Abstract
Adipogenesis is a key differentiation process relevant to obesity and associated diseases such as type 2 diabetes. This process involves temporally regulated genes controlled by a set of transcription factors, CCAAT/enhancer-binding proteins (C/EBP) β, C/EBPδ, and C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ). Currently, PPARγ is universally accepted as the master regulator that is necessary and sufficient to induce adipogenesis as no known factor can induce adipogenesis without PPARγ. We present evidence that a novel zinc finger protein, MCP-1-induced protein (MCPIP), can induce adipogenesis without PPARγ. Classical adipogenesis-inducing medium induces MCP-1 production and expression of MCPIP in 3T3-L1 cells before the induction of the C/EBP family of transcription factors and PPARγ. Knockdown of MCPIP prevents their expression and adipogenesis as measured by expression of adipocyte markers and lipid droplet accumulation. Treatment of 3T3-L1 cells with MCP-1 or forced expression of MCPIP induces expression of C/EBPβ, C/EBPδ, C/EBPα, and PPARγ and adipogenesis without any other inducer. Forced expression of MCPIP induces expression of the C/EBP family of transcription factors and adipogenesis in PPARγ−/− mouse embryonic fibroblasts. Thus, MCPIP is a newly identified protein that can induce adipogenesis without PPARγ.
The incidence of obesity and associated diseases is increasing dramatically throughout the world. The global incidence of type 2 diabetes is projected to double to 350 million cases by the year 2030 (1). Because adipose tissue serves as a crucial integrator of glucose homeostasis, understanding of adipocyte biology is crucial for understanding the pathophysiological basis of obesity and related metabolic diseases (2, 3). Adipogenesis and angiogenesis, which supply blood to the growing adipose tissue, occur in the growing adipose tissue when the caloric intake significantly exceeds caloric expenditure. Adipogenesis involves a temporally regulated set of gene expression controlled by a set of transcription factors (3, 4). C/EBPβ2 and C/EBPδ are induced early, followed by C/EBPα and PPARγ. It is widely accepted that PPARγ is the master regulator of adipogenesis (2, 4). This conclusion is based mainly on the finding that forced expression of PPARγ in C/EBPα−/− fibroblasts induces adipogenesis (5), whereas forced expression of C/EBPα in PPARγ−/− fibroblasts does not induce adipogenesis (6). Thus, the current concept is that PPARγ is both necessary and sufficient for adipogenesis. No other factor is known that can induce adipogenesis in the absence of PPARγ (7). Here we report that a newly discovered zinc finger protein, MCP-1-induced protein (MCPIP) (8), induces adipogenesis in the absence of PPARγ. In an engineered tissue model, MCP-1 treatment was reported to enhance adipose tissue volume (9). In 3T3-L1 cells undergoing adipogenesis in the commonly used adipogenesis-inducing medium, containing dexamethasone, 3-isobutyl-1-methylxanthine, and insulin (DMI), MCP-1 is produced, and MCPIP is induced before the induction of the C/EBP family of transcription factors and PPARγ. Knockdown of MCPIP with siRNA inhibits induction of these genes and adipogenesis. Treatment of 3T3-L1 cells with MCP-1 or forced expression of MCPIP induces adipogenesis without the adipogenesis-inducing DMI medium. Forced expression of MCPIP induces adipogenesis in PPARγ−/− mouse embryonic fibroblasts (MEF). Thus, MCPIP is a newly identified inducer of adipogenesis.
EXPERIMENTAL PROCEDURES
Cell Culture
The 3T3-L1 fibroblast cell line was obtained from the American Type Culture Collection (ATCC). PPARγ−/− MEF cell line was obtained from Dr. Bruce Spiegelman, Harvard University. Cells were maintained in Dulbecco's modified Eagle's medium with 1% penicillin/streptomycin and 10% fetal bovine serum until experimentation. Cells growing on plates at 60% confluency were treated with or without 10 ng/ml MCP-1 for 7 days. Adipocyte differentiation was induced by replacing Dulbecco's modified Eagle's medium with media containing DMI (Zen-Bio, Inc.). Construction of the MCPIP-GFP expression plasmid has been described previously (8). Cells were transfected with 100 nmol/liter of a chemically synthesized siRNA targeted for MCPIP (Ambion) or with 100 nmol/liter nonspecific siRNA (Ambion) 12 h prior to treatment with DMI. All cells types were transfected using FuGENE HD (Roche Applied Science).
Oil Red-O
3T3-L1 or PPARγ−/− MEF cells were fixed with 10% formalin for 30 min at room temperature 8 days after experimental treatment. Cells were then washed with 60% isopropyl alcohol followed by treatment with Oil Red-O (2.1 mg/ml) for 20 min at room temperature. Samples were then washed four times with H2O. Images of sample plates stained with Oil Red-O were taken and analyzed using morphometric software analysis. Oil Red-O was evaluated by measuring total objects stained, area of plate stained, and total intensity of stained area. All three methods yielded similar results. After examining the plates microscopically, they were treated with 100% isopropyl alcohol to extract Oil Red-O. The solution was then measured for absorbance at 520 nm.
RT-PCR
Total RNA was isolated from treated fibroblasts using TRIzol (Invitrogen). First-strand cDNA was synthesized using 1 μg of total RNA (DNase-treated) using the RT cDNA synthesis kit (High Capacity, Applied Biosystems). β-Actin served as internal control. The primer set forward 5-ctccaatgttctcaaacttac-3′ and reverse 5′-gtattcctatggcttccagtgc-3′ was used to detect a 400-bp product in the PPARγ−/− MEF cell line to confirm the deletion of exon 2 in PPARγ (9).
Immunoblot Analysis
3T3-L1 and PPARγ−/− MEFs were treated with cell lysis buffer (20% glycerol, 0.1% Triton X-100, 8% 0.5 m EDTA, and 1% 1 m dithiothreitol), and protein samples were collected and subjected to immunoblot using polyclonal antibodies specific for MCPIP (1:2000), C/EBPα (1:2000), C/EBPβ (1:2000, Abcam), C/EBPδ (1:2000, Abcam), PPARγ (1:2000, Abcam), adiponectin (1:3000, Abcam), LPL (1:2000, Abcam), aP2 (1:2000, Abcam), and apelin (1:3000, Abcam). Rabbit secondary antibody was used at a concentration of 1:5000 except for aP2, which used a chicken secondary antibody at a concentration of 1:5000.
RESULTS
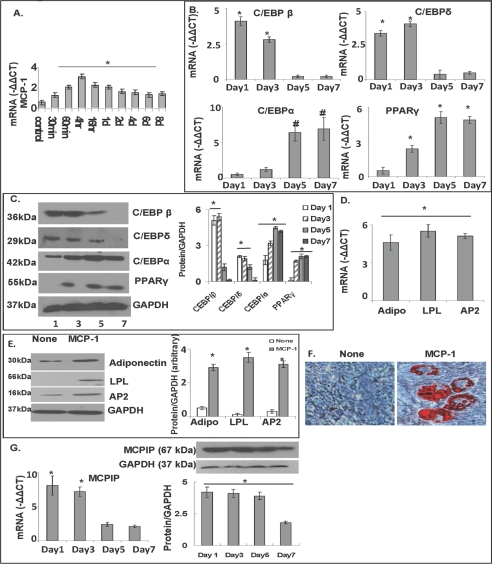
We recently reported that MCP-1 binding to CCR2 leads to the induction of a novel zinc finger protein, called MCPIP, that can induce cell death (8) or differentiation leading to angiogenesis (10). MCP-1 production by preadipocytes has been detected (11), and in a tissue engineering model of adipogenesis MCP-1 treatment was reported to increase adipose tissue mass in vivo (9). In view of these observations that suggest the possibility of a role for MCP-1 in adipogenesis, we tested whether differentiation of 3T3-L1 cells into adipocytes involves MCP-1. First, we tested whether 3T3-L1 cells were induced to produce MCP-1 by DMI medium. Real time RT-PCR showed that MCP-1 transcript level reached maxima 4 h after treatment with DMI medium compared with non-DMI-containing medium (Fig. 1A). We then tested whether MCP-1 treatment could induce differentiation of 3T3L-1 cells into adipocytes in a non-DMI-containing medium. MCP-1 treatment induced expression of the C/EBP family of transcription factors and PPARγ that is known to be induced during differentiation of 3T3-L1 cells into adipocytes (Fig. 1B). C/EBPβ and C/EBPδ were induced early followed by induction of C/EBPα and PPARγ. Induction of these adipogenesis-associated transcription factors was clearly seen at the transcript level determined by real time PCR analysis and at the protein level as determined by immunoblot analysis (Fig. 1, B and C). MCP-1 treatment induced adipocyte markers. Induction of adiponectin, LPL, and AP2 was clearly seen at the transcript level determined by real time PCR and protein level as determined by immunoblot analysis (Fig. 1, D and E). Microscopic examination revealed lipid body accumulation. Oil Red-O staining revealed robust accumulation of lipid bodies (Fig. 1F). We tested whether MCP-1-induced adipogenesis is mediated via MCPIP. Upon MCP-1 treatment, MCPIP was induced to a maximal level during the 1st day (Fig. 1G). This induction was clear from real time PCR measurement of the transcript level and from the immunoblot analysis of MCPIP protein level. After the 2nd day MCPIP levels decreased. This result is consistent with the involvement of MCPIP in adipogenesis.
FIGURE 1.
3T3-L1 fibroblasts produced MCP-1 when treated with DMI, and MCP-1 treatment of 3T3-L1 fibroblasts induced MCPIP expression and adipogenesis. A, 3T3-L1 cells were treated with or without DMI. RNA was isolated, and MCP-1 transcript levels were measured using real time RT-PCR (* = p < 0.02). −ΔΔCt values of treated/untreated are displayed. B, 3T3-L1 cells grown to 60% confluency were treated with or without 10 ng/ml MCP-1 protein for 7 days. RNA and cell lysate were collected 1, 3, 5, and 7 days after treatment. Transcript levels of the adipogenesis markers C/EBPβ, C/EBPδ, C/EBPα, and PPARγ were measured using real time RT-PCR (* = p < 0.02; # = p < 0.03). C, protein levels of C/EBPβ, C/EBPδ, C/EBPα, and PPARγ were measured using immunoblot analysis. Results were quantified against GAPDH. D, RNA and cell lysate were collected 7 days after MCP-1 treatment. Real time RT-PCR was used to measure the adipogenesis markers adiponectin (Adipo), LPL, and AP2 (normalized to β-actin, −ΔΔCt values of treated/untreated are displayed; * = p < 0.02). E, protein levels of adiponectin, LPL, and AP2 were measured using immunoblot analysis. Results were quantified against GAPDH (* = p < 0.02). F, cells were stained with Oil Red-O 8 days after MCP-1 treatment. G, MCPIP transcript levels were measured using real time RT-PCR (normalized to β-actin, −ΔΔCt values of treated/untreated are displayed). Protein levels of MCPIP were measured using immunoblot analysis. Results were quantified against GAPDH (* = p < 0.02; # = p < 0.03). All experiments were repeated three times.
If MCPIP is a key player in adipogenesis, it should be induced early during the adipocyte differentiation and induced in 3T3-L1 cells by the commonly used adipogenesis-inducing DMI medium. The levels of MCPIP, C/EBPβ, and C/EBPδ reached maximal levels during the first 2 days. Induction of PPARγ and C/EBPα followed as expected (Fig. 2). That MCPIP plays a critical role in adipogenesis is indicated by the finding that knockdown of MCPIP expression with siRNA specific for MCPIP inhibited adipogenesis induced by DMI medium, whereas nonspecific (scrambled) siRNA had very little effect (Fig. 3, A–H). This inhibition was demonstrated by the inhibition of appearance of adipocyte markers adiponectin and LPL at the transcript level as indicated by real time RT-PCR (Fig. 3A) and at the protein level as measured by immunoblot analysis (Fig. 3, B and C). In addition, real time RT-PCR measurements of transcript levels showed that knockdown of MCPIP expression resulted in inhibition of induction of the known adipogenesis-associated transcription factors C/EBPβ, C/EBPδ, C/EBPα, and PPARγ (Fig. 3, D–G). Staining of the cells with Oil Red-O after 8 days of differentiation in DMI medium showed that knockdown of MCPIP by treatment with siRNA, specific for MCPIP, drastically inhibited lipid droplet accumulation, whereas nonspecific siRNA had little effect (Fig. 3H). Measurement of Oil Red-O extracted from stained cells also shows that knockdown of MCPIP inhibited lipid accumulation (data not shown). These results suggest that MCPIP plays a critical role in adipogenesis induced by DMI.
FIGURE 2.
DMI-induced adipocyte differentiation of 3T3-L1 fibroblast cells elicited an early increase in MCPIP expression. 3T3-L1 fibroblasts were treated with or without DMI. RNA was collected at 1–4 days after treatment. Real time RT-PCR was used to measure MCPIP (A), and the known adipogenesis markers C/EBPβ (B), C/EBPδ (C), C/EBPα (D), and PPARγ (E). Results were normalized to β-actin; −ΔΔCt values of treated/untreated are displayed (* = p < 0.02). F, immunoblot analysis was performed with cell lysate collected at the same time points with antibodies against MCPIP, C/EBPβ, C/EBPδ, C/EBPα, and PPARγ, G–K, results in F were quantified against GAPDH (* = p < 0.02; # = p < 0.03; error bars = p < 0.05). All experiments were repeated three times.
FIGURE 3.
Knockdown of MCPIP inhibited DMI-induced adipogenesis. 3T3-L1 fibroblasts were treated with siRNA specific for MCPIP for 24 h before treatment with DMI. A, RNA was isolated from all samples 7 days after DMI treatment and was evaluated for transcript levels for the adipocyte markers, adiponectin, and LPL by using RT-PCR; β-actin served as a control. B, immunoblot analysis was performed with cell lysate collected from all samples 7 days after DMI treatment. The samples were evaluated for the adipocyte markers adiponectin and LPL. C, results in B were quantified against GAPDH (* = p < 0.02; # = p < 0.03). NS siRNA, nonspecific (scrambled) siRNA. D–G, RNA was isolated from samples 1–4 days after DMI treatment. Real time RT-PCR was used to measure transcript levels of C/EBPβ, C/EBPδ, C/EBPα, and PPARγ. β-Actin served as a control; −ΔΔCt values of treated/untreated are displayed. * = p < 0.02; # = p < 0.03. H, cells were stained with Oil Red-O 8 days after treatment with DMI. All experiments were repeated three times.
If MCPIP is one of the critical regulators involved in adipogenesis, forced expression of MCPIP in 3T3-L1 cells might be expected to cause induction of adipogenesis in the absence of adipogenesis-inducing DMI mixture. Transfection of 3T3-L1 cells with an expression construct for MCPIP-GFP caused the induction of adipogenesis in the absence of DMI medium, whereas GFP controls did not show differentiation (Fig. 4). Forced expression of MCPIP induced the expression of the well established cascade of gene expression known to occur in DMI medium. MCPIP expression induced C/EBPβ, C/EPBδ, C/EBPα, and PPARγ, the transcription factors well known to be involved in adipogenesis. The induction of these transcription factors was seen both at the transcript levels measured by real time RT-PCR (Fig. 4, B–E) and at a protein level measured by immunoblot analyses (Fig. 4, F–K). C/EBPβ and C/EBPδ levels reached maximal levels before C/EBPα and PPARγ levels reached maximal levels as seen in DMI medium. MCPIP-induced adipogenesis was demonstrated by the induction of adipocyte markers, adiponectin and LPL, both at the transcript level as indicated by real time RT-PCR and at protein level as measured by immunoblot analysis (Fig. 4, L–O). Staining of the cells transfected with MCPIP-GFP expression plasmid, after 8 days in non-DMI medium showed strong staining of lipid bodies, whereas GFP controls showed no staining (Fig. 4P). Thus it is clear that MCPIP expression can induce adipogenesis in 3T3-L1 cells in the absence of the usual adipogenesis-inducing factors in the medium. MCP-1 treatment and forced expression of MCPIP in non-DMI medium caused accumulation of lipid droplets comparable with that observed in the DMI medium, although the DMI medium induced the most robust degree of lipid droplet accumulation. This conclusion is based on morphometric quantitative analysis of the stained plates (Fig. 5).
FIGURE 4.
Forced expression of MCPIP-induced adipogenesis without DMI in 3T3-L1 cells. 3T3-L1 fibroblasts were transfected with MCPIP-GFP or GFP alone. RNA and cell lysate were collected 0.5, 1, 3, and 5 days after transfection. Transcript levels of MCPIP (A), and the adipogenesis markers C/EBPβ (B), C/EBPδ (C), C/EBPα (D), and PPARγ (E) were measured using real time RT-PCR (normalized to β-actin; −ΔΔCt values of MCPIP-GFP/GFP are displayed; * = p < 0.02; # = p < 0.03; error bars = p < 0.05). F, protein levels of MCPIP, C/EBPβ, C/EBPδ, C/EBPα, and PPARγ were measured using immunoblot analysis. G–K, results in F were quantified against GAPDH (* = p < 0.02). RNA and cell lysate were collected 7 days after transfection. L, transcript levels of the adipocyte markers, adiponectin and LPL, were measured using RT-PCR; β-actin served as a control; −ΔΔCt values of MCPIP-GFP/GFP are displayed. M, protein levels of the adipocyte markers, adiponectin and LPL, were measured using immunoblot analysis. N and O, results in M were quantified against GAPDH (* = p < 0.02). P, cells were stained with Oil Red-O 8 days after transfection. All experiments were repeated three times.
FIGURE 5.
Treatment of 3T3-L1 cells with MCP-1 or transfection with MCPIP-GFP resulted in oil droplet accumulation comparable with 3T3-L1 cells treated with the classical mixture DMI. 3T3-L1 fibroblasts were treated with DMI or MCP-1 or were transfected with MCPIP-GFP. Cells were stained with Oil Red-O 8 days after treatment. Images of plates were captured, and staining was measured using morphometric software analysis. Quantitation of lipid accumulation in the entire plate was done on the basis of total stained area, number of cells stained, and intensity of staining. All three methods yielded similar results. The results obtained on the basis of total area stained are displayed.
In view of the currently held view that adipogenesis cannot be induced in the absence of PPARγ, we tested whether forced expression of MCPIP can induce adipogenesis in PPARγ−/− MEFs. We transfected PPARγ−/− cells with MCPIP-GFP expression plasmids or GFP control and incubated them in DMI medium. Forced expression of MCPIP in PPARγ−/− cells caused induction of the C/EBP family of transcription factors, C/EBPβ, C/EBPδ, and C/EBPα, as seen both at the transcript level as measured by real time PCR (Fig. 6A) and at protein level as measured by immunoblot analysis (Fig. 6, B–E). The MCPIP-GFP-transfected cells exhibited adipogenesis as indicated by induction of adipocyte markers, Ap2, adiponectin, and LPL, as measured by immunoblot analysis (Fig. 6, F and G), whereas GFP controls showed very little induction. Microscopic examination of the Oil Red-O-stained cells showed robust accumulation of lipid droplets in MCPIP-GFP-transfected PPARγ−/− cells, whereas GFP controls showed little lipid accumulation (Fig. 6H). Because this result is contrary to the currently accepted view that PPARγ is necessary for adipogenesis, we confirmed that the PPARγ−/− MEFs we used were, in fact, PPARγ−/− as reported by the authors who generated this PPARγ null cell line (12) and as indicated under “Experimental Procedures.” Immunoblot analysis confirmed the absence of PPARγ protein (Fig. 6I). Thus, MCPIP expression can induce adipogenesis in the absence of PPARγ.
FIGURE 6.
Forced expression of MCPIP in PPARγ−/− fibroblasts induced adipogenesis. PPARγ−/− fibroblasts were transfected with MCPIP-GFP or GFP alone and were evaluated for adipogenesis. A, RNA was collected from PPARγ−/− fibroblasts 1, 3, and 5 days after transfection with MCPIP-GFP or GFP alone. Transcript levels of C/EBPβ, C/EBP δ, and C/EBPα were evaluated using real time RT-PCR; β-actin served as a control; −ΔΔCt values of MCPIP-GFP/GFP are displayed (* = p < 0.02). B, cell lysate was collected from the same samples and were evaluated via immunoblot for C/EBPβ, C/EBPδ, and C/EBPα. C–E, results in B were quantified against GAPDH (* = p < 0.02; # = p < 0.03). F, immunoblot analysis was performed with cell lysate collected from all samples 1, 3, 5, and 7 days after transfection. Samples were evaluated for the adipocyte markers AP2, adiponectin, and LPL. G, results in A were quantified against GAPDH (* = p < 0.02). H, cells were treated with Oil Red-O 8 days after transfection and were microscopically evaluated. I, PPARγ+/+ or PPARγ−/− fibroblasts were transfected with MCPIP-GFP. Samples were evaluated for PPARγ using immunoblot analysis. All experiments were repeated three times.
DISCUSSION
The results presented here constitute the discovery of a previously unknown key player that can induce adipogenesis in the absence of PPARγ that is currently thought to be necessary and sufficient for adipogenesis. The central role of MCPIP in adipogenesis was demonstrated by the finding that MCP-1 production was induced very early after 3T3-L1 cells were placed in DMI medium. Furthermore, MCP-1 treatment of 3T3-L1 cells induced adipogenesis. This direct demonstration of MCP-1 induction of adipogenesis is consistent with a recent report that MCP-1 enhanced new adipose tissue formation in vivo (9). Adipogenic precursor cells are known to have CCR2 (13). Because MCPIP is induced by the signaling process resulting from MCP-1 interaction with its receptor CCR2 (8), the present results have implications on the role of MCP-1/CCR2 in obesity and type 2 diabetes. In diet-induced insulin resistance, mcp-1 gene expression in adipose tissue increases in ob/ob and db/dp mice (14). Adipocyte-specific overexpression of MCP-1 in mice results in increased macrophage recruitment to adipose tissue and causes hepatic steatosis and insulin resistance in liver and muscle as well as fat tissue. In CCR2−/− mice, recruitment of monocytes/macrophages into adipose tissue is inhibited, and development of obesity-induced insulin resistance is attenuated (15). Macrophage recruitment and consequent inflammatory response in adipose tissue result in secretion of more MCP-1, tumor necrosis factor-α, and interleukin-β (16). These agents cause increase in lipolysis and decrease in triacylglycerol synthesis leading to increased levels of fatty acids and in the availability of triacylglycerols for uptake directly into skeletal muscle (3). These excess circulating lipids cause accumulation of fatty acyl-CoA in skeletal muscle, liver, and β cells leading to insulin resistance and type 2 diabetes (17, 18). The inflammatory stress causes interleukin-6 secretion from the adipose tissue that leads to expression of suppressor of cytokine signaling expression that is known to induce hepatic insulin resistance (19). Thus, there is strong evidence for a key role that MCP-1/CCR2 system plays in obesity and type 2 diabetes. The role of MCP-1 in this process has been questioned on the basis that macrophage accumulation in adipose tissue was not significantly attenuated in MCP-1-deficient mice (20). However, MCP-5 can substitute as a CCR2 ligand in mice (21).
The exact source of MCP-1 in adipose tissue is not known, although there are several possibilities. Obesity is well known to cause elevation of MCP-1 levels. The resident macrophages and macrophages infiltrating the adipose tissue could produce MCP-1. MCP-1 production could also be caused by endotoxemia from gut flora elevated by feeding (22). Death of adipocytes could release MCP-1, as dying cells are known to release MCP-1 (23). Adipose tissue in obese animals is reported to have localized hypoxia that could lead to hypoxia-inducible factor induction and MCP-1 production (24, 25). We have found that under hypoxia human macrophages secrete MCP-1. In adipose tissue macrophage-containing areas were found to be hypoxic, and thus these macrophages would also produce MCP-1.
We show that MCP-1 binding to CCR2, which is known to be present in fibroblasts and preadipocytes (13, 26), caused MCPIP induction before C/EBPβ and C/EBPδ were induced. Furthermore, knockdown of MCPIP expression inhibited the induction of these transcription factors and drastically inhibited adipogenesis. Forced expression of MCPIP induced the cascade of transcription factors well known to be induced by DMI medium leading to robust adipogenesis. Thus MCPIP expression induced adipogenesis via the already known sequence of induction of transcription factors and expression of adipogenesis markers. MCPIP induction is one of the earliest events in adipogenesis, and MCPIP-induced adipogenesis was associated with PPARγ induction. What was surprising was that MCPIP expression could induce adipogenesis in PPARγ−/− cells in which no other factor has previously been able to induce adipogenesis. This finding shows that MCPIP is a key regulator of adipogenesis.
How exactly MCPIP induces the expression of the C/EBP family of transcription factors that would lead to adipogenesis remains to be elucidated. Considering the finding that MCPIP is induced before the induction of the C/EBP family, it appears possible that the role of MCPIP is to induce the early transcriptions factors C/EBPβ and C/EBPδ, which would then lead to the induction of subsequent factors required for adipogenesis. C/EBPα and PPARγ can induce each other. However, C/EBPα cannot induce adipogenesis without PPARγ. Our results demonstrate that MCPIP can induce C/EBPα and adipogenesis without PPARγ. Thus, MCPIP can be considered a critical regulator of adipogenesis.
There is overwhelming evidence that demonstrates a key role for CCR2-mediated events in obesity and development of type 2 diabetes. Our results show that the MCP-1/CCR2 system plays a direct role in adipogenesis. There is experimental evidence that MCP-1 can promote angiogenesis (27, 28) and that this MCP-1-induced angiogenesis is mediated via MCPIP (10). Thus, MCPIP induced by MCP-1/CCR2 interaction promotes adipogenesis and angiogenesis to supply blood to growing adipose tissue. These findings help provide a molecular basis for the role of the MCP-1/CCR2 system in the development of obesity and consequent development of type 2 diabetes.
Acknowledgments
We thank Dr. Bruce Spiegelman from Harvard University for the PPARγ−/− cell line.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-69458.
- C/EBP
- CCAAT/enhancer-binding protein
- PPARγ
- peroxisome proliferator-activated receptor γ
- CCR2
- C-C motif chemokine receptor 2
- DMI
- dexamethasone, 3-isobutyl-1-methylxanthine
- LPL
- lipoprotein lipase
- MCPIP
- MCP-1-induced protein
- MEF
- mouse embryonic fibroblasts
- RT
- reverse transcription
- siRNA
- small interfering RNA
- GFP
- green fluorescent protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
REFERENCES
- 1.Ogden C. L., Carroll M. D., Curtin L. R., McDowell M. A., Tabak C. J., Flegal K. M. (2006) JAMA 295, 1549–1555 [DOI] [PubMed] [Google Scholar]
- 2.Rosen E. D., Spiegelman B. M. (2006) Nature 444, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilherme A., Virbasius J. V., Vishwajeet P., Czech M. P. (2008) Nat. Rev. Mol. Cell Biol. 9, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen E. D., MacDougald O. A. (2006) Nat. Rev. Mol. Cell Biol. 7, 885–896 [DOI] [PubMed] [Google Scholar]
- 5.Wu Z., Rosen E. D., Brun R., Hauser S., Adelmant G., Troy A. E., McKeon C., Darlington G. J., Spiegelman B. M. (1999) Mol. Cell 3, 151–158 [DOI] [PubMed] [Google Scholar]
- 6.Rosen E. D., Hsu C. H., Wang X., Sakai S., Freeman M. W., Gonzalez F. J., Spiegelman B. M. (2002) Genes Dev. 16, 22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefterova M. I., Lazar M. A. (2009) Trends Endocrinol. Metab. 20, 107–114 [DOI] [PubMed] [Google Scholar]
- 8.Zhou L., Azfer A., Niu J., Graham S., Choudhury M., Adamski F. M., Younce C., Binkley P. F., Kolattukudy P. E. (2006) Circ. Res. 98, 1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmrich K., Thomas G. P., Abberton K. M., Thompson E. W., Rophael J. A., Penington A. J., Morrison W. A. (2007) Obesity 15, 2951–2957 [DOI] [PubMed] [Google Scholar]
- 10.Niu J., Azfer A., Zhelyabovska O., Fatma S., Kolattukudy P. E. (2008) J. Biol. Chem. 283, 14542–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B., Jenkins J. R., Trayhurn P. (2005) Am. J. Physiol. Endocrinol. Metab. 288, E731–E740 [DOI] [PubMed] [Google Scholar]
- 12.Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. (1999) Mol. Cell 4, 611–617 [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt C. C., Romero I. A., Cancello R., Camoin L., Strosberg A. D. (2001) Mol. Cell. Endocrinol. 175, 81–92 [DOI] [PubMed] [Google Scholar]
- 14.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. (2006) J. Clin. Invest. 116, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagathu C., Yvan-Charvet L., Bastard J. P., Maachi M., Quignard-Boulangé A., Capeau J., Caron M. (2006) Diabetologia 49, 2162–2173 [DOI] [PubMed] [Google Scholar]
- 17.Unger R. H. (1995) Diabetes 44, 863–870 [DOI] [PubMed] [Google Scholar]
- 18.Unger R. H. (2002) Annu. Rev. Med. 53, 319–336 [DOI] [PubMed] [Google Scholar]
- 19.Sabio G., Das M., Mora A., Zhang Z., Jun J. Y., Ko H. J., Barrett T., Kim J. K., Davis R. J. (2008) Science 322, 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye K. E., Shi H., Howard J. K., Daly C. H., Lord G. M., Rollins B. J., Flier J. S. (2007) Diabetes 56, 2242–2250 [DOI] [PubMed] [Google Scholar]
- 21.Sarafi M. N., Garcia-Zepeda E. A., MacLean J. A., Charo I. F., Luster A. D. (1997) J. Exp. Med. 185, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., Waget A., Delmée E., Cousin B., Sulpice T., Chamontin B., Ferrières J., Tanti J. F., Gibson G. R., Casteilla L., Delzenne N. M., Alessi M. C., Burcelin R. (2007) Diabetes 56, 1761–1772 [DOI] [PubMed] [Google Scholar]
- 23.Kobara M., Sunagawa N., Abe M., Tanaka N., Toba H., Hayashi H., Keira N., Tatsumi T., Matsubara H., Nakata T. (2008) J. Appl. Physiol. 104, 601–609 [DOI] [PubMed] [Google Scholar]
- 24.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., Shimomura I. (2007) Diabetes 56, 901–911 [DOI] [PubMed] [Google Scholar]
- 25.Ye J., Gao Z., Yin J., He Q. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 [DOI] [PubMed] [Google Scholar]
- 26.Hogaboam C. M., Bone-Larson C. L., Lipinski S., Lukacs N. W., Chensue S. W., Strieter R. M., Kunkel S. L. (1999) J. Immunol. 163, 2193–2201 [PubMed] [Google Scholar]
- 27.Salcedo R., Ponce M. L., Young H. A., Wasserman K., Ward J. M., Kleinman H. K., Oppenheim J. J., Murphy W. J. (2000) Blood 96, 34–40 [PubMed] [Google Scholar]
- 28.Hong K. H., Ryu J., Han K. H. (2005) Blood 105, 1405–1407 [DOI] [PubMed] [Google Scholar]