivated fetal bovine serum and 100 μg/ml of kanamycin and were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
Immunoblot Analysis
Cells were lysed in a 1× SDS sample buffer, and protein concentrations of the lysates were measured with a Bio-Rad protein assay kit. To analyze secreted proteins, culture medium (serum-free) was concentrated with Microcon YM-30 (Millipore; Billerica, MA) and then boiled in a 1× SDS buffer. Equal amounts of proteins were resolved on a 10% SDS-polyacrylamide gel and transferred by electroblotting to a nitrocellulose membrane. Membranes were probed with the indicated antibodies, and the specific signals were detected using an enhanced chemiluminescence detection system (GE Healthcare).
Immunoprecipitation
Cells were washed with ice-cold PBS and lysed in 50 mm Tris-HCl (pH 8.0), 1% Triton X-100, 150 mm NaCl, 1 mm EDTA, supplemented with a protease inhibitor mixture (Sigma). The lysates were cleared by centrifugation at 13,000 × g for 10 min at 4 °C and immunoprecipitated by anti-FLAG beads (Sigma) in lysis buffer. Immunoprecipitates were washed three times with lysis buffer and eluted by boiling in an SDS sample buffer for immunoblot analysis.
Immunocytochemistry
Cells on a polylysine-coated coverslip were fixed and permeabilized for 10 min in PBS containing 4% paraformaldehyde and 0.1% Triton X-100. After blocking for 1 h in PBS with 10% bovine serum albumin, we incubated the cells with primary (mouse anti-FLAG M2 (1:1000), rabbit anti-V5 (1:1000), mouse anti-Golgin-97 (1:1000); or mouse anti-KDEL (1:1000)) and subsequent secondary antibodies (Alexa-fluor 488-conjugated anti-rabbit Ig (1:1000) or Alexa-fluor 568-conjugated anti-mouse Ig (1:1000); Invitrogen)) in PBS with 1.5% bovine serum albumin for 1 h at room temperature. The coverslips were mounted on microscope slides and observed under Olympus IX71 microscope at ×400 magnification with appropriate filters to detect fluorescence.
Plasmids
Expression vectors for V5-tagged NUCB1, RCN1, Calumenin, Cab45, and Sar1 (pcDNA3.1 TOPO V5/His; Invitrogen) were constructed by TA-cloning each cDNA amplified by reverse transcription-PCR into pcDNA3.1 TOPO V5/His (Invitrogen). FLAG-tagged NUCB1 was constructed by subcloning into the HindIII/NotI site of pFLAG-CMV-5c (Sigma) from pNUCB1(pcDNA3.1). Non-tagged NUCB1 was constructed by ligating PCR-amplified NUCB1 into the HindIII/NotI site of pcDNA3 (Invitrogen). Each of the NUCB1 deletion mutants (Mut1, Mut2, Mut3, Mut4, and Mut5) was produced by TA cloning of each segment of NUCB1 amplified by PCR and using the pNUCB1-V5 plasmid (pcDNA3.1) as the template. p1–35aa-EGFP was produced by ligating a PCR-amplified EGFP gene immediately downstream of the DNA sequence encoding the N-terminal 35 amino acids in pcDNA3. pSec13-DsRed was produced by ligating a PCR-amplified DsRed gene immediately downstream of the human sec13 DNA sequence subcloned in pcDNA3.1. GalT-YFP was purchased from TAKARA Bio, Japan (pEYFP Golgi vector). For point mutation, site-directed mutagenesis was carried out using a QuikChange mutagenesis kit (Agilent Technologies, Santa Clara, CA). The proper construction of plasmids was confirmed by DNA sequencing.
Transfection
Transient transfections were performed using either FuGENE6 Transfection Reagent (Roche Applied Science) for HT1080 cells or Lipofectamine 2000 (Invitrogen) for 293T, according to each manufacturer's protocol. At 24 h after transfection, the cells were used for each assay.
In Vitro Translation
In vitro translation was done using the TNT Quick Coupled Transcription/Translation system (Promega, Madison, WI). Reaction mixtures containing 1 μg of plasmid DNA were incubated in the presence or absence of canine pancreatic microsomal membrane fractions at 30 °C for 60 min. The reactions were stopped by adding a 1× SDS sample buffer and analyzed by immunoblotting.
Pulse-Chase Analysis
293T cells transfected transiently with a vector that encodes FLAG-tagged NUCB1-WT or -P28A (4 × 105/well in a 6-well plate) were incubated for 10 min in Met/Cys-free Dulbecco's modified Eagle's medium supplemented with 2 mm glutamine, 10% dialyzed fetal bovine serum. Cells were then pulse-labeled for 10 min, using the 300 μCi/ml easy tag EXPRE35S35S protein labeling mix (PerkinElmer Life Sciences) dissolved in 400 μl of the above mentioned medium. To chase 35S-labeled proteins, cells were washed with fresh medium and incubated in fresh, complete medium for various time periods. After medium was removed, cells were lysed (Tris buffer with 1% Triton X-100 and protease inhibitor mixture) and immunoprecipitated with anti-FLAG antibody-conjugated beads. Then each sample was boiled for 5 min in SDS buffer and subjected to SDS-PAGE. After gel drying, the incorporated [35S]Met/Cys was visualized with Typhoon9410 image analyzer (GE Healthcare).
Glycosidase Treatment
The lysate prepared from HT1080 cells using Triton X-100 were boiled in the presence of 0.5% SDS and 0.5% 2-mercaptoethanol for 5 min before digestion by endoglycosidase H or peptide N-glycosidase F (Glyko, San Leandro, CA). After the addition of 5× reaction buffer (Glyko), we incubated the samples for 20 h at 37 °C in the presence of 20 milliunits of sialidase C (Glyko), 0.5 milliunits of endoglycosidase H, or 2 milliunits of peptide N-glycosidase F.
Live Cell Imaging
Fluorescence images were obtained with an Olympus Fluoview 500 confocal microscope using 488-nm laser excitation for EGFP or 543 nm for DsRed.
Determination of Signal Peptide Cleavage Sites of CREC Proteins
293T cells were transiently transfected with RCN1, Cab45, or calumenin plasmids (FLAG tag). Cells were washed with ice-cold PBS and lysed in 50 mm Tris-HCl (pH 8.0), 1% Triton X-100, 150 mm NaCl, 1 mm EDTA, supplemented with a protease inhibitor mixture (Sigma). The lysates were immunopurified with anti-FLAG beads. Each sample was subjected to SDS-PAGE and then transferred to polyvinylidene difluoride membrane. After staining the membrane with Ponceau S (0.1% (w/v) Ponceau, 1% acetic acid (v/v) in distilled water), the sample bands were cut out for peptide sequence. N-terminal amino acids up to the fifth were determined by standard Edman degradation using a Procise 494 HT (Applied Biosystems).
RESULTS
The N-terminal Region (aa 27–35) of NUCB1 Is Involved in the Golgi Localization
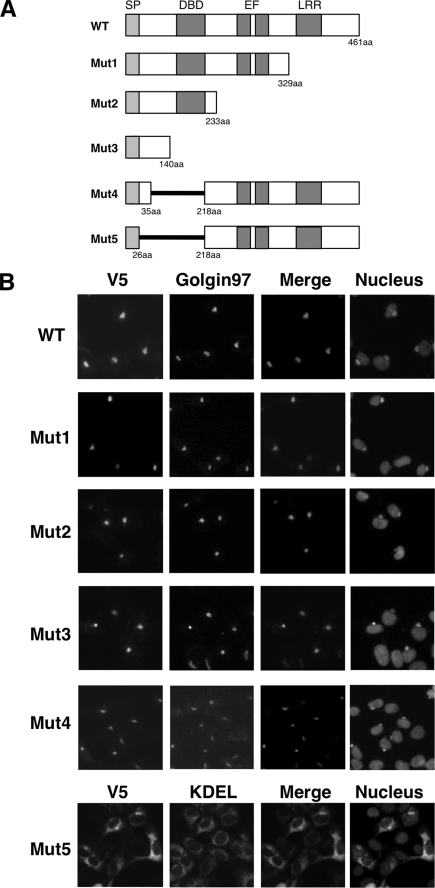
To determine the region necessary for the Golgi localization, we constructed five deletion mutants of NUCB1 (Fig. 1A). As we reported previously, when NUCB1-WT was expressed in HT1080 cells, Golgi localization was confirmed by colocalization of NUCB1 with the Golgi marker Golgin-97 (Fig. 1B, top) (12). The mutants that deleted a large part of the C terminus (Mut1 to -3) also exhibited Golgi localization (Fig. 1B, Mut1 to -3). To examine whether the remaining N-terminal region was important for Golgi localization, we constructed Mut-4 and -5, which lacked aa 35–218 or 27–218 (Fig. 1A). Mut4 (del 35–218) still had the ability to localize in the Golgi, but Mut5 (del 27–218) was largely localized at the ER, as shown by co-localization with the ER marker GRP78 (Fig. 1B, bottom). These results indicate that N-terminal residues 27–35 of NUCB1 are involved in the Golgi localization.
FIGURE 1.
The N-terminal aa 27–35 of NUCB1 is involved in Golgi localization. A, schematic representation of each NUCB1 construct. SP, signal peptide; DBD, DNA-binding domain; EF, EF-hand motif; LRR, leucine-rich repeat. B, after transfection with each plasmid (V5 tag), as shown in A, HT1080 cells were fixed and double stained with anti-V5, anti-Golgin-97 (Golgi marker), or anti-KDEL (ER marker) antibodies. Nuclei were counterstained with Hoechst 33342. Images of the nuclei were merged with each V5 image.
Mutation of Proline at the 28-Position Results in ER Localization of NUCB1
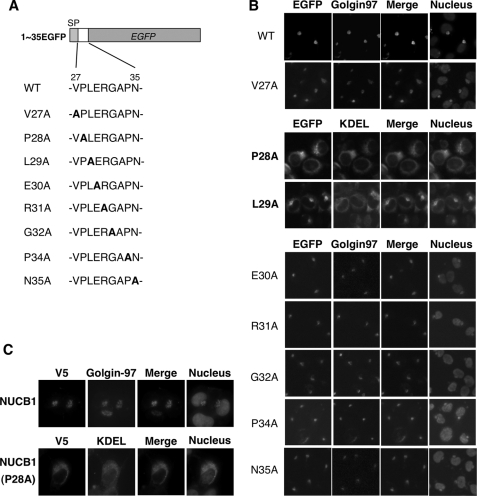
To determine whether the N-terminal aa 27–35 region of NUCB1 was sufficient for Golgi localization of the protein, we constructed 1–35EGFP, which fused the aa 1–35 region of NUCB1 to the N terminus of EGFP (Fig. 2A). The N-terminal aa 1–26 region of the NUCB1 protein is known to function as an ER signal peptide, which is critical for ER targeting of newly synthesized polypeptides. Therefore, we fused EGFP with the aa 1–35 region but not the aa 27–35 alone. As we had expected, 1–35EGFP was localized in the Golgi as the NUCB1 protein was (Fig. 2B, top). Using alanine exchange mutants of the aa 27–35 region, we determined the amino acid motifs that were important for Golgi localization. Surprisingly, an alanine exchange of Pro28 or Leu29 weakened the Golgi staining pattern. Instead, they exhibited an ER staining pattern. The P28A mutant, especially, showed a striking ER distribution (Fig. 2B). A similar staining pattern was observed in the NUCB1-P28A mutant (Fig. 2C). These observations indicated that Pro28 of NUCB1 is important for the Golgi localization.
FIGURE 2.
Pro28 mutation leads to ER localization of NUCB1. A, schematic representation of aa 1–35/EGFP fusion protein and sequential alanine exchange in the aa 27–35 segment. B, after transfection with each of the expression vectors, as shown in A, HT1080 cells were fixed and stained with anti-Golgin-97 (Golgi marker) or anti-KDEL (ER marker) antibodies. Nuclei were counterstained with Hoechst 33342. GFP was analyzed by its fluorescence. Images of the nuclei were merged with each EGFP image. C, after transfection with pNUCB1-V5 or pNUCB1(P28A)-V5, HT1080 cells were fixed and double-stained with anti-V5, anti-Golgin-97 (Golgi marker), or anti-KDEL (ER marker) antibodies. Nuclei were counterstained with Hoechst 33342. Images of the nuclei were merged with each V5 image.
Functional Conservation of Pro+2 in Other Golgi Proteins
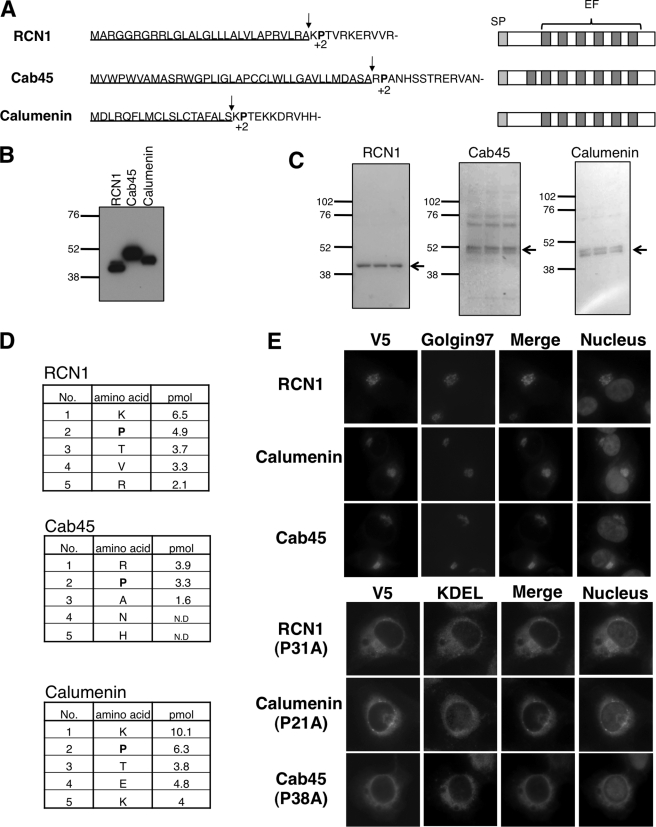
Pro28 is located at the +2 position from the N-terminal signal peptide of NUCB1. Interestingly, we found that other ER/Golgi-localized proteins, RCN1 (reticulocalbin 1), calumenin, and Cab45, possess proline at the +2-position from their putative signal peptide cleavage site, using a commonly used signal peptide prediction system, SignalP 3.0 (available on the World Wide Web) (Fig. 3A). These are called CREC (Cab45, reticulocalbin, ERC-45, calumenin) family genes. Although these proteins contain multiple EF-hand motifs, like NUCB1, their functions are largely unknown (21).
FIGURE 3.
Functional conservation of Pro+2 in CREC family genes. A, N-terminal sequences (left) and functional domains (right) of CREC family proteins. Signal peptide sequences are underlined. Signal peptide cleavage sites and Pro+2 are shown by arrows and boldface type, respectively. SP, signal peptide; EF, EF-hand motif. B, 293T cells were transiently transfected with RCN1, Cab45, or calumenin plasmids (FLAG tag). Each cell lysate was immunopurified with anti-FLAG beads. The samples were subjected to SDS-PAGE and then underwent immunoblot analysis with anti-FLAG antibody. C, the transferred polyvinylidene difluoride membrane was stained with Ponceau S. Each band indicated by an arrow was analyzed for the purpose of peptide sequence. D, N-terminal amino acid sequence determined by standard Edman degradation. The table shows the detected order and amounts of amino acids. ND, not detected. E, after transfection with each of RCN1 (WT or P31A), calumenin (WT or P21A), and Cab45 (WT or P38A) plasmids (V5 tag), the cells were fixed and double-stained with anti-V5, anti-Golgin-97 (Golgi marker), or anti-KDEL (ER marker) antibodies. Nuclei were counterstained with Hoechst 33342. Images of the nuclei were merged with each V5 image.
To confirm actual signal peptide cleavage sites of these proteins, we analyzed the N-terminal sequences of FLAG-tagged proteins expressed in 293T cells. The expression of each protein was confirmed by both immunoblotting with anti-FLAG antibody and Ponceau S staining (Fig. 3, B and C). The sample bands (Fig. 3C) were processed for N-terminal sequencing. We detected amino acid sequences that were completely identical to the sequence beginning from the +1-position from each putative signal peptide cleavage site without overlapping at any other positions (Fig. 3D). Although both Cab45 and calumenin proteins were consistently detected as a doublet band in SDS-PAGE (Fig. 3C), the detected amino acids showed the single peptide sequence. Therefore, the doublet bands may result from posttranslational modifications of these proteins, like NUCB1 (see Fig. 4A). These results demonstrated that RCN1, calumenin, and Cab45 have proline at the +2-position (Pro+2) from the signal peptide cleavage site.
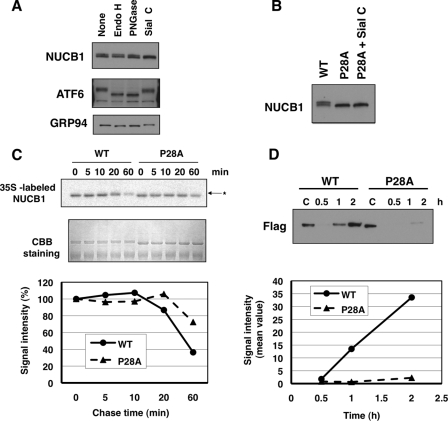
FIGURE 4.
Inefficient export of the P28A mutant from the ER. A, HT1080 cells were lysed in 1% Triton X-100 buffer. The lysates were digested with the indicated glycosidase for 20 h. Each sample was subjected to SDS-PAGE and then underwent immunoblot analysis with anti-NUCB1, anti-ATF6, and anti-KDEL antibodies. B, HT1080 cells were transiently transfected with pNUCB1 or pNUCB1-P28A plasmid (non-tag). The prepared samples, as shown in A, underwent immunoblot analysis with an anti-NUCB1 antibody. C, after transfection with pNUCB1-WT or -P28A (FLAG tag), 293T cells were pulse-labeled for 10 min with [35S]Met/Cys. Then the cells were incubated for the indicated periods in fresh medium to chase 35S-labeled proteins. Each cell lysate was immunopurified with anti-FLAG beads. The samples were subjected to SDS-PAGE, and the gel image was visualized using typhoon (top). The gel was Coomassie Brilliant Blue (CBB)-stained for loading control. Signal intensity was counted using Image J (bottom). D, 293T cells were transiently transfected with the NUCB1-WT or P28A plasmid. After changing to serum-free, fresh medium, each culture medium was collected at the indicated time period. The samples were condensed by an Amicon YM-30 centrifugal ultrafilter and boiled in SDS sample buffer. Then the samples were subjected to SDS-PAGE and immunoblot analysis with an anti-FLAG antibody (top). Signal intensity was counted using Image J (bottom).
As to intercellular localization, both RCN1 and calumenin have been reported to localize in the ER (22, 23), but we observed Golgi localization of these proteins at steady state (Fig. 3E). Cab45 was localized in the Golgi, as reported previously (24). An alanine substitution of Pro+2 in these proteins led to a striking ER distribution, as observed with NUCB1-P28A (Fig. 3E). These results indicated that Pro+2 determines the Golgi localization of several proteins.
Inefficient Export of P28A Mutant from the ER
To elucidate whether ER distribution of the NUCB1-P28A mutant was due to inefficient transport from the ER to the Golgi, we monitored the oligosaccharide modification state of the NUCB1 protein as a transportation indicator. The NUCB1 protein is known to specifically undergo sialylation at the Golgi, which can be monitored by a slight shift in molecular weight in SDS-PAGE (Fig. 4A) (11, 12). Indeed, NUCB1 was selectively deglycosylated by sialidase C treatment but not endoglycosidase H and peptide N-glycosidase F, whereas ATF6 and GRP94, which are ER proteins that contain N-glycosylation sites, were deglycosylated by endoglycosidase H and peptide N-glycosidase F treatments, as shown by mobility shifts to lower molecular weight (Fig. 4A). On the other hand, NUCB1-P28A was detected as only a single band and was resistant to sialidase C treatment (Fig. 4B). Thus, NUCB1-P28A seemed not to reach the Golgi.
To examine turnover kinetics after synthesis of NUCB1-WT or NUCB1-P28A protein, we performed a pulse-chase assay with [35S]Met/Cys labeling (Fig. 4C). After 20–60 min, sialylation of NUCB1-WT was indicated by a shift in molecular weight, suggesting that it was normally transported to the Golgi (Fig. 4C, asterisk). In addition, at 60 min of chase, signal intensity of the intracellular WT protein was reduced by less than 40%. By contrast, NUCB1-P28A exhibited no band shift, and the signal intensity remained more than 70% at that time point. Furthermore, we compared time-dependent secretion into the culture medium of NUCB1-WT with that of NUCB1-P28A by immunoblot analysis (Fig. 4D). After changing to fresh medium, the WT protein was detected at 1 h and correlated with the reduction of intracellular [35S]Met signal intensity. On the other hand, the P28A mutant was only marginally detected even after 2 h. Thus, the P28A mutant remained in the ER for a prolonged period. These results suggested that P28A had an abnormality in its ER export step and a defect in subsequent events, such as Golgi transport and secretion.
Pro28 Mutation Has Little Effect on Protein Maturation
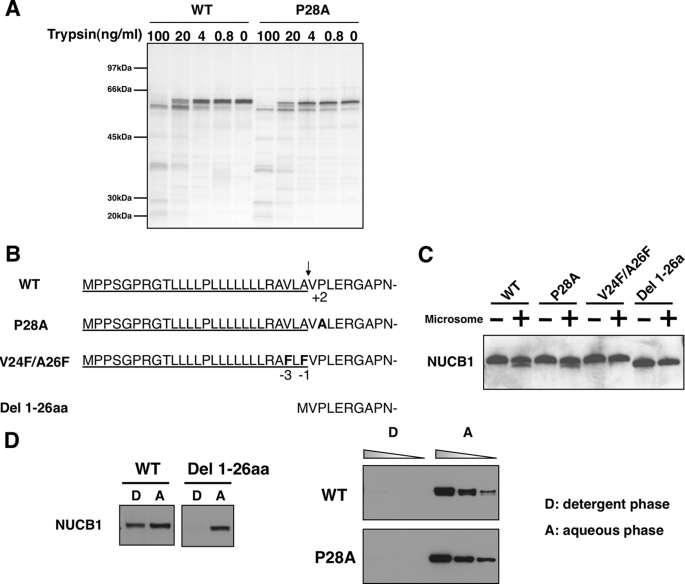
In general, misfolded proteins remain in the ER because of its quality control system. A single amino acid mutation or deletion can potentially affect protein folding within the ER, resulting in a defective export from the ER (25, 26). As shown in Fig. 2B, 1–35EGFP(P28A) emitted strong luminescence, as observed with the wild type. Additionally, in a trypsin digestion assay, we saw no apparent difference in the fragment patterns between NUCB1-WT and -P28A (Fig. 5A). Therefore, the Pro28 mutation probably had little effect on the overall protein conformation.
FIGURE 5.
Pro28 mutation had little effect on protein maturation. A, 293T cells were transiently transfected with pNUCB1-WT or -P28A (FLAG). Each cell lysate was immunopurified with anti-FLAG beads and then eluted using FLAG peptides. The samples were digested with the indicated dose of trypsin at room temperature for 10 min and then subjected to SDS-PAGE. The gel was silver-stained. B, N-terminal amino acid sequence of each NUCB1 construct. The signal peptide is underlined, and its cleavage site is shown by an arrow. Boldface type represents mutated amino acids. C, in vitro transcription/translation was performed using pNUCB1wt, P28A, V24F/A26F, and Del 1–26aa plasmids (non-tag) in the presence or absence of a dog microsome fraction. The reaction samples were boiled in SDS sample buffer and then underwent immunoblot analysis with anti-NUCB1 antibody. D, phase separation assay using Triton X-114. In vitro synthesized NUCB1-WT or Del 1–26aa protein (non-tag), as shown in B, was suspended in a 1% Triton X-114 solution. The samples were phase-partitioned and subjected to immunoblot analysis (left). 293T transfected with pNUCB1-WT, P28A (FLAG-tag) plasmids were lysed in a 1% Triton X-114 solution, including a protease inhibitor mixture. The phase-separated samples were immunopurified with anti-FLAG antibody and phase-partitioned. Graded amounts of lysate (2, 6, and 18 μl) were subjected to immunoblot analysis (right). D and A, detergent phase and aqueous phase, respectively.
Next, we examined whether the mutation of Pro28 affected processing of the N-terminal ER signal peptide, which is an initial step for protein maturation at the ER (27). The signal peptide targets the newly synthesized polypeptide to the ER, and it is cleaved rapidly after entering the ER. In fact, the N-terminal peptide sequencing of the human NUCB1 protein purified from cell or culture medium detected only the sequence corresponding to amino acids beginning at position 27 (data not shown), indicating that cleavage of the signal peptide indeed occurs between 26 and 27. Pro28 is located at the +2-position from the cleavage site, and such an amino acid residue can affect cleavage efficiency (28, 29). Therefore, we performed in vitro a signal peptide cleavage assay using the constructs shown in Fig. 5B. In vitro translation of NUCB1-WT in the presence of the dog microsome fraction, which contains signal peptidase, produced a new lower band corresponding to the Del 1–26aa mutant, which lacks a signal peptide. NUCB1-P28A also produced the lower band at the same level as the wild-type (Fig. 5C). In contrast, in vitro translated V24F/A26F, which was a mutant resistant to signal peptide cleavage due to phenylalanine substitution at −1 and −3-positions, known as sites having a strong effect on signal peptide cleavage (28, 29), showed no production of the lower band despite the presence of microsome fraction (Fig. 5C). Thus, it is unlikely that the Pro28 mutation affected the signal peptide cleavage process in vitro.
Next, we performed a phase separation assay using Triton X-114 to confirm that the Pro28 mutation also had little effect on signal peptide cleavage within the cell. In this assay, hydrophilic proteins are found exclusively in the aqueous phase, and membrane proteins with an amphiphilic nature are recovered in the detergent phase (30). As shown in Fig. 5D (left), in vitro translated NUCB1 protein, which is a proNUCB1 protein containing a hydrophobic signal peptide, was found in both the detergent and aqueous phases, whereas in vitro translated Del 1–26aa was detected only in the aqueous phase (Fig. 5D, left). To examine in vivo signal peptide processing, we used NUCB1-WT or -P28A protein immunopurified from transiently transfected cells. Neither was detected in the detergent phase (Fig. 5D, right), strongly suggesting that the signal peptide of P28A was processed successfully within the cell. Thus, mutation of Pro28 had little effect on signal peptide cleavage in vivo. Taken together, we concluded that the Pro28 mutation had little effect on the protein maturation process.
Significant Role of Pro28 in ER Export of Cargo Protein
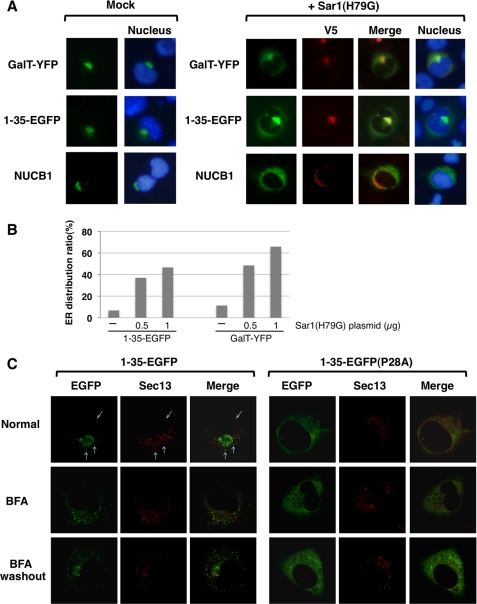
Newly synthesized soluble or membrane cargo proteins are sorted into export vesicles for transport after folding within the ER. Membrane cargo proteins are known to be concentrated in the COPII vesicle via its cytoplasmic region, whereas the transport mechanism of soluble cargo proteins is largely unknown (31, 32). We first examined whether ER export of NUCB1 is dependent on the COPII system. For this purpose, we used Sar1(H79G), a dominant negative form of Sar1 GTPase that is an essential component for COPII coat assembly (33). The Sar1(H79G) mutant is restricted to the GTP-bound state and biochemically functions as a competitive inhibitor of wild-type Sar1 recruitment. As shown in Fig. 6A (top), Sar1(H79G) expression prevented Golgi localization of GalT-YFP, which was a fusion protein of the N-terminal Golgi localization segment of β-1,4-galactosyltransferase and yellow fluorescent protein (YFP) and led to ER-like perinuclear distribution of GalT-YFP. Likewise, overexpression of Sar1(H79G) led to perinuclear distribution of both 1–35EGFP and NUCB1 (Fig. 6A, bottom). Counting YFP- or EGFP-positive cells revealed that the ratio of perinuclear distribution to Golgi localization was increased in the cells cotransfected with Sar1(H79G) (Fig. 6B). These results showed that ER export of NUCB1 was mediated by COPII-dependent vesicle transport.
FIGURE 6.
Significant role of Pro28 in the ER export of cargo protein. A, after transfection with GalT-YFP, p1–35EGFP, pNUCB1 alone or together with pSar1(H79G) plasmid, HT1080 cells were fixed and double-stained with anti-V5 (for Sar1) and anti-NUCB1 antibody. GFP and YFP were analyzed by its fluorescence. The nuclei were counterstained with Hoechst 33342. B, HT1080 cells were co-transfected with GalT-YFP or p1–35EGFP together with pSar1(H79G). The ratio of ER distribution was determined by counting more than 100 EGFP- or YFP-positive cells. C, live cell imaging with confocal microscope. p1–35EGFP or p1–35EGFP (P28A) was transiently co-transfected with a Sec13-DsRed vector in HT1080 cells. The cells were observed under three different conditions (normal, treatment with 5 μg/ml BFA for 2 h, overnight culture after BFA washout).
Next, we examined 1–35EGFP-WT or -P28A concentration at the ERES, where COPII vesicle formation occurs. To visualize the ERES, we constructed a vector that encoded the Sec13-DsRed fusion protein. Sec13 is a component of the COPII complex and is known as an ERES marker (15). When 1–35EGFP and Sec13-DsRed vectors were transiently cotransfected into the cells, 1–35EGFP largely exhibited a Golgi distribution pattern at the steady state but minor fraction colocalized with Sec13 in punctate structures (Fig. 6B, top left three panels, arrow). The colocalization of 1–35EGFP with Sec13 was augmented in cells treated with brefeldin A (BFA), a reversible ER-to-Golgi traffic inhibitor, which strongly triggers ER redistribution of the Golgi matrix, whereas BFA had little effect on ERES formation, represented by Sec13-positive punctate structure (Fig. 6B, left middle panels), as reported previously (35). After BFA washout, 1–35EGFP recovered the Golgi distribution pattern (Fig. 6B, left bottom panels). These observations indicated that 1–35EGFP was concentrated in the ERES to be transported to the Golgi. On the other hand, the P28A mutant showed a stably diffused pattern within the ER and was not detected in Sec13-positive punctate structures under any conditions examined (Fig. 6B, right). Essentially the same results were obtained using HT1080 cells that stably expressed the Sec13-DsRed fusion protein (data not shown). These observations strongly suggest that Pro28 functions as an ER export signal to concentrate proteins in the ERES.
DISCUSSION
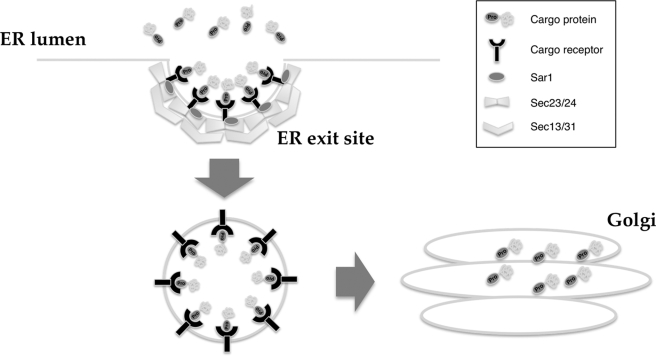
The ER export signal of soluble cargo has been poorly understood, although the specific export signals of some membrane cargoes, such as the diacidic or dihydrophobic motifs (DXE, LL, and FF), have been reported recently (14). In this study, we describe how proline at the +2-position from the signal peptide cleavage site plays an important role in NUCB1 protein export from the ER. The fusion of the N-terminal 35 amino acids of the NUCB1 protein, including the Pro+2 site, into EGFP was sufficient to localize the fusion protein in the Golgi, and mutation of the Pro+2 residue led to a striking ER distribution. A similar result was obtained in other Golgi-localized CREC family proteins with Pro+2, which was confirmed by N-terminal sequencing (Fig. 3). In addition, ER export of 1–35EGFP depended on the COPII machinery. Mutation of Pro+2 disrupted the protein's concentration at the ERES. Taken together, our results demonstrate that Pro+2 can function as a novel ER export signal of soluble Golgi proteins (Fig. 7).
FIGURE 7.
ER export model via Pro+2 signal. Pro+2 signal-containing proteins, such as NUCB1 and CREC, were concentrated in the ER exit site by a putative, unidentified cargo receptor in the ER lumen. Then these proteins were transported to the Golgi via the formed vesicle.
Previous examination of amino acid frequency in the region following signal peptide cleavage site of 270 secreted or membrane proteins showed a preference of Pro at many sites, excluding the +1-position after the cleavage site (36). Indeed, in the case of NUCB1, Pro appears at the +2- and +8-positions. However, an analysis by single amino acid substitution at each of the two positions revealed that only Pro+2 was a determinant for Golgi localization (Fig. 2). Likewise, among the CREC family proteins, Pro is only present at +2 in the downstream vicinity of the signal peptide cleavage site, and Pro+2 was important for Golgi localization (Fig. 3). Furthermore, Pro+2 of the NUCB1 and CREC family proteins have been conserved across species (human, mouse, and rat). Thus, the presence of Pro at the +2-position is likely to be very important as an ER export signal of these soluble cargo proteins.
Some membrane cargo proteins, such as vesicular stomatitis virus glycoprotein and ERGIC53, have specific ER export signals within their cytosolic regions, which are important in direct interaction with the coat protein complex (16, 17). However, soluble cargo proteins, including NUCB1, cannot be directly captured by coat subunits, and little is known about their export signals. Until now, two export models, “bulk flow” and “receptor-mediated,” have been described in studies of soluble cargo protein export (37–40). In the bulk flow model, soluble cargo proteins depart in vesicles at a concentration equal to that found in the ER lumen. In the receptor-mediated export model, receptor-like proteins, such as Erv29, ERGIC53, Emp24, and Erv26, assist in concentrative sorting of specific soluble cargo proteins into the COPII vesicle (14, 38). In the case of NUCB1 proteins, concentration at the ERES was Pro+2-dependent, suggesting that the ER export is mediated by receptor-like proteins. A recent study indicated that ERv29 could recognize and bind to a specific ER export signal contained within target soluble cargo proteins (19). Emp24 was involved in transport of several soluble cargoes, including the glycosylphosphatidylinositol-anchored protein Gas1p (41). ERGIC53, which possesses lectin activity, has been reported to mediate transport of glycoproteins with N-glycosylation (20). However, NUCB1 is neither an N-glycosylated protein nor a glycosylphosphatidylinositol-anchored protein. In addition, target proteins of the above mentioned cargo receptors have no Pro+2 signal. Therefore, another unknown receptor may recognize the Pro+2 signal and may mediate transport of the Golgi proteins, including NUCB1 (Fig. 7).
NUCB1 and many ER-to-Golgi transport genes, including erv29, are up-regulated during the unfolded protein response (11, 42, 43). Our previous report showed that NUCB1 negatively regulated the unfolded protein response by inhibiting ATF6 in the Golgi (12). Despite export restriction of the general cargo proteins under ER stress conditions, ATF6 is transported, via the COPII machinery, from the ER to the Golgi, where it is converted to active form by specific proteases that induce the expression of many stress-responsive genes (34). NUCB1 was also able to localize in the Golgi under ER stress conditions and to prevent ATF6 activation at the Golgi (12). It is important to note that the mutation of the NUCB1 Pro+2 abolished both Golgi localization and inhibitory effect on ATF6 activation (12). The transport mechanism via Pro+2 might be important for successful ER export of NUCB1 during the unfolded protein response, and such an export system would allow the cell to respond properly under stress conditions.
In conclusion, we have shown that Pro+2 of NUCB1, as well as CREC proteins, is a determinant for whether proteins are exported from the ER. Interestingly, computational prediction or validation of amino acid sequence following the signal peptide cleavage site in a previous study showed that many growth factors or cell surface proteins also have proline residues at the +2-position (36). Our study provides information to elucidate the mechanism by which a large number of soluble cargo and membrane cargo proteins are efficiently and properly sorted into the ER vesicles.
This work was supported in part by Grant-in-aid for Cancer Research 21-3-1 from the Ministry of Health, Labour, and Welfare (to A. T.), a Grant-in-aid for scientific research on priority areas for cancer (to T. T. and A. T.), a grant from the New Energy and Industrial Technology Development Organization (NEDO) of Japan, and aid from the Ishizu Scholarship Foundation (to Y. T.).
- ER
- endoplasmic reticulum
- COPII
- coat protein complex II
- ERES
- ER exit site
- PBS
- phosphate-buffered saline
- GFP
- green fluorescent protein
- EGFP
- enhanced GFP
- YFP
- yellow fluorescent protein
- WT
- wild type
- aa
- amino acid(s)
- BFA
- brefeldin A.
REFERENCES
- 1.Miura K., Titani K., Kurosawa Y., Kanai Y. (1992) Biochem. Biophys. Res. Commun. 187, 375–380 [DOI] [PubMed] [Google Scholar]
- 2.Kanai Y., Miura K., Uehara T., Amagai M., Takeda O., Tanuma S., Kurosawa Y. (1993) Biochem. Biophys. Res. Commun. 196, 729–736 [DOI] [PubMed] [Google Scholar]
- 3.Lin P., Le-Niculescu H., Hofmeister R., McCaffery J. M., Jin M., Hennemann H., McQuistan T., De Vries L., Farquhar M. G. (1998) J. Cell Biol. 141, 1515–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura K., Hirai M., Kanai Y., Kurosawa Y. (1996) Genomics 34, 181–186 [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki N., Hibi M., Kanai Y., Insel P. A. (1995) FEBS Lett. 373, 155–158 [DOI] [PubMed] [Google Scholar]
- 6.Ballif B. A., Mincek N. V., Barratt J. T., Wilson M. L., Simmons D. L. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5544–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendel M., Sommarin Y., Bergman T., Heinegård D. (1995) J. Biol. Chem. 270, 6125–6133 [DOI] [PubMed] [Google Scholar]
- 8.Kanai Y., Tanuma S. (1992) Immunol. Lett. 32, 43–48 [DOI] [PubMed] [Google Scholar]
- 9.Lin P., Yao Y., Hofmeister R., Tsien R. Y., Farquhar M. G. (1999) J. Cell Biol. 145, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S. N., Miyauchi M., Koshikawa N., Maruyama K., Kubota T., Miura K., Kurosawa Y., Awaya A., Kanai Y. (1994) Pathol. Int. 44, 844–849 [DOI] [PubMed] [Google Scholar]
- 11.Lavoie C., Meerloo T., Lin P., Farquhar M. G. (2002) Mol. Endocrinol. 16, 2462–2474 [DOI] [PubMed] [Google Scholar]
- 12.Tsukumo Y., Tomida A., Kitahara O., Nakamura Y., Asada S., Mori K., Tsuruo T. (2007) J. Biol. Chem. 282, 29264–29272 [DOI] [PubMed] [Google Scholar]
- 13.Petersson U., Somogyi E., Reinholt F. P., Karlsson T., Sugars R. V., Wendel M. (2004) Bone 34, 949–960 [DOI] [PubMed] [Google Scholar]
- 14.Barlowe C. (2003) Trends. Cell Biol. 13, 295–300 [DOI] [PubMed] [Google Scholar]
- 15.Hammond A. T., Glick B. S. (2000) Mol. Biol. Cell 11, 3013–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura N., Balch W. E. (1997) Science 277, 556–558 [DOI] [PubMed] [Google Scholar]
- 17.Kappeler F., Klopfenstein D. R., Foguet M., Paccaud J. P., Hauri H. P. (1997) J. Biol. Chem. 272, 31801–31808 [DOI] [PubMed] [Google Scholar]
- 18.Sato K., Nakano A. (2002) Mol. Biol. Cell 13, 2518–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otte S., Barlowe C. (2004) Nat. Cell Biol. 6, 1189–1194 [DOI] [PubMed] [Google Scholar]
- 20.Appenzeller C., Andersson H., Kappeler F., Hauri H. P. (1999) Nat. Cell Biol. 1, 330–334 [DOI] [PubMed] [Google Scholar]
- 21.Honoré B., Vorum H. (2000) FEBS Lett. 466, 11–18 [DOI] [PubMed] [Google Scholar]
- 22.Ozawa M., Muramatsu T. (1993) J. Biol. Chem. 268, 699–705 [PubMed] [Google Scholar]
- 23.Yabe D., Nakamura T., Kanazawa N., Tashiro K., Honjo T. (1997) J. Biol. Chem. 272, 18232–18239 [DOI] [PubMed] [Google Scholar]
- 24.Scherer P. E., Lederkremer G. Z., Williams S., Fogliano M., Baldini G., Lodish H. F. (1996) J. Cell Biol. 133, 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskowski R. A., Thornton J. M. (2008) Nat. Rev. Genet. 9, 141–151 [DOI] [PubMed] [Google Scholar]
- 26.Cheung J. C., Deber C. M. (2008) Biochemistry 47, 1465–1473 [DOI] [PubMed] [Google Scholar]
- 27.Martoglio B., Dobberstein B. (1998) Trends Cell Biol. 8, 410–415 [DOI] [PubMed] [Google Scholar]
- 28.Perlman D., Halvorson H. O. (1983) J. Mol. Biol. 167, 391–409 [DOI] [PubMed] [Google Scholar]
- 29.Shen L. M., Lee J. I., Cheng S. Y., Jutte H., Kuhn A., Dalbey R. E. (1991) Biochemistry 30, 11775–11781 [DOI] [PubMed] [Google Scholar]
- 30.Bordier C. (1981) J. Biol. Chem. 256, 1604–1607 [PubMed] [Google Scholar]
- 31.Kuehn M. J., Herrmann J. M., Schekman R. (1998) Nature 391, 187–190 [DOI] [PubMed] [Google Scholar]
- 32.Aridor M., Weissman J., Bannykh S., Nuoffer C., Balch W. E. (1998) J. Cell Biol. 141, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aridor M., Bannykh S. I., Rowe T., Balch W. E. (1995) J. Cell Biol. 131, 875–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadanaka S., Yoshida H., Kano F., Murata M., Mori K. (2004) Mol. Biol. Cell 15, 2537–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward T. H., Polishchuk R. S., Caplan S., Hirschberg K., Lippincott-Schwartz J. (2001) J. Cell Biol. 155, 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z., Henzel W. J. (2004) Protein Sci. 13, 2819–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. (1987) Cell 50, 289–300 [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Menárguez J. A., Geuze H. J., Slot J. W., Klumperman J. (1999) Cell 98, 81–90 [DOI] [PubMed] [Google Scholar]
- 39.Malkus P., Jiang F., Schekman R. (2002) J. Cell Biol. 159, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno M., Singer S. J. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 5732–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñiz M., Nuoffer C., Hauri H. P., Riezman H. (2000) J. Cell Biol. 148, 925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., Walter P. (2000) Cell 101, 249–258 [DOI] [PubMed] [Google Scholar]
- 43.Caldwell S. R., Hill K. J., Cooper A. A. (2001) J. Biol. Chem. 276, 23296–23303 [DOI] [PubMed] [Google Scholar]