Abstract
Myosins are cytoskeletal motors critical for generating the forces necessary for establishing cell structure and mediating actin-dependent cell motility. In each cell type a multitude of myosins are expressed, each myosin contributing to aspects of morphogenesis, transport, or motility occurring in that cell type. To examine the roles of myosins in individual retinal cell types, we first used polymerase chain reaction (PCR) screening to identify myosins expressed in retina and retinal pigmented epithelium (RPE), followed by immunohistochemistry to examine the cellular and subcellular localizations of seven of these expressed myosins. In the myosin PCR screen of cDNA from striped bass retina and striped bass RPE, we amplified 17 distinct myosins from eight myosin classes from retinal cDNA and 11 distinct myosins from seven myosin classes from RPE cDNA. By using antibodies specific for myosins IIA, IIB, IIIA, IIIB, VI, VIIA, and IXB, we examined the localization patterns of these myosins in retinas and RPE of fish, and in isolated inner/outer segment fragments of green sunfish photoreceptors. Each of the myosins exhibited unique expression patterns in fish retina. Individual cell types expressed multiple myosin family members, some of which colocalized within a particular cell type. Because much is known about the functions and properties of these myosins from studies in other systems, their cellular and subcellular localization patterns in the retina help us understand which roles they might play in the vertebrate retina and RPE.
Indexing terms: myosin, retina, photoreceptor, actin, fish, RPE
Although myosin mutations have been linked to sensory defects in vision, hearing, and vestibular function over the past two decades (Brown and Bridgman, 2004), specific details of what roles myosins might play in sensory cell function are just beginning to emerge. Members of the large myosin superfamily are characterized by possession of a motor domain that mediates movement along actin filaments. Currently 24 classes of myosins have been recognized, and at least 11 of these classes are found among vertebrates (Foth et al., 2006). By using polymerase chain reaction (PCR) analysis, Bement and coworkers (1994) showed that as many as 11 different unconventional myosin family members can be expressed within a single cell type. By using a similar PCR strategy, our lab has amplified sequences from 18 different myosins representing nine myosin classes from fish retina and retinal pigmented epithelium (RPE) cDNA. This report summarizes the findings of our PCR screen and provides information about the immunocytochemical localization of a subset of the identified myosins in retinas of several fish species.
Localization studies in the retina offer an interesting opportunity to compare the cellular expression patterns of different myosins in five different types of neurons, Müller glial cells, and the RPE. Three of these retinal cell types (i.e., photoreceptors, Müller cells, and RPE cells) possess well-characterized and elaborate actin cytoskeletons that feature specialized membrane projections supported underneath by core bundles of actin filaments. In photoreceptors, actin is concentrated in the inner segment, connecting cilium, and synapse (Chaitin and Bok, 1986; Nagle et al., 1986). Long parallel arrays of actin filament bundles form the core of microvillar calycal processes that embrace the base of the outer segment, course through the inner segment beneath the plasma membrane, and extend into the myoid. Müller cells span the width of the retina, forming adherens junctions with the photoreceptors at their distal ends to create the outer limiting membrane (OLM) and elaborating foot processes that form the internal limiting membrane (ILM) at the vitreal surface of the retina. At their apical ends, Müller cells extend numerous microvilli into the subretinal space. These apical microvilli and the OLM both stain very brightly with phalloidin, indicating that filamentous actin is highly concentrated at these sites in Müller cells. In RPE cells, filamentous actin is found along the basolateral membranes, in apical circumferential microfilament bundles, and in the apical microvilli that interdigitate with photoreceptor outer segments (Burnside and Bost-Usinger, 1998).
In this study, we examine the expression and localization of unconventional myosins in the retinas of several fish species. Fish have useful experimental advantages for studies of the retinal cytoskeleton. Retina and RPE are readily obtainable from large fish in sufficient quantities for molecular and biochemical analyses, and many aspects of the cell biology of different retinal cell types have been well characterized. Fish cones are very large in comparison with rodent and avian photoreceptors, making them especially useful for immunohistochemistry, and both cones and rods can be readily isolated from fish retina for higher resolution of subcellular detail and biochemical analysis. Furthermore, many mutants have been identified by mutagenesis screens in zebrafish, providing excellent animal models for elucidating gene function and understanding human diseases.
In our study seven different myosin family members (myosins IIA, IIB, IIIA, IIIB, VI, VIIA, and IXB) were examined in the retina by immunohistochemistry. Of these different myosins, only myosin VIIA has been associated with retinal disease in humans (Weil et al., 1995). We chose myosin III because mutation or deletion of myosin III has been shown to cause photoreceptor degeneration in flies. In Drosophila, the myosin III mutant ninaC compromises phototransduction and leads to light-induced photoreceptor degeneration (Montell and Rubin, 1988). However, in human patients with mutations in myosin IIIA that produce deafness, no effects on retinal morphology or function have been reported (Walsh et al., 2002). Thus, it is unclear whether myosin III is important for vertebrate photoreceptors.
Each class of myosin displays unique molecular features that are likely to mediate distinct functions in different cell types. For example, the tails of class II myosins possess a long coiled coil region that mediates dimerization, whereas class III myosins have N-terminal kinase domains. Class VI myosins exhibit minus end-directed movement along actin filaments in contrast to all other myosins, which are plus end-directed. Class VII myosins can associate with membranes via specific tail domains, and class IX myosins possess a GTPase-activating protein (GAP) tail domain. We report here that the expression patterns of these different myosin family members in the fish retina are unique for each myosin, and that specific retinal cell types express multiple myosins.
Materials and Methods
Animals
All experiments and procedures involving animals for this study were approved by the University of California at Berkeley Animal Care and Use Committee (ACUC). Adult zebrafish (Danio rerio) were obtained from the zebrafish colony maintained at University of California at Berkeley. Albino trout (Oncorhynchus mykiss) retinal tissue was obtained from fish raised at the Kamas Fish Hatchery (Kamas, UT). Green sunfish (Lepomis cyanellus) were obtained from Stillwater AquaFarms (Pleasant Grove, CA). Striped bass (Morone saxatilis) were obtained from Chico Game Fish Farm (Chico, CA).
RNA isolation from tissue and cDNA synthesis
The dissection of retinal and RPE tissue from dark-adapted adult striped bass and the subsequent RNA isolation and cDNA synthesis have been previously described (Breckler et al., 2000).
Polymerase chain reaction (PCR) and cloning
The PCR-based screen using degenerate primers designed against the conserved nucleotide binding subdomain of the myosin motor domain has already been described elsewhere (Breckler et al., 2000; Dosé et al., 2003). In summary, an upstream GE-GAGKT primer [GGIGA(A/G)(A/T)(G/C)IGGIGCIGGAXAA(A/G)AC] and a downstream EAFGNAKT primer [GT(T/C)TTIGC(A/G)TTICC(A/G)AAIGC(T/C)TC] that were identical to primers used in a previous myosin survey (Bement et al., 1994) were synthesized by Operon Technologies (Huntsville, AL) and used to amplify myosin fragments from retinal and RPE cDNA. Approximately 5 ng cDNA was used in a 50-μl reaction containing 60 mM Tris-HCl, pH 8.5, 15 mM NH4SO4, 2.0 mM MgCl2, 250 μM dNTPs, 1 μM GE-GAGKT, and 1 μM EAFGNAKT. Then 1.25 U of AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) was added to the reaction at 90°C. The primary PCR amplification consisted of 35 cycles of 94°C for 1 minute, 55°C for 2 minutes, and 72°C for 2 minutes with a 10-minute final extension step. For the RPE sample, a second round of amplification (14 cycles) was performed on 10 ng of gel-purified DNA from the primary amplification.
Analyses of RPE- and retina-specific clones
RPE PCR products were cloned into pBluescript SK+ (Stratagene, La Jolla, CA), and 64 of the 96 resulting clones were sequenced. Eleven of the 64 clones represented unique sequences; each was radiolabeled and used sequentially to screen cDNA clones of 170 retinal PCR products in order to classify 145 retinal clones. The remaining unidentified retina-specific clones were sequenced.
Northern blot analysis
The Northern blot used in this study has already been described elsewhere (Breckler et al., 2000). The same Northern blot was stripped and used with radiolabeled probes for different myosin family members, generated from cloned PCR products as described previously (Breckler et al., 2000).
Antibodies
The myosin IIIB antibody was made in rabbits against a glutathione-S-transferase fusion to the tail region of striped bass MyoIIIB downstream of the first IQ motif and upstream of the PEDS sequence at the C-terminus corresponding to amino acids 1,143–1,234 (Bethyl Laboratories, Montgomery, TX). The resulting serum was affinity purified with a maltose binding protein (MBP) fusion to the MyoIIIB tail peptide. The other antibodies used for Western blots and immunohistochemistry were: actin mouse monoclonal antibody made against an SGPSIVHRKCF peptide (Sigma-Aldrich, St. Louis, MO), myosin IIA (made against the human GKADGAEAKPAE peptide) and IIB (made against the human SDVNETQPPQSE peptide) affinity-purified polyclonal rabbit antibodies from Robert Adelstein (Rochlin et al., 1995), affinity-purified polyclonal myosin IIIA antibody made against the striped bass NPYDFRHLLRKTSQRRKLIKQY peptide in rabbits (Dosé et al., 2003), affinity-purified rabbit polyclonal myosin VI fusion antibody made against amino acids 1,049–1,254 of the porcine myosin VI tail (Hasson and Mooseker, 1994), affinity-purified rabbit polyclonal myosin VIIA antibody made against amino acids 800–1,077 of the human myosin VIIA tail (Hasson et al., 1995), and affinity-purified rabbit polyclonal myosin IXB antibody made against amino acids 1,252–2,022 of the human myosin IXB tail (Post et al., 1998). Normal rabbit IgG (Jackson ImmunoResearch, West Grove, PA) was used as a control.
For myosin IIIB antibodies, myosin IIIB-depleted and control myosin IIIB-depleted sera were generated as follows: MBP fusions to the myosin IIIB tail region used to produce the IIIB antibody and to zebrafish fascin 2B, an unrelated protein, were purified, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to Immobilon-P membrane (Millipore, Bedford, MA). Strips containing the fusion proteins were excised, blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS), pH 7.4, and incubated with the myosin IIIB diluted in 3% bovine serum albumin (BSA)/PBS overnight at 4°C.
Immunoblots
Retinas with some contaminating RPE were dissected from dark-adapted zebrafish in 1X Hanks' Balanced Salt Solution (Invitrogen, Carlsbad, CA) containing 1 μM calpeptin (Sigma-Aldrich). Retinal tissue was homogenized in homogenization buffer made of 50 mM MOPS, pH 7.2; 1 mM EDTA; 1 mM EGTA; 160 mM KCl; 1 mM dithiothreitol (DTT); 10 mM ATP; 2 μg/ml aprotinin; 100 mM leupeptin; 10 μM pepstatin; 1 μM calpeptin; 5 μM calpain inhibitor III; 400 μM PMSF; 1 mM benzamidine; 2 mM phenanthroline; 10 mg/ml TAME; and 5 μl/ml protease cocktail P8340 (Sigma-Aldrich). Retinas with attached RPE were dissected from light-adapted striped bass in a similar manner. For the zebrafish sample, whole cells and pigment granules were removed from the homogenate with a low-speed spin at 800g for 10 minutes at 4°C. The low-speed supernatant was centrifuged at 100,000g for 30 minutes at 4°C. For the striped bass retina/RPE extract, the homogenized extract was spun at 14,000 rpm in a microfuge at 4°C for 20 minutes, and the supernatant was used for SDS-PAGE.
The low- and high-speed supernatants of the fish retinal/RPE extracts were loaded onto NuPAGE 7% Tris-acetate or 4–12% Bis-Tris gels (Invitrogen) and blotted onto Immobilon-P membrane in NuPAGE transfer buffer containing 10% methanol and 0.05% SDS for the Tris-acetate gels. Blots were rinsed in PBS and blocked for 1 hour in 5% nonfat dry milk in PBS at room temperature. Protein blots were incubated at 4°C overnight with primary antibodies diluted in 3% BSA in PBS at the following concentrations: 1.3 μg/ml myosin IIA antibody, 2 μg/ml myosin IIB antibody, 0.1 μg/ml myosin IIIA antibody, 0.1 μg/ml myosin IIIB antibody, 1 μg/ml myosin VI antibody, 0.2 μg/ml myosin VIIA antibody, and 0.9 μg/ml myosin IXB antibody. Detection of primary antibody was carried out by using horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG diluted 1:3,000 (BioRad, Hercules, CA) and ECL reagent (GE Healthcare Bio-Sciences, Piscataway, NJ) according to the manufacturer's recommendations. Myosin IIIB sera depleted with myosin IIIB or fascin 2B fusion proteins were diluted to the same concentration as blots incubated with myosin IIIB antibody.
Immunohistochemistry
Eyecups of albino trout were dissected (Allen and Hallows, 1997) at the Kamas Fish Hatchery, fixed in −20°C 100% methanol or 80% methanol/3.2% paraformaldehyde, and shipped on dry ice overnight. Eyecups from light-adapted zebrafish and retina/RPE tissue from light-adapted striped bass were dissected and fixed in −20°C Cytoskelfix (Sigma-Aldrich) overnight at −20°C. Fixed tissue was rinsed twice for 5 minutes in 4°C PBS, and then cryoprotected by equilibration in 15% sucrose in PBS for more than 1 hour at 4°C followed by 1-hour to overnight incubation in 30% sucrose in PBS at 4°C. The albino trout sclera was removed from the retina/choroid/RPE, and small slices of the retina/choroid/RPE were oriented in OCT compound and frozen in liquid nitrogen. Zebrafish eyecups in OCT were frozen in liquid nitrogen. The striped bass retina/RPE was removed from the vitreous and small pieces were frozen in OCT in liquid nitrogen. The retinal tissue was cut into 8–10-μm sections on a Leica cryostat, and sections were placed on poly-l-lysine-coated slides for immunostaining.
For cone inner segment/cone outer segment (CIS/COS) preparations, green sunfish were dark-adapted for 30 minutes to 1 hour. Retinal shake-off cells were prepared according to Pagh-Roehl and Burnside (1995), except that cells were dissociated into modified Earle's Ringer's solution (Burnside et al., 1993). After attaching retinal shake-off cells onto poly-l-lysine-coated coverslips for 5 minutes, CIS/COS preps were fixed either by the addition of an equal volume of 8% paraformaldehyde in modified Earle's Ringer's solution or by submerging the coverslips in −20°C methanol for 5 minutes. After fixation, coverslips were rinsed in PBS. Formaldehyde-fixed coverslips were treated three times with fresh 0.5 mg/ml sodium borohydride in PBS for 5 minutes, rinsed three times in PBS, permeabilized with 0.1% Triton X-100 in PBS for 4 minutes, and rinsed again three times in PBS. Coverslips were blocked overnight at 4°C in PBS containing 20% normal goat serum, 4% BSA, and 0.05 M glycine.
Immunofluorescence on albino trout, zebrafish, and striped bass cryosections and CIS/COS preparations was carried out according to Hoang et al. (1999), except that Cy3 goat anti-rabbit secondary antibodies (Jackson ImmunoResearch) were used for visualization. For immunostaining, primary antibodies were diluted in 2% normal goat serum, 2% BSA, and 0.05 M glycine in PBS and used at the following range of concentrations for the different preparations: 13 μg/ml actin antibody, 0.26–0.74 μg/ml myosin IIA antibody, 0.2–5.2 μg/ml myosin IIB antibody, 0.1–0.26 μg/ml myosin IIIA antibody, 0.1–0.5 μg/ml myosin IIIB antibody, 0.45–1.58 μg/ml myosin VI antibody, 0.1–2.0 μg/ml myosin VIIA antibody, and 0.61–7.32 μg/ml myosin IXB antibody. Alexa-488 phalloidin (Invitrogen) was used at a dilution of 1:40. Myosin IIIB sera depleted with myosin IIIB or fascin 2B fusion proteins were diluted identically to the myosin IIIB antibody.
Images of albino trout immunofluorescence were taken on a Zeiss Axiovert (Zeiss, Thornwood, NY) with a Hamamatsu Orca II CDC camera with OpenLab Image acquisition software (Improvision, Lexington, MA) or on the confocal microscope at the University of California at Berkeley Molecular Imaging Facility. Images of cone photoreceptor inner segment/outer segment preparations were created by merging images from z-section series generated by deconvolution by using OpenLab software or by confocal microscopy. Images of all zebrafish retinal sections were acquired on the confocal microscope at the University of California at Berkeley Molecular Imaging Facility. Captured images were processed for figures by using Openlab and Adobe Photoshop CS3 to adjust brightness and contrast for greater clarity.
Results
Multiple classes of myosins expressed in fish retina and RPE
To identify myosins expressed in fish retina and RPE, we used degenerate PCR primers to amplify myosin fragments from striped bass retinal and RPE cDNA. The 64 RPE and 170 retinal PCR clones obtained were then classified according to their homology to other vertebrate myosins (Table 1). From RPE cDNA, 11 distinct myosin family members from seven myosin classes were amplified. Myosins IIIA, IXB, and XA comprised nearly half (46.8%) of the myosin clones obtained from the fish RPE screen, each represented by ∼15% of the clones obtained. Retinal cDNA also contained all 11 myosins amplified from RPE cDNA. The retinal screen also identified seven additional myosins (from classes I, II, VI, and XIX) that were not detected in RPE. The most abundant myosin clones amplified from retina were myosin VIIA (22.4% of clones).
Table 1.
Myosin Family Members Identified in Teleost Retinal Pigmented Epithelium (RPE) and Retina
| Percent of clones2 | ||||||
|---|---|---|---|---|---|---|
| Fish sequence | Myosin class1 | Identity (%) | Similarity (%) | RPE | Retina | Predominant mRNA (kb) |
| Class 1 | 15.6 | 27.6 | ||||
| FM1b | Myosin IB | 94.1 | 97.1 | 7.8 | 5.9 | 6.3 |
| FM1c | Myosin IC | 91.4 | 97.1 | 7.8 | 18.2 | 6.3 |
| FM1d | Myosin ID | 97.2 | 97.2 | — | 1.2 | |
| FM1e | Myosin IE | 97.0 | 100 | — | 1.8 | |
| FM1h | Myosin IH | 73.5 | 85.2 | — | 0.6 | |
| Class 2 | 4.7 | 3.5 | ||||
| FM2 | Myosin IIA | 94.6 | 100 | 4.7 | 2.9 | 7.8 |
| FM11 | MHC 8 (perinatal) | 76.9 | 89.7 | — | 0.6 | |
| Class 3 | 21.9 | 20.6 | ||||
| FM16A | Myosin IIIA | 97.0 | 100 | 15.6 | 8.8 | 6.6 |
| FM16B | Myosin IIIB | 97.0 | 97.0 | 6.3 | 11.8 | 4.0 |
| Class 5 | 10.9 | 2.4 | ||||
| FM5A | Myosin VA | 100 | 100 | 10.9 | 2.4 | 10.4 |
| Class 6 | — | 6.5 | ||||
| FM6A | Myosin VI | 93.5 | 97.0 | — | 1.8 | 7.5 |
| FM6B | Myosin VI | 100 | 100 | — | 4.7 | 5.5 |
| Class 7 | 6.3 | 22.4 | ||||
| FM7A | Myosin VIIA | 100 | 100 | 6.3 | 22.4 | 9.9 |
| Class 9 | 20.3 | 10.6 | ||||
| FM9A | Myosin IXA | 97.0 | 100 | 4.7 | 4.7 | 10.2 |
| FM9B | Myosin IXB | 97.0 | 97.0 | 15.6 | 5.9 | 9.0 |
| Class 10 | 20.3 | 5.3 | ||||
| FM10A | Myosin X | 65 | 82.5 | 15.6 | 1.2 | 8.8 |
| FM10B | Myosin X | 75 | 95.0 | 4.7 | 4.1 | |
| Class 19 | — | — | ||||
| FM41 | Myosin XIX | 62.5 | 80 | — | 1.2 | |
| Total no. of clones | 64 | 170 | ||||
Myosin class for each FM PCR sequence was determined by using the translated amino acid sequence to BLAST the protein database.
Boldface type indicates the total percentage of clones in each myosin class in RPE versus retina.
Two distinct clones amplified from fish retina and RPE exhibited the greatest homology with class X myosin. Only a single myosin X member has been reported in other vertebrate species. Sequence database searches for matches to the two striped bass myosin sequences identified two genes for myosin X in zebrafish (Danio rerio) and in pufferfish (Tetraodon nigroviridis). We have arbitrarily designated the two striped bass clones as myosins XA and XB, because it is unclear from sequence comparisons which of the two fish myosin X genes is the orthologue of the single myosin X gene found in other species. When all the fish myosin X sequences are compared, the sequences of the different fish XB myosins are more conserved (i.e., similar to each other) than are the sequences of the XA myosins (data not shown). Two different genes for myosin VI have also been reported in fish (Breckler et al., 2000), whereas other vertebrates have only one myosin VI gene (Seiler et al., 2004). The two sets of fish genes for myosins VI and X likely result from a genome duplication hypothesized to have occurred during fish evolution (Meyer and Van de Peer, 2005). In contrast two distinct genes for myosin III have been found in all vertebrates examined.
The tissue expression and relative transcript abundance of different myosins in retina and RPE were examined by Northern blot analysis of striped bass tissues by using PCR-generated sequences for striped bass myosins (Fig. 1). In total RNA from striped bass RPE, transcripts for eight distinct myosins were detected (myosin IIA, IIIA, VIA, VIB, VIIA, IXA, IXB, and XB), with myosins VIIA and IXB present in greatest abundance. In total RNA from striped bass retina, transcripts for nine myosins were detected (myosins IC, IIA, IIIA, VA, VIA, VIB, VIIA, IXA, and IXB), with myosins IC, IIIA, and VIB expressed in greatest abundance. Although myosins IB, ID, XB, and XIX were amplified from retina by PCR, no transcripts were detected for these myosins by Northern blot, suggesting that their transcripts were relatively less abundant than those of the other myosins. Myosin IIIB transcripts were not detected in retinal Northern blots in spite of the fact that PCR clones of myosin IIIB were quite abundant in the retinal PCR screen. In a previous study using more sensitive Northern blots of mRNA rather than total RNA, a 4-kb myosin IIIB transcript was detected in striped bass retina (Dosé and Burnside, 2002). The sizes of the different myosin transcripts from the Northern blot observations are shown in Table 1. We have previously reported Northern blot results for bass myosins VIA and VIB (Breckler et al., 2000) but have included them again here to permit comparison with the other myosins.
Figure 1.
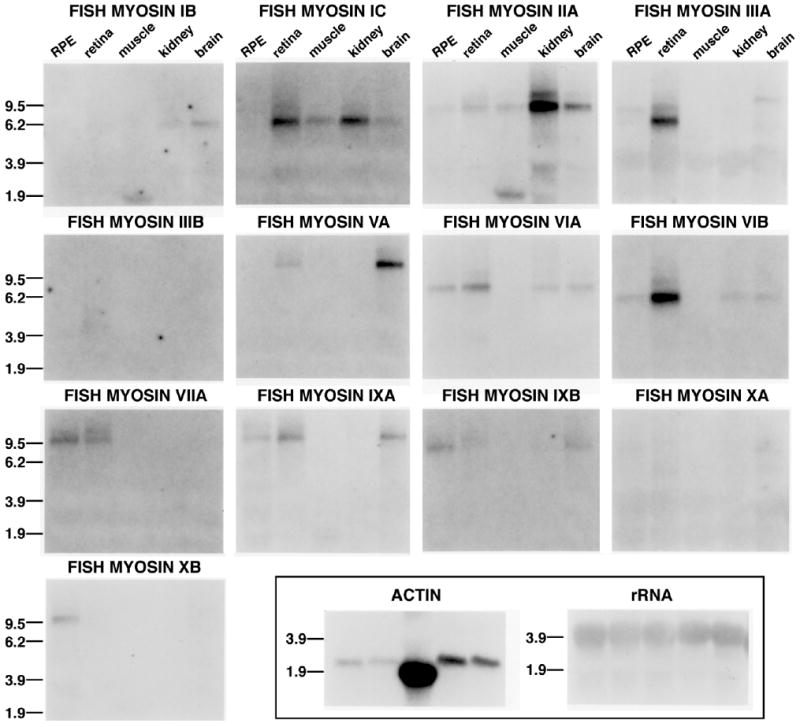
Tissue expression of different myosins in striped bass tissue. A Northern blot containing 15 μg of total RNA extracted from RPE, retina, skeletal muscle, kidney, and brain was sequentially probed with radiolabeled myosin sequences derived from a degenerate PCR screen. Expression of myosins IC, IIIA, and VIB were enhanced in striped bass retina, whereas myosins IIA, VA, VIA, VIIA, IXA, and IXB had lower levels of transcripts in retina. Striped bass RPE expressed myosin IIA, IIIA, VIA, VIB, VIIA, IXA, IXB, and XB transcripts, with myosin VIIA and IXB containing the greatest levels. Results with an actin probe were used for comparison with myosins in the different tissues and with an rRNA probe to check for equal sample loading.
To examine myosin protein expression, we tested an array of antibodies specific for various myosin class members (myosins IIA, IIB, IIIA, IIIB, VI, VIIA, and IXB) by Western blot analysis of fish retinal extracts (Fig. 2). Myosins IIA, IIB, IIIA, VI, VIIA, and IXB were examined in zebrafish retinal extracts (Fig. 2A), but myosin IIIB was analyzed in striped bass (Fig. 2B), because the region of the striped bass myosin IIIB tail used to make this antibody is not highly conserved in zebrafish.
Figure 2.
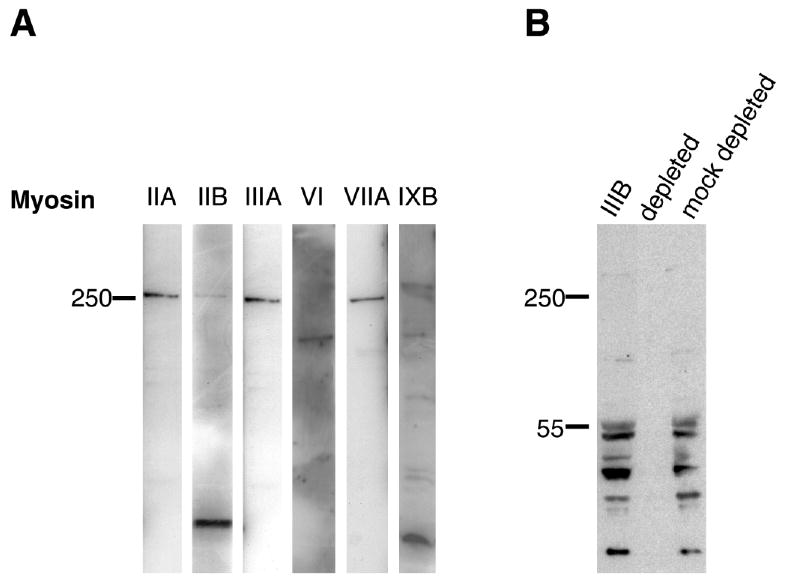
Western blot survey of myosins expressed in zebrafish retinal and striped bass retinal/retinal pigmented epithelial extracts. A: Antibodies specific for different classes of myosins were used to examine adult zebrafish retinal extracts. Myosin IIA, IIB, IIIA, VIIA antibodies reacted with single bands running with similar mobility to the 250-kDa protein standard. A single band at ∼140 kDa is observed with the myosin VI antibody. Myosin IXB antibody reacts with a high molecular weight band that is greater than 250 kDa as well as smaller molecular weight bands less than 150 kDa in size. B: Myosin III antibodies bind to multiple bands in striped bass retina/RPE extracts. No bands were observed using myosin IIIB sera depleted with a fusion protein to the myosin IIIB tail antigen (depleted). Myosin IIIB sera depleted with an unrelated fusion protein (mock depleted) as a control bound to a similar pattern of bands as untreated myosin IIIB sera.
A single high molecular weight band running with similar mobility to the 250-kDa protein standard was observed in retinal extracts with antibodies to myosins IIA, IIIA, and VIIA. A high molecular weight band of this size was also seen with myosin IIB antibody; the darkly stained low molecular weight band observed with the myosin IIB antibody most likely represents a proteolytic fragment, because the intensity of this band varied in different fish retinal extracts. For zebrafish, the predicted molecular weights for these myosins from cloned and database sequences are 205 kDa for myosin IIA, 230 kDa for myosin IIB, 202 kDa for myosin IIIA, and 251 kDa for myosin VIIA. Thus, the mobilities for some myosins in our Western blots were slightly slower than would be predicted for their size; however, determination of precise molecular weights is limited by the SDS-PAGE resolution of such large proteins.
Two myosin VI genes are found in fish; for zebrafish the predicted molecular weights are 149 kDa (VIA) and 147 kDa (VIB). However, only a single band of ∼149 kDa is resolved in zebrafish retinal extracts.
Myosin IXB antibody bound to a high molecular weight band that ran with slower mobility than the 250-kDa protein standard, and also bound to smaller bands with molecular weights less than 150 kDa. These latter bands are likely the result of protein degradation, because their intensity varied in different retinal extracts. There are two different zebrafish myosin IXB genes in the sequence database: one is predicted to be 221 kDa, whereas the size of the second is uncertain because there appear to be sequence anomalies. Analysis of our myosin IXB Western blot was further complicated by the possibility that the antibody may cross-react with zebrafish myosin IXA or recognize splice variants, because human myosin IXB gene is known to be subject to alternative splicing. The size of the high molecular weight band in the myosin IXB blot is larger than would be predicted; however, many of the other classes of myosins with predicted molecular weights in the 200–230-kDa range exhibited slower than expected mobility, running close to or above the 250-kDa standard.
In striped bass retina/RPE extracts (Fig. 2B), only bands smaller than the predicted ∼140-kDa molecular weight for striped bass myosin IIIB bind specifically to the myosin IIIB antibody. An almost identical banding pattern has also been observed with green sunfish retinal extracts, suggesting that the myosin IIIB antibodies cross-react with green sunfish tissue (data not shown). When depleted with a MBP fusion to the myosin 3B tail region used to make the antibody, the antibody fails to bind any detectable bands, but when control sera depleted with an unrelated fusion protein (MBP:fascin 2B) is used, a ladder of low molecular weight bands is observed that is similar to the results with the myosin IIIB antibody, suggesting that antibody binding to myosin IIIB is specific. The specificity of the myosin IIIB antibody is also supported by results from Western blots of purified myosin IIIB fusion proteins expressed in bacteria and of HeLa cell extracts expressing myosin IIIB fusions to green fluorescent protein; the myosin IIIB antibody incubated with and without competing myosin IIIB fusion proteins display specific and robust binding to bands of the predicted sizes (data not shown). The presence of multiple lower molecular weight bands in retinal extracts suggests that proteolysis of myosin IIIB has occurred and that myosin IIIB is very labile, as we have found previously in our experiments with myosin IIIA in fish retinal extracts.
Myosin localization patterns in fish retina
To identify which myosins are expressed within specific retinal cells and RPE, we used antibodies specific to the different myosin classes for fluorescent immunohistochemistry of fish eyes (Fig. 3). In describing localization patterns in retina, we use “distal” to refer to the part of the retina facing the RPE and “proximal” to refer to the part of the retina facing the vitreous. Expression for myosins IIA, IIB, IIIA, VI, VIIA, and IXB was examined in zebrafish retina, but because the myosin IIIB likely does not cross-react in zebrafish, myosin IIIB immunostaining was performed on striped bass retina.
Figure 3.
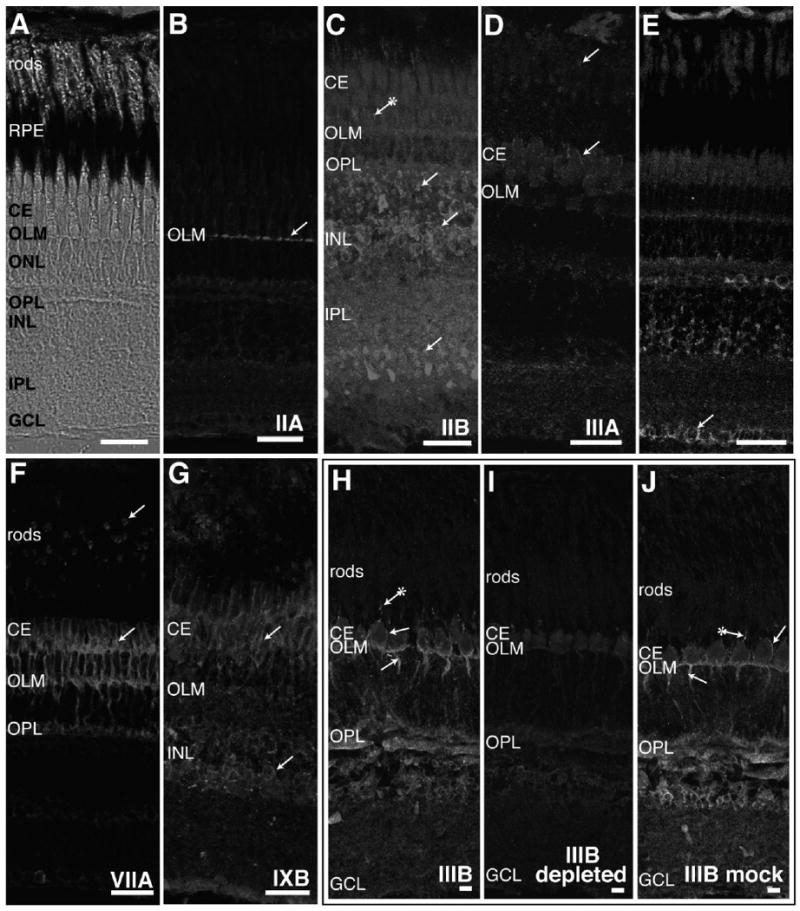
Immunohistochemistry of adult fish retina with antibodies to different classes of myosins. A: The laminar arrangement of the adult zebrafish retina is seen with Nomarski optics. Light-adapted zebrafish retinal cryosections were stained with antibodies specific to myosins IIA (B), IIB (C), IIIA (D), VI (E), VIIA (F), and IXB (G). Light-adapted striped bass retinal sections were used for myosin IIIB immunostaining (H–J). B: Myosin IIA antibody stains only the outer limiting membrane (OLM) formed by termini of Müller cell processes. C: Myosin IIB antibodies brightly label cell bodies in the stratum containing predominantly bipolar cells in the inner nuclear layer (INL) and termini of cell processes found in the inner plexiform layer (IPL) and at the outer plexiform layer (OPL). Fluorescence can sometimes be seen in cone accessory segments with myosin IIB antibodies (indicated by an arrow with an attached asterisk). D: Rod inner segments and the distal region of cone ellipsoids are labeled with myosin IIIA antibodies. E: Staining with myosin VI antibodies is found in horizontal cells in the INL and ganglion cells (GCL). Some myosin VI immunostaining is also exhibited in the OLM and OPL. F: Myosin VIIA antibody fluorescence is found throughout cone inner segments and in rod inner segments. In cones, myosin VIIA staining is also located in accessory outer segments, axons, and synapses. Weaker labeling of myosin VIIA occurs in horizontal, amacrine, and ganglion cells. G: Myosin IXB is expressed in cone ellipsoids and accessory outer segments and in the amacrine cell region of the INL. H: Myosin IIIB antibody stains cone accessory outer segments, inner segments, and axons. Additional myosin IIIB fluorescence is localized to the OLM, OPL, and INL. I: Greatly reduced staining is observed when myosin IIIB antibody was preincubated with a fusion protein containing the myosin IIIB tail prior to incubation with striped bass retinal sections. J: Preincubation of myosin IIIB antibodies with a control fascin 2B fusion protein does not change the staining pattern in striped bass retina. RPE, retinal pigmented epithelium; CE, cone ellipsoid. Scale bar = 20 μm in A–J.
Each of the different myosin classes displayed a unique expression pattern in the different subsets of retinal cell types. Antibodies to myosin IIA localized exclusively to the OLM, where distal termini of Müller cells form adherens junctions with photoreceptors (Fig. 3B, arrow). Antibodies to myosin IIB produced much more widespread staining of the retina (Fig. 3C). Although there was some staining in the OLM, myosin IIB antibody localized more strongly to the inner nuclear layer (INL) in the stratum containing bipolar cells, down to the bipolar axon terminals in the inner plexiform layer (IPL) and to bipolar dendritic synapses in the outer plexiform layer (OPL; Fig. 3C, arrows). Some staining was also observed in the cone accessory outer segments in the photoreceptor layer (Fig. 3C, arrow with attached asterisk).
Myosin IIIA labeling was highly specific to the extreme distal region of the cone and rod ellipsoids and in the calycal processes (Fig. 3D, arrows). Calycal processes are microvillus-like projections that extend from the distal ellipsoid to cup the base of the outer segment.
Myosin VI staining localized most strongly in the OLM, the horizontal cells underlying the OPL, ganglion cells (GCL), and probably Müller cells of the INL (Fig. 3E, arrows). Myosin VIIA staining was localized strongly to the distal ellipsoids of rods and cones, cone myoids and axons, the OLM, and the photoreceptor synapses of the OPL (Fig. 3F, arrows). In retinal sections stained with myosin IXB, fluorescence was localized to cone ellipsoids and accessory outer segments and the amacrine cell area of the INL (Fig. 3G, arrows). Minimal background from autofluorescence or fluorescent secondary antibody was observed in control sections of zebrafish retina incubated in the absence of primary antibody (data not shown).
Striped bass retina stained with myosin IIIB antibodies exhibited fluorescence in cone accessory outer segments (Fig. 3H, arrows with asterisk), ellipsoids (arrow), myoid, and axons (arrow). Myosin IIIB immunostaining was also present in the OLM, OPL, and INL. The specificity of the myosin IIIB immunostaining in fish retina was tested by using myosin IIIB sera preabsorbed to a myosin IIIB tail fusion protein; staining with myosin IIIB-depleted sera eliminated or greatly reduced signal in the cones and the other retinal layers (Fig. 3I, IIIB depleted). In contrast, if control sera were depleted with an unrelated fusion protein (zebrafish fascin 2B) prior to incubation with striped bass retinal sections, the pattern was identical to staining with myosin IIIB antibody (Fig. 3J, IIIB mock-depleted).
Localization of myosins in the retinal pigmented epithelium and retina of albino trout
Immunocytochemistry of the RPE in fish retinal sections is difficult, because the fluorescence is obscured by RPE pigment granules. Therefore, retinas from albino (i.e., nonpigmented) trout were used to determine which myosins were expressed in the RPE and for comparative purposes to assess the consistency of expression patterns in a second fish species. Like zebrafish, trout is classified in a different phylogenetic order from striped bass and green sunfish. Therefore the myosin IIIB antibody was not used to analyze albino trout, because the region used to make this antibody is not well conserved in zebrafish.
In albino trout retina, myosin IIA labeling was prominent in photoreceptors, rather than the OLM, as observed in zebrafish. The cone inner segment (both ellipsoid and myoid) and synapse in the OPL were all brightly stained (Fig. 4A). The cone accessory outer segment was also strongly labeled.
Figure 4.
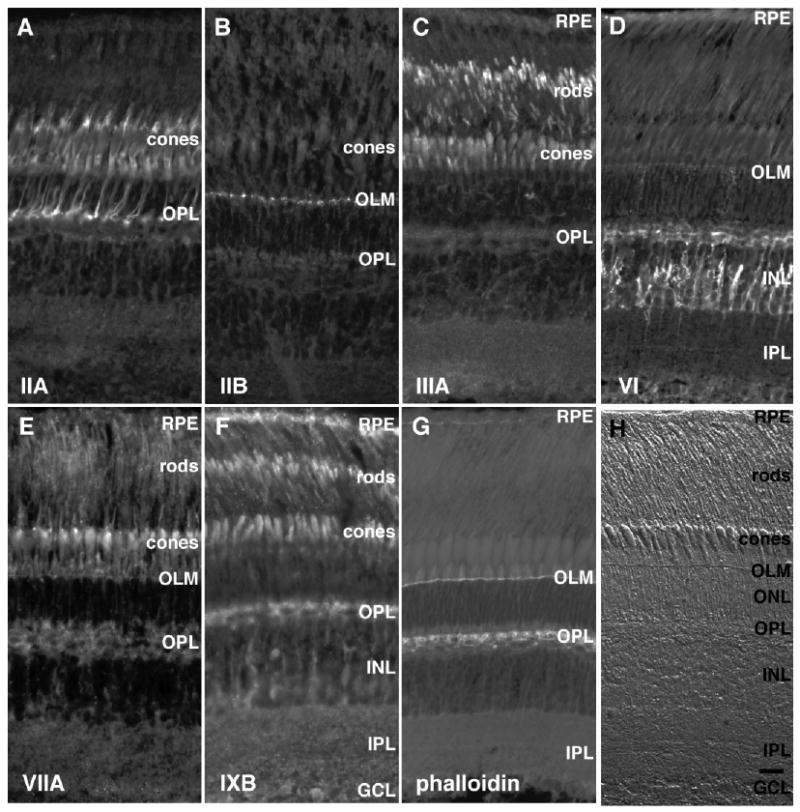
Immunohistochemistry of light-adapted adult albino trout retina with antibodies to different classes of myosins. Methanol-fixed adult albino trout retinas were stained with antibodies to different members of the myosin superfamily. A: Myosin IIA stains cone accessory segments, inner segments, axons, and synapses. Faint myosin IIA signal is detected in the horizontal cell layer beneath the outer plexiform layer (OPL). B: Myosin IIB immunostaining is restricted to the outer limiting membrane (OLM). C: Myosin IIIA labels rod and cone inner segments; in cones, myosin IIIA is confined to the distal end of the ellipsoid and in the calycal processes located near the proximal outer segment. Some faint myosin IIIA staining is observed in the retinal pigmented epithelium (RPE). D: Myosin VI antibody stains horizontal cells and cell bodies and processes in the inner nuclear layer (INL). Lower levels of myosin VI labeling are seen in the RPE. E: Myosin VIIA antibody stains rod inner segments, cone accessory segments, and ellipsoid and basal bodies lying proximal to the inner/outer segment junction. F: Myosin IXB immunostains the RPE, rod inner segments, cone ellipsoids, horizontal cells, and ganglion cells. There is also staining of cell bodies and processes of an undetermined cell type in the INL. G: Phalloidin labeling of formaldehyde-fixed retina reveals fluorescence of actin filaments found in the RPE, the OLM, and synapses of the OPL. I: Nomarski image of a section from a light-adapted adult albino trout retina showing the various retinal layers. GCL, ganglion cell layer; IPL, inner plexiform layer. Scale bar = 10 μm in H (applies to A–H).
In trout, myosin IIB was highly localized to the outer limiting membrane (Fig. 4B), similar to the pattern of myosin IIA staining in zebrafish retina. Myosin IIB staining was below the level of detection in all other retinal areas.
As seen in zebrafish, trout retinas labeled with myosin IIIA antibody displayed intense staining in the photoreceptor inner segments; staining was particularly concentrated in the distal ellipsoid region containing the calycal processes (Fig. 4C). Fainter myosin IIIA staining was seen in the RPE.
In trout, myosin VI antibodies produced the brightest signal in INL cell bodies and cellular processes extending bidirectionally into the IPL and distally into the horizontal cell layer lying beneath the OPL (Fig. 4D). Although more intense in the INL, this pattern of myosin VI localization resembles that observed in zebrafish. Both RPE and horizontal cells exhibited significant levels of myosin VI fluorescence. With higher antibody dilutions or longer exposures, myosin VI was also observed in the OLM, cone ellipsoids, and cone accessory segments.
In trout as in zebrafish, myosin VIIA labeling was concentrated in distinct bright spots in the region of the photoreceptor inner/outer segment junctions where the outer segment basal bodies are located (Fig. 4E). Fluorescence was also seen in cone ellipsoids and accessory outer segments. Fainter myosin VIIA staining was visible in the rod inner segments and RPE.
Myosin IXB antibodies labeled the RPE, photoreceptor inner segments, horizontal cells, and ganglion cells (Fig. 4F). INL cell bodies and processes and the IPL stained less brightly with the myosin IXB antibody.
In control albino trout sections incubated with rabbit immunoglobulins substituted for primary antibody, followed by fluorescent secondary antibody incubation, little fluorescence was observed (data not shown). There was also no background fluorescence from the secondary antibody on sections incubated with only secondary antibody (data not shown).
To display the actin cytoskeleton, paraformaldehyde-fixed albino trout sections were incubated with phalloidin to stain F-actin filaments. Actin filament staining was most prominent in the OLM and synapses of the OPL (Fig. 4G). Actin filament staining was also observed more faintly in the RPE and IPL.
The immunostained albino trout retina sections shown in Figure 4 were fixed in methanol. To determine the effect of fixation on retention of antigenicity and hence antibody staining patterns, we also performed immunolocalizations with an alternative fixation procedure comprised of a paraformaldehyde/methanol mixture (data not shown). Staining patterns similar to those in methanol-fixed retinas were observed with each of the myosin antibodies except for the following discrepancies: in paraformaldehyde/methanol-fixed albino trout retina, myosins IIA and VI exhibited enhanced staining in the ONL compared with the methanol-fixed retinas, and myosins VIIA and IIB antibodies did not yield any detectable staining in retinas fixed with the paraformaldehyde/methanol mixture.
In fish, dramatic changes in the retina occur with changing light conditions, such as changes in cone morphology and RPE pigment localization. Our albino trout data in Figure 4 were obtained from light-adapted fish. In order to determine whether myosin expression patterns were affected by the light-dark cycle, immunostaining of light- and dark-adapted striped bass retinas fixed with either methanol or paraformaldehyde were compared (data not shown). Only myosin IIA exhibited a significant change in signal intensity in dark-adapted retina, cone myoid labeling decreased, and OPL labeling increased compared with light-adapted retina.
Subcellular localization of myosins in isolated photoreceptor inner and outer segments
Isolated photoreceptor inner segment/photoreceptor outer segment (PIS/POS) fragments from green sunfish retina were used to visualize subcellular myosin immunolocalization in the inner segment ellipsoids and attached outer segments in greater detail (Fig. 5). In control preparations incubated without primary antibody but with secondary fluorescent antibodies, only faint diffuse background ellipsoidal labeling was present from autofluorescence or nonspecific fluorescent secondary antibody binding (Fig. 5A2). However, in control preparations incubated with purified rabbit IgG substituted for the primary antibody in the normal protocol, bright and distinct labeling of cone accessory outer segments (small arrows) was observed well above the background levels seen in the cone ellipsoid (Fig. 5A4,A5). Thus the bright accessory outer segment labeling seen in the myosin localization studies is an artifact produced by nonspecific binding of IgG to this structure.
Figure 5.
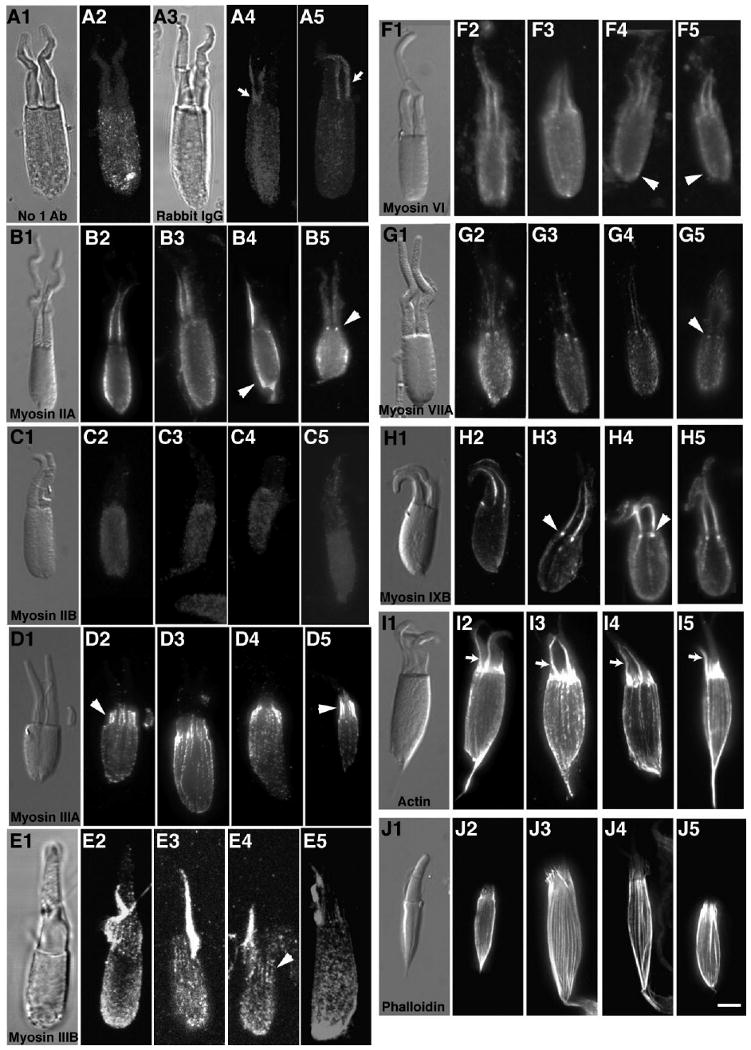
Cone inner/outer segment preparations stained with antibodies to different classes of myosins. Preparations consisting of cone inner segment ellipsoids and attached outer segments from green sunfish were immunostained for various myosin family members and actin. A2: Faint background staining in the cone ellipsoid with the fluorescently tagged secondary antibody was observed in controls incubated without primary antibody. A4,5: Substitution of rabbit IgG for primary antibodies gave some nonspecific staining in the cone ellipsoid but resulted in brighter fluorescence located in the accessory outer segment, which was also observed in preps treated with other antibodies (indicated by small arrows). B2–5: Myosin IIA antibodies stained the ellipsoid surface membranes and basal bodies (arrowheads). C2–5: Myosin IIB antibody staining was the same as controls (i.e., low-level stain). D2–5: Myosin IIIA localized in a distinct pattern to the distal region of inner segment actin filaments, extending into the calycal processes surrounding the proximal outer segment (arrowheads). E2–5: Like myosin IIIA, some actin filament bundle staining in the calycal processes is exhibited near the proximal outer segment with myosin IIIB antibody (arrowhead), but there is additional staining of the ellipsoid. F2–5: Myosin VI antibodies stain the cone ellipsoids (arrowheads). G2–5: Myosin VIIA immunostains the two basal bodies found in double cones (arrowheads). H2–5: Myosin IXB antibodies also stain the basal bodies at the base of the outer segment (arrowheads). A monoclonal actin antibody (I2–5) and phalloidin (J2–5) brightly stain the actin filament bundles found running the length of the ellipsoid and terminating near the proximal end of the outer segments in the calycal processes. Scale bar = 5 μm in J5 (applies to A1–J5).
Myosin IIA antibodies labeled the cone ellipsoid (arrowhead) and the basal bodies associated with the outer segment axoneme (Fig. 5B2–5); in twin cones the basal bodies were sometimes seen as two bright dots at the base of the accessory outer segments (arrowhead). No myosin-specific staining was detected with myosin IIB antibodies (Fig. 5C2–5); the staining pattern was similar to that of the negative control using rabbit IgGs.
Consistent with a previous report (Dosé et al., 2003), myosin IIIA labeling was concentrated in the distal portions of actin filament bundles that form the core bundles of the calycal processes (Fig. 5D2–5, arrowheads). The accessory outer segment is not labeled by the myosin IIIA antibody, probably because the extreme dilution we used with this very high affinity antibody did not produce enough nonspecific IgG binding.
As with myosin IIIA, myosin IIIB antibodies stained the cone inner segment bundles, although the filament bundle signal was not as clear in the IIIB preparations because it was somewhat obscured by general myosin IIIB fluorescence in the ellipsoid (Fig. 5E2–5). Myosin IIIB clearly localizes to calycal process actin filament bundles distal to the inner segment (Fig. 5E2–5, arrowhead). There is intense nonspecific staining of the cone accessory outer segment with myosin IIIB, consistent with the higher concentration of primary antibody used.
Myosin VI antibodies labeled the ellipsoid of isolated cone preparations (Fig. 5F2–5, arrowheads). Consistent with previous reports, antibodies to myosin VIIA strongly label basal bodies in the isolated cones (Fig. 5G2–5, arrowhead). Myosin IXB was also localized to basal bodies in the isolated cones (Fig. 5H2–5, arrowheads).
To compare the localization of myosins with the distribution of actin filaments in the cone fragments, isolated preparations were stained with monoclonal actin antibodies (Fig. 5I2–5) and phalloidin (Fig. 5J2–5). Both phalloidin and the actin antibody clearly labeled the inner segment actin filament bundles that are the core of the calycal processes and extend proximally through the ellipsoid toward the myoid. The actin antibody brightly labeled the accessory outer segment (arrows), as observed with myosin antibodies. The accessory outer segment was not labeled with phalloidin. We attribute this difference in accessory outer segment staining with the actin antibody and not phalloidin to nonspecific IgG binding to this structure, which has also been observed with many of the myosin antibodies and control rabbit IgG's.
Although the much smaller size of fish rods does not allow such precise localization in rod fragments, we found the immunofluorescence patterns of the studied myosins in rods to be essentially similar to those observed in cones. Myosins IIA, IIIA, IIIB, and IXB were localized to rod ellipsoids, and myosin VIIA antibodies labeled the rod basal body near the inner/outer segment junction (data not shown).
Discussion
We have identified members of the myosin superfamily expressed in fish retina and RPE. In our immunohistochemical analysis of seven myosins we observe that each myosin is expressed in unique patterns within different subsets of retinal cell types. We also find that multiple myosins are expressed in a specific retinal cell type and that subcellular localizations may overlap, consistent with the possibility of functional redundancy.
Myosins expressed in photoreceptors
All the myosin classes examined in this study by immunohistochemistry were detected in photoreceptors except myosin IIB. Myosins IIA, IIIA, IIIB, VI, VIIA, and IXB were localized in actin-rich ellipsoids of fish cones. The myosin IIA and VI immunostaining of the peripheral cytoplasm in the cone ellipsoids reported here is similar to that observed in striped bass (Breckler et al., 2000) and myosin VI in inner segments of mouse photoreceptors (Kitamoto et al., 2005). Myosins IIIA and IIIB localized to calycal processes and distal ends of ellipsoidal actin filament bundles; however, none of the myosins colocalized with more proximal portions of ellipsoidal actin filament bundles.
Observations to date strongly suggest that myosin VIIA found in the mammalian connecting cilium participates in protein transport between the inner and outer segments (Liu et al., 1997, 1991; Wolfrum and Schmitt, 1999). Unfortunately, bright nonspecific AOS staining obscured the connecting cilium in our inner/outer segment preparations and made it impossible to detect myosin staining in fish connecting cilia.
The distal inner segment localization of myosin IIIA in zebrafish and albino trout photoreceptors described here is consistent with that previously reported for striped bass (Dosé et al., 2003). Both myosins IIIA and IIIB localized to cone calycal processes in inner/outer segment preparations. This colocalization suggests that functional redundancy of these two class III myosins might occur in photoreceptors.
We report here the localization to cone myoids of myosin VIIA in zebrafish, myosin IIIB in striped bass, and myosin IIA in albino trout. Myosin VIIA localization in this region has not been reported in mammalian retinas. In fish, but not mammals, photoreceptors undergo specialized retinomotor movements in which rod and cone myoids elongate and contract in response to changes in light conditions. Cytochalasin studies have shown that rod myoid elongation and contraction and cone myoid contraction are actin dependent (Burnside, 1976; O'Connor and Burnside, 1981). Thus in fish, myosins found in photoreceptor myoids could play a role in retinomotor movements.
Photoreceptors synapse with biopolar and horizontal cells in the OPL. Myosin IIIB is localized to the OPL in striped bass. Myosin VIIA is also localized in the OPL in zebrafish and albino trout and may play a specialized role in fish synapses, because mammalian myosin VIIA has not been reported in this region. In albino trout, myosin IIA expression is found throughout the cone inner segment and axon, extending into the synapse, similar to the expression of myosin VIIA in zebrafish. Myosin IIB is found in the zebrafish OPL but is most likely localized to postsynaptic bipolar dendrites, because myosin IIB appears to be specific for bipolar cells.
Myosin V was detected in fish retina and RPE by PCR, but its protein localization was not examined. However, myosin V localization to rat rod synapses (Schlamp and Williams, 1996) and abnormal ribbon synapses and electroretinographs in dilute lethal mice with myosin VA mutations (Libby et al., 2004) suggest a role in synapse function.
Myosins expressed in glial Müller cells
Glial Müller cell bodies are located in the INL, whereas their cellular projections extend radially across the retina and form the OLM and ILM. Myosin VI was found in the OLM of zebrafish and albino trout. Myosin IIA was detected in the OLM in zebrafish. Myosin IIA was also localized to the OLM in striped bass, where it was found in the cone inner segment and axon as well (Breckler et al., 2000), similar to the myosin IIB staining reported here for albino trout. In contrast, myosin IIB was detected in the OLM in trout, but myosin IIA was not. Results from transgenic knockout mice in which myosin IIA was substituted for myosin IIB suggest that myosin IIA has only a limited ability to compensate for the absence of myosin IIB in mice (Bao et al., 2007). However, the similarities in myosin IIA and IIB OLM immunostaining in different fish species suggest that in fish redundancy might occur. Myosin IIIB was also localized to the OLM in striped bass, suggesting that several myosins may be active in this actin-rich domain. Myosin IXB is localized in Müller cell bodies and processes in zebrafish and albino trout, suggesting that it also participates in cytoskeletal or motile activities in these long cells.
Myosins expressed in RPE cells
RPE cells possess actin-rich apical projections that interdigitate with photoreceptor outer segments. The RPE carries out critical functions such as phagocytosis of shed photoreceptor outer segments and pigment granule movement that are actin dependent (Besharse and Dunis, 1982; King-Smith et al., 1997). Four of the 11 myosins identified by PCR in striped bass RPE immunolocalized to RPE in albino trout. Immunolocalization results suggest that the RPE has abundant levels of myosins IIIA, VI, and IXB and lower levels of myosin VIIA. Myosin VI was detected in albino trout RPE by immunostaining but not by PCR of striped bass RPE cDNA. Whereas myosins IIA and IIIB were amplified from striped bass RPE cDNA, transcripts of these myosins were not detected in RPE by Northern blot, and myosin IIA antibodies did not label albino trout RPE, suggesting that myosins IIA and IIIB are expressed in RPE at very low levels.
Only for myosin VIIA has a functional role in the RPE been demonstrated. In shaker-1 mice mutant for myosin VIIA, pigment granules fail to move into RPE apical processes (Liu et al., 1998). Phagocytosis is also defective in RPE from shaker mutants (Gibbs et al., 2003), suggesting that myosin VIIA plays a dual role in RPE. Myosin VI localizes to the cell bodies of mouse RPE cells in situ and colocalizes with lysosomal markers in cultured RPE cells (Kitamoto et al., 2005), suggesting it might play a role in vesicle traffic or organelle morphology. Although myosins VI and IIIA localized to RPE in fish in this and previous studies (Breckler et al., 2000), no RPE defects have been reported in Snell's walzer mice defective for myosin VI or in humans with nonsyndromic deafness associated with defective myosin IIIA (Walsh et al., 2002). It will be of interest to examine myosin IIIA and VI mutants in other systems for subtle alterations in retinal or RPE morphology and function. Because myosin IIIB is also expressed in the RPE, it may compensate for the absence of myosin IIIA.
Although myosin IC was amplified from RPE cDNA, no transcripts were detected on Northern blots, suggesting low message levels. However, myosin IC immunolocalization to lateral membranes was observed in cultured human RPE cells (Breckler and Burnside, 1994).
Possible functions of myosins in retina and RPE
Our study is the first to examine unconventional class II myosin expression in retina, but critical roles for these myosins have been reported in other vertebrate cells. A myosin IIA gene knockout in mice causes early embryonic lethality that is likely due to problems in cytokinesis and cell adhesion (Conti et al., 2004). Human myosin IIA mutations have been associated with hearing loss (Lalwani et al., 2000; Heath et al., 2001), and myosin IIA localizes to mouse hair cell stereocilia (Mhatre et al., 2006). In our study of zebrafish retina, myosin IIA was localized exclusively in the OLM, where it may contribute to the adherens junctional complexes between Müller cells and photoreceptors. In albino trout and striped bass, myosin IIA was found in the cone inner segment, axon, and synapse.
Mice with a myosin IIB gene knockout die around birth due to cardiac failure (Tullio et al., 1997). Mice defective for myosin IIB exhibit rosettes of cells in the retina, suggesting a role for myosin IIB in adhesion. Myosin IIB is concentrated in the OLM in retinas of albino trout and striped bass; this contrasts with zebrafish, in which myosin IIA localizes to the OLM. In zebrafish, myosin IIB appears to be relatively enhanced in bipolar cells, particularly in synaptic terminals. In rat retina, myosin IIB was found in the IPL and OPL and in cell bodies of a subpopulation of INL cells (Schlamp and Williams, 1996); this localization is consistent with our findings for zebrafish. Note that whereas there is exclusive expression of myosins IIA and IIB in the OLM of zebrafish and albino trout, respectively, myosins IIB and IIA are expressed in different cell types in the retinas of zebrafish and trout. These observations suggest that the two myosin II family members may play different roles in the retinas of different species.
Mutations in Drosophila myosin III (ninaC) lead to an altered electroretinogram and photoreceptor degeneration, suggesting a role for myosin III in photoreceptor function and survival (Montell and Rubin, 1988). However, mutations in human myosin IIIA produce progressive deafness but do not affect vision (Walsh et al., 2002). Drosophila ninaC is a single gene that is alternatively spliced to produce long and short isoforms. In vertebrates, long and short class III myosins are encoded by two separate genes. Myosins IIIA and IIIB immunolocalize to fish cone inner segments, although myosin IIIB is distributed in additional subcellular compartments. The colocalization of myosins IIIA and IIIB in the distal actin filament bundles of calycal processes suggests that myosin IIIB may compensate for the absence of photoreceptor myosin IIIA in human mutants. Myosin IIIB immunostaining of photoreceptor inner segments has also been observed in mouse (B. Battelle, personal communication). An N-terminal kinase found in all class III myosins suggests a role in signaling (Dosé et al., 2004).
Heterologous expression of fish myosin IIIA in transgenic Xenopus rods using a strong rod opsin promoter disrupts calycal processes and results in rod degeneration. The production of enlarged calycal processes in these transgenics suggests a role for myosin IIIA in the formation and maintenance of calycal processes (Lin-Jones et al., 2004). However, transgenics expressing Xenopus myosin IIIA did not exhibit defects in calycal processes, suggesting that effects on calycal process morphology depend on specific properties of fish myosin IIIA (J. Lin-Jones, unpublished observations).
Myosin VI is the only myosin known to display minus end-directed motility along actin filaments (Wells et al., 1999). Roles in endocytosis, Golgi transport and structure, and cell migration have been described for myosin VI in mammalian cells (Buss et al., 2004). However, mutations of myosin VI in humans, mice, and zebrafish are characterized primarily by auditory and vestibular defects (Avraham et al., 1995; Melchionda et al., 2001; Ahmed et al., 2003; Seiler et al., 2004; Kappler et al., 2004). In hair cells, myosin VI is important for stereociliary structure, anchoring the stereocilia to the underlying cuticular plate (Self et al., 199; Kappler et al., 2004; Seiler et al., 2004). In mouse mutants of myosin VI, no morphological changes were detected in retina, but electroretinograms of the mice displayed reduced amplitudes (Kitamoto et al., 2005).
Dissection of myosin VI function is complicated by splice variants and in fish by the existence of two myosin VI genes. Myosin VIA and VIB genes documented in zebrafish and striped bass (Breckler et al., 2000; Kappler et al., 2004; Seiler et al., 2004) likely result from a genome duplication occurring before these species split into different phylogenetic orders. Zebrafish myosin VIB expression is restricted to hair cells and the retina and may have a specific function in sensory neurons (Seiler et al., 2004). Myosin VIA has broader tissue expression and may participate in more generalized cellular functions. The myosin VI antibody used in this study likely binds both myosin VIA and VIB, because the same antibody labeled two bands on Western blots with striped bass retina and RPE samples (Breckler et al., 2000). Results in striped bass suggest that both myosin VI isoforms are expressed in retina and RPE with greater levels of myosin VIB in retina.
Usher's syndrome type Ib patients with myosin VIIA mutations are characterized by deafness and retinitis pigmentosa (Weil et al., 1995). However, only auditory defects caused by abnormal hair cell stereocilia were initially described in zebrafish mariner mutants defective for myosin VIIA (Ernest et al., 2000). More recently retinal abnormalities have been found in mariner mutants (Biehlmaier et al., 2007), suggesting similar roles for fish myosin VIIA in connecting cilium transport, RPE phagocytosis of shed outer segment disks, and RPE melanosome transport, as has been documented in mammals (Williams, 2008). Our data in fish retina confirm myosin VIIA expression in RPE and photoreceptors, consistent with its postulated functional roles in vision.
The GTPase-activating protein (GAP) tail domain is the distinctive feature of class IX myosins. In mammals, myosin IXB GAP activity regulates Rho, a G-protein affecting the actin cytoskeleton (Wirth et al., 1996; Müller et al., 1997). In studies of cultured mouse melanoma cells, myosin IXB localized to areas of actin polymerization (van den Boom et al., 2007). Myosin IXB immunostaining of fish cone inner/outer segments was not associated with inner segment actin filament bundles but instead localized to the basal body and peripheral cytoplasm of the ellipsoid. Myosin IXB expression in RPE and Müller cells suggests a functional role in these cell types.
In summary, we have surveyed the myosin superfamily members expressed in fish retina and RPE, and described the subcellular localizations of seven of these myosins. Different subsets of myosins are expressed in the various retinal cell types in different species, and several myosin family members are expressed in a specific retinal cell type. Specific myosins are likely to play critical roles in morphogenesis, structural scaffolding, motility, protein and vesicle transport, and signaling in these cells. Our finding that myosins sometimes colocalize in retinal cells suggests that functional redundancies could preserve visual function in cases of myosin gene mutation. Also, the species-specific expression of myosins in some retinal cell types suggests that retinal cells of different species may use different myosins to perform the same cellular function.
Acknowledgments
The authors thank Robert Adelstein, Mark Mooseker, and Tama Hasson for their generous gifts of antibodies, as well as Ed Parker and Jennifer Fogarty for their expert technical assistance.
Grant sponsor: National Eye Institute, National Institutes of Health; Grant number: EY03575 (to B.B.); Grant sponsor: Research Infrastructure in Minority Institutions (RIMI); Grant number: 5 P20 RR11805 (to J.B. and L.S.).
Literature Cited
- Ahmed ZM, Morell RJ, Riazuddin S, Gropman A, Shaukat S, Ahmad MM, Mohiddin SA, Fananapazir L, Caruso RC, Husnain T, Khan SN, Riazuddin S, Griffith AJ, Friedman TB, Wilcox ER. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–1322. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DM, Hallows TE. Solar pruning of retinal rods in albino rainbow trout. Vis Neurosci. 1997;14:589–600. doi: 10.1017/s0952523800012244. [DOI] [PubMed] [Google Scholar]
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- Bao J, Ma X, Liu C, Adelstein RS. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem. 2007;282:22102–22111. doi: 10.1074/jbc.M702731200. [DOI] [PubMed] [Google Scholar]
- Bement WM, Hasson T, Wirth JA, Cheney RE, Mooseker MS. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc Natl Acad Sci U S A. 1994;91:6549–6553. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Rod photoreceptor disk shedding in vitro: inhibition by cytochalasins and activation by colchicine. In: Hollyfield JG, editor. The structure of the eye. New York: Elsevier Biomedical; 1982. pp. 85–96. [Google Scholar]
- Biehlmaier O, Hodel C, Neuhauss SCF. Localization and function of myosin VIIA in the retina of wild-type and mutant zebrafish. Ft. Lauderdale, FL: ARVO; 2007. [Google Scholar]
- Breckler J, Burnside B. Myosin-I in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:2489–2499. [PubMed] [Google Scholar]
- Breckler J, Au K, Cheng J, Hasson T, Burnside B. Novel myosin VI isoform is abundantly expressed in retina. Exp Eye Res. 2000;70:121–134. doi: 10.1006/exer.1999.0758. [DOI] [PubMed] [Google Scholar]
- Brown ME, Bridgman PC. Myosin function in nervous and sensory systems. J Neurobiol. 2004;58:118–130. doi: 10.1002/neu.10285. [DOI] [PubMed] [Google Scholar]
- Burnside B. Microtubules and actin filaments in teleost visual cone elongation and contraction. J Supramol Struct. 1976;5:257–275. doi: 10.1002/jss.400050302. [DOI] [PubMed] [Google Scholar]
- Burnside B, Bost-Usinger L. The RPE cytoskeleton. In: Marmor MF, Wolfensberger TJ, editors. The retinal pigment epithelium: function and disease. New York: Oxford University Press; 1998. [Google Scholar]
- Burnside B, Wang E, Pagh-Roehl K, Rey H. Retinomotor movements in isolated teleost retinal cone inner-outer segment preparations (CIS-COS): effects of light, dark and dopamine. Exp Eye Res. 1993;57:709–722. doi: 10.1006/exer.1993.1179. [DOI] [PubMed] [Google Scholar]
- Buss F, Spudich G, Kendrick-Jones J. Myosin VI: cellular functions and motor properties. Annu Rev Cell Dev Biol. 2004;20:649–676. doi: 10.1146/annurev.cellbio.20.012103.094243. [DOI] [PubMed] [Google Scholar]
- Chaitin MH, Bok D. Immunoferritin localization of actin in retinal photoreceptors. Invest Ophthalmol Vis Sci. 1986;27:1764–1767. [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- Dosé AC, Burnside B. A class III myosin expressed in the retina is a potential candidate for Bardet-Biedl syndrome. Genomics. 2002;79:621–624. doi: 10.1006/geno.2002.6749. [DOI] [PubMed] [Google Scholar]
- Dosé AC, Hillman DW, Wong C, Sohlberg L, Lin-Jones J, Burnside B. Myo3A, one of two class III myosin genes expressed in vertebrate retina, is localized to the calycal processes of rod and cone photoreceptors and is expressed in the sacculus. Mol Biol Cell. 2003;14:1058–1073. doi: 10.1091/mbc.E02-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosé A, Lin-Jones J, Burnside B. Myosin III in photoreceptors: what does it do? In: Williams DS, editor. Photoreceptor cell biology and inherited retinal degenerations. River Edge, NJ: World Scientific Publications; 2004. pp. 133–162. [Google Scholar]
- Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc Natl Acad Sci U S A. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci U S A. 2003;100:6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Mooseker MS. Porcine myosin-VI: characterization of a new mammalian unconventional myosin. J Cell Biol. 1994;127:425–440. doi: 10.1083/jcb.127.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath KE, Campos-Barros A, Toren A, Rozenfeld-Granot G, Carlsson LE, Savige J, Denison JC, Gregory MC, White JG, Barker DF, Greinacher A, Epstein CJ, Glucksman MJ, Martignetti JA. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am J Hum Genet. 2001;69:1033–1045. doi: 10.1086/324267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang E, Bost-Usinger L, Burnside B. Characterization of a novel C-kinesin (KIFC3) abundantly expressed in vertebrate retina and RPE. Exp Eye Res. 1999;69:57–68. doi: 10.1006/exer.1999.0671. [DOI] [PubMed] [Google Scholar]
- Kappler JA, Starr CJ, Chan DK, Kollmar R, Hudspeth AJ. A nonsense mutation in the gene encoding a zebrafish myosin VI isoform causes defects in hair-cell mechanotransduction. Proc Natl Acad Sci U S A. 2004;101:13056–13061. doi: 10.1073/pnas.0405224101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith C, Paz P, Lee CW, Lam W, Burnside B. Bidirectional pigment granule migration in isolated retinal pigment epithelial cells requires actin but not microtubules. Cell Motil Cytoskeleton. 1997;38:229–249. doi: 10.1002/(SICI)1097-0169(1997)38:3<229::AID-CM2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kitamoto J, Libby RT, Gibbs D, Steel KP, Williams DS. Myosin VI is required for normal retinal function. Exp Eye Res. 2005;81:116–120. doi: 10.1016/j.exer.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Goldstein JA, Kelley MJ, Luxford W, Castelein CM, Mhatre AN. Human nonsyndromic hereditary deafness DFNA17 is due to a mutation in nonmuscle myosin MYH9. Am J Hum Genet. 2000;67:1121–1128. doi: 10.1016/s0002-9297(07)62942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Lillo C, Kitamoto J, Williams DS, Steel KP. Myosin Va is required for normal photoreceptor synaptic activity. J Cell Sci. 2004;117:4509–4515. doi: 10.1242/jcs.01316. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J, Parker E, Wu M, Dosé A, Burnside B. Myosin 3A transgene expression produces abnormal actin filament bundles in transgenic Xenopus laevis rod photoreceptors. J Cell Sci. 2004;117:5825–5834. doi: 10.1242/jcs.01512. [DOI] [PubMed] [Google Scholar]
- Liu X, Vansant G, Udovichenko IP, Wolfrum U, Williams DS. Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil Cytoskeleton. 1997;37:240–252. doi: 10.1002/(SICI)1097-0169(1997)37:3<240::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Liu X, Ondek B, Williams DS. Mutant myosin VIIa causes defective melanosome distribution in the RPE of shaker-1 mice. Nat Genet. 1998;19:117–118. doi: 10.1038/470. [DOI] [PubMed] [Google Scholar]
- Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda S, Ahituv N, Bisceglia L, Sobe T, Glaser F, Rabionet R, Arbones ML, Notarangelo A, Di Iorio E, Carella M, Zelante L, Estivill X, Avraham KB, Gasparini P. MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- Mhatre AN, Li Y, Atkin G, Maghnouj A, Lalwani AK. Expression of Myh9 in the mammalian cochlea: localization within the stereocilia. J Neurosci Res. 2006;84:809–818. doi: 10.1002/jnr.20993. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. The Drosophila ninaC locus encodes two photoreceptor cell specific proteins with domains homologous to protein kinases and the myosin heavy chain head. Cell. 1988;52:757–772. doi: 10.1016/0092-8674(88)90413-8. [DOI] [PubMed] [Google Scholar]
- Müller RT, Honnert U, Reinhard J, Bahler M. The rat myosin myr 5 is a GTPase-activating protein for Rho in vivo: essential role of arginine 1695. Mol Biol Cell. 1997;8:2039–2053. doi: 10.1091/mbc.8.10.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle B, Okamoto C, Taggart B, Burnside B. The teleost cone cytoskeleton. Invest Ophthalmol Vis Sci. 1986;27:689–701. [PubMed] [Google Scholar]
- O'Connor P, Burnside B. Actin-dependent cell elongation in teleost retinal rods: requirement for actin filament assembly. J Cell Biol. 1981;89:517–524. doi: 10.1083/jcb.89.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagh-Roehl K, Burnside B. Preparation of teleost rod inner and outer segments. Methods Cell Biol. 1995;47:83–92. doi: 10.1016/s0091-679x(08)60794-3. [DOI] [PubMed] [Google Scholar]
- Post PL, Bokoch GM, Mooseker MS. Human myosin-IXb is a mechanochemically active motor and a GAP for rho. J Cell Sci. 1998;111:941–950. doi: 10.1242/jcs.111.7.941. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, Williams DS. Myosin V in the retina: localization in the rod photoreceptor synapse. Exp Eye Res. 1996;63:613–619. doi: 10.1006/exer.1996.0155. [DOI] [PubMed] [Google Scholar]
- Seiler C, Ben-David O, Sidi S, Hendrich O, Rusch A, Burnside B, Avraham KB, Nicolson T. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272:328–338. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Self T, Sobe T, Copeland NG, Jenkins NA, Avraham KB, Steel KP. Role of myosin VI in the differentiation of cochlear hair cells. Dev Biol. 1999;214:331–341. doi: 10.1006/dbio.1999.9424. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci U S A. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom F, Dussmann H, Uhlenbrock K, Abouhamed M, Bahler M. The Myosin IXb motor activity targets the myosin IXb RhoGAP domain as cargo to sites of actin polymerization. Mol Biol Cell. 2007;18:1507–1518. doi: 10.1091/mbc.E06-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Walsh V, Vreugde S, Hertzano R, Shahin H, Haika S, Lee MK, Kanaan M, King MC, Avraham KB. From flies' eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc Natl Acad Sci U S A. 2002;99:7518–7523. doi: 10.1073/pnas.102091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, Kelley PM, Kimberling WJ, Wagenaar M, Levi-Acobas F, Larget-Piet D, Munnich A, Steel KP, Brown DMB, Petit C. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Williams DS. Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vis Res. 2008;48:433–441. doi: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth JA, Jensen KA, Post PL, Bement WM, Mooseker MS. Human myosin-IXb, an unconventional myosin with a chimerin-like rho/rac GTPase-activating protein domain in its tail. J Cell Sci. 1996;109:653–661. doi: 10.1242/jcs.109.3.653. [DOI] [PubMed] [Google Scholar]
- Wolfrum U, Schmitt A. Evidence for myosin VIIa-driven transport of rhodopsin in the plasma membrane of the photoreceptor-connecting cilium. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Retinal degenerative diseases and experimental therapy. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 3–14. [Google Scholar]


