Abstract
Among older persons, sleep complaints in the form of insomnia and daytime drowsiness are highly prevalent and associated with adverse outcomes. The underlying mechanisms are linked to age-related declines in physiology, i.e., normal aging, and age-related increases in disease prevalence, i.e., usual aging. In this monograph, we describe how normal aging leads to less restorative sleep, characterized by reductions in homeostatic and circadian sleep, and to phase advancement of the sleep-wake cycle, characterized by older persons being more alert in the early morning but drowsier in the early evening. We also describe how usual aging leads to sleep complaints through reductions in health status, loss of physical function, and primary sleep disorders. Psychosocial influences are likewise described and their relevance to sleep complaints is discussed. We subsequently incorporate these aging-related changes into a conceptual model that describes sleep complaints as a consequence of multiple and interdependent predisposing, precipitating, and perpetuating factors, akin to a geriatric syndrome. We conclude our discussion by applying our conceptual model to the sleep-related care of an older person with insomnia and daytime drowsiness, and suggest that the diagnostic assessment consider, in addition to primary sleep disorders, multiple domains including medical, physical, cognitive, psychological, and social issues with the intent of developing an overall therapeutic plan and establishing long-term follow-up.
Keywords: insomnia, drowsiness, senescence
INTRODUCTION
Ms. K is a community-living 79-year old female who presents with lifelong difficulties in initiating and maintaining sleep, which have worsened since the death of her husband three months ago. She also reports the recent onset of anorexia, fatigue and, at times, abrupt sleepiness even when engaged in conversation. Her medical history includes Parkinson’s disease, congestive heart failure, chronic obstructive pulmonary disease, hypertension, diabetes mellitus, depression, osteoarthritis, and obesity. She is taking several medications and has limited mobility, with multiple falls reported in the past year. Given her long-standing struggles with insomnia, she clings to the belief she could do more if only her sleep quality were improved. She therefore requests a bedtime sleep aid, as well as “something” to perk her up during the course of the day.
The scenario described above presents a daunting public health challenge.1 Population studies have established that sleep complaints in older persons are highly prevalent,2,3 and characterized by high levels of concurrent morbidity2–17 and increased risk for adverse outcomes.2,18–32 Given the complexity of geriatric sleep complaints, their likely multifactorial etiology,2,3,6,7,17,18,33,34 and the need for more effective interventions,19,22,25 underlying sleep mechanisms need to be better defined, as these ultimately determine the requisite preventive, diagnostic and therapeutic strategies.
In this monograph, we review geriatric sleep epidemiology and propose a conceptual model that illustrates how aging-related mechanisms lead to the development of sleep complaints in older persons, including Ms. K. While our comments are directed primarily to older persons who are living in the community, many of the principles are applicable to those who are residing in institutional settings. To enhance readability, common sleep terminology is summarized in the appendix. Unless otherwise specified, “geriatric” and “older persons” refer to those aged 65 years or older.
GERIATRIC SLEEP EPIDEMIOLOGY
Epidemiologic surveys of sleep among older persons typically evaluate symptoms related to insomnia and/or drowsiness, which are collectively referred to as sleep complaints.2 For insomnia, symptoms are based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, which include difficulties in sleep onset or maintenance, early morning awakenings, and/or nonrestorative sleep.35,36 This is in contrast to a clinical diagnosis of insomnia, which requires a determination of the duration and daytime consequences of symptoms.35,36 Drowsiness is most often established by self-reported napping behavior and, less often, by the Epworth Sleepiness Scale. The latter is a brief self-administered questionnaire with scores ranging from 0 to 24, with higher scores denoting greater drowsiness.37
In the “Established Populations for Epidemiologic Studies of the Elderly” (EPESE), involving 9,282 community-living persons aged 65 years or older, 43% reported difficulty in sleep onset or maintenance, while 25% napped.2 These findings were confirmed in the National Sleep Foundation’s (NSF) 2003 Sleep in America Poll, which reported one or more symptoms of insomnia in 46% of persons aged 65–74 years and 50% of those aged 75–84 years, with corresponding rates for napping of 39% and 46%.3 In general, insomnia symptoms are more prevalent in older women,2,3,5 with gender differences in napping being less clear.38
Sleep complaints in older persons are characterized by high levels of concurrent morbidity. Insomnia symptoms are cross-sectionally associated with reduced self-reported health status, medication-use, cognitive impairment, visual and hearing impairments, depression, behavioral disturbances, reduced activity, prior falls, and disability in instrumental activities of daily living (ADL)2–13 Similarly, daytime drowsiness, either by napping behavior or Epworth scale, is cross-sectionally associated with functional and cognitive impairment.13–17 In general, older persons demonstrate stronger cross-sectional associations between insomnia and impaired quality of life than younger persons.39
Adverse outcomes in the setting of sleep complaints are also common in older persons. Insomnia symptoms are longitudinally associated with reduced self-reported health status, cognitive decline, depression, disability in basic ADLs, missed work-days, poorer quality of life, and institutionalization.18–26 Drowsiness is likewise detrimental, with longitudinal studies documenting napping as a risk factor for incident cardiovascular disease, falls, and death.2,27–32 Napping that is unscheduled, prolonged, or daily imparts the greatest risk,14,16,29,30,32 but this is attenuated upon exclusion of individuals with chronic conditions.30
SENESCENT SLEEP MODEL
Conventional wisdom postulates that sleep complaints in older persons are not the consequence of “aging per se”.18,40–43 This supposition emphasizes the importance of a detailed evaluation to identify the underlying sleep disorder. However, such an approach runs the risk of discounting the impact of senescence. Senescence is a biologically active, postmaturational process leading to diminished homeostasis and vulnerability to disease.44,45 It results from expected age-related declines in physiology, i.e., normal aging, and age-related increases in disease prevalence, i.e., usual aging.44,45
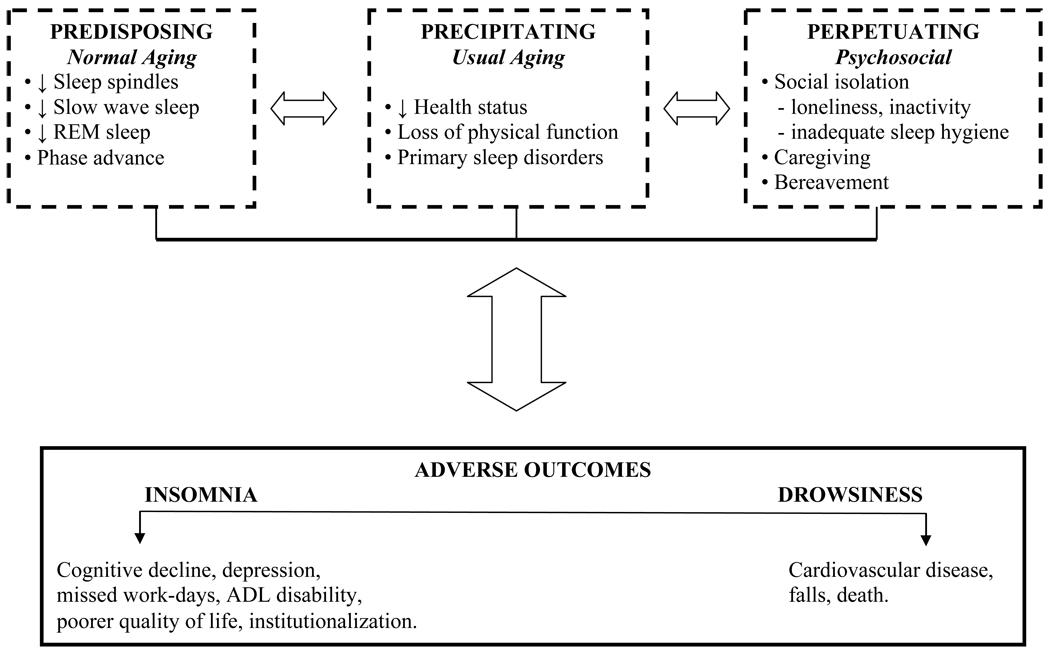
We propose that senescence in the form of normal and usual aging is predisposing, precipitating, and perpetuating to sleep complaints and adverse outcomes, and ultimately defines geriatric sleep epidemiology. This is illustrated in Figure 1, a geriatric version of an insomnia model previously described by Spielman and colleagues.46 Predisposing factors refer to intrinsic changes in sleep-wake physiology, resulting from normal aging. These changes render the older person vulnerable to subsequent sleep complaints and adverse outcomes, but are not by themselves causal. The more pervasive the intrinsic changes, the greater the vulnerability. Precipitating factors are directly and temporally causal to sleep complaints and adverse outcomes, but are extrinsic to sleep-wake physiology. Such factors result from usual aging, and typically occur in multiple domains, including medical, physical, cognitive, and psychological. Perpetuating factors are largely psychosocial influences that adversely impact the sleep-wake cycle, emerging principally as a consequence of aging-related sequelae. Such influences magnify the impact of predisposing or precipitating factors, and may perpetuate a sleep complaint, even after resolution of other reversible factors. When especially severe, psychosocial influences may also lead to adverse outcomes. The noted predisposing, precipitating, and perpetuating factors are multiple and interdependent, suggesting that sleep complaints in older persons resemble a multifactorial geriatric syndrome.
Figure 1. Mechanisms underlying sleep complaints and associated adverse outcomes.
Adapted from an insomnia model previously described by Spielman and colleagues.46 Sleep complaints present as various combinations of insomnia and drowsiness, and are a consequence of multiple predisposing, precipitating, and perpetuating factors. The postulated relationships are interdependent, as denoted by the bi-directional arrows, and are characterized by interindividual variability, as denoted by the dashed boundaries. See text for discussion.
NORMAL AGING
Developmentally, sleep physiology undergoes significant changes across the lifespan. Ohayon et al47 have most effectively demonstrated this in a meta-analysis of quantitative sleep parameters in otherwise healthy individuals, from childhood to old age. We summarize their adult findings in Table 1. To more completely illustrate these aging-related changes in sleep, we focus next on the physiology underlying two forms of sleep (NonREM and REM) and the sleep-wake cycle, first in the young adult (non-senescent sleep) and, thereafter, in older persons (senescent sleep).
Table 1.
Normal aging-related changes in sleep*
| Sleep Parameter | Adult Lifespan † |
|---|---|
| Total sleep time | Decreases |
| Time to fall asleep | No Change |
| Time awake after sleep onset | Increases |
| Sleep efficiency | Decreases |
| Stage 1 NonREM | Increases |
| Stage 2 NonREM | Increases |
| Slow wave sleep | Decreases |
| REM sleep | Decreases |
Based on a meta-analysis of quantitative sleep parameters from Ohayon et al;47
These changes are most notable across the ages of 18–40 and 40–60 years, with persons older than 60 years demonstrating only continued reductions in sleep efficiency.
Non-Senescent Sleep
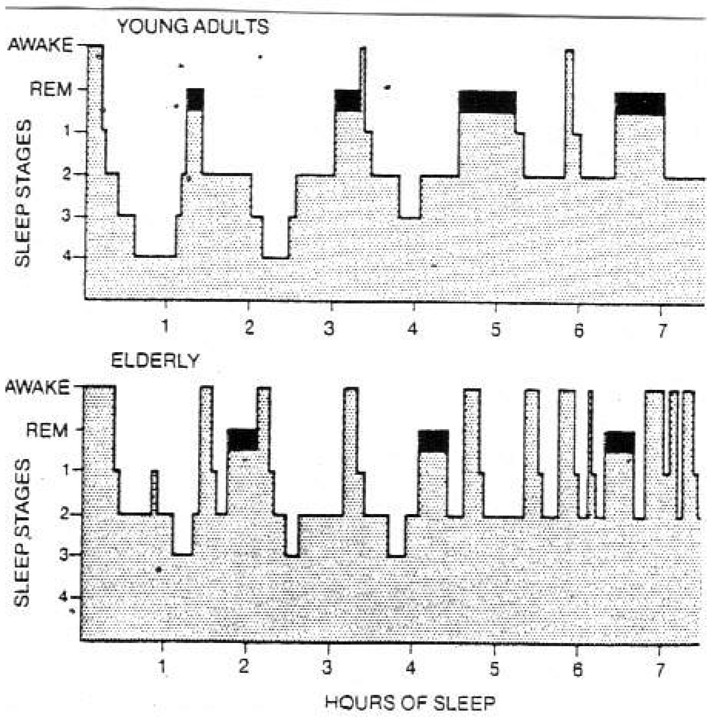
In the young adult (age 18–40 years), sleep is normally entered through NonREM sleep, with a normal night’s sleep consisting of repetitive cycles, each lasting 90–110 minutes.48–50 As shown in Figure 2, a cycle begins with NonREM sleep and ends with REM sleep. Typically, 75–80% of total sleep time is spent in NonREM sleep and 20–25% in REM sleep.48–50
Figure 2. NonREM-REM sleep cycles in young adults and older persons.
Reproduced with permission from Kales.50 REM sleep is represented by blackened area; sleep stages refer to NonREM sleep. See text for discussion.
NonREM Sleep
NonREM sleep typically progresses through four stages.48–51 These are identified in the laboratory by a pattern on the electroencephalogram (EEG) of gradual synchronization, initially consisting of low amplitude, mixed frequency waveforms, i.e., NonREM Stages 1 and 2, and later progressing to high amplitude and slower frequency waveforms termed delta waves, i.e., NonREM Stages 3 and 4. The latter two stages are categorized as slow wave sleep (SWS), and are also referred to as homeostatic sleep.
The physiological drive for NonREM sleep is wake-dependent.52,53 The more prolonged the duration of wakefulness, the greater the need to enter NonREM sleep.52,53 A potential mediator is adenosine, a byproduct of adenosine triphosphate metabolism.54–57 Heightened neuronal metabolism during prolonged wakefulness leads to energy (glycogen) depletion, with consequent increases in extracellular adenosine.54–58 Adenosine, in turn, exerts a global effect on the cerebral cortex, which suppresses waking neuronal activity; alternatively, it may act locally as a “metabolic switch” on sleep-promoting nuclei.57 The net-effect is the onset of NonREM sleep. Supporting the role of adenosine as a sleep mediator is the observation that adenosine receptor antagonists like caffeine are known to promote alertness.59
Prolonged wakefulness leads to progressive increases in the duration of the deepest form of NonREM sleep, namely SWS.52,53 In other words, slow wave activity in NonREM sleep depends only on prior waking and is not influenced by circadian phase.53 This implies that SWS is homeostatic, in that it may foster metabolic and cognitive recovery in response to the day’s wake-related activities.60–64 For example, prior research suggests that SWS is associated with increases in cerebral protein synthesis and energy (glycogen) conservation, and that the onset of SWS is associated with a reproducible growth hormone pulse, which in men represents the largest daily secretory release.60–64 Additionally, SWS, by diminishing synaptic activity, may increase the cortical signal-to-noise ratio while minimizing saturation; this, in turn, could lead to enhanced execution in neural circuits, possibly enhancing memory.64 These attributes may explain why SWS is strategically most prevalent in the first third of the night, which makes possible a prompt sleep-based homeostatic response to prolonged wakefulness, and why the first decade of life, a time in human development characterized by tremendous physical and cognitive growth, is distinguished by the greatest amount of SWS.48,49
Stage 2 NonREM sleep may also contribute to homeostasis. Although often thought of as a transitional state that progresses to either SWS or REM sleep,65 Stage 2 NonREM sleep may facilitate memory.66 For example, sleep spindle activity, an EEG marker of this sleep stage, is associated with increased recall performance, i.e., declarative memory.66
REM Sleep
REM sleep is identified in the laboratory by a combination of episodic rapid eye movements, muscle atonia, and a desynchronized EEG pattern of low voltage, fast frequency waveforms.48,49,51 In the young adult, the first REM period is brief and is entered after 70–90 minutes of NonREM sleep.48–50 REM sleep is temporally related to circadian rhythms, hence the term circadian sleep, and is popularly referred to as “dream” sleep. In contrast to SWS, the largest amount of REM sleep occurs in the last third of the night, around 4 AM, a time period termed the circadian sleep propensity zone.48–50 The latter is concurrent with the trough of the body temperature and nadir of the blood cortisol level, which are both under circadian regulation.48,49,52,67,68
The modulation of REM sleep occurs primarily in the brainstem and is characterized by a reciprocal interactive cycle of pontine cholinergic REM-On and monoaminergic REM-Off neurons.69–71 REM-On neuronal activity is facilitated by GABAergic interneurons within the brainstem but inhibited by input from pontine-mesencephalic tegmental nuclei (dopaminergic and norepinephric) and hypothalamic nuclei (histaminergic and hypocretinergic). The dynamic balance of inhibitory versus excitatory activity of REM-On relative to REM-Off neurons contributes to the cycling of REM and Non-REM sleep.
Several lines of evidence suggest that REM sleep may enhance memory and learning. First, REM sleep is an activated state of arousal characterized within the central nervous system by an increase in temperature, metabolism and blood flow.48,49,52,67,68 Second, hippocampal theta rhythms, thought to represent information storage, are observed during both wakefulness and REM sleep.72–78 Third, there is a positive and significant correlation between learning efficiency and increases in REM sleep.79 Fourth, donepezil, a cholinesterase inhibitor used in Alzheimer’s Disease, appears to be most beneficial when REM sleep is increased.80
REM sleep is also relevant to human development. Infants average a total of 16 to 18 hours of sleep per 24-hour period, of which 50–80% is REM, and they enter sleep through REM.48,49 This emphasis on REM sleep is unique to infants and is concurrent with the rapid development of motor skills, language, and socialization that is characteristic of infancy.
Sleep-Wake Cycle
The circadian system plays a pivotal role in the timing of sleep-wake states, namely the ability to fall asleep and/or to maintain wakefulness. The suprachiasmatic nucleus, located in the anterior hypothalamus, is the circadian oscillator, popularly referred to as the biologic clock.52,67,68 It receives photic and nonphotic input, synchronizing, i.e., entraining, circadian rhythms to environmental cycles. The suprachiasmatic nucleus ultimately establishes the temporal hierarchy that regulates behavioral states such as the sleep-wake cycle and physiologic responses such as the 24-hour pattern of the core body temperature and serum cortisol.
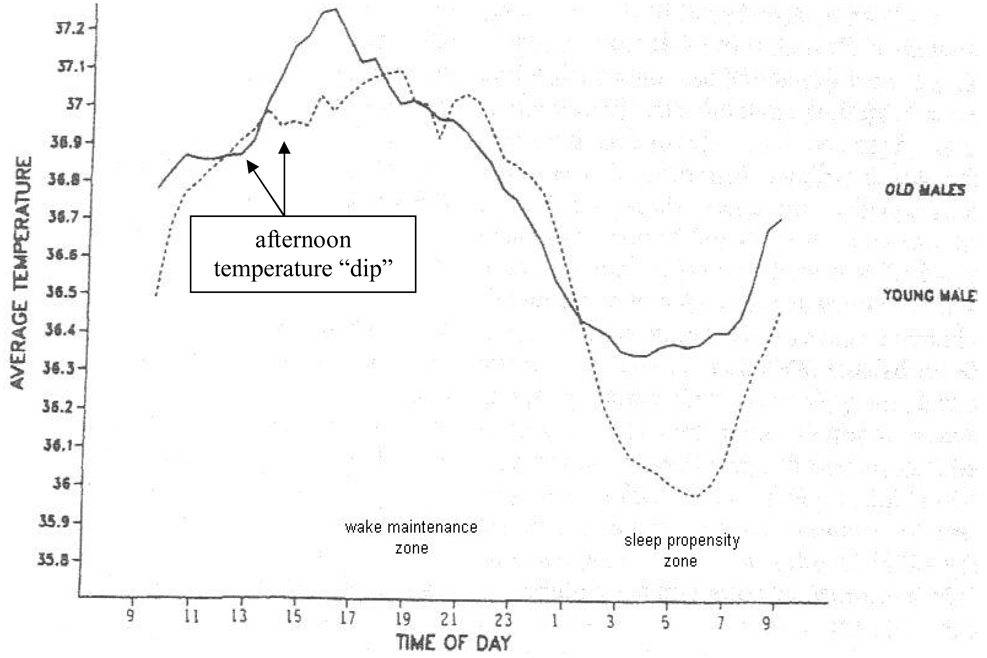
Under normal conditions of entrainment, i.e., in the presence of environmental cues such as the light-dark cycle, the circadian rhythm of body temperature parallels the sleep-wake cycle.52,67,68 As temperature rises, we become more alert. As it declines, we become sleepy. Characteristically, the temperature curve changes in a sinusoidal pattern with an approximate 24-hour periodicity, as shown in Figure 3. The temperature curve crests between 7 PM to 10 PM, which corresponds to the circadian wake maintenance zone, i.e., a time period conferring peak circadian alertness, but nadirs between 4 AM to 7 AM, which corresponds to the circadian sleep propensity zone, i.e., a time period conferring heightened circadian sleepiness. Otherwise, between 4 AM and 10 PM, the temperature rises, with the exception being a small, transient dip occurring at approximately 2 PM to 4 PM. Between 10 PM and 4 AM, the temperature again decreases, although more precipitously. These declines in body temperature correspond to the peak incidence of motor vehicle accidents, which occurs between 2 PM to 5 PM and midnight to 5 AM.81–83
Figure 3. Circadian temperature curve under typical conditions for persons living in the community.
Reproduced with permission from Monk.100
Senescent Sleep
In healthy older persons, sleep is likewise entered through NonREM sleep and consists of the same NonREM-REM cycles, as previously described and as shown in Figure 2. However, normal aging-related changes across the adult lifespan result in increased awakenings and reductions in sleep spindle activity, SWS, and REM sleep.47,84,85 As shown in Table 1, these changes in sleep parameters are most notable across the ages 18–40 and 40–60 years, with persons older than 60 years demonstrating only continued reductions in sleep efficiency (percent of time in bed spent asleep).47 Daytime wakefulness is affected as well, with older persons being more alert in the early morning but more drowsy in the early evening.86–91 In general, normal aging-related changes in sleep parameters appear to affect men more than women,47,86,92 although recent evidence is mixed regarding SWS.47,92–94
NonREM Sleep
In the fifth decade, most notably in men, there is a reduction in SWS and nocturnal growth hormone release, as well as a temporal dissociation of the previous linkage of growth hormone secretion with SWS.47,84,87,92 In addition, although Stage 2 NonREM sleep increases with age, sleep spindle activity declines.85 These changes may contribute to the aging-associated manifestations of frequent nocturnal awakenings, nonrestorative sleep, cognitive decline, truncal obesity, reduced lean body mass, and impaired exercise response.84,85
The evidence for age-related gender differences in NonREM sleep is somewhat conflicting, and may be related to how SWS is measured. Two large polysomnography-based studies involving older women with visual sleep staging have reported little to no age-related changes in SWS, with older women having increased amounts of SWS relative to age-matched men.47,92 However, the more effective method for quantifying the intensity of SWS is to measure slow wave activity by spectral analysis of the EEG.93,94 When EEG spectral power in the slow wave band is normalized for activity in REM sleep, slow wave activity during NonREM sleep is reduced in older women relative to older men.93,94 This is concurrent with increases in alpha activity, an EEG pattern of wakefulness, and a lower sleep-onset release of growth hormone in older women than older men.93,94 These EEG spectral power findings may explain, in part, the female-specific vulnerability to developing insomnia. 94
REM Sleep
Normal aging-related changes, which become evident in the 6th decade, are characterized by decreased REM sleep and eye movement density, as well as a higher evening cortisol.47,84,87,92 These changes are present in both men and women across the adult life span.47,84,92 Some investigators postulate that higher evening cortisol levels may foster sleep fragmentation, insulin-resistance, hippocampal atrophy, and deficits in hippocampus-dependent learning and memory.95–99 A reduction in REM sleep, below 16.1% of total sleep time, has been shown to increase the risk of death in healthy older adults by more than 70%.26
Sleep-Wake Cycle
In contrast to young adults, older persons sleep less during the circadian sleep propensity zone,89,90 corresponding to aging-related complaints of increased nocturnal and early morning awakenings,88–91 but sleep more during the circadian wake maintenance zone,89 corresponding to aging-related increases in daytime napping and an earlier bedtime.88
The sleep-wake patterns of older persons correspond to normal aging-related alterations in the temperature curve, as shown in Figure 3. Under conditions of normal entrainment, such as in a community setting, older persons demonstrate a phase advance, i.e., a shift in the peak and trough temperature positions to an earlier time.100,101 These circadian changes parallel the normal aging-related observations of an earlier bedtime and arise time and, possibly, a reduced ability to tolerate phase shifts due to jet travel or night work.87,88,101 An aging-related reduction in amplitude of the temperature curve has also been postulated, but the evidence for this is conflicting.101
The mechanisms underlying these changes in the sleep-wake cycle and temperature curve are not attributable to an alteration in the periodicity of the circadian system, which at 24.18 hours does not change with age.91 Thus, despite having the same circadian periodicity, older persons demonstrate an earlier entrained circadian phase, which is most likely due to changes in homeostatic sleep and in the interaction of circadian and entrainment processes.90,91 The collective effect of such changes is for sleep to be less restorative, with increased awakenings and reductions in homeostatic and circadian forms of sleep, and for the sleep-wake cycle to be phase advanced, with older persons being more alert in the early morning but drowsier in the early evening.
USUAL AGING
Senescence in the form of usual aging is characterized by a reduction in health status, loss of physical function, and primary sleep disorders, all of which are capable of precipitating sleep complaints and adverse outcomes.
Health Status
In the National Follow-up Survey of Self-Care and Aging, 32.6% of the 937 community-dwelling persons aged 65 years or older reported only fair-to-poor health status.102 Similar findings were noted in the National Sleep Foundation’s (NSF) 2003 poll of community-dwelling persons aged 65 to 84 years.3 In the NSF poll, 71% of those who rated their health status as fair-to-poor also reported insomnia, while 29% reported drowsiness. Likewise, in EPESE, 36% of community-dwelling persons aged 65 years or older reported fair-to-poor health status,2 which was significantly associated with the development of insomnia symptoms.18 Reductions in health status are often attributable to an increase in the burden of chronic conditions, medication use, and neuropsychological impairment, each of which can precipitate insomnia and/or drowsiness.
Chronic Conditions
Chronic conditions are highly prevalent in older persons. In the NSF poll, 43% reported two to three chronic conditions, while 24% had four or more; only 10% had none.3 The six most prevalent conditions were hypertension (47%), arthritis (46%), enlarged prostrate (22% of men), heart disease (18%), depression (16%) and diabetes mellitus (15%). Each of these conditions may be linked to a sleep disorder. For example, hypertension, diabetes mellitus, and heart disease are associated with sleep disordered breathing;103 depression is associated with insomnia;104 and hypertension, arthritis, depression, and diabetes mellitus are associated with sleep-related movement disorders such as restless legs syndrome.105,106 In the NSF poll, the prevalence of self-reported snoring, apnea, insomnia, and restless legs rose as the number of chronic conditions increased.3 Similarly, in EPESE, chronic conditions such as heart disease, stroke, and diabetes were significantly associated with the development of insomnia symptoms.18
Several chronic conditions can also directly lead to sleep-disruptive symptoms. In the NSF cohort, nocturia was the most disruptive symptom to sleep, with 65% of men and women reporting that it fragments sleep at least a few nights a week.3 In the National Follow-up Survey of Self-Care and Aging, daily arthritic pain, as experienced by 48% of the cohort, was associated with insomnia and reduced activity.102 In EPESE, the risk of developing insomnia at 3 years of follow-up was significantly elevated in participants who reported having “any respiratory symptom” at baseline (32% of the cohort).2,18
Medications
Medication use in older persons is likewise highly prevalent. Among non-institutionalized Medicare enrollees, the mean number of filled medication prescriptions in 2000 was thirty.107 Of the 200 million office/clinic visits made by older persons in 2000, one-third resulted in one or two medications being prescribed or renewed, while another third resulted in three or more.108,109 If nonprescription drugs such as herbals/supplements, vitamins, and minerals are also considered, 44% of community-living older Americans 65 years or older report taking an average of five or more medications in a given week.110
One contributor to the high rate of medication use in older persons is patient-perceived impairment in sleep quality. In the National Follow-Up Survey of Self-Care and Aging, 48.1% of participants used prescription medications and 65.9% used over-the-counter medications for arthritic pain, most often to relieve pain-related sleep disruptions.102 In the NSF poll, 20% of participants used a bedtime hypnotic at least a few nights a week to improve their sleep.3
Counter to its intended benefit, medication use in older persons often results in substantial toxicity. In 2000, inappropriate medications were prescribed during 8% of office/clinic visits made by older persons.108 The major culprits included analgesics (e.g. propoxyphene) and other centrally active agents (e.g. hydroxyzine, diazepam, amitriptyline, oxybutinin).108,109 In a separate study of community-dwelling older persons who were prescribed five or more medications, the annual rate of an adverse drug event was 35%, with central nervous system side effects occurring commonly.111,112 In general, medications with central nervous system side effects, including sleep aids, may impart an increased risk for falls, health care utilization, and death, disrupt sleep architecture through reductions in slow wave and REM sleep, exacerbate sleep disordered breathing and sleep-related movement disorders, and foster daytime drowsiness.25,48,113–116 The persons most vulnerable to these side effects are the elderly and individuals with multiple comorbidities.115 In EPESE, the use of sedatives was significantly associated with development of insomnia symptoms.18
Neuropsychologic Impairments
Depression and cognitive impairment are also associated with sleep complaints.3,18,104 In the NSF poll, 70% of older persons who reported being depressed had insomnia, while 32% were drowsy.3 In EPESE, depressed mood was significantly associated with development of insomnia symptoms.18 Older persons who are hospitalized or in long-term care settings are especially vulnerable to depressive symptoms, with prevalence rates of 23% and 35%, respectively.117 Geriatric depression merits prompt medical attention given that older persons, who represent 12% of the US population, account for 17.6% of suicide deaths.118
In the NSF poll, 62% of older persons who reported memory problems also had insomnia, while 24% were drowsy.3 Similarly, in another study involving an elderly community-based population, self-reported excessive daytime sleepiness was independently associated with cognitive impairment in multiple domains, including attention-concentration, praxis, delayed recall, orientation (person and temporal), and prospective memory.14 When cognitive impairment progresses to dementia, a nonrestorative form of sleep often develops that is characterized by increased Stage 1 NonREM sleep, reduced number of sleep spindles, decreased slow wave and REM sleep, and frequent nocturnal awakenings.119,120 The latter may be additionally accompanied by disorientation, referred to as confusional arousals, as well as agitation and wandering.119,120 Dementia is also associated with other conditions that are disruptive to sleep and daytime alertness such as sleep disordered breathing, depression, psychosis, REM Behavior Disorder, and sundowning.119,120 REM Behavior Disorder (discussed further below) is characterized by dream-directed behaviors that are causally linked to a dissociated form of REM sleep. The importance of dementia as an underlying cause of sleep complaints will likely escalate, given the advancing age of the American population.121,122 In community-dwelling older persons, the prevalence of Alzheimer’s Disease, the most common cause of dementia, increases from 3% in those 65–74 years of age to 19% in 75–84 year olds, and to 47% in those over the age of 85 years.123
Loss of Function
Loss of physical function occurs commonly in older persons. In 1999, 6.8 million Americans aged 65 years or older were chronically disabled.107 In a survey involving community-dwelling persons 80 years or older, 35.9% needed assistance or were unable to walk ¼ mile, 40.6% needed assistance or were unable to climb a flight of stairs, and 27% reported visual impairment, denoted by “any difficulty seeing the words or letters in ordinary newspaper print”.122 In the 2000 census, 20.4% of persons aged 65 years or older reported difficulty going outside the home.121
Loss of physical function may have an adverse effect on zeitgebers, which are environmental cues that entrain circadian rhythms to a 24-hour cycle length and, in turn, promote normal sleep-wake scheduling.68 Zeitgebers may be photic (light-based) as in the day-night cycle, or nonphotic as in exercise activities, scheduled meals, or social cues. Because loss of physical function often leads to inactivity, inadequate outdoor light exposure, and social isolation, it may result in absent or inconsistent exposure to normal zeitgebers, with the net-effect being irregular sleep-wake patterns that engender insomnia and/or drowsiness. Thus, it is not surprising that a high proportion of community-dwelling older persons with mobility disability3 report insomnia (66%) and drowsiness (28%). In EPESE, physical disability was significantly associated with the development of insomnia symptoms.18
Primary Sleep Disorders
Usual aging is associated with an increased prevalence of primary sleep disorders, namely sleep disordered breathing (SDB), sleep-related movement disorders, dream-directed behaviors, and circadian rhythm disorders.
Sleep Disordered Breathing
The standard for establishing a diagnosis of SDB is an apneahypopnea index (AHI) of ≥ 5.124–126 The laboratory severity of SDB is commonly categorized as mild, moderate, or severe based on an AHI of 5 to 14, 15 to 29, ≥ 30, respectively.125 The Centers for Medicare and Medicaid Services (CMS) recognizes an AHI of 5 to 14 (mild) as an indication for treatment only if clinically accompanied by excessive daytime drowsiness, impaired cognition, mood disorder, insomnia, hypertension, ischemic heart disease, or prior stroke.126 Symptoms are not required, however, for an AHI ≥ 15.126
The laboratory prevalence of SDB is known to increase with age.127 In a study of 741 randomly selected men aged 20 to 100 years, SDB defined by an AHI of ≥ 5 was highest in those aged 65 years or older (at 30.5%), and involved both obstructive and central SDB.127 The higher laboratory-based prevalence of SDB requires clinical correlation, especially as it relates to obstructive SDB, commonly known as the obstructive sleep apnea hypopnea syndrome (OSAHS). For example, when OSAHS is clinically defined in a manner similar to the CMS criteria, its prevalence actually declines with age to 1.7% in persons 65 years or older, and its severity, as characterized by sleep-induced hypoxemia, is milder in older persons than younger persons.127 Moreover, one study has found that severe SDB, even at an AHI > 50, does not confer excess mortality in older persons.128 Such findings underscore the importance of basing treatment decisions on clinical grounds, rather than solely on laboratory findings. Further, therapeutic goals in geriatric OSAHS need to be clearly defined, with efficacy periodically assessed relative to daytime sleepiness, quality of life, driving safety, mood, and/or hypertension.103 Conversely, chronological age should not be a contraindication to pursuing treatment such as continuous positive airway pressure (CPAP), as long as it is clinically indicated and the affected individual can physically and cognitively participate. Indeed, up to 70% of older persons are able to achieve CPAP compliance for an average of 6.5-hours/night, 6.5-nights/week.129
The approach to central SDB in older persons likewise requires clinical correlation. When mild and asymptomatic, i.e., AHI < 15, central SDB might not warrant treatment since it may simply represent a laboratory event that is attributable to normal aging or sleep-wake transitions. Nonetheless, central SDB can be associated with increased morbidity and mortality, especially if it is symptomatic and characterized by an AHI > 15.130,131 For example, the prevalence of atrial fibrillation is higher in middle aged to older adults with idiopathic central sleep apnea (27%) than in those with OSAHS (1.7%) or no sleep apnea (3.3%).132 Further, Cheyne-Stokes respirations, a variant of central sleep apnea, is strongly associated with cardiovascular disease, most often in the setting of a low cardiac output state (cardiomyopathy).133 Central SDB may also reflect a destabilized breathing response due to numerous sleep-wake transitions (e.g. insomnia), hypoxemia (e.g. high altitude), respiratory-suppressant medications (e.g. opiates), metabolic disturbances (e.g. hypothyroidism), neurological disorders (e.g. especially those associated with autonomic dysfunction or neuromuscular disease) and, paradoxically, nasal obstruction.131 Finally, central SDB in the elderly is postulated to be a sleep-related cause of death, a consequence of changes in the medullary ventral respiratory group neurons.134 Thus, depending on the clinical presentation, central SDB in an older person may require a detailed medical evaluation and consultation with a sleep specialist, cardiologist, and/or neurologist.
Movement Disorders
Sleep-related movement disorders, namely restless legs syndrome (RLS) and periodic limb movements in sleep (PLMS), are prevalent in older persons. Approximately 10% of persons aged 65 years or older have RLS,105 rising to 19% in those 80 years or older.135 RLS is a sensorimotor disorder that is characterized by an urge to move, typically in response to unpleasant sensations involving the legs or other parts of the body, and that worsens with inactivity and in the evening hours.136–138 While the unpleasant sensations may be partially or totally relieved by movement, such as walking or stretching, they can result in disruptions in sleep onset and maintenance.3,136–138 Among persons with RLS, 80% also have PLMS, which is characterized by cyclical leg kicking while asleep, occurring approximately every 20 to 40 seconds and leading at times to arousals.136–138
RLS may be familial or secondary to iron deficiency or uremia.136–138 It may be exacerbated by antidepressants, caffeine, nicotine, alcohol, and inactivity, and is often associated with concurrent morbidity, including medical and psychiatric conditions (diabetes mellitus, arthritis, depression, anxiety, and peripheral neuropathy), restricted mobility, primary sleep disorders (sleep apnea), drowsy driving, and reduced cognition.3,105,106,136–139 RLS may pose special challenges in older persons. For example, a cognitively impaired older person who is agitated may respond poorly to physical restraints because the imposed inactivity may worsen restless legs; similarly, because restlessness is less severe during the day, daytime napping may be favored in individuals with RLS, but such a practice could adversely delay sleep onset at the evening bedtime.138
PLMS is the most common movement disorder in older persons, with a prevalence of 34%.137 Although there is considerable overlap with RLS, PLMS usually occurs more commonly in the absence of RLS, i.e., approximately 70% of the time. Because nocturnal leg movements may be exacerbated by other factors, including obstructive sleep disordered breathing, uremia, medications (e.g. antidepressants), and iron deficiency, PLMS is often a secondary phenomenon with therapy best directed at the underlying cause.137 Nevertheless, PLMS may be idiopathic and, when associated with sleep fragmentation, i.e., a PLMS arousal index > 15 per hour, may result in insomnia symptoms or daytime fatigue that warrant targeted treatment (e.g. dopamine-agonist). 137 In this setting, the idiopathic form of PLMS is termed periodic limb movement disorder.137
Advanced Sleep Phase Syndrome
This circadian disorder, which is more prevalent in the elderly, is characterized by an extreme phase advancement in the sleep-wake cycle, beyond that which can be attributable to normal aging alone.137,140 Affected individuals fall asleep as early as 6 to 9 PM, awakening at 2 to 5 AM, and are thereafter unable to resume sleep.137 This pattern reflects a diminished ability to phase-delay the circadian system in response to normal environmental cues (light-dark cycle). Advanced sleep phase syndrome is a diagnosis of exclusion, since early morning awakenings are commonly observed in depression.
Narcolepsy
Although onset characteristically occurs in the teenage years, narcolepsy remains in the differential diagnosis of daytime drowsiness in an older person, as there may be a delayed presentation due to misdiagnosis, mild disease severity, or late-life onset of symptoms.137,141
REM Behavior Disorder
Caregivers often cite sleep complaints as a reason for pursuing long-term care of older persons who are cognitively impaired.24 On occasion, these sleep complaints may include nocturnal behaviors such as loud talking, eating, drinking, and wandering.24 Whenever an older person presents with such sleep-related manifestations, the possibility of dream-directed behaviors need to be considered, in particular REM Behavior Disorder (RBD).
RBD is a dissociated form of REM sleep that lacks the normal occurrence of muscle atonia.137,142 Patients with RBD complain of sleep disruption or vivid dreams, and at times physically act out violent dream-directed behaviors as if they were awake.137,142 Onset is in the 6th to 7th decade, with a male preponderance.137,142 RBD often results from a neurodegenerative disease (48 to 75%), e.g. Parkinson’s and Lewy Body Disease, although it can be idiopathic (25 to 60%).137,142 RBD may also be precipitated by treatment with an antidepressant, or occur in the setting of withdrawal from alcohol or sedative-hypnotics.137,142 Although the prevalence of RBD is unknown, it is becoming more frequently recognized and needs to be considered in older persons presenting with dream-directed behaviors.
PSYCHOSOCIAL INFLUENCES
Psychosocial influences such as caregiving, social isolation, and bereavement are prevalent in older persons. These can lead to sleep complaints by magnifying the impact of predisposing or precipitating factors, but may also perpetuate a sleep complaint, even after resolution of other reversible factors. When especially severe, such as with high levels of caregiver burden and prolonged bereavement, psychosocial influences might themselves lead directly to adverse outcomes.
Caregiving
The high prevalence of chronic condition and loss of function in older persons, largely a consequence of usual aging, imposes a substantial caregiving burden for older partners. Caring for an ailing elder entails significant physical, emotional, and financial responsibility.143 Debilitating conditions that are especially labor-intensive such as dementia, stroke, or hip fracture confer the highest caregiving burden,143,144 and can lead to highly disrupted sleep-wake cycles, e.g. middle-of-the-night caregiving. In fact, caregivers specifically identify night-time caregiving responsibilities as a reason for pursuing institutionalization of a debilitated older person.24 In addition, caregivers who experience mental or emotional strain in response to this burden are at especially high risk for depression, anxiety, use of psychotropic medications, and poorer health.144–147
Social Isolation
Old age denotes retirement and, for many, social isolation. In the 2000 census, 19% of men and 40% of women 65 years or older lived alone.121 Loss of a work schedule and social isolation can foster sleep complaints through loneliness, inactivity, and boredom, and can result in prolonged bedroom stays with poor sleep-wake habits. Often referred to as inadequate sleep hygiene,137 these habits include napping, and irregular bedtimes, arise times, and meal times, as well as napping. Although napping need not be pathologic,14,38,148 and may, in fact, enhance evening performance,148 it becomes problematic in older persons when it is unintentional, prolonged, occurs late in the day or early evening, represents a new pattern, or is described as irresistible.14,16,29,30,32 Inadequate sleep hygiene disrupts zeitgebers and can elicit abnormal sleep-wake cycles, with the net effect being an escalation in sleep complaints. In the NSF poll, older persons who felt socially isolated were more likely to report insomnia and drowsiness.3 In another study, activity and satisfaction with social life protected against insomnia at any age, including those older than 65 years.5
Bereavement-Effect
Persons bound by social ties are at increased risk for reductions in their own health in response to a partner’s illness or death, a phenomenon known as the bereavement-effect. 144,147 Among older persons, the risk for adverse health consequences attributable to bereavement may persist for up to two years and, when accompanied by depression, may lead to subsequent sleep complaints.144,149 In the 2000 census, 32% of older persons were widowed.121 In EPESE, widowhood was significantly associated with the development of insomnia symptoms.18
INTERDEPENDENCE
We have proposed that sleep complaints in older persons are attributable to multiple and interdependent predisposing, precipitating, and perpetuating factors, akin to a geriatric syndrome. To illustrate this interdependence, we provide two examples, linking two common aging-related conditions, namely menopause and nocturia, to sleep complaints and sleep disorders. In addition, we describe how sleep disorders are bi-directional and explain their possible impact on the aging process.
Menopause is a mid-life event with long-lasting consequences on the sleep patterns of older women, resulting in an increased prevalence of insomnia, relative to men.150 The mechanisms underlying menopausal-associated insomnia relate to both normal and usual aging. Normal aging renders older women more vulnerable to insomnia because menopause is characterized by a decline in endocrine physiology that is associated with a greater reduction in slow wave activity in NonREM sleep.93,94 Usual aging likewise renders older women more vulnerable to insomnia and drowsiness because menopause is characterized by an increased prevalence of depression, coronary artery disease, and SDB.150 Therapies that prevent such aging-related sequelae may be of value, as they could mitigate adverse outcomes specific to menopausal-associated insomnia. Although hormone replacement therapy was once considered promising, its adverse risk profile,151,152 conflicting findings in SDB,153 and lack of efficacy in coronary heart disease154 suggest the need for alternative treatments.
Among older persons, sleep is often disrupted by nocturia,3 which reflects an interdependence between normal and usual aging. Normal aging-related mechanisms render older persons vulnerable to nocturia through reductions in bladder volume, displacement of urinary production to later in the day, and decreased ability to postpone voiding.155 Usual aging-related mechanisms include a higher prevalence of benign prostatic hyperplasia, atrophic urethritis, and diuretic use.155
Once established, geriatric sleep disorders are likewise highly interdependent. For example, depression is a common cause of insomnia and imparts a worse prognosis in heart disease;156,157 while insomnia at baseline is a risk factor for subsequent depression and heart disease.18,23 Finally, the association of impaired sleep with subsequent insulin resistance, reduced immunity, and memory decrements suggest that sleep disorders may also accelerate senescence.158–160 Such sleep-related sequelae can lead to diminished homeostasis and vulnerability to disease, and may accelerate age-related declines in physiology, i.e., normal aging, and age-related increases in disease prevalence, i.e., usual aging.
CLINICAL APPLICATION
Returning to Ms. K’s case, her requests for a bedtime hypnotic and daytime stimulant are premature. Such medications simply target symptoms, rather than the underlying problem, and often lead to adverse outcomes. What is needed instead is a more detailed description of Ms. K’s insomnia and drowsiness, which can be readily obtained through patient-completed instruments such as a sleep log and Epworth Sleepiness Scale.35,37
The results of her assessment suggest that Ms. K’s sleep-related presentation is consistent with a diagnosis of chronic insomnia. This is on the basis of her insomnia symptoms having lasted longer than 6 months and being accompanied by daytime impairment, namely sleepiness and fatigue.137 The underlying sleep disruptions related to Ms. K’s insomnia are likely to be multifactorial and will necessitate a comprehensive approach, as shown in Table 2. Based on the observed reductions in her health status, the first priority should be to optimize the management of her medical and neuropsychiatric conditions and enhance her physical and cognitive function. A careful review of her medications is similarly required to determine their potential contribution to her insomnia and daytime drowsiness. An important goal would be to increase physical activity, as well as the physical and cognitive capacity to adhere to potential interventions such as CPAP or insomnia-related cognitive-behavioral therapy. Ms. K also has multiple risk factors for several primary sleep disorders, and would benefit from a consultation with a sleep specialist and a laboratory-based polysomnography. Polysomnography is indicated for diagnosing sleep disorder breathing, sleep-related movement disorders, narcolepsy, and unusual sleep-related behaviors, and for titrating positive airway pressure or assessing treatment response in established sleep disordered breathing.166 Lastly, referral to a case manager could help to identify community-based programs that are relevant to her physical and social needs, e.g. nutrition-services, home-based nursing, aide, and/or physical and occupational therapy, senior center activities, transportation, etc. These services could further improve Ms. K’s social and physical activity levels, and reestablish zeitgebers germane to a normal sleep-wake cycle.
Table 2.
Clinical features of Ms. K that disrupt sleep, with related therapeutic recommendations*
| Clinical Feature | Sleep Disruption † | Therapeutic Recommendations |
|---|---|---|
| Predisposing Factors (Normal Aging) | ||
| 79 years old | Reduced slow wave and REM sleep, phase advancement of sleep-wake cycle, nocturia. | Physical activity and outdoor light exposure in the afternoon; weight control; avoid large meal and alcohol within 4 hours of bedtime; avoid caffeine after 10 am. |
| Postmenopausal | At risk for insomnia, depression and SDB | Insomnia-related cognitive-behavioral therapy;161–163 exercise; targeted therapy for depression; and/or SDB. |
| Precipitating Factors (Usual Aging) | ||
| Depression | Insomnia | Treat depression, rule out suicidal ideations; may benefit from cognitive-behavioral therapy and an exercise program. |
| Medications | Adverse effect on slow wave and REM sleep, sleep-wake cycle, SDB, restless legs, periodic limb movements | Consider alternate medications that do not aversely affect sleep quality and daytime alertness, e.g. avoid centrallyacting agents. |
| Daytime sleepiness | Medications (e.g. dopamine-agonists164), SDB, narcolepsy | Consider alternate medications (see above); therapy for SDB and narcolepsy (daytime napping, stimulants). |
| Osteoarthritis | Pain-related insomnia | Pharmacologic and non-pharmacologic related therapy for arthritis |
| Hypertension, obesity, cardiovascular disease | Obstructive and central forms of SDB | Weight control; avoid alcohol within 4 hours of bedtime; avoid sedative/hypnotics; maximize oronasal patency (rhinitis); maintain euthyroid state; positional therapy; and CPAP. If central SDB, evaluate underlying cause– see text. |
| Parkinson’s Disease | Depression, obstructive and/or central SDB, restless legs, periodic limb movements, REM Behavior Disorder. | Targeted therapy. |
| COPD | Insomnia – depression, anxiety, hypoxia165 | Targeted therapy. |
| Loss of physical function | Disrupted zeitgebers, inadequate sleep hygiene, irregular sleep-wake patterns | Physical/occupational therapy, home safety evaluation. |
| Perpetuating Factors (Psychosocial Influences) | ||
| Social isolation, caregiving, bereavement | Disrupted zeitgebers, inadequate sleep hygiene, irregular sleep-wake patterns. | Insomnia-related cognitive-behavioral therapy; exercise; social services consult – see text. |
SDB= sleep disordered breathing; CPAP= Continuous Positive Airway Pressure; COPD= chronic obstructive pulmonary disease;
Refers to changes in sleep parameters and/or sleep disorders (e.g. primary or secondary forms) that are related to Ms. K’s clinical presentation. These sleep disruptions lead to sleep complaints and adverse outcomes, as conceptualized in Figure 1.
As shown in Table 3, two types of treatment offer benefit for managing the symptoms of insomnia in an older person like Ms. K. Cognitive-behavioral therapy provides several strategies that have proven efficacy in older persons, including those with comorbid-associated and hypnotic-dependent insomnia.161–163 Pharmacologic sleep aids also have a role, especially when insomnia symptoms persists despite behavioral interventions and targeted treatment of secondary factors.35,161,162,167,168,170–172 While there are risks associated with sleep aids (Table 3),35,116,169 these may be minimized in several ways.115 First, the pharmacokinetics of a sleep aid need to match the desired effect, e.g., a sleep aid with a rapid onset and short duration of action such as zaleplon or rozerem is preferred for sleep onset insomnia, while a longer duration of action as provided by zolpidem and eszopiclone is preferred for sleep maintenance insomnia. Second, the sleep aid needs to be initiated at the lowest dose, while avoiding dose escalations beyond that which is recommended. Third, dosage adjustments initiated by the patient should be discouraged. Finally, prescriptions lasting longer than one-month should be avoided, with subsequent renewals based on serial clinical assessments of the benefits and risks.
Table 3.
Symptom-management of insomnia in older persons.
| Treatment | Benefit | Risk |
|---|---|---|
| Cognitive-Behavioral Therapy161–163 | ||
|
Restores sleep-wake scheduling and the association of the bedroom with sleep | None known to date, but implementing these behavioral strategies require expertise, which may not be readily available in many communities; additionally, an appropriate level of motivation, commitment, and understanding are required of the patient. |
|
Improves sleep efficiency (the percent of time in bed that is spent asleep) | |
|
Reduce somatic tension and intrusive thoughts at bedtime that are sleep disruptive | |
|
Reduces excessive mentation or worrying about insomnia | |
|
Improves health practices and environmental factors that adversely affect sleep | |
| Pharmacotherapy35,116,167–174 | ||
|
|
|
In general, sleep aids as an initial intervention in geriatric insomnia is discouraged but, when required, nonbenzodiazepine hypnotics, such as zaleplon, zolpidem, or eszopiclone, appear to have the best safety profile.35, 167, 168, 170–172 A sedating antidepressant such as trazadone may also be used as a sleep aid, especially if the insomnia is associated with depressive symptoms and/or anxiety.173,174 Otherwise, barbiturates, antihistamines, antipsychotics, or alternative/complimentary therapies do not have an established efficacy as sleep aids in older persons.35 For our patient, Ms. K, we would not consider a sleep aid as an initial treatment given her history of chronic obstructive pulmonary disease, restricted mobility, and prior falls. Instead, we would implement cognitive-behavioral therapy concurrent with the measures targeting secondary factors, as described earlier.
One challenge to Ms. K’s sleep-related care is the paucity of interventions specifically designed to target the normal aging-related factors that predispose to sleep complaints. Such factors, when left untreated, render an older person persistently vulnerable to suboptimal outcomes and could, over time, lead to even more complex sleep disorders. In Table 2, non-pharmacologic measures are suggested that may, at least, temporize the normal-aging related changes of reduced slow wave and REM sleep, sleep phase advancement, and nocturia-induced sleep disruptions.
CONCLUSION
Sleep complaints in older persons are highly prevalent and often lead to adverse outcomes. The underlying mechanisms are senescence-based and characterized by an interdependent convergence of multiple predisposing, precipitating, and perpetuating factors, akin to a geriatric syndrome. Accordingly, the requisite diagnostic and therapeutic strategies for managing sleep complaints in older persons should be based on a comprehensive geriatric assessment that considers relevant sleep-related physiological changes, reductions in health status, loss of physical function, primary sleep disorders, and psychosocial influences.
ACKNOWLEDGMENTS
We thank Drs. Leo Cooney and H. Klar Yaggi of Yale University School of Medicine for their insightful recommendations on earlier drafts of the manuscript. We also express our gratitude to the reviewers and editorial staff of JAGS for their likewise insightful recommendations.
Dr. Fragoso is a postdoctoral research fellow in geriatric clinical epidemiology and is supported by a training grant from the National Institute on Aging (T32AG1934). Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-oriented Research (K24AG021507) from the National Institute on Aging.
Appendix
Common sleep-related terminology and abbreviations
- Apnea-Hypopnea Index (AHI) -
- The average number of apneas (cessation of airflow lasting at least 10 seconds) and hypopneas (reduced airflow lasting at least 10 seconds and associated with a ≥ 4% oxygen desaturation) per hour of sleep.
- Advanced Sleep Phase Syndrome (ASPS) -
- Circadian rhythm disorder resulting in a phase advance of the sleep-wake cycle.
- Central Sleep Apnea (CSA) -
- Transient episodes of cessation in respiratory effort while asleep resulting in cessation of airflow.
Continuous positive airway pressure (CPAP)
- Non Rapid-Eye-Movement Sleep (NonREM sleep) -
- Consists of four stages, 1–4.
- Obstructive Sleep Apnea-Hypopnea Syndrome (OSAHS) -
- Transient episodes of complete or partial upper airway obstruction while asleep, resulting in cessation or reduction of airflow despite continued respiratory effort.
- Periodic Limb Movements in Sleep (PLMS) -
- A motor movement disorder potentially disruptive to sleep continuity.
- Rapid-Eye-Movement Sleep (REM sleep) -
- Also known as circadian or dream sleep.
- REM Behavior Disorder (RBD) -
- An abnormal form of REM sleep that results in dream-directed behaviors.
- Restless Legs Syndrome (RLS) -
- A sensorimotor movement disorder especially disruptive when inactive such as bedtime, often delaying sleep onset.
- Sleep Disordered Breathing (SDB) -
- Defined in the sleep laboratory by an AHI of ≥ 5.
- Slow Wave Sleep (SWS) -
- Combined stages 3 and 4 of NonREM sleep, also referred to as homeostatic sleep. It is popularly known as “deep sleep”.
Footnotes
The authors were involved in all aspects of this manuscript and report no conflicts of interest.
REFERENCES
- 1.Institute of Medicine Report. [Accessed November 27, 2006];Sleep disorders and sleep deprivation: an unmet public health problem. 2006 April; At: www.iom.edu. [PubMed]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.National Sleep Foundation. [Accessed December 4, 2005];Sleep in America Poll. 2003 At: www.sleepfoundation.org.
- 4.Phillips B, Mannino D. Correlates of sleep complaints in adults: The ARIC study. J Clin Sleep Med. 2005;1:277–283. [PubMed] [Google Scholar]
- 5.Ohayon MM, Zulley J, Guilleminault C, Smirne S, Priest RG. How age and daytime activities are related to insomnia in the general population: Consequences for older people. J Am Geriatr Soc. 2001;49:360–366. doi: 10.1046/j.1532-5415.2001.49077.x. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto R, Yoshida O, Oka Y, Takagi Y. Risk factors for insomnia in community-dwelling older persons. Geriatrics and Gerontology International. 2004;4:163–168. [Google Scholar]
- 7.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW, et al. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: The cardiovascular health study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 8.Schubert CR, Cruikshanks KJ, Dalton DS, Klein BEK, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002;25:48–52. [PubMed] [Google Scholar]
- 9.Brassington GS, King AC, Bliwise DL. Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64–99 years. J Am Geriatr Soc. 2000;48:1234–1240. doi: 10.1111/j.1532-5415.2000.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 10.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Medicine. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Pollack CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- 12.Motivala SJ, Levin MJ, Oxman MN, Irwin MJ. Impairments in health functioning and sleep quality in older adults with a history of depression. J Am Geriatr Soc. 2006;54:1184–1191. doi: 10.1111/j.1532-5415.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, et al. Poor sleep is associated with impaired cognitive function in older women: The study of osteoporotic fractures. J Gerontol. 2006;61A:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, Vecchierini M-F. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–208. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 15.Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE, Boeve BF. A distinctive interaction between memory and chronic daytime somnolence in asymptomatic APOE e4 homozygotes. Sleep. 2002;25:447–453. [PubMed] [Google Scholar]
- 16.Campos H, Xinia S. Siesta and the risk of coronary heart disease: results from a population-based, case-controlled study in Costa Rica. Int J Epidemiol. 2000;29:429–437. [PubMed] [Google Scholar]
- 17.Gooneratne NS, Weaver TE, Cater JR, Pack FM, Arner HM, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–649. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 18.Foley DJ, Monjan AA, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: An epidemiologic study of 6,800 persons over three years. Sleep. 1999;22:S366–S372. [PubMed] [Google Scholar]
- 19.Byles JE, Mishra GD, Harris MA, Nair K. The problems of sleep for older women: changes in health outcomes. Age and Ageing. 2003;32:154–163. doi: 10.1093/ageing/32.2.154. [DOI] [PubMed] [Google Scholar]
- 20.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 21.Eriksen W, Natvig, Bruusgaard D. Sleep problems: A predictor of long-term work disability? A four year prospective study. Scand J Public Health. 2001;29:23–31. [PubMed] [Google Scholar]
- 22.Byles JE, Mishra GD, Harris MA. The experience of insomnia among older women. Sleep. 2005;28:972–979. doi: 10.1093/sleep/28.8.972. [DOI] [PubMed] [Google Scholar]
- 23.Ayas NT, White DP, Manson JE, Stampfler MJ, Speizer FE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 24.Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. International J Geriatr Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to19 years of follow-up. Psychosomatic Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 27.Bursztyn M, Ginsberg G, Hammerman-Rozenberg R, Stessman J. The siesta in the elderly. Risk factor for mortality? Arch Intern Med. 1999;159:1582–1586. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Spiekerman CF, Enright P, Lefkowicz D, Manolio T, Reynolds CF, Robbins J The cardiovascular health study research group. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 29.Bursztyn M, Ginsberg G, Stessman J. The siesta and mortality in the elderly: Effect of rest without sleep and daytime sleep duration. Sleep. 2002;25:187–191. doi: 10.1093/sleep/25.2.187. [DOI] [PubMed] [Google Scholar]
- 30.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: A community study in Jerusalem. Sleep. 2003;26:578–584. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 31.Bursztyn M, Stessman J. The siesta and mortality: Twelve years of prospective observations in 70-year-olds. Sleep. 2005;28:345–347. [PubMed] [Google Scholar]
- 32.Stone KL, Ewing SK, Lui L-Y, Ensrud KE, Ancoli-Israel S, et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1177–1183. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 33.Ancoli-Israel S. Sleep problems in older adults: putting myths to bed. Geriatrics. 1997;52:20–30. [PubMed] [Google Scholar]
- 34.Vitiello MV. Sleep disorders and aging: understanding the causes. J Gerontol A Biol Sci Med Sci. 1997;52:M189–M191. doi: 10.1093/gerona/52a.4.m189. [DOI] [PubMed] [Google Scholar]
- 35.National Institutes of Health State of the Science Conference Statement. Manifestations and management of chronic insomnia in adults June 13–15, 2005. Sleep. 2005;28:1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 36.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 37.Johns M. Sleepiness in different situations measured by the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Martin JL. Insomnia and daytime napping in older adults. J Clin Sleep Med. 2006;2:333–342. [PubMed] [Google Scholar]
- 39.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 40.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 national sleep foundation sleep in America survey. J Psychosomatic Research. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Ancoli-Israel S. Sleep disorders in older adults: a primary care guide to assessing 4 common sleep problems in geriatric patient- sleep. Geriatrics. 2004;59:37–40. [PubMed] [Google Scholar]
- 42.Kryger M, Monjan A, Bliwise D, Ancoli-Israel S. Sleep, health, and aging. Bridging the gap between science and clinical practice. Geriatrics. 2004;59:24–26–29–30. [PubMed] [Google Scholar]
- 43.Shochat T, Loredo J, Ancoli-Israel S. Sleep disorders in the elderly. Current Treatment Options In Neurology. 2001;3:19–36. doi: 10.1007/s11940-001-0021-x. [DOI] [PubMed] [Google Scholar]
- 44.The Aging Process. Hospital Physician Board Review Manual. Geriatric Medicine. 2000;1:2–12. [Google Scholar]
- 45.Drachman DA. Aging and the brain: A new frontier. Ann Neurol. 1997;42:819–828. doi: 10.1002/ana.410420602. [DOI] [PubMed] [Google Scholar]
- 46.Spielman AJ, Caruso L, Glovinsky PB. A behavioral perspective on insomnia. Psychiatry Clin North Am. 1987;10:541–553. [PubMed] [Google Scholar]
- 47.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 48.Chokroverty S. An overview of sleep. In: Chokoverty S, editor. Sleep Disorders Medicine. 2nd Edition. Worcester, MA: Butterworth-Heinemann; 1999. pp. 7–20. [Google Scholar]
- 49.Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 13–23. [Google Scholar]
- 50.Kales A, Kales D. Sleep disorders: recent findings in the diagnosis and treatment of disturbed sleep. N Engl J Med. 1974;290:487–499. doi: 10.1056/NEJM197402282900905. [DOI] [PubMed] [Google Scholar]
- 51.Rechtschaffen A, Kales, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages in human subjects. Los Angeles: BIS/BRI, UCLA; 1968. [DOI] [PubMed] [Google Scholar]
- 52.Kilduff TS, Kushida CA. Circadian regulation of sleep. In: Chokoverty S, editor. Sleep Disorders Medicine. 2nd Edition. Worcester, MA: Butterworth-Heinemann; 1999. pp. 135–147. [Google Scholar]
- 53.Dijk DJ, Brunner DP, Beersma DGM, et al. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–440. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 54.Murillo-Rodriguez E, Blanco-Centurion C, Sanchez C, Piomelli D, Shiromani PJ. Anandamide enhances extracellular levels of adenosine and induces sleep: An in vivo microdialysis study. Sleep. 2003;26:943–947. doi: 10.1093/sleep/26.8.943. [DOI] [PubMed] [Google Scholar]
- 55.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeitzer JM, Morales-Villagran A, Maidment NT, Behnke EJ, Ackerson LC, et al. Extracellular adenosine in the human brain during sleep and sleep deprivation: An in vivo microdialysis study. Sleep. 2006;29:455–461. doi: 10.1093/sleep/29.4.455. [DOI] [PubMed] [Google Scholar]
- 57.Dinges DF. Forum on critical topics – sleep, adenosine, and the basal forebrain. Sleep. 2006;29:1381–1389. [Google Scholar]
- 58.Kong J, Shepel PN, Holden CP, et al. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wyatt JK, Cajochen C, Ritz-De Cecco A, et al. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 60.Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, et al. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur J Neurosci. 1997;9:271–279. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 61.Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990;48:749–753. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- 62.Berger RJ, Phillips NH. Energy conservation and sleep. Behavioural brain research. 1995;69:65–73. doi: 10.1016/0166-4328(95)00002-b. [DOI] [PubMed] [Google Scholar]
- 63.Van Cauter E. Endocrine physiology. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 266–282. [Google Scholar]
- 64.Walker MP. A refined model of sleep and the time course of memory formation. Behavioral and Brain Sciences. 2005;28:51–104. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- 65.Brandenberger G, Ehrhart JE, Buchheit M. Sleep stage 2: An electroencephalographic, autonomic, and hormonal duality. Sleep. 2005;28:1535–1540. doi: 10.1093/sleep/28.12.1535. [DOI] [PubMed] [Google Scholar]
- 66.Mu Q, Nahas Z, Johnson KA, Yamanaka K, Mishory A, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- 67.Czeisler CA, Buxton OM, Khalsa SBS. The human circadian timing system and sleep-wake regulation. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 375–394. [Google Scholar]
- 68.Mistlemerger RE, Rusak B. Circadian rhythms in mammals: formal properties and environmental influences. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 321–334. [Google Scholar]
- 69.McCarley RW. Sleep neurophysiology: basic mechanisms underlying control of wakefulness and sleepf. In: Chokoverty S, editor. Sleep Disorders Medicine. 2nd Edition. Worcester, MA: Butterworth-Heinemann; 1999. pp. 21–50. [Google Scholar]
- 70.Siegel JM. REM sleep. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 120–135. [Google Scholar]
- 71.Pal D, Madan V, Mallick BN. Neural mechanism of rapid eye movement sleep generation: Cessation of locus coeruleus neurons is a necessity. Acta Physiologica Sinica. 2005;57:401–413. [PubMed] [Google Scholar]
- 72.Roehrs T, Roth T. Sleep-wake states and memory function. Sleep. 2000;23:S64–S68. [PubMed] [Google Scholar]
- 73.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 74.Crick F, Mitchison G. REM sleep and neural nets. Behav Brain Res. 1995;69:147–155. doi: 10.1016/0166-4328(95)00006-f. [DOI] [PubMed] [Google Scholar]
- 75.Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep: Relevance for memory. Behav Brain Res. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- 76.Smith C. Sleep states and memory process. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 77.Karni A, Tanne D, Rubenstein BS, Askenazy JJM, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 78.Rauchs G, Bertran F, Guillery-Girard B, Desgranges B, Kerrouche N, et al. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep. 2004;27:395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- 79.De Koninck J, Lorrain D, Christ G, et al. Intensive language learning and increases in rapid eye movement sleep: evidence of a performance factor. Int J Psychophysiol. 1989;8:43–47. doi: 10.1016/0167-8760(89)90018-4. [DOI] [PubMed] [Google Scholar]
- 80.Dos Santos Maraes W, Poyares DR, Guillemenault C, Ramos LR, Bertolucci HF, Tufik S. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer’s Disease: A double-blind placebo controlled study. Sleep. 2006;29:199–205. doi: 10.1093/sleep/29.2.199. [DOI] [PubMed] [Google Scholar]
- 81.Garbarino S, Nibili L, Beeke M, et al. The contributing role of sleepiness in highway vehicle accidents. Sleep. 2001;24:203–206. [PubMed] [Google Scholar]
- 82.Horne JA, Reyner LA. Sleep related vehicle accidents. BMJ. 1995;310:565–567. doi: 10.1136/bmj.310.6979.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.George CFP. Driving and automobile crashes in patients with obstructive sleep apnea/hypopnea syndrome. Thorax. 2004;59:804–807. doi: 10.1136/thx.2003.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 85.Nicolas A, Petit D, Rompre S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol. 2001;112:521–527. doi: 10.1016/s1388-2457(00)00556-3. [DOI] [PubMed] [Google Scholar]
- 86.Bliwise DL. Normal Aging. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 24–38. [Google Scholar]
- 87.Chokoverty S. Sleep Disorders in the Elderly. In: Chokoverty S, editor. Sleep Disorders Medicine. 2nd Edition. Worcester, MA: Butterworth-Heinemann; 1999. pp. 661–681. [Google Scholar]
- 88.Bliwise DL, Ansari FP, Straight L-B, Parker KP. Age changes in timing and 24-hour distribution of self-reported sleep. Am J Geriatr Psychiatry. 2005;13:1077–1082. doi: 10.1176/appi.ajgp.13.12.1077. [DOI] [PubMed] [Google Scholar]
- 89.Haimov I, Lavie P. Circadian characteristics of sleep propensity function in healthy elderly: A comparison with young adults. Sleep. 1997;20:294–300. doi: 10.1093/sleep/20.4.294. [DOI] [PubMed] [Google Scholar]
- 90.Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–1376. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- 91.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, et al. Stability, precision, and near 24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 92.Redline S, Kirchner HL, Quan SF, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 93.Latta F, Leproult R, Tasali E, Hofmann E, L’Hermite-Baleriaux M, et al. Sex differences in nocturnal growth hormone and prolactin secretion in healthy older adults: Relationships with sleep EEG variables. Sleep. 2005;28:1519–1524. doi: 10.1093/sleep/28.12.1519. [DOI] [PubMed] [Google Scholar]
- 94.Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E. Sex differences in delta and alpha EEG activities in older adults. Sleep. 2005;28:1525–1534. doi: 10.1093/sleep/28.12.1525. [DOI] [PubMed] [Google Scholar]
- 95.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 96.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, et al. Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 97.Poter NM, Landfield PW. Stress hormones and brain aging: Adding injury to insult? Nat Neurosi. 1998;1:3–4. doi: 10.1038/196. [DOI] [PubMed] [Google Scholar]
- 98.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosi. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 99.Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psych. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 100.Monk TH. Sleep and circadian rhythms. Experimental Gerontology. 1991;26:233–243. doi: 10.1016/0531-5565(91)90015-e. [DOI] [PubMed] [Google Scholar]
- 101.Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Bio Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- 102.Jordan JM. National follow-up survey of self-care and aging. Arch Fam Med. 2000;9:143–149. doi: 10.1001/archfami.9.2.143. [DOI] [PubMed] [Google Scholar]
- 103.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173:7–15. doi: 10.1164/rccm.200509-1478OE. [DOI] [PubMed] [Google Scholar]
- 104.Buysse D, Reynolds C, Kupfer D, Thorpy MJ, Bixler E, et al. Clinical diagnosis in 216 insomnia patients using the international classification of sleep disorders (ICSD), DSM IV, and ICD-10 categories: A report from the APA/NIMH DSM-IV field trial. Sleep. 1994a;17:630–637. doi: 10.1093/sleep/17.7.630. [DOI] [PubMed] [Google Scholar]
- 105.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K The MEMO study. Prevalence and risk factors of RLS in an elderly population. Neurology. 2000;54:1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 106.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 107. [Accessed May 9, 2006];Federal Interagency Forum on Aging Related Statistics. At: www.agingstats.gov.
- 108. [Accessed August 5, 2006];National Center for Health Statistics. At: www.cdc.gov.
- 109.Goulding MR. Inappropriate medication prescribing for elderly ambulatory care patients. Arch Intern Med. 2004;164:305–312. doi: 10.1001/archinte.164.3.305. [DOI] [PubMed] [Google Scholar]
- 110.Kaufman DW, Kelly JP, Rosenberg L, Andersen TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 111.Hanlon JT, Shimp LA, Semla TP. Advances in geriatrics drug-related problems in the elderly. Ann Pharmacotherapy. 2000;34:360–365. doi: 10.1345/aph.19140. [DOI] [PubMed] [Google Scholar]
- 112.Ghandi TK, Weingart SN, Borus J, Seger AC, Peterson J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 113.Byles JE, Mishra GD, Harris MA. The experience of insomnia among older women. Sleep. 2005;28:972–979. doi: 10.1093/sleep/28.8.972. [DOI] [PubMed] [Google Scholar]
- 114.Robinson A, Guilleminault C. Obstructive sleep apnea syndrome. In: Chokoverty S, editor. Sleep Disorders Medicine. 2nd Edition. Worcester, MA: Butterworth-Heinemann; 1999. pp. 331–354. [Google Scholar]
- 115.Walsh JK, Roehrs T, Roth T. Pharmacologic treatment of primary insomnia. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 749–760. [Google Scholar]
- 116.Tinetti ME. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 117.Blazer DG. Depression in late life: Review and commentary. J Gerontol. 2003;58A:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 118.Arias E, Anderson RN, Kung HC, Murphy SL, Kochanek KD. Deaths: Final data for 2001: National vital statistics reports 52(3) Hyatsville, Md: National Center for Health Statistics; DHHS publication (PHS) 2003–1120. [PubMed] [Google Scholar]
- 119.Petit D, Montplaisir J, Boeve BF. Alzheimer’s disease and other dementias. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 853–862. [Google Scholar]
- 120.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Medicine. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 121.US Census Bureau. [Accessed April 10, 2006];Decennial Census and Projections. At: www.census.gov.
- 122. [Accessed January 19, 2006];Research Highlights in the Demography of Older Americans. 1999 March;(Issue No 5) At: http://agingmeta.psc.isr.umich.edu/resHigh5.pdf.
- 123.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, et al. Prevalence of Alzheimer’s Disease in a community population of older persons: Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 124.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 125.Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin sleep cohort study. Sleep. 1998;21:701–706. [PubMed] [Google Scholar]
- 126. [Accessed November 27, 2006];CMS Manual System (Publication 100-03). 240-4 - Continuous Positive Airway Pressure therapy for obstructive sleep apnea. 2005 April; At: www.cms.gov.
- 127.Bixler EO, Vgontzas AN, Ten HT, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 128.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnea syndrome: Declining mortality rates with age. Eur Respir J. 2005;25:514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 129.Parish JM, Lyng PJ, Wisbey J. Compliance with CPAP in elderly patients with OSA. Sleep Medicine. 2000;1:209–214. doi: 10.1016/s1389-9457(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 130.Arzt M, Bradley TD. Treatment of sleep apnea in heart failure. Am J Respir Crit Care Med. 2006;173:1300–1308. doi: 10.1164/rccm.200511-1745PP. [DOI] [PubMed] [Google Scholar]
- 131.White DP. Central sleep apnea. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 969–982. [Google Scholar]
- 132.Leung RST, Heuber MA, Rogge T, Maimon N, Chiu K-L. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28:1543–1546. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 133.Javaheri S, Parker TJ, Wexler L, Michaels SE, Stanberry E, Nishyama H, Roselle GA. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med. 1995;122:487–492. doi: 10.7326/0003-4819-122-7-199504010-00002. [DOI] [PubMed] [Google Scholar]
- 134.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nature Neuroscience. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Int Med. 2000;160:2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 136.Restless Legs Syndrome Foundation. [Accessed February 17, 2007];RLS Medical Bulletin. 2005 At: www.rls.org.
- 137.International classification of sleep disorders, Second Edition: Diagnostic and coding manual. Westchester, Illinois: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 138.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. Sleep Medicine. 2003;1:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 139.Pearson V, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome. Sleep Medicine. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 140.Baker SK, Zee PC. Circadian disorders of the sleep-wake cycle. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th Edition. Philadelphia: Elsevier Saunders; 2005. pp. 691–701. [Google Scholar]
- 141.Rye DB, Dihenia B, Weissman JD, Epstein CM, Bliwise DL. Presentation of narcolepsy after age 40. Neurology. 1998;50:459–465. doi: 10.1212/wnl.50.2.459. [DOI] [PubMed] [Google Scholar]
- 142.Schenck CH, Mahowald MW. REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification. Sleep. 2002;25:120–138. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 143.Dunkin JJ, Anderson-Hanley C. Dementia caregiver burden: A review of the literature and guidelines for assessment and intervention. Neurology. 1998;51:S53–S60. doi: 10.1212/wnl.51.1_suppl_1.s53. [DOI] [PubMed] [Google Scholar]
- 144.Christakis NA, Allison PD. Mortality after hospitalization of a spouse. N Engl J Med. 2006;354:719–730. doi: 10.1056/NEJMsa050196. [DOI] [PubMed] [Google Scholar]
- 145.Schulz R, Beach SR. Caregiving as a risk factor for mortality. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 146.Kiecolt-Glaser JK, Glaser R. Chronic stress and mortality among older adults. JAMA. 1999;282:2259–2260. doi: 10.1001/jama.282.23.2259. [DOI] [PubMed] [Google Scholar]
- 147.Martikainen P, Valkonen T. Mortality after the death of a spouse: Rates and causes of death in a large Finnish cohort. Am J Public Health. 1996;86:1087–1093. doi: 10.2105/ajph.86.8_pt_1.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monk TH, Buysse DJ, Carrier J, et al. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 149.Pasternak RE, Reynolds CF, 3rd, Houck PR. Sleep in bereavement-related depression during and after pharmacotherapy with nortriptyline. J Geriatr psychiatry Neurol. 1994;7:69–73. doi: 10.1177/089198879400700201. [DOI] [PubMed] [Google Scholar]
- 150.Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Medicine Reviews. 2003;7:155–177. doi: 10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- 151.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 152.Lacey JV, Mink PJ, Lubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 153.Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Ballard RD, Magalang UJ. Medical therapy for obstructive sleep apnea: a review by the medical therapy for obstructive sleep apnea task force of the standards of practice committee of the American academy of sleep medicine. Sleep. 2006;29:1036–1044. doi: 10.1093/sleep/29.8.1036. [DOI] [PubMed] [Google Scholar]
- 154.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 155.Dubeau CE. Urinary incontinence. In: Cobbs EL, Duthie EH, Murphy JB, editors. Geriatric Review Syllabus. 5th Edition. Malden, MA: Blackwell Publishing; 2002. pp. 139–418. [Google Scholar]
- 156.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 157.Rumsfeld JS, Ho PM. Depression and Cardiovascular Disease: A Call For Recognition. Circulation. 2005;111:250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 158.Spiegel K, Leproult R, Van Caulter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 159.Spiegel K, Sheridan J, Van Caulter E. Effect of Sleep Deprivation on Response to Immunization. JAMA. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 160.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 161.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 162.Morin CM, Colecchi, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia. A randomized controlled trial. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 163.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2:403–406. [PubMed] [Google Scholar]
- 164.Homann CN, Wenzel K, Suppan K, et al. Sleep attacks in patients taking dopamine agonists: review. BMJ. 2002;324:1483–1487. doi: 10.1136/bmj.324.7352.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fragoso C, Kotch A. Sleep related issues in COPD. Emphysema/COPD: The Journal of Patient-Centered Care. 2005;2:14–22. [Google Scholar]
- 166.Kushida CA, Littner MR, Morgenthaler TM, et al. Practice parameters for the indications of polysomnography and related procedures: an update for 2005. Sleep. 28:499–521. doi: 10.1093/sleep/28.4.499. 20005. [DOI] [PubMed] [Google Scholar]
- 167.Witmans M. Systematic review of the efficacy and safety of drug treatments and combination treatments in the management of chronic insomnia in adults. J Clin Sleep Med. 2005;1:488–489. [Google Scholar]
- 168.Wohlgemuth WK, Krystal AD. Hypnotics should be considered for the initial treatment of chronic insomnia. J Clin Sleep Med. 2005;1:120–124. [PubMed] [Google Scholar]
- 169.Stepanski EJ. Hypnotics should not be considered for the initial treatment of chronic insomnia. J Clin Sleep Med. 2005;1:125–128. [PubMed] [Google Scholar]
- 170.Escopiclone, a new hypnotic. The Medical Letter. 2005;47:17–19. [Google Scholar]
- 171.Ramelteon for insomnia. The Medical Letter. 2005;47:89–91. [PubMed] [Google Scholar]
- 172.Ambien CR for insomnia. The Medical Letter. 2005;47:97–98. [PubMed] [Google Scholar]
- 173.Kaynak H, Kaynak D, Gozukirmizi E, Guilleminault C. The effects of trazodone on sleep in patients treated with stimulant antidepressants. Sleep Med. 2004;5:15–20. doi: 10.1016/j.sleep.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 174.Becker PM. Trazodone as a hypnotic in major depresssion. Sleep Med. 2004;5:7–8. doi: 10.1016/j.sleep.2003.08.007. [DOI] [PubMed] [Google Scholar]