Abstract
During early adulthood, a phase in which the central nervous system displays considerable plasticity and in which important cognitive traits are shaped, the effects of exercise on cognition remain poorly understood. We performed a cohort study of all Swedish men born in 1950 through 1976 who were enlisted for military service at age 18 (N = 1,221,727). Of these, 268,496 were full-sibling pairs, 3,147 twin pairs, and 1,432 monozygotic twin pairs. Physical fitness and intelligence performance data were collected during conscription examinations and linked with other national databases for information on school achievement, socioeconomic status, and sibship. Relationships between cardiovascular fitness and intelligence at age 18 were evaluated by linear models in the total cohort and in subgroups of full-sibling pairs and twin pairs. Cardiovascular fitness, as measured by ergometer cycling, positively associated with intelligence after adjusting for relevant confounders (regression coefficient b = 0.172; 95% CI, 0.168–0.176). Similar results were obtained within monozygotic twin pairs. In contrast, muscle strength was not associated with cognitive performance. Cross-twin cross-trait analyses showed that the associations were primarily explained by individual specific, non-shared environmental influences (≥80%), whereas heritability explained <15% of covariation. Cardiovascular fitness changes between age 15 and 18 y predicted cognitive performance at 18 y. Cox proportional-hazards models showed that cardiovascular fitness at age 18 y predicted educational achievements later in life. These data substantiate that physical exercise could be an important instrument for public health initiatives to optimize educational achievements, cognitive performance, as well as disease prevention at the society level.
Keywords: aerobic fitness, intelligence, muscular strength, twin analysis, exercise
The ability of the brain to adapt to a new situation, environment, or consequences of an injury is often referred to as brain plasticity. Physical exercise, as indexed by cardiovascular fitness, is a factor that strongly affects brain plasticity (1). In rodents, physical exercise improves memory function and structural parameters such as synapse density, neuronal complexity, and hippocampal neurogenesis (2–5). In the injured brain, exercise induces neuroprotection in animal models of stroke (6), traumatic brain injury (7), and Parkinson disease (8). We have recently shown that voluntary running significantly restores the neural stem cell pool, hippocampal neurogenesis, and behavioral deficits following a clinically relevant, moderate dose of irradiation (9). These experimental studies indicate the importance of physical exercise for cognitive performance.
Positive cognitive effects of exercise have also been demonstrated in humans. Meta-analyses demonstrate a positive association between cardiovascular (or “aerobic”) fitness and cognitive performance in elderly subjects (10–14). Higher levels of cardiovascular fitness are associated with increased hippocampal volume as well as better memory function (15). Physical activity during midlife appears to protect against dementia and to improve cognitive performance in older adults with memory impairment (16, 17). At the other end of the age spectrum, physical activity and academic achievement display positive correlation, as indicated by meta-analysis of smaller cohort studies of school children (18). However, the relationship between physical exercise and neurocognitive function in young adults remains unknown because of conflicting data. Acutely, physical exercise seems to have little effect on memory and cognition; executive function processes involved in working memory remain unaltered, although aspects of delayed long-term memory improve (19, 20). Long-term physical exercise appears to have a slight effect on reaction time in young people (21). Based on small sample size, an 8-week training program resulted in improved reaction time (n = 20) and executive function (n = 37) (22, 23). Cross-sectional studies demonstrated weak associations between cardiovascular fitness and cognitive performance in young adults (24, 25). However, frequent or strenuous physical activity in young people has been suggested to negatively affect cognitive achievement during adolescence (12, 20). In view of these conflicting data, a larger scale, population-wide analysis of young adults is warranted.
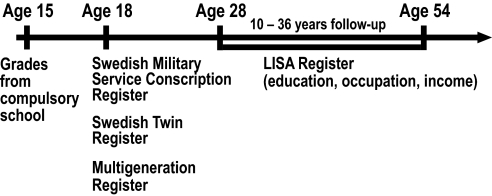
The present study analyzed compulsory screening results for military service at 18 years of age from all Swedish men born from 1950 through 1976. The study aimed to determine the association between physical activity and cognitive performance, as well as the specific interactions of cardiovascular fitness and muscular strength on cognitive performance. In addition, the roles of genetic and familial influences were assessed. Moreover, longitudinal associations between physical training and midlife indicators of cognitive performance, such as educational and occupational status, were investigated. For study design, please see Fig. 1.
Fig. 1.
Study design. The conscription register data were linked with the National Swedish Board of Education school records database to obtain grades from the final year of compulsory school (age 15 y), the Multi-Generation Register for data on full brothers, the Swedish Twin Register for information on zygosity, and Statistics Sweden National Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA) for information on education and occupation.
Results
Cross-Sectional Analyses.
Cardiovascular fitness, not muscular strength, at age 18 y is associated with cognitive performance.
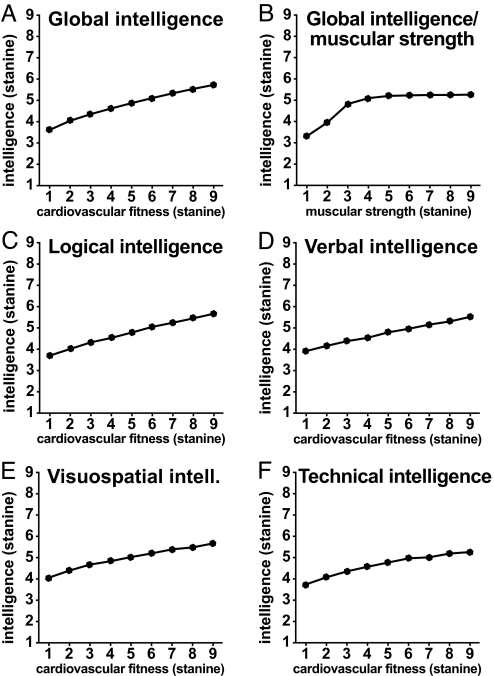
Associations between cardiovascular fitness and global intelligence scores, as well as logical, verbal, visuospatial, and technical scores, were determined (see Fig. 2 A and C–F and Table 1). Increased cardiovascular fitness, as measured by Wmax/kg by using an ergometer cycle, was associated with better cognitive scores at age 18 y. In contrast, muscular strength weakly associated with global intelligence, and was significant only for the lower scores (Fig. 2B). Therefore, 2 additional linear regression analyses were performed for separate segments of the curve, i.e., muscular strength score 1 to 4 and muscular strength score 4 to 9 (see Table 1). Similar results were observed for intelligence score subcategories.
Fig. 2.
Mean levels of intelligence stanine scores by cardiovascular fitness or muscular strength at age 18 y. For each cognitive measure, all means significantly differed from the others, with the exception of cardiovascular fitness scores 6 vs. 7 and 8 vs. 9 in F. In B, the means of global intelligence score for muscular strength scores 4–9 were not significantly separated from each other. The SDs were 1.8–2.0 (A), 1.8–2.3 (B), and 0.8–1.3 (C–F). The P value was <0.0001 for all associations. For regression and correlation coefficients, see Table 1.
Table 1.
Associations between cardiovascular fitness and intelligence scores as well as muscular strength and intelligence scores at age 18 y
| Parameter | Global intelligence | Logical intelligence | Verbal intelligence | Visuospatial intelligence | Technical intelligence |
|---|---|---|---|---|---|
| Cardiovascular fitness | |||||
| n | 1,214,472 | 1,159,011 | 1,158,146 | 1,158,148 | 1,146,662 |
| b | 0.222 (0.220–0.224) | 0.221 (0.219–0.223) | 0.189 (0.187–0.190) | 0.161 (0.159–0.163) | 0.141 (0.139–0.143) |
| r | 0.20 | 0.20 | 0.18 | 0.15 | 0.13 |
| Muscular strength | |||||
| n | 1,217,584 | 1,161,545 | 1,160,685 | 1,160,687 | 1,149,185 |
| b | 0.056 (0.054–0.057) | 0.014 (0.012–0.016) | −0.003 (−0.005 to −0.001) | 0.045 (0.043–0.047) | 0.129 (0.128–0.131) |
| r | 0.054 | 0.01 | −0.003 | 0.04 | 0.13 |
| b1 | 0.333 (0.320–0.345) | 0.237 (0.225–0.250) | 0.159 (0.147–0.171) | 0.277 (0.265–0.289) | 0.429 (0.417–0.441) |
| b2 | 0.030 (0.028–0.032) | −0.006 (−0.008 to −0.004) | −0.017 (−0.019 to −0.015) | 0.023 (0.021–0.025) | 0.100 (0.098–0.102) |
Values in parentheses are 95% CIs. b=regression coefficient presented with 95% confidence intervals. r=Pearson correlation coefficients. b1, linear regression analysis for muscular strength score 1–4; b2, muscular strength score 4–9.
Associations between cognitive performance and cardiovascular fitness remained in adjusted models.
In linear multiple regression models, in which adjustment for conscription year, conscription test center, father's education, and mother's education was performed, the associations remained between cardiovascular fitness and global intelligence scores (b = 0.168; 95% CI, 0.166–0.171; R2 = 0.163; n = 948,078), as well as logical (b = 0.176; CI 0.174–0.178; R2 = 0.142; F = 24,033; n = 899,474), verbal (b = 0.133; CI 0.131–0.135; R2 = 0.148; F = 15,632; n = 898,667), visuospatial (b = 0.114; CI, 0.112–0.116; R2 = 0.089; F = 9,666; n = 898,668), and technical scores (b = 0.113; CI, 0.111–0.115; R2 = 0.100; F = 9,809; n = 888,021).
To determine whether cardiovascular fitness was differentially correlated with one domain of cognitive performance more than others, we repeated the adjusted models with the exact same set of individuals who had complete records in all domains (n = 883,740). This analysis changed the associations very little (b and R2 values were identical within 0.001) and it thus appears that aerobic capacity was associated most strongly with logic and verbal intelligence.
Associations between cardiovascular fitness and cognition within brother and twin pairs.
To assess potential effects of familial and genetic factors, regression analyses were performed among brother pairs, as well as dizygous (DZ) and monozygous (MZ) twin pairs, using global intelligence, logical, verbal, visuospatial, and technical scores as dependent variables and cardiovascular fitness as an independent variable (Table 2). Brothers' scores were included as covariates. If the association between cardiovascular fitness and cognition was entirely explained by genetic and upbringing conditions, the association would be substantially reduced or even disappear within MZ twin pairs. However, the association remained strong (as indicated by the b value), even within MZ twin pairs, indicating that the association was predominantly caused by environmental factors.
Table 2.
Brother and twin pair analyses
| Analysis | Global intelligence | Logical intelligence | Verbal intelligence | Visuospatial intelligence | Technical intelligence |
|---|---|---|---|---|---|
| Brother pairs | |||||
| n | 268,496 | 257,591 | 257,352 | 257,351 | 255,635 |
| b | 0.172 | 0.177 | 0.143 | 0.114 | 0.113 |
| 95% CI | 0.168–0.176 | 0.173–0.181 | 0.140–0.147 | 0.110–0.118 | 0.108–0.117 |
| R2 | 0.27 | 0.23 | 0.20 | 0.16 | 0.18 |
| DZ twin pairs | |||||
| n | 1,715 | 1,676 | 1,676 | 1,676 | 1,672 |
| b | 0.136 | 0.152 | 0.104 | 0.054 | 0.096 |
| 95% CI | 0.089–0.182 | 0.103–0.202 | 0.057–0.152 | 0.005–0.104 | 0.047–0.144 |
| R2 | 0.30 | 0.25 | 0.21 | 0.15 | 0.19 |
| MZ twin pairs | |||||
| n | 1,432 | 1,391 | 1,394 | 1,394 | 1,377 |
| b | 0.128 | 0.114 | 0.113 | 0.099 | 0.060 |
| 95% CI | 0.084–0.172 | 0.062–0.166 | 0.059–0.166 | 0.039–0.159 | 0.003–0.118 |
| R2 | 0.66 | 0.53 | 0.46 | 0.37 | 0.43 |
| Bivariate heritability | |||||
| r (DZ) | 0.14 | 0.14 | 0.13 | 0.08 | 0.08 |
| r (MZ) | 0.20 | 0.21 | 0.20 | 0.13 | 0.13 |
| Heritability | 0.14 | 0.15 | 0.14 | 0.10 | 0.10 |
| Shared environment | 0.07 | 0.06 | 0.06 | 0.03 | 0.03 |
| Non-shared environment | 0.79 | 0.79 | 0.80 | 0.87 | 0.87 |
The association between cardiovascular fitness and cognitive performance within brother and twin pairs. r2=adjusted coefficient of determination; b=regression coefficient presented with 95% confidence intervals. Bivariate heritability: By comparing correlation coefficients (r) between dizygotic (DZ) and monozygotic (MZ) twin pairs, bivariate heritability and contribution of shared and non-shared environment for associations between cardiovascular fitness and intelligence scores wee calculated. env=environment.
Importance of genetic influences for the measures: univariate heritability.
The correlation coefficients for the 5 intelligence scores were similar for brother pairs (r = 0.32–0.48) and DZ twins (r = 0.33–0.52), but those for cardiovascular fitness differed in full brother pairs (r = 0.26) and DZ twins (r = 0.39). In contrast, MZ twin pairs exhibited considerably greater correlation coefficients for cardiovascular fitness (r = 0.67) and intelligence scores (r = 0.61–0.80). The heritability of cardiovascular fitness was 56%; the shared environmental component explained 11% and the non-shared environmental component 33% of the variance. Heritabilities for cognitive measures ranged between 52% and 56%, shared environmental component between 5% and 24%, and non-shared environment between 20% and 39%.
Genetic and environmental influences on the associations: bivariate heritability.
To quantify the importance of genetic and environmental factors in associations between cardiovascular fitness and cognitive performance, “cross-twin cross-trait” correlations were compared within MZ and DZ pairs. Results yielded a cross-trait heritability (i.e., bivariate heritability). Consistent with the sibling regression analyses (Table 2), the non-shared environment predominantly explained the associations (≥80%), whereas genetic factors explained <15% of the covariation between cardiovascular fitness and cognitive measures (Table 2).
Longitudinal Analyses.
Prediction of cognitive performance from changes in cardiovascular fitness.
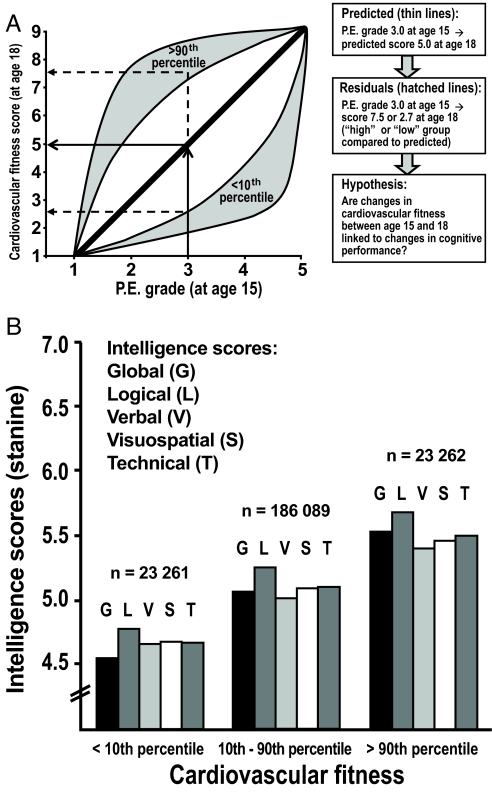
In this analysis, we tried to evaluate whether young male subjects whose cardiovascular fitness had improved between age 15 y and age 18 y had significantly higher intelligence scores than those whose cardiovascular fitness declined (Fig. 3A). Overall, the regression coefficient of cardiovascular fitness score at age 18 y on physical education grades at age 15 y was 0.638 (95% CI, 0.633–0.643; R2 = 0.16). Cognitive performance was then compared among high, low, and middle groups of cardiovascular fitness; interpreted as increased (i.e., the average deviation from the regression line was +2.5; SD, 0.4), decreased (average deviation, −2.3; SD, 0.6), and approximately unchanged (average deviation, −0.03; SD, 0.8) cardiovascular fitness, respectively. Those with increased fitness between 15 and 18 y of age exhibited significantly higher global intelligence scores than those with decreased fitness (Fig. 3B). Similar results were obtained for logical, verbal, visuospatial, and technical scores. These findings indicated that changes in cardiovascular fitness were linked to changes in cognitive performance during adolescence.
Fig. 3.
Change in cardiovascular fitness between age 15 y and 18 y predicts intelligence scores. (A) Schematic presentation of the model. Note that the regression line is not perfectly straight in reality, but it has essentially this appearance in the various analyses. (B) From all subjects with physical education grades at age 15 y and cardiovascular fitness scores at age 18 y, the 10% of subjects with the highest and lowest changes in fitness scores compared with predicted scores were selected (<10th percentile; 10% lowest vs. predicted scores; >90th percentile, 10% highest vs. predicted scores; 10th-90th percentile, remaining 80%). Mean global intelligence, logical, verbal, visuospatial, and technical scores were compared among the 3 percentile groups and significant differences were found among all groups (P < 0.0001). The SDs were 1.81–1.97.
Prediction of education and occupation.
Table 3 lists the adjusted hazard ratios for cardiovascular fitness relation at age 18 y and subsequent education (university vs. high school) and occupation (professions ranked with high vs. low socioeconomic index). We found that better cardiovascular fitness at age 18 y was associated with a higher educational attainment. A similar pattern, but of greater magnitude, was observed for occupational outcome.
Table 3.
Hazard ratios for relationships of cardiovascular fitness at age 18 y with education and occupational outcomes in Swedish men enlisted for military service in 1968–1994
| Event | Cardiovascular fitness | No. of events | Adjusted HR (95% CI) |
|---|---|---|---|
| Education/university outcome | Per stanine score | 230,567 | 1.09 (1.09–1.10) |
| Scores 6–9 vs. 1–4 | 103,444 | 1.78 (1.75–1.81) | |
| Occupation/SEI 3 outcome | Per stanine score | 56,697 | 1.13 (1.12–1.13) |
| Scores 6–9 vs. 1–4 | 48,459 | 1.51 (1.47–1.55) |
The relationships between cardiovascular fitness at age 18 and subsequent time dependent events; obtaining a university degree (compared to pre-high school or high school) or achieving an occupation with a high socioeconomic index; SEI 3 (compared to an occupation with a low socioeconomic index; SEI 1). SEI 1, unskilled/semi-skilled worker in manufacturing sector; skilled worker in manufacturing sector; unskilled/semi-skilled worker in service sector; or skilled worker in service sector. SEI 2, lower-level non-manual employees (education <2 y high school), lower-level non-manual employees (education 2 y high school), intermediate-level non-manual employees, farmers, and other self-employed. SEI 3, self-employed with academic education, manager, higher civil servants, and senior salaried employees.
Discussion
Our data demonstrate that cardiovascular fitness and cognitive performance at age 18 y are positively associated, even after adjusting for relevant confounders. Change in physical achievement between ages 15 y and 18 y predicted cognitive performance at age 18 y. Moreover, cardiovascular fitness during early adulthood predicted socioeconomic status and educational attainment later in life. To our knowledge, this is the first study to demonstrate a clear positive association between cardiovascular fitness and cognitive performance in a large population of young adults. These results have implications for the influence of exercise on plasticity and the cardiovascular fitness hypothesis.
In animal studies, a number of mechanisms have been shown to play a role in exercise-induced cognition and memory improvements. From structural and functional MRI, as well as cognitive tests and neurophysiology, it appears that the same effects are present in corresponding brain regions in humans, and it is likely that the same neurobiological mechanisms are responsible (1). Specifically, increased physical exercise appears to decrease activation of the anterior cingulated cortex, whereas increased activation is observed in the middle frontal gyrus and superior parietal cortex (26). In addition, correlations existed for performance in a selective-attention task. Interestingly, in the hippocampus, increased cerebral blood volume has been observed in the dentate gyrus following a program of long-term physical exercise (27). This has also been observed in animals, in which hippocampal angiogenesis (28), neurogenesis (4), and synaptic plasticity (29) increase in response to cardiovascular exercise. Mechanistically, there are several potential biochemical mediators of exercise effects on the brain, e.g., IGF1, BDNF, and VEGF, which exhibit similar or complementary effects in the hippocampus (30). Circulating levels of these substances increase in response to exercise (31–33), and to various extents, they cross the blood–brain barrier (30).
The cardiovascular fitness hypothesis suggests that cardiovascular (i.e., aerobic) fitness is the physiological mediator that explains the relationship between physical exercise and improved cognitive performance (34). Vigorous aerobic-based exercise intervention studies in children reported enhanced cognitive performance (35, 36), in contrast to studies targeted at moderate levels of physical activity (37, 38). In elderly people, meta-analyses (10, 12) did not fully support the cardiovascular fitness hypothesis, although it was confirmed that physical activity and cognitive performance are associated. The present data provide support for the cardiovascular fitness hypothesis in early adulthood, a period that was previously not studied. Furthermore, similar to a recent study of school children (39), the present study demonstrates that positive associations with intelligence scores were restricted to cardiovascular fitness and not muscular strength (see Fig. 2B). These findings support the notion that cardiovascular exercise improved cognition through increased amounts of circulating factors that positively influence brain plasticity and cognitive function (30).
Magnitude of Associations.
The strength of this study was the ability to include information from all young men in Sweden born from 1950 through 1976 at the time of compulsory military conscription (N > 1,200,000). This conferred 2 disadvantages. Because only male subjects were analyzed, these results might not be applicable to women. Moreover, because the statistical power was so large, even very small associations (i.e., effect sizes) were statistically significant. Thus, it is important to put the magnitude of regression coefficients into perspective. A regression coefficient b of 0.22 for global intelligence demonstrates that an increase of 1 stanine unit in cardiovascular fitness was associated with a change in global intelligence score of 0.22 stanine units. Assuming a 70-kg young male subject, one stanine unit of cardiovascular fitness corresponded roughly to 20 W in maximal load on an ergonometer cycle (40). Thus, 5 points in Wechsler Adult Intelligence Scale correspond to 60 W ergonometer cycle load (assuming intercept in global intelligence score of 100).
The present study assessed logical, verbal, spatial, and technical aspects of intelligence; however, information regarding more specific neuropsychological functions, in particular executive control functions, was lacking. Executive function involves scheduling, response inhibition, planning, and working memory. Our data were collected between 1968 and 1994, during which more complex neurocognitive measurements were still under development. Moreover, in a population-based study with more than 1.2 million subjects, such detailed psychological analyses are technically very difficult to implement. Nevertheless, others have shown that executive functions display strong association to physical exercise. A review by Hall and colleagues (41), as well as a meta-analysis by Colcombe and Kramer (11), indicate that the exercise effect was particularly strong for executive function tests. Among children, the effect of physical activity on cognition is task-dependent (42), and there is also evidence for a selective facilitation effect of aerobic fitness on executive function (35). Although we lacked specific tests of executive control function, we found that cardiovascular fitness was more strongly associated with 2 domains: logical and verbal performance. As mentioned earlier, exercise induced specific functional improvements, in particular in the hippocampus and frontal lobe. Interestingly, both logical reasoning (which includes executive components) and verbal intelligence are domains considered to be linked to these brain areas (43, 44).
Predictors of Change.
Results demonstrated that male subjects with improved predicted cardiovascular fitness between 15 and 18 y of age exhibited significantly greater intelligence scores than subjects with decreased cardiovascular fitness. This indicates that changes in cardiovascular fitness are linked to changes in cognitive performance during adolescence. Nevertheless, direct causality cannot be established. The reverse, i.e., better cardiovascular fitness is a consequence of greater intelligence, is also possible. However, the fact that we demonstrated associations between cognition and cardiovascular fitness but not muscle strength, the differential link to some domains over others, and the longitudinal prediction by cardiovascular fitness at age 18 y on subsequent academic achievement speak in favor of a cardiovascular effect on brain function. It is important to note that differences between short-term (i.e., weeks to months) and long-term (i.e., years) effects of increased physical exercise on aerobic capacity might exist. However, to our knowledge, no studies have assessed these relationships.
Genetic Influences on Associations.
The importance of genetic effects on intelligence (45–47), as well as on physical activity (48, 49), is well established. The present study determined high heritability for cardiovascular fitness. However, according to brother pair comparisons and modeling cross-twin cross-trait correlations, the associations were predominantly explained by the non-shared environment. Thus, factors other than heredity and upbringing are important for the association.
In summary, in a large population-based analysis, cardiovascular fitness was positively associated with cognitive performance at age 18 y. Longitudinal analyses of age showed that improved physical fitness between 15 and 18 y was associated with better cognitive performance and that physical fitness at age 18 y predicted occupational status and educational achievement later in life. More studies addressing causality are needed. However, we believe the present results provide scientific support for educational policies to maintain or increase physical education in school curricula as a means to stem the growing trend toward a sedentary lifestyle, which is accompanied by an increased risk for diseases and perhaps intellectual and academic underachievement.
Methods
For a full description of all materials and methods, see SI Methods.
Participants.
A cohort of 18-y-old Swedish male subjects who were enlisted for military service between 1968 and 1994 (N = 1,221,727) and represented approximately 97% of the male Swedish population born between 1950 and 1976, was compiled from the Swedish Military Service Conscription Register.
Physical and Cognitive Tests.
Cardiovascular fitness was assessed by using a cycle ergonometry test. Isometric muscle strength was measured by knee extension, elbow flexion, and hand grip. Four cognitive tests were used covering the following areas: logical performance test, verbal test of synonyms and opposites, test of visuospatial/geometric perception, and technical/mechanical skills including mathematical/physics problems (50). Performance on all 4 tests were combined to obtain a global intelligence score, which was regarded as a measure of general cognitive ability (50). To provide long-term stability of the data sets across test centers, all physical and cognitive test results were standardized as stanine scores from 1 (low) to 9 (high) against data from previous years.
Links to the Swedish Multi-Generation Register and Twin Register enabled the identification of full brothers and twins. Education and occupation information was obtained from the longitudinal LISA database. The Ethics Committee of Sahlgrenska Academy at the University of Gothenburg and the Secrecy Clearance at Statistics Sweden approved the study.
Statistical Analysis.
All statistical calculations were performed with SAS software (version 8.1; SAS Institute). Because of the large number of observations, the majority of P values and SEMs were very small. Therefore, P values <0.0001 and SEMs in tables and figures were not reported unless otherwise stated. As a measure of variation, SDs are included in the legends.
Cross-Sectional Analysis.
Linear regression models.
Linear regression was analyzed with PROC GLM using intelligence scores as dependent variables and cardiovascular fitness and muscle strength scores as independent variables. To determine if intelligence score means were significantly separated from each other, the Student-Newman-Keuls post-hoc test was used. Effect sizes are presented as regression coefficients (b) with 95% CIs in all models.
Adjusted models.
The associations between cardiovascular fitness and intelligence scores was tested in multiple regression models adjusted for multiple confounders. Because differences over time as well as among the 6 test centers could introduce bias, conscription year and conscription test center were considered as possible confounders. In addition, parental educational level was included as a confounder. The proportion of the variation explained by the adjusted model is given by the adjusted coefficient of determination (R2). Because our statistical software did not present P values below 0.0001, which was achieved for virtually every analysis as a result of the large numbers, here we present the F-values indicating the strength of the analysis.
We also performed the adjusted models using the identical number of observations, and the identical set of explanatory variables. In this analysis, the coefficients of regression and degrees of determinations are fully comparable among the models. Therefore, the coefficients of determination indicate which response has the highest degree of variation explained by the factors included in the models, whereas the coefficients of regression or subgroup means show the magnitude of importance for each intelligence score.
Brother and twin analysis.
Associations were evaluated within brother pairs to assess familial factors, adjusting for conscription year and test center, as well as brothers' cardiovascular and cognitive performance. One brother was randomly selected to provide dependent variable values and the other brother's scores formed the independent variables. In the case of several brothers, the median served as the proxy for familial or heritable effects. The same analyses were repeated within DZ and MZ twin pairs, although without adjustment for conscription year, resulting in a co-twin control analysis (51).
Pearson correlation coefficients (r) within brother pairs, as well as DZ and MZ twin pairs, were used to assess univariate heritability. Similarly, a cross-twin cross-trait analysis (52) was performed to yield bivariate heritabilities. By comparing the cross-correlation coefficients between DZ and MZ twin pairs, the influence of genetic, shared environmental, and non-shared environmental factors on the associations was calculated (53).
Longitudinal Analysis.
Prediction of cognitive performance from changes in cardiovascular fitness.
Regression modeling was performed with physical education grades at age 15 y as the independent variable and the cardiovascular fitness score at age 18 y as the dependent variable. The rationale is shown in Fig. 3A. Individuals deviating from the regression line were identified as residuals (i.e., outliers) in this model, and 3 groups were defined according to cardiovascular fitness at age 18 y: the “increased” group comprising the 90th percentile (i.e., 10% highest cardiovascular fitness vs. predicted), the “decreased” group comprising the 10th percentile (i.e., 10% lowest fitness vs. predicted) and the “unchanged” group representing the 10th to 90th percentile (i.e., remaining 80%). Cognitive performance at age 18 y was compared among the 3 groups, adjusting for conscription year. The analysis was based on the 232,612 individuals with complete records of final year grades, cardiovascular fitness score, intelligence scores, and conscription year.
Prediction of education and occupation.
The relationships between cardiovascular fitness at age 18 y and subsequent academic and educational achievements were determined using Cox proportional-hazards regression models. Further details are described in SI Methods.
Supplementary Material
Acknowledgments.
The authors acknowledge the important contribution to this study of Professor Peter S. Eriksson, who unexpectedly died in August 2007. We thank Dr. Leif Samuelsson, Dr. Berit Carlstedt, and Dr. Johan Lothigius (National Service Administration) for practical help and advice, and Dr. Charles Taft, Mr. Stephen Ordway, and Dr. Michelle Anderson for comments on the manuscript. This study was supported by grants from the Swedish Medical Research Council, the Regional Developmental Board in Western Sweden, Sahlgrenska Academy, the Swedish Society of Medicine, the STENA foundation, the Söderberg foundation, Hjärnfonden, Barncancerfonden, Swedish Research Council for Worklife and Social Science (FAS), and the Swedish government under the LUA/ALF agreement for biomedical research. The Swedish Twin Registry is supported by grants from the Swedish Research Council, the Ministry for Higher Education, and grants AG 08724, DK 066134, and CA 085739 from the National Institutes of Health. The funding sources did not read or comment on any version of the manuscript, nor influence the analyses in any way.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905307106/DCSupplemental.
References
- 1.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 2.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 3.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu CW, et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- 6.Hayes K, et al. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griesbach GS, Gómez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 8.Yoon MC, et al. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson's rats. Neurosci Lett. 2007;423:12–17. doi: 10.1016/j.neulet.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Naylor AS, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci USA. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;3:CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 12.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Etnier JL, et al. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. J Sport Exerc Psychol. 1997;19:249–277. [Google Scholar]
- 14.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Erickson KI, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andel R, et al. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol Series A Biol Sci Med Sci. 2008;63:62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- 17.Lautenschlager NT, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 18.Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr Exerc Sci. 2003;15:243–256. [Google Scholar]
- 19.Coles K, Tomporowski PD. Effects of acute exercise on executive processing, short-term and long-term memory. J Sports Sci. 2008;26:333–344. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- 20.Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood DE, Selder DJ. Cardiorespiratory health, reaction time and aging. Med Sci Sports. 1979;11:186–189. [PubMed] [Google Scholar]
- 22.Hansen AL, Johnsen BH, Sollers JJ, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur J Appl Physiol. 2004;93:263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- 23.Hascelik Z, Basgöze O, Türker K, Narman S, Ozker R. The effects of physical training on physical fitness tests and auditory and visual reaction times of volleyball players. J Sports Med Phys Fitness. 1989;29:234–239. [PubMed] [Google Scholar]
- 24.Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med Sci Sports Exerc. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- 25.Shay KA, Roth DL. Association between aerobic fitness and visuospatial performance in healthy older adults. Psychol Aging. 1992;7:15–24. doi: 10.1037//0882-7974.7.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Colcombe SJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Praag H, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 30.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Asano M, et al. Increase in serum vascular endothelial growth factor levels during altitude training. Acta Physiol Scand. 1998;162:455–459. doi: 10.1046/j.1365-201X.1998.0318e.x. [DOI] [PubMed] [Google Scholar]
- 32.Castellano V, White LJ. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J Neurol Sci. 2008;269:85–91. doi: 10.1016/j.jns.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–3497. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- 34.North TC, McCullagh P, Tran ZV. Effect of exercise on depression. Exerc Sport Sci Rev. 1990;18:379–415. [PubMed] [Google Scholar]
- 35.Davis CL, et al. Effects of aerobic exercise on overweight children's cognitive functioning: a randomized controlled trial. Res Q Exerc Sport. 2007;78:510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuckman BW, Hinkle JS. An experimental study of the physical and psychological effects of aerobic exercise on schoolchildren. Health Psychol. 1986;5:197–207. doi: 10.1037//0278-6133.5.3.197. [DOI] [PubMed] [Google Scholar]
- 37.Coe DP, Pivarnik JM, Womack CJ, Reeves MJ, Malina RM. Effect of physical education and activity levels on academic achievement in children. Med Sci Sports Exerc. 2006;38:1515–1519. doi: 10.1249/01.mss.0000227537.13175.1b. [DOI] [PubMed] [Google Scholar]
- 38.Shepard RJ, et al. Required physical activity and academic grades: A controlled longitudinal study. In: Ilmarinen J, Valimaki I, editors. Children and Sport. Berlin: Springer; 1984. pp. 58–63. [Google Scholar]
- 39.Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. J Sport Exerc Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- 40.Åstrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol. 1954;7:218–221. doi: 10.1152/jappl.1954.7.2.218. [DOI] [PubMed] [Google Scholar]
- 41.Hall CD, Smith AL, Keele SW. The impact of aerobic activity on cognitive function in older adults: A new synthesis based on the concept of executive control. Eur J Cognitive Psychol. 2001;13:279–300. [Google Scholar]
- 42.Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children's intelligence, cognition, and academic achievement. Educ Psychol Rev. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prado J, Noveck IA. Overcoming perceptual features in logical reasoning: A parametric functional magnetic resonance imaging study. J Cogn Neurosci. 2007;19:642–657. doi: 10.1162/jocn.2007.19.4.642. [DOI] [PubMed] [Google Scholar]
- 44.Whitney C, et al. Task-dependent modulations of prefrontal and hippocampal activity during intrinsic word production. J Cogn Neurosci. 2009;21:697–712. doi: 10.1162/jocn.2009.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkel D, Pedersen NL, McGue M, McClearn GE. Heritability of cognitive abilities in adult twins: comparison of Minnesota and Swedish data. Behav Genet. 1995;25:421–431. doi: 10.1007/BF02253371. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen NL, Reynolds CA, Gatz M. Sources of covariation among Mini-Mental State Examination scores, education, and cognitive abilities. J Gerontol B Psychol Sci Soc Sci. 1996;51:55–63. doi: 10.1093/geronb/51b.2.p55. [DOI] [PubMed] [Google Scholar]
- 47.Stromswold K. Why aren't identical twins linguistically identical? Genetic, prenatal and postnatal factors. Cognition. 2006;101:333–384. doi: 10.1016/j.cognition.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Eriksson M, Rasmussen F, Tynelius P. Genetic factors in physical activity and the equal environment assumption—the Swedish young male twins study. Behav Genet. 2006;36:238–247. doi: 10.1007/s10519-005-9018-7. [DOI] [PubMed] [Google Scholar]
- 49.Frederiksen H, Christensen K. The influence of genetic factors on physical functioning and exercise in second half of life. Scand J Med Sci Sports. 2003;13:9–18. doi: 10.1034/j.1600-0838.2003.20219.x. [DOI] [PubMed] [Google Scholar]
- 50.Carlstedt B. Gothenburg, Sweden: Univ of Gothenburg; 2000. Cognitive abilities—aspects of structure, process and measurement. Thesis. [Google Scholar]
- 51.Lichtenstein P, et al. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 52.Plomin R, DeFries JC. Multivariate behavioral genetics and development: twin studies. Prog Clin Biol Res. 1981;69:25–33. [PubMed] [Google Scholar]
- 53.Heiser P, et al. Twin study on heritability of activity, attention, and impulsivity as assessed by objective measures. J Atten Disord. 2006;9:575–581. doi: 10.1177/1087054705284298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.