Abstract
Retinal degenerations are a class of neurodegenerative disorders that ultimately lead to blindness due to the death of retinal photoreceptors. In most cases, death is the result of long-term exposure to environmental, inflammatory, and genetic insults. In age-related macular degeneration, significant vision loss may take up to 70–80 years to develop. The protracted time to develop blindness suggests that retinal neurons have an endogenous mechanism for protection from chronic injury. Previous studies have shown that endogenous protective mechanisms can be induced by preconditioning animals with sublethal bright cyclic light. Such preconditioning can protect photoreceptors from a subsequent damaging insult and is thought to be accomplished through induced expression of protective factors. Some of the factors shown to be associated with protection bind and activate the signal transducing receptor gp130. To determine whether stress-induced endogenous protection of photoreceptors requires gp130, we generated conditional gp130 knockout (KO) mice with the Cre/lox system and used light-preconditioning to induce neuroprotection in these mice. Functional and morphological analyses demonstrated that the retina-specific gp130 KO impaired preconditioning-induced endogenous protection. Photoreceptor-specific gp130 KO mice had reduced protection, although the Müller cell KO mice did not, thus gp130-induced protection was restricted to photoreceptors. Using an animal model of retinitis pigmentosa, we found that the photoreceptor-specific gp130 KO increased sensitivity to genetically induced photoreceptor cell death, demonstrating that gp130 activation in photoreceptors had a general protective role independent of whether stress was caused by light or genetic mutations.
Keywords: IL6 signal transducing receptor, neuroprotection, conditional gp130 knockout, inherited retinal degeneration, light damage
The signal-transducing receptor gp130 is a common receptor for the IL-6 family of cytokines, such as ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), and cardiotrophin-like cytokine (CLC). Use of these cytokines has been shown to be neuroprotective in both the central and peripheral nervous systems, suggesting that this receptor and its signal transduction pathways may be important for the design of neuroprotective therapeutics (1–4).
Up-regulation of protective cytokines by acute or chronic stress has been observed in several studies. Mild light stress has been shown to induce endogenous protection of photoreceptors from a subsequent light damage, and protection coincided with prolonged up-regulation of neurotrophic factors (5). bFGF mRNA expression was up-regulated in mice homozygous for the rd1 mutation in the PDE6b gene (a model of retinitis pigmentosa) (6). Elevated levels of bFGF and/or CNTF expression in the retina were also found in light-induced as well as inherited models of photoreceptor degeneration in both mice and rats (7, 8). LIF and CLC were also up-regulated by stress from constant light exposure in the mouse retina (9). CNTF and bFGF expression were induced after a single mechanical lesion to the retina and subretinal space in rats (10) and mice (11), respectively. Collectively, these studies demonstrate that stress in the retina caused by light, mechanical injury, and genetic mutations can induce endogenous up-regulation of neurotrophic factors. Up-regulation of these factors is likely functional, since a number of studies have shown that injection or viral-mediated expression of these factors can protect photoreceptors (12–19).
Determining the mechanism of preconditioning-induced protection has been hampered because multiple factors are induced, which can activate different receptors located on different cell types. The ligands or receptors that are required for preconditioning-induced protection have yet to be identified. In addition, it is not known which cell type is responsible for protection. Thus, the purpose of this study was to determine the role of gp130 activation in endogenous protection induced by bright light preconditioning and by chronic stress caused by genetic mutations. We also sought to determine whether protection of photoreceptors requires activation of gp130 in photoreceptors or in Müller cells. To accomplish this, we generated mice with conditional gp130 KO in the retina using the Cre/lox system. Our data clearly show that chronic stress such as preconditioning and genetic mutations induced endogenous protection of photoreceptors and that loss of gp130 in either the whole retina or specifically in photoreceptors impairs stress-induced protection, although Müller cells do not. This study demonstrates that gp130 activation in photoreceptors is required to prevent death of neurons under stressful conditions and suggests that gp130 may help prevent or delay cell death in neurodegenerative diseases or chronic injury.
Results
Cre-Mediated Deletion of gp130 Abolished STAT3 Activation in Response to a gp130 Ligand in a Cell-Specific Manner.
To study the cell-specific roles of gp130 in endogenous photoreceptor protection, we generated murine models of conditional gp130 KO in the retina using the Cre/lox system. We mated mice carrying the floxed gp130 allele (gp130f/f) (20) with Chx10-cre (21), rod-cre (22), or VMD2-cre (23) transgenic lines. The resultant conditional gp130 KO mice had gp130 deletion in almost all retinal cells, rod photoreceptors, or Müller glial cells, respectively. All mice were viable and had no apparent phenotypic abnormalities. To test for toxicity induced by Cre expression or by floxed alleles, we used electroretinography (ERG) and histology to examine the retinas of 6-week-old mice for functional and morphological alterations. We did not observe any unexpected abnormalities in these mice. Data also show that gp130 deletion did not cause any detectable effect on retinal development, function, and integrity in unstressed animals.
To assess the extent of cell-specific gp130 deletion in the retina, we initially tested multiple commercially available antibodies against gp130 by using Western blot analysis; however, high nonspecific binding produced multiple bands, preventing the use of immunohistochemistry to demonstrate the loss of gp130. To assess the extent of the gp130 deletion, we measured STAT3 phosphorylation, a known downstream event of gp130 (24). We intravitreally injected 6- to 7-week-old gp130 KO mice with recombinant human LIF. At 30 min and 1 day, we performed immunohistochemistry for phosphorylated STAT3 on the retinal sections (Fig. 1). Similar to the retinas from WT mice (19), the gp130f/f; cre− retinas had a LIF-induced, robust STAT3 activation in Müller cells 30 min postinjection (Fig. 1B) and in all retinal cells 1 day postinjection (Fig. 1G). Although injection of PBS did not induce detectable STAT3 activation in any of our gp130 conditional KO retinas (Fig. 1 A and F), 80% of the retinal cells, including Müller cells (Fig. 1 C and H, arrows) and photoreceptors (Fig. 1H, arrowheads) in the gp130f/f; Chx10-cre+ mice lost STAT3 activation in response to LIF due to gp130 deletion. The gp130 deletion in gp130f/f; rod- cre+ mice abolished STAT3 activation in 30% of rod photoreceptors without affecting STAT3 activation in the other cell types (Fig. 1 D and I). In gp130f/f; VMD2-cre+ mice, STAT3 activation was lost in 50% of Müller cells (Fig. 1 E and J, arrows) and in <5% of the photoreceptors (Fig. 1J, arrowheads) (23).
Fig. 1.
Retinal cell-specific deletion of gp130 resulted in LIF-induced loss of STAT3 activation. Immunohistochemical detection of STAT3 activation (pSTAT3; green) was used to localize cells that did not responded to LIF 30 min or 1 day after intravitreal injection of LIF. Injection of PBS did not induce detectable STAT3 activation at 30 min A or 1 day (F). pSTAT3 was detected in Müller cells and all retinal cells 30 min B and 1 day (G) post-LIF injection in gp130f/f; cre− retinas, respectively. In gp130f/f; Chx10-cre+ retina (C and H), 80% of the cells lost STAT3 activation including photoreceptors (arrowheads) and Müller cells (arrows). In gp130f/f; rod-cre+ retina, 30% of photoreceptors lost STAT3 activation (arrowheads in I), while other cell types were unaffected (D and I). In gp130f/f; VMD2-cre+ retina, 50% of Müller cells (arrows in E and J) and <5% of photoreceptors lost STAT3 activation (arrowheads in J). Nuclei were counterstained with DAPI (blue). onl, outer nuclear layer; inl, inner nuclear layer.
We used Western blots to quantify the loss of STAT3 activation in the whole retina due to gp130 deletion in each conditional KO line (Fig. S1). Retinas from gp130f/f; Chx10-cre+ mice had 80% reduction in LIF-induced signaling at all time points when compared to retinas from gp130f/f; Chx10-cre− mice. The retinas from gp130f/f; VMD2-cre+ mice had significantly less signaling activation than the retinas from gp130f/f; VMD2-cre− mice when LIF-induced signaling activation was only detected in Müller cells (30 min) (P < 0.05). The retinas from the gp130f/f; rod-cre+ mice had less STAT3 activation than those from the gp130f/f; rod-cre− mice when photoreceptors were most active (1 day), but no effect on early STAT3 activation when only Müller cells were active (30 min).
Loss of gp130 in the Retina Significantly Impaired Endogenous Protection of Photoreceptor Function and Accelerated Light-Induced Photoreceptor Cell Death.
It was previously shown that preconditioning with bright cyclic light can induce endogenous mechanisms of photoreceptor protection against subsequent exposure to damaging light (light damage; LD) (5, 25–27). Ligands of gp130, such as CNTF, are up-regulated in response to light exposure, suggesting that gp130 might play a protective role (8, 9). We found previously that a 6-day exposure to sublethal bright cyclic light (600 lux) was sufficient to induce endogenous protection against subsequent LD in WT albino mice (28). We used this preconditioning model (Fig. 2) to analyze the effect of gp130 loss on endogenous protection of photoreceptors in gp130f/f; Chx10-cre mice (Figs. 3 and 4).
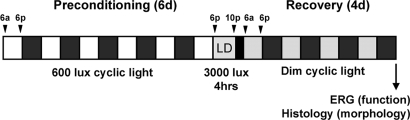
Fig. 2.
Bright light preconditioning model. Mice were exposed to moderately bright cyclic light (600 lux; 12 h on/12 h off) for 6 days to induce endogenous protection in the retina. The mice were exposed to 3,000 lux light to induce LD and then to 50 lux cyclic light (12 h on/12 h off) for 4 days to recover. Photoreceptor function and morphology were subsequently assessed by electroretinography and histology.
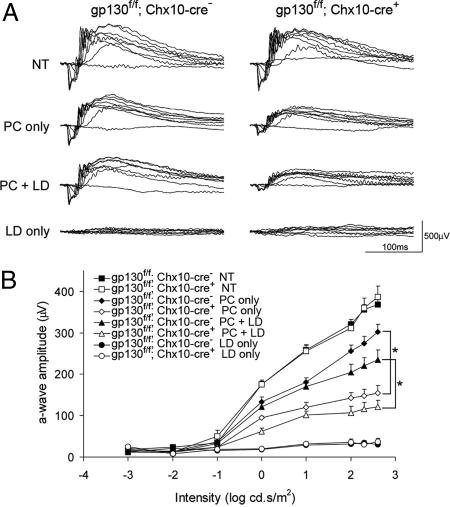
Fig. 3.
Loss of gp130 in gp130f/f; Chx10-cre+ mice significantly impaired preconditioning-induced endogenous protection of photoreceptor function. (A) Representative ERG traces of gp130f/f; Chx10-cre− (Left) and gp130f/f; Chx10-cre+ (Right) before preconditioning (NT), after preconditioning (PC only), preconditioning and then LD (PC + LD), and LD (LD only). (B) Photoreceptor response (a-wave amplitude) at different intensities of light flashes. *, P < 0.05 with t test (n = at least 6 per group). Error bars represent SEM.
Fig. 4.
Loss of gp130 in gp130f/f; Chx10-cre+ mice significantly impaired endogenous protection of photoreceptors. The degree of photoreceptor loss was quantified by counting the number of rows of photoreceptor nuclei along the retina from the optic nerve head (ONH). (A) Representative histological sections are from the superior retina at 0.48 mm from the ONH. (B) The mean number of rows of photoreceptor nuclei in the gp130f/f; Chx10-cre+ retinas was significantly fewer than the number in the gp130f/f; Chx10-cre− retinas after preconditioning and light damage (PC + LD) (open and closed triangles, respectively). onl, outer nuclear layer. Error bars represent SEM (n = at least 6 per group).
To evaluate the effect of gp130 loss on protection of photoreceptor function, we performed electroretinography (ERG) (Fig. 3). Decreased a-waves in scotopic ERG recordings are an indication of reduced photoreceptor function. Both wild-type (gp130f/f; Chx10-cre−) and gp130 KO retinas (gp130f/f; Chx10-cre+) had normal responses to light stimulation, indicating that neither the gp130 floxed allele (Fig. 3B, closed squares) nor retina-specific loss of gp130 by Chx10-cre (Fig. 3B, open squares) affected photoreceptor function under normal conditions. LD without preconditioning resulted in complete loss of photoreceptor function in both gp130f/f; Chx10-cre− and gp130f/f; Chx10-cre+ retinas (Fig. 3B, circles), showing susceptibility of nonpreconditioned photoreceptors to light-induced oxidative damage. Retinas from gp130f/f; Chx10-cre− mice were significantly protected from LD after preconditioning (Fig. 3B, closed triangles). This response was similar to the preconditioning-induced protection of normal mice that we described in ref. 28. In gp130f/f; Chx10-cre+ retinas, LD resulted in significant reduction in photoreceptor function (Fig. 3B, triangles). In contrast, preconditioning-induced protection of photoreceptors was not impaired in gp130f/+; Chx10-cre+ retinas compared to gp130f/+; Chx10-cre− retinas (Fig. S2), demonstrating that the expression of Cre was not toxic to photoreceptors nor making photoreceptors more susceptible to light exposure. These data suggest that loss of gp130 in the retina abolishes endogenous protection of photoreceptor function against light-induced oxidative damage.
To determine whether loss of function was the result of photoreceptor cell death, we quantified photoreceptor survival by morphometric analyses (Fig. 4). Representative histological sections of the superior retina, a region most susceptible to light damage, are shown in Fig. 4A. The number of rows of photoreceptors was counted at 0.16-mm intervals from the optic nerve head (Fig. 4B). LD after preconditioning resulted in loss of photoreceptor cells in gp130f/f; Chx10-cre+ retinas (PC + LD; Fig. 4B, open triangles), but not in gp130f/f; Chx10-cre− retinas (Fig. 4B, closed triangles), suggesting that gp130 expression in the retina is essential for preconditioned-induced protection.
Unexpectedly, we observed a significant loss of photoreceptor function in gp130f/f; Chx10-cre+ retinas after preconditioning (Fig. 3B, open diamonds). Despite the loss of function, the number of photoreceptors did not differ significantly between gp130f/f; Chx10-cre− and gp130f/f; Chx10-cre+ retinas after preconditioning (Fig. 4B, diamonds). This suggests that the reduction in function after preconditioning was not due to cell loss. The mechanism for loss of function in preconditioned gp130f/f; cre+ mice is unknown, but the results suggest that loss of gp130 alters photoreceptor responses to light stress.
gp130 Expression in Rod Photoreceptors, But Not Müller Cells, Is Essential for Endogenous Protection of Photoreceptors Against Light-Induced Oxidative Stress.
While our data from the gp130f/f; Chx10-cre mice demonstrate that gp130 is essential for stress-induced protection (Figs. 3 and 4), it is not clear whether protection is an effect of gp130 activation in photoreceptors or in Müller cells. In mice with photoreceptor specific deletion of gp130 (gp130f/f; rod-cre+), preconditioning-induced protection was dramatically reduced (Fig. 5A, compare triangles). In agreement with ERG functional analysis, histological analysis revealed significantly more photoreceptor loss in the gp130f/f; rod-cre+ retinas after LD compared to gp130f/f; rod-cre− retinas (Fig. 5C, Left, and D, triangles). Expression of Cre in rod-cre+ retinas did not show impairment in preconditioning-induced protection (Fig. S3), further supporting that the loss of protection observed in gp130f/f; rod-cre+ retinas can be attributed to loss of gp130 in photoreceptors rather than potential toxicity associated with Cre expression. Both functional and histological data demonstrate that gp130 in rod photoreceptors was essential for endogenous protection of photoreceptors from light-induced oxidative stress.
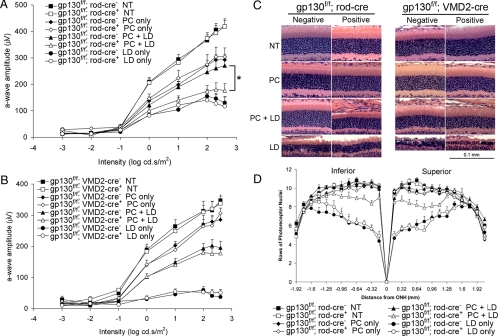
Fig. 5.
Loss of gp130 in rod photoreceptors, but not Müller cells, significantly impaired preconditioning-induced endogenous protection of photoreceptors. (A) The mean a-wave amplitude was significantly lower for gp130f/f; rod-cre+ mice than for gp130f/f; rod-cre− mice after preconditioning followed by light damage (PC + LD) (triangles). (B) Loss of gp130 in 50% of Müller cells in the gp130f/f; VMD2-cre+ retina did not affect gp130-mediated endogenous protection of photoreceptor function (open and closed triangles). (C) Representative histological sections of the outer nuclear layer at the superior retina 0.48 mm from the ONH. (D) Mean number of rows of photoreceptors after PC + LD in gp130f/f; rod-cre+ retina was significantly less than in gp130f/f; rod-cre− retina after PC + LD (open and closed triangles, respectively). Error bars represent SEM (n = at least 8 per group). *, P < 0.05 with t test.
Preconditioning-induced protection of photoreceptor function was not impaired in gp130f/f; VMD2-cre+ retinas (Fig. 5B, triangles). Histological analyses support these findings and revealed the loss of gp130 in Müller cells did not reduce preconditioning-induced protection of photoreceptors from LD (Fig. 5C, Right). These results demonstrate that deletion of gp130 in rod photoreceptors, but not Müller cells, impairs stress-induced endogenous protection of photoreceptors, and suggests gp130 activation in photoreceptors rather than in Müller cells is essential for endogenous protection of photoreceptors.
gp130 in Rod Photoreceptors Plays an Essential Role in Endogenous Protection of Photoreceptors from Genetically Induced Cell Death.
Light damage is an experimental model of retinal degeneration that may or may not reflect mechanisms of inherited retinal degeneration. Preconditioning studies suggest retinas under stress induced gp130-mediated endogenous protection. Inherited mutations likely induce chronic stress in which gp130 may also play a role in delaying or reducing photoreceptor cell death. To test this, we mated gp130f/f; rod-cre+ mice with VPP transgenic mice (29), which express a mutant rhodopsin and display photoreceptor degeneration occurring after 2 weeks of age (Fig. 6). Deletion of gp130 (VPP+; gp130f/f; rod-cre+) resulted in accelerated degeneration compared to VPP mice with gp130 (VPP+; gp130f/f; rod-cre−) (Fig. 6 A and B). The accelerated degeneration was the result of gp130 loss and not the result of Cre toxicity since the rate of degeneration caused by the VPP transgene was the same in Cre+ and Cre− mice, which had one wild-type allele of gp130 (Fig. S4). These results clearly demonstrate gp130 in photoreceptors plays a role in preventing or delaying cell death from genetically induced chronic stress.
Fig. 6.
Loss of gp130 in rod photoreceptors accelerated the rate of photoreceptor cell death in an inherited model of retinal degeneration. Rod photoreceptor-specific gp130 KO mice were crossed with transgenic mice expressing a mutant from of opsin (VPP) to determine whether loss of gp130 accelerated retinal degeneration. (A) Representative histological sections are from the superior retina at 0.32 mm from the ONH. Progressive loss of photoreceptors was observed in VPP+ retina (Left column). (B) Mean number of photoreceptors in the region shown in panel A is reported. Loss of gp130 in 30% of rod photoreceptors (VPP+; gp130f/f; rod-cre+) (open triangles) significantly accelerated the rate of genetically induced photoreceptor cell death. onl, outer nuclear layer. Error bars represent SEM (n = at least 5 per group). *, P < 0.001 with t test.
Discussion
We previously showed that sublethal light stress (preconditioning) induces endogenous neuroprotection, which is highly effective at protecting photoreceptors from a subsequent exposure to LD (28). Therefore, identifying the mechanism for endogenous protection may lead to the development of neuroprotective therapies. We and others have previously shown that application of exogenous gp130 ligands promotes photoreceptor survival in light-induced and inherited models of retinal degeneration in many species (12–19). Thus, gp130 activation could be one of the key events mediating endogenous neuroprotection. Our conditional gp130 KO data clearly show that gp130 plays an essential role in endogenous neuroprotection and that this protection is mediated by activation of gp130 in photoreceptors. Data further suggest that the indirect effect of gp130 activation in Müller glial cells is not as critical, since inactivating gp130 in 30% of rod photoreceptors reduced protection while inactivating gp130 in 50% of Müller glial cells did not.
The light history of an animal greatly affects the susceptibility of its retinas to subsequent light damage (25, 30, 31). For example, albino rats raised in darkness were more susceptible to light-induced retinal degeneration than albino rats raised in cyclic light (30). Previous studies demonstrated that moderate bright light stress induced endogenous protection of photoreceptors from subsequent LD in both mice and rats (5, 26, 27, 32). In addition, preconditioning has been shown to coincide with prolonged up-regulation of neurotrophic factors (5). Elevated levels of bFGF, CNTF, LIF, and CLC have been found in mouse and rat retinas in light-induced and inherited models of photoreceptor degeneration (7–9).
We have previously measured preconditioning-induced expression of several factors including FGF2, BDNF, LIF, oncostatin M (OSM), and CLC (28). Several of the induced cytokines including LIF, CNTF, OSM, and CLC signal through the common receptor gp130, suggesting that this cytokine/gp130 system may be important for neuroprotection. However, other factors that do not activate gp130 were also up-regulated, suggesting the possibility that other factors or receptors may also be involved. Since multiple cytokines and growth factors are up-regulated by stress, it has been difficult until now to determine which ligand-receptor systems are essential for stress-induced protection. We previously used antagonists to the LIF receptor (LIFR) and found that ligands, including LIF, CNTF, OSM, and CLC, that used LIFR are the most likely candidates for involvement (28). A recent study using LIF KO mice suggests that LIF is the key cytokine for protection from inherited and light-induced retinal degenerations (33, 34). LIF's role in preconditioning-induced protection is yet to be determined, but based on its induction and the requirement for LIFR, LIF is the most logical candidate (28). In the current study, loss of gp130 in 80% of all retinal cells in the gp130f/f; Chx10-cre+ mice resulted in near complete loss of preconditioning-induced protection (Figs. 3 and 4). Our data clearly demonstrate that stress-induced neuroprotection requires the signal-transducing receptor gp130.
Activation of the PI3K/Akt pathway can promote cell survival in many tissues, including the retina. Li et al. found that Akt2, one of the three isoforms of Akt kinases, has a role in protecting photoreceptors from acute light-induced cell death, suggesting an essential neuroprotective role of PI3K/Akt pathway during acute insults (35). Acute bright light stress can activate the insulin receptor (IR), which in turn activates the PI3K/Akt pathway in photoreceptors (36). Moreover, rod-specific deletion of the IR increased acute light-induced photoreceptor apoptosis, suggesting a neuroprotective role of the IR/PI3K/Akt signaling during acute light insults (37). We observed no increase in sensitivity to LD in the absence of preconditioning in any of the conditional gp130 KO mice (Figs. 3, 4, and 5, LD-only group). This was expected since induction of gp130 ligands requires exposure to chronic stress. Without ligands, loss of the receptor should have no effect. This suggests that there are two nonredundant pathways for protection of photoreceptors; one is the acute response that involves the IR and signaling through Akt2 (35–37) and the other, as shown in this current study, is induced when ligands of gp130 are elevated after prolonged light stress or by genetic mutations (5, 8, 9, 28).
Our data showing that this receptor can induce protection suggests that gp130 ligands may be effective at preventing or delaying neurodegeneration in human diseases. However, little is known regarding the mechanism of protection. In addition, several adverse consequences of overstimulation of this receptor have been reported, including gliosis (38), inhibition of neural function (17, 19, 39, 40), and altered developmental states (41–43). Therefore, specific knowledge of the mechanism for gp130-induced protection is essential for the development of neuroprotective therapies to avoid these side effects. Several studies have suggested that photoreceptor protection is mediated by gp130 activation in Müller cells through an indirect mechanism (44, 45). Evidence for this model has been based solely on activation of STAT3 and/or ERK1/2 signaling in Müller cells. This model suggests that Müller cells are activated by gp130 ligands and in response release other factors that would protect photoreceptors. We previously demonstrated that LIF activates signaling in all retinal cells, suggesting the possibility of direct protection by gp130 activation in photoreceptors (19). In our current study, loss of gp130 in only 30% of rod photoreceptors significantly impaired stress-induced photoreceptor protection (Figs. 5 A, C, and D, and 6). On the other hand, loss of gp130 activation in 50% of Müller cells (gp130f/f; VMD2-cre mice) did not affect endogenous protection (Fig. 5 B and C). These two observations suggest that protection through gp130 activation in Müller cells is not as critical as it is in rod photoreceptors. Other recent studies have shown that Müller cells are the source of LIF in stressed retinas (33, 34). Together, these studies suggest that Müller cells respond to an unknown stress signal to produce protective factors including LIF. LIF then directly activates gp130 on photoreceptors to induce their survival.
In summary, we provide direct evidence for the essential role of gp130 in stress-induced endogenous protection of photoreceptors. These data support the findings of recent studies showing that photoreceptors with inherited retinal degeneration die faster in the absence of LIF (33) or in the presence of a LIFR antagonist (28). The current study extends these findings by demonstrating that photoreceptor protection by gp130 activation is mediated by direct mechanism in photoreceptors. Thus, we hypothesize that photoreceptors under stress stimulate Müller cells to express LIF (33, 34), which then activates LIFR (28) and gp130 signaling in photoreceptors to induce protection. The mechanism by which stressed photoreceptors signal to Müller cells has not yet been identified. The stress-induced endogenous protection by gp130 activation may be playing an important role to help prolong neuronal survival in neurodegenerative and neuroinflammatory diseases in which neurons are under chronic stress.
Methods
Mice.
All animal procedures followed the guidelines of the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the IACUC at the University of Oklahoma Health Sciences Center and the Dean A. McGee Eye Institute. gp130flox/flox mice (20) were obtained and mated with Chx10-cre (Jackson Laboratory), rod-cre (22), or VMD2-cre (23) mice to generate gp130f/f; Chx10-cre+, gp130f/f; rod-cre+, or gp130f/f; VMD2-cre+ mice, respectively. VPP transgenic mice (29) were mated with gp130f/f; rod-cre+ to obtain rod-specific conditional gp130 KO carrying the VPP transgene. All mice used in this study were albino and are homozygous for the L450 variant of RPE65 (46).
Intravitreal Injection.
Recombinant human LIF (gp130 ligand) was produced in house and injected intravitreally as described in ref. 19. Briefly, 0.5 μg rLIF (1 μL final volume) was injected into one eye of 6- to 7-week-old mice to induce gp130-dependent signaling activation. PBS (vehicle) was injected in the other eye of each mouse as a control.
Immunohistochemistry.
Loss of STAT3 activation was used as a marker for loss of gp130. After 30 min and 1 day, the cornea and lens were removed from LIF- and PBS-injected retinas, and the eye cups were fixed with 2% paraformaldehyde/1× PBS for 30 min. Eye cups were then cryoprotected with sucrose solution (10%, 20%, and then 30%), and cross-sections of the retina were obtained by using a cryostat. Retinal cross-sections were immunostained by using the anti-pSTAT3 antibody (Cell Signaling Technology), and imaging was performed by using a FluoView FV500 confocal laser scanning microscope (Olympus). Imaging and microscope settings were identical in all samples.
Bright Light Preconditioning.
Unanesthetized mice were exposed to diffuse, cool, white fluorescent light coming from the top of the cage. Food and water were provided ad libitum, but were placed inside the cage to avoid blocking exposure to light. The light preconditioning model (Fig. 2) was used to induce endogenous protection (600 lux; 12 h on/12 h off for 6 days). Mice were then exposed to 3,000 lux light for 4 h to induce LD, which was followed by a 4-day recovery in 50-lux cyclic light (12 h on/12 h off).
Electroretinogram.
Photoreceptor function was measured by using a Colordome ERG instrument (Diagnosxys). After dark adaptation, mice were deeply anesthetized with a single i.p. injection of xylazine (7 mg/g) and ketamine (40 mg/g). Pupils were dilated with tropicamide and phenilepherine, and gold wire electrodes were placed centrally on the cornea to record full-field scotopic ERGs for both eyes.
Morphometric Analysis.
After the ERG recordings, mice were killed by CO2 asphyxiation, and the eyes were collected for histology. Conditional gp130 KO mice carrying the VPP transgene were collected at the postnatal weeks 2, 4, 6, and 8. Paraffin sections of the eye through the optic nerve head were stained with H&E. The number of photoreceptors lying in a single column spanning the outer nuclear layer (at 0.16-mm intervals) was counted under light microscopy.
Supplementary Material
Acknowledgments.
The authors thank the outstanding technical assistance from Louisa Williams and Linda Boone. This work is supported by National Eye Institute, National Institutes of Health Grant R01 EY016459, and core facilities provided by Grants P20 RR017703 and P30 EY012190. Additional funding was from an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906156106/DCSupplemental.
References
- 1.Ip NY, et al. Ciliary neurotrophic factor enhances neuronal survival in embryonic rat hippocampal cultures. J Neurosci. 1991;11:3124–3134. doi: 10.1523/JNEUROSCI.11-10-03124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheema SS, Richards L, Murphy M, Bartlett PF. Leukemia inhibitory factor prevents the death of axotomised sensory neurons in the dorsal root ganglia of the neonatal rat. J Neurosci Res. 1994;37:213–218. doi: 10.1002/jnr.490370207. [DOI] [PubMed] [Google Scholar]
- 3.Mittoux V, et al. Corticostriatopallidal neuroprotection by adenovirus-mediated ciliary neurotrophic factor gene transfer in a rat model of progressive striatal degeneration. J Neurosci. 2002;22:4478–4486. doi: 10.1523/JNEUROSCI.22-11-04478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji JZ, et al. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: The possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–1344. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao H, Hollyfield JG. Basic fibroblast growth factor in retinal development: Differential levels of bFGF expression and content in normal and retinal degeneration (rd) mutant mice. Dev Biol. 1995;169:168–184. doi: 10.1006/dbio.1995.1135. [DOI] [PubMed] [Google Scholar]
- 7.Gao H, Hollyfield JG. Basic fibroblast growth factor: Increased gene expression in inherited and light-induced photoreceptor degeneration. Exp Eye Res. 1996;62:181–189. doi: 10.1006/exer.1996.0022. [DOI] [PubMed] [Google Scholar]
- 8.Wen R, et al. Continuous exposure to bright light upregulates bFGF and CNTF expression in the rat retina. Curr Eye Res. 1998;17:494–500. doi: 10.1076/ceyr.17.5.494.5186. [DOI] [PubMed] [Google Scholar]
- 9.Samardzija M, et al. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20:2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- 10.Wen R, et al. Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J Neurosci. 1995;15:7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao W, et al. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp Eye Res. 1997;65:241–248. doi: 10.1006/exer.1997.0328. [DOI] [PubMed] [Google Scholar]
- 12.LaVail MM, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayouette M, et al. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaVail MM, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 15.Chong NH, et al. Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest Ophthalmol Vis Sci. 1999;40:1298–1305. [PubMed] [Google Scholar]
- 16.Liang FQ, et al. AAV-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol Ther. 2001;3:241–248. doi: 10.1006/mthe.2000.0252. [DOI] [PubMed] [Google Scholar]
- 17.Bok D, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, et al. Photoreceptor protection by cardiotrophin-1 in transgenic rats with the rhodopsin mutation s334ter. Invest Ophthalmol Vis Sci. 2003;44:4069–4075. doi: 10.1167/iovs.02-1130. [DOI] [PubMed] [Google Scholar]
- 19.Ueki Y, Wang J, Chollangi S, Ash JD. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008;105:784–796. doi: 10.1111/j.1471-4159.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- 20.Betz UA, et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955–1965. doi: 10.1084/jem.188.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowan S, Cepko CL. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev Biol. 2004;271:388–402. doi: 10.1016/j.ydbio.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Le YZ, et al. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- 23.Ueki Y, et al. Expression of Cre recombinase in retinal Muller cells. Vis Res. 2009;49:615–621. doi: 10.1016/j.visres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrich PC, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penn JS, Naash MI, Anderson RE. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987;44:779–788. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- 26.Kaldi I, et al. Bright cyclic rearing protects albino mouse retina against acute light-induced apoptosis. Mol Vis. 2003;9:337–344. [PubMed] [Google Scholar]
- 27.Li F, Cao W, Anderson RE. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Invest Ophthalmol Vis Sci. 2003;44:4968–4975. doi: 10.1167/iovs.03-0140. [DOI] [PubMed] [Google Scholar]
- 28.Chollangi S, et al. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol Dis. 2009;34:535–544. doi: 10.1016/j.nbd.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto Y, et al. Rod phototransduction in transgenic mice expressing a mutant opsin gene. J Opt Soc Am A Opt Image Sci Vis. 1996;13:577–585. doi: 10.1364/josaa.13.000577. [DOI] [PubMed] [Google Scholar]
- 30.Noell WK, Albrecht R. Irreversible effects on visible light on the retina: Role of vitamin A. Science. 1971;172:76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- 31.Penn JS, Anderson RE. Effect of light history on rod outer-segment membrane composition in the rat. Exp Eye Res. 1987;44:767–778. doi: 10.1016/s0014-4835(87)80040-4. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Cao W, Anderson RE. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp Eye Res. 2001;73:569–577. doi: 10.1006/exer.2001.1068. [DOI] [PubMed] [Google Scholar]
- 33.Joly S, et al. Leukemia inhibitory factor extends the lifespan of injured photoreceptors in vivo. J Neurosci. 2008;28:13765–13774. doi: 10.1523/JNEUROSCI.5114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgi S, Samardzija M, Grimm C. Endogenous leukemia inhibitory factor protects photoreceptor cells against light-induced degeneration. Mol Vis. 2009;15:1631–1637. [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, et al. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J Biol Chem. 2002;277:43319–43326. doi: 10.1074/jbc.M206355200. [DOI] [PubMed] [Google Scholar]
- 37.Rajala A, et al. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winter CG, Saotome Y, Levison SW, Hirsh D. A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc Natl Acad Sci USA. 1995;92:5865–5869. doi: 10.1073/pnas.92.13.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang FQ, et al. Long-term protection of retinal structure but not function using RAAV. CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- 40.Schlichtenbrede FC, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 41.Ezzeddine ZD, et al. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 42.Graham DR, Overbeek PA, Ash JD. Leukemia inhibitory factor blocks expression of Crx and Nrl transcription factors to inhibit photoreceptor differentiation. Invest Ophthalmol Vis Sci. 2005;46:2601–2610. doi: 10.1167/iovs.05-0129. [DOI] [PubMed] [Google Scholar]
- 43.Sherry DM, et al. Leukemia inhibitory factor inhibits neuronal development and disrupts synaptic organization in the mouse retina. J Neurosci Res. 2005;82:316–332. doi: 10.1002/jnr.20619. [DOI] [PubMed] [Google Scholar]
- 44.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 46.Wenzel A, et al. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.